Increased sucrose accumulation is required for regulating Fe deficiency responses in plants, with auxins acting downstream in transmitting the Fe deficiency signal.
Abstract
Previous studies have identified that auxins acts upstream of nitric oxide in regulating iron deficiency responses in roots, but the upstream signaling molecule of auxins remains unknown. In this study, we showed that Fe deficiency increased sucrose (Suc) level in roots of Arabidopsis (Arabidopsis thaliana). Exogenous application of Suc further stimulated Fe deficiency-induced ferric-chelate-reductase (FCR) activity and expression of Fe acquisition-related genes FRO2, IRT1, and FIT in roots. The opposite patterns were observed in the dark treatment. In addition, FCR activity and expression of Fe acquisition-related genes were higher in the Suc high-accumulating transgenic plant 35S::SUC2 but were lower in the Suc low-accumulating mutant suc2-5 compared with wild-type plants under Fe-deficient conditions. Consequently, Fe deficiency tolerance was enhanced in 35S::SUC2 but was compromised in suc2-5. Exogenous Suc also increased root β-glucuronidase (GUS) activity in auxin-inducible reporter DR5-GUS transgenic plants under Fe deficiency. However, exogenous Suc failed to increase FCR activity and expression of Fe acquisition-related genes in the auxin transport-impaired mutants aux1-7 and pin1-1 as well as in the wild-type plants treated with an auxin transport inhibitor under Fe deficiency. In summary, we found that increased Suc accumulation is required for regulating Fe deficiency responses in plants, with auxins acting downstream in transmitting the Fe deficiency signal.
Iron (Fe) is an essential micronutrient for living organisms. Despite the fact that Fe is abundant in soils, its bioavailability is often limiting for the growth of plants, especially in calcareous soils, which represent 30% of arable soils (Guerinot and Yi, 1994). To counteract lower Fe bioavailability, plants have evolved a range of responses to increase their capacity for Fe acquisition from soils. These responses are classified as strategy I in nongraminaceous monocots and dicots and strategy II in graminaceous monocots (Romheld and Marschner, 1986). In strategy I plants, Fe deficiency promotes increased reduction-based Fe uptake in roots by stimulating the activity of the plasma membrane ferric chelated reductase (FCR), which catalyzes the reduction of ferric iron chelates to Fe2+ (Robinson et al., 1999), and induces expression of a high-affinity ferrous Fe transporter (IRT1), which is responsible for transporting the Fe2+ into the root cells (Vert et al., 2002). Although the above Fe deficiency responses have been well documented, the signals involved in the regulatory cascade leading to their activation are not well understood to date. In tomato (Lycopersicon esculentum) plants, the basic helix-loop-helix (bHLH) transcription factor FER was identified to be a key component of root Fe signaling in response to Fe deficiency (Ling et al., 2002). Subsequently, FIT, a homolog of the tomato FER protein, was also shown to be essential for the regulation of Fe deficiency responses in Arabidopsis (Arabidopsis thaliana) plants (Colangelo and Guerinot, 2004; Jakoby et al., 2004). To function, FIT forms heterodimers with one of the four subgroups of Ib bHLH proteins: bHLH 038, bHLH 039, bHLH 100, and bHLH 101 (Yuan et al., 2008; Wu et al., 2012a; Wang et al., 2013).
In addition to the above transcription factors, we and others showed that the chemical molecules, auxins and nitric oxide (NO), both of which accumulate to higher levels in Fe-deficient plants, are also two general regulators controlling the initiation of adaptations to Fe deficiency in Strategy I plants (Graziano and Lamattina, 2007; Jin et al., 2009, 2011; Chen et al., 2010b; Bacaicoa et al., 2011; Wu et al., 2012b). The NO acts upstream of FIT to trigger Fe deficiency responses through a mechanism of reducing the proteasomal degradation of FIT (Meiser et al., 2011), while the elevation of auxin levels is required for the regulation of increased synthesis of NO under Fe-deficient conditions (Chen et al., 2010b; Jin et al., 2011). Therefore, we previously suggested an auxin→NO→FIT signaling cascade in transmitting Fe deficiency information (Chen et al., 2010b), but the identity of the upstream signal of auxins remains unknown. Suc might be a promising candidate, since the amount in roots is increased by Fe deficiency (Jiménez et al., 2011; Rellán-Álvarez et al., 2010) and it often functions as an auxin-like signaling molecule involved in regulating various physiological processes (León and Sheen, 2003; Rolland et al., 2006). In addition, numerous studies have shown that Suc regulates auxin biosynthesis, transport, and metabolism (Meir et al., 1985, 1989; Leclere et al., 2010; Lilley et al., 2012). Furthermore, our previous studies showed that elevated atmospheric carbon dioxide (CO2), which facilitates increased Suc production, significantly enhanced FCR activity and expression of FER, LeFRO1, and LeIRT1 in roots of Fe-deficient tomato plants compared with ambient CO2 (Jin et al., 2009). From these results, it is reasonable to assume that Suc may act as an upstream signal of auxins in modulating Fe deficiency responses in Strategy I plants.
In this study, we used Arabidopsis plants as a model system to investigate the above hypothesis. Our results revealed that increased Suc accumulation is required for the regulation of Fe deficiency responses in roots, with auxins acting downstream in transmitting the Fe deficiency information.
RESULTS
Suc Level in Roots Increased under Fe Deficiency and Exogenous Suc Enhanced Fe Deficiency Responses
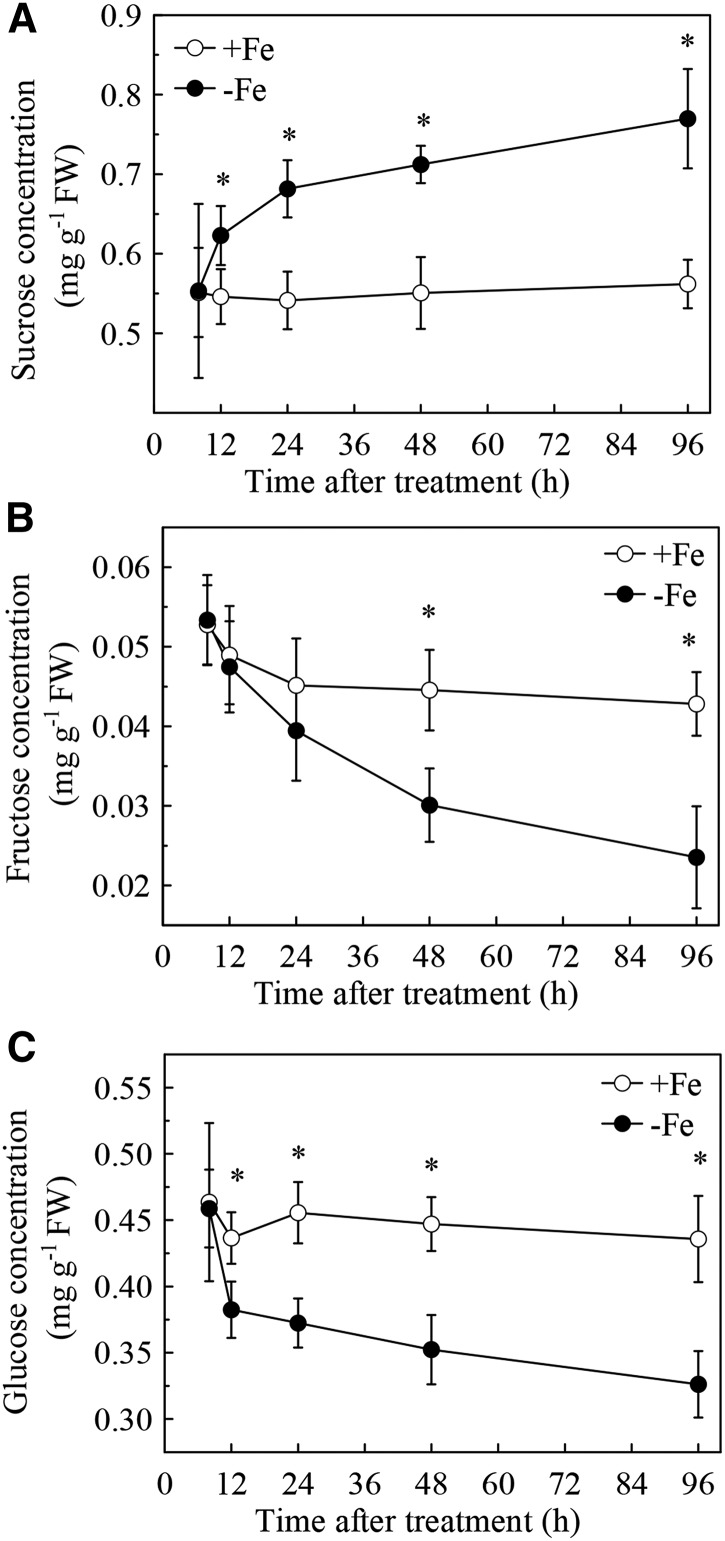
To study the role of Suc in Fe nutrition in plants, we first investigated the effect of Fe deficiency on the level of Suc in roots of Columbia ecotype (Col-0) Arabidopsis plants. As shown in Figure 1A, 12 h of treatment of Fe deficiency resulted in a clear increase in Suc levels in roots. After 12 h of treatment, this increase became progressively more pronounced. Another two sugars, Glc and Fru, are also often suggested to be signaling molecules and may also have an association with auxins in the regulation of various physiological processes in plants (Sairanen et al., 2012; Sheen, 2014; Yuan et al., 2014). However, the levels of Fru and Glc in roots were decreased after 48 h and 12 h of Fe deficiency treatment, respectively (Fig. 1, B and C), which indicated that Fe deficiency increased Suc accumulation in a relatively specific manner.
Figure 1.
Effect of Fe deficiency on sugar concentration in the roots of Col-0 in Arabidopsis. Five-week-old plants were grown in either complete (+ Fe) or Fe-free (-Fe) nutrient solutions. A, Concentrations of Suc, Fru (B), and Glc (C) in the roots were analyzed at 8, 12, 24, 48, and 96 h after each treatment. Data are expressed as mean ± sd (n = 4). An asterisk indicates significant differences between two treatments at each time point (one-way ANOVA, P < 0.05).
