A Synthase impacts ester cross-links of tomato cutin.
Abstract
Cuticle function is closely related to the structure of the cutin polymer. However, the structure and formation of this hydrophobic polyester of glycerol and hydroxy/epoxy fatty acids has not been fully resolved. An apoplastic GDSL-lipase known as CUTIN SYNTHASE1 (CUS1) is required for cutin deposition in tomato (Solanum lycopersicum) fruit exocarp. In vitro, CUS1 catalyzes the self-transesterification of 2-monoacylglycerol of 9(10),16-dihydroxyhexadecanoic acid, the major tomato cutin monomer. This reaction releases glycerol and leads to the formation of oligomers with the secondary hydroxyl group remaining nonesterified. To check this mechanism in planta, a benzyl etherification of nonesterified hydroxyl groups of glycerol and hydroxy fatty acids was performed within cutin. Remarkably, in addition to a significant decrease in cutin deposition, mid-chain hydroxyl esterification of the dihydroxyhexadecanoic acid was affected in tomato RNA interference and ethyl methanesulfonate-cus1 mutants. Furthermore, in these mutants, the esterification of both sn-1,3 and sn-2 positions of glycerol was impacted, and their cutin contained a higher molar glycerol-to-dihydroxyhexadecanoic acid ratio. Therefore, in planta, CUS1 can catalyze the esterification of both primary and secondary alcohol groups of cutin monomers, and another enzymatic or nonenzymatic mechanism of polymerization may coexist with CUS1-catalyzed polymerization. This mechanism is poorly efficient with secondary alcohol groups and produces polyesters with lower molecular size. Confocal Raman imaging of benzyl etherified cutins showed that the polymerization is heterogenous at the fruit surface. Finally, by comparing tomato mutants either affected or not in cutin polymerization, we concluded that the level of cutin cross-linking had no significant impact on water permeance.
Cuticles are ubiquitous hydrophobic barriers at the surfaces of aerial plant organs. These complex hydrophobic assemblies consist of a biopolymer, cutin, coated and filled with waxes and can also comprise embedded cell wall polysaccharides. Waxes comprise solvent-soluble aliphatic molecules with long hydrocarbon chains, terpenes, and steroids (Kunst and Samuels, 2003; Nawrath, 2006; Pollard et al., 2008; Samuels et al., 2008; Schreiber, 2010; Lee and Suh, 2015). Cutin is an insoluble polyester of ω- and mid-chain hydroxy C16 and C18 fatty acids. Glycerol has also been described as a ubiquitous cutin monomer (Graça et al., 2002; Pollard et al., 2008). In some cuticles, a hydrophobic polymer that is resistant to alkaline hydrolysis (i.e. cutan) has been observed (Gupta et al., 2006; Li-Beisson et al., 2010).
Cutin fulfills multiple functions in plants, such as the control of nonstomatal water loss (Sieber et al., 2000) and the permeation of gases and solutes (Kersteins, 1996; Schreiber, 2010). Cutin also plays an essential role in the regulation of cell adhesion during plant development by preventing organ fusion, as observed in Arabidopsis (Arabidopsis thaliana) mutants with cuticle defects (Sieber et al., 2000; Nawrath, 2006), or by participating in hull adhesion in grains (Taketa et al., 2008). Finally, it is generally accepted that plant cuticle and its polymeric skeleton, cutin, are primary barriers to pathogens and that cutin monomers released by fungal cutinase are signaling molecules for both the pathogen and plants (Gilbert et al., 1996; Schweizer et al., 1996; Iwamoto et al., 2002; Yeats and Rose, 2013).
The biological functions of cutin are closely controlled by its structure, which is determined by its monomer composition and by the number and position of its ester bonds. Cutin monomer composition can vary according to plant species, developmental stage (Baker et al., 1982; Peschel et al., 2007; Mintz-Oron et al., 2008), organs, and environmental stress (Espelie et al., 1979; Li-Beisson et al., 2009; Panikashvili et al., 2009; Bessire et al., 2011). Actually, cutin monomer composition determines the total number of hydroxyl (OH) groups that are potentially available for the formation of ester bonds and, therefore, the cross-linking of the polyester (Bonaventure et al., 2004; Franke et al., 2005; Peschel et al., 2007). The nonesterified OH groups enhance the hydrophilic character of the cutin polymer, increasing its elasticity (Bargel and Neinhuis, 2005).
Whereas cutin monomer composition has been described extensively for different plant species, organs, and development stages, the macromolecular structure of the cutin polyester has been much less thoroughly investigated. In particular, the connectivity between the monomers is a key point for understanding the three-dimensional expansion of the polyester in relation to the polymerization process. Different approaches have been proposed to delineate the polymeric architecture of cutin. Linear dimers were identified after partial alkaline hydrolysis of tomato (Solanum lycopersicum) cutin (Osman et al., 1995). NMR and mass spectrometry analyses of oligomers released after partial depolymerization revealed primary and secondary ester linkages between cutin monomers (Graça et al., 2002; Stark and Tian, 2006) as well as covalent linkages between some cutin OH fatty acids and oligosaccharides (Tian et al., 2008). However, it has been shown that partial hydrolysis does not necessarily release all of the representative building blocks of the entire polymer (Deshmukh et al., 2003). Spectrometric analyses have also been developed for the polymer. Attenuated total reflectance (ATR)-Fourier transform infrared (FTIR) spectroscopy analyses of the methylene and carbonyl stretching vibrations allowed the estimation of an ester cross-linking index for cutin but could not differentiate the primary from the secondary ester linkages (Girard et al., 2012; Heredia-Guerrero et al., 2014). NMR studies have provided evidence of both ω- and mid-chain esters in tomato (Deshmukh et al., 2003).
In this regard, tomato fruit has proved to be an interesting model for structural studies of the cutin polymer. Indeed, its astomatous cuticle can be easily isolated and is devoid of cutan. Moreover, tomato cutin composition is dominated by a monomer with two OH groups, 9(10),16-dihydroxyhexadecanoic acid (Baker et al., 1982; Osman et al., 1999; Deshmukh et al., 2003). Accordingly, the cutin monomers can be linked by either a linear (on the primary OH) or a branched (with a secondary OH) pattern. Previous studies have demonstrated that both linear and branched cross-links occur in tomato cutin. However, the relative proportion of the linear versus branched esters remains a matter of debate. Oxidation experiments have indicated that almost all of the primary cutin OH groups (94%) were involved in ester bonds, whereas only 44% of the secondary OH groups were esterified in the cutin polymer (Deas and Holloway, 1977; Kolattukudy, 1977). Conversely, partial depolymerization coupled with NMR studies of the released oligomers indicated that the branched secondary esters were the major form of tomato cutin (Graça and Lamosa, 2010). In addition, none of these studies could decipher the ester links of the glycerol OH groups.
Additionally, the role of a GDSL lipase, involved in cutin polymerization, was recently reported using two different experimental approaches (Girard et al., 2012; Yeats et al., 2012). The corresponding GDSL lipase, named SlGDSL1 (Girard et al., 2012) or SlGDSL2 (Yeats et al., 2012), is now named CUTIN SYNTHASE1 (SlCUS1; Yeats et al., 2014). Different mutants affected in the expression of SlCUS1 have been generated and constitute attractive tools to delineate the structure of the cutin polymer (Girard et al., 2012; Petit et al., 2014).
It has been further demonstrated that 2-monoacylglycerol (2-MAG), a putative precursor of the cutin polymer in Arabidopsis (Yang et al., 2010), can be used by a heterologously expressed SlCUS1 (Yeats et al., 2012) to produce in vitro linear oligomers in aqueous solution (Yeats et al., 2014). Nevertheless, the question of the mechanism of cutin polymerization in planta is still open. Indeed, SlCUS1 is specifically localized within the cutin matrix (i.e. a hydrophobic environment; Girard et al., 2012; Yeats et al., 2012), which could impact the acyltransferase activity of the enzyme as observed previously for lipases (Sharma et al., 2001).
By coupling O-alkylation of the nonesterified OH groups of glycerol and fatty acids in an isolated cutin matrix and by further analyses of O-alkylated and nonalkylated monomers released after depolymerization, we elucidated the ester cross-link pattern of tomato cutin. We also showed at two stages of fruit development and in two different genetic backgrounds that the modulation of SlCUS1 protein level, either through RNA interference (cherry tomato ‘West Virginia 106’ [WVa106]) or mutagenesis (miniature tomato ‘Micro-Tom’), resulted in a strong alteration of the cutin ester cross-link pattern. These results give new insights into the polyester structure. In addition, while CUS1 esterification involves mostly primary OH groups in vitro (Yeats et al., 2014), our data here indicate that, in planta, deficiencies in CUS1 also affect the secondary OH group of 9(10),16-dihydroxyhexadecanoic acid and both the primary and secondary OH groups of glycerol.
