Aphid and caterpillar feeding induce different resistance mechanisms against subsequent feeding by a specialist caterpillar, and these entail different costs in terms of plant growth.
Abstract
Plants respond to herbivory with the induction of resistance, mediated by distinct phytohormonal signaling pathways and their interactions. Phloem feeders are known to induce plant resistance via the salicylic acid pathway, whereas biting-chewing herbivores induce plant resistance mainly via the jasmonate pathway. Here, we show that a specialist caterpillar (biting-chewing herbivore) and a specialist aphid (phloem feeder) differentially induce resistance against Pieris brassicae caterpillars in Arabidopsis (Arabidopsis thaliana) plants. Caterpillar feeding induces resistance through the jasmonate signaling pathway that is associated with the induction of kaempferol 3,7-dirhamnoside, whereas aphid feeding induces resistance via a novel mechanism involving sinapoyl malate. The role of sinapoyl malate is confirmed through the use of a mutant compromised in the biosynthesis of this compound. Caterpillar-induced resistance is associated with a lower cost in terms of plant growth reduction than aphid-induced resistance. A strong constitutive resistance against P. brassicae caterpillars in combination with a strong growth attenuation in plants of a transfer DNA (T-DNA) insertion mutant of WRKY70 (wrky70) suggest that the WRKY70 transcription factor, a regulator of downstream responses mediated by jasmonate-salicylic acid signaling cross talk, is involved in the negative regulation of caterpillar resistance and in the tradeoff between growth and defense. In conclusion, different mechanisms of herbivore-induced resistance come with different costs, and a functional WRKY70 transcription factor is required for the induction of low-cost resistance.
Herbivory is a common biotic stress that terrestrial plants frequently encounter during their life cycle. In their natural habitat, plants are continuously challenged by the same or different herbivore species. Hence, the ability to mount rapid and effective responses against subsequent herbivores is essential for plant fitness (Karban and Baldwin, 1997; Kessler and Baldwin, 2004; Bruce et al., 2007; Frost et al., 2008; Gális et al., 2009; Vos et al., 2013; Stam et al., 2014). To tailor their responses against subsequent herbivore feeding, plants can benefit from mechanisms that utilize the information from previous herbivore attack to modify their defense responses (Baldwin and Schmelz, 1996; De Vos et al., 2006; Bruce et al., 2007; Vos et al., 2013). Based on current understanding, plants mount responses to caterpillar feeding via intricate signaling events that integrate and synchronize various stress signals to orchestrate the elicitation of cost-effective defense responses (Heil and Silva Bueno, 2007; Frost et al., 2008). Among the various signaling pathways, phytohormones, especially jasmonic acid (JA), salicylic acid (SA), and their cross talk, are known to mediate plant responses to herbivory (Gols et al., 2003; Frost et al., 2008; Gális et al., 2009; Pieterse et al., 2012; Thaler et al., 2012; Vos et al., 2013; Menzel et al., 2014).
Jasmonates, a group of plant hormones including JA and its derivatives such as methyl jasmonate and jasmonic acid-isoleucine (JA-Ile), are important regulators of plant defenses against tissue-chewing herbivores and necrotrophic pathogens (Wasternack and Hause, 2013). To activate defense against herbivory, JA-Ile binds to the CORONATINE INSENSITIVE1 (COI1) receptor protein, which results in ubiquitination and subsequent degradation of JAZ proteins that act as repressors of JA signaling by binding to transcription factors (TFs) such as MYC2 that regulate JA-responsive genes (Chini et al., 2007; Thines et al., 2007; Wasternack, 2007; Browse and Howe, 2008; Chung et al., 2008; Yan et al., 2009). To be able to mount earlier and stronger defense responses against subsequent caterpillar feeding, an ability to enhance JA biosynthesis is required. Indeed, several studies have reported an earlier increased expression of JA biosynthetic genes after exogenous application of JA, wounding, or oral secretions to mimic caterpillar feeding in different model plants, including Arabidopsis (Arabidopsis thaliana) and Nicotiana attenuata, suggesting the existence of a regulatory network that augments JA biosynthesis in response to continuous or recurring biotic stresses (Reymond et al., 2000; Wasternack, 2007; Chung et al., 2008; Stork et al., 2009).
The major plant hormone that regulates and activates plant defenses in response to phloem-feeding herbivores and biotrophic pathogens is SA (Walling, 2008; Vlot et al., 2009). SA mediates transcriptional reprogramming through the NONEXPRESSOR OF PATHOGENESIS-RELATED GENE1 (NPR1) protein, which is a master regulator that synchronizes the activation of SA-responsive TFs and downstream up-regulation of defense-related genes (Després et al., 2003; Vlot et al., 2009; Wu et al., 2012; Seyfferth and Tsuda, 2014). In addition to the widely proposed role as an activator of plant resistance against phloem-feeding herbivores such as aphids, a role of SA as a negative regulator of plant defense against tissue-chewing herbivores has also been reported. For instance, Cui et al. (2002) showed that the SA-deficient mutant (sid2-1) and the SA-insensitive mutant (npr1) of Arabidopsis were highly resistant to a generalist tissue-chewing caterpillar (Trichoplusia ni). Moreover, tomato (Solanum lycopersicum) and Brussels sprouts (Brassica oleracea var gemmifera) plants that had been exposed to a 7-d aphid infestation, resulting in a significant increase in SA, were more susceptible to subsequent caterpillar feeding (Rodriguez-Saona et al., 2005; Soler et al., 2012). The results from these studies indicate that antagonistic interactions between SA and JA signaling may interfere with JA-induced responses against caterpillar feeding in Arabidopsis.
The underlying molecular mechanisms of SA-JA antagonistic interactions or cross talk have been well studied in Arabidopsis. For instance, Spoel et al. (2003) demonstrated that a functional NPR1 protein is indispensable for the regulation of downstream transcript reprogramming mediated by SA-JA cross talk. Furthermore, Li et al. (2004) identified the WRKY70 TF, which acts downstream of NPR1 and COI1, as a transcriptional regulator that positively regulates SA-dependent genes but negatively regulates a subset of JA-inducible genes in response to pathogen infection. Recently, Shim et al. (2013) reported that the MYB44 TF regulates WRKY70 in a regulatory network parallel to NPR1, thus facilitating SA-JA cross talk in plant-pathogen interactions. Based on the available information, it is now clear that the WRKY70 TF is a transcriptional regulator acting downstream of NPR1 and COI1 in mediating plant responses against necrotrophic pathogen infection (Li et al., 2004; Cui et al., 2005; De Vos et al., 2006; Mur et al., 2006; Van der Does et al., 2013; Caarls et al., 2015; Vos et al., 2015). However, the role of WRKY70 in plant defenses against biting-chewing herbivores such as caterpillars, particularly when they arrive as a second herbivore, remains to be elucidated. Interestingly, Kroes et al. (2015) reported a negative correlation between WRKY70 and MYC2 transcription in Arabidopsis plants that were simultaneously infested with specialist aphids (Brevicoryne brassicae) at a high density and specialist caterpillars (Plutella xylostella). Moreover, Li et al. (2015) demonstrated the involvement of OsWRKY70 in rice (Oryza sativa) resistance against a chewing herbivore and in the tradeoff between growth and defense. These studies strongly indicate a role of WRKY70 in plant responses to herbivore attack in both dicot and monocot plants.
In this study, we investigated the role of the WRKY70 TF in plant resistance against secondary Pieris brassicae caterpillar feeding in Arabidopsis. We found that a short (24-h) exposure to feeding by P. brassicae caterpillars or B. brassicae aphids rendered plants more resistant to subsequent P. brassicae caterpillars. However, enhanced resistance after previous exposure to herbivore feeding was only minor in a transfer DNA (T-DNA) insertion mutant of WRKY70 (wrky70) that already had a strong constitutive resistance to P. brassicae caterpillars. Analysis of phytohormone and defense metabolite levels revealed that fundamentally different mechanisms, which incurred different costs in terms of plant growth, were responsible for an enhanced resistance in plants with prior exposure to caterpillar or aphid feeding. A strong growth reduction after subsequent caterpillar feeding in all treatments of wrky70 plants suggests that the WRKY70 TF functions as a negative regulator of induced resistance against P. brassicae caterpillars and is also involved in optimizing plant fitness in response to herbivory in Arabidopsis. This study signifies the underlying mechanisms of the very different induced resistance mechanisms that are effective against the same specialist herbivore (i.e. P. brassicae caterpillars) but come with very different fitness costs.