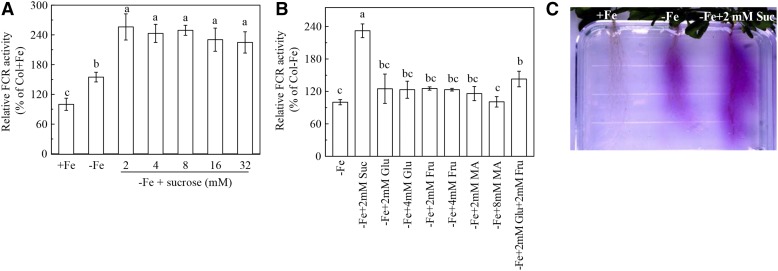
We then investigated the possible role of Suc in the regulation of Fe deficiency responses by studying the effect of exogenous Suc on root FCR activity. FCR is one of the most typical responses to Fe-deficient conditions in plants, and its activity is often used as a biomarker for Fe deficiency in plants (Chen et al., 2010b; Li et al., 2013). We found that 2 mm of Suc treatment was sufficient to significantly increase the FCR activity in Fe-deficient roots (Fig. 2A). A similar result was observed by using in vivo staining of Fe (II)-ferrozine complex in agar medium (Fig. 2C). However, application of Suc with concentrations >2 mm did not result in a further increase in FCR activity (Fig. 2A). Since Suc is an osmoticum for plant cells, we also investigated the effect of another well-known osmoticum, mannitol, on Fe deficiency-induced FCR activity. However, neither 4 mm nor 8 mm mannitol resulted in a significant effect on the FCR activity of roots during Fe deficiency (Supplemental Fig. S1). The result suggests that the Suc-increased FCR activity is not associated with an osmotic effect.
Figure 2.
Effects of carbon metabolites on the activity of root FCR in Col-0 Arabidopsis. The 5-week-old plants were grown in either complete (+ Fe) or Fe-free (- Fe) nutrient solutions. On day 3, the above nutrient solutions were supplied with or without various carbon metabolites, and the plants were continuously grown for 24 h. A, Relative root FCR activity of the plants in response to varying doses of exogenous Suc. B, Relative FCR activity in roots of the plants in response to various carbon metabolites. C, Visualization of FCR activity in roots with ferrozine in the agar medium. The concentrations of carbon metabolites used in experiments A and B are indicated in the figures. The concentration of Suc used in experiment C is 2 mM. Data are means ± sd (n = 5). Different letters indicate significant differences among treatments (one-way ANOVA, P < 0.05).
Besides being a signaling molecule, Suc also serves as an energy source in plant metabolism. In addition, it was proposed that activation of Fe deficiency responses may necessitate an increased demand for energy to keep these responses working at the necessary rates (Espen et al., 2000; Zocchi, 2006). Therefore, a question arises as to whether the Suc-mediated enhancement of FCR activity is attributed to an alteration in the signaling process or due to an increase in energy generation. Accordingly, we also examined the effects of other carbon metabolites, including Glc, Fru, and malic acid, on root FCR activity. Glc and Fru are hydrolysis products of Suc, while malic acid is one of the metabolites of Suc. These three compounds also serve as carbon resources to provide energy in plants. However, none of the above compounds was able to noticeably enhance the root FCR activity under Fe-deficient conditions (Fig. 2B). The above results indicate that the enhancement of root FCR activity by exogenous Suc in Fe-deficient plants is probably attributed to the signaling function of Suc rather than its energy function. Therefore, we examined the effect of a nonmetabolizable Suc analog, turanose, on root FCR activity. Treatment with 2 mm turanose also significantly increased root FCR activity under Fe deficiency, although this increase was less pronounced than that of the Suc treatment (Supplemental Fig. S1), providing evidence that the signaling function of Suc helps to enhance Fe deficiency-induced root FCR activity.
The effect of exogenous Suc on the expression of genes encoding FCR (AtFRO2) and the high-affinity ferrous Fe transporter (AtIRT1) were also measured. As shown in Supplemental Fig. S2, the transcript levels of the above two genes in roots were significantly up-regulated in the 2 mm Suc treatment under Fe-deficient conditions. This result, combined with the observation that exogenous Suc enhances root FCR activity in Fe-deficient plants, suggests that Fe deficiency responses may be integratively regulated by Suc.
Decreased Endogenous Suc by Dark Treatment or SUC2 Mutation Repressed Fe Deficiency Responses
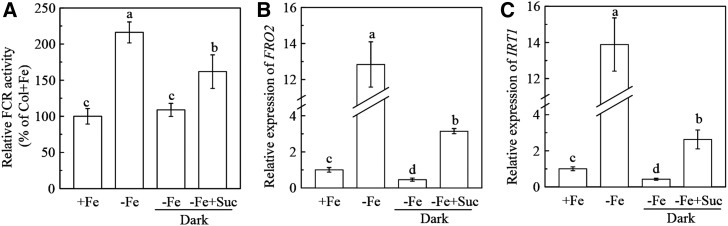
Given that exogenous Suc enhances Fe deficiency responses, it is of interest to test the effect of decreased endogenous Suc on Fe deficiency responses. Dark treatment has been widely used to decrease endogenous Suc in studies investigating Suc signaling behaviors in plants (Uemura and Steponkus, 2003; Price et al., 2004; Kozuka et al., 2005; Liu et al., 2005; Karthikeyan et al., 2007). Here we found that 24 h of dark growth resulted in a significant Suc decrease in roots of Fe-deficient plants (Supplemental Fig. S3). Consequently, following the dark treatment, root FCR activity and expression of AtIRT1 and AtFRO2 in Fe-deficient plants after 24 h of dark growth, were repressed to levels similar to or even lower than those in Fe-sufficient plants (Fig. 3). These repressions were partially reversed by exogenous Suc application (Fig. 3), which increased the root Suc level in the dark-grown plants under Fe deficiency (Supplemental Fig. S3). These results indicate that an increased level of endogenous Suc is required for the regulation of Fe deficiency responses.
Figure 3.
Effects of dark treatment and exogenous Suc application on Fe deficiency responses in roots of Col-0 Arabidopsis. The 5-week-old plants were cultured in either complete (+ Fe) or Fe-free (- Fe) nutrient solutions. On day 3, a part of the plants was covered using a black box, and the above nutrient solutions were supplied with or without 2 mm Suc and the plants were continuously grown for 24 h. A, Relative FCR activity. B, Expression of FRO2. C, Expression of IRT1. Gene expression was analyzed by real-time qPCR. Transcript level of UBQ10 was used as an internal control. Data are means ± sd (n = 7). Different letters indicate significant differences among treatments (one-way ANOVA, P < 0.05).
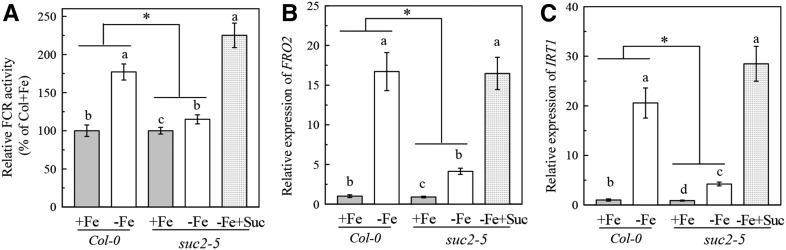
Previous studies showed that mutation of SUC2, a gene encoding a phloem-specific Suc transporter that controls the export of Suc from leaves to roots, results in a significant decrease in the Suc level in roots (Gottwald et al., 2000; Lei et al., 2011). Therefore, we also used the suc2 mutant to examine the effect of a decrease in endogenous Suc on Fe deficiency responses. Consistent with previous reports, the root Suc levels in T-DNA insertion mutant suc2-5 were significantly lower than those in Col-0 plants under both Fe-sufficient and Fe-deficient conditions (Supplemental Fig. S4). Although Fe deficiency also significantly up-regulated root FCR activity and expression of AtIRT1 and AtFRO2 in the suc2-5 mutant, these Fe deficiency responses were greatly compromised compared with the Col-0 plants. Nevertheless, exogenous application of 2 mm Suc to the suc2-5 mutant increased its root FCR activity and expression of AtIRT1 and AtFRO2 to levels comparable with or even more than those in Col-0 plants under Fe deficiency (Fig. 4, A–C). These results further support the notion that the elevation of endogenous Suc is required for the induction of Fe deficiency responses.
Figure 4.
Comparison of Fe deficiency responses in roots between Col-0 plants and the suc2-5 mutants. The plants were precultured as described in “Materials and Methods.” Then the 20-d-old plants were transferred to either complete (+Fe) or Fe-free (- Fe) agar medium supplied with or without 2 mm Suc for 5 d. A, Relative FCR activity. B, Expression of FRO2. C, Expression of IRT1. Gene expression was analyzed by real-time qPCR. Transcript level of UBQ10 was used as an internal control. Data are means ± sd (n = 5-7). Different letters indicate significant differences among treatments with in a genotype (one-way ANOVA, P < 0.05). An asterisk shows a significant genotype by treatment interaction (two-way ANOVA, P < 0.05).