RESULTS
Labeling of the Nonesterified OH Groups from Cutin in the Wild Type and cus1 Mutants
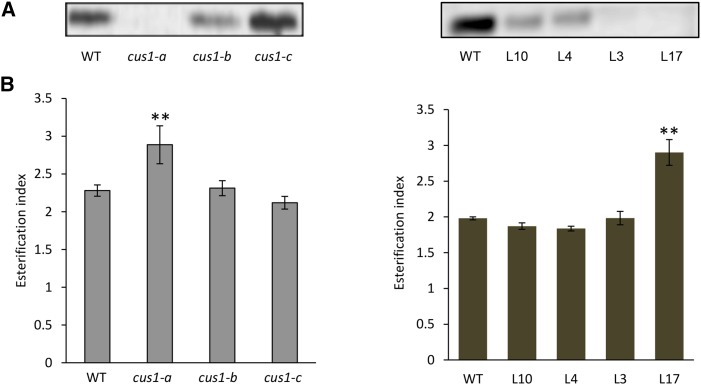
To investigate the function of SlCUS1 in cutin polymerization, we used a series of transgenic and mutant lines obtained in two different tomato genetic backgrounds. The first set of lines was generated as described previously (Girard et al., 2012) in the cherry tomato ‘WVa106’. Cherry tomatoes (var cerasiforme) are considered close to the ancestral form of the cultivated tomato (Ranc et al., 2008). The series consists of four selected transgenic lines in which the down-regulation of SlCUS1 using RNA interference results in mild to severe alterations in cuticle thickness and cutin monomer deposition (Pro-35S:SlCUS1RNAi lines L4, L10, L3, and L17; Girard et al., 2012). The second set of lines was generated in the miniature cv Micro-Tom by ethyl methanesulfonate (EMS) mutagenesis (Just et al., 2013; Petit et al., 2014). The cus1-a mutant isolated previously by map-based cloning carries a splicing site mutation in the SlCUS1 gene, resulting in a strong reduction in fruit cuticle thickness and cutin monomer deposition (Petit et al., 2014). Two other cus1 mutants, cus1-b and cus1-c, were identified by TILLING (Okabe et al., 2011). The cus1-b mutant carries an Arg-229 to Leu-229 missense mutation, whereas the cus1-c mutant carries an Asp-264 to Asn-264 missense mutation (Supplemental Fig. S1). None of these mutations, which could potentially affect the function of the protein, are located within the five GDSL lipase conserved domains. In addition, they did not affect fruit cuticle thickness (data not shown) or the amount of cutin monomers (Table I) and, therefore, could be used as negative controls. Analysis of SlCUS1 protein levels in the cuticle of growing fruits at 20 DPA confirmed that the most affected Pro-35S:SlCUS1RNAi lines were L3 and L17 and that the only cus1 mutant lacking SlCUS1 was cus1-a (Fig. 1A).
Table I. Cutin monomer composition of 20-DPA and red ripe fruits in the wild type, Pro-35S:SlCUS1RNAi lines (L4, L10, L3, and L17), and cus1 mutants (cus1-a, cus1-b, and cus1-c).
Values ± sd are expressed in µg cm−2 and are means of at least three biological replicates. Significant differences for glycerol and 9(10),16-dihydroxyhexadecanoic acid (boldface) from the corresponding wild type are indicated with asterisks (Student’s t test; *, P < 0.05; **, P < 0.01; and ***, P < 0.001).
| Compounds | cv Micro-Tom |
cv WVa106 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild Type | cus1-a | cus1-b | cus1-c | Wild Type | L4 | L10 | L3 | L17 | |
| 20 DPA | |||||||||
| Glycerol | 4.5 ± 2.0 | 7.9 ± 0.7* | 5.2 ± 0.5 | 3.1 ± 0.7 | 2.4 ± 0.1 | 3.5 ± 0.6 | 2.6 ± 1.0 | 3.9 ± 0.2** | 3.8 ± 0.8 |
| p-Coumaric acid | 2.5 ± 0.2 | 1.9 ± 0.0 | 2.5 ± 0.5 | 2.3 ± 0.2 | 2.7 ± 1.8 | 1.1 ± 0.0 | 0.9 ± 0.2 | 0.5 ± 0.0 | 0.3 ± 0.2 |
| m-Coumaric acid | 5.2 ± 0.5 | 3.4 ± 0.9 | 6.2 ± 0.4 | 5.0 ± 0.4 | 2.7 ± 0.7 | 1.7 ± 0.1 | 1.4 ± 0.4 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Hexadecanoic acid | 1.8 ± 0.8 | 0.8 ± 0.2 | 2.0 ± 0.6 | 1.7 ± 0.6 | 1.2 ± 1.1 | 1.1 ± 0.5 | 0.6 ± 0.1 | 0.6 ± 0.2 | 1.0 ± 0.3 |
| 1,16-Hexadecanedioic acid | 1.8 ± 0.8 | 0.2 ± 0.1 | 1.3 ± 1.7 | 1.4 ± 0.3 | 0.9 ± 0.1 | 2.1 ± 0.3 | 0.5 ± 0.3 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| 16-Hydroxyhexadecanoic acid | 22.2 ± 0.2 | 0.9 ± 0.0 | 23.6 ± 1.2 | 20.5 ± 2.9 | 18.4 ± 0.8 | 13.0 ± 0.2 | 9.8 ± 4.7 | 1.9 ± 0.1 | 1.1 ± 0.0 |
| 9(10),16-Dihydroxyhexadecanoic acid | 537.8 ± 29.1 | 93.4 ± 0.4** | 609.9 ± 39.5 | 511.4 ± 16.4 | 523.0 ± 28.2 | 450.1 ± 22.5 | 348.7 ± 37.4* | 141.9 ± 0.6** | 83.4 ± 9.9** |
| 9-Hydroxy-1,16-hexadecanedioic acid | 35.1 ± 1.5 | 5.5 ± 0.0 | 22.8 ± 2.3 | 27.4 ± 4.9 | 44.8 ± 7.6 | 37.1 ± 0.9 | 32.4 ± 3.3 | 9.9 ± 0.2 | 5.4 ± 1.8 |
| Octadecanoic acid | 2.7 ± 1.1 | 1.1 ± 0.3 | 2.5 ± 0.4 | 2.9 ± 1.1 | 1.4 ± 0.8 | 2.1 ± 1.2 | 1.1 ± 0.5 | 0.8 ± 0.3 | 1.3 ± 0.6 |
| 18-Hydroxyoctadecanoic acid | 0.8 ± 0.2 | 0.1 ± 0.0 | 1.1 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.4 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 9(10),18-Dihydroxyoctadecanoic acid | 4.9 ± 1.5 | 1.1 ± 0.2 | 8.5 ± 2.6 | 4.0 ± 0.7 | 3.5 ± 0.0 | 3.2 ± 0.3 | 2.3 ± 0.3 | 0.8 ± 0.0 | 0.7 ± 0.3 |
| 9,10-Epoxy-18-hydroxyoctadecenoic acid | 4.0 ± 0.9 | 0.9 ± 0.1 | 6.1 ± 2.1 | 6.1 ± 1.4 | 1.5 ± 1.6 | 1.7 ± 0.0 | 0.4 ± 0.2 | 0.2 ± 0.0 | 0.1 ± 0.1 |
| 9,10-Epoxy-18-hydroxyoctadecanoic acid | 8.4 ± 1.8 | 5.8 ± 0.4 | 9.5 ± 2.7 | 10.6 ± 1.9 | 4.3 ± 0.4 | 5.9 ± 0.4 | 4.9 ± 1.5 | 7.0 ± 0.2 | 5.7 ± 1.6 |
| 9,10,18-Trihydroxyoctadecanoic acid | 6.1 ± 3.4 | 2.8 ± 0.8 | 3.9 ± 1.9 | 5.0 ± 2.0 | 3.5 ± 0.2 | 2.1 ± 0.2 | 1.8 ± 0.0 | 1.6 ± 0.0 | 2.4 ± 0.5 |
| 24-Hydroxytetracosanoic acid | 0.0 ± 0.0 | 1.9 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.1 | 0.5 ± 0.0 | 0.9 ± 0.9 | 1.9 ± 0.2 | 1.8 ± 0.4 |
| Unidentified compounds | 17.9 ± 3.8 | 8.1 ± 2.7 | 17.3 ± 11.4 | 22.2 ± 10.4 | 33.8 ± 10.2 | 27.2 ± 0.3 | 22.3 ± 0.9 | 10.1 ± 0.1 | 3.4 ± 0.5 |
| Red Ripe | |||||||||
| Glycerol | 4.2 ± 1.4 | 7.3 ± 0.9* | 4.5 ± 0.7 | 3.6 ± 1.4 | 1.9 ± 0.0 | 2.1 ± 0.0 | 2.3 ± 0.7 | 1.8 ± 0.2 | 1.9 ± 0.1 |
| p-Coumaric acid | 4.8 ± 0.9 | 4.0 ± 1.2 | 3.0 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.5 | 1.9 ± 0.3 | 1.3 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.0 |
| m-Coumaric acid | 15.1 ± 4.7 | 13.5 ± 2.1 | 13.1 ± 1.0 | 9.3 ± 2.4 | 4.5 ± 0.7 | 5.4 ± 1.1 | 4.2 ± 0.2 | 0.7 ± 0.1 | 0.5 ± 0.0 |
| Hexadecanoic acid | 1.8 ± 0.8 | 4.1 ± 0.3 | 2.1 ± 0.2 | 1.7 ± 0.4 | 2.1 ± 0.2 | 2.1 ± 0.2 | 1.0 ± 0.0 | 1.1 ± 0.2 | 1.0 ± 0.0 |
| 1,16-Hexadecanedioic acid | 3.3 ± 0.1 | 0.3 ± 0.2 | 2.2 ± 0.1 | 1.7 ± 0.2 | 2.0 ± 0.1 | 1.6 ± 0.2 | 0.6 ± 0.2 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| 16-Hydroxyhexadecanoic acid | 21.1 ± 2.2 | 1.5 ± 0.4 | 23.3 ± 1.3 | 12.9 ± 0.5 | 23.0 ± 3.3 | 23.2 ± 1.2 | 11.4 ± 3.1 | 1.1 ± 0.3 | 0.6 ± 0.0 |
| 9(10),16-Dihydroxyhexadecanoic acid | 588.4 ± 89.5 | 135.1 ± 7.4* | 730.2 ± 45.2 | 511.5 ± 10.3 | 571.5 ± 6.4 | 550.5 ± 27.4 | 459.1 ± 16.4* | 114.9 ± 11.2*** | 86.0 ± 1.8*** |
| 9-Hydroxy-1,16-hexadecanedioic acid | 19.7 ± 4.4 | 6.4 ± 1.0 | 23.5 ± 2.8 | 11.3 ± 2.6 | 32.5 ± 10.0 | 32.9 ± 0.4 | 24.8 ± 3.0 | 3.6 ± 0.6 | 2.4 ± 0.1 |
| Octadecanoic acid | 1.6 ± 0.1 | 3.0 ± 1.7 | 2.0 ± 0.2 | 1.6 ± 0.6 | 2.2 ± 0.1 | 1.5 ± 0.3 | 1.5 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.1 |
| 18-Hydroxyoctadecanoic acid | 1.0 ± 0.3 | 0.3 ± 0.1 | 1.1 ± 0.1 | 0.6 ± 0.0 | 1.3 ± 0.1 | 1.4 ± 0.2 | 0.8 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 9(10),18-Dihydroxyoctadecanoic acid | 10.4 ± 1.5 | 2.7 ± 0.0 | 11.5 ± 0.0 | 6.6 ± 0.2 | 7.5 ± 1.3 | 5.9 ± 0.7 | 7.4 ± 1.7 | 1.7 ± 0.1 | 1.4 ± 0.2 |
| 9,10-Epoxy-18-hydroxyoctadecenoic acid | 1.2 ± 0.4 | 0.8 ± 0.2 | 1.6 ± 0.2 | 0.9 ± 0.0 | 0.8 ± 0.1 | 1.3 ± 0.2 | 0.9 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 |
| 9,10-Epoxy-18-hydroxyoctadecanoic acid | 0.8 ± 0.1 | 3.8 ± 1.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.0 | 1.4 ± 0.2 | 1.8 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 9,10,18-Trihydroxyoctadecenoic acid | 1.6 ± 0.3 | 0.0 ± 0.0 | 1.9 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.7 | 2.0 ± 0.3 | 1.8 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 9,10,18-Trihydroxyoctadecanoic acid | 2.9 ± 0.4 | 2.8 ± 0.1 | 4.9 ± 0.2 | 2.5 ± 0.1 | 4.9 ± 1.0 | 4.9 ± 2.7 | 4.6 ± 0.2 | 3.5 ± 0.6 | 2.9 ± 1.1 |
| 24-Hydroxytetracosanoic acid | 1.4 ± 1.3 | 4.2 ± 0.9 | 0.6 ± 0.0 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.0 | 12.3 ± 0.8 | 11.1 ± 0.7 |
| Unidentified compounds | 42.2 ± 2.2 | 25.5 ± 5.7 | 50.7 ± 0.2 | 33.3 ± 0.0 | 34.4 ± 14.8 | 39.7 ± 1.5 | 36.6 ± 2.3 | 31.3 ± 1.0 | 25.4 ± 0.2 |
Figure 1.
