Abstract
Cisplatin and its derivatives are widely used chemotherapeutic drugs for cancer treatment. However, they have debilitating side-effects in normal tissues and induce ototoxicity, neurotoxicity, and nephrotoxicity. In kidneys, cisplatin preferentially accumulates in renal tubular cells causing tubular cell injury and death, resulting in acute kidney injury (AKI). Recent studies have suggested that DNA damage and the associated DNA damage response (DDR) is an important pathogenic mechanism of AKI following cisplatin treatment. Activation of DDR may lead to cell cycle arrest and DNA repair for cell survival or, in the presence of severe injury, kidney cell death. Modulation of DDR may provide novel renoprotective strategies for cancer patients undergoing cisplatin chemotherapy.
Keywords: Cisplatin, Nephrotoxicity, Apoptosis, Kidney, DNA damage, p53
Introduction
Platinum-based anti-cancer therapeutics, including cisplatin, are widely used for the treatment of various human cancers, including lung, bladder, head and neck, ovarian, and testicular cancer. It was first synthesized in 1845 by Michele Peyrone, and in the 1960s, researchers found that platinum compounds can suppress cell division, which led to the development of cisplatin and other platinum-based chemotherapeutics for anti-cancer therapy (Basu and Krishnamurthy 2010). Being one of the few metal-based cancer therapeutics, cisplatin has since become one of the most widely used and successful anti-cancer drugs. However, as seen with most anti-cancer drugs, treatment resistance and side-effects in normal tissues are the two major challenges in the use of cisplatin. In particular, nephrotoxicity associated with cisplatin remains a major problem (Dasari and Tchounwou 2014; Miller et al. 2010). For years, efforts have been made to develop cisplatin derivatives with less side effects during cancer therapy, resulting in the formulation of carboplatin and oxaliplatin, which nevertheless have a much narrower therapeutic spectrum (Kruger et al. 2015). The other strategy to limit cisplatin nephrotoxicity is extensive hydration to clear cisplatin from the kidneys. Despite these efforts, almost one third of patients treated with cisplatin still develop AKI (Dasari and Tchounwou 2014; Jiang and Dong 2008; Miller et al. 2010).
Although multiple mechanisms contribute to the pathogenesis of cisplatin nephrotoxicity, evidence has been accumulating to suggest that DNA damage and the associated DDR play a critical role (Basu and Krishnamurthy 2010; Dasari and Tchounwou 2014; Pabla and Dong 2008). Cisplatin is known to cause inter- and intra-strand DNA crosslinks, which perturbs DNA replication and transcription, thereby inducing a replication stress and DDR, which may eventually result in cell cycle arrest and cell apoptosis(Fig. 1) (Dasari and Tchounwou 2014; Fujikawa et al. 2014; Kruger et al. 2015; Lando et al. 2014; Roos and Kaina 2013; Wang and Lippard 2005). This review will elucidate DDR, its regulatory mechanisms, and pathological role in cisplatin-AKI. It will also highlight renoprotective strategies by targeting DDR to reduce nephrotoxicity during cisplatin chemotherapy.
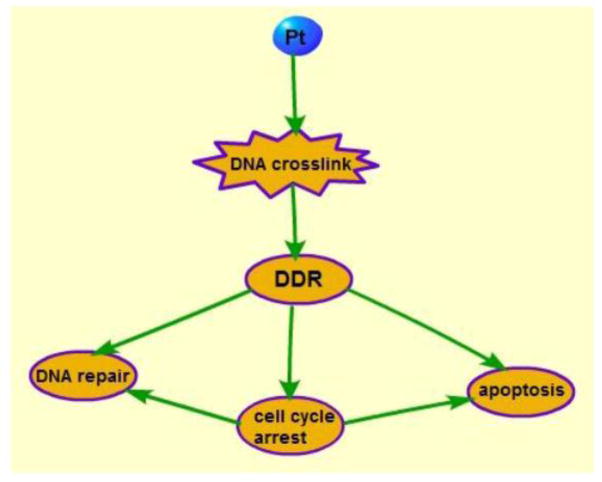
Figure 1.