RESULTS
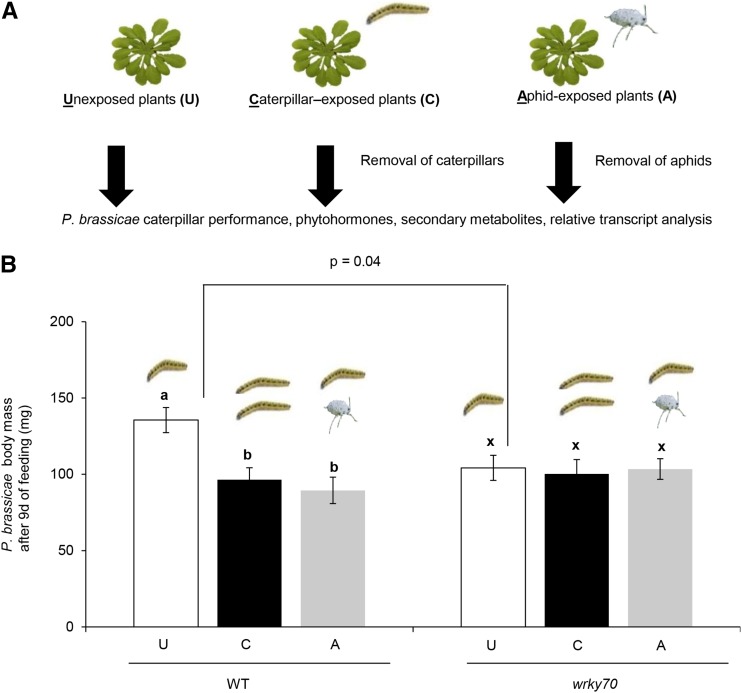
Prior Feeding by Caterpillars or Aphids Enhances Plant Resistance against Subsequent P. brassicae Caterpillars in Arabidopsis
A previous exposure of plants to either caterpillar or aphid feeding, resulting in an alteration of JA or SA levels, respectively, may have a positive or negative effect on the resistance against subsequent caterpillar attack (Cui et al., 2002; Kessler and Baldwin, 2004; Rodriguez-Saona et al., 2005; De Vos et al., 2006; Soler et al., 2012; Rasmann et al., 2015). Here, we observed that short feeding (24 h) by P. brassicae caterpillars or B. brassicae aphids on Arabidopsis wild-type, ecotype Columbia-0 plants significantly induced an accumulation of JA or SA, respectively (Supplemental Fig. S1, A and D; Student’s t test, P[JA] = 0.01 and P[SA] = 0.05). To investigate the effect of this short feeding by caterpillars or aphids on plant resistance against subsequent feeding by specialist caterpillars (i.e. P. brassicae), we assessed the body mass of P. brassicae caterpillars that had fed on wild-type plants that had been exposed previously to either P. brassicae caterpillars or B. brassicae aphids for 24 h. Plants without prior herbivore exposure were used as a control (Fig. 1A). After 9 d of feeding, P. brassicae caterpillars had gained significantly less body mass when feeding on caterpillar-exposed (ANOVA, P = 0.01) or aphid-exposed (ANOVA, P ≤ 0.01) plants than caterpillars that had fed on plants without prior herbivore exposure (Fig. 1B). Intriguingly, P. brassicae caterpillar body mass did not differ between caterpillars that had fed on caterpillar-exposed or aphid-exposed wild-type plants (Fig. 1B; ANOVA, P = 0.84). As we recorded a significant increase in SA level after 24 h of aphid feeding (Supplemental Fig. S1D; Student’s t test, P = 0.05), enhanced resistance against subsequent P. brassicae caterpillars on aphid-exposed wild-type plants was unexpected, because SA signaling is known to suppress JA-induced responses to caterpillar feeding (Cui et al., 2002; Soler et al., 2012). It has been demonstrated that SA-JA cross talk affects plant resistance against pathogens through the WRKY70 TF that functions downstream of NPR1 and COI1 (Van der Does et al., 2013; Caarls et al., 2015). Moreover, the effect of WRKY70 on plant resistance against pathogens is independent of subsequent changes in SA and JA levels (Li et al., 2004). Therefore, we investigated whether the herbivore-enhanced plant resistance phenotypes recorded here are mediated by SA-JA cross talk and WRKY70 regulation. To this end, we measured the body mass of P. brassicae caterpillars that had fed on unexposed, caterpillar-exposed, or aphid-exposed plants of a T-DNA insertion mutant of the WRKY70 gene (wrky70 plants). We observed enhanced resistance to P. brassicae caterpillars but increased susceptibility to B. brassicae aphids in wrky70 plants, which indicates a role of WRKY70 in plant resistance against specialist caterpillars and aphids (Supplemental Fig. S2, A and B; Student’s t test, P[caterpillar] = 0.04 and P[aphid] = 0.01). Unlike the situation in wild-type plants, caterpillar body mass after 9 d of feeding did not differ among caterpillars that had fed on unexposed, caterpillar-exposed, or aphid-exposed wrky70 plants (Fig. 1B; ANOVA, P = 0.74). Although a significantly lower body mass was recorded for caterpillars that had fed on unexposed wrky70 plants than for caterpillars that had fed on unexposed wild-type plants (Fig. 1B; Student’s t test, P = 0.04), there was no significant difference in body mass between caterpillars that had fed on caterpillar-exposed wild-type or wrky70 plants (Fig. 1B; Student’s t test, P = 0.51) or between caterpillars that had fed on aphid-exposed wild-type or wrky70 plants (Fig. 1B; Student’s t test, P = 0.63). Moreover, similar trends of herbivore-induced plant resistance against subsequent P. brassicae caterpillars to that shown in wkry70 plants were observed in SA-deficient mutant (sid2-1) plants that are impaired in SA biosynthesis (Supplemental Fig. S3). This indicates that an induced biosynthesis of SA was not required for the herbivore-enhanced resistance phenotypes observed in our study (Supplemental Fig. S3). Collectively, these results suggest that enhanced resistance against P. brassicae caterpillars in plants with prior exposure to feeding by P. brassicae caterpillars or B. brassicae aphids is not regulated through SA-JA signaling cross talk and WRKY70 TF regulation (Fig. 1B). To address this hypothesis, the transcript expression of biosynthetic genes and phytohormone levels were analyzed in wild-type or wrky70 plants upon caterpillar feeding.
Figure 1.
Plant resistance to P. brassicae caterpillars and effect of previous plant exposure to feeding by caterpillars or aphids. A, Wild-type and wrky70 mutant plants were infested with five first instar P. brassicae caterpillars or five adult B. brassicae aphids for 24 h to create caterpillar-exposed (C) or aphid-exposed (A) plants. B, Effect of a prior exposure to caterpillar or aphid feeding on plant resistance against subsequent P. brassicae caterpillar feeding is determined by measuring caterpillar body mass (means ± se) of 20 individual caterpillars feeding on caterpillar-exposed and aphid-exposed wild-type (WT) or wrky70 mutant plants for 9 d. Caterpillar body mass from the group fed on plants without prior herbivore experience or unexposed (U) plants was used as a control to assess the positive or negative effect of previous herbivore exposure on plant resistance. Caterpillar body mass after 9 d of feeding on unexposed, caterpillar-exposed, and aphid-exposed wild-type or wrky70 mutant plants was compared within the same genotype by one-way ANOVA followed by Tukey’s honestly significant difference (HSD) posthoc test. Different letters indicate significant differences within a plant genotype among treatments (P ≤ 0.05).
Impact of Herbivory History on JA and SA Signaling Pathways in Wild-Type and wrky70 Plants
To assess the effect of caterpillar feeding on JA and SA signaling in plants that had been previously exposed to caterpillars or aphids, we analyzed the relative transcript levels of selected biosynthetic and responsive genes in the JA and SA signaling pathways as well as the phytohormone levels for unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants. In addition, the transcript levels of WRKY70 upon subsequent caterpillar attack were also analyzed. The relative transcript levels of WRKY70 were undetectable before and after subsequent caterpillar feeding in wrky70 plants of all three treatments (Supplemental Fig. S4D), which indicates that WRKY70 was fully nonfunctional in the wrky70 mutant plants. Interestingly, while subsequent caterpillar feeding hardly increased the expression of WRKY70 in previously unexposed wild-type plants, WRKY70 expression was distinctively increased in wild-type plants previously exposed to caterpillars or aphids after subsequent caterpillar feeding (Supplemental Fig. S4A).
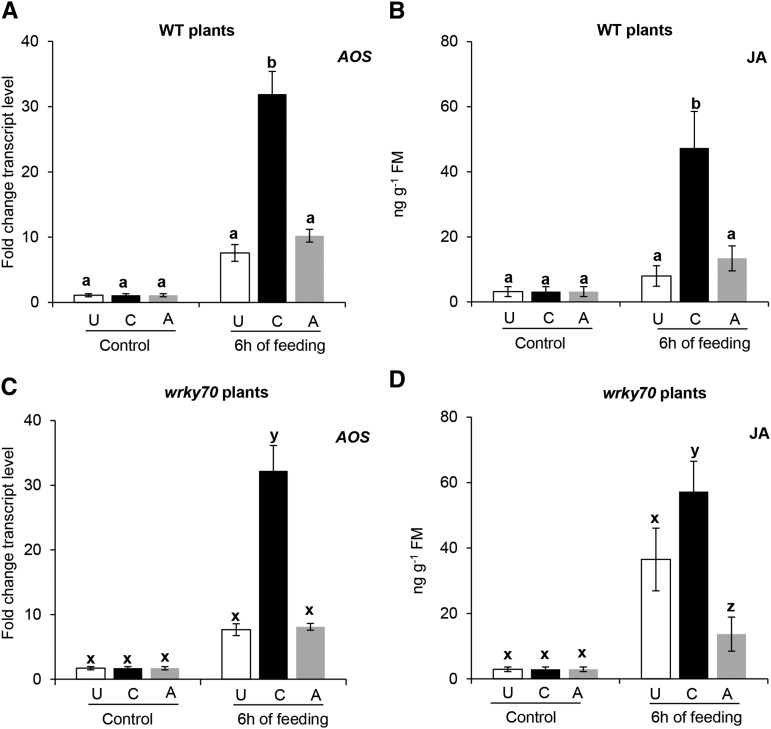
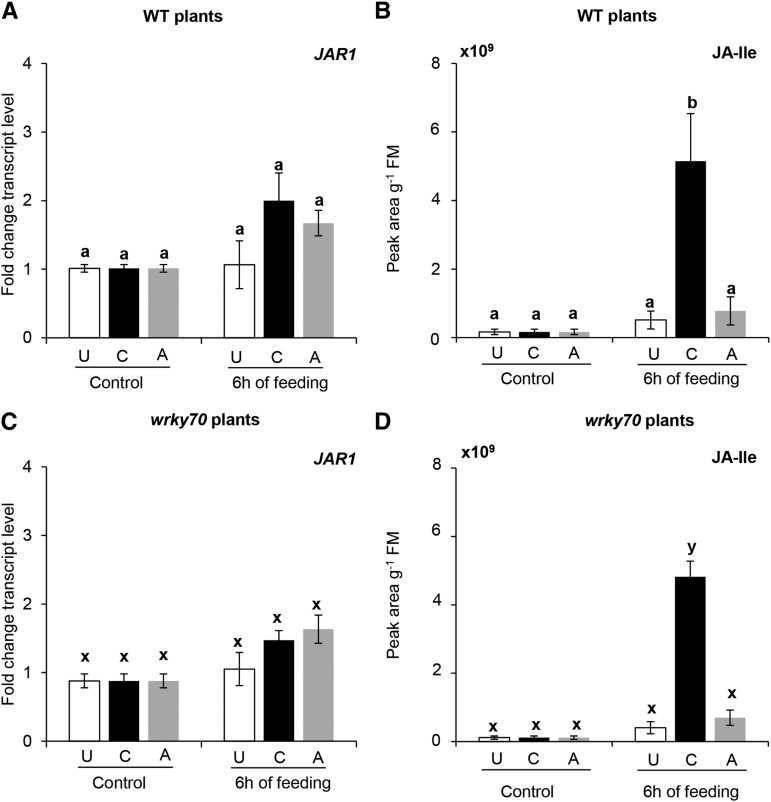
The relative transcript levels of a JA biosynthetic gene, ALLENE OXIDE SYNTHASE (AOS), were transiently induced after 24 h of caterpillar feeding in unexposed and aphid-exposed wild-type and wrky70 plants (Supplemental Fig. S4, B and E). In contrast, in wild-type and wrky70 plants that had been previously exposed to caterpillar feeding, AOS expression was already significantly induced after 6 h of subsequent feeding by caterpillars (Fig. 2, A and C; ANOVA, P[wild type] = 0.01 and P[wrky70] = 0.01). Consistent with induced AOS expression, JA levels were significantly increased after 6 h of subsequent caterpillar feeding on caterpillar-exposed wild-type and wrky70 plants (Fig. 2, B and D; ANOVA, P[wild type] = 0.01 and P[wrky70] = 0.01). Furthermore, JA-Ile levels in caterpillar-exposed wild-type and wrky70 plants were also significantly induced after 6 h of subsequent caterpillar feeding (Fig. 3; ANOVA, P[wild type-JA-Ile] = 0.01 and P[wrky70-JA-Ile] = 0.01), whereas JA-Ile levels showed similar patterns for unexposed and aphid-exposed wild-type and wrky70 plants (Fig. 3). It is noteworthy that the relative expression of JAR1, a JA-Ile biosynthetic gene, was higher in caterpillar-exposed wild-type and wrky70 plants after 6 h of subsequent feeding; however, these expression levels were not significantly different from the levels in unexposed or aphid-exposed wild-type or wrky70 plants (Fig. 3, A and C; ANOVA, P[wild type-JAR1] = 0.20 and P[wrky70-JAR1] = 0.15). It is interesting that the expression of VEGETATIVE STORAGE PROTEIN2 (VSP2), a well-established marker gene for JA-regulated defense responses, was higher in wrky70 plants than in wild-type plants in unexposed, caterpillar-exposed, and aphid-exposed plants after 48 or 72 h of subsequent caterpillar feeding (Supplemental Fig. S5, A–C). These results suggest that the WRKY70 TF is a negative regulator of JA-induced responses.