Characterization of tomato cutin in cus1 mutants and Pro-35S:SlCUS1RNAi lines. A, Immunoblot analysis of the SlCUS1 protein in 20-DPA fruits. B, Nonnormalized areas of methylene (CH2; 2,978–2,838 cm−1) and carbonyl (CO; 1,750–1,690 cm−1) bands, and esterification index, the ratio of CH2 to CO areas in red ripe fruits. Left, cus1 mutants; right, Pro-35S:SlCUS1RNAi lines. WT, Wild type. Mean values ± sd were calculated from 32 independent measurements (four biological samples and eight technical replicates each). Significant differences from the wild type are indicated by asterisks (Student’s t test; **, P < 0.01).
ATR-FTIR spectroscopic analysis of the cutin matrix isolated from red ripe fruits returned a ratio of the intensities of methylene and carbonyl stretching vibrations, also called the esterification index (Girard et al., 2012; Heredia-Guerrero et al., 2014). Actually, SlCUS1 down-regulation induced a decrease in cutin density that resulted in a decrease in both methylene and carbonyl bands (Girard et al., 2012). Consequently, the esterification index of L3, a less affected transgenic line than L17, was not altered significantly. Conversely, in the most affected lines (L17 and cus1-a), this ratio was higher for these SlCUS1 mutants than for the corresponding wild types and the nonphenotypically affected SlCUS1 mutants, cus1-b and cus1-c (Fig. 1B). These spectrometric analyses suggest a lower proportion of ester cross-linking for the most affected lines L17 and cus1-a cutins.
Free (i.e. nonesterified) OH groups were chemically labeled, by O-alkylation, in the cutin of tomato skins of the different wild-type and mutant fruits. By using 2-benzyloxypyridine alkylation, the OH groups are transformed to benzyl ether adducts (Lopez and Dudley, 2008). The corresponding benzyl ether derivatives of glycerol and major tomato hydroxy fatty acids can be separated, identified, and quantified by gas chromatography-mass spectrometry (GC-MS; Supplemental Fig. S2). This procedure revealed the esterification levels of the primary and secondary OH groups of the major cutin monomers of the cutin polyester in various cus1 mutants and their corresponding wild-type lines.
CUS1 Protein Knockdown and Glycerol Esterification of the Cutin Polyester
Glycerol is a ubiquitous cutin monomer (Graça et al., 2002; Graça and Lamosa, 2010). Whatever the developmental stage, the glycerol content of cutin varies from about 2 μg cm−2 in cv WVa106 to about 4 μg cm−2 in cv Micro-Tom (Table I). These glycerol contents are slightly below the range found previously (i.e. from 5 to 8 μg cm−2) in the cuticle of different plants, including tomato fruit (Graça et al., 2002). However, an unreported significant variability of glycerol content within tomato cultivars must be mentioned here, even though glycerol is still a minor cutin component in both tomato cultivars. Regarding the formation and structure of the cutin polyester, it is important to also consider the molar ratio between glycerol and the major hydroxy fatty acids, such as 9(10),16-dihydroxyhexadecanoic acid (Table II). Indeed, this ratio is less variable between genetic backgrounds and developmental stages, suggesting a common polymerization mechanism.
Table II. Molar ratio of glycerol to 9(10),16-dihydroxyhexadecanoic acid within cutin from 20-DPA and red ripe fruits from the wild type, Pro-35S:SlCUS1RNAi lines (L4, L10, L3, and L17), and cus1 mutants (cus1-a, cus1-b, and cus1-c).
Values are multiplied by 100. Significant differences from the corresponding wild type are indicated by asterisks (Student’s t test; *, P < 0.05; **, P < 0.01; and ***, P < 0.001).
| Cultivar | Line | 20 DPA | Red Ripe |
|---|---|---|---|
| WVa106 | Wild type | 1.5 ± 0.0 | 1.0 ± 0.1 |
| L4 | 2.5 ± 0.4 | 1.2 ± 0.1 | |
| L10 | 2.4 ± 1.2 | 1.6 ± 0.4 | |
| L3 | 8.8 ± 0.4** | 5.1 ± 0.1*** | |
| L17 | 14.9 ± 5.3* | 6.6 ± 0.1*** | |
| Micro-Tom | Wild type | 2.9 ± 1.3 | 2.5 ± 0.1 |
| cus1-a | 26.4 ± 3.1* | 15.9 ± 0.6** | |
| cus1-b | 2.6 ± 0.2 | 1.8 ± 0.1* | |
| cus1-c | 2.1 ± 0.3 | 1.8 ± 0.6 |
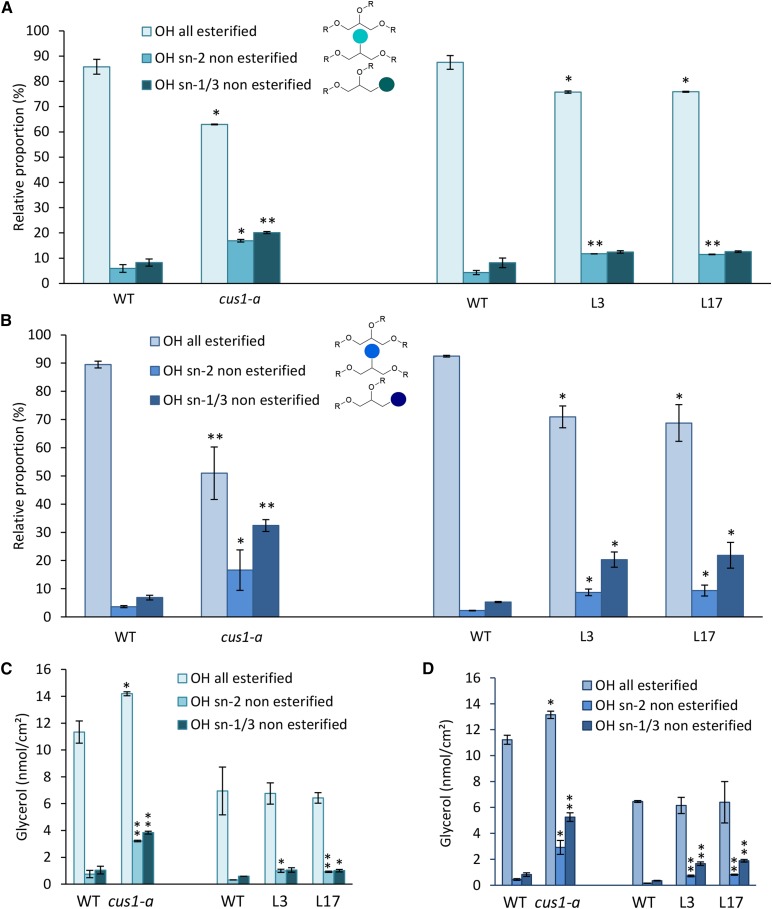
Furthermore, we observed in both cultivars that nearly 90% of the OH groups from glycerol are esterified in wild-type 20-DPA and red ripe fruit cutins (Fig. 2; Supplemental Fig. S3). Although glycerol is a minor cutin monomer, this result is in agreement with a significant role of glycerol in the structure of the cutin polymer, as suggested previously (Graça et al., 2002; Pollard et al., 2008). In addition, approximately 10% of residual nonesterified OH groups were observed in both the sn-1,3 and sn-2 positions of glycerol in wild-type tomato fruit cutins. In CUS1 tomato mutants, the amount of glycerol per surface area is less affected than the hydroxy fatty acid content, which decreases sharply (Table I).
Figure 2.