Increased Endogenous Suc in 35S::SUC2 Transgenic Plants Lead to Intensified Fe Deficiency Responses
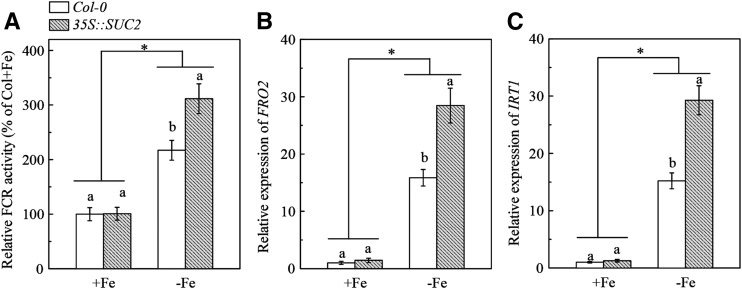
We then asked whether an increase in endogenous Suc had a positive effect on Fe deficiency responses in roots. Therefore, we used 35S::SUC2 transgenic plants that overexpress the SUC2 gene to address the above question. A significantly higher accumulation of Suc in roots was observed in 35S::SUC2 transgenic plants compared with the Col-0 plants under both Fe-sufficient and Fe-deficient conditions (Supplemental Fig. S5). As a result, root FCR activity and expression of AtIRT1 and AtFRO2 in 35S::SUC2 transgenic plants were significantly higher than those in Col-0 plants in response to Fe deficiency (Fig. 5), suggesting that increase in endogenous Suc facilitates the up-regulation of Fe deficiency responses. Nevertheless, in Fe-sufficient conditions, the 35S::SUC2 transgenic plant did not show higher FCR activity or increased expression of AtIRT1 and AtFRO2 in roots compared with the Col-0 plants (Fig. 5). These results provided further evidence that an Fe-deficient condition of plants is a prerequisite for Suc regulating the Fe deficiency responses in roots.
Figure 5.
Comparison of Fe deficiency responses in roots between Col-0 plants and the 35S::SUC2 transgenic plants. The indicated plants were treated as in Figure 1. A, Relative FCR activity. B, Expression of FRO2. C, Expression of IRT1. Gene expression was analyzed by real-time qPCR. Transcript level of UBQ10 was used as an internal control. Data are means ± sd (n = 5-7). Different letters indicate significant differences between two genotypes with in a treatment (one-way ANOVA, P < 0.05). An asterisk shows a significant genotype by treatment interaction (two-way ANOVA, P < 0.05).
Regulation of Fe Deficiency Tolerance by Suc
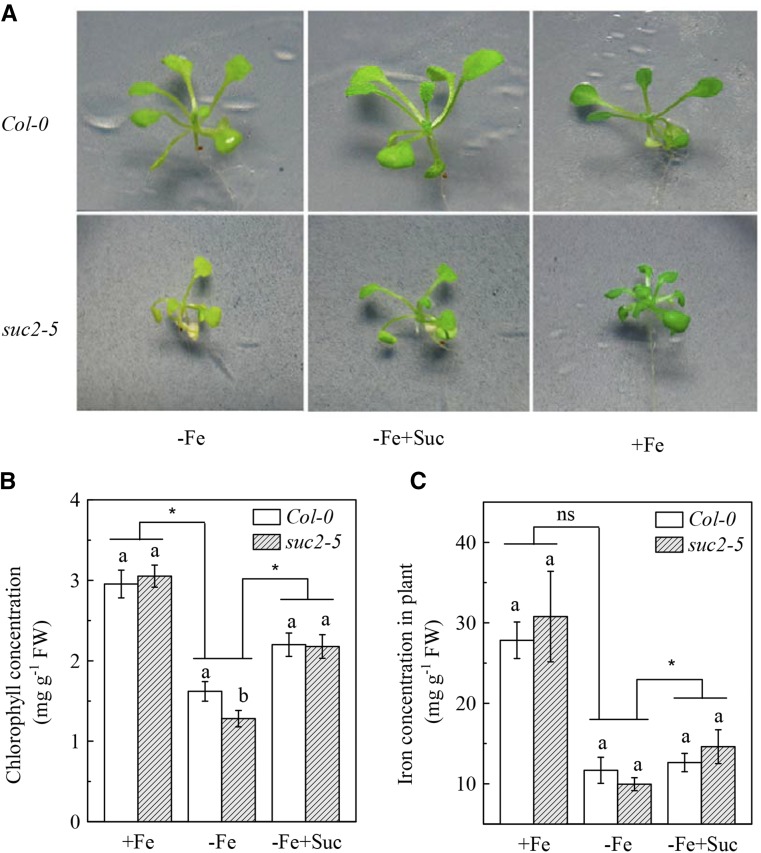
As already mentioned above, the iron uptake of strategy I plants primarily depends on the reduction of ferric iron chelates by FCR and the subsequent acquisition of the resulting Fe2+ into root cells by the IRT1 transporter (Robinson et al., 1999; Vert et al., 2002). Accordingly, the regulatory role of Suc in up-regulation of Fe deficiency responses indicates that the increase in endogenous Suc may also be required for regulating Fe deficiency tolerance in Arabidopsis plants. We compared chlorosis symptoms of leaves between Col-0 plants and the Suc low-accumulating mutant suc2-5. As shown in Figure 6A, after 8-d growth in Fe-deficient medium, the leaves of suc2-5 plants exhibited more severe chlorosis symptoms than those of the Col-0 plants; consistent with this result, the chlorophyll concentration in leaves of the suc2-5 plants was significantly lower than that of the Col-0 plants (Fig. 6B). In addition, Fe concentration in suc2-5 plants was also lower, although not statistically significant, than that in Col-0 plants under Fe deficiency (Fig. 6C). However, in Fe-sufficient conditions, the leaf chlorophyll concentrations and Fe level were similar between Col-0 and suc2-5 plants (Fig. 6A). Application of Suc to the Fe-deficient growth medium increased the Fe concentration in suc2-5 plants (Fig. 6C). As a result, leaf chlorosis in Fe-deficient suc2-5 plants was clearly ameliorated by the Suc application (Fig. 6, A and B). These results indicate that the Fe deficiency tolerance of plants is regulated by Suc.
Figure 6.
Fe deficiency tolerance of Col-0 plants and the suc2-5 mutants. The indicated plants were treated as in Figure 4. A, Photographs of shoots. B, Chlorophyll concentration of leaves. C, Fe level of plants. Data are means ± sd (n = 5). Different letters indicate significant differences between two genotypes with in a treatment (one-way ANOVA, P < 0.05). Asterisk and ns indicate that the genotype by treatment interactions are significant and not significant, respectively (two-way ANOVA, P < 0.05).
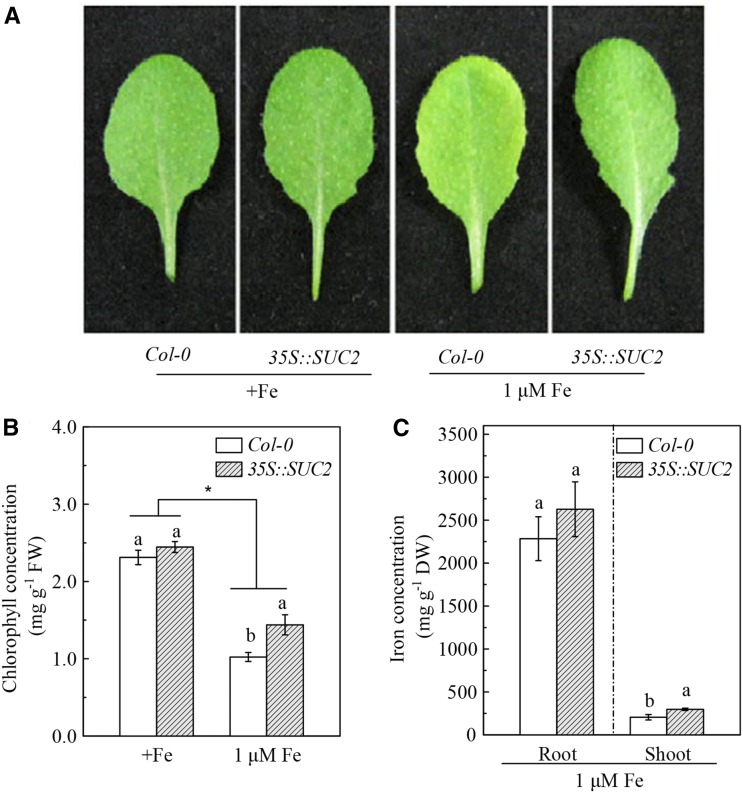
We also examined the Fe deficiency tolerance in the Suc high-accumulating transgenic plant 35S::SUC2. After 10-d growth in Fe-limited growth conditions (1 μM), both Col-0 and 35S::SUC2 plants developed chlorotic symptom in leaves, whereas the symptoms in the 35S::SUC2 plants were less pronounced than those in the Col-0 plants (Fig. 7A). This result was confirmed by the measurement of chlorophyll concentration (Fig. 7B). In accordance with the chlorotic phenotype, the Fe concentration in shoots and roots was also higher in 35S::SUC2 plants than in Col-0 plants in the Fe-limited growth condition, although the root Fe concentration did not show any statistically significant difference between these two plants (Fig. 7C). However, when the plants were grown with Fe-sufficient medium, the chlorophyll concentrations in both Col-0 and 35S::SUC2 plants were higher and were also nearly the same between the two plants (Fig. 7B). Collectively, the above results further confirmed that Suc plays an important role in the regulation of Fe deficiency tolerance in plants.
Figure 7.
Fe deficiency tolerance of Col-0 plants and the 35S::SUC2 transgenic plants. The 5-week-old plants were grown in nutrient solutions containing either 50 μm (+Fe) or 1 μm Fe-EDTA for 10 d. A, Photographs of leaves. B, Chlorophyll concentration of leaves. C, Fe level in roots and shoots. Data are means ± sd (n = 5). Different letters indicate significant differences between two genotypes with in a treatment (one-way ANOVA, P < 0.05). An asterisk shows a significant genotype by treatment interaction (two-way ANOVA, P < 0.05).