Figure 2.
Transcript levels of AOS, a JA biosynthetic gene, and JA levels in previously unexposed (U), caterpillar-exposed (C), and aphid-exposed (A) wild-type (WT) or wrky70 plants after 6 h of subsequent P. brassicae caterpillar feeding. A and C, AOS transcript levels in unexposed, caterpillar-exposed, and aphid-exposed plants of the wild type and the wrky70 mutant after subsequent feeding by five first instar P. brassicae caterpillars. Leaves were harvested just before caterpillar introduction and after 6 h of caterpillar feeding. Relative transcript levels were analyzed by quantitative real-time PCR (RT-qPCR). Values for each time point represent means ± se (n = 5) of AOS transcript levels relative to those of the ELONGATION FACTOR-1α (EF1α) gene. B and D, JA levels in unexposed, caterpillar-exposed, and aphid-exposed plants of the wild type and the wrky70 mutant after subsequent caterpillar feeding. The same leaf samples used for AOS transcript analysis were extracted, and JA levels were quantified by liquid chromatography quadrupole tandem mass spectrometry (LC-MS) using deuterium-labeled JA as an internal standard. Each graph shows means ± se (n = 5) of the JA level in wild-type and wrky70 plants. JA levels in wild-type and wrky70 plants from the same treatment were compared by Student’s t test. AOS expression and JA levels were compared among unexposed, caterpillar-exposed, and aphid-exposed treatments within the same genotype (wild-type or wrky70 plants) using one-way ANOVA followed by Tukey’s HSD posthoc test; different letters indicate significant differences within a plant genotype (P ≤ 0.05). FM, Fresh mass.
Figure 3.
Transcript levels of JASMONIC ACID AMIDO SYNTHETASE1 (JAR1), a JA-Ile biosynthetic gene, and JA-Ile levels in response to subsequent caterpillar feeding in unexposed (U), caterpillar-exposed (C), and aphid-exposed (A) wild-type (WT) and wrky70 plants. A and C, Means ± se (n = 5) of JAR1 relative transcript levels in unexposed, caterpillar-exposed, and aphid-exposed wild type and wrky70 mutant plants before and after 6 h of subsequent feeding of five first instar larvae of P. brassicae. Relative transcript levels of JAR1 were determined by RT-qPCR. The value at each time point represents the expression of JAR1 relative to the EF1α gene in wild-type and wrky70 plants. B and D, Means ± se (n = 5) of JA-Ile levels in unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 mutant plants before and after 6 h of subsequent feeding by five first instar larvae of P. brassicae. JA-Ile levels were determined by LC-MS followed by quantifying peak areas. Mean values of JAR1 relative transcripts and JA-Ile levels from unexposed, caterpillar-exposed, and aphid-exposed treatment were compared among treatments within the same plant background (wild-type and wrky70 plants) by one-way ANOVA by Tukey’s HSD posthoc test; different letters indicate significant differences within a plant genotype (P ≤ 0.05). FM, Fresh mass.
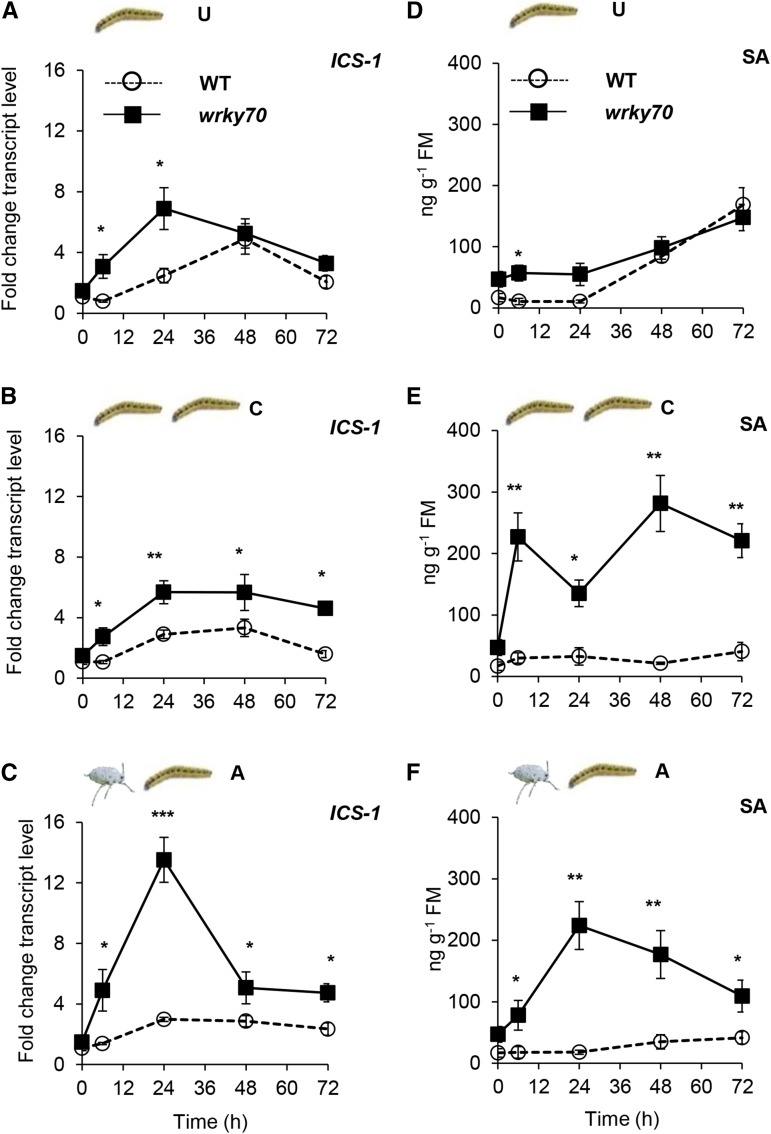
The transcript levels of the SA biosynthetic gene ISOCHORISMATE SYNTHASE1 (ICS1) and SA levels after subsequent caterpillar feeding in aphid-exposed plants were not significantly different from those in unexposed and caterpillar-exposed wild-type plants (Fig. 4; ANOVA, P[0 h] = 0.85, P[6 h] = 0.13, P[24 h] = 053, P[48 h] = 0.16, and P[72 h] = 0.36). In contrast, in wrky70 plants, levels of ICS1 expression and SA were significantly higher than in wild-type plants, regardless of the type of prior herbivory (Fig. 4). Furthermore, the expression of PATHOGENESIS-RELATED GENE1 (PR1), an SA-responsive marker gene, was consistent with an increased expression of ICS1 and SA levels in wrky70 plants (Supplemental Fig. S5, D–F). The negative correlation between WRKY70 expression on the one hand and ICS1 expression and SA level on the other suggests that the WRKY70 TF is a negative regulator of the SA biosynthetic pathway, which supports data by Wang et al. (2006).
Figure 4.
Expression profile of an SA biosynthetic gene, ICS1, and SA levels in unexposed (U), caterpillar-exposed (C), and aphid-exposed (A) wild-type (WT) and wrky70 plants after subsequent caterpillar feeding. A to C, Kinetics of ICS-1 expression relative to the EF1α gene from unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants after subsequent feeding by five first instar P. brassicae caterpillars; graphs represent means ± se (n = 5). D to F, Free SA levels at the designated time points after subsequent P. brassicae caterpillar feeding on unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants. Free SA levels were determined by LC-MS using deuterium-labeled SA as an internal standard. Mean values of ICS1 relative transcripts and SA levels at each time point from unexposed, caterpillar-exposed, and aphid-exposed treatment were compared between wild-type and wrky70 plants from the same treatment by Student’s t test; asterisks indicate significant differences: *, P ≤ 0.05, **, P ≤ 0.01 and ***, P ≤ 0.001. FM, Fresh mass.
Thus, in comparison with unexposed plants, prior exposure to caterpillar feeding results in an earlier (6-h) induction of JA and JA-Ile in wild-type and wrky70 plants upon subsequent caterpillar feeding (Figs. 2 and 3). However, because JA and JA-Ile levels in unexposed, caterpillar-exposed, and aphid-exposed wrky70 plants are similar to the levels in wild-type plants, this suggests that WRKY70 does not exert control on JA and JA-Ile biosynthesis (Figs. 2 and 3). In contrast, prior exposure of wild-type plants to aphid feeding hardly affected JA, JA-Ile, and SA levels during subsequent caterpillar feeding in comparison with unexposed wild-type plants (Figs. 2–4) and, importantly, did not correlate with enhanced resistance to subsequent caterpillar feeding in aphid-exposed wild-type plants (Fig. 1B). These results indicate that an earlier induction of JA and JA-Ile in caterpillar-exposed plants that is correlated with an enhanced resistance against subsequently feeding caterpillars is not affected by a nonfunctional WRKY70. Thus, aphid-enhanced plant resistance against subsequent caterpillar feeding seems to be mediated through a novel mechanism that does not depend on SA signaling, SA-JA cross talk, and WRKY70 regulation.
