The cuticular transpiration barrier is formed by very-long-chain acyls rather than alicyclic wax compounds, primarily in the intracuticular layer and complemented by epicuticular wax in some species.
Abstract
Plant cuticular waxes play a crucial role in limiting nonstomatal water loss. The goal of this study was to localize the transpiration barrier within the layered structure of cuticles of eight selected plant species and to put its physiological function into context with the chemical composition of the intracuticular and epicuticular wax layers. Four plant species (Tetrastigma voinierianum, Oreopanax guatemalensis, Monstera deliciosa, and Schefflera elegantissima) contained only very-long-chain fatty acid (VLCFA) derivatives such as alcohols, alkyl esters, aldehydes, and alkanes in their waxes. Even though the epicuticular and intracuticular waxes of these species had very similar compositions, only the intracuticular wax was important for the transpiration barrier. In contrast, four other species (Citrus aurantium, Euonymus japonica, Clusia flava, and Garcinia spicata) had waxes containing VLCFA derivatives, together with high percentages of alicyclic compounds (triterpenoids, steroids, or tocopherols) largely restricted to the intracuticular wax layer. In these species, both the epicuticular and intracuticular waxes contributed equally to the cuticular transpiration barrier. We conclude that the cuticular transpiration barrier is primarily formed by the intracuticular wax but that the epicuticular wax layer may also contribute to it, depending on species-specific cuticle composition. The barrier is associated mainly with VLCFA derivatives and less (if at all) with alicyclic wax constituents. The sealing properties of the epicuticular and intracuticular layers were not correlated with other characteristics, such as the absolute wax amounts and thicknesses of these layers.
The plant cuticle is one of the major adaptations of vascular plants for life in the atmospheric environment. Accordingly, the primary function of cuticles is to limit nonstomatal water loss and, thus, to protect plants against drought stress (Burghardt and Riederer, 2006). However, plant cuticles also play roles in minimizing the adhesion of dust, pollen, and spores (Barthlott and Neinhuis, 1997), protecting tissues from UV radiation (Krauss et al., 1997; Solovchenko and Merzlyak, 2003), mediating biotic interactions with microbes (Carver and Gurr, 2006; Leveau, 2006; Hansjakob et al., 2010, 2011; Reisberg et al., 2012) as well as insects (Eigenbrode and Espelie, 1995; Müller and Riederer, 2005), and preventing deleterious fusions between different plant organs (Tanaka and Machida, 2013).
Cuticles are composite (nonbilayer) membranes consisting of an insoluble polymer matrix and solvent-soluble waxes. The polymer matrix (MX) is mainly made of the hydroxy fatty acid polyester cutin (Nawrath, 2006) and also contains polysaccharides and proteins (Heredia, 2003). In contrast, cuticular waxes are complex mixtures of aliphatic compounds derived from very-long-chain fatty acids (VLCFAs) with hydrocarbon chains of C20 and more (Jetter et al., 2007). Wax quantities and compositions vary greatly between plant species and, in many cases, even between organs and developmental stages. Diverse VLCFA derivatives can be present, including free fatty acids, aldehydes, ketones, primary and secondary alcohols, alkanes, and alkyl esters. Besides, the cuticular waxes of many plant species also contain cyclic compounds such as triterpenoids and aromatics.
In order to characterize the physiological function of cuticular waxes, methods have been developed for the isolation of astomatous cuticles and the measurement of transpiration rates under exactly controlled conditions, so that well-defined physical transport parameters such as permeances and resistances can be determined and compared across species and organs (Schönherr and Lendzian, 1981; Kerstiens, 1996; Riederer and Schreiber, 2001; Lendzian, 2006). With these methods, it was demonstrated that the cuticular water permeance increases by up to 3 orders of magnitude upon wax removal, thus showing the central role of waxes as a transpiration barrier (Schönherr, 1976). Permeances for water determined so far with astomatous isolated leaf cuticular membranes (CMs) or in situ leaf cuticles range over 2.5 orders of magnitude, from 3.63 × 10−7 m s−1 (Vanilla planifolia) to 7.7 × 10−5 m s−1 (Maianthemum bifolium; Riederer and Schreiber, 2001).
The species-dependent differences of both wax composition and permeance led to a search for correlations between cuticle structure and function. If such a structure-function relationship could be established, then it would become possible to select or alter wax composition in order to improve cuticle performance in crop species (Kosma and Jenks, 2007). However, all attempts to understand cuticle permeance based on cuticle composition have failed so far: correlations between wax amounts and permeances could not be established, contrary to the common assumption that thicker wax layers must provide better protection against desiccation (Schreiber and Riederer, 1996; Riederer and Schreiber, 2001). Similarly, a correlation between wax quality (i.e. the relative portions of its constituents) and permeance could also not be established to date (Burghardt and Riederer, 2006). It is not clear how certain wax components contribute to the vital barrier function of the cuticle.
Previous attempts to establish wax structure-function relationships may have failed because only bulk wax properties were studied and important effects of substructures were averaged out. However, distinct compartments of wax exist within the cuticle, most prominently as a layer of intracuticular wax embedded within the MX and a layer of epicuticular wax deposited on the outer surface of the polymer (Jeffree, 2006). Over the last years, methods have been developed that allow the selective removal of epicuticular wax by adhesive surface stripping, followed by equally selective extraction of intracuticular wax (Jetter et al., 2000; Jetter and Schäffer, 2001). Chemical analyses showed that, for most plant species investigated to date, both wax layers have distinct compositions (Buschhaus and Jetter, 2011). The most pronounced differences between the layers were found for the triterpenoids, which were localized predominantly (or even exclusively) in the intracuticular wax. These findings raised the possibility that the chemically distinct wax layers might also have distinct functions, leading back to the long-standing question of whether the water barrier function is exerted by the intracuticular and/or the epicuticular wax. There are only scant data to answer this question so far, mainly because methods allowing a distinction between epicuticular and intracuticular waxes were established only recently. Using these sampling techniques, it was recently found that, for leaves of Prunus laurocerasus, the epicuticular wax layer does not contribute to the transpiration barrier (Zeisler and Schreiber, 2016). In contrast, it had been reported that removal of the epicuticular wax layer from tomato (Solanum lycopersicum) fruit caused an approximately 2-fold increase in transpiration, suggesting that, in this species, the epicuticular layer constitutes an important part of the barrier (Vogg et al., 2004). Based on these conflicting reports, it is not clear to what extent the intracuticular or the epicuticular waxes contribute to the sealing function of the plant skin.
The goal of this study was to localize the transpiration barrier within the cuticular membrane of selected plant species and to put the physiological function into context with the chemical composition of both the epicuticular and intracuticular wax layers. To this end, we selected eight species from which leaf cuticles could be isolated and methods for step-wise wax removal could be applied without damaging the cuticle. Preliminary studies had shown that the adaxial cuticles on leaves of Citrus aurantium (Rutaceae), Euonymus japonica (Celastraceae), Clusia flava (Clusiaceae), Garcinia spicata (Clusiaceae), Tetrastigma voinierianum (Vitaceae), Oreopanax guatemalensis (Araliaceae), Monstera deliciosa (Araceae), and Schefflera elegantissima (Araliaceae) were astomateous and showed wide chemical diversity. Therefore, these eight species were selected to address the following questions: (1) What are the amounts of epicuticular and intracuticular waxes? (2) Do compositional differences exist between the layers? (3) Where are the cuticular triterpenoids located? (4) How much do the epicuticular and intracuticular waxes contribute to the transpiration barrier? (5) Is the barrier associated with certain components of the intracuticular or epicuticular waxes?
RESULTS
This investigation aimed at comparing the chemical composition and physiological properties of cuticular membranes between plant species. In particular, we studied (1) cuticle thickness and weight per unit of surface area, (2) wax amount and composition, (3) epicuticular and intracuticular locations of wax constituents, and (4) cuticular water permeance for eight selected species.