Esterification levels of glycerol OH groups of tomato fruit cutin isolated from the cus1-a mutant and Pro-35S:SlCUS1RNAi lines. A and C, Fruits at 20 DPA. B and D, Red ripe fruits. Left, cus1-a; right, Pro-35S:SlCUS1RNAi lines. WT, Wild type. Mean values ± sd were calculated from nine independent measurements (three fruits and three technical replicates each). Significant differences from the corresponding wild type are indicated by asterisks (Student’s t test; *, P < 0.05 and **, P < 0.01).
However, considering the molar content of cutin monomers, it is noteworthy that the glycerol-to-hydroxy fatty acid molar ratio is higher in the cus1-a mutant and in most affected Pro-35S:SlCUS1RNAi lines compared with the corresponding wild-type plants (about 6- to 10-fold; Table II). Furthermore, glycerol esterification is impacted, as evidenced by an increase of up to 5-fold in the amount of nonesterified glycerol OH in the mutant lines. Moreover, the increase in the free OH groups of glycerol was closely related to the severity of SlCUS1 protein knockdown (Figs. 1A and 2).
Effect of SlCUS1 Protein Knockdown on Mid-Chain OH Esterification of Cutin Fatty Acids
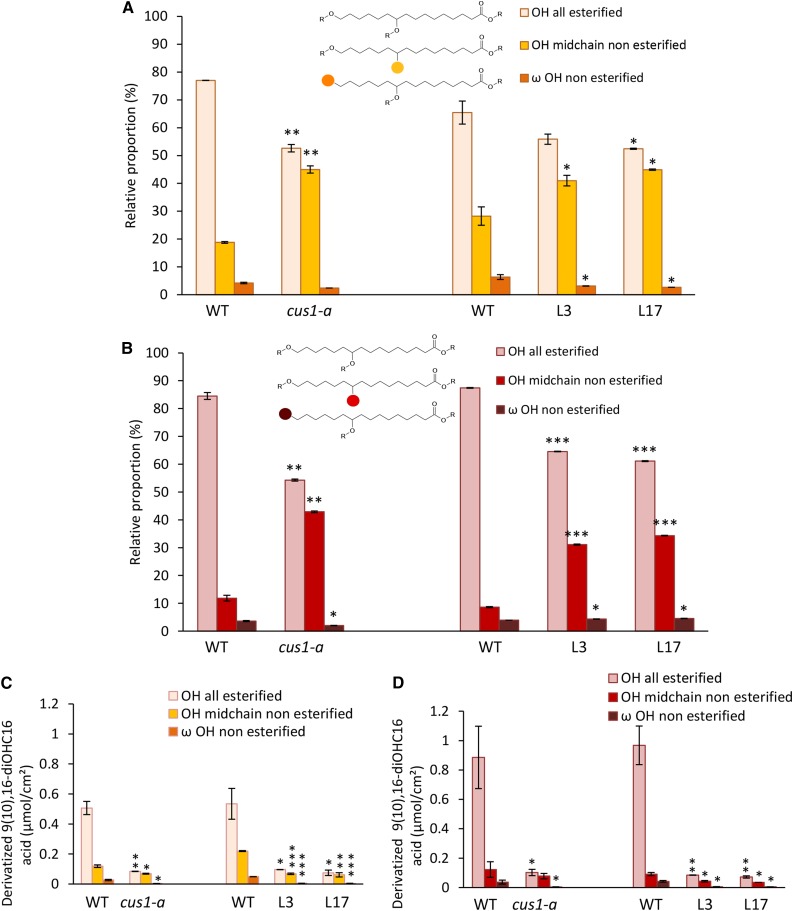
The 9(10),16-dihydroxyhexadecanoic acid is the main cutin monomer in both tomato cultivars, which constitutes more than 80% of fruit cutin hydroxy fatty acids (Table I). Consequently, we focused on this fatty acid in this study (Fig. 3; Supplemental Fig. S4). Similar esterification levels were observed for both cv WVa106 and cv Micro-Tom. Indeed, the primary OH group of this fatty acid, in the ω-position, is preferentially esterified in accordance with previous structural data (Deshmukh et al., 2003; Fig. 3). A noticeable increase in the esterification of the secondary OH groups was observed between the 20-DPA (Fig. 3A) and red ripe (Fig. 3B) stages, especially in cv WVa106. This result is in accordance with previous FTIR and NMR data, suggesting an increase in ester bond cross-links during tomato fruit development (Benítez et al., 2004).
Figure 3.
Esterification levels of 9(10),16-dihydroxyhexadecanoic acid [9(10),16-diOHC16 acid] OH groups of tomato fruit cutin from the cus1-a mutant and Pro-35S:SlCUS1RNAi lines. A and C, Fruits at 20 DPA. B and D, Red ripe fruits. Left, cus1-a; right, Pro-35S:SlCUS1RNAi lines. WT, Wild type. Mean values ± sd were calculated from nine independent measurements (three fruits and three technical replicates each). Significant differences from the corresponding wild type are indicated by asterisks (Student’s t test; *, P < 0.05; **, P < 0.01; and ***, P < 0.001).
Remarkably, whatever the cultivar, SlCUS1 knockdown resulted, in planta, in a specific reduction of mid-chain OH esterification of the 9(10),16-dihydroxyhexadecanoic acid, whereas esterification of the ω-OH group was not affected.
Surface Distribution of the Nonesterified OH Groups in the Cutin Polymer
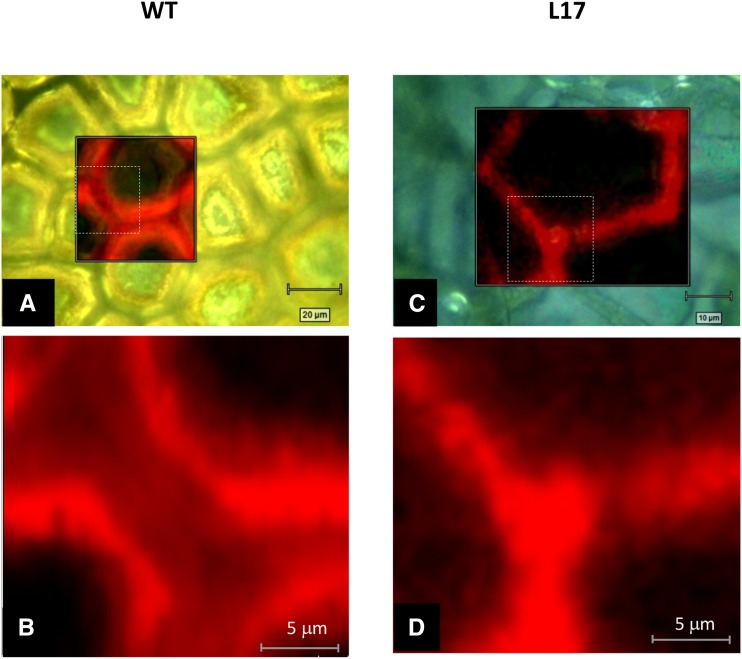
Benzyl etherification of free OH groups gives rise to a specific Raman band at 1,001 cm−1 that was not retrieved in the nonlabeled cutin (Supplemental Fig. S5). Moreover, Raman microspectroscopy has a high resolution that fits with structural characterization of cuticle surfaces (Littlejohn et al., 2015). Therefore, Raman imaging at the specific 1,001-cm−1 band was used to monitor the spatial distribution of free OH groups within the cutin polymer. High-resolution confocal images revealed that free OH groups were not evenly distributed (Fig. 4, A and B) within the wild-type cutin polymer. Indeed, a weaker signal (i.e. a higher cross-link level) was observed in the cutin ridge at the midpoint in the cell junctions than at its external part. Interestingly, in a previous work (Girard et al., 2012) using SlCUS1 immunolocalization, we observed a sharp protein signal in the region that corresponded to the central part of the cutin ridge. In contrast, in the Pro-35S:SlCUS1RNAi line L17, a sharper and homogenous signal was observed (Fig. 4, C and D).
Figure 4.
Raman mapping of nonesterified OH groups within cutin polyester from tomato. Cutin from wild-type (WT; A and B) and Pro-35S:SlCUS1RNAi line L17 (D and D) red ripe fruit were benzyl etherified and analyzed by Raman microspectroscopy. Each image was obtained by mapping the characteristic band of aromatic groups at 1,001 cm−1. Images B and D correspond to higher magnification views (dashed squares) of the Raman images (A and C, respectively).
Water Permeance of Cutins from Wild-Type and cus1 Tomato
Water permeability is an important feature related to the biological function of the cuticle. Waxes and, in particular, wax composition have been described as major factors involved in cuticle permeability (Riederer and Schreiber, 2001; Leide et al., 2007), although the cutin matrix contributes to the transport properties of the cuticle. In particular, it was suggested that a decrease in the permeability of chloroform-extracted fruits observed during the development of cv Micro-Tom fruit could be due to an increase in ester cross-links of the cutin monomer (Leide et al., 2007).