Enhanced Resistance in Caterpillar-Exposed Plants Is Associated with a Significant Increase in Kaempferol 3,7-Dirhamnoside
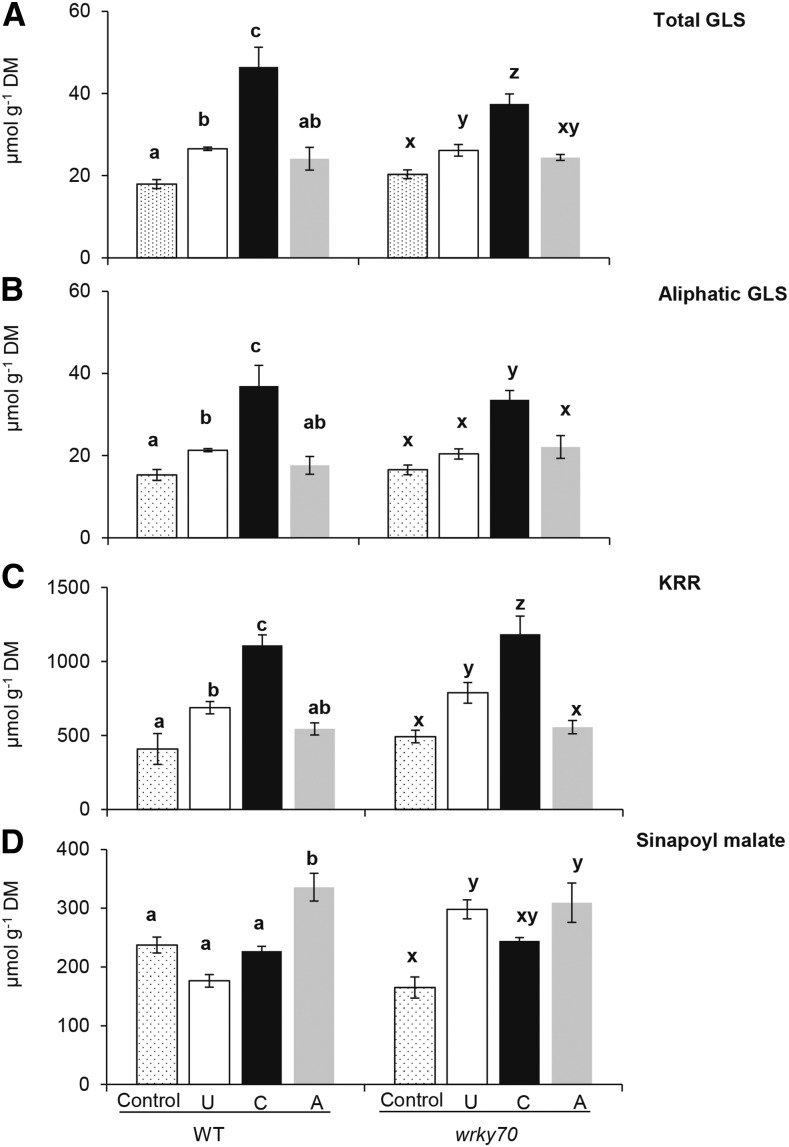
In order to examine the effects of an earlier induction (6 h) of JA and JA-Ile in caterpillar-exposed plants on defense metabolite accumulations that consequently may enhance plant resistance against subsequent caterpillar feeding, we quantified levels of kaempferol 3,7-dirhamnoside (KRR), a defense metabolite against specialist P. brassicae caterpillars (Onkokesung et al., 2014), in previously unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants. In addition, levels of glucosinolates (GLSs), common defense metabolites effective against generalist herbivores (Mewis et al., 2005; Halkier and Gershenzon, 2006), were also analyzed. After 4 d of caterpillar feeding, total GLS and KRR levels were significantly higher in previously caterpillar-exposed than in previously unexposed and aphid-exposed wild-type or wrky70 plants (Fig. 5, A–C; ANOVA, P[GLS-wild type] ≤ 0.01, P[KRR-wild type] ≤ 0.01, P[GLS-wrky70] ≤ 0.01, and P[KRR-wrky70] ≤ 0.01). Moreover, GLS and KRR levels did not differ between unexposed and aphid-exposed plants from both genotypes (Fig. 5, A–C). In addition, the accumulation patterns of other flavonol glycosides showed similar trends to those observed for KRR accumulation (Supplemental Fig. S6). Importantly, a significantly increased KRR level in caterpillar-exposed plants corresponds with enhanced resistance against subsequent caterpillar feeding in caterpillar-exposed wild-type and wkry70 plants. From these results, we infer that caterpillar-enhanced plant resistance is associated with an early transient induction of JA and JA-Ile at 6 h of caterpillar feeding that contributes to an increase in KRR levels upon subsequent caterpillar feeding.
Figure 5.
GLS, KRR, and sinapoyl malate (SM) production after 4 d of P. brassicae caterpillar feeding on previously unexposed (U), caterpillar-exposed (C), and aphid-exposed (A) wild-type (WT) and wrky70 plants. A and B, Means ± se (n = 5) levels of total and aliphatic GLSs in unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants. GLS was determined by HPLC using the peak areas at 229 nm relative to the peak area of the internal standard. C and D, Means ± se (n = 5) of KRR (C) and SM (D) in unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 mutant plants after 4 d of subsequent feeding by five first instar P. brassicae caterpillars. KRR levels were quantified by HPLC based on an external standard curve of an authentic standard of KRR. SM levels were determined by HPLC based on an external standard curve of an authentic standard of sinapic acid. GLS, KRR, and SM levels in unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 mutant plants were compared by one-way ANOVA followed by Tukey’s HSD posthoc test; different letters indicate significant differences (P ≤ 0.05) among treatments within the same genotype. DM, Dry mass.
Aphid-Enhanced Plant Resistance against Subsequent Caterpillar Feeding Is Associated with Alteration of Sinapoyl Ester Levels
Besides GLS and KRR, sinapate esters, a group of flavonoid metabolites, play a role in plant resistance to caterpillars and necrotrophic pathogens (Leiss et al., 2011; Demkura and Ballaré, 2012). As we did not observe a correlation between KRR levels and aphid-enhanced plant resistance against subsequent caterpillar feeding in wild-type and wrky70 plants (Figs. 1B and 5D), we investigated whether sinapate esters play a role in an enhanced resistance against specialist caterpillars in our system. The levels of two sinapate esters that are abundant in Arabidopsis, namely sinapoyl glucose (SG) and sinapoyl malate (SM), were analyzed in unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants. SG levels in wild-type and wrky70 plants were reduced after 4 d of subsequent caterpillar feeding on previously unexposed, caterpillar-exposed, and aphid-exposed plants (Supplemental Fig. S7B). While SM levels in unexposed and caterpillar-exposed wild-type plants hardly changed from the basal levels, a significant increase in SM level was observed in aphid-exposed wild-type plants (Fig. 5D; ANOVA, P ≤ 0.01). In contrast, in the wrky70 mutant, SM levels increased from the basal level after subsequent caterpillar feeding in previously unexposed, caterpillar-exposed, and aphid-exposed plants (Fig. 5D; ANOVA, P ≤ 0.01). Consistent with an increase in SM levels, the relative transcript level of SINAPOYLGLUCOSE:MALATE SINAPOYLTRANSFERASE (SMT), a gene coding for an SM biosynthetic enzyme, was significantly higher in aphid-exposed than in unexposed and caterpillar-exposed wild-type or wrky70 plants (Supplemental Fig. S8, B and E). In contrast, the transcript levels of SINAPATE-1-GLUCOSYL TRANSFERASE (SGT), an SG biosynthetic gene, did not correlate with SG levels in previously unexposed, caterpillar-exposed, and aphid-exposed of wild-type or wkry70 plants (Supplemental Fig. S8, C and F). Importantly, the significant increase in SM corresponded with an enhanced resistance against subsequent caterpillar feeding in aphid-exposed wild-type plants, and in wrky70 plants this was the case regardless of prior herbivory history (Fig. 1B). It is noteworthy that a significant increase in SM levels did not correlate with phytohormone profiles in aphid-exposed wild-type plants and in wrky70 plants of all three treatments (Figs. 2–5). Taken together, our results suggest that SM is a defense metabolite effective against P. brassicae caterpillars and that SM biosynthesis is likely to be regulated through an independent mechanism rather than the JA or SA signaling cascade. We further investigated the role of SM in plant resistance against subsequent caterpillar feeding in an SM-deficient mutant plant.
SM Plays a Role in Enhanced Resistance to P. brassicae Caterpillars Feeding on Arabidopsis
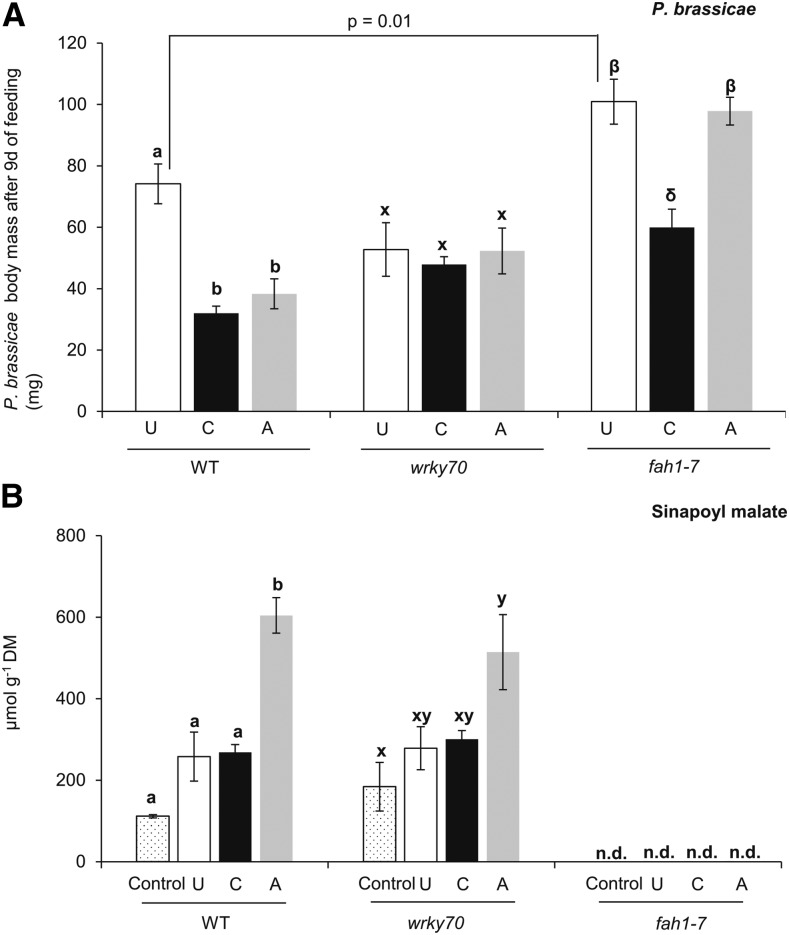
A mutant plant silenced in ferulic acid 5-hydroxylase (fah1), a major enzyme in sinapate ester biosynthesis, has been reported to lack sinapate ester accumulation, including SM (Ruegger et al., 1999; Hagemeier et al., 2001). To examine the role of SM in enhanced resistance against subsequent caterpillar feeding in aphid-exposed wild-type plants, we compared P. brassicae caterpillar performance on fah1-silenced (fah1-7) wild-type and wrky70 plants. On unexposed fah1-7 plants, P. brassicae caterpillars gained significantly higher body mass than on unexposed wild-type or wrky70 plants (Fig. 6A; ANOVA, P = 0.01). As expected, enhanced resistance against subsequently feeding caterpillars, as observed in aphid-exposed wild-type plants, was not observed in fah1-7 plants (Fig. 6A; ANOVA, P[fah1-7] = 0.40). It is noteworthy that while constitutive and induced KRR levels in fah1-7 plants were significantly lower compared with the levels in wild-type and wrky70 plants after subsequent caterpillar feeding, enhanced resistance against subsequent P. brassicae caterpillars in caterpillar-exposed plants was still observed in fah1-7 mutant plants (Fig. 6A; Supplemental Fig. S9D). These results suggest an involvement of other defense metabolites in mediating the caterpillar-enhanced resistance phenotype in fah1-7 plants. Nevertheless, our results indicate that SM is a metabolite that provides resistance against P. brassicae caterpillars in Arabidopsis plants that had been previously exposed to aphid feeding.
Figure 6.
Biomass of P. brassicae caterpillars and SM production in previously unexposed (U), caterpillar-exposed (C), and aphid-exposed (A) wild-type, wrky70, and fah1-7 plants. A, P. brassicae caterpillar body mass (means ± se; n = 20) after 9 d of feeding on unexposed, caterpillar-exposed, and aphid-exposed plants of the wild type and the wrky70 and fah1-7 mutants. Caterpillar body mass was compared among treatments within the same genotype by one-way ANOVA followed by Tukey’s HSD posthoc test; different letters indicate significant differences within a plant genotype (P ≤ 0.05). In addition, caterpillar body mass from the same treatment was compared between wild-type and wrky70 plants or between wild-type and fah1-7 plants by Student’s t test. B, SM levels (means ± se; n = 5) after 4 d of feeding by five first instar P. brassicae caterpillars on previously unexposed, caterpillar-exposed, and aphid-exposed wild-type, wrky70, and fah1-7 plants. SM levels were quantified by HPLC using an external standard curve of an authentic sinapic acid standard. Mean values of SM levels were compared among unexposed, caterpillar-exposed, and aphid-exposed treatments within the respective genotypes (wild-type, wrky70, or fah1-7 plants) by one-way ANOVA followed by Tukey’s HSD posthoc test; different letters indicate significant differences within the same genotype (P ≤ 0.05). DM, Dry mass; n.d., not detected.