Cuticle Thickness and Gravimetrically Determined Coverages
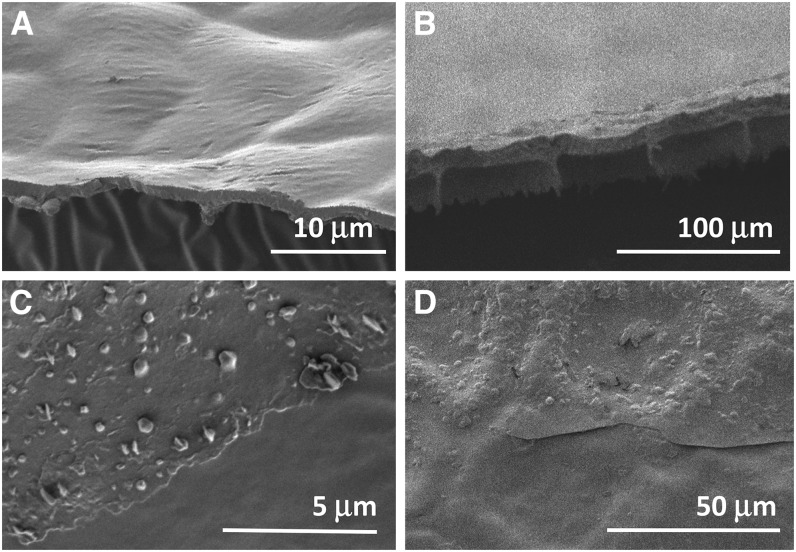
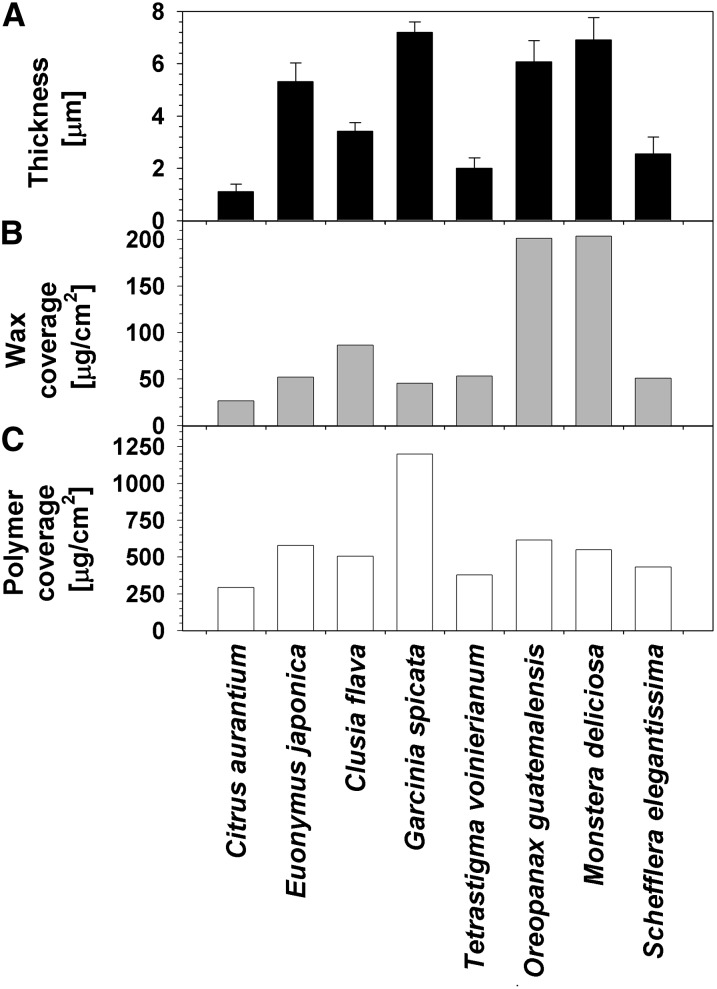
In order to assess the overall membrane characteristics that might affect the transpiration barrier, the thickness and weight of the adaxial leaf cuticle of all eight species were first measured. CMs were isolated, cryofractured, and viewed in cross section by scanning electron microscopy (SEM; Fig. 1). All cuticles showed relatively straight outlines on the outer surface and pronounced ridges protruding on the opposite side. The network of the latter resembled the outlines of leaf epidermal cells of each species and, therefore, was interpreted as thicker cuticle zones that mirrored the indentations over anticlinal cell walls of the epidermal cells. These ridges were 1 to 2 μm high and approximately 1 μm wide at their base, thus adding little to the cuticle thickness and covering less than 5% of the average surface area of 300 to 500 μm2 per epidermis cell. The average thickness of the periclinal parts of the cuticles varied widely, from 1 μm for Citrus aurantium to 7 μm for G. spicata and M. deliciosa (Fig. 2).
Figure 1.
Scanning electron micrographs of isolated cuticles. A and B, Side views of cryofractured cuticles from leaves of Citrus aurantium (A) and M. deliciosa (B), illustrating species with relatively thin and thick cuticles, respectively. C and D, Top-down views of the outer surface of cuticles of Citrus aurantium (C) and M. deliciosa (D). The bottom portions of the images show areas from which the epicuticular wax film was removed with adhesive, while the top portions show the native cuticle surface.
Figure 2.
Thickness and material coverages of isolated adaxial cuticles from leaves of eight plant species. A, CMs were isolated enzymatically, dried, cut transversally, and viewed by SEM to assess their cross section. Average values (n ≥ 15; ±sd) are given for the thickness of cuticle areas above the outer periclinal cell walls of normal epidermal cells. B, Wax coverages (μg cm−2) were calculated from weight differences of isolated cuticular membranes before and after extraction with chloroform. C, Cutin coverages (μg cm−2) were determined gravimetrically after waxes had been extracted from isolated cuticles.
To further compare the physical properties of the cuticles of the eight species, their weights per unit of surface area were determined gravimetrically. To assess the coverages of waxes and cutin separately, the isolated membranes were weighed both before and after exhaustive wax removal using hot chloroform. From the difference in weight before and after extraction, the wax coverages of the isolated membranes could be calculated. These gravimetrically determined wax coverages ranged from 25 μg cm−2 for Citrus aurantium to approximately 50 μg cm−2 for S. elegantissima, E. japonica, G. spicata, and T. voinierianum, approximately 100 μg cm−2 for Clusia flava, and approximately 200 μg cm−2 for O. guatemalensis and M. deliciosa (Fig. 2).
The coverages of the remaining cutin polymer matrices ranged from approximately 300 μg cm−2 for Citrus aurantium, 400 μg cm−2 for T. voinierianum, 500 μg cm−2 for S. elegantissima, 600 μg cm−2 for Clusia flava and E. japonica, and 800 μg cm−2 for O. guatemalensis and M. deliciosa up to 1,200 μg cm−2 for G. spicata (Fig. 2). For all eight species, the MX thus largely dominated cuticle composition, with weight percentages ranging from approximately 75% in M. deliciosa and O. guatemalensis to more than 90% in Citrus aurantium, E. japonica, and G. spicata. Accordingly, waxes contributed between less than 10% and 25% to the mass of the cuticles of these species. Values for total cuticle weights per unit area were roughly proportional to cuticle thicknesses measured in the previous experiment (Fig. 2).
Finally, the measured CM coverages were combined with the corresponding thickness values to calculate coverage-thickness ratios representing apparent cuticle density for each species. The resulting values varied from approximately 1 g cm−3 for M. deliciosa, O. guatemalensis, and E. japonica, to approximately 2 g cm−3 for Clusia flava, G. spicata, T. voinierianum, and S. elegantissima, and to almost 3 g cm−3 for Citrus aurantium.
Gas Chromatography-Determined Wax Coverages
To study the overall amounts and the relative compositions of the epicuticular and intracuticular layers, waxes were sampled from the adaxial leaf surfaces for chemical analyses by gas chromatography (GC)-flame ionization detection (FID) and GC-mass spectrometry. In the first experiment, gum arabic was used as an adhesive to mechanically remove surface waxes (Fig. 1). For all species tested here, this glue could be administered three times to the same area without damaging the leaves, and the resulting three wax samples were analyzed separately. Wax yields for the first treatment ranged from 2 μg cm−2 for O. guatemalensis to 15 μg cm−2 for G. spicata (Table I). For all eight species, the wax amounts liberated by consecutive gum arabic treatments declined steadily, reaching values of 0.1 to 0.8 μg cm−2 in the third round (Table I). The latter values represent 1% to 15% of the cumulative gum arabic yields for the different species. Based on these results, it can be extrapolated that a fourth glue treatment would not have yielded significant further wax amounts and, therefore, that the three sampling rounds had exhaustively removed the mechanically accessible wax. However, when the samples that had been treated three times with gum arabic were then extracted with chloroform (instead of a fourth glue treatment), the extraction yielded wax amounts ranging from 1 μg cm−2 for M. deliciosa to 27 μg cm−2 for E. japonica (Table I).
Table I. Amounts of cuticular waxes sampled from adaxial leaf surfaces of eight plant species.
In two independent experiments, waxes were removed either in four consecutive treatments (three times with gum arabic and then by a final extraction) or in one step (total extraction). Wax amounts (μg cm−2) were quantified by GC-FID, and results are given as means ± sd (n = 5) for each treatment and for the sum of gum arabic and extraction steps. tr, Trace.
| Species | Gum Arabic |
Final Extraction | Gum Arabic Treatments Plus Final Extraction | Total Extraction | ||
|---|---|---|---|---|---|---|
| First Treatment | Second Treatment | Third Treatment | ||||
| Citrus aurantium | 4.2 ± 1.8 | 0.6 ± 0.4 | 0.3 ± 0.2 | 1.9 ± 0.6 | 7.0 ± 2.2 | 7.0 ± 0.7 |
| E. japonica | 3.3 ± 1.3 | 0.4 ± 0.2 | 0.2 ± 0.2 | 26.6 ± 5.2 | 30.5 ± 4.5 | 29.2 ± 4.6 |
| Clusia flava | 5.8 ± 1.0 | 0.2 ± 0.1 | 0.1 ± tr | 8.9 ± 1.0 | 15.0 ± 1.5 | 12.5 ± 1.2 |
| G. spicata | 14.9 ± 3.3 | 0.6 ± 0.1 | 0.2 ± 0.1 | 3.4 ± 1.7 | 19.1 ± 3.5 | 19.8 ± 2.6 |
| T. voinierianum | 5.8 ± 3.0 | 0.2 ± tr | 0.1 ± tr | 3.3 ± 0.5 | 9.4 ± 2.5 | 11.7 ± 1.3 |
| O. guatemalensis | 1.8 ± 0.5 | 0.4 ± 0.2 | 0.4 ± 0.2 | 6.0 ± 2.3 | 8.6 ± 1.8 | 7.6 ± 1.3 |
| M. deliciosa | 2.6 ± 0.7 | 1.2 ± 0.6 | 0.6 ± 0.1 | 0.7 ± 0.04 | 5.1 ± 1.0 | 4.9 ± 0.7 |
| S. elegantissima | 10.7 ± 2.9 | 1.4 ± 0.2 | 0.8 ± 0.4 | 3.7 ± 0.7 | 16.6 ± 2.6 | 18.4 ± 5.1 |
In a separate experiment, the waxes on the adaxial leaf surfaces of the eight plant species were extracted directly with chloroform (without prior gum arabic treatments). The resulting wax yields varied widely, from 5 μg cm−2 for M. deliciosa to 30 μg cm−2 for E. japonica (Table I). In all cases, the wax amounts released by direct extraction in a single treatment were not significantly different from the summed wax quantities released by four consecutive treatments in the previous experiment (three gum arabic treatments plus consecutive extraction; Table I).