Suc-Regulating Induction of Fe Deficiency Responses Required the FIT Protein
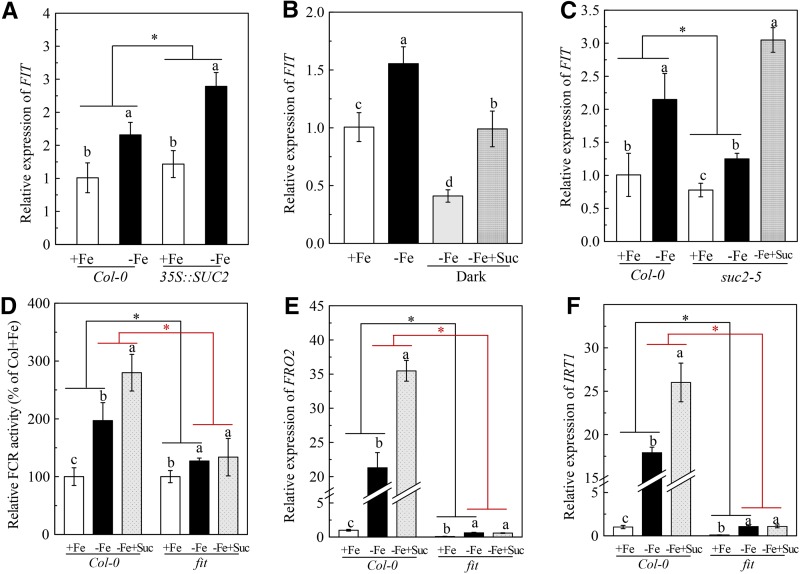
Since the bHLH transcription factor FIT is a master regulator of Fe deficiency responses in Arabidopsis (Colangelo and Guerinot 2004; Jakoby et al., 2004), it is important to understand the linkage between Suc and FIT in regulating Fe deficiency responses. We investigated the role of Suc in regulation of AtFIT expression. As shown in Supplemental Figure S2C, Fe deficiency significantly elevated the AtFIT expression in roots of Col-0 plants compared with plants under Fe-sufficient conditions, and this elevation was further increased by the application of 2 mm Suc. In addition, the Fe deficiency-induced AtFIT expression in roots was also higher in Suc high-accumulating transgenic plants 35S::SUC2 than in Col-0 plants (Fig. 8A). On the contrary, the AtFIT expression in Fe-deficient Col-0 plant was inhibited by the 24-h dark treatment (Fig. 8B). Furthermore, although Fe deficiency also increased the AtFIT expression in the Suc low-accumulating mutant suc2-5, the increase was clearly less than that in Col-0 plants (Fig. 8C). The application of 2 mm Suc to the dark-grown Col-0 plants and the suc2-5 mutants significantly increased the AtFIT expression in their roots under Fe deficiency (Fig. 8B). These results suggest that Suc also plays an important role in regulating AtFIT expression under Fe deficiency. However, under Fe-sufficient conditions, application of 2 mm Suc had little effect on the expression of AtFIT expression in roots of Col-0 plants (Supplemental Fig. S2C).
Figure 8.
The role of FIT in exogenous Suc regulation of Fe deficiency responses in roots. A, Comparison of FIT expression between Col-0 plant and the 35S::SUC2 transgenic plants. B, Effects dark treatment on FIT expression in Col-0 plant. C, Comparison of FIT expression between Col-0 plants and the suc2-5 mutants. D-F, Effects of exogenous Suc on FCR activity and the expression of FRO2 and IRT1 in Col-0 plants and the fit mutants. For the experiments in A-C, the plants were treated as in Figures 5, 3, and 4, respectively. For the experiments in D-F, the plants were treated as in Figure 2C. Gene expression was analyzed by real-time qPCR. Transcript level of UBQ10 was used as an internal control. Data are means ± sd (n = 5-7). Different letters indicate significant differences among treatments with in a genotype (one-way ANOVA, P < 0.05). An asterisk shows a significant genotype by treatment interaction (two-way ANOVA, P < 0.05).
We then investigated the role of FIT in the process of Suc regulation of root FCR activity and expression of AtIRT1 and AtFRO2 under Fe deficiency. The fit mutant was used to address this issue. In agreement with previous reports (Séguéla et al., 2008; Maurer et al., 2011), induction of root FCR activity and expression of AtIRT1 and AtFRO2 by Fe deficiency were severely compromised in the fit mutant compared with those in Col-0 plants (Fig. 8, D-F). The application of 2 mm Suc failed to increase root FCR activity and expression of AtIRT1 and AtFRO2 in the fit mutant under Fe deficiency, which was different from the results observed in Col-0 plants (Fig. 8, D-F), indicating that the process of Suc regulation of the Fe deficiency response depends on the FIT function.
Another bHLH transcription factor, POPEYE (PYE), has been previously characterized as a key regulator in controlling iron homeostasis in plants (Long et al., 2010). Therefore, it is interesting to clarify whether there is a link between Suc and PYE in the regulation of Fe deficiency responses. As shown in Supplemental Figure S6, although Fe deficiency increased the expression of PYE in roots of Col-0 plants compared with the Fe-sufficient treatment, this increase was not affected by the application of Suc. Furthermore, the Suc treatment significantly enhanced the activity of FCR and the expression of AtIRT1 and AtFRO2 in the roots of pye-1 mutants under Fe-deficient conditions, which is similar to patterns observed in Col-0 plants (Supplemental Fig. S7). Therefore, the Suc-mediated regulation of Fe deficiency responses is probably independent of PYE. We also investigated the effect of Suc application on the expression of three PYE-regulated genes, FRO3, NAS4, and ZIF1, which are known to encode for proteins involved in metal ion homeostasis (Long et al., 2010). The expression of these three genes was induced by Fe deficiency, but Suc application did not affect induction (Supplemental Fig. S6), indicating that the Suc-conferred tolerance of Fe deficiency may not be related to PYE.
Suc-Modulated Fe Deficiency Responses Depend on the Auxin Signaling Process
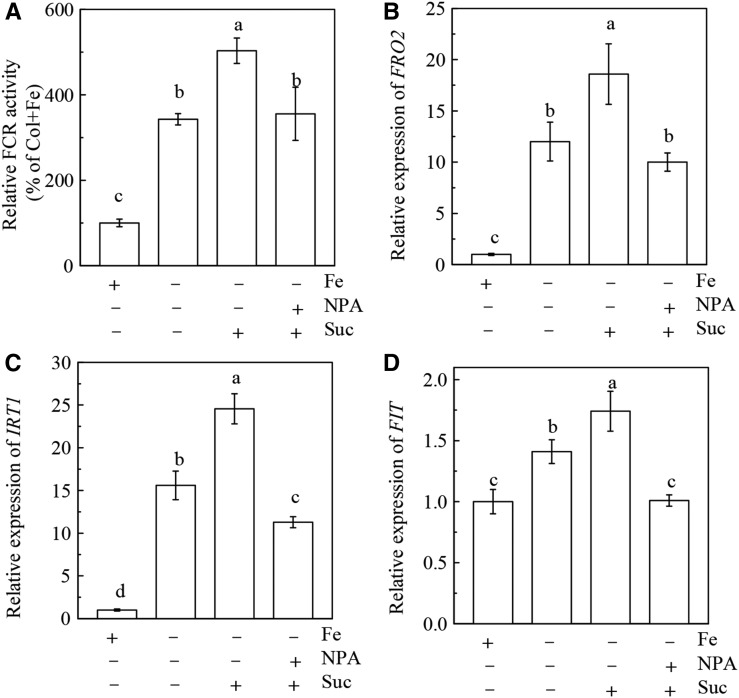
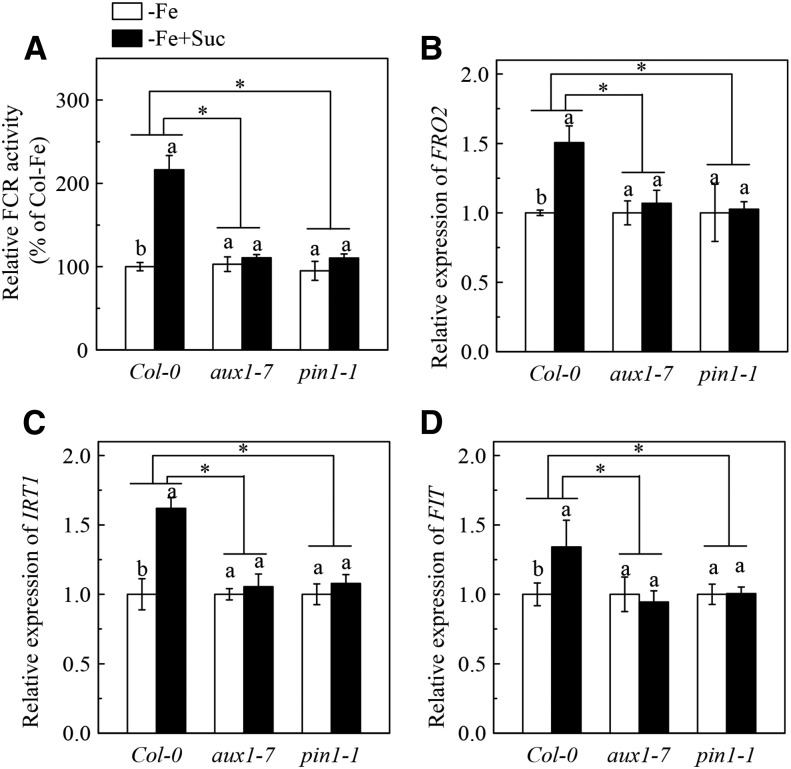
Studies have shown that Suc positively regulates auxin accumulation in plants in normal growth conditions (Meir et al., 1985, 1989; LeClere et al., 2010; Lilley et al., 2012). Auxin-inducible reporter DR5-GUS transgenic plants are frequently used to indicate the level of auxins in plant tissues (Chen et al., 2010a; Song et al., 2013). We found that the GUS staining in roots was more intense in Fe-deficient conditions than in Fe-sufficient conditions, indicating that Fe deficiency increases auxin levels in roots (Supplemental Fig. S8). The result is consistent with previous reports (Chen et al., 2010b; Jin et al., 2011). Application of 2 mm Suc further increased the GUS staining in roots of Fe-deficient plants. These results suggest that Suc may also have a positive effect on auxin accumulation in plants under Fe-deficient growth conditions. Since previous studies have shown that auxins play a critical role in the regulation of Fe deficiency responses (Chen et al., 2010b; Jin et al., 2011; Wu et al., 2012b), it is therefore necessary to clarify whether auxins have an association with Suc in the regulation of Fe deficiency responses. As shown in Figure 9, in the presence of the auxin transport inhibitor NPA, the stimulating effect of Suc on root FCR activity and expression of IRT1, FRO2, and FIT in Fe-deficient Col-0 plants were completely terminated. Furthermore, in two auxin transport mutants, aux1-7 and pin1-1, application of 2 mm had little effect on root FCR activity and expression of AtFRO2, AtIRT1, and AtFIT under Fe-deficient conditions (Fig. 10). This result is in contrast with the results from the Col-0 plants and suggests that the regulation of Fe deficiency responses by Suc requires the signaling function of auxins. However, in the Fe-deficient suc2-5 mutant, application of the auxin analog 1-naphthylacetic acid could increase the FCR activity (Supplemental Fig. S9). This effect is similar with the results obtained in Col-0 plants, indicating that the regulation of Fe deficiency responses by auxins does not depend on Suc. Collectively, we conclude that Suc acts upstream of auxins to regulate Fe deficiency responses.