Cisplatin induces DNA crosslinks and adducts, activating DDR, which may lead to cell cycle arrest, DNA repair, and apoptosis.
1. DNA damage caused by cisplatin
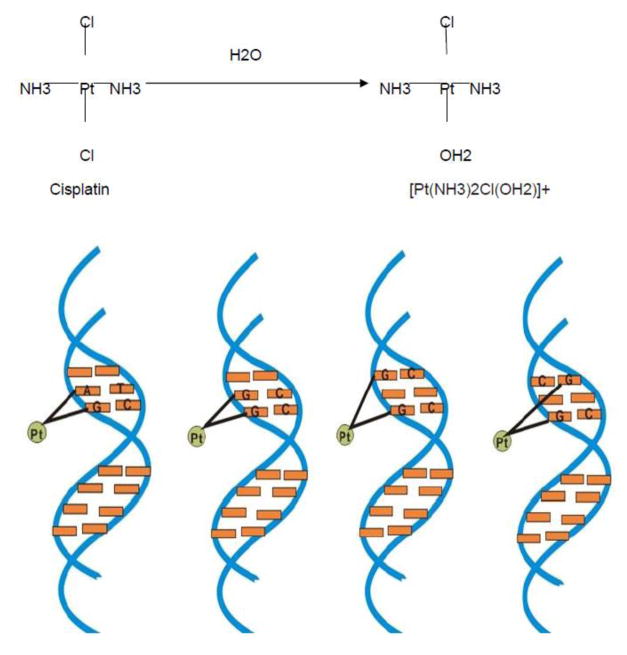
After entering the cell, cisplatin loses its chloride ligand due to the low intracellular chloride anionic concentration, resulting in the aquated forms of [Pt(NH3)2Cl(OH2)]+ and [Pt(NH3)2(OH2)2]2+ (Cepeda et al. 2007; Fujikawa et al. 2014; Wang and Lippard 2005). Aquated cisplatin has a high affinity with DNA and binds DNA through the interaction of platinum atom and N7 position of purine bases leading to the formation of intra-strand crosslinks and inter-strand crosslinks (Basu and Krishnamurthy 2010; Fujikawa et al. 2014; Wang and Lippard 2005). 1,2-intrastrand crosslinks can form by adjacent guanines(1,2-GpG) or adjacent adenine and guanine(1,2-ApG), whereas 1,3-intrastrand crosslinks are formed by nonadjacent guanines(1,3-GpNpG) and interstrand crosslinks by guanines (Fig. 2). Among them, guanine-guanine intrastrand crosslinks (1,2-GpG) are the most common adducts caused by cisplatin. Altogether, these DNA crosslinks induce DNA double helix disruption and further blockage of DNA replication and transcription (Ciccia and Elledge 2010; Fujikawa et al. 2014; Kruger et al. 2015; Roos and Kaina 2013; Wang and Lippard 2005).
Figure 2.
a. After entering cells, cisplatin loses chloride ligands to form [Pt(NH3)2Cl(OH2)]+ and [Pt(NH3)2(OH2)2]2+. b. Aquated cisplatin interact with DNA to form 1,2-intrastrand crosslink (including 1,2-GpG and 1,2-ApG), 1,3-intrastrand crosslink and interstrand crosslink.