All combined, these results show that the gum arabic treatments selectively removed mechanically accessible material; hence, the three glue samples together give the composition of the epicuticular wax layer for each species. As the repeated glue treatments achieved an exhaustive removal of mechanically accessible material, these samples also give quantitative information on the epicuticular wax amounts. Conversely, the waxes remaining after three rounds of gum arabic treatment must have been localized (almost) entirely within the MX, and analyses of the fourth, extractive treatment will reflect the composition of the intracuticular wax layers of the species. This material was also removed exhaustively and can be used to quantify wax amounts in this layer, as confirmed by the second experiment, where direct extraction of the total wax mixture yielded exactly the same amounts as the epicuticular and intracuticular samples from the first experiment taken together.
Compound Class Compositions
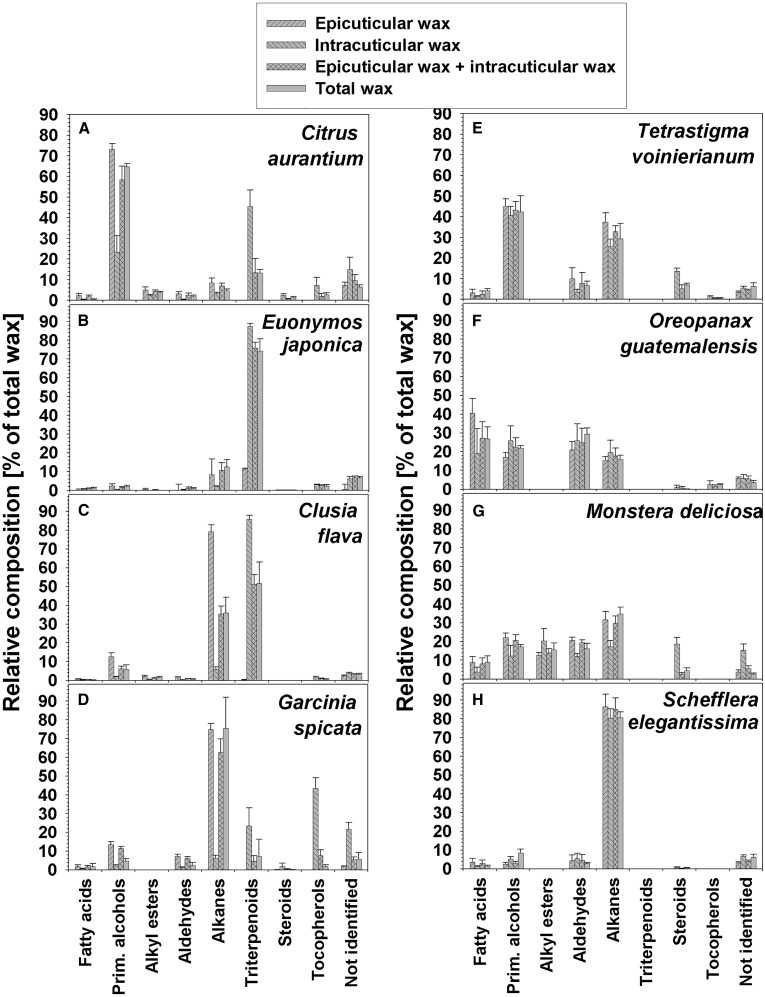
The total wax extracts from the second experiment were found to contain relative amounts of compound classes differing widely between the eight species (Fig. 3; Supplemental Tables S1 and S2). Typical wax constituents were identified in all samples, including VLCFAs and their primary alcohol, alkyl ester, aldehyde, and alkane derivatives. In addition, varying amounts of pentacyclic triterpenoids, steroids, and tocopherols were identified (Supplemental Tables S3 and S4). The wax mixtures of Citrus aurantium, G. spicata, and S. elegantissima were dominated by single VLCFA derivative classes, namely by primary alcohols in the first case and by alkanes in the latter two. E. japonica wax also contained one predominant compound class, but here the triterpenoids prevailed instead of VLCFA derivatives. In the remaining species, two or more compound classes were found at equally high abundance, with both alkanes and triterpenoids dominating for Clusia flava and various VLCFA derivatives in the wax mixtures of T. voinierianum, O. guatemalensis, and M. deliciosa.
Figure 3.
Wax composition in isolated adaxial cuticles from leaves of eight plant species. Epicuticular waxes were sampled using gum arabic as an adhesive, and intracuticular waxes were extracted after gum arabic treatments. In a separate experiment, total cuticular waxes were extracted without prior adhesive treatment. Relative compositions are given as percentages of compound classes in respective wax mixtures (n = 5; ±sd).
The compound classes described above for the direct wax extraction experiment were also identified in the wax mixtures sampled separately either by gum arabic treatment or the following extraction step (Fig. 3; Supplemental Tables S1 and S2). For O. guatemalensis and M. deliciosa, both experiments gave not only qualitatively but also quantitatively matching results, with all compound classes present at similar percentages in the gum arabic samples and the final extracts. In contrast, the relative compositions differed between the epicuticular and intracuticular wax layers in all other species (Fig. 3; Supplemental Tables S1 and S2). The cyclic compounds (triterpenoids, steroids, and tocopherols) exhibited drastic gradients, with percentages in the intracuticular waxes always much higher than in the epicuticular layer. The relative amounts of cyclic compound classes in the intracuticular wax mixtures varied from trace levels in O. guatemalensis and S. elegantissima, over moderate levels (10%–20%) in T. voinierianum and M. deliciosa, to large percentages (greater than 50%) in the other four species. The corresponding epicuticular waxes contained less than 10% of cyclic compounds in all eight species. These gradients of cyclic wax constituents were balanced by countergradients of VLCFA derivatives, with particularly high primary alcohol concentrations in the epicuticular wax of Citrus aurantium and high alkane percentages in the epicuticular layer of Clusia flava and G. spicata.
Chain Length Distribution of VLCFA Derivatives
All VLCFA derivatives identified in the waxes of the eight species were found to have unbranched and fully saturated hydrocarbon structures (Supplemental Tables S5 and S6). The homologous series of fatty acids, primary alcohols, alkyl esters, and aldehydes showed very pronounced predominance of compounds with even carbon numbers, while odd-numbered homologs prevailed in the alkane series. One homolog was dominating the compound classes in most cases, with few exceptions where bimodal chain length distributions were found (e.g. for E. japonica epicuticular wax). Chain length distributions were relatively similar between compound classes within the same species. In contrast, the chain length ranges and predominant chain lengths differed substantially between species.
In the waxes of T. voinierianum, O. guatemalensis, M. deliciosa, and S. elegantissima, the complete homologous series of VLCFA derivatives could be accurately quantified and were found to be very similar for epicuticular and intracuticular wax layers (Supplemental Table S6). These chain length distributions were also confirmed by analyses of the corresponding total wax extracts generated in the second experiment. In the waxes of Citrus aurantium, E. japonica, Clusia flava, and G. spicata, where triterpenoids and steroids were present in high concentration, a few VLCFA derivatives with C28 and C30 chain lengths could not be completely separated and quantified (Supplemental Table S5). As this affected mainly the chemical analyses of intracuticular wax samples, their chain length distribution can only partially be compared with the corresponding epicuticular wax composition of the same species and also with the other species.
Transpiration Barrier Properties
In order to characterize the transpiration barriers of the adaxial leaf cuticles of all eight plant species, the water permeances were measured first for entire cuticles, then again after removing the epicuticular wax layer, and finally after also removing the intracuticular wax. Permeances of intact isolated cuticles ranged from 1.3 × 10−6 m s−1 for M. deliciosa to 1.4 × 10−5 m s−1 for E. japonica (Table II). From these permeances, corresponding transpiration resistances could be calculated as inverse values (R = 1/P). Corresponding water transport resistances of CMs varied among the eight species from 9.8 × 104 to 8.8 × 105 s m−1 (Fig. 4).
Table II. Permeances of isolated cuticular membranes before and after wax removal.
Initially, the water transpiration permeances of intact CMs containing both epicuticular and intracuticular waxes were measured. Then the epicuticular wax was removed (by peeling with gum arabic) and permeances were determined again. Finally, intracuticular waxes too were removed (by extraction with chloroform) and permeances of the remaining cutin MX were measured.
| Species | Permeance of Intact Cuticular Membrane | Permeance of Cuticular Membrane Minus Epicuticular Wax | Permeance of Cuticular Membrane Minus Epicuticular and Intracuticular Wax |
|---|---|---|---|
| m s−1 | |||
| Citrus aurantium | 1.22 × 10−5 ± 8.81 × 10−6 | 2.10 × 10−5 ± 1.07 × 10−5 | 1.72 × 10−3 ± 1.90 × 10−4 |
| E. japonica | 1.37 × 10−5 ± 7.70 × 10−6 | 2.11 × 10−5 ± 9.93 × 10−6 | 7.93 × 10−4 ± 1.17 × 10−4 |
| Clusia flava | 7.22 × 10−6 ± 8.34 × 10−6 | 2.17 × 10−5 ± 1.32 × 10−5 | 7.50 × 10−4 ± 4.23 × 10−4 |
| G. spicata | 5.44 × 10−6 ± 4.00 × 10−6 | 9.32 × 10−6 ± 8.48 × 10−6 | 4.07 × 10−4 ± 7.74 × 10−5 |
| T. voinierianum | 1.36 × 10−6 ± 4.33 × 10−7 | 1.78 × 10−6 ± 7.59 × 10−7 | 7.32 × 10−4 ± 1.94 × 10−4 |
| O. guatemalensis | 4.12 × 10−6 ± 3.14 × 10−6 | 5.92 × 10−6 ± 4.42 × 10−6 | 1.07 × 10−3 ± 1.33 × 10−3 |
| M. deliciosa | 1.29 × 10−6 ± 4.88 × 10−7 | 1.49 × 10−6 ± 6.15 × 10−7 | 3.65 × 10−4 ± 1.02 × 10−4 |
| S. elegantissima | 1.83 × 10−6 ± 7.89 × 10−7 | 2.71 × 10−6 ± 1.57 × 10−6 | 3.92 × 10−4 ± 1.13 × 10−4 |
Figure 4.