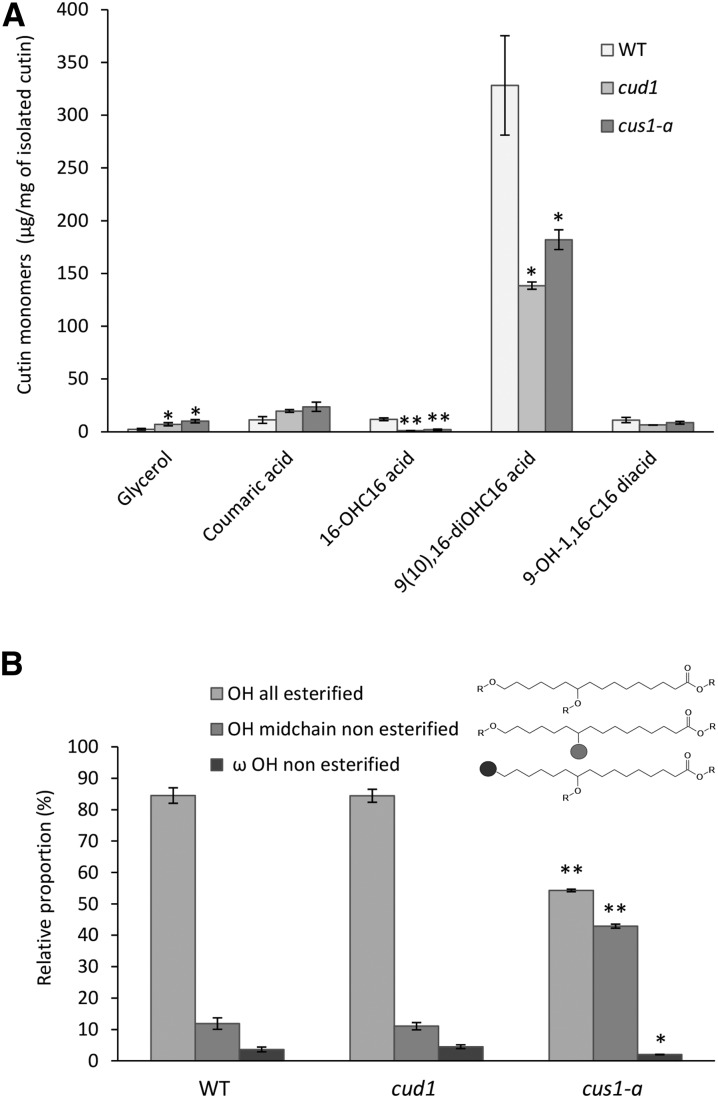
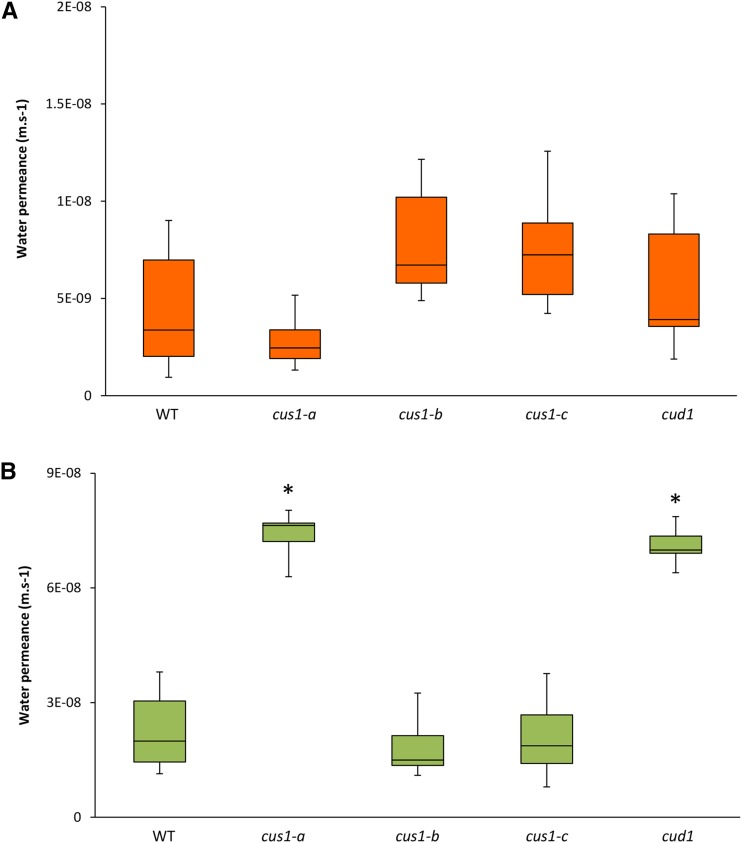
Additionally, in a previous study, cv M82 tomato fruits affected in SlCUS1 expression had a higher transpiration rate than its background genotype (Isaacson et al., 2009). This experiment was conducted on whole fruit, and it could not be deciphered whether this higher water permeability was associated with the observed modifications of cutin or with the wax composition. Accordingly, the water permeance of isolated and enzyme-treated cuticle and cutin from wild-type and EMS mutant fruit were compared to assess the impact of polymerization. The tomato mutant cus1-a had a sharply reduced thickness. While comparing cuticle from different species, it was concluded that there is no direct correlation between cuticle permeability and cuticle thickness (Riederer and Schreiber, 2001). Nevertheless, when comparing cutin with similar monomer composition, a thickness effect could not be ruled out. Another EMS tomato mutant, cutin-deficient1 (cud1), highlighted a thinner cutin with similar cutin composition than that shown previously (Petit et al., 2014) and confirmed in this study (Fig. 5A), without a defect in ester cross-linking according to the labeling of the free OH derivatization (Fig. 5B). Accordingly, cud1 was used as a control in the water permeance experiments. The water permeance of the isolated enzyme-treated cuticle from the different cutin mutants, either affected (cus1-a) or not (cud1) in cutin cross-linking, was compared (Fig. 6A), and no significant differences were observed between the different cv Micro-Tom mutants and the wild type. Conversely, the median water permeance of the enzyme-treated dewaxed cuticle of the cus1-a mutant was significantly (P < 0.05) higher than the permeance of the wild type as well as the cus1-b and cus1-c cutin (Fig. 6B). However, the water permeances of the cud1 and cus1-a cutins were not statistically different, while both mutants highlighted a reduced cutin thickness compared with the wild-type fruits (Petit et al., 2014). These data suggest that modification of the polymerization pattern of cus1-a cutin is not the major factor that explains its higher cutin water permeance.
Figure 5.
The cutin-deficient mutant cud1 is not affected in the polymerization of 9(10),16-dihydroxyhexadecanoic acid. A, Quantification of major cutin monomers. 16-OHC16 acid, 16-Hydroxyhexadecanoic acid; 9(10),16-diOHC16 acid, 9(10),16-dihydroxyhexadecanoic acid; 9-OH-1,16-C16 diacid, 9-hydroxy-1,16-hexadecanedioic acid. B, Esterification level of 9(10),16-dihydroxyhexadecanoic acid OH groups from cud1 cutin compared with wild-type (WT) and cus1-a cutins. Data are expressed as mean values, and error bars represent sd (n = 3). Significant differences from the wild type are indicated by asterisks (Student’s t test; *, P < 0.05 and **, P < 0.01).
Figure 6.
Comparison of the water permeance of tomato cutin in red ripe fruits from wild-type and tomato EMS mutant plants. Data are shown as median values ± minimum/maximum (n = 8–10). Differences between the wild type (WT), cus1 mutants (cus1-a, cus1-b, and cus1-c), and cud1 were tested with the Mann-Whitney U test. Asterisks indicate significant differences from the wild type (*, P < 0.05).
DISCUSSION
SlCUS1 Catalysis Impacts the Ester Cross-Linking of Fruit Cutin
The cutin polyester is essential for the functional properties of plant cuticles (Nawrath, 2006; Schreiber, 2010; Yeats and Rose, 2013). While cutin monomer biosynthesis is now relatively well described (Li-Beisson et al., 2010, 2013; Beisson et al., 2012), the extracellular assembly of the monomers in a three-dimensional network is less documented. In this regard, the role of SlCUS1, a member of the GDSL lipase, expressed in the tomato fruit exocarp, on cutin polymerization, has been elucidated by two concomitant independent studies (Girard et al., 2012; Yeats et al., 2012). GDSL lipases are ubiquitous proteins in the plant kingdom suggesting a CUS1-like conserved pathway for cutin polymerization (Yeats et al., 2014). With the discovery of the key function of glycerol-3-phosphate acyltransferases in cutin deposition (Li et al., 2007), it has been hypothesized that 2-MAG could be involved in glycerol incorporation within cutin polymer. Besides, it has been shown, in vitro, that these 2-MAGs produced by glycerol-3-phosphate acyltransferases are substrates of SlCUS1 (Yeats et al., 2012, 2014; Yeats and Rose, 2013). Consequently, a mechanism involving the self-transesterification of 2-MAG precursors by CUS1 has been proposed, leading first to the formation of a 9(10),16-dihydroxyhexadecanoyl dimer and then, in a cascade, to oligomers of higher molecular mass. This mechanism implies the release of glycerol and is consistent with the low level of glycerol in tomato cuticles and, indeed, in most plant cutins (Yeats et al., 2014). This catalysis involves only the primary OH group of the dihydroxy fatty acid and leaves the medium chain OH nonesterified, as demonstrated by the NMR analysis of the oligomers produced in vitro.
In this study, we observed a lower molar proportion of glycerol and, in particular, a lower molar ratio of glycerol to OH fatty acids in the wild-type cutin than in the SlCUS1 cutin of knockdown lines and cus1-a mutants. Considering the proposed polymerization mechanism, in which the residual glycerol is due to a residual monoglyceride unit during polyester growth (Yeats et al., 2014), these results may indicate that polyesters of shorter chain length are formed in the cutins of cus1-a mutants than in those of wild-type fruits. Remarkably, in these mutants, we also observed a significant decrease in the esterification level of the secondary OH group of the dihydroxy fatty acid. This decrease was associated with a decrease of cutin polyester deposition, which means that, from a quantitative point of view, both primary and secondary OH group contents decreased significantly in the cus1 mutants. Therefore, these results strongly support that, in planta, SlCUS1 is involved in cutin polymer branching by carrying out the esterification of both primary and secondary OH groups. This mechanism was verified in both 20-DPA and mature fruits and whatever the genetic background of the mutant lines (i.e. cv Micro-Tom or cv WVa106). While cv Micro-Tom belongs to the domesticated tomato group, the cherry-type tomato cv WVa106 clusters with the wild species Solanum pimpinellifolium (Ranc et al., 2008). These results suggest that the function of SlCUS1 can be conserved across tomato cultivars and species displaying diverse cuticle architecture and composition (Ranc et al., 2008; Yeats et al., 2014). It is noteworthy that the involvement of both the primary and secondary OH groups is quite compatible with the release of glycerol during the catalytic process and, therefore, with a low proportion of glycerol in the cutin polyester.