2, DNA Damage Response
In response to the formation of cisplatin-DNA crosslinks and adducts, the cell mounts a rapid DNA damage response-DDR. The current understanding of DDR is mainly based on the extensive studies on Ataxia telangiectasia mutated (ATM) and ATM and Rad-3 related (ATR) kinases, which are the key upstream regulators of cellular response to DNA damage (Cuadrado et al. 2006; Hurley and Bunz 2007; Jiang and Dong 2008; Ma et al. 2014; Myers et al. 2009; Pabla et al. 2008; Pabla et al. 2011b; Zannini et al. 2014). Essentially, these two protein kinases act as the “sensors” of DNA lesions, which, upon activation, can phosphorylate the “mediator” proteins (Ciccia and Elledge 2010; Cuadrado et al. 2006; Hurley and Bunz 2007; Kemp et al. 2011; Roos and Kaina 2013). The mediators further recruit “transducer” kinases such as checkpoint kinase 1 (Chk1) and checkpoint kinase 2 (Chk2) for phosphorylation and activation (Hurley and Bunz 2007; Woods and Turchi 2013). Finally, the “transducer” kinases phosphorylate their substrate proteins, called “effectors”, which regulate multiple cellular responses, including DNA repair, cell cycle arrest, and cell death. One of the key effectors in DDR response is p53 (Jiang and Dong 2008; Pabla and Dong 2008; Wang et al. 2006; Wei et al. 2007). P53 induces cell-cycle arrest and/or apoptosis in response to DNA damage by transcriptional and non-transcriptional mechanisms (Ciccia and Elledge 2010; Jiang and Dong 2008; Wang and Lippard 2005).
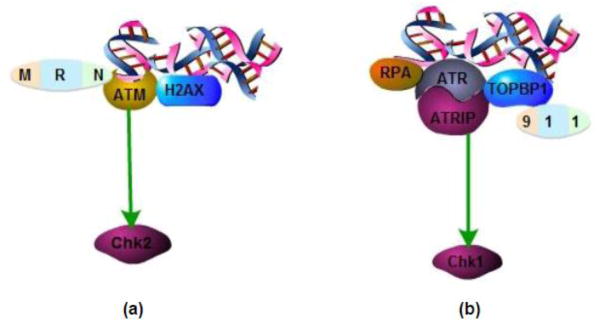
In general, ATM responds to DNA double strand break (Lovejoy and Cortez 2009). ATM is recruited to DNA damage sites via MRN complex (mre11-rad50-nbs1) and then activates H2AX (a member of H2A histone family) (Guo et al. 2008; Jaamaa et al. 2010; Koike et al. 2008; Lovejoy and Cortez 2009; Paull and Lee 2005; Thomasova and Anders 2014; Woods and Turchi 2013). With the help of activated H2AX, ATM phosphorylate Chk2, the cell cycle checkpoint kinase that induces G1/S and G2/M arrest (Fig. 3 a)(Hurley and Bunz 2007; Thomasova and Anders 2014; Woods and Turchi 2013; Zannini et al. 2014). The occurrence of G1/S phase arrest is mainly mediated by p53. After phosphorylation and activation by Chk2, p53 induces the expression of p21, a well-known cyclin dependent kinase inhibitors (CDKI) that inactivates cyclin dependent kinase 2 (CDK2) to prevent G1/S phase progression (Jiang and Dong 2008; Miller et al. 2010; Oh et al. 2014; Pabla and Dong 2008; Price et al. 2004a; Price et al. 2009; Zannini et al. 2014). Alternatively, Chk2 induces cell division cycle 25 homolog C (cdc25C) phosphorylation and translocation from nuclei to cytosol and, as a result, cdc25C can no longer activate CDK1 for G2/M phase progression and result in cell cycle arrest at G2/M (Silva et al. 2014; Thomasova and Anders 2014; Zannini et al. 2014).
Figure 3.
a. ATM is recruited to DNA damage sites via MRN complex (mre11-rad50-nbs1) and then activates H2AX. With the help of activated H2AX, ATM phosphorylate Chk2. b. RPA identifies DNA lesions and recruits ATR to the DNA damage site with the help of ATRIP. Meanwhile, 9-1-1 complex activates and recruits TOPBP1. Together with TOPBP1, ATR-ATRIP complex induces Chk1 phosphorylation and activation.