Transpiration resistances of isolated adaxial cuticles from eight species. Water transport was measured gravimetrically under controlled conditions for intact CMs after removing the epicuticular wax layer and again after also removing the remaining intracuticular waxes. Based on these data, the transpiration resistance of the total cuticular material and the MX could be calculated directly, and the resistances of the epicuticular and intracuticular waxes were inferred as differences of resistances before and after removing these waxes (n ≥ 15; ±sd).
After the removal of epicuticular waxes, the permeances of the eight species ranged from 1.8 × 10−6 to 2.2 × 10−5 m s−1 (Table II). The corresponding transpiration resistances may be compared with the overall cuticular resistances of the same species. It can be assumed that the epicuticular and intracuticular resistances act in series and are thus additive. Consequently, the differences in resistances before and after the removal of epicuticular waxes corresponded to water transport resistances of the epicuticular layer alone. Respective epicuticular resistances ranged from 3.9 × 104 s m−1 for E. japonica to 1.8 × 105 s m−1 for Clusia flava (Fig. 4). In a control experiment, the same CMs were tested after opening, closing, and refilling the transpiration chambers as in the experiment described above, but without wax removal. There was no significant difference between the permeances before and after these manipulations (data not shown), demonstrating that the effects described above were due to the removal of the epicuticular waxes alone and not caused by mechanical damage during treatments or by the aging of the cuticle mounted in the transpiration chamber over the time of the experiments.
After further removal of the intracuticular waxes, the permeances of the membranes of all eight species increased drastically, to values between 3.6 × 10−4 m s−1 (M. deliciosa) and 1.7 × 10−3 m s−1 (Citrus aurantium; Table II). On the one hand, the difference of permeances before and after removing the intracuticular waxes can be used to calculate the water transport resistance of this wax fraction. The results varied between relatively small values of approximately 6 × 104 s m−1 for Citrus aurantium, E. japonica, and Clusia flava to more than 10-fold higher intracuticular resistances for M. deliciosa (Fig. 4). On the other hand, these residual permeances can be used to assess the resistance of the MX devoid of waxes. The resulting values ranged from 5.9 × 103 to 2.9 × 104 s m−1 (Fig. 4) and, thus, were approximately 2 orders of magnitude smaller than those for corresponding CMs.
Tests for Correlations between Barrier Properties and Structure
In order to link cuticle barrier properties and structure, comprehensive correlation analyses were carried out for combinations between the data described above. In particular, structural parameters (thickness, matrix amount, and wax coverage) of the isolated cuticular membranes of the eight species were selected together with chemical parameters (amounts and percentages of each compound class in either the epicuticular or the intracuticular wax layers) and physiological parameters (transpiration resistances of the CM, the epicuticular wax, the intracuticular wax, and the cutin matrix). All possible combinations between these parameters were tested in pair-wise regression analyses (Supplemental Table S7; significance threshold Bonferroni-adjusted for 18 independent parameter families to P < 0.0028). In the following, the results for the most important parameter pairs will be summarized.
Within the chemical subset of data, a few correlations were found between compound classes either within a wax layer or between layers. Most notably, epicuticular alkane amounts (in μg cm−2) were proportional to the overall aliphatic contents of the epicuticular waxes (r2 = 0.92) as well as the total epicuticular wax amounts (r2 = 0.92). The overall aliphatic contents were strongly correlated with the amounts of epicuticular wax (r2 = 1). In particular, the amounts of epicuticular and intracuticular esters were correlated, both when expressed in μg cm−2 (r2 = 0.93) and as percentages within each layer (r2 = 0.9). Similarly, the percentages of acids in the epicuticular and intracuticular waxes were correlated (r2 = 0.91), as were aldehyde percentages in both layers (r2 = 0.73). Finally, the amounts of total intracuticular waxes, of intracuticular alicyclics, and of epicuticular alicyclics were all correlated with each other (r2 = 0.75–0.95).
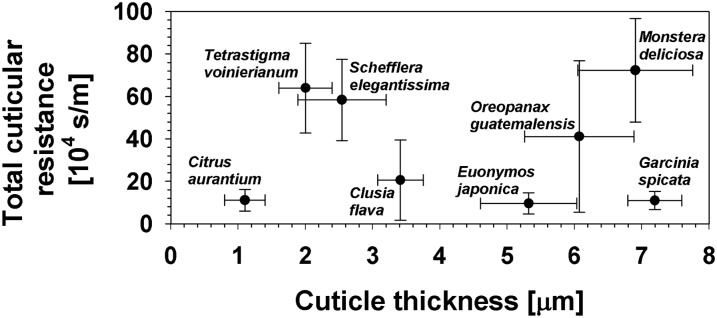
Total cuticular resistances of the eight species investigated here were found not to be correlated with overall cuticle thickness (r2 = 0.01; Fig. 5), with cuticle weight (r2 = 0.03), or with total wax amounts (r2 = 0.17). Similarly, correlations did not exist between the weight and the resistance of the MX (r2 = 0.21; Fig. 6), between the amounts and resistances of the epicuticular waxes (r2 = 0.002), or between the amounts and resistances of the intracuticular waxes (r2 = 0.24). However, the intracuticular resistances were found to be correlated with the total resistances (r2 = 0.98).
Figure 5.
Total cuticular resistances plotted against cuticle thicknesses. A correlation could not be found between total cuticular resistances and cuticle thicknesses, two parameters describing the overall structure and physiological properties of the cuticles of eight tested species.
Figure 6.
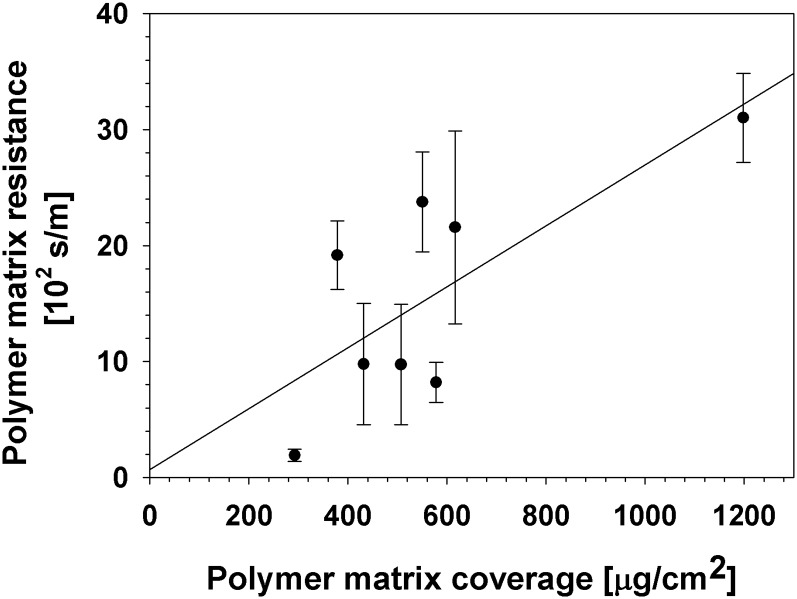
Polymer matrix resistances plotted against matrix coverages. Two parameters describing the amount and physiological properties of the cutin matrix were selected. For the eight species investigated here, the resistance of the MX and its coverage were not correlated (P = 0.11, r2 = 0.21).
Strong correlations between any of the compositional parameters and resistances could not be found. As one notable exception, both the intracuticular and the total resistances were somewhat correlated with the percentage of aliphatic compounds within the intracuticular wax mixtures (r2 = 0.57). Conversely, relatively low contributions of the intracuticular resistance were associated in our species with relatively high amounts of alicyclic compounds in the intracuticular wax (Fig. 7), expressed as percentages either of the triterpenoids alone (r2 = 0.56) or the sum of all three classes (triterpenoids, steroids, and tocopherols; r2 = 0.52). The absolute amounts (μg cm−2) of the alicyclic compounds were not proportional to the absolute resistances (s m−1) of the intracuticular compartments (r2 = 0.26).
Figure 7.
Comparison of relative transpiration resistances and relative composition of intracuticular waxes. The contributions of the intracuticular waxes to the overall barrier properties of the cuticular membranes were negatively correlated with the relative amounts of alicyclic compounds in the intracuticular wax mixtures.
There were also no correlations between the relative composition and absolute amounts of VLCFA derivatives in the epicuticular wax mixture and the transpiration resistance of this layer (data not shown). For example, G. spicata and S. elegantissima had very similar composition and amounts of epicuticular waxes but very different epicuticular barrier properties. Conversely, Citrus aurantium and G. spicata had similar epicuticular barriers but very different epicuticular wax compositions and amounts.
DISCUSSION
The principal goal of this study was to quantify to which degree the intracuticular and epicuticular waxes contribute to the barrier against nonstomatal water loss. To address this question, water permeances from eight plant species were measured and corresponding transpiration resistances were calculated. To further distinguish between the barrier properties of the MX, the intracuticular wax, and the epicuticular layer within the cuticles of each of these species, transpiration resistances of each part of the cuticle were determined.