Different Costs of Enhanced Resistance to Subsequent P. brassicae Caterpillar Feeding
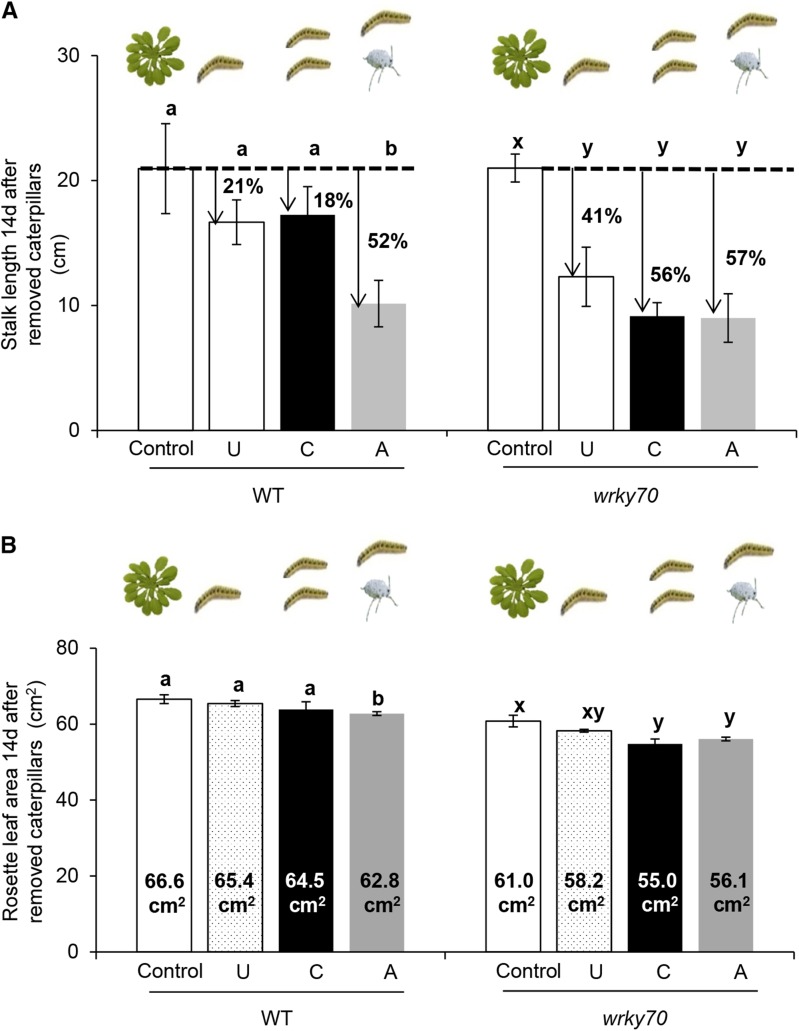
An increased accumulation of distinct defense metabolites upon caterpillar feeding on either caterpillar-exposed plants (KRR) or aphid-exposed plants (SM) suggests that plants employ different defense mechanisms depending on previous herbivory history to enhance their defense against subsequent feeding by P. brassicae caterpillars (Fig. 5). As defense mechanisms are costly for plant growth and development (Gulmon and Mooney, 1986; van Hulten et al., 2006; Zavala and Baldwin, 2006; Onkokesung et al., 2010), we investigated the effect of the different enhanced-resistance mechanisms on plant growth in caterpillar-exposed and aphid-exposed wild-type and wrky70 plants upon subsequent exposure to caterpillars. To determine plant growth, we measured stalk length and rosette area at 14 d after removing caterpillars for previously unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants and compared this with the respective control (undamaged) plants.
A slight, nonsignificant reduction in stalk length and rosette area was observed for previously unexposed and previously caterpillar-exposed wild-type plants; however, a large reduction of stalk length (approximately 50%) and a small but significant reduction in rosette area (approximately 6%) were observed in wild-type plants previously exposed to aphids compared with control plants (Fig. 7; ANOVA, P[stalk] = 0.02 and P[rosette] = 0.01). In contrast, a strong reduction of stalk length was observed in previously unexposed (41%), caterpillar-exposed (56%), and aphid-exposed (57%) wrky70 plants (Fig. 7A; ANOVA, P = 0.01). Consistent with the stalk length reduction, rosette area was significantly reduced in wrky70 plants regardless of prior herbivory conditions (Fig. 7B; ANOVA, P = 0.02). It is interesting that there was no significant difference in stalk length between undamaged control wild-type and wrky70 plants (Fig. 7A; Student’s t test, P = 0.99); however, the rosette area of undamaged wrky70 plants was significantly smaller than the rosette area of undamaged wild-type plants (Fig. 7B; Student’s t test, P = 0.01). In addition, although leaf area damaged after 4 d of caterpillar feeding showed no significant difference among previously unexposed, caterpillar-exposed, and aphid-exposed wild-type plants or among wrky70 plants of these three treatments, the caterpillar-damaged leaf areas were significantly smaller in wrky70 plants than in wild-type plants for unexposed and caterpillar-exposed plants (Supplemental Fig. S10; Student’s t test, P[unexposed] = 0.007 and P[caterpillar] = 0.04). Taken together, our results indicate that enhanced resistance to caterpillars in wild-type plants that had previously been exposed to aphid feeding is more costly than for wild-type plants that had previously been exposed to caterpillars. A significant attenuation of plant growth after subsequent caterpillar feeding in wrky70 plants, which have a high constitutive resistance against P. brassicae caterpillars, indicates an involvement of the WRKY70 TF in suppressing herbivore-induced resistance to maintain an acceptable plant growth during herbivory conditions.
Figure 7.
Plant growth in unexposed (U), caterpillar-exposed (C), and aphid-exposed (A) wild-type (WT) and wrky70 plants. A, Stalk length (means ± se; n = 20) of unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants at 14 d after removal of P. brassicae caterpillars. Five first instar P. brassicae caterpillars were removed from unexposed, caterpillar-exposed, and aphid-exposed plants of the wild type and the wrky70 mutant after 4 d of feeding. The percentage of stalk length reduction in unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants was calculated based on the stalk length of control (undamaged) wild-type plants. B, Rosette area (means ± se; n = 20) at 14 d after P. brassicae caterpillars had been removed from unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants. Five first instar P. brassicae caterpillars were allowed to feed freely for 4 d before removal from unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants. The entire rosette area of each plant from undamaged control, unexposed, caterpillar-exposed, and aphid-exposed treatments was photographed, and total rosette area was analyzed using ImageJ software. Mean values of stalk length and total rosette area were compared among control (undamaged), unexposed, caterpillar-exposed, and aphid-exposed plants within the respective plant type (wild type or wrky70) by one-way ANOVA followed by Tukey’s HSD posthoc test; different letters indicate significant differences within a plant genotype (P ≤ 0.05).
DISCUSSION
A prior exposure to herbivore attack has been proposed to influence plant responses to subsequent attack from a similar or different herbivore species (Karban and Baldwin, 1997; Kessler and Baldwin, 2004; Rodriguez-Saona et al., 2005; De Vos et al., 2006; Poelman et al., 2008, 2010; Zhang et al., 2013a, 2013b; Rasmann et al., 2015). Here, we show that an enhanced resistance to P. brassicae caterpillars, which involves an early induction of JA signaling in wild-type plants previously exposed to caterpillar feeding, is more cost effective than an enhanced resistance via an alternative mechanism (SM) in wild-type plants that had been previously exposed to aphid feeding. A combination of enhanced JA responses and an increase in SM after P. brassicae feeding on wrky70 plants, which occurs independent of herbivory history and which results in a caterpillar-resistant phenotype, indicates that the WRKY70 TF plays an important role in this. Subsequently, the fact that caterpillar feeding on wrky70 plants results in growth reduction, compared with the effect of caterpillar feeding on wild-type plants, indicates that this TF, which is a negative regulator of JA-induced resistance, also mediates a tradeoff between plant growth and defense.
WRKY70 TF Regulation Is Involved in Suppressing Plant Resistance against P. brassicae Caterpillars
Jasmonates are major signaling molecules mediating the defense against caterpillars in various plant species, including Arabidopsis (Wasternack and Hause, 2013). A significant increase in susceptibility to generalist caterpillars (Spodoptera exigua) in an Arabidopsis genotype with an overexpressor of a negative regulator of JA (JAZ1Δ3A) indicated an important role for this negative regulator in the induced resistance against caterpillars (Chung et al., 2008). Because the WRKY70 TF has been identified as a negative regulator of JA-induced responses in Arabidopsis (Li et al., 2004), we hypothesized that wrky70 plants would become resistant to P. brassicae caterpillars due to a lack of this negative regulator. Indeed, our data show that wrky70 plants are more resistant to P. brassicae caterpillars than wild-type plants (Fig. 1B; Supplemental Fig. S2A). Although similar levels of JA and JA-Ile were observed in wild-type and wrky70 plants upon caterpillar feeding, the transcript level of the JA-responsive marker gene VSP2 was significantly higher in wrky70 plants than in wild-type plants after caterpillar feeding (Figs. 2 and 3; Supplemental Fig. S4, A–C). Our results are consistent with previous studies on a T-DNA insertion mutant of WRKY70 that reported an increased expression of JA-responsive genes and increased JA sensitivity in wrky70 mutant plants, while the transcript expression of JA biosynthetic genes and JA levels in wrky70 plants remained at the same levels as in wild-type plants in response to necrotrophic pathogens (Li et al., 2004, 2006). Altogether, our data indicate that the WRKY70 TF is a negative regulator of JA-induced responses that lead to plant resistance against P. brassicae caterpillars feeding on Arabidopsis. It is interesting that in the monocot species rice, OsWRKY70 mediated plant resistance against chewing herbivores via positive regulation of JA biosynthesis rather than suppressing JA-induced responses (Li et al., 2015). Together with our results, this suggests a different evolution of WRKY70 functions in plant resistance against chewing herbivores in dicot and monocot plants.
