Abstract
Epigenetic mechanisms have been implicated in the pathogenesis of renal diseases, including acute kidney injury. Mar et. al. now unravel the acetylation and methylation at histones that are associated with the transcription of key genes in AKI. Notably, histone modifications display a remarkable heterogeneity in ischemic and endotoxic AKI. Targeting epigenetic programs may offer novel strategies to protect kidneys from AKI and enhance kidney repair and recovery.
Keywords: acute kidney injury, epigenetics, histone, ischemia-reperfusion, sepsis
AKI is manifested by a rapid decline of renal function that is associated with high morbidity and mortality. It may also lead to end stage renal disease and contribute to the initiation and progression of chronic kidney disease. Clinically, the main causes of AKI include sepsis, ischemia/reperfusion (I/R), and nephrotoxicity. Pathologically, AKI is characterized by the damage of renal tubules, vascular dysfunction, and a robust inflammatory response.1 Despite the pathological characterization, the underlying molecular basis of AKI remains poorly understood. Recent studies have suggested an emerging role of epigenetic regulation in AKI.2 However, systematic analysis of epigenetic response in AKI was lacking. Mar and colleagues3 (this issue) have now unveiled heterogeneous patterns of epigenetic regulation at several relevant genes in AKI induced by I/R, the endotoxin LPS, and I/R in conjunction with LPS.
Epigenetics refers to heritable changes in gene expression that does not involve changes in the nucleotide sequence. DNA methylation and histone modifications are two important epigenetic mechanisms. DNA methylation refers to the addition of a methyl group to the 5 position of a cytosine ring in the CpG dinucleotides catalyzed by DNA methyltransferases, which in general represses gene transcription. In contrast, post-translational modifications of histone proteins may alter chromatin structure and the docking sites for transcription regulators, leading to transcriptionally permissive or repressive states. There are several types of histone modification, such as methylation, acetylation, phosphorylation, and ubiquitination. The acetylation of histones at specific lysine residuals is catalyzed by histone acetyltransferases and in general, favors gene transcription, whereas histone methylation may promote or suppress gene transcription depending on the gene and sites of modification. In addition to DNA methylation and histone modifications, noncoding RNAs, such as long non-coding RNAs and microRNAs, are also considered as important epigenetic modulators. In kidneys, epigenetic mechanisms have been implicated in renal development and emerging studies have further suggested an important role of epigenetic regulation in the pathogenesis of renal diseases.4
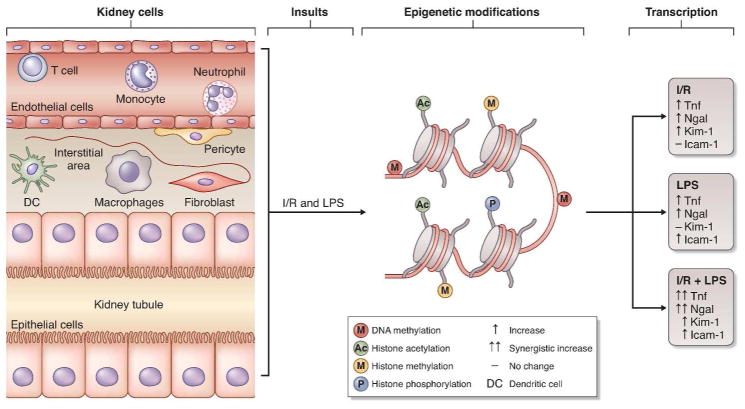
In their study3, Mar and colleagues profiled the transcription of 56 AKI-associated genes in kidney tissues during I/R, LPS, or LPS+I/R treatment. mRNA expression of these genes was highly different across various types of injury and also at different time-points of the same injury. Among them, Tnf and Ngal were induced by LPS, I/R, and synergistically by I/R+LPS, whereas, Kim-1 and Icam-1 were only induced by I/R and LPS, respectively. Based on their well-documented roles in AKI and the distinct temporal and injury-specific transcription patterns, Tnf, Kim-1, Ngal and Icam-1 were chosen as representative genes for further analysis. Binding of RNA polymerase II to these genes correlated well with their mRNA expression, supporting a critical role of transcriptional induction of these genes during AKI. What is responsible for transcriptional activation in these genes? Classically, one would focus on specific transcription factors, including both activators and repressors. However, as alluded above, epigenetic mechanisms may play a significant role as well. By re-shaping the chromatin structure, epigenetic modifications may expose the key docking sites for transcription factors on specific genes, resulting in the assembly of efficacious transcription complexes and ensuing gene transcription. Mar and Colleagues have now shed significant lights in this novel area by revealing the regulation of AKI-associated genes via histone modifications. 3