Different from ATM, ATR is generally activated in response to single strand DNA breaks and replication stress (Lovejoy and Cortez 2009; Thomasova and Anders 2014). Here DNA lesions are initially detected by replication protein A (RPA), which then recruits ATR to the DNA damage site with the help of ATR interacting protein (ATRIP). Meanwhile, there is a formation and activation of the 9-1-1 complex (containing Rad9, Hus1, and Rad1), which leads to the recruitment of topoisomerase II β binding protein 1 (TOPBP1). Together with TOPBP1, ATR-ATRIP complex induces Chk1 phosphorylation and activation (Fig. 3 b)(Lovejoy and Cortez 2009; Pabla et al. 2008; Roos and Kaina 2013; Wang et al. 2013; Zannini et al. 2014). Chk1 regulate DNA replication in S phase and G2/M transition (Pabla et al. 2012; Patil et al. 2013b; Wang et al. 2013; Woods and Turchi 2013). In addition to phosphorylation, Chk1 may also be subjected to the regulation by alternative splicing forms. Recent work has discovered a short splicing form of Chk1, named Chk1-S, which interacts with and antagonizes Chk1. Interestingly, Chk1/Chk1-S interaction is disrupted by Chk1 phosphorylation. Therefore, in response to DNA damage, Chk1 is phosphorylated preventing the association of Chk1-S for a sustained activation of Chk1 ensuring cell cycle arrest and apoptosis (Pabla et al. 2012; Patil et al. 2013b), then G2/M phase arrest occurs; besides, Chk1 and also activates cdc25A to induce CDK1 and CDK2 inhibition, leading to G1/S, S and G2/M phase arrest (Pabla et al. 2012; Pabla and Dong 2012; Patil et al. 2013b; Thomasova and Anders 2014). Recently, the NIMA-related kinases (Neks) family member Nek1 and Nek8 are found to play roles in ATR/Chk1 pathway to induce cell cycle arrest (Choi et al. 2013; Jackson 2013; Patil et al. 2013a).
3, DNA repair
DNA repair is a mechanism contributing to the cisplatin resistance of many tumor types, such as ovarian, testicular, head and neck and non-small cell lung carcinomas (NSCLCs) (Bowden 2014), and numerous stuides show that nucleotide excision repair (NER) is an important DNA repair mechanism participate in DNA repair of cancer cells (Basu and Krishnamurthy 2010; Bowden 2014; Hlavin et al. 2010; Roos and Kaina 2013). NER can recognize DNA lesion, then excise the abnormal bases and replace them with newly-synthesized bases. According to the repair sites, NER can be classified into two types: one is transcription-coupled repair (TCR) that prefers to repair the transcribed strand to ensure transcription efficiency, and the other is global genomic repair that repairs the whole DNA strand (Bowden 2014; Wang and Lippard 2005). In kidneys, DNA repair following cisplatin treatment has been rarely studied. The only available information is the expression of some proteins that have been implicated in DNA repair. For example, proliferating cell nuclear antigen (PCNA), GADD 45 and GADD153 are induced during cisplatin treatment of kidney cells. GADD45 plays a role in DDR and overexpression of GADD45 was reported to have a cytoprotective role (Wang and Lippard 2005). The Growth Arreat and DNA Damage-inducible 45 (GADD45) and GADD153 can prevent cells from entering S phase and induce DNA excision repair. PCNA was shown to accumulate in the same tubular cells in the outer stripe of the outer medulla with p27, a protein that mediate G1/S and G2/M phase arrest by preventing CDK2, CDK4 and CDK6 activation (Zhou et al. 2004), These observations suggest a possible link of DDR, DNA repair, and cell cycle arrest.