Caterpillar-Induced Plant Resistance
An enhanced resistance to caterpillar feeding on plants previously exposed to the same caterpillar species has been reported in the Solanaceae and Brassicaceae families (Rodriguez-Saona et al., 2005; De Vos et al., 2006; Soler et al., 2012; Rasmann et al., 2015). Here, we report that a short-term (24-h) exposure to feeding by P. brassicae caterpillars was sufficient to significantly enhance plant resistance against subsequent P. brassicae caterpillar feeding in wild-type Arabidopsis plants (Fig. 1B). The induction of JA, caused by prior caterpillar attack, or an exogenous application of JA or methyl jasmonate can facilitate earlier and stronger responses to subsequent herbivory (Gols et al., 2003; Cui et al., 2005; Rasmann et al., 2012; Menzel et al., 2014). Indeed, we observed an early increase in the transcript expression of AOS and JAR1 and the accumulation of JA and JA-Ile (at 6 h of subsequent feeding) in wild-type plants that had previously been exposed to caterpillars or that had been previously unexposed (Figs. 2 and 3). Moreover, GLS and KRR levels after 4 d of caterpillar feeding were significantly higher in wild-type plants that had previously been exposed to caterpillar feeding (approximately 2-fold) than in previously unexposed wild-type plants (Fig. 5, A and C). Many studies on Arabidopsis showed that specialist herbivores such as P. brassicae caterpillars are highly adapted to GLS (Hopkins et al., 2009; Müller et al., 2010; Winde and Wittstock 2011). Therefore, plant resistance against specialist caterpillars is unlikely to depend on an individual group of defense metabolites such as GLS (Rasmann et al., 2015). We have previously demonstrated that KRR is an effective defense metabolite against P. brassicae caterpillars (Onkokesung et al., 2014). The data presented here show that a short-term exposure of Arabidopsis to caterpillar feeding is sufficient to induce an early accumulation of JA and JA-Ile that is associated with an increase in the levels of KRR and an enhanced resistance against subsequent caterpillar feeding. Although unexposed wrky70 plants were more resistant to P. brassicae caterpillars than unexposed wild-type plants, a comparable level of resistance against subsequent caterpillar feeding was observed in caterpillar-exposed wild-type and wrky70 plants (Fig. 1B). Furthermore, the accumulation of KRR was similar in caterpillar-exposed wild-type and wrky70 plants (Fig. 5C). These results indicate that the lack of a negative regulator of JA (i.e. the WRKY70 TF) is unlikely to further enhance plant resistance against subsequent caterpillar feeding beyond the level represented in unexposed wrky70 plants. Recently, it has been shown for Arabidopsis natural accessions that an enhanced resistance against subsequent caterpillar feeding was minor in the accessions with high constitutive resistance against caterpillars (Rasmann et al., 2015). Together, our results suggest a constraint of JA-induced plant resistance against caterpillars that restricts an induction of defense mechanisms to the level that allows enough resources to be allocated to other processes, particularly growth. The effect of enhanced resistance in herbivore-exposed wild-type plants and a highly resistant phenotype in wrky70 plants on plant growth will be discussed below.
Aphid-Induced Plant Resistance
Aphid infestation is commonly known to increase the accumulation of SA, a major phytohormone in plant responses to phloem feeders (Zhu-Salzman et al., 2004; Walling, 2008). Previous studies have suggested that defense against caterpillars was suppressed after long-term (7-d) exposure to aphid feeding due to antagonistic interactions between SA and JA signaling (Rodriguez-Saona et al., 2005; Soler et al., 2012). Although a short-term (24-h) exposure to B. brassicae aphid feeding in wild-type plants was sufficient to significantly increase SA levels (Supplemental Fig. S1D), an unexpected enhanced resistance against subsequent caterpillar feeding was observed in aphid-exposed wild-type plants (Fig. 1B). Moreover, a similarly low body mass was recorded for P. brassicae caterpillars after 9 d of subsequent feeding on aphid-exposed wild-type, wrky70, or sid2-1 plants (Fig. 1B; Supplemental Fig. S3). These results indicate that neither a functional WRKY70 TF nor an increase in SA level is required for the regulation of aphid-enhanced plant resistance to P. brassicae caterpillars. Furthermore, the levels of JA, JA-Ile, and KRR were similar for previously unexposed and previously aphid-exposed wild-type and wrky70 plants, which suggests that a novel mechanism, independent of SA, JA, or their cross talk, regulates aphid-enhanced plant resistance against subsequent feeding by a specialist caterpillar (Figs. 2, 3, and 5).
Besides an increase in SA accumulation, aphid infestation is also known to induce oxidative stress in plant cells (Moran et al., 2002; Kempema et al., 2007). Demkura and Ballaré (2012) reported that a prior mild UV light treatment, which induced oxidative stress, made Arabidopsis plants highly resistant to (necrotrophic) pathogens that are sensitive to JA-dependent defenses through an induction of sinapate esters. Interestingly, we found a correlation between high SM accumulation and enhanced resistance to P. brassicae caterpillars in aphid-exposed wild-type plants as well as in wrky70 plants regardless of herbivory history (Figs. 1B and 5D). An increased susceptibility to P. brassicae caterpillars and a lack of enhanced resistance against subsequent caterpillar feeding in aphid-exposed SM-deficient plants (fah1-7) further support the function of SM in plant defense against P. brassicae caterpillars in Arabidopsis. It is interesting that the accumulation of KRR, a metabolite that provides resistance against P. brassicae caterpillars (Onkokesung et al., 2014), was much lower (approximately 50%) in fah1-7 plants than in wild-type and wrky70 plants. Furthermore, caterpillar feeding failed to induce KRR levels in fah1-7 mutant plants (Supplemental Fig. S6D). These results indicate that the combination of a lack of SM accumulation and a low KRR level contribute to the susceptibility to P. brassicae caterpillars in previously unexposed fah1-7 plants (Fig. 6; Supplemental Fig. S9D). Further investigation of P. brassicae caterpillar resistance in Arabidopsis mutant plants silenced in SMT should provide additional information on the function of SM in plant resistance against specialist caterpillars.
SM induction after subsequent caterpillar feeding is a unique phenomenon in aphid-exposed wild-type plants. It is interesting that feeding by either P. brassicae caterpillars or B. brassicae aphids did not induce SM in wild-type plants, suggesting that the mechanism that regulated SM accumulation might act independently from JA- or SA-signaling pathways (Supplemental Fig. S11). Although we observed a tentatively negative correlation between SMT and WRKY70 expression in unexposed, caterpillar-exposed, and aphid-exposed wild-type plants (Supplemental Fig. S8, A and B), the lack of an increased transcript level of SMT and SM accumulation at the basal level in wrky70 mutant plants prevent us from drawing the conclusion that the WRKY70 TF is a negative regulator of SM biosynthesis. Moreover, Kim et al. (2015) reported that the level of indole-3-acetaldoxime, a precursor in indole GLS, auxin, and camalexin biosynthesis, negatively affected SM level in Arabidopsis. Based on available information on the SM biosynthetic pathway (Clauss et al., 2011) and our results, we speculate that SM biosynthesis is regulated through a novel molecular mechanism that may involve reactive oxygen species. Future studies using an integrative analysis of transcriptomics and metabolomics are required to identify the regulatory network of SM biosynthesis from signaling to metabolite biosynthesis.
It is noteworthy that KRR and SM both are flavonoid metabolites that are active defense metabolites against specialist caterpillars. Therefore, it is likely that other flavonoid metabolites might also be an active defense metabolite against specialist herbivores of Arabidopsis. Studying plant resistance against specialist herbivores in Arabidopsis mutant plants defective in transcriptional regulators of flavonoid biosynthesis pathways may result in the identification of novel defense metabolites against specialist herbivores.
Differential Costs of JA-Dependent and JA-Independent Herbivore-Induced Plant Resistance
The concept that plants utilize information of a previous encounter with biotic or abiotic stress in phytohormonal networks and enhance resistance against subsequent stress conditions in a cost-effective manner has been proposed previously (van Hulten et al., 2006; Bruce et al., 2007; Conrath, 2011; Vos et al., 2013). We found that an enhanced resistance against subsequent P. brassicae caterpillar feeding through a JA-independent mechanism involving SM biosynthesis in aphid-exposed wild-type plants strongly reduced plant growth. In contrast, enhanced resistance through JA-dependent mechanisms via the induction of GLS and KRR in caterpillar-exposed wild-type plants did not have a significant growth reduction compared with previously unexposed plants or control uninfested wild-type plants (Fig. 7). Although the induction of defense metabolites that is mediated by JA signaling after caterpillar feeding has been shown to incur costs in terms of plant growth reduction in Arabidopsis (Kliebenstein et al., 2001; Paul-Victor et al., 2010; Züst et al., 2011; Bekaert et al., 2012), this mechanism appears to be more cost effective than the induction of aphid-induced defense involving the metabolite SM. Nevertheless, we cannot rule out that a direct negative impact of aphid feeding on plant growth (Züst et al., 2011) leads to a stronger growth reduction upon caterpillar feeding in aphid-exposed wild-type plants.
Although the induction of JA signaling and defense metabolites in caterpillar-exposed or aphid-exposed wrky70 plants were similar to those in wild-type plants, a strong growth reduction that followed subsequent caterpillar feeding was observed in wrky70 plants (Fig. 7). Wang et al. (2006) reported a dual function of the WRKY70 TF as a negative and a positive regulator of SA biosynthesis and perception, respectively. It has been reported that high SA accumulation can suppress plant growth in Arabidopsis (Rivas-San Vicente and Plasencia, 2011). Indeed, wrky70 plants had a significantly higher level of SA at the constitutive and induced levels than wild-type plants (Fig. 4). It is interesting that, although stalk length was comparable between undamaged control wild-type and wrky70 plants, undamaged control wrky70 plants had a smaller rosette than undamaged control wild-type plants (Fig. 7). These results suggest a possible negative effect of high SA level on plant growth in wrky70 mutant plants. However, in comparison with undamaged control wrky70 plants, a further and significant reduction of stalk length and rosette area was observed after subsequent caterpillar feeding on wrky70 plants (Fig. 7). Taken together, we infer from our results that the growth attenuation in wrky70 plants is a consequence of a high SA level and induced defense against caterpillars. Furthermore, a highly caterpillar-resistant phenotype that might derive from a strong induction of JA-dependent responses coincides with a strong growth reduction after caterpillar feeding on wrky70 plants regardless of a prior herbivory history (Figs. 1B and 7). These results suggest that the WKRY70 TF is involved in a negative regulatory network that represses JA-inducible resistance against caterpillars to maintain an acceptable plant growth during herbivory stress conditions. More studies, for instance on the interaction between WRKY70 TF and other negative regulators of JA, particularly JAZ repressor proteins, are required to understand the role of the WRKY70 TF in a JA negative regulatory network that might also be involved in the regulation of a tradeoff between growth and defense during herbivory stress.
CONCLUSION
Our study shows that, although a prior exposure to short-term aphid or caterpillar feeding enhances plant resistance to subsequent caterpillar feeding, fundamentally different underlying defense mechanisms are involved. An enhanced resistance through the JA signaling network in caterpillar-exposed plants is more cost effective than an enhanced resistance through an alternative mechanism involving an increase in SM level, as observed in aphid-exposed plants. The constitutive caterpillar-resistant phenotype in wrky70 plants coincides with a strong growth reduction, which indicates that plants utilize negative regulators of JA-induced responses, such as the WRKY70 TF, to restrict JA-induced resistance against caterpillar feeding while maintaining plant growth during subsequent herbivory.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 was used as the wild type. Seeds of a T-DNA insertion mutant of WRKY70 (wrky70; SALK_025198; Li et al., 2006), a sinapate ester mutant (fah1-7; N8604; Ruegger et al., 1999), and an SA induction-deficient mutant (sid2-1; N16438; Wildermuth et al., 2001) in the Columbia-0 background were obtained from the European Arabidopsis Stock Centre. Surface-sterilized seeds as described previously (Onkokesung et al., 2014) were germinated on one-half-strength Murashige and Skoog medium containing 3% (w/v) Suc. Plates were kept in the dark at 4°C for 2 d before transfer to a growth chamber at 21°C ± 1°C, 60% ± 5% relative humidity (RH), 120 μmol m−2 s−1 light intensity, and an 8/16-h (light/dark) photoperiod. Fourteen-day-old seedlings were subsequently transplanted into round plastic pots (diameter, 4.5 cm) containing sterilized substrate mix (Horticoop) and kept under environmental conditions as described above. Four- to 5-week-old plants were used in all experiments.