Figure 9.
Effects of exogenous Suc on Fe deficiency responses in roots of Col-0 plant treated with auxin transport inhibitor. The 5-week-old plants were cultured in either complete (+ Fe) or Fe-free (- Fe) nutrient solution with or without addition of auxin transport inhibitor NPA (5 μM). On day 3, the above nutrient solutions were supplied with or without 2 mm Suc, and the plants were continuously grown for 24 h. A, Relative FCR activity. B-D, Expression of FRO2, IRT1, and FIT. Gene expression was analyzed by real-time qPCR. Transcript level of UBQ10 was used as an internal control. Data are means ± sd (n = 5-7). Different letters indicate significant differences among treatments (one-way ANOVA, P < 0.05).
Figure 10.
Effect of exogenous Suc on Fe deficiency responses in roots of Col-0 plant and the aux1-7 and pin1-1 mutants. The 5-week-old plants were grown in Fe-free (- Fe) nutrient solution. On day 3, the above nutrient solution was supplied with or without 2 mm Suc, and the plants were continuously grown for 24 h. A, Relative FCR activity. B-D, Expression of FRO2, IRT1, and FIT. Gene expression was analyzed by real-time qPCR. Transcript level of UBQ10 was used as an internal control. Data are means ± sd (n = 5-7). Different letters indicate significant differences between two treatments with in a genotype (one-way ANOVA, P < 0.05). An asterisk shows a significant genotype by treatment interaction (two-way ANOVA, P < 0.05).
DISCUSSION
Identification of regulators to bridge the gap between the Fe-deficient status and the induction of specific root responses is crucial if we wish to improve growth of crops in marginal soils, where Fe deficiency frequently limits crop growth (Walker and Connolly, 2008). Therefore, in recent decades, plant nutritionists have made great efforts to understand the signaling process in transmitting Fe deficiency information. As already mentioned, several bHLH proteins and chemical molecules have been identified to be the components involved in the above process. In this study, we revealed that Suc is also a regulator involved in the regulation of Fe deficiency responses in roots, namely that Fe deficiency results in an increase in Suc accumulation in roots, which consequently up-regulates the FCR activity and the expression of Fe acquisition-related genes through an auxin-dependent manner.
Suc is a carbohydrate compound and was originally recognized as an energy source for metabolism in plants. However, recently, several studies showed that Suc also functions as a signaling molecule involved in regulation of various physiological processes in plants such as root growth, fruit development and ripening, and hypocotyl elongation (Kircher and Schopfer, 2012; Lilley et al., 2012; Jia et al., 2013; Ruan, 2014). In this study, we found that Fe deficiency-induced root FCR activity and expression of FRO2 and IRT1 were significantly further up-regulated by exogenous Suc (Fig. 2; Supplemental Fig. S2). In addition, elevation of endogenous Suc levels in roots by overexpression of SUC2 also increased the above Fe deficiency responses, whereas a decrease in endogenous Suc by dark treatment or by mutation of SUC2 repressed the Fe deficiency responses (Fig. 4 and Fig. 5). Rhizosphere acidification mediated by proton ATPase AHA2 is another important Fe deficiency response (Santi and Schmidt, 2009). Interestingly, exogenous Suc treatment significantly stimulated the activity of proton ATPase by inducing the phosphorylation of AHA2 proteins (Niittylä et al., 2007). Therefore, we conclude that Suc plays a critical role in the regulation of Fe deficiency responses. Since Suc is the major sugar that plants assimilate in photosynthesis and transport to various nonphotosynthetic tissues, its accumulation in plant tissues could be affected by stress conditions. Interestingly, several recent studies have suggested that Suc could also act in regulation of stress responses of some other nutrients (Liu et al., 2009). For instance, increased accumulation of Suc in roots with deficiency of either nitrate or phosphate is a critical requirement for the adaptation of plants to these stresses (Hermans et al., 2006; Hammond and White, 2008). In addition, exogenous Suc not only up-regulates the expression of genes involved in nitrate and phosphate uptake in plants but also increases the uptake of the corresponding nutrients (Lejay et al., 2003; Karthikeyan et al., 2007). These results, combined with our present finding, demonstrate that the requirement of Suc in response to nutrient deficiencies to coordinate the nutrient uptake activity in roots is likely shared by different nutrients (Lejay et al., 2003; Liu et al., 2009).
As already mentioned, Suc not only acts as an energy source in metabolism but also functions as a signaling molecule (Dasgupta et al., 2014). However, it seems that the signaling function rather than the energy function is the mechanism for Suc regulating Fe deficiency responses, on the basis of the following five findings: (1) exogenous application of Glc, Fru, and malic acid, which are downstream metabolites of Suc and could also serve as carbon resources to provide energy, failed to have a noticeable increase in root FCR activity under Fe deficiency; (2) Fe deficiency relatively specifically increased the Suc level in roots but decreased the levels of Glc and Fru, which is consistent with the findings of Rellán-Álvarez et al. (2010) and Jiménez et al. (2011) in Adesoto (Prunus insititia) and Beta vulgaris plants; (3) the time-course expression of FIT, FRO2, and IRT1 correlated well with the time-course accumulation of Suc in roots during Fe-deficient treatment (Fig. 1A; Supplemental Fig. S10); (4) exogenous Suc application >2 mm did not result in a further increase in the root FCR activity of Fe-deficient plants compared with the 2-mm Suc treatment (Fig. 2A); and (5) application of the nonmetabolizable Suc analog, turanose, also increased root FCR activity under conditions of Fe deficiency (Supplemental Fig. S1), although this increase was less pronounced than that of the Suc treatment, which most likely owes to the fact that Suc enters plant tissues more efficiently than turanose via Suc transporters (Chandran et al., 2003). In fact, Suc-specific signaling has already been suggested to play a critical role in various physiological processes (Chiou and Bush, 1998; Nagaraj et al., 2001; Solfanelli et al., 2006; Tognetti et al, 2013). For instance, it was demonstrated that the starch synthase was specifically induced by Suc and not by its downstream metabolites in sweet potato (Wang et al., 2001). Likewise, sugar-dependent up-regulation of the anthocyanin and fructan synthesis pathway is Suc specific, as the efficiency of Glc or Fru on the synthesis of these compounds is much lower than that of Suc (Nagaraj et al., 2001; Solfanelli et al., 2006). It is worth noting that since the disaccharide turanose could increase the Fe deficiency-induced FCR activity (Supplemental Fig. S1), other disaccharides that are structurally similar to Suc may also have a role in the regulation of Fe deficiency responses. It is necessary to clarify this assumption in future studies.
The up-regulation of root FCR activity and expression of FRO2 and IRT1 might be expected to increase the Fe acquisition of roots, thus strengthening the plant tolerance to Fe deficiency. The fact that there was more leaf chlorosis in the Suc low-accumulating mutant suc2-5 (Fig. 6A), but less leaf chlorosis in the Suc high-accumulating transgenic plant 35S::SUC2 than in the wild-type plant under Fe-limited growth conditions (Fig. 7A), provided evidence that increases in endogenous Suc in roots facilitates plant tolerance to Fe deficiency. Nevertheless, although there is a distinct difference in Fe deficiency-induced leaf chlorosis among the above three plants, the Fe levels in these plants did not differ noticeably. One explanation is that the Fe level used in this study is very low and can be quickly depleted from the growth medium. This may be somewhat similar to the phenomenon observed in the study of Graziano and Lamattina (2007) where the increased NO in roots of tomato plants by GSNO (a NO donor) clearly enhanced both FCR activity and LeFRO1 and LeIRT1 expression and thus alleviated leaf chlorosis under extremely low Fe conditions (0.1 μm Fe-EDTA) but did not contribute to a noticeable increase in the Fe level in plant tissues. However, in our previous study, also in tomato plants, the increased root NO levels under elevated CO2 not only enhanced the Fe deficiency responses but also resulted in a clear increase in Fe levels in plants tissues when the plants were grown with a hydrous Fe(III)-oxide medium that could continuously release low levels of soluble Fe for plant growth (Jin et al., 2009). Therefore, if the above explanation is correct, then the Suc-regulated Fe deficiency response may also significantly confer a noticeable improvement in Fe nutrition in plants grown in calcareous soils that have low levels of soluble Fe that could also be continuously released from the Fe (III)-oxide minerals.
A question arises as to how Suc regulates the Fe deficiency responses. Thus far, several components have been proposed to be involved in various Suc signaling processes. For instance, the protein kinase Suc nonfermenting related kinase-1 (SnRK1) is required for Suc signal transduction, leading to starch synthesis and Suc synthase induction in potato (Tiessen et al., 2003); the REDUCED SUC RESPONSE 1 (RSR1) was identified as a critical factor for Suc signaling during seed germination and early seedling development (Funck et al., 2012); the SUC UNCOUPLED 6 (SUN6) is involved in the Suc-mediated down-regulation of photosynthesis genes (Dijkwel et al., 1997); and the IMPAIRED SUC INDUCTION 4 (ISI4) is required for Suc-mediated growth inhibition (Laby et al., 2000). Here, we investigated whether the regulation of Fe deficiency responses by Suc was associated with the aforementioned Suc signaling components by using snrk1.1, snrk1.2, rsr1, sun6, and isi4 mutants. However, the results showed that in the latter four mutants, FCR activity and the expression of Fe uptake-related genes were induced by Fe deficiency and were further enhanced by exogenous Suc, similar to the results observed in Col-0 plants (Supplemental Fig. S11). This indicates that the Suc-mediated regulation of Fe deficiency responses may not be associated with SnRK1.2, RSR1, SUN6, and ISI4. Interestingly, we found that the Fe-deficient treatment induced less FCR activity and Fe uptake-related gene expression in snrk1.1 mutants compared with those in Col-0 plants. Therefore, it is possible that SnRK1.1 may be involved in the regulation of Fe deficiency responses. Nevertheless, the effects of exogenous Suc on the above Fe deficiency responses in snrk1.1 mutants were not significantly different from those in Col-0 plants, suggesting that the Suc-mediated regulation of Fe deficiency responses may be independent of SnRK1.1.