Total Wax Composition and Barrier Properties
The amounts and compositions of total cuticular wax mixtures of the eight species investigated here, comprising both epicuticular and intracuticular waxes together, fall well within the range of compositions described previously for various plant waxes. Among the present species, only three had been investigated before, with similar results. Most notably, a detailed analysis of Citrus aurantium leaf wax had shown very similar compound class and chain length profiles (Riederer and Schneider, 1989). The overall amounts of Citrus aurantium leaf waxes, extracted by short surface immersion, were also nearly identical with our results (Table I). However, much higher wax amounts could be extracted from isolated CMs with hot chloroform in a Soxhlet apparatus (Fig. 2), similar to previous reports for Citrus aurantium showing that wax yields largely depend on extraction conditions (Riederer and Schneider, 1989; Schreiber and Riederer, 1996). In particular, it had been reported that surface extraction with chloroform yields wax mixtures with reproducible amounts and composition free of cellular lipids but discriminates against longer chain compounds, mainly due to limited solubility (Riederer and Schneider, 1989). This may also explain, at least in part, the consistently higher wax amounts released by prolonged hot chloroform extraction from isolated CMs of all species tested here (Fig. 2), as compared with short surface dipping of corresponding leaf material (Table I). This conclusion is further supported by previous literature, since the wax yields reported before for exhaustive extraction of E. japonica and M. deliciosa CMs also match our findings here (Schreiber and Riederer, 1996). Finally, it should be noted that our results for Clusia flava also fit previous reports on the amounts and compositions of wax mixtures extracted from other Clusia spp. (Medina et al., 2004).
The permeances of intact CMs had been reported before for three of the species investigated here, with similar results. In particular, 12 investigations using methods comparable to ours showed P values for Citrus aurantium ranging by more than 1 order of magnitude, from 5.5 × 10−6 to 1.7 × 10−4 m s−1 (Schönherr, 1976; Haas and Schönherr, 1979; Schönherr and Schmidt, 1979; Schönherr and Lendzian, 1981; Becker et al., 1986; Lendzian et al., 1986; Kerstiens and Lendzian, 1989; Geyer and Schönherr, 1990; Schreiber and Riederer 1996; Riederer, 2006; Ballmann et al., 2011), with a median of 3.2 × 10−5 m s−1, fairly similar to the value 1.2 × 10−5 m s−1 found here. Similarly, the permeances determined here for M. deliciosa and E. japonica (1.3 × 10−6 and 0.4 × 10−5 m s−1, respectively) deviated somewhat from the literature (1.9 × 10−5 and 1.6 × 10−4 m s−1, respectively; Schreiber and Riederer, 1996), the differences being similar to those reported before for repeat permeance measurements of other species.
Comparisons of Epicuticular and Intracuticular Wax Compositions
Among the eight species investigated here, the epicuticular wax amounts varied widely, from 3 to 16 μg cm−2. Accordingly, and assuming a wax density of 0.8 to 1 × 106 g m−3 (Le Roux, 1969; Small, 1984), the thickness of the epicuticular layer can be estimated to vary from 30 nm for O. guatemalensis to 200 nm for G. spicata. At the same time, the intracuticular wax amounts ranged from 1 to 27 μg cm−2 in the species investigated here. Consequently, the ratios between wax amounts in both layers varied from 5:1 to 1:7, further illustrating the diversity in absolute and relative quantities of material in the different layers within the cuticles of these species. In previous studies, epicuticular layers had been found to vary in thickness from approximately 10 nm on Arabidopsis (Arabidopsis thaliana) leaves to 130 nm on mature P. laurocerasus leaves, while ratios of epicuticular and intracuticular waxes ranged from 9:1 to 1:1 (Jetter et al., 2000; Buschhaus and Jetter, 2012). The species investigated here thus had similar epicuticular and intracuticular wax amounts and proportions to previously studied plants, but they showed a wider range of layer dimensions. This presented an opportunity to test for correlations between wax amounts and barrier properties. However, such correlations could not be found. The permeances of the epicuticular and intracuticular wax layers are independent of their thickness, and wax resistances must instead be determined by more subtle effects, including wax composition within both layers.
All eight species investigated here had epicuticular waxes containing large percentages of VLCFA derivatives and only little or no alicyclic compounds (Fig. 4). Depending on the species, between one and four VLCFA compound classes were present at high concentrations in this layer forming the true surface of the leaves. In many cases, alkanes dominated, but primary alcohols, fatty acids, and aldehydes also accumulated to levels above 20% in some epicuticular wax mixtures. More subtle differences among the species were found in the exact chain length distributions within these epicuticular wax compound classes (Supplemental Tables S5 and S6). Overall, these findings are similar to previous reports for the composition of the epicuticular wax on other plant species that are also characterized by smooth surfaces lacking epicuticular wax crystals (Buschhaus and Jetter, 2011). For example, tomato fruits and mature P. laurocerasus leaves have epicuticular waxes dominated by alkanes (Jetter et al., 2000; Vogg et al., 2004), whereas young P. laurocerasus leaves and Macaranga tanarius leaves have predominantly primary alcohols in their surface waxes (Jetter and Schäffer, 2001; Guhling et al., 2005).
In this study, four species (T. voinierianum, O. guatemalensis, M. deliciosa, and S. elegantissima) had relatively small differences between the composition of their epicuticular and intracuticular wax layers. The bulk wax analyses closely matched those of both the separate epicuticular and intracuticular wax samples, further confirming that no transversal gradients existed within the cuticles of these species. It should be noted that the same species contained no (or only very small amounts of) alicyclic wax compounds. In the cuticular wax mixtures of the other species (Citrus aurantium, E. japonica, Clusia flava, and G. spicata), triterpenoids, steroids, or tocopherols were present at relatively high concentrations. These alicyclic compounds were in all cases largely (or entirely) restricted to the intracuticular wax layer; consequently, drastic compositional gradients existed within the cuticular waxes: alicyclic concentrations decreased outward, whereas VLCFA derivative percentages increased in the same direction. These findings are very similar to previous reports on plant species with smooth epicuticular wax surfaces (i.e. lacking surface wax crystals) and alicyclic wax constituents (Buschhaus and Jetter, 2011). In five cases, namely for tomato fruits and for leaves of P. laurocerasus, Rosa canina, Ligustrum vulgare, and M. tanarius, pentacyclic triterpenoids had been found at high concentrations in the intracuticular wax only (Jetter et al., 2000; Jetter and Schäffer, 2001; Vogg et al., 2004; Guhling et al., 2005; Buschhaus et al., 2007a, 2007b; Zeisler and Schreiber, 2016).
Model Underlying the Analysis of Cuticular Resistances
In order to calculate values for the contribution of various structural components to the overall cuticular resistance, it can be assumed that (1) the epicuticular wax layer and the intracuticular compartment (polymer matrix plus waxes) act as two resistances in series and (2) the major resistance within the intracuticular layer is due to intracuticular waxes, whereas the matrix contributes relatively little. The first part of this model is based on the finding that the majority of water molecules are absorbed into and cross through the cuticular wax (as opposed to larger, polar solutes that pass through a limited number of aqueous channels extending from the epidermal cell wall to the tissue surface; Riederer and Schreiber, 2001; Schönherr and Schreiber, 2004; Beyer et al., 2005; Popp et al., 2005; Schreiber, 2005; Schönherr, 2006; Weichert and Knoche, 2006; Arand et al., 2010). The epicuticular wax film must consequently impose a resistance on water movement, and this resistance acts in series with the resistance(s) imposed on inner pathway sections.
The second part of this model is based on previous results that the MX alone imposes only a relatively small resistance (Schönherr, 1976). This is confirmed by our finding that, for all eight species investigated here, cuticular water transport resistances increased by 2 orders of magnitude when the intracuticular wax was removed from the cutin matrix. Therefore, it is likely that the major part of the intracuticular resistance is due to wax and not cutin. However, it must be noted that the intracuticular wax is likely associated with the MX on a molecular scale (Nawrath, 2006); therefore, cutin will have an indirect effect on the barrier properties of the wax. While this indirect contribution of the MX cannot be assessed at present, we assume that it will be relatively small in comparison with that of the intracuticular wax and can be neglected in a first approximation.
Correlations between Cuticle Structure and Barrier Localization
High levels of intracuticular alicyclics were found for four of the species investigated here, and in the same species the intracuticular wax layers formed only approximately 50% of the cuticular transpiration barrier (Fig. 7). The second half of the overall barrier was contributed by the epicuticular wax in these species, consisting entirely of VLCFA derivatives. This is in contrast to the other four species, with predominantly fatty acid derivatives in their intracuticular waxes (and lacking cyclic compounds), where the barrier was localized entirely within the intracuticular layer. It should be noted that, among the species tested here, the presence of intracuticular alicyclics coincided not only with combined epicuticular and intracuticular barriers but also with relatively low overall transpiration resistances. In summary, the transpiration barrier may be formed either by the intracuticular wax alone or by both the intracuticular and epicuticular wax layers together, depending on the species and cuticular wax composition.
Our findings can be corroborated by the few previous studies in which permeances were measured separately for epicuticular and intracuticular wax layers. In one example, the permeances of wax layers on cherry (Prunus avium) fruits were reported, and the epicuticular contribution to the wax resistance can be inferred to be approximately 50% (Knoche et al., 2000). However, cellulose acetate, an adhesive typically applied in organic solution, was used to remove the epicuticular film in that study, and the solvent may have disturbed the intracuticular layer and, consequently, caused an overestimation of the epicuticular resistance (Jetter et al., 2000; Zeisler and Schreiber, 2016). Furthermore, it seems plausible that the intracuticular wax of the cherry fruit contains high concentrations of triterpenoids and only low amounts of fatty acid derivatives, but quantitative data on its composition are missing to date. Overall, the current information on cherry fruit matches our findings here qualitatively but will need to be backed up by more quantitative data.