Insects
Caterpillars (Pieris brassicae) and aphids (Brevicoryne brassicae) from their respective stock colonies (Laboratory of Entomology, Wageningen University) were reared on Brussels sprouts plants (Brassica oleracea var gemmifera ‘Cyrus’) at 22°C ± 1°C, 60% ± 5% RH, and a 16/8-h (light/dark) photoperiod.
Herbivore Treatments
Four- to 5-week-old Arabidopsis plants were transferred from short (8/16-h light/dark) to long (16/8-h light/dark) photoperiod conditions, at 21°C ± 1°C and 60% ± 5% RH, 24 h before herbivore treatments.
For caterpillar-exposed plants, five first instar P. brassicae caterpillars were placed on each Arabidopsis plant and allowed to feed freely for 24 h before being removed from the plants.
For aphid-exposed plants, five adult B. brassicae aphids were placed on individual Arabidopsis plants, and the plants were kept in cylindrical plastic containers (diameter 8 cm × height 14 cm) covered with fine-mesh gauze under the same growth conditions as for the caterpillar-exposed treatment. After 24 h of feeding, the aphids were removed from the plants.
Plants without prior herbivore exposure (unexposed plants) were kept under the same growth conditions as caterpillar- and aphid-exposed plants for 24 h. After 24 h of exposure to caterpillars or aphids, unexposed, caterpillar-exposed, and aphid-exposed plants were subjected to P. brassicae caterpillar feeding.
P. brassicae Caterpillar Performance
To determine the effects of prior herbivory on the performance of P. brassicae caterpillars, a freshly hatched neonate caterpillar was placed on unexposed, caterpillar-exposed, or aphid-exposed plants (one caterpillar per plant). The larvae were allowed to continuously feed on the plants for 9 d, after which their body mass was determined. The fresh weights of 20 individual caterpillars fed on unexposed, caterpillar-exposed, and aphid-exposed plants were assessed.
Phytohormone Analysis
Five first instar larvae of P. brassicae were placed on each unexposed, caterpillar-exposed, and aphid-exposed plant. After removal of the caterpillars, caterpillar-damaged leaves were harvested after 6, 24, 48, or 72 h of feeding and pooled from two individual plants to obtain one biological replicate. Leaf tissue from undamaged plants was used as a control. Five biological replicates for each time point were harvested for unexposed, caterpillar-exposed, and aphid-exposed plants, flash frozen in liquid nitrogen, and kept at −80°C until analysis.
Approximately 0.1 g of finely pulverized leaf tissue from control, unexposed, caterpillar-exposed, and aphid-exposed plants was extracted and analyzed for JA, JA-Ile, and SA levels by LC-MS (Varian) as described in detail by Onkokesung et al. (2014). JA, JA-Ile, and SA were detected in the electrospray ionization (ESI) negative mode. JA was quantified based on the deuterium-labeled JA internal standard (C/D/N Isotope). SA and JA-Ile levels were quantified based on peak areas.
GLS Analysis
Each of 10 unexposed, caterpillar-exposed, and aphid-exposed plants were damaged by five first instar P. brassicae larvae for 4 d. Control (undamaged) plants were also kept under similar environmental conditions (16/8-h light/dark photoperiod, 21°C ± 1°C, and 60% ± 5% RH) to caterpillar-damaged plants. After removal of the caterpillars, damaged leaf tissues were harvested and pooled from two individual plants to obtain one biological replicate. Leaf tissue from undamaged plants was used as a control. Five biological replicates of unexposed, caterpillar-exposed, and aphid-exposed plants were harvested, flash frozen in liquid nitrogen, and kept at −80°C until analysis.
Approximately 20 mg of lyophilized tissue was used for GLS extraction and analyzed by HPLC and UV light detection; GLSs from the 80% (v/v) methanol extracts were bound to DEAE-Sephadex and converted to desulfoglucosinolates by the use of Helix pomatia sulfatase (Burow et al., 2006). An HPLC instrument (Agilent 1100 series), equipped with a C-18 reverse-phase column (Nucleodur Sphinx RP; 250 × 4.6 mm, 5-µm particle size; Macherey-Nagel), was used as described by Burow et al. (2006). Desulfoglucosinolates were identified based on comparison of retention times and UV light absorption spectra with those of known standards. GLS levels (μmol g−1 dry weight) were calculated from the peak areas at 229 nm relative to the peak area of the internal standard para-hydroxybenzyl GLS using the relative response factors 2 for aliphatic and 0.5 for indolic GLSs (Burow et al., 2006; Onkokesung et al., 2014).
Flavonol and Sinapate Ester Analysis
The same tissue samples from unexposed, caterpillar-exposed, and aphid-exposed plants that were used for GLS analysis were also used for flavonol and sinapate ester analysis. Approximately 20 mg of lyophilized tissue was extracted and analyzed by HPLC (Agilent HP1100 series) in conjunction with a C-18 reverse-phase column (Nucleodur Sphinx RP; 250 × 4.6 mm, 5-μm particle size; Macherey-Nagel) as described by Onkokesung et al., (2014). KRR (High-Purity Compound Standard), quercetin-3-glucoside (Sigma-Aldrich), and an authentic standard of sinapic acid (Fluka) were used as external standards for the quantification of kaempferol glycoside, quercetin glycoside, SG, and SM (Onkokesung et al., 2014).
RT-qPCR
Approximately 100 mg of finely ground frozen leaf tissue was used for total RNA isolation using the NucleoSpin RNA Plant Kit (Macherey-Nagel). Total RNA samples were treated with RQ1 DNase (Promega) followed by ethanol precipitation. Complementary DNA (cDNA) was synthesized from 1 μg of RNA using an iScript cDNA synthesis kit for RT-qPCR (Bio-Rad) in a 20-μL reaction volume. RT-qPCR was performed in a CFX96 Touch Real Time PCR Detection System (Bio-Rad) in a total volume of 20 μL containing 1.5 μL of cDNA from 1 μg of RNA, 10 μL of iQ SYBR Green supermix (Bio-Rad), and 1.2 μL of 5 μm forward and reverse gene-specific primers. The primer sequences are listed in Table I. The reactions were run in a three-step program including melting curve analysis: preincubation at 95°C for 10 min, amplification for 40 cycles (95°C for 15 s, 59°C for 30 s, and 72°C for 45s), and melting analysis from 72°C to 95°C. For normalization, specific primers of EF1α from Arabidopsis (accession no. NM_001125992) were used. All reactions were performed with five biological replicates. Relative gene expression (fold change) was calculated based on an efficiency-corrected model (Pfaffl, 2001).
Table I. Specific primers used for RT-qPCR.
| Gene | Sequence (5′→3′) |
|---|---|
| WRKY70-Forward | ACCCGTTAAGGGTAAAAGAGGA |
| WRKY70-Reverse | CTTGGGTTCGAGCTCAACCT |
| AOS-Forward | TCCACCCAAAAACCGTACGA |
| AOS-Reverse | TGAAGAACTCTTCAGCTCCTTG |
| JAR1-Forward | GAAGCTGCTCACACCTAACC |
| JAR1-Reverse | CAATCCATCCTTCCGAGCTAC |
| ICS1-Forward | CACTAGATTCTCCCGCAAGAAG |
| ICS1-Reverse | TGGTCAATTGGAACCTGTAACC |
| VSP2-Forward | TCAGTGACCGTTGGAAGTTGTG |
| VSP2-Reverse | GTTCGAACCATTAGGCTTCAATATG |
| SMT-Forward | GAATGTTGGGCTAACGACGA |
| SMT-Reverse | CTTATCCAGGCTTGAGTTGCA |
| EF-Forward | TGAGCACGCTCTTCTTGCTTTCA |
| EF-Reverse | GGTGGTGGCATCCATCTTGTTACA |
Plant Growth Analysis
The effect of enhanced resistance to P. brassicae caterpillars on plant growth was determined by measuring stalk length and rosette leaf area. Five first instar P. brassicae larvae were allowed to continuously feed on each unexposed, caterpillar-exposed, and aphid-exposed plant for 4 d. Caterpillars were removed from the plants after 4 d of feeding, and unexposed, caterpillar-exposed, and aphid-exposed plants were kept under 16/8-h light/dark photoperiod, 21°C ± 1°C, and 60% ± 5% RH conditions. Fourteen days after removal of caterpillars, stalk lengths were measured and rosette leaf area was determined from digital image analysis using ImageJ software (http://imagej.nih.gov/ij/index.html; Schneider et al., 2012). Undamaged plants were used as controls. In addition, the total damaged leaf area after 4 d of caterpillar feeding on unexposed, caterpillar-exposed, and aphid-exposed plants was quantified from digital image analysis by ImageJ software.
Statistical Analysis
The data were analyzed by Student’s t test or ANOVA followed by Tukey’s HSD posthoc test using SPSS 22 (IBM).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AT3G56400 (WRKY70), AT5G42650 (AOS), AT2G46370 (JAR1), AT1G74710 (ICS1), AT5G24770 (VSP2), AT2G22990 (SMT), AT3G21560 (SGT1), AT4G36220 (FAH1), AT2G14610 (PR1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Phytohormone levels in wild-type and wrky70 mutant plants after feeding by P. brassicae caterpillars or B. brassicae aphids.
Supplemental Figure S2. Effect of functional WRKY70 TF on plant resistance against specialist caterpillars or specialist aphids in Arabidopsis.
Supplemental Figure S3. Effect of previous exposure to feeding by caterpillars or aphids on wild-type and SA-deficient mutant plants.
Supplemental Figure S4. Kinetics of transcript expression of WRKY70, AOS, and ICS1 in unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 mutant plants after subsequent P. brassicae caterpillar feeding.
Supplemental Figure S5. VSP2 and PR1 relative transcript levels in previously unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants after subsequent P. brassicae caterpillar feeding.
Supplemental Figure S6. Flavonol glycoside levels after 4 d of feeding by P. brassicae caterpillars on previously unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants.
Supplemental Figure S7. Indole GLS and SG levels after feeding by P. brassicae caterpillars on previously unexposed, caterpillar-exposed, and aphid-exposed wild-type or wrky70 plants.
Supplemental Figure S8. Relative transcript levels of WRKY70, SMT, and SGT after feeding by P. brassicae caterpillars on previously unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants.
Supplemental Figure S9. GLS and KRR levels upon P. brassicae caterpillar feeding on previously unexposed, caterpillar-exposed, and aphid-exposed wild-type, wrky70, and fah1-7 plants.
Supplemental Figure S10. Percentage of total damaged leaf area after 4 d of feeding by P. brassicae caterpillars on unexposed, caterpillar-exposed, and aphid-exposed wild-type and wrky70 plants.
Supplemental Figure S11. SM levels after 4 d of P. brassicae caterpillar feeding or after 7 d of B. brassicae aphid feeding on wild-type Arabidopsis.
Acknowledgments
We thank André Gidding, Frans van Aggelen, and Léon Westerd for insect rearing.
Glossary
- JA
jasmonic acid
- JA-Ile
jasmonic acid-isoleucine
- TF
transcription factor
- T-DNA
transfer DNA
- KRR
kaempferol 3,7-dirhamonoside
- GLS
glucosinolate
- SG
sinapoyl glucose
- SM
sinapoyl malate
- RH
relative humidity
- LC-MS
liquid chromatography quadrupole tandem mass spectrometry
- cDNA
complementary DNA
- RT-qPCR
quantitative real-time PCR
- HSD
honestly significant difference
Footnotes
This work was supported by the Netherlands Organization for Scientific Research (Spinoza Award to M.D.).