To analyze histone modifications relating to a specific gene, Mar and colleagues used a microplate-based chromatin immunoprecipitation assay, called Matrix CHIP. In this assay, the antibody against a specific histone modification is immobilized in a well of the microplate and then incubated with samples for immunoprecipitation and chromatin binding, followed by real-time PCR analysis of specific genes. Matrix CHIP is a powerful technique as it can simultaneously detect the association of various histone modifications with multiple genes. By Matrix CHIP, Mar and colleagues examined and compared histone modification patterns at Tnf, Ngal, Kim-1 and Icam-1 genes in AKI. While histone modifications at these genes showed some similarities, remarkable heterogeneities were detected among the modifications in different genes and AKI models, and at different time-points or stages of AKI. 3 For example, repressive histone methylation marks were attenuated at all four genes within 26–74 hours post I/R, whereas permissive histone acetylation was induced by I/R only at the Tnf gene. Epigenetic response at histones was also profoundly different for I/R and LPS. As such, I/R increased permissive histone methylation and histone phosphorylation at the locus of Tnf, Ngal, and Kim-1 genes, but LPS had no effects; instead, LPS reduced repressive histone methylation only at Icam-1 gene.3 Histone modifications at these four genes also exhibited distinct time-dependent changes in response to I/R and LPS. For example, repressive histone phosphorylation mark H4pSer1 at the all four genes increased at 3 hours and decreased at 74 hours post I/R.3 Together, these findings demonstrate a highly dynamic change in epigenetic regulation in AKI. Moreover, they further reinforce the idea that the pathogenesis of AKI is multifactorial and may vary significantly according to the initial cause(s) of the disease: sepsis, ischemia, nephrotoxicity, either alone or in combination.
The pathogenesis of I/R and septic (endotoxic LPS) AKI may be profoundly different.1,5 In I/R, ischemia impairs the delivery of oxygen and nutrients to kidney tissues and cells, resulting in a drastic metabolic or bioenergetic stress followed by cellular ATP depletion, leading to sublethal and lethal damage to tubular cells. In sharp contrast, septic AKI occurs as a consequence of systemic inflammatory response and may be independent of hypoperfusion in kidneys.5 Pathologically, despite the signs of tubular cell stress and dysfunction, septic AKI is not always associated with the characteristic tubular damage observed in I/R. The differences between these two types of AKI have yet to be investigated systematically at the cellular and molecular levels. The results by Mar and colleagues unravel distinct features in epigenetic regulation in I/R and LPS models, providing new mechanistic insights into the differences between these two types of AKI.
Mar and colleagues demonstrated a general correlation between histone modifications and gene expression, supporting a role of these epigenetic modifications in triggering gene transcription (Figure 1). Especially, increased mRNA expression of Tnf, Ngal, Kim-1 and Icam-1 was generally associated with an increase in the level of transcription-permissive histone modifications and a decrease in the level of repressive histone modifications. 3 Such a conclusion is further supported by closer examination of the correlation between histone modifications and mRNA expression of a specific gene. For example, repressive histone methylation marks were only diminished by LPS treatment at the Icam-1 locus, which was accompanied by an increase of Icam-1 expression. In addition, LPS increased the transcription elongation marks of H3K36m3 at both Tnf and Ngal genes, but not Kim-1; the latter did not respond to LPS treatment either. Of note, there are multiple types of epigenetic mechanisms and, histone modification, depending on the cellular context, can occur at numerous sites (Figure 1). Therefore, it is not surprising that some of the epigenetic modifications of histones did not correlate with gene expression in AKI. 3
Figure 1.
Epigenetic regulation of AKI-associated gene transcription.