In a second study, gum arabic was used to distinguish the permeances of epicuticular and intracuticular layers in tomato fruits, and transpiration resistances of 1.9 × 10−6 and 2.5 × 10−6 s m−1 were calculated, respectively (Vogg et al., 2004; Leide et al., 2007). With this roughly equal split of resistances between both layers, the tomato fruit is another system where the epicuticular wax contributes substantially to the transpiration barrier. The tomato fruit cuticle was shown to contain a large percentage of intracuticular triterpenoids (Vogg et al., 2004), thus further confirming the conclusion that these alicyclics are not directly involved in forming the cuticular transpiration barrier.
Very recently, the composition and barrier properties of the epicuticular and intracuticular wax layers on leaves of P. laurocerasus were investigated (Zeisler and Schreiber, 2016). Three different methods for the removal of epicuticular wax were compared, along with control experiments testing the effects of the solvents involved in each of them. Two of these methods, employing gum arabic or collodion (applied in water or 1:1 ether:ethanol, respectively), were shown to remove epicuticular wax without releasing triterpenoids known to be intracuticular components in this species (Jetter et al., 2000; Jetter and Schäffer, 2001). Removal of the epicuticular material with either of these methods had no significant effect on the transport of water through the isolated CM, leading the authors to conclude that, on P. laurocerasus leaves, the epicuticular wax does not contribute to the transpiration barrier (Zeisler and Schreiber, 2016). It should be noted that the methods used for assessing water transport in P. laurocerasus (short-term kinetics of radioactive water diffusion instead of gravimetry) and for data analysis (calculation of relative effects instead of serial resistances) were fairly different from those employed in our study, even though the protocols for epicuticular wax removal were similar. Therefore, the results of both studies may not be directly comparable across methods and species until they are corroborated in future studies employing the same methods on identical plant material. However, it is interesting that the intracuticular wax mixtures extracted from P. laurocerasus leaves after treatments with gum arabic or collodion contained substantial amounts (approximately 6 and 15 μg cm−2, respectively) of fatty acid derivatives (Zeisler and Schreiber, 2016). In comparison, the four species in our study resembling P. laurocerasus in their substantial amounts of intracuticular alicyclics had much lower coverages of VLCFA compounds (approximately 1 μg cm−2 for Citrus aurantium, Clusia flava, and G. spicata and approximately 2.5 μg cm−2 for E. japonica). These differences in intracuticular wax composition might account, at least in part, for the different barrier qualities of the intracuticular wax layers in P. laurocerasus and the species investigated here.
Only two studies so far have tested the effect of specific wax components on cuticular transpiration by manipulating wax composition within a single species rather than comparing between different compositions across species. Each of these studies compared two plant lines differing in the concentration of intracuticular triterpenoids. In one case, a tomato mutant deficient in VLCFA derivatives was found to have strongly reduced transpiration resistance, even though the amounts of (intracuticular) triterpenoids were significantly increased over wild-type levels (Vogg et al., 2004). In a second study, the ectopic expression of a triterpenoid synthase gene led to the accumulation of small percentages of the triterpenoid β-amyrin in leaves of Arabidopsis, an organ devoid of cuticular triterpenoids in wild-type lines (Buschhaus and Jetter, 2012). The triterpenoid was found only in the intracuticular wax, and this change in composition led to a decrease of the intracuticular transpiration resistance.
Thus, the two manipulative investigations confirm our finding that intracuticular triterpenoids do not contribute directly to the transpiration barrier; instead, in their presence, the barrier is split between the intracuticular and epicuticular layers. This study puts this conclusion into a broader context by analyzing multiple species with a wider range of triterpenoid concentrations. All data taken together, we conclude that, at least when both VLCFA derivatives and aliphatics together form the cuticular wax mixture, the former compounds alone form the cuticular transpiration barrier and the (intracuticular) triterpenoids do not contribute directly to the physiological function. This establishes, to our knowledge, a first, albeit crude, structure-function relationship for the composition and barrier properties of cuticular waxes.
The structure-function relationship thus far is in accordance with a long-standing model for the spatial arrangement of wax molecules and the resulting transport resistances (Riederer and Schreiber, 2001). The model stipulates crystalline domains of VLCFA derivatives with narrow chain length distribution, surrounded by amorphous zones containing alicyclic compounds and wide arrays of VLCFA homologs. The tight packing of the crystalline domains excludes water molecules, effectively forcing water molecules to follow a tortuous path through the amorphous domains and, thus, establishing the transpiration barrier. Consequently, this model predicts that the transpiration barrier is associated with the VLCFA wax components rather than the alicyclics. To our knowledge, our experiments test this hypothesis for the first time, and our results are in good overall accordance with the domain model. Conversely, we predict that in species with intracuticular wax rich in VLCFA derivatives, the overall transpiration barrier will be located largely in the intracuticular layer alone, whereas in species with lower concentrations of intracuticular fatty acid derivatives, the barrier will be formed by the epicuticular and intracuticular layers together.
The importance of individual VLCFA compounds cannot be assessed in more detail at this point. In this context, it should be noted that, for some of the species investigated here, the epicuticular and intracuticular layers had similar VLCFA compositions, and yet the two layers showed dramatically different resistances. It is currently not clear how similar compositions can cause different physiological properties in these cases. Low intracuticular water diffusion resistances were not only correlated with high intracuticular alicyclic concentrations but also with a low overall cuticular transpiration barrier. This second effect cannot be explained by a small resistance contribution from the triterpenoids alone but must be due to other factors. It seems unlikely that differences in the relative or absolute composition of VLCFA derivatives caused the low overall resistances in some species, as the VLCFA amounts were not correlated with barrier properties in species comparisons or between wax layers within species. Instead, the physical structure of the waxes may be a more important factor determining barrier properties. The link between low overall resistances and relatively small intracuticular barrier contributions suggests that the physical structure of the intracuticular wax is of central importance and that the epicuticular layer adds to the barrier function only where the intracuticular structure is imperfect.
CONCLUSION
We have shown that, depending on the plant species, the cuticular transpiration barrier may be located entirely in the intracuticular wax or distributed up to 1:1 between the epicuticular and intracuticular wax layers. In species where the intracuticular wax does not constitute the entire barrier, high concentrations of intracuticular triterpenoids or other alicyclic compounds were detected, and the overall transpiration resistance was found to be relatively low. Therefore, we conclude that the majority of the resistance against cuticular transpiration is formed by the VLCFA derivative compounds in the waxes, either entirely in the intracuticular wax or by a combination of epicuticular and intracuticular compartments. Overall, these findings, to our knowledge for the first time, link cuticle substructures and their composition with the physiological function of water transport control. Our results are compatible with previous models describing cuticular structure and transpiration, now enabling the deduction of more detailed models for the causal relationships between wax composition and physiological function. These models may be tested using the methods established here, providing quantitative information on the composition and barrier properties of the intracuticular and epicuticular waxes separately. It will be very interesting to thus compare further plant species selected for subtle differences in alicyclic composition or VLCFA profiles or to investigate series of transgenics with systematically varying wax composition.
MATERIALS AND METHODS
Plant Material
Small individuals of Citrus aurantium, Euonymus japonica, Clusia flava, Garcinia spicata, Tetrastigma voinierianum, Oreopanax guatemalensis, Monstera deliciosa, and Schefflera elegantissima were continuously grown in pots in the greenhouses of the Botanical Garden of the University of Würzburg. Mature leaves were harvested randomly from three individual plants and pooled before using them either for the isolation of adaxial CMs or for wax removal and analysis.
In order to isolate CMs, the method of Schönherr and Riederer (1986) was adapted to these species. Circular discs of 20 mm diameter were cut from harvested leaves of the eight species, marked with a felt pen on the abaxial side, and then vacuum infiltrated with a sodium citrate buffer containing 2% (w/w) pectinase (Roth), 2% (w/w) cellulase (Roth), and 1 mm sodium azide to prevent bacterial growth. The enzyme solution was kept at room temperature and changed every 2 to 3 d until the tissue was largely dissolved or at least the CMs were completely removed from it. Adaxial CMs were identified by the absence of pen marks, spread out onto Teflon discs, washed thoroughly with distilled water, air dried, and transferred into glass vials for storage. Isolated CMs were either cut with a hand microtome for cross-sectional viewing by SEM or treated with gum arabic to remove epicuticular waxes.
SEM and Determination of Cuticle Thickness and Weight
Samples were mounted on aluminum stubs using double-sided adhesive tape, coated with 5 nm of gold using a Cressington Sputter Coater 208HR (Ted Pella), and investigated with a Hitachi S4700 field emission SEM device (Nissei Sangyo America) using a 1-kV accelerating voltage and a 12-mm working distance.
In order to determine cutin and wax amounts in isolated cuticular membranes, a specified number of circular CMs (diameter, 20 mm) were weighed before and after the extraction of cuticular waxes. Exhaustive wax removal was achieved by extraction with chloroform for at least 4 h in a Soxhlet apparatus.
Wax Sampling
A polymer film of gum arabic was employed for the selective preparation and analysis of epicuticular waxes. Commercial gum arabic (Roth) was extracted exhaustively with hot chloroform to remove any soluble lipids, and residual organic solvent was allowed to evaporate completely. Approximately 0.1 mL of a 90% (w/w) aqueous solution of delipidated gum was applied per cm2 of leaf surface using a small paintbrush. After 1 to 2 h, a dry and stable polymer film could be broken off in pieces. These were collected and transferred into a vial containing 7 mL each of chloroform and water. The polymer films from five 3.1-cm2 discs were pooled into the same two-phase system. Tetracosane was added as an internal standard, after vigorous agitation and phase separation the organic solution was removed, and the solvent was evaporated under reduced pressure. After treatment with gum arabic, the leaf discs were still physically intact and could be used in repeated mechanical removal experiments or in a final solvent extraction step to remove the remaining cuticular waxes.