It is worth noting that the activation of Fe deficiency responses by Suc requires a low level of Fe to be effective, because neither the exogenous Suc nor the increase of endogenous Suc by overexpression of SUC2 could affect root FCR activity and expression of FRO2 and IRT1 under Fe-sufficient conditions (Fig. 5; Supplemental Fig. S2). This regulation manner is similar to that of auxins and NO, both of which also require an Fe-deficient status to up-regulate the FCR activity and expression of FRO2 and IRT1 (Graziano and Lamattina, 2007; Chen et al., 2010b). In addition, by measuring the effects of exogenous Suc, darkness treatment, and modulations of SUC2 expression on the expression of FIT in roots, we showed that Suc is also required for the up-regulation of FIT under Fe-deficient conditions (Fig. 8A-C). Nevertheless, exogenous Suc had little effect on root FCR activity and expression of FRO2 and IRT1 in Fe-deficient fit mutants (Fig. 8D-F), indicating that Suc-mediated enhancement of Fe deficiency responses was FIT dependent. This regulation pattern is also similar to those of auxins and NO, neither of which had a stimulation effect on the Fe deficiency responses in fit mutants (Graziano and Lamattina, 2007; Chen et al., 2010b). The above two similarities indicate that Suc, auxins, and NO may have synergistic effects on the regulation of Fe deficiency responses. As stated above, several studies have shown that Suc has positive effects on auxin accumulation. For instance, Suc stimulated the degradation of auxin conjugates, which lead to an increase in the auxin level (Meir et al., 1985; Meir et al., 1989); Suc positively regulated the expression of auxin biosynthesis gene YUCCA, consequently increasing auxin synthesis (LeClere et al., 2010; Lilley et al., 2012); Suc also enhanced root-ward auxin transport (Lilley et al., 2012). Therefore, we intended to demonstrate a clear association between Suc and auxins in the regulation of Fe deficiency responses. We found that exogenous Suc elevated the root auxin level under Fe deficiency (Supplemental Fig. S8). However, when the root-ward auxin transport was impaired by pharmacological methods or mutation of auxin transport genes, the exogenous Suc failed to increase FCR activity and expression of IRT1 and FRO2 in roots under Fe-deficient conditions (Fig. 9 and Fig. 10). These results indicate that the process of Suc regulation of Fe deficiency responses depends on the auxin signaling, i.e. Suc acts upstream of auxins to regulate the Fe deficiency responses.
The question of how the Fe deficiency increases the Suc level in roots remains open. Theoretically, the level of Suc in roots should be controlled by Suc translocation from shoots to roots and by enzymatic reactions responsible for Suc metabolism in roots. The Suc symporter, SUC2, is necessary for efficient transport of Suc from mature leaves (source) to sink tissues (roots and young leaves) in Arabidopsis (Lalonde et al.., 2004). Interestingly, real-time quantitative PCR (qPCR) analysis showed that the expression of SUC2 in mature leaves was continuously induced by Fe deficiency (Supplemental Fig. S12), indicating that Fe deficiency may elevate the SUC2-mediated transport of Suc from mature leaves to sink tissues. This assumption was supported by the observation that Fe deficiency decreased Suc concentration in mature leaves and increased Suc concentration in young leaves and roots (Supplemental Fig. S1 and Supplemental Fig. S13B). In addition, we observed that Fe deficiency increased the intensity of GUS staining in the young leaves and roots of DR5::GUS transgenic plants (Supplemental Fig. S8 and Supplemental Fig. S13A). These observations are consistent with the previous finding that Suc facilitates auxin accumulation (Lilley et al., 2012). Therefore, we suggested that Fe deficiency increased the SUC2-mediated transport of Suc from mature leaves to young leaves and roots, thus resulting in an increase of auxin levels in roots, which ultimately enhanced the Fe deficiency responses in the epidermis of the root. We also investigated the effects of Fe deficiency on the activities of Suc synthase and neutral cytosolic invertase, which have been reported to play an important role in regulating Suc fluxes in sink tissues (Koch, 2004; Qazi et al., 2012). However, Fe deficiency had little effect on the activities of these two enzymes, suggesting that neither Suc synthase nor neutral cytosolic invertase confer Fe deficiency-induced Suc accumulation in roots.
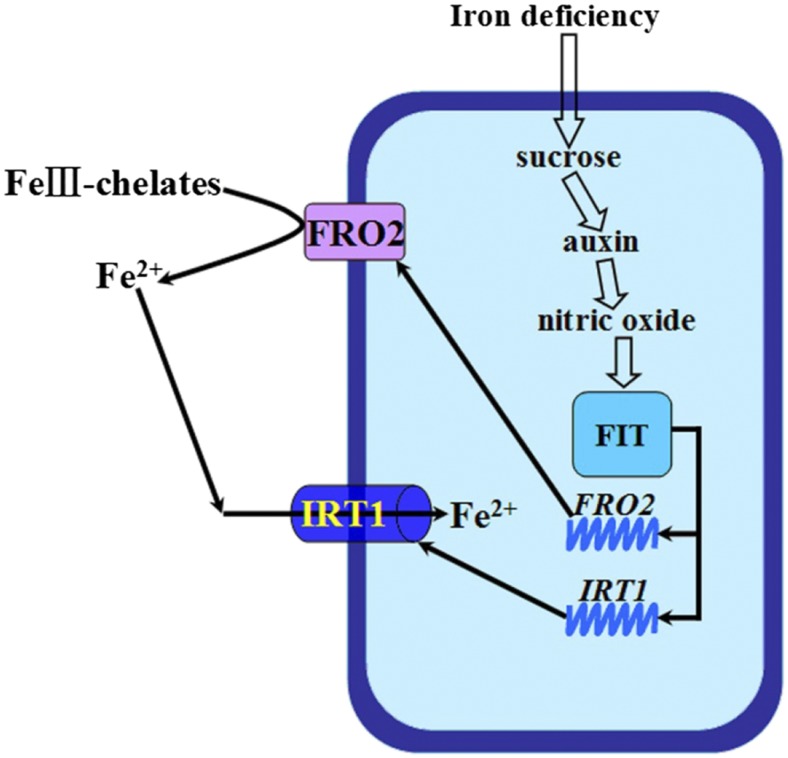
In conclusion, although previous studies have identified an auxin→NO→FIT signaling cascade in transmitting Fe deficiency information (Chen et al., 2010b), the upstream signal that controls auxins remains unknown. Our results indicate that Suc acts upstream of auxins in regulating Fe deficiency responses in a FIT-dependent manner. By combining the results of this study and previous studies, we proposed the following model: Fe deficiency increases Suc in roots, which elevates auxin levels in roots and subsequent induction of NO accumulation, thereby activating reduction-based Fe uptake via the FIT-mediated transcriptional regulation of FRO2 and IRT1 (Fig. 11)
Figure 11.
Schematic model of Suc in the regulation of Fe deficiency responses.
MATERIALS AND METHODS
Plant Material
The mutants suc2-5 (SALK_087046), fit, pin1-1 and aux1-7, snrk1.1 (CS86777), snrk1.2 (SALK_139618C), rsr1, isi4 (CS6147), sun6 (CS8104) and the transgenic plant lines 35S::SUC2 and DR5::GUS were on the Col-0 background. The SALK_087046, SALK_139618C, CS86777, CS6147, and CS8104 seeds were purchased from the Arabidopsis Biological Resource Center (ARBC). The insertion in SALK lines were verified using the primers listed in Supplemental Table S1. The pin1-1, rsr1, pye-1 and 35S::SUC2 seeds were, respectively, a kind gift from Dr. Yong Song Zhang (Zhejiang University, China), Dr. Dietmar Funck (University Konstanz, Germany), Dr. Terri A. Long (NC State University) and Dr. Dong Liu (Tsinghua University, China). The aux1-7 and DR5::GUS seeds were kindly provided by Dr. Philippe Nacry (Biochimie et Physiologie Moléculaire des Plantes, France).
Plant Culture
The seeds were germinated on a nylon net that was suspended in a one-half strength nutrient solution. The nutrient solution had the following composition: NaH2PO4 750 μM, MgSO4 500 μM, KNO3 3,000 μM, CaCl2 1,000 μM, H3BO3 10 μM, MnSO4 0.5 μM, ZnSO4 0.5 μM, CuSO4 0.1 μM, (NH4)6Mo7O24 0.1 μM, and Fe-EDTA 50 μM, pH 5.5. On day 7, uniform seedlings were transferred to sand supplemented with complete nutrient solution. After 10 d, batches of four seedlings were transplanted into 0.3-L pots filled with complete nutrient solution, which was renewed daily. At 5 weeks, these plants were used in studies and treated as indicated in the figure legends. For all experiments involving the supply of carbon metabolites, 50 mg/L ampicillin was added to the nutrient solution as described by Lejay et al., 2003, and control plants (Col-0) were transferred to the nutrient solution supplemented with the antibiotic only.
Because the suc2-5 mutants grow poorly in the nutrient solution, all experiments comparing Col-0 and suc2-5 were performed on agar medium. Briefly, seeds were germinated and grown on agar medium containing 1% Suc. The composition of nutrients was identical to the above complete nutrient solution. On day 12, the seedlings were transferred to either complete or Fe-free agar medium lacking Suc for an additional 8 d to minimize the accumulation of Suc, which was previously acquired from the agar medium containing Suc. Then, various treatments were initiated as indicated in the figure legends.