Considering the contribution of glycerol to the structure of cutin polymer, its OH groups are mostly involved in the formation of ester bonds in wild-type plants, whereas its esterification level decreases significantly in the Pro-35S:SlCUS1RNAi and cus1-a mutant. The nonesterified OH groups of glycerol were observed at both the sn-2 and sn-1,3 positions, and especially in the mutants, the esterification of both positions was impacted. Furthermore, a significantly higher impact was observed on the sn-1,3 positions than on the sn-2 position. First, this means that the primary OH groups of glycerol are involved, as expected, in the transesterification process and agree with the presence of 1-MAG in the cutin building blocks (Graça et al., 2002). Second, these results strengthen the hypothesis that, in tomato, 2-MAGs are precursors of the cutin polymer as they are in Arabidopsis and, as discussed above, that the polymer sizes of the polyesters are lower in cus1-a mutants than in the wild type. Finally, an isomerization from 2-MAG to 1,3-monoacylglycerol cannot be excluded, as it was observed previously in vitro for 2-MAG (Lyubachevskaya and Boyle-Roden, 2000; Compton et al., 2012). The apparent discrepancy between these results and previous in vitro experiments (Yeats et al., 2014) can be easily explained by differences in the environment of CUS1. Indeed, in vitro catalysis (Yeats et al., 2014) was performed in an aqueous medium, while in planta, the enzyme is specifically localized within the cutin matrix (Girard et al., 2012; Yeats et al., 2012), which means that the catalysis occurs in its native hydrophobic environment. As observed for lipases in organic water-free solvents (Sharma et al., 2001), this hydrophobic environment could favor the transesterification process, thereby leading to the synthesis of high molecular mass polyesters. These lipase-catalyzed esterifications are well documented for both the synthesis of small esters and the synthesis of high-Mr polyesters (Wescott and Klibanov, 1994; Krishna et al., 2002; Kobayashi, 2010). These studies have clearly shown that the lipase-catalyzed esterification can involve both primary and secondary alcohol groups, depending on the source of lipase and environmental conditions (e.g. the temperature and the type of organic solvent), and lipases immobilized on polymer beads (e.g. acrylic resin) or grafted with polyoxyethylene detergent. In particular, the impact of solvent hydrophobicity on the regiospecificity of lipase-catalyzed transesterification has already been reported. Indeed, in the case of Pseudomonas cepacia lipase, a shift from toluene to acetonitrile modified the regioselectivity of the butanolysis of 1,4-dibutyryloxy-2-octylbenzene, leading to either a 1-hydroxy or a 4-hydroxy compound (Rubio et al., 1991). Moreover, although CUS1 is structurally different from these microbial lipases, at least on the basis of the position of the catalytic amino acid (i.e. Asp-Ser-Pro catalytic triad) in the sequence, it is interesting to note that CUS1 is localized in a hydrophobic environment controlled by wax deposition in which it is more or less immobilized on polysaccharides.
Besides, the involvement of another protein activating CUS1, as occurs in the lipase-colipase system of the pancreatic enzyme (Lowe, 1997), cannot be excluded.
Finally, although the same results were obtained in two different genetic backgrounds and with two different ways to modulate CUS1 expression, the hypothesis of an indirect effect of CUS1 down-regulation, such as an adaptation phenomenon, on cutin structure cannot be totally ruled out.
Changes in the Esterification in cus1 Mutants Reveal Another Polymerization Mechanism
Other interesting and unexpected results include (1) the sharp increase in the molar ratio of glycerol to dihydroxy fatty acid in the SlCUS1-depleted mutants compared with the corresponding wild-type fruit cutins and (2) the lower esterification level of the secondary OH groups in both glycerol and dihydroxy fatty acids. As suggested above, this means that a polyester of lower molecular size is formed in the mutants than in the wild-type plants. This suggests that, in the absence of SlCUS1, another mechanism is involved in the formation of the cutin polymer. Indeed, GDSL lipases form a multigenic family of proteins, and more than 100 genes are found in the tomato genome (http://solgenomics.net/). Therefore, additional GDSL lipases with CUS activity could be expressed in the fruit epidermis, as already shown during the early stages of fruit development (Yeats et al., 2010, 2014; Matas et al., 2011). These enzymes would have a higher regiospecificity toward primary OH groups. Since nonenzymatic transesterification processes preferentially use primary OH groups (Benítez et al., 2015), such reactions cannot be excluded. A self-assembly process of cutin monomers has already been suggested for cutin formation (Domínguez et al., 2015). Whatever the mechanisms involved in the absence of SlCUS1 (i.e. enzymatic or nonenzymatic), our results suggest that, in the wild-type plants, different mechanisms should participate in the formation of the cutin polymer.
Benzyl Etherification: A Tool to Probe Cutin Ester Cross-Link Heterogeneity
Derivatization within cutin polymers coupled with Raman imaging is also a new tool to elucidate the structural heterogeneity within the cutin biopolymer. This heterogeneity has been suggested previously in the literature (Stark and Tian, 2006) and was fully confirmed by our data here. The most highly cross-linked areas are localized at the ridge between the cells and coincide with high CUS1 immunolabeling (Girard et al., 2012). This strengthens the role of CUS1 in the extracellular polymerization of the cutin polymer. In addition, this labeling procedure could also be applied to the study of cutin formation. Indeed, according to different biochemical and spectrometric studies, modification of the cross-links within the cutin polymer may be associated with the changes in cutin composition observed during fruit development (Baker et al., 1982; Benítez et al., 2004; Matas et al., 2011). Such studies would indeed benefit from this imaging approach.
The Ester Cross-Link Does Not Affect the Water Permeance of Cutin
The question of the impact of cutin cross-linking on the functional properties of cutin is still open. The tomato mutants with different levels of cross-linking that were characterized in our study should be valuable tools for further studies on the biological function of the cutin.
A previous study has compared the water permeance of intact, dewaxed, and peeled cv Micro-Tom red ripe tomato in whole fruit (Leide et al., 2007). It was estimated that cutin accounted for approximatively 20% of cuticle permeance. Although minor, this is a significant contribution to cuticle permeability.
From our results, it can be concluded that the cross-linking pattern of the tomato cutin has a limited impact on the water permeance of the polymer. Rather, cutin water permeance seems to be associated with polymer thickness. It is noteworthy that in both cus1-a and cud1 mutants, the molar ratio between glycerol and hydroxy fatty acids is significantly higher than in the wild-type plants (i.e. 0.159 ± 0.006, 0.170 ± 0.027, and 0.025 ± 0.001, respectively). A potential effect of the esterified glycerol on cutin permeability is not documented in the literature but cannot be ruled out.
The water permeance of isolated cuticle was not correlated with the cutin permeance, as no significant difference in water permeance was highlighted between the cus1-a, cud1, and wild-type cuticles. The median water permeances of the cuticles were much lower than that of cutin, which fits with a major impact of waxes on water permeance. It was shown previously that the wax load was higher in cus1-a (8.9 ± 1.3 μg cm−2) and cud1 (10.6 ± 1.9 μg cm−2) than in wild-type (6.2 ± 0.7 μg cm−2) red ripe tomato fruits (Petit et al., 2014). This result suggests that the wax load could have compensated for the higher cutin water permeance of cus1-a and cud1. Waxes are either deposited on the outer surface of the cuticle (epicuticular waxes) or embedded in the cutin polymer (intracuticular waxes), and a recent study has established that epicuticular waxes from Prunus laurocerasus leaves were not significantly involved in the water-loss barrier properties of the cuticle (Zeisler and Schreiber, 2016). In this study, the ratio of the intracuticular and epicuticular waxes of the isolated tomato cuticles was not monitored but could explain how the water permeance of cuticle was not associated directly with the wax load.
CONCLUSION
The benzyl etherification of nonesterified OH groups in cutin is a procedure that probes cutin polymerization in planta by combining complementary GC-MS and microspectroscopy imaging analyses of cutin building blocks. The results confirm the major role for SlCUS1 in cutin polymerization in planta. SlCUS1 is involved in a transesterification process that is compatible with 2-MAG as the enzyme substrate and involves both primary and secondary OH groups of 9(10),16-dihydroxyhexadecanoic acid and glycerol. This enzymatic catalysis leads to glycerol release and, therefore, is consistent with the low glycerol content of cutin polyester. This enzymatic process leads to a highly reticulated network but, surprisingly, without a major relationship with the water permeance of the corresponding cutins. Finally, in the absence of SlCUS1, another enzymatic or nonenzymatic mechanism that produces cutins with a higher proportion of glycerol could take place. In wild-type plants, this mechanism could coexist with the SlCUS1-catalyzed formation of the cutin polyester.
MATERIALS AND METHODS
Plant Materials and Isolation of Fruit Cuticles
The Pro-35S:SlCUS1RNAi lines generated in the cherry tomato (Solanum lycopersicum var cerasiforme ‘WVa106’; L10, L4, L3, and L17) have been described previously (Girard et al., 2012). The cus1 cv Micro-Tom mutants were identified from a tomato EMS mutant collection either by phenotypic screening for P15C12 and renamed cus1-a (Petit et al., 2014) or by TILLING (P13E2 renamed cus1-b and P4H12 renamed cus1-c; Supplemental Fig. S1). The cutin-deficient mutant P23F12 was identified from the same collection by phenotypic screening (Petit et al., 2014) and was renamed cud1. All plants were grown in a greenhouse under previously described conditions (Girard et al., 2012; Petit et al., 2014).
Fruit skins were peeled and enzymatically digested in a solution of 1% (v/v) cellulase and 2% (v/v) pectinase containing 1 mm NaN3. After drying, the cuticles were exhaustively delipidated in CH3OH:CH2Cl2 (1:2) and dried for analysis. These enzyme-treated and dewaxed cuticles are referred to as cutins here.
For SlCUS1 immunoblot analysis, the proteins were extracted from 10 mg of 20-DPA fruit pericarps ground in liquid nitrogen. Proteins were extracted with a 50 mm Tris-HCl buffer (pH 8) containing 2% SDS and 1% mercaptoethanol. After electroblotting, SlCUS1 was detected with an Immuno-Star AP kit (Bio-Rad) as described previously (Girard et al., 2012).