Selective superficial extraction of intracuticular waxes or total cuticular waxes from the adaxial surface was achieved by placing the intact leaf onto a flexible rubber mat, gently pressing a glass cylinder of 10 mm diameter onto the exposed surface, and filling the cylinder with approximately 1.5 mL of chloroform. The solvent was agitated for 30 s by pumping with a Pasteur pipette and removed. This procedure was repeated once, and both extracts were combined. When any solvent leaked between cylinder and leaf surface, the sample was discarded. Extracts from approximately 10 individual leaves were pooled for further analysis. Tetracosane was immediately added to all the extracts of cuticular waxes as an internal standard, and the solvent was removed under reduced pressure.
Chemical Characterization
Prior to analysis by GC, hydroxyl-containing compounds in all samples were transformed to the corresponding trimethylsilyl derivatives by reaction with bis-N,O-trimethylsilyltrifluoroacetamide (Macherey-Nagel) in pyridine (30 min at 70°C). Wax mixtures were analyzed using a capillary GC apparatus (5890N, Agilent; column 30 m HP-1, 0.32-mm i.d., film thickness = 0.1 μm) with helium carrier gas inlet pressure programmed for constant flow of 1.4 mL min−1 with a mass spectrometric detector (5973N; Agilent). GC was carried out with temperature-programmed on-column injection and oven temperature set at 50°C for 2 min, raised by 40°C min−1 to 200°C, held for 2 min at 200°C, raised by 3°C min−1 to 320°C, and held for 30 min at 320°C. Individual wax components were identified by comparing their mass spectra with those of authentic standards and literature data. Quantitative analysis of wax mixtures was carried out using capillary GC with FID under the same conditions as above, but with H2 carrier gas inlet pressure regulated for constant flow of 2 mL min−1. Wax loads were determined by comparing GC-FID peak areas against an internal standard and dividing by the leaf surface area from which the corresponding sample had been taken. These surface areas were calculated for the direct wax extraction steps of both experiments (final extraction in the first experiment and total extraction in the second experiment) using the i.d. of the glass cylinders and assuming circular geometry. The gum arabic sampling steps were also restricted to circular areas with known diameters, in this case marked prior to glue application and wax removal.
Cuticular Transpiration Measurements
Isolated CMs (20 mm in diameter) were used in gravimetric transpiration measurements using methods adapted from Schönherr and Lendzian (1981). All CMs were carefully handled with blunt spring steel tweezers and had been stored for at least 6 weeks after isolation. Three consecutive experiments were performed as described below on each individual CM to measure paired values of permeances before and after wax-removal steps.
First, intact CMs were attached to stainless steel transpiration chambers by vacuum grease (Wacker), then a ring-like lid was placed on top and fastened by adhesive tape. The chambers were filled with deionized water through a small opening in the bottom of the chambers, which was then sealed with two layers of adhesive tape. The chambers were incubated upside down at 25°C ± 1°C over dried silica beads in a closed plastic container. After overnight preincubation, the water loss from the chambers was determined at five to eight defined time points. After the transpiration measurements had been carried out on the intact CMs, the chamber lids were carefully removed and a gum arabic solution (1 g mL−1 water) was applied by a soft brush onto the exposed CM surfaces as described above for chemical analyses; the gum arabic film was dried for at least 1 h and then stripped off in order to remove adhering epicuticular waxes. The transpiration chambers were then reassembled by installing the lids, refilling the water reservoirs, and sealing with adhesive tape. Using this setup, transpiration rates were determined for the cuticles without epicuticular waxes by gravimetrically determining water loss at five defined time points. Finally, the water was removed from the chambers, the setup was dried for several hours, and the remaining cuticular waxes were removed by immersing the chambers together with the still mounted cuticles in CHCl3. The chambers were then refilled with water, and transpiration rates of the MX (i.e. dewaxed cuticular matrices) were determined gravimetrically (five time points). In a control experiment, CMs were measured twice, once in the original setup and a second time after disassembling the chambers and refilling them but omitting the wax-removal steps in between.
The transpiration rates were used to calculate permeances according to the equation:
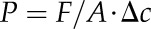 |
where F is the measured flux of water in g s−1, A is the transpiring cuticle surface area in m2, and Δc is the difference between the (vapor-based) water concentration on the inner (Ci = 23.074 g m−3) and outer (Co = 0 g m−3) side of the cuticle (silica beads adsorb all moisture in the container around the transpiration chambers). From the three consecutive experiments (see above), permeances resulted for the untreated CM (Pu), for cuticles from which epicuticular waxes had been stripped off with gum arabic (Pg), and for dewaxed cuticles (Pd). Permeance values deviating from the sample average by more than three times the sd were removed as outliers. Data were tested for normal or log-normal distribution (Kolmogoroff-Smirnov) and for significant differences (Student’s t test) using Statistica software (6.0; StatSoft).
Resistances of the three different types of samples against water transport (Ru, Rg, and Rd) were calculated as inverse permeances (resistance R = 1/P). By correcting for the additional resistance imposed by water surface tension and diffusion within the container (determined in control experiments with open transpiration chambers; R0 = 3.37 × 10−6 s m−1), the total resistance of the intact membrane (CM) and the resistance of the MX could be calculated:
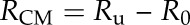 |
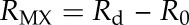 |
Finally, the resistance of individual membranes after epicuticular wax had been stripped off (RES) was calculated for each sample by correcting the values after gum arabic treatment for the control resistance (RES = Rg – R0). The resulting resistances were compared with those before the initial gum arabic treatment (RCM) and after the ensuing wax extraction (RMX) to assess the contributions of the epicuticular and the intracuticular wax, respectively:
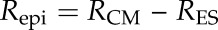 |
 |
Resistance values for individual specimens were then averaged for each compartment within the cuticle. In all experiments, permeance and resistance data showed normal distributions. Multiple regression analysis was performed using SPSS (13.0; SPSS) with default settings. In the overall data set, 18 independent families of parameters were recognized, and Bonferroni adjustments were used for reporting significant correlations (Supplemental Table S7).
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Compound class composition of the cuticular waxes from adaxial leaf sides of Citrus aurantium, E. japonica, Clusia flava, and G. spicata.
Supplemental Table S2. Compound class composition of the cuticular waxes from adaxial leaf sides of T. voinierianum, O. guatemalensis, M. deliciosa, and S. elegantissima.
Supplemental Table S3. Relative composition of the alicyclic compounds in the cuticular waxes from adaxial leaf sides of Citrus aurantium, E. japonica, Clusia flava, and G. spicata.
Supplemental Table S4. Relative composition of the alicyclic compounds in the cuticular waxes from adaxial leaf sides of T. voinierianum, O. guatemalensis, M. deliciosa, and S. elegantissima.
Supplemental Table S5. Chain length composition of VLCFA derivative classes in the cuticular waxes from adaxial leaf sides of Citrus aurantium, E. japonica, Clusia flava, and G. spicata.
Supplemental Table S6. Chain length composition of VLCFA derivative classes in the cuticular waxes from adaxial leaf sides of T. voinierianum, O. guatemalensis, M. deliciosa, and S. elegantissima.
Supplemental Table S7. Multivariate correlation matrix of parameters describing the structure, composition, and transpiration barrier properties for the investigated species.
Supplementary Material
Acknowledgments
We thank Cornelia Vermeer for skillfully conducting the experiments, the Botanical Garden of the University of Würzburg for maintaining the plants, and the Bio-imaging Facility at the University of British Columbia for providing microscopy and technical support.
Glossary
- MX
polymer matrix
- VLCFA
very-long-chain fatty acid
- CM
cuticular membrane
- SEM
scanning electron microscopy
- GC
gas chromatography
- FID
flame ionization detection
Footnotes
This work was supported by the German Science Foundation (to M.R. and R.J.), the Natural Sciences and Engineering Research Council of Canada (to R.J.), the Canada Foundation for Innovation (to R.J.), the Canada Research Chairs Program (to R.J.), and the Chinese Academy of Sciences, Visiting Professorship for Senior International Scientists (grant no. 2011T2S31 to M.R.).
Articles can be viewed without a subscription.