Root FCR Activity Determination
FCR activity was determined according to (Grusak, 1995). Briefly, the whole excised roots were placed in 5 mL assay solution (pH 5.5) consisting of 0.5 mm CaSO4, 0.1 mm 4-morpho-lineethanesulfonic acid, 0.1 mm ferrozine, and 100 μm Fe-EDTA. Reduction activity was measured by following the changes in A562. Reduction rates were calculated using an extinction coefficient of 27.9 mm−1 cm−1. Data were expressed as the means of relative root FCR activity, which was calculated as the percentage of FCR activity of different lines with various treatments to that of the wild type with sufficient Fe supply, unless otherwise indicated. Images of FCR activity were taken by embedding the roots in an agar (0.7%, w/v) medium containing the assay solution (Schmidt et al., 2000). Photographs were taken after 60 min.
Real-Time Reverse Transcription-PCR Analysis
Total RNA in roots was extracted using RNAisoPlus (Takara, Otsu, Shiga, Japan). All RNA samples were checked for DNA contamination before cDNA synthesis. Then, the real-time reverse transcription-PCR analyses were performed as described previously (Jin et al., 2009).
Quantitative Analysis of Sugar Concentrations
Sugar concentrations were determined according to Guignard et al., 2005. Briefly, the roots were ground in liquid nitrogen. Sugar was extracted with 80% (v/v) ethanol twice for 1 h. After centrifugation at 10,000g at 4°C for 10 min, the supernatant was pooled and dried by vacuum centrifugation at 40°C. Samples were resuspended in water and filtered through a 0.45-μm-pore size filter prior to the analysis. Ion chromatography with Pulsed Amperometric Detection analyses were carried out on a Dionex DX-SC3000 chromatograph (Dionex) consisting of a Spark Midas auto-sampler, a GP-50 gradient pump, and an ED-50 electrochemical detector. Two different sets of columns and precolumns were used for carbohydrate separation. A first set, combining a Carbopac PA10 (4 mm id × 250 mm) and Carbopac PA10 (4 mm id × 50 mm; Dionex) was used for the separation of Suc, Glc, and Fru. Then the elution was performed as described previously (Guignard et al., 2005).
Fe Level Determination
For Fe determination, roots were desorbed in a 50-mm CaCl2 solution for 30 min and were subsequently rinsed with ultrapure water. The shoots were directly rinsed with ultrapure water. After that, plant samples were blot-dried and weighted and then dried at 80°C for 48 h. The samples were wet-digested as described previously (Jin et al., 2009), the digests diluted with ultrapure water, and the concentration of iron was measured using inductively coupled plasma-mass spectrometry (Agilent 7500a, Agilent, Santa Clara, CA).
Chlorophyll Quantification and GUS Staining Assay
Chlorophyll of leaves (0.1 g fresh weight) was extracted with 2 mL of 80% (v/v) acetone until complete bleaching was achieved. The total chlorophyll concentration was quantified by absorbances at 663 nm and 645 nm according to the method described previously (Arnon, 1949).
Histochemical assay of GUS gene expression in roots of DR5::GUS transgenic plants was performed as described in our previous study (Mao et al., 2014), and the distribution and intensity of the blue product observed under a microscope (Nikon Eclipse E600, Nikon).
Measurements of Activities of Suc Synthase and Neutral Invertase
Suc synthase was extracted according to Jiang et al. (2012). Briefly, approximately 200 mg of fresh roots was ground to a fine powder in liquid N2. Grinding continued for 5 min in cold extraction buffer (25 mm HEPES–KOH, pH 7.3, 5 mm EDTA, 1 mm dithiothreitol, 0.1% soluble polyvinylpyrrolidone, 20 mm β-mercaptoethanol, 1 mm phenylmethylsulfonyl fluoride, and 0.01 mm leupeptin). The homogenate was separated by centrifugation (10,000g for 5 min at 4°C), and the supernatant was used as the crude extract for assays. The activity of Suc synthase was then assayed according to Ruan et al. (2003).
Neutral invertase was extracted and determined according to Nägele et al. (2010). Briefly, approximately 100 mg of fresh roots was ground to fine powder in liquid N2 and then homogenized in 50 mm HEPES-KOH (pH 7.4), 5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm phenylmethylsulfonyl fluoride, 5 mm dithiothreitol, 0.1% Triton X-100, and 10% glycerin. Suspensions were centrifuged at 6,000 rpm for 25 min at 4°C. Neutral invertase activity was measured in 20 mm HEPES-KOH (pH 7.5) using 100 mm Suc as a substrate. Controls for each assay were boiled for 3 min after the addition of the enzyme extract. Reactions were incubated for 60 min at 30°C and stopped by boiling for 3 min. The concentration of Glc was determined spectrophotometrically.
Statistical Analysis
The data were subjected to ANOVA, and the Duncan's test was employed to determine differences among the treatments at P < 0.05 levels.
Accession Numbers
The sequences of genes examined in this study can be found in The Arabidopsis Information Resource (TAIR) data library under the following accession numbers: SUC2 (At1g22710), FIT (At2g28160), FRO2 (At1g01580), IRT1 (At4g19690), PYE (At3g47640), FRO3 (At1g23020), NAS4 (At1g56430), and ZIF1 (At5g13710).
Supplemental Data
Supplemental Figure S1. Effects of Suc, mannitol, and turanose on the activity of root FCR in Col-0 Arabidopsis.
Supplemental Figure S2. Effect of exogenous Suc on expression of FRO2 (A), IRT1 (B), and FIT (C) in the roots of Col-0 plants.
Supplemental Figure S3. Effects of dark treatment and exogenous Suc application on the Suc concentration in roots of Col-0 Arabidopsis plants.
Supplemental Figure S4. Effect of exogenous Suc application on the Suc concentration in roots of Col-0 plants and the suc2-5 mutants.
Supplemental Figure S5. Effect of Fe deficiency on the Suc concentration in roots of Col-0 and 35S::SUC2 transgenic plants.
Supplemental Figure S6. Effects of exogenous Suc on expression of PYE, FRO3, NAS4, and ZIF1 in the roots of Col-0 plants.
Supplemental Figure S7. Effects of Suc on Fe deficiency responses in roots of Col-0 plants and pye-1 mutants.
Supplemental Figure S8. Effects of Fe deficiency and exogenous Suc application on GUS staining in roots of DR5-GUS transgenic plants.
Supplemental Figure S9. Effect of the 1-naphthylacetic acid treatment on root FCR activity in Col-0 plants and the suc2-5 mutants.
Supplemental Figure S10. Time-course expression of Fe deficiency-responsive genes in the roots of Col-0 plants.
Supplemental Figure S11. Effects of Suc on Fe deficiency responses in the roots of Col-0 plants and Suc signaling mutants.
Supplemental Figure S12. Time-course effect of Fe deficiency on AtSUC2 expression in the leaves of Col-0 plants.
Supplemental Figure S13. Effects of Fe deficiency on GUS staining and Suc concentration in DR5-GUS transgenic plants.
Supplemental Figure S14. Effects of Fe deficiency on the activities of Suc synthase and neutral invertase in the roots of Col-0 plants
Supplemental Table S1. Primers used in this work.