ATR-FTIR and Atomic Force Microscopy
FTIR spectra (200 scans) were recorded by ATR as described previously (Girard et al., 2012) on a Nicolet Magna IR 550 spectrometer using a single reflection accessory fitted with a thermostatted diamond crystal with a 45° angle of light incidence. Two spectra were acquired on four different parts of the fruit cutin, and four fruits per line were analyzed. Surface calculations were conducted from the nonnormalized spectra after a baseline correction, which was established in the same conditions using Galactic software (Thermo Scientific). The surfaces of the methylene (CH2; 2,978–2,838 cm−1) and the carbonyl (CO; 1,750–1,695 cm−1) bands were measured and used to calculate the ratio RICH2/ICO.
Derivatization of Free OH Groups within Cutin Polyester
Free OH groups were derivatized by benzyl etherification by a procedure adapted from a procedure developed for organic alcohols (Lopez and Dudley, 2008). Isolated cutins (3 mg) were mixed in a screw-capped glass tube for 24 h with 15 mg of 2-benzyloxy-1-methylpyridinium triflate (Sigma-Aldrich) and 1.68 mg of magnesium oxide in 1 mL of trifluorotoluene at 90°C overnight. Cutin was then washed extensively with CH2Cl2 and dried. By comparing different sizes of cutin samples and cutin powders, we verified that the same amount of derivatized cutin monomer was obtained, indicating that cutin diffusion barriers did not hinder the reaction.
Determination of Cutin Monomer Composition
Tomato cutins were depolymerized via methanolysis according to a previously described procedure (Molina et al., 2006). The reaction was performed in dry methanol containing 15% (v/v) methyl acetate and 6% (v/v) sodium methoxide, and 15-hydroxypentadecanoic acid was added to the reaction medium as an internal standard. Complete depolymerization was obtained after 6 h of heating at 60°C. Afterward, the mixture was cooled, and it was adjusted to pH 5 with acetic acid. Fatty acids were extracted from the aqueous mixture with methylene chloride, and the nonbenzylated OH groups of the depolymerized fatty acids were silylated with 1% N,O-bis(trimethylsilyl)trifluoroacetamide/trimethyl chlorosilane and analyzed by GC-MS and gas chromatography-flame ionization detection as described previously (Girard et al., 2012).
The mass spectrometry spectra of derivatized cutin monomers were characterized by their fragmentation and the ion of mass 91 corresponding to the benzyloxy group (Supplemental Fig. S2).
Glycerol Quantification
Glycerol was released by mild methanolysis using a modified procedure (Graça et al., 2002). Isolated cutin slices were stirred at room temperature in a mixture of 50 mm sodium methoxide in dry methanol with the internal standard 1,2,3-butanetriol (Shen and Xu, 2013). We used different reaction times to determine the time necessary to attain a plateau for glycerol release. After 20 h, the extract was dried with a nitrogen flow, silylated with 1% N,O-bis(trimethylsilyl)trifluoroacetamide/trimethyl chlorosilane, and analyzed by GC-MS and gas chromatography-flame ionization detection.
Raman Microspectrometry Imaging
Cutin samples (approximately 2 cm2) were placed on aluminum supports that were mounted on the motorized xyz stage of the InVia Raman spectrometer (Renishaw) equipped with confocal capability, a 785-nm diode laser (50 mW), and a 1,200 L mm−1 grating. The instrument wavelength was calibrated with silicon at 520 cm−1. Spectra were collected with an accumulation time of 0.5 s over a fixed window of 1,065 cm−1 (ranging from 838 to 1,902 cm−1) every 2 mm over a line map of 100 to 200 mm. The instrument was operated using a 50× or 100× objective, a 65-μm slit, and a 1,040- × 256-pixel Renishaw CCD camera. Each data set was analyzed using the Renishaw WIRE 4.1 software.
Measurement of Water Permeance of Tomato Fruit Cutin
Purified tomato fruit cutins (approximately 1.5 cm2) from wild-type and EMS mutant cv Micro-Tom plants were used for water permeability experiments using a gravimetric method as described previously (Schreiber and Riederer, 1996; Riederer and Schreiber, 2001). Isolated cuticles or cutin (eight to 10 samples of each mutant) were placed in transpiration chambers filled with 300 μL of deionized water. The amount of evaporated water across the cuticle was monitored as a function of time to achieve a linear regression. Permeances (m s−1) were calculated from the slope of this linear regression. Water permeances were presented as medians with 25% and 75% quartiles.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers solyc11g006250; Solgenomics, SGN-U585129; NCBI Gene ID, LOC101254153.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. EMS MicroTom mutants used this study.
Supplemental Figure S2. Benzyl etherification of non esterified OH groups in cutin monomers.
Supplemental Figure S3. Esterification level of glycerol in cus1mutants and Pro35S:SlCUS1RNAi cutin fruits.
Supplemental Figure S4. Esterification levels of 9(10),16-dihydroxyhexadecanoic acid of cus1 mutants and Pro35S:SlCUS1RNAi fruit cutin.
Supplemental Figure S5. Typical Raman spectra of non-alkylated and alkylated cutin, and alkylation reagent.
Supplementary Material
Glossary
- OH
hydroxyl
- ATR
attenuated total reflectance
- FTIR
Fourier transform infrared
- 2-MAG
2-monoacylglycerol
- WVa106
West Virginia 106
- EMS
ethyl methanesulfonate
- GC-MS
gas chromatography-mass spectrometry
Footnotes
This work was supported by the Institut National de la Recherche Agronomique and the Région Pays de la Loire to G.P.
References
- Baker EA, Bukovac MJ, Hunt GM (1982) Composition of tomato fruit cuticle as related to fruit growth and development. In Cutler DF, Alvin KL, Price CE, eds, The Plant Cuticle. Academic Press, London, pp 33–44 [Google Scholar]
- Bargel H, Neinhuis C (2005) Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J Exp Bot 56: 1049–1060 [DOI] [PubMed] [Google Scholar]
- Beisson F, Li-Beisson Y, Pollard M (2012) Solving the puzzles of cutin and suberin polymer biosynthesis. Curr Opin Plant Biol 15: 329–337 [DOI] [PubMed] [Google Scholar]
- Benítez JJ, Heredia-Guerrero JA, Guzmán-Puyol S, Domínguez E, Heredia A (2015) Polyester films obtained by noncatalyzed melt-condensation polymerization of aleuritic (9,10,16-trihydroxyhexadecanoic) acid in air. J Appl Polym Sci 132: 41328 [Google Scholar]
- Benítez JJ, Matas AJ, Heredia A (2004) Molecular characterization of the plant biopolyester cutin by AFM and spectroscopic techniques. J Struct Biol 147: 179–184 [DOI] [PubMed] [Google Scholar]
- Bessire M, Borel S, Fabre G, Carraça L, Efremova N, Yephremov A, Cao Y, Jetter R, Jacquat AC, Métraux JP, et al. (2011) A member of the PLEIOTROPIC DRUG RESISTANCE family of ATP binding cassette transporters is required for the formation of a functional cuticle in Arabidopsis. Plant Cell 23: 1958–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure G, Beisson F, Ohlrogge J, Pollard M (2004) Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6,cis-9-diene-1,18-dioate as the major component. Plant J 40: 920–930 [DOI] [PubMed] [Google Scholar]
- Compton D, Laszlo J, Appell M, Vermillion K, Evans K (2012) Influence of fatty acid desaturation on spontaneous acyl migration in 2-monoacylglycerols. J Am Oil Chem Soc 89: 2259–2267 [Google Scholar]
- Deas AHB, Holloway PJ (1977) The intermolecular structure of some plant cutins. In Tevini M, Lichtenthaler H, eds, Lipids and Lipid Polymers in Higher Plants. Springer Verlag, Berlin, pp 293–299 [Google Scholar]
- Deshmukh AP, Simpson AJ, Hatcher PG (2003) Evidence for cross-linking in tomato cutin using HR-MAS NMR spectroscopy. Phytochemistry 64: 1163–1170 [DOI] [PubMed] [Google Scholar]
- Domínguez E, Heredia-Guerrero JA, Heredia A (2015) Plant cutin genesis: unanswered questions. Trends Plant Sci 20: 551–558 [DOI] [PubMed] [Google Scholar]
- Espelie KE, Dean BB, Kolattukudy PE (1979) Composition of lipid-derived polymers from different anatomical regions of several plant species. Plant Physiol 64: 1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L (2005) Apoplastic polyesters in Arabidopsis surface tissues: a typical suberin and a particular cutin. Phytochemistry 66: 2643–2658 [DOI] [PubMed] [Google Scholar]
- Gilbert RD, Johnson AM, Dean RA (1996) Chemical signals responsible for appressorium formation in the rice blast fungus Magnaporthe grisea. Physiol Mol Plant Pathol 48: 335–346 [Google Scholar]
- Girard AL, Mounet F, Lemaire-Chamley M, Gaillard C, Elmorjani K, Vivancos J, Runavot JL, Quemener B, Petit J, Germain V, et al. (2012) Tomato GDSL1 is required for cutin deposition in the fruit cuticle. Plant Cell 24: 3119–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graça J, Lamosa P (2010) Linear and branched poly(omega-hydroxyacid) esters in plant cutins. J Agric Food Chem 58: 9666–9674 [DOI] [PubMed] [Google Scholar]
- Graça J, Schreiber L, Rodrigues J, Pereira H (2002) Glycerol and glyceryl esters of omega-hydroxyacids in cutins. Phytochemistry 61: 205–215 [DOI] [PubMed] [Google Scholar]
- Gupta NS, Michels R, Briggs DE, Evershed RP, Pancost RD (2006) The organic preservation of fossil arthropods: an experimental study. Proc Biol Sci 273: 2777–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia-Guerrero JA, Benítez JJ, Domínguez E, Bayer IS, Cingolani R, Athanassiou A, Heredia A (2014) Infrared and Raman spectroscopic features of plant cuticles: a review. Front Plant Sci 5: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson T, Kosma DK, Matas AJ, Buda GJ, He Y, Yu B, Pravitasari A, Batteas JD, Stark RE, Jenks MA, et al. (2009) Cutin deficiency in the tomato fruit cuticle consistently affects resistance to microbial infection and biomechanical properties, but not transpirational water loss. Plant J 60: 363–377 [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Takeuchi Y, Takada Y, Yamaoka N (2002) Coleoptile surface cuticle of barley is involved in survival and penetration of Blumeria graminis. Physiol Mol Plant Pathol 60: 31–38 [Google Scholar]