References
- Baldwin IT, Schmelz EA (1996) Immunological “memory” in the induced accumulation of nicotine in wild tobacco. Ecology 77: 236–246 [Google Scholar]
- Bekaert M, Edger PP, Hudson CM, Pires JC, Conant GC (2012) Metabolic and evolutionary costs of herbivory defense: systems biology of glucosinolate synthesis. New Phytol 196: 596–605 [DOI] [PubMed] [Google Scholar]
- Browse J, Howe GA (2008) New weapons and a rapid response against insect attack. Plant Physiol 146: 832–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173: 603–608 [Google Scholar]
- Burow M, Müller R, Gershenzon J, Wittstock U (2006) Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J Chem Ecol 32: 2333–2349 [DOI] [PubMed] [Google Scholar]
- Caarls L, Pieterse CMJ, Van Wees SCM (2015) How salicylic acid takes transcriptional control over jasmonic acid signaling. Front Plant Sci 6: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Chung HS, Koo AJK, Gao X, Jayanty S, Thines B, Jones AD, Howe GA (2008) Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol 146: 952–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss K, von Roepenack-Lahaye E, Böttcher C, Roth MR, Welti R, Erban A, Kopka J, Scheel D, Milkowski C, Strack D (2011) Overexpression of sinapine esterase BnSCE3 in oilseed rape seeds triggers global changes in seed metabolism. Plant Physiol 155: 1127–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U. (2011) Molecular aspects of defence priming. Trends Plant Sci 16: 524–531 [DOI] [PubMed] [Google Scholar]
- Cui J, Bahrami AK, Pringle EG, Hernandez-Guzman G, Bender CL, Pierce NE, Ausubel FM (2005) Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc Natl Acad Sci USA 102: 1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Jander G, Racki LR, Kim PD, Pierce NE, Ausubel FM (2002) Signals involved in Arabidopsis resistance to Trichoplusia ni caterpillars induced by virulent and avirulent strains of the phytopathogen Pseudomonas syringae. Plant Physiol 129: 551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demkura PV, Ballaré CL (2012) UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol Plant 5: 642–652 [DOI] [PubMed] [Google Scholar]
- Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15: 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Van Zaanen W, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, Pieterse CMJ (2006) Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol 142: 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146: 818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gális I, Gaquerel E, Pandey SP, Baldwin IT (2009) Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant Cell Environ 32: 617–627 [DOI] [PubMed] [Google Scholar]
- Gols R, Roosjen M, Dijkman H, Dicke M (2003) Induction of direct and indirect plant responses by jasmonic acid, low spider mite densities, or a combination of jasmonic acid treatment and spider mite infestation. J Chem Ecol 29: 2651–2666 [DOI] [PubMed] [Google Scholar]
- Gulmon SL, Mooney HA (1986). Costs of defense and their effect on plant productivity. In Givnish TJ, ed, On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, UK, pp 681–699 [Google Scholar]
- Hagemeier J, Schneider B, Oldham NJ, Hahlbrock K (2001) Accumulation of soluble and wall-bound indolic metabolites in Arabidopsis thaliana leaves infected with virulent or avirulent Pseudomonas syringae pathovar tomato strains. Proc Natl Acad Sci USA 98: 753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57: 303–333 [DOI] [PubMed] [Google Scholar]
- Heil M, Silva Bueno JC (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA 104: 5467–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RJ, van Dam NM, van Loon JJA (2009) Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol 54: 57–83 [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago [Google Scholar]
- Kempema LA, Cui X, Holzer FM, Walling LL (2007) Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs: similarities and distinctions in responses to aphids. Plant Physiol 143: 849–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2004) Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant J 38: 639–649 [DOI] [PubMed] [Google Scholar]
- Kim JI, Doland WL, Anderson NA, Chapple C (2015) Indole glucosinolate biosynthesis limits phenylproponoid accumulation in Arabidopsis thaliana. Plant Cell 27: 1529–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Lambrix VM, Reichelt M, Gershenzon J, Mitchell-Olds T (2001) Gene duplication in the diversification of secondary metabolism: tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 13: 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes A, van Loon JJA, Dicke M (2015) Density-dependent interference of aphids with caterpillar-induced defenses in Arabidopsis: involvement of phytohormones and transcription factors. Plant Cell Physiol 56: 98–106 [DOI] [PubMed] [Google Scholar]
- Leiss KA, Choi YH, Verpoorte R, Klinkhamer PGL (2011) An overview of NMR-based metabolomics to identify secondary plant compounds involved in host plant resistance. Phytochem Rev 10: 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Brader G, Kariola T, Palva ET (2006) WRKY70 modulates the selection of signaling pathways in plant defense. Plant J 46: 477–491 [DOI] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16: 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhang J, Li J, Zhou G, Wang Q, Bian W, Erb M, Lou Y (2015) Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. eLife 4: e04805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel TR, Weldegergis BT, David A, Boland W, Gols R, van Loon JJA, Dicke M (2014) Synergism in the effect of prior jasmonic acid application on herbivore-induced volatile emission by lima bean plants: transcription of a monoterpene synthase gene and volatile emission. J Exp Bot 65: 4821–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC (2005) Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol 138: 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran PJ, Cheng Y, Cassell JL, Thompson GA (2002) Gene expression profiling of Arabidopsis thaliana in compatible plant-aphid interactions. Arch Insect Biochem Physiol 51: 182–203 [DOI] [PubMed] [Google Scholar]
- Müller R, de Vos M, Sun JY, Sønderby IE, Halkier BA, Wittstock U, Jander G (2010) Differential effects of indole and aliphatic glucosinolates on lepidopteran herbivores. J Chem Ecol 36: 905–913 [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onkokesung N, Gális I, von Dahl CC, Matsuoka K, Saluz HP, Baldwin IT (2010) Jasmonic acid and ethylene modulate local responses to wounding and simulated herbivory in Nicotiana attenuata leaves. Plant Physiol 153: 785–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onkokesung N, Reichelt M, van Doorn A, Schuurink RC, van Loon JJA, Dicke M (2014) Modulation of flavonoid metabolites in Arabidopsis thaliana through overexpression of the MYB75 transcription factor: role of kaempferol-3,7-dirhamnoside in resistance to the specialist insect herbivore Pieris brassicae. J Exp Bot 65: 2203–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Victor C, Züst T, Rees M, Kliebenstein DJ, Turnbull LA (2010) A new method for measuring relative growth rate can uncover the costs of defensive compounds in Arabidopsis thaliana. New Phytol 187: 1102–1111 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28: 489–521 [DOI] [PubMed] [Google Scholar]
- Poelman EH, Broekgaarden C, Van Loon JJA, Dicke M (2008) Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol Ecol 17: 3352–3365 [DOI] [PubMed] [Google Scholar]
- Poelman EH, van Loon JJA, van Dam NM, Vet LEM, Dicke M (2010) Herbivore-induced plant responses in Brassica oleracea prevail over effects of constitutive resistance and result in enhanced herbivore attack. Ecol Entomol 35: 240–247 [Google Scholar]
- Rasmann S, Chassin E, Bilat J, Glauser G, Reymond P (2015) Trade-off between constitutive and inducible resistance against herbivores is only partially explained by gene expression and glucosinolate production. J Exp Bot 66: 2527–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmann S, De Vos M, Casteel CL, Tian D, Halitschke R, Sun JY, Agrawal AA, Felton GW, Jander G (2012) Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol 158: 854–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62: 3321–3338 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Saona C, Chalmers JA, Raj S, Thaler JS (2005) Induced plant responses to multiple damagers: differential effects on an herbivore and its parasitoid. Oecologia 143: 566–577 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Meyer K, Cusumano JC, Chapple C (1999) Regulation of ferulate-5-hydroxylase expression in Arabidopsis in the context of sinapate ester biosynthesis. Plant Physiol 119: 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfferth C, Tsuda K (2014) Salicylic acid signal transduction: the initiation of biosynthesis, perception and transcriptional reprogramming. Front Plant Sci 5: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JS, Jung C, Lee S, Min K, Lee YW, Choi Y, Lee JS, Song JT, Kim JK, Choi YD (2013) AtMYB44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant J 73: 483–495 [DOI] [PubMed] [Google Scholar]
- Soler R, Badenes-Perez FR, Broekgaarden C, Zheng SJ, David A, Boland W, Dicke M (2012) Plant-mediated facilitation between a leaf-feeding and a phloem-feeding insect in a brassicaceous plant: from insect performance to gene transcription. Funct Ecol 26: 156–166 [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, et al. (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam JM, Kroes A, Li Y, Gols R, van Loon JJA, Poelman EH, Dicke M (2014) Plant Interactions with Multiple Insect Herbivores: From Community to Genes. Annu Rev Plant Biol 65: 689–713 [DOI] [PubMed] [Google Scholar]
- Stork W, Diezel C, Halitschke R, Gális I, Baldwin IT (2009) An ecological analysis of the herbivory-elicited JA burst and its metabolism: plant memory processes and predictions of the moving target model. PLoS ONE 4: e4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17: 260–270 [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Van der Does D, Leon-Reyes A, Koornneef A, Van Verk MC, Rodenburg N, Pauwels L, Goossens A, Körbes AP, Memelink J, Ritsema T, et al. (2013) Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell 25: 744–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103: 5602–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Vos IA, Moritz L, Pieterse CMJ, Van Wees SCM (2015) Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front Plant Sci 6: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos IA, Pieterse CMJ, van Wees SCM (2013) Costs and benefits of hormone-regulated plant defences. Plant Pathol 62: 43–55 [Google Scholar]
- Walling LL. (2008) Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol 146: 859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Amornsiripanitch N, Dong X (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C. (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot (Lond) 111: 1021–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Winde I, Wittstock U (2011) Insect herbivore counteradaptations to the plant glucosinolate–myrosinase system. Phytochemistry 72: 1566–1575 [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Reports 1: 639–647 [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, et al. (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21: 2220–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT (2006) Jasmonic acid signalling and herbivore resistance traits constrain regrowth after herbivore attack in Nicotiana attenuata. Plant Cell Environ 29: 1751–1760 [DOI] [PubMed] [Google Scholar]
- Zhang PJ, Broekgaarden C, Zheng SJ, Snoeren TAL, van Loon JJA, Gols R, Dicke M (2013a) Jasmonate and ethylene signaling mediate whitefly-induced interference with indirect plant defense in Arabidopsis thaliana. New Phytol 197: 1291–1299 [DOI] [PubMed] [Google Scholar]
- Zhang PJ, Li WD, Huang F, Zhang JM, Xu FC, Lu YB (2013b) Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J Chem Ecol 39: 612–619 [DOI] [PubMed] [Google Scholar]
- Zhu-Salzman K, Salzman RA, Ahn JE, Koiwa H (2004) Transcriptional regulation of sorghum defense determinants against a phloem-feeding aphid. Plant Physiol 134: 420–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Züst T, Joseph B, Shimizu KK, Kliebenstein DJ, Turnbull LA (2011) Using knockout mutants to reveal the growth costs of defensive traits. Proc Biol Sci 278: 2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]