Supplementary Material
Glossary
- bHLH
basic helix-loop-helix
- Col-0
Columbia ecotype
- FCR
Fe deficiency-induced ferric-chelate-reductase
References
- Arnon DI. (1949) Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacaicoa E, Mora V, Zamarreño ÁM, Fuentes M, Casanova E, García-Mina JM (2011) Auxin: a major player in the shoot-to-root regulation of root Fe-stress physiological responses to Fe deficiency in cucumber plants. Plant Physiol Biochem 49: 545–556 [DOI] [PubMed] [Google Scholar]
- Chandran D, Reinders A, Ward JM (2003) Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. J Biol Chem 278: 44320–44325 [DOI] [PubMed] [Google Scholar]
- Chen D, Ren Y, Deng Y, Zhao J (2010a) Auxin polar transport is essential for the development of zygote and embryo in Nicotiana tabacum L. and correlated with ABP1 and PM H+-ATPase activities. J Exp Bot 61: 1853–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ (2010b) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154: 810–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95: 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo EP, Guerinot ML (2004) The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16: 3400–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta K, Khadilkar AS, Sulpice R, Pant B, Scheible WR, Fisahn J, Stitt M, Ayre BG (2014) Expression of sucrose transporter cDNAs specifically in companion cells enhances phloem loading and long-distance transport of sucrose but leads to an inhibition of growth and the perception of a phosphate limitation. Plant Physiol 165: 715–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel PP, Huijser C, Weisbeek PJ, Chua NH, Smeekens SC (1997) Sucrose control of phytochrome A signaling in Arabidopsis. Plant Cell 9: 583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espen L, Dell’Orto M, De Nisi P, Zocchi G (2000) Metabolic responses in cucumber (Cucumis sativus L.) roots under Fe-deficiency: a 31P-nuclear magnetic resonance in-vivo study. Planta 210: 985–992 [DOI] [PubMed] [Google Scholar]
- Funck D, Clauß K, Frommer WB, Hellmann HA (2012) The Arabidopsis CstF64-like RSR1/ESP1 protein participates in glucose signaling and flowering time control. Front Plant Sci 3: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97: 13979–13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M, Lamattina L (2007) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52: 949–960 [DOI] [PubMed] [Google Scholar]
- Grusak MA. (1995) Whole -root iron(III)-reductase activity throughout the life cycle of iron-grown Pisum sativum L. (Fabaceae) relevance to the iron nutrition of developing seeds. Planta 197: 111–117 [Google Scholar]
- Guerinot ML, Yi Y (1994) Iron: nutritious, noxious, and not readily available. Plant Physiol 104: 815–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignard C, Jouve L, Bogéat-Triboulot MB, Dreyer E, Hausman JF, Hoffmann L (2005) Analysis of carbohydrates in plants by high-performance anion-exchange chromatography coupled with electrospray mass spectrometry. J Chromatogr A 1085: 137–142 [DOI] [PubMed] [Google Scholar]
- Hammond JP, White PJ (2008) Sucrose transport in the phloem: integrating root responses to phosphorus starvation. J Exp Bot 59: 93–109 [DOI] [PubMed] [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11: 610–617 [DOI] [PubMed] [Google Scholar]
- Jakoby M, Wang HY, Reidt W, Weisshaar B, Bauer P (2004) FRU (BHLH029) is required for induction of iron mobilization genes in Arabidopsis thaliana. FEBS Lett 577: 528–534 [DOI] [PubMed] [Google Scholar]
- Jia H, Wang Y, Sun M, Li B, Han Y, Zhao Y, Li X, Ding N, Li C, Ji W, Jia W (2013) Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol 198: 453–465 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Guo W, Zhu H, Ruan YL, Zhang T (2012) Overexpression of GhSusA1 increases plant biomass and improves cotton fiber yield and quality. Plant Biotechnol J 10: 301–312 [DOI] [PubMed] [Google Scholar]
- Jiménez S, Ollat N, Deborde C, Maucourt M, Rellán-Álvarez R, Moreno MÁ, Gogorcena Y (2011) Metabolic response in roots of Prunus rootstocks submitted to iron chlorosis. J Plant Physiol 168: 415–423 [DOI] [PubMed] [Google Scholar]
- Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ (2009) Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiol 150: 272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Du ST, Shamsi IH, Luo BF, Lin XY (2011) NO synthase-generated NO acts downstream of auxin in regulating Fe-deficiency-induced root branching that enhances Fe deficiency tolerance in tomato plants. J Exp Bot 62: 3875–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG (2007) Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta 225: 907–918 [DOI] [PubMed] [Google Scholar]
- Kircher S, Schopfer P (2012) Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc Natl Acad Sci USA 109: 11217–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7: 235–246 [DOI] [PubMed] [Google Scholar]
- Kozuka T, Horiguchi G, Kim GT, Ohgishi M, Sakai T, Tsukaya H (2005) The different growth responses of the Arabidopsis thaliana leaf blade and the petiole during shade avoidance are regulated by photoreceptors and sugar. Plant Cell Physiol 46: 213–223 [DOI] [PubMed] [Google Scholar]
- Laby RJ, Kincaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23: 587–596 [DOI] [PubMed] [Google Scholar]
- Lalonde S, Wipf D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55: 341–372 [DOI] [PubMed] [Google Scholar]
- LeClere S, Schmelz EA, Chourey PS (2010) Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiol 153: 306–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Liu Y, Zhang B, Zhao Y, Wang X, Zhou Y, Raghothama KG, Liu D (2011) Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis. Plant Physiol 156: 1116–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejay L, Gansel X, Cerezo M, Tillard P, Müller C, Krapp A, von Wirén N, Daniel-Vedele F, Gojon A (2003) Regulation of root ion transporters by photosynthesis: functional importance and relation with hexokinase. Plant Cell 15: 2218–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8: 110–116 [DOI] [PubMed] [Google Scholar]
- Li H, Song JB, Zhao WT, Yang ZM (2013) AtHO1 is involved in iron homeostasis in an NO-dependent manner. Plant Cell Physiol 54: 1105–1117 [DOI] [PubMed] [Google Scholar]
- Lilley JLS, Gee CW, Sairanen I, Ljung K, Nemhauser JL (2012) An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol 160: 2261–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling HQ, Bauer P, Bereczky Z, Keller B, Ganal M (2002) The tomato fer gene encoding a bHLH protein controls iron-uptake responses in roots. Proc Natl Acad Sci USA 99: 13938–13943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Samac DA, Bucciarelli B, Allan DL, Vance CP (2005) Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. Plant J 41: 257–268 [DOI] [PubMed] [Google Scholar]
- Liu TY, Chang CY, Chiou TJ (2009) The long-distance signaling of mineral macronutrients. Curr Opin Plant Biol 12: 312–319 [DOI] [PubMed] [Google Scholar]
- Long TA, Tsukagoshi H, Busch W, Lahner B, Salt DE, Benfey PN (2010) The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell 22: 2219–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao QQ, Guan MY, Lu KX, Du ST, Fan SK, Ye YQ, Lin XY, Jin CW (2014) Inhibition of nitrate transporter 1.1-controlled nitrate uptake reduces cadmium uptake in Arabidopsis. Plant Physiol 166: 934–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer F, Müller S, Bauer P (2011) Suppression of Fe deficiency gene expression by jasmonate. Plant Physiol Biochem 49: 530–536 [DOI] [PubMed] [Google Scholar]
- Meir S, Philosoph-Hadas S, Epstein E, Aharoni N (1985) Carbohydrates stimulate ethylene production in tobacco leaf discs: I. Interaction with auxin and the relation to auxin metabolism. Plant Physiol 78: 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir S, Riov J, Philosoph-Hadas S, Aharoni N (1989) Carbohydrates stimulate ethylene production in tobacco leaf discs: III. Stimulation of enzymic hydrolysis of indole-3-acetyl-l-alanine. Plant Physiol 90: 1246–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J, Lingam S, Bauer P (2011) Posttranslational regulation of the iron deficiency basic helix-loop-helix transcription factor FIT is affected by iron and nitric oxide. Plant Physiol 157: 2154–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj VJ, Riedl R, Boller T, Wiemken A, Meyer AD (2001) Light and sugar regulation of the barley sucrose: fructan 6-fructosyltransferase promoter. J Plant Physiol 158: 1601–1607 [Google Scholar]
- Nägele T, Henkel S, Hörmiller I, Sauter T, Sawodny O, Ederer M, Heyer AG (2010) Mathematical modeling of the central carbohydrate metabolism in Arabidopsis reveals a substantial regulatory influence of vacuolar invertase on whole plant carbon metabolism. Plant Physiol 153: 260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittylä T, Fuglsang AT, Palmgren MG, Frommer WB, Schulze WX (2007) Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Mol Cell Proteomics 6: 1711–1726 [DOI] [PubMed] [Google Scholar]
- Price J, Laxmi A, St Martin SK, Jang JC (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16: 2128–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi HA, Paranjpe S, Bhargava S (2012) Stem sugar accumulation in sweet sorghum - activity and expression of sucrose metabolizing enzymes and sucrose transporters. J Plant Physiol 169: 605–613 [DOI] [PubMed] [Google Scholar]
- Rellán-Alvarez R, Andaluz S, Rodríguez-Celma J, Wohlgemuth G, Zocchi G, Alvarez-Fernández A, Fiehn O, López-Millán AF, Abadía J (2010) Changes in the proteomic and metabolic profiles of Beta vulgaris root tips in response to iron deficiency and resupply. BMC Plant Biol 10: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397: 694–697 [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Romheld V, Marschner H (1986) Mobilization of iron in the rhizosphere of different plant species. Adv Plant Nutr 2: 155–204 [Google Scholar]
- Ruan YL, Llewellyn DJ, Furbank RT (2003) Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. Plant Cell 15: 952–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL. (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol 65: 33–67 [DOI] [PubMed] [Google Scholar]
- Sairanen I, Novák O, Pěnčík A, Ikeda Y, Jones B, Sandberg G, Ljung K (2012) Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 24: 4907–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S, Schmidt W (2009) Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol 183: 1072–1084 [DOI] [PubMed] [Google Scholar]
- Schmidt W, Tittel J, Schikora A (2000) Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol 122: 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla M, Briat JF, Vert G, Curie C (2008) Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. Plant J 55: 289–300 [DOI] [PubMed] [Google Scholar]
- Sheen J. (2014) Master regulators in plant glucose signaling networks. J Plant Biol 57: 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140: 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Sun H, Li J, Gong X, Huang S, Zhu X, Zhang Y, Xu G (2013) Auxin distribution is differentially affected by nitrate in roots of two rice cultivars differing in responsiveness to nitrogen. Ann Bot (Lond) 112: 1383–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG, Geigenberger P (2003) Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant J 35: 490–500 [DOI] [PubMed] [Google Scholar]
- Tognetti JA, Pontis HG, Martínez-Noël GM (2013) Sucrose signaling in plants: a world yet to be explored. Plant Signal Behav 8: e23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Steponkus PL (2003) Modification of the intracellular sugar content alters the incidence of freeze-induced membrane lesions of protoplasts isolated from Arabidopsis thaliana leaves. Plant Cell Environ 26: 1083–1096 [Google Scholar]
- Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14: 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EL, Connolly EL (2008) Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Curr Opin Plant Biol 11: 530–535 [DOI] [PubMed] [Google Scholar]
- Wang N, Cui Y, Liu Y, Fan H, Du J, Huang Z, Yuan Y, Wu H, Ling HQ (2013) Requirement and functional redundancy of Ib subgroup bHLH proteins for iron deficiency responses and uptake in Arabidopsis thaliana. Mol Plant 6: 503–513 [DOI] [PubMed] [Google Scholar]
- Wang SJ, Yeh KW, Tsai CY (2001) Regulation of starch granule-bound starch synthase I gene expression by circadian clock and sucrose in the source tissue of sweet potato. Plant Sci 161: 635–644 [Google Scholar]
- Wu H, Chen C, Du J, Liu H, Cui Y, Zhang Y, He Y, Wang Y, Chu C, Feng Z, Li J, Ling HQ (2012a) Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis-enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol 158: 790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Zhang HT, Wang Y, Jia WS, Xu XF, Zhang XZ, Han ZH (2012b) Induction of root Fe(lll) reductase activity and proton extrusion by iron deficiency is mediated by auxin-based systemic signalling in Malus xiaojinensis. J Exp Bot 63: 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan TT, Xu HH, Zhang KX, Guo TT, Lu YT (2014) Glucose inhibits root meristem growth via ABA INSENSITIVE 5, which represses PIN1 accumulation and auxin activity in Arabidopsis. Plant Cell Environ 37: 1338–1350 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Wu H, Wang N, Li J, Zhao W, Du J, Wang D, Ling HQ (2008) FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res 18: 385–397 [DOI] [PubMed] [Google Scholar]
- Zocchi G. (2006) Metabolic changes in iron-stressed dicotyledonous. In Barton LL, Abadía J, eds, Iron Nutrition in Plants and Rhizospheric Microorganisms, Ed 1 Springer, Dordrecht, pp 359–370 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.