DDR, as the early cellular response to DNA damage, is known to trigger DNA repair, cell cycle arrest as well as cell death. In kidney ischemia-reperfusion injury, ATM was recently shown to play a cytoprotective role through activating Chk2 (Zhou et al. 2004), an observation that is consistent with the finding that ATM-deficient kidney tubule cells are more sensitive to cisplatin injury (Ma et al. 2014), During cisplatin treatment of mice, ATM activity showed a marginal decrease, whereas ATR was activated, suggesting that ATM-mediated DNA repair may be inactivated during severe injury in kidneys. Although DNA repair in cisplatin-AKI remains elusive, we speculate that DNA repair mechanisms are induced in the early phase of injury but inactivated in the presence of severe or prolonged insult (Pabla et al. 2011b).
4, Renal cell death
If the DNA damage is very severe, cells initiate the activation of apoptotic pathways to induce cell death (Thomasova and Anders 2014). The downstream pathways can be classified into : p53 dependent pathways and p53 independent pathways.
4.1, P53 dependent apoptosis
4.1.1, upstream activators of p53
As is discussed above, ATM and ATR can activate Chk2 and Chk1 to modulate cell cycle arrest. Do they also play a role in cisplatin induced renal cell apoptosis?
In rat kidney proximal tubular cell line (RPTC), ATM is cleaved in the late stage of cisplatin treatment (Wang et al. 2006). Consistently, ATM degradation or activity decrease was shown in kidney tissues after cisplatin treatment (Pabla et al. 2008). By sharp contrast, ATR is activated during cisplatin treatment and participates in renal cell apoptosis. In this regard, we reported that hMSH2, a mismatch repair protein, accumulates to DNA damage site during cisplatin treatment and further recruits ATR-ATRIP complex to activate Chk2, which then phosphorylate p53 to induce further apoptosis (Pabla et al. 2011b). In other cell types, very recent work also demonstrated MSH2 induction by cisplatin (Zhang et al. 2013). Classically, ATR is known to be a upstream regular of Chk1 and the ATR-Chk1 signaling axis induces S-phase arrest under DNA damage to play a cytoprotective role (Hurley and Bunz 2007; Lovejoy and Cortez 2009; Wang et al. 2013), Therefore the recent studies indicate that there are two ATR pathways—hMSH2-dependent pathway that activates Chk2 and RPA-dependent pathway that activates Chk1. Notably, these two pathways lead to different outcomes—the first one leads to apoptosis whereas the second leads to cell cycle arrest followed by DNA repair and resistance to cell death (Pabla et al. 2011b).
4.1.2, Downstream effector of p53 pathway
The role of p53, a well-known tumor suppressor protein, in cisplatin-AKI has been well established by various pharmacological and genetic inhibitory studies (Basu and Krishnamurthy 2010; Cummings and Schnellmann 2002; Jiang and Dong 2008; Jiang et al. 2006; Kutuk et al. 2010; Nakano and Vousden 2001; Pabla and Dong 2008; Price et al. 2009; Seth et al. 2005; Tsuruya et al. 2008; Zannini et al. 2014). Moreover, several downstream effectors of p53 have been identified (Jiang and Dong 2008).
PUMA (p53-upregulated modulator of apoptosis) is a proapoptotic protein that has been shown to be induced via p53 in both in vitro and in vivo models of cisplatin-AKI (Jiang et al. 2006; Tsuruya et al. 2008; Zhang et al. 2014). Upon induction, PUMA accumulates in mitochondria, where it interacts and suppress the antiapoptotic protein Bcl-xl. Leading to the activation of the proapoptotic protein Bax, followed by cytochrome C release from mitochondria to activate caspases for apoptosis (Basu and Krishnamurthy 2010; Jiang and Dong 2008; Jiang et al. 2006; Nakano and Vousden 2001; Tsuruya et al. 2008).
Other than PUMA, PIDD (p53-induced protein with death domain) was also reported to play a role downstream of p53 to induce kidney cell apoptosis. PIDD was shown to be induced by cisplatin via p53 and then interacts with RIP-associated Ich-1/Ced-3 homologous protein with a death domain (RAIDD) to recruit procaspase 2 to trigger the release of apoptosis-inducing factor(AIF) and Endo G from mitochondria. AIF then translocates to nucleus to induce cell death in a caspase independent way (Jiang and Dong 2008; Price et al. 2009; Seth et al. 2005).