References
- Arand K, Stock D, Burghardt M, Riederer M (2010) pH-dependent permeation of amino acids through isolated ivy cuticles is affected by cuticular water sorption and hydration shell size of the solute. J Exp Bot 61: 3865–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmann C, De Oliveira S, Gutenberger A, Wassmann F, Schreiber L (2011) A radioactive assay allowing the quantitative measurement of cuticular permeability of intact Arabidopsis thaliana leaves. Planta 234: 9–20 [DOI] [PubMed] [Google Scholar]
- Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202: 1–8 [Google Scholar]
- Becker M, Kerstiens G, Schönherr J (1986) Water permeability of plant cuticles: permeance, diffusion and partition coefficients. Trees (Berl) 1: 54–60 [Google Scholar]
- Beyer M, Lau S, Knoche M (2005) Studies on water transport through the sweet cherry fruit surface, IX. Comparing permeability in water uptake and transpiration. Planta 220: 474–485 [DOI] [PubMed] [Google Scholar]
- Burghardt M, Riederer M (2006) Cuticular transpiration. In Riederer M, Müller C, eds, Biology of the Plant Cuticle, Vol 23. Blackwell, Oxford, pp 292–311 [Google Scholar]
- Buschhaus C, Herz H, Jetter R (2007a) Chemical composition of the epicuticular and intracuticular wax layers on adaxial sides of Rosa canina leaves. Ann Bot (Lond) 100: 1557–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschhaus C, Herz H, Jetter R (2007b) Chemical composition of the epicuticular and intracuticular wax layers on the adaxial side of Ligustrum vulgare leaves. New Phytol 176: 311–316 [DOI] [PubMed] [Google Scholar]
- Buschhaus C, Jetter R (2011) Composition differences between epicuticular and intracuticular wax substructures: how do plants seal their epidermal surfaces? J Exp Bot 62: 841–853 [DOI] [PubMed] [Google Scholar]
- Buschhaus C, Jetter R (2012) Composition and physiological function of the wax layers coating Arabidopsis leaves: β-amyrin negatively affects the intracuticular water barrier. Plant Physiol 160: 1120–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver TLW, Gurr SJ (2006) Filamentous fungi on plant surfaces. In Riederer M, Müller C, eds, Biology of the Plant Cuticle, Vol 23. Blackwell, Oxford, pp 368–397 [Google Scholar]
- Eigenbrode SD, Espelie KE (1995) Effects of plant epicuticular lipids on insect herbivores. Annu Rev Entomol 40: 171–194 [Google Scholar]
- Geyer U, Schönherr J (1990) The effect of the environment on the permeability and composition of Citrus leaf cuticles. I. Water permeability of isolated cuticular membranes. Planta 180: 147–153 [DOI] [PubMed] [Google Scholar]
- Guhling O, Kinzler C, Dreyer M, Bringmann G, Jetter R (2005) Surface composition of myrmecophilic plants: cuticular wax and glandular trichomes on leaves of Macaranga tanarius. J Chem Ecol 31: 2323–2341 [DOI] [PubMed] [Google Scholar]
- Haas K, Schönherr J (1979) Composition of soluble cuticular lipids and water permeability of cuticular membranes from Citrus leaves. Planta 146: 399–403 [DOI] [PubMed] [Google Scholar]
- Hansjakob A, Bischof S, Bringmann G, Riederer M, Hildebrandt U (2010) Very-long-chain aldehydes promote in vitro prepenetration processes of Blumeria graminis in a dose- and chain length-dependent manner. New Phytol 188: 1039–1054 [DOI] [PubMed] [Google Scholar]
- Hansjakob A, Riederer M, Hildebrandt U (2011) Wax matters: absence of very-long-chain aldehydes from the leaf cuticular wax of the glossy11 mutant of maize compromises the prepenetration processes of Blumeria graminis. Plant Pathol 60: 1151–1161 [Google Scholar]
- Heredia A. (2003) Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochim Biophys Acta 1620: 1–7 [DOI] [PubMed] [Google Scholar]
- Jeffree CE. (2006) The fine structure of the plant cuticle. In Riederer M, Müller C, eds, Biology of the Plant Cuticle, Vol 23. Blackwell, Oxford, pp 11–144 [Google Scholar]
- Jetter R, Kunst L, Samuels AL (2007) Composition of plant cuticular waxes. In Riederer M, Müller C, eds, Biology of the Plant Cuticle, Vol 23. Blackwell, Oxford, pp 145–181 [Google Scholar]
- Jetter R, Schäffer S (2001) Chemical composition of the Prunus laurocerasus leaf surface: dynamic changes of the epicuticular wax film during leaf development. Plant Physiol 126: 1725–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetter R, Schäffer S, Riederer M (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23: 619–628 [Google Scholar]
- Kerstiens G. (1996) Cuticular water permeability and its physiological significance. J Exp Bot 47: 1813–1832 [Google Scholar]
- Kerstiens G, Lendzian KJ (1989) Interactions between ozone and plant cuticles. II. Water permeability. New Phytol 112: 21–27 [Google Scholar]
- Knoche M, Peschel S, Hinz M, Bukovac MJ (2000) Studies on water transport through the sweet cherry fruit surface: characterizing conductance of the cuticular membrane using pericarp segments. Planta 212: 127–135 [DOI] [PubMed] [Google Scholar]
- Kosma DK, Jenks MA (2007) Eco-physiological and molecular-genetic determinants of plant cuticle function in drought and salt stress tolerance. In Jenks MA, ed, Advances in Molecular Breeding towards Salinity and Drought Tolerance. Springer, Heidelberg, Germany, pp 91–120 [Google Scholar]
- Krauss P, Markstädter C, Riederer M (1997) Attenuation of UV radiation by plant cuticles from woody species. Plant Cell Environ 20: 1079–1085 [Google Scholar]
- Leide J, Hildebrandt U, Reussing K, Riederer M, Vogg G (2007) The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: effects of a deficiency in a β-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiol 144: 1667–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendzian KJ. (2006) Survival strategies of plants during secondary growth: barrier properties of phellems and lenticels towards water, oxygen, and carbon dioxide. J Exp Bot 57: 2535–2546 [DOI] [PubMed] [Google Scholar]
- Lendzian KJ, Nakajima A, Ziegler H (1986) Isolation of cuticular membranes from various conifer needles. Trees (Berl) 1: 47–53 [Google Scholar]
- Le Roux JH. (1969) Fischer-Tropsch waxes. II. Crystallinity and physical properties. J Appl Chem 19: 86–88 [Google Scholar]
- Leveau JHJ. (2006) Microbial communities in the phyllosphere. In Riederer M, Müller C, eds, Biology of the Plant Cuticle, Vol 23. Blackwell, Oxford, pp 334–367 [Google Scholar]
- Medina E, Aguiar G, Gomez M, Medina JD (2004) Patterns of leaf epicuticular waxes in species of Clusia: taxonomical implications. Interciencia 29: 579–582 [Google Scholar]
- Müller C, Riederer M (2005) Plant surface properties in chemical ecology. J Chem Ecol 31: 2621–2651 [DOI] [PubMed] [Google Scholar]
- Nawrath C. (2006) Unraveling the complex network of cuticular structure and function. Curr Opin Plant Biol 9: 281–287 [DOI] [PubMed] [Google Scholar]
- Popp C, Burghardt M, Friedmann A, Riederer M (2005) Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticular membranes: permeation of water and uncharged organic compounds. J Exp Bot 56: 2797–2806 [DOI] [PubMed] [Google Scholar]
- Reisberg EE, Hildebrandt U, Riederer M, Hentschel U (2012) Phyllosphere bacterial communities of trichome-bearing and trichomeless Arabidopsis thaliana leaves. Antonie van Leeuwenhoek 101: 551–560 [DOI] [PubMed] [Google Scholar]
- Riederer M. (2006) Thermodynamics of the water permeability of plant cuticles: characterization of the polar pathway. J Exp Bot 57: 2937–2942 [DOI] [PubMed] [Google Scholar]
- Riederer M, Schneider G (1989) Comparative study of the composition of waxes extracted from isolated leaf cuticles and from whole leaves of Citrus: evidence for selective extraction. Physiol Plant 77: 373–384 [Google Scholar]
- Riederer M, Schreiber L (2001) Protecting against water loss: analysis of the barrier properties of plant cuticles. J Exp Bot 52: 2023–2032 [DOI] [PubMed] [Google Scholar]
- Schönherr J. (1976) Water permeability of isolated cuticular membranes: the effect of cuticular waxes on diffusion of water. Planta 131: 159–164 [DOI] [PubMed] [Google Scholar]
- Schönherr J. (2006) Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. J Exp Bot 57: 2471–2491 [DOI] [PubMed] [Google Scholar]
- Schönherr J, Lendzian KJ (1981) A simple and inexpensive method of measuring water permeability of isolated plant cuticular membranes. Z Pflanzenphysiol 102: 321–327 [Google Scholar]
- Schönherr J, Riederer M (1986) Plant cuticles sorb lipophilic compounds during enzymatic isolation. Plant Cell Environ 9: 459–466 [Google Scholar]
- Schönherr J, Schmidt HW (1979) Water permeability of plant cuticles: dependence of permeability coefficients of cuticular transpiration on vapor pressure saturation deficit. Planta 144: 391–400 [DOI] [PubMed] [Google Scholar]
- Schönherr J, Schreiber L (2004) Size selectivity of aqueous pores in astomatous cuticular membranes isolated from Populus canescens (Aiton) Sm. leaves. Planta 219: 405–411 [DOI] [PubMed] [Google Scholar]
- Schreiber L. (2005) Polar paths of diffusion across plant cuticles: new evidence for an old hypothesis. Ann Bot (Lond) 95: 1069–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L, Riederer M (1996) Ecophysiology of cuticular transpiration: comparative investigation of cuticular water permeability of plant species from different habitats. Oecologia 107: 426–432 [DOI] [PubMed] [Google Scholar]
- Small DM. (1984) Lateral chain packing in lipids and membranes. J Lipid Res 25: 1490–1500 [PubMed] [Google Scholar]
- Solovchenko A, Merzlyak M (2003) Optical properties and contribution of cuticle to UV protection in plants: experiments with apple fruit. Photochem Photobiol Sci 2: 861–866 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Machida Y (2013) The cuticle and cellular interactions. In Riederer M, Müller C, eds, Biology of the Plant Cuticle, Vol 23. Blackwell, Oxford, pp 312–333 [Google Scholar]
- Vogg G, Fischer S, Leide J, Emmanuel E, Jetter R, Levy AA, Riederer M (2004) Tomato fruit cuticular waxes and their effects on transpiration barrier properties: functional characterization of a mutant deficient in a very-long-chain fatty acid β-ketoacyl-CoA synthase. J Exp Bot 55: 1401–1410 [DOI] [PubMed] [Google Scholar]
- Weichert H, Knoche M (2006) Studies on water transport through the sweet cherry fruit surface. 10. Evidence for polar pathways across the exocarp. J Agric Food Chem 54: 3951–3958 [DOI] [PubMed] [Google Scholar]
- Zeisler V, Schreiber L (2016) Epicuticular wax on cherry laurel (Prunus laurocerasus) leaves does not constitute the cuticular transpiration barrier. Planta 243: 65–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.