- Just D, Garcia V, Fernandez L, Bres C, Mauxion JP, Petit J, Jorly J, Assali J, Bournonville C, Ferrand C, et al. (2013) Micro-Tom mutants for functional analysis of target genes and discovery of new alleles in tomato. Plant Biotechnol 30: 225–231 [Google Scholar]
- Kersteins G. (1996) Plant Cuticles: An Integrated Approach. BIOS Scientific Publisher, Oxford [Google Scholar]
- Kobayashi S. (2010) Lipase-catalyzed polyester synthesis: a green polymer chemistry. Proc Jpn Acad Ser B Phys Biol Sci 86: 338–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE. (1977) Lipid polymers and associated phenols, their chemistry, biosynthesis, and role in pathogenesis. Recent Adv Phytochem 77: 185–246 [Google Scholar]
- Krishna SH, Srinivas ND, Raghavarao KS, Karanth NG (2002) Reverse micellar extraction for downstream processing of proteins/enzymes. Adv Biochem Eng Biotechnol 75: 119–183 [DOI] [PubMed] [Google Scholar]
- Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42: 51–80 [DOI] [PubMed] [Google Scholar]
- Lee SB, Suh MC (2015) Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep 34: 557–572 [DOI] [PubMed] [Google Scholar]
- Leide J, Hildebrandt U, Reussing K, Riederer M, Vogg G (2007) The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: effects of a deficiency in a beta-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiol 144: 1667–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Beisson F, Koo AJ, Molina I, Pollard M, Ohlrogge J (2007) Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc Natl Acad Sci USA 104: 18339–18344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y, Pollard M, Sauveplane V, Pinot F, Ohlrogge J, Beisson F (2009) Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc Natl Acad Sci USA 106: 22008–22013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, et al. (2010) Acyl-lipid metabolism. The Arabidopsis Book 8: e0133 doi: 10.1199/tab.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, et al. (2013) Acyl-lipid metabolism. The Arabidopsis Book 11: e0161 doi: 10.1199/tab.0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn GR, Mansfield JC, Parker D, Lind R, Perfect S, Seymour M, Smirnoff N, Love J, Moger J (2015) In vivo chemical and structural analysis of plant cuticular waxes using stimulated Raman scattering microscopy. Plant Physiol 168: 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez SS, Dudley GB (2008) Convenient method for preparing benzyl ethers and esters using 2-benzyloxypyridine. Beilstein J Org Chem 4: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe ME. (1997) Structure and function of pancreatic lipase and colipase. Annu Rev Nutr 17: 141–158 [DOI] [PubMed] [Google Scholar]
- Lyubachevskaya G, Boyle-Roden E (2000) Kinetics of 2-monoacylglycerol acyl migration in model chylomicra. Lipids 35: 1353–1358 [DOI] [PubMed] [Google Scholar]
- Matas AJ, Yeats TH, Buda GJ, Zheng Y, Chatterjee S, Tohge T, Ponnala L, Adato A, Aharoni A, Stark R, et al. (2011) Tissue- and cell-type specific transcriptome profiling of expanding tomato fruit provides insights into metabolic and regulatory specialization and cuticle formation. Plant Cell 23: 3893–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz-Oron S, Mandel T, Rogachev I, Feldberg L, Lotan O, Yativ M, Wang Z, Jetter R, Venger I, Adato A, et al. (2008) Gene expression and metabolism in tomato fruit surface tissues. Plant Physiol 147: 823–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina I, Bonaventure G, Ohlrogge J, Pollard M (2006) The lipid polyester composition of Arabidopsis thaliana and Brassica napus seeds. Phytochemistry 67: 2597–2610 [DOI] [PubMed] [Google Scholar]
- Nawrath C. (2006) Unraveling the complex network of cuticular structure and function. Curr Opin Plant Biol 9: 281–287 [DOI] [PubMed] [Google Scholar]
- Okabe Y, Asamizu E, Saito T, Matsukura C, Ariizumi T, Brès C, Rothan C, Mizoguchi T, Ezura H (2011) Tomato TILLING technology: development of a reverse genetics tool for the efficient isolation of mutants from Micro-Tom mutant libraries. Plant Cell Physiol 52: 1994–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman SF, Gerard HC, Fett WF, Moreau RA, Dudley RL (1995) Method for the production and characterization of tomato cutin oligomers. J Agric Food Chem 43: 2134–2137 [Google Scholar]
- Osman SF, Irwin P, Fett WF, O’Connor JV, Parris N (1999) Preparation, isolation, and characterization of cutin monomers and oligomers from tomato peels. J Agric Food Chem 47: 799–802 [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Shi JX, Schreiber L, Aharoni A (2009) The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiol 151: 1773–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel S, Franke R, Schreiber L, Knoche M (2007) Composition of the cuticle of developing sweet cherry fruit. Phytochemistry 68: 1017–1025 [DOI] [PubMed] [Google Scholar]
- Petit J, Bres C, Just D, Garcia V, Mauxion JP, Marion D, Bakan B, Joubès J, Domergue F, Rothan C (2014) Analyses of tomato fruit brightness mutants uncover both cutin-deficient and cutin-abundant mutants and a new hypomorphic allele of GDSL lipase. Plant Physiol 164: 888–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard M, Beisson F, Li Y, Ohlrogge JB (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13: 236–246 [DOI] [PubMed] [Google Scholar]
- Ranc N, Muños S, Santoni S, Causse M (2008) A clarified position for Solanum lycopersicum var. cerasiforme in the evolutionary history of tomatoes (Solanaceae). BMC Plant Biol 8: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer M, Schreiber L (2001) Protecting against water loss: analysis of the barrier properties of plant cuticles. J Exp Bot 52: 2023–2032 [DOI] [PubMed] [Google Scholar]
- Rubio E, Fernandez-Mayorales A, Klibanov AM (1991) Effect of solvent on enzyme regioselectivity. J Am Chem Soc 113: 695–696 [Google Scholar]
- Samuels L, Kunst L, Jetter R (2008) Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu Rev Plant Biol 59: 683–707 [DOI] [PubMed] [Google Scholar]
- Schreiber L. (2010) Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci 15: 546–553 [DOI] [PubMed] [Google Scholar]
- Schreiber L, Riederer M (1996) Ecophysiology of cuticular transpiration: comparative investigation of cuticular water permeability of plant species from different habitats. Oecologia 107: 426–432 [DOI] [PubMed] [Google Scholar]
- Schweizer P, Felix G, Buchala A, Muller C, Metraux JP (1996) Perception of free cutin monomers by plant cells. Plant J 10: 331–341 [Google Scholar]
- Sharma R, Chisti Y, Banerjee UC (2001) Production, purification, characterization, and applications of lipases. Biotechnol Adv 19: 627–662 [DOI] [PubMed] [Google Scholar]
- Shen Y, Xu Z (2013) An improved GC-MS method in determining glycerol in different types of biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 930: 36–40 [DOI] [PubMed] [Google Scholar]
- Sieber P, Schorderet M, Ryser U, Buchala A, Kolattukudy P, Métraux JP, Nawrath C (2000) Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell 12: 721–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark RE, Tian S (2006) The cutin biopolymer matrix. In Riederer M, Mueller C, eds, Biology of the Plant Cuticle. Blackwell Publishing, Oxford, pp 126–144 [Google Scholar]
- Taketa S, Amano S, Tsujino Y, Sato T, Saisho D, Kakeda K, Nomura M, Suzuki T, Matsumoto T, Sato K, et al. (2008) Barley grain with adhering hulls is controlled by an ERF family transcription factor gene regulating a lipid biosynthesis pathway. Proc Natl Acad Sci USA 105: 4062–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Fang X, Wang W, Yu B, Cheng X, Qiu F, Mort AJ, Stark RE (2008) Isolation and identification of oligomers from partial degradation of lime fruit cutin. J Agric Food Chem 56: 10318–10325 [DOI] [PubMed] [Google Scholar]
- Wescott CR, Klibanov AM (1994) The solvent dependence of enzyme specificity. Biochim Biophys Acta 1206: 1–9 [DOI] [PubMed] [Google Scholar]
- Yang W, Pollard M, Li-Beisson Y, Beisson F, Feig M, Ohlrogge J (2010) A distinct type of glycerol-3-phosphate acyltransferase with sn-2 preference and phosphatase activity producing 2-monoacylglycerol. Proc Natl Acad Sci USA 107: 12040–12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats TH, Howe KJ, Matas AJ, Buda GJ, Thannhauser TW, Rose JK (2010) Mining the surface proteome of tomato (Solanum lycopersicum) fruit for proteins associated with cuticle biogenesis. J Exp Bot 61: 3759–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats TH, Huang W, Chatterjee S, Viart HM, Clausen MH, Stark RE, Rose JK (2014) Tomato Cutin Deficient 1 (CD1) and putative orthologs comprise an ancient family of cutin synthase-like (CUS) proteins that are conserved among land plants. Plant J 77: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats TH, Martin LB, Viart HM, Isaacson T, He Y, Zhao L, Matas AJ, Buda GJ, Domozych DS, Clausen MH, et al. (2012) The identification of cutin synthase: formation of the plant polyester cutin. Nat Chem Biol 8: 609–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats TH, Rose JK (2013) The formation and function of plant cuticles. Plant Physiol 163: 5–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisler V, Schreiber L (2016) Epicuticular wax on cherry laurel (Prunus laurocerasus) leaves does not constitute the cuticular transpiration barrier. Planta 243: 65–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.