It is important to point out that p53 may also activate mechanisms of cell survival (Jiang and Dong 2008; Pabla and Dong 2008; Price et al. 2009; Zannini et al. 2014). This is well exemplified by the induction of p21, a CDK2 inhibitor. By antagonizing CDK2, p21 is known to be a cell cycle inhibitor(Miyaji et al. 2001; Price et al. 2006). However, the induction of p21 by p53 has also been shown to be cytoprotective(Megyesi et al. 1998). Essentially, p21 may antagonize CDK2 to block its apoptotic signaling (Hodeify et al. 2011; Price et al. 2004b), Interestingly, there is a novel feedback mechanism whereby CDK2 may phosphorylate p21 to block its inhibitory effect on CDKs (Hodeify et al. 2011), Therefore, the fine balance between p21 and CDK2 plays an important role in determining cell death or survival in cisplatin-AKI. In addition to p21, p53 may also induce autophagy during cisplatin-AKI (Periyasamy-Thandavan et al. 2008), Autophagy is a critical mechanism for kidney cell survival and protection in AKI (Livingston and Dong 2014), Thus, by inducing autophagy p53 may activate another mechanism of kidney protection. It remains unclear how and why p53 activates both mechanisms of cell survival and cell death. We speculate that the role of p53 in cisplatin-AKI depends on the experimental condition and cellular context: in early stage of injury or under mild to moderate injury, p53 may mainly activate the mechanism of cell survival; however when the injury is severe and prolonged, cell death mechanisms become activates and predominant.
4.2, c-Abl dependent apoptosis
Besides p53, cisplatin can also activate c-Abl via the mismatch repair (MMR) system to induce cell death (Basu and Krishnamurthy 2010; Kim et al. 2007; Li 2008; Shimodaira et al. 2003). MMR is a highly conserved pathway generally known for DNA repair by detecting and binding to DNA lesion site for cytoprotection (Li 2008; Shimodaira et al. 2003; Stojic et al. 2004), However, recent studies suggest that MMR may also participate in cisplatin-induced cell apoptosis (Nehme et al. 1999; Wang and Lippard 2005).
During cisplatin treatment, c-Abl may be activated via ATM-mediated phosphorylation. It has been shown that MutSα can identify DNA lesion site and bind to it under the help of MutLα, recruiting the MMR complex with ATM, which then phosphorylate c-Abl (Guggenheim et al. 2009; Preyer et al. 2007; Tang et al. 2012; Topping et al. 2009; Vasquez 2010; Wang 2000). Upon phosphorylation, c-Abl is transported into the nucleus to function. In this regard, genetic disruption of c-Abl nuclear import decreases renal cell death in cisplatin therapy (Preyer et al. 2007; Sridevi et al. 2013). Intriguingly, normally c-Abl is sequestered in the cytosol by 14-3-3 proteins and upon cisplatin treatment, c-Jum N-terminal kinase (JNK) is activated to interact with 14-3-3 proteins resulting in the release c-Abl for its import to the nucleus (Jiang et al. 2011; Levy et al. 2008; Toh et al. 2004; Yoshida 2008; Yoshida and Miki 2005).
How does c-Abl induce cell death? Several studies have suggested the involvement of p73 (a p53 family member) in mediating the effect of c-Abl (Di et al. 2013; Shimodaira et al. 2003; Stojic et al. 2004). C-Abl may activate p73 by different pathways. It has been shown that c-Abl can phosphorylate yes kinase associate protein 1 (YAP-1), which then interacts with p63 to release p73 for activation (Gonfloni et al. 2009; Levy et al. 2007; Levy et al. 2008; Yuan et al. 2010). In addition, c-Abl may activate p73 in a p38 mitogen-activated protein kinase (p38 MAPK) dependent manner, c-Abl may also directly phosphorylate and activate p73 (Sanchez-Prieto et al. 2002). Finally, nuclear c-Abl may associate with MEK kinase 1 (MEKK-1) to activate JNK for p73 activation (Francescato et al. 2007; Kharbanda et al. 2000; Sanchez-Prieto et al. 2002). It is noteworthy that the role and regulation of the c-Abl pathway is largely unknown in cisplatin-AKI.
5. Targeting DNA damage response for renoprotection during cisplatin treatment
Cisplatin and related platinum compounds bind to DNA to induce crosslinks and adducts, resulting in DNA damage and replication stress. In response, the affected cells activate DDR. On one hand, DDR may arrest cell cycle and promote DNA repair; on the other hand, it activates various signaling pathways to culminate in cell death. In kidneys, cisplatin-induced DDR has been delineated, which consists of the sequential activation of ATR, Chk2, p53, and downstream target genes. However, the information remains partial. It remains unclear if and to what extents ATM and Chk1 contribute. Also DNA repair may be activated, at least at the early stage of cisplatin treatment or in response to mild to moderate levels of injury. Little is known about how DNA repair is activated and regulated. In addition, it is elusive as to how the DNA damage and DNA repair pathways interact and are tilted to dictate the fate of the affected cell.
Therapeutically, a number of pharmacological and genetic approaches have been shown to block DDR at various signaling levels for the protection of kidney cells and tissues during cisplatin treatment (Hodeify et al. 2011; Jiang et al. 2007; Jiang et al. 2006; Kang et al. 2011; Sahu et al. 2013; Sridevi et al. 2013), Currently, the focus is mainly at preventing kidney cells from injury and death. But, as alluded above, it would also be possible to prevent cisplatin-AKI by enhancing DNA repair, which apparently depends on further investigation and elucidation of the underlying signaling pathways.
As we proposed in 2008 (Pabla and Dong 2008), an effective renoprotective approach has to be evaluated in tumor-bearing conditions. This is because many renoprotective agents may also protect tumor or cancer cells and, as a result, attenuate the chemotherapy effect of cisplatin, making it an invalid approach for cancer patients. This thought has to be applied, when considering targeting DDR for renoprotection during cisplatin therapy. In cancer cells, cisplatin stimulates DDR; therefore an overall or blunt inhibition of DDR would block cell death in tumors and reduce or eliminate the cancer therapy effect of cisplatin. Then, how to make use of the information of DDR for renoprotection during cisplatin chemotherapy? First, it may be possible to locally deliver DDR inhibitory agents to kidneys. Experimentally, it can be done by invasive methods including direct injection to kidneys, which nevertheless are hard to apply to patients. Alternatively, it is possible to engineer kidney-directed agents by conjugating DDR inhibitors with kidney-specific molecules. Second, some of the genes in DDR are deleted or mutated in cancers. For example, p53 is deleted or mutated in over a half of cancers. In patients with these cancers, blockade of p53 may provide renoprotection without affecting cisplatin therapy in cancers. Finally, it is important to further investigate and identify the critical differences between the DDRs in cancer cells and kidney cells. With that information, we will be able to design strategies that specifically suppress the cell killing signaling in kidney cells without blocking that in cancer cells. In this aspect, recent studies have offered interesting insights. For example, blockade of protein kinase C δ (PKCδ) has been shown to protect kidney cells without diminishing the chemotherapy efficacy of cisplatin; as a mater of fact, the chemotherapy effect is enhanced in several types of experiemntal tumors (Pabla et al. 2011a)
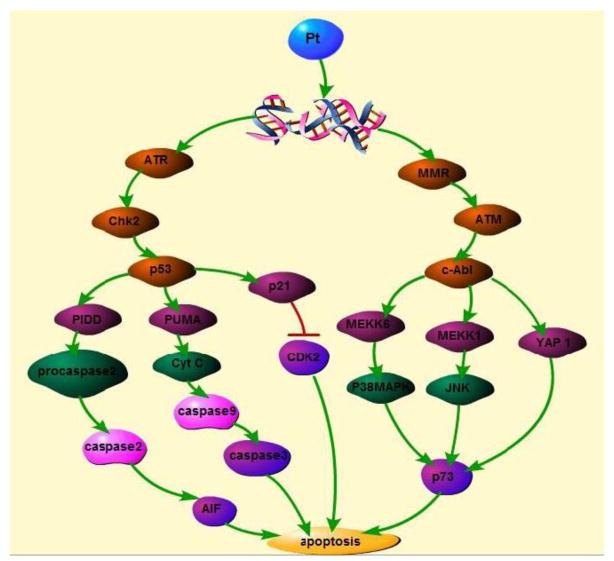
Figure 4.
cisplaitn treatment induce DNA damage, the damaged DNA is recognized by ATR and MMR. ATR activates p53, MMR activates c-Abl. P53 and c-Abl eventually mediate apoptosis through many pathways.
Acknowledgments
The study was supported in part by grants from National Natural Science Foundation of China [81430017], National Basic Research Program of China 973 Program No. 2012CB517601, and the National Institutes of Health and Department of Veterans Administration of USA.
References
- Basu A, Krishnamurthy S. Cellular responses to Cisplatin-induced DNA damage. Journal of nucleic acids. 2010;2010 doi: 10.4061/2010/201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden NA. Nucleotide excision repair: why is it not used to predict response to platinum-based chemotherapy? Cancer letters. 2014;346:163–171. doi: 10.1016/j.canlet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anti-cancer agents in medicinal chemistry. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- Choi HJ, et al. NEK8 links the ATR-regulated replication stress response and S phase CDK activity to renal ciliopathies. Molecular cell. 2013;51:423–439. doi: 10.1016/j.molcel.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Molecular cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado M, Martinez-Pastor B, Murga M, Toledo LI, Gutierrez-Martinez P, Lopez E, Fernandez-Capetillo O. ATM regulates ATR chromatin loading in response to DNA double-strand breaks. The Journal of experimental medicine. 2006;203:297–303. doi: 10.1084/jem.20051923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. The Journal of pharmacology and experimental therapeutics. 2002;302:8–17. doi: 10.1124/jpet.302.1.8. [DOI] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. European journal of pharmacology. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di C, et al. Mechanisms, function and clinical applications of DNp73. Cell cycle. 2013;12:1861–1867. doi: 10.4161/cc.24967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francescato HD, Costa RS, Junior FB, Coimbra TM. Effect of JNK inhibition on cisplatin-induced renal damage. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:2138–2148. doi: 10.1093/ndt/gfm144. [DOI] [PubMed] [Google Scholar]
- Fujikawa Y, Kawanishi M, Kuraoka I, Yagi T. Frequencies of mutagenic translesion DNA synthesis over cisplatin-guanine intra-strand crosslinks in lacZ plasmids propagated in human cells Mutation research. Genetic toxicology and environmental mutagenesis. 2014;770:23–28. doi: 10.1016/j.mrgentox.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Gonfloni S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nature medicine. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- Guggenheim ER, Xu D, Zhang CX, Chang PV, Lippard SJ. Photoaffinity isolation and identification of proteins in cancer cell extracts that bind to platinum-modified DNA. Chembiochem : a European journal of chemical biology. 2009;10:141–157. doi: 10.1002/cbic.200800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, et al. Aven-dependent activation of ATM following DNA damage. Current biology : CB. 2008;18:933–942. doi: 10.1016/j.cub.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavin EM, Smeaton MB, Miller PS. Initiation of DNA interstrand cross-link repair in mammalian cells. Environmental and molecular mutagenesis. 2010;51:604–624. doi: 10.1002/em.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodeify R, Tarcsafalvi A