Tnf, Ngal, Kim-1, and Icam-1 are known to play distinct roles in the pathogenesis of AKI. Tnf is a pro-inflammatory cytokine, and Icam-1 is an adhesion molecule required for leukocyte adhesion to endothelia during inflammation.6 As such, they both are inflammatory and may contribute to tissue damage and renal dysfunction in AKI.6 On the contrary, Ngal and Kim-1 may be protective in AKI. Ngal is a member of the lipocalin superfamily that has bacteriostatic effects and also acts as an inducer of growth and differentiation in a variety of cell types, including renal epithelia.7 KIM-1 is a type 1 transmembrane protein with an immunoglobulin and mucin domain that was recently shown to protect against AKI by mediating the phagocytosis of apoptotic and necrotic cell debris.8 The induction of both injurious and protective genes in AKI verifies the complexity of the pathogenesis of the disease. Presumably, kidney cells respond to AKI by mounting a rapid protective response and, when the insult is prolonged or becomes overwhelming, the injury mechanisms kick in and then take over. It remains poorly understood how the protective and injurious mechanisms are activated and tilted. In this regard, epigenetic regulation of AKI-associated genes may be part of the answer.
Despite these interesting findings and ideas, a number of questions remain open. First, where and in what cells do the epigenetic modifications take place in AKI? Kidney is a complex organ with several compartments that contain more than 20 types of cells. Importantly, depending on the cause, degree of severity, and the stage of AKI, different cell types may respond differently. In the genes examined, while Icam-1 is preferentially expressed in endothelial cells, Tnf and Ngal may be expressed by infiltrating neutrophils and macrophages as well as renal tubular cells.8 Consistently, different cell types demonstrated distinct gene transcription profiles in I/R.9 The histone modifications observed by Mar and colleagues in AKI 3 is a combined result of the responses of various kidney cells, without informing about the specific changes in specific cell types (Figure 1). Second, in addition to histone modifications, other forms of epigenetic mechanisms may be activated as well in AKI. These, at least, include DNA methylation and the regulation by noncoding RNAs. Analysis of these mechanisms would provide a more comprehensive understanding of epigenetic regulation in AKI. In addition, even for histones, there are several types of modifications at different sites. Does each of the modifications contribute to gene transcription, and to kidney injury and renal dysfunction in AKI? Thus, as much as its novelty and significance are appreciated, the study by Mar and colleagues is just the start-point of a long journal for the pursuit of epigenetic regulation in AKI. Further investigation needs to profile cell specific gene transcription, map relevant epigenetic patterns, and delineate their pathologic roles, leading to novel therapeutic strategies for AKI.
Acknowledgments
The work of the authors was supported in part by grants from National Natural Science Foundation of China [81430017, 81370791], National Basic Research Program of China 973 Program No. 2012CB517601, and the National Institutes of Health and Department of Veterans Administration of USA.
Footnotes
DISCLOSURES
The authors declared no competing interests.
References
- 1.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bomsztyk K, Denisenko O. Epigenetic alterations in acute kidney injury. Sem Nephrol. 2013;33:327–340. doi: 10.1016/j.semnephrol.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mar D, Gharib SA, Zager RA, Johnson A, Denisenko O, Bomsztyk K. Heterogeneity of epigenetic changes at ischemia/reperfusion- and endotoxin-induced AKI genes. Kidney Int. doi: 10.1038/ki.2015.164. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susztak K. Understanding the epigenetic syntax for the genetic alphabet in the kidney. J Am Soc Nephrol. 2014;25:10–17. doi: 10.1681/ASN.2013050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrell ED, Kellum JA, Pastor-Soler NM, et al. Septic acute kidney injury: molecular mechanisms and the importance of stratification and targeting therapy. Critical Care. 2014;18:501. doi: 10.1186/s13054-014-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11:88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Brooks CR, Xiao S, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125:1620–1636. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Krautzberger AM, Sui SH, et al. Cell-specific translational profiling in acute kidney injury. J Clin Invest. 2014;124:1242–1254. doi: 10.1172/JCI72126. [DOI] [PMC free article] [PubMed] [Google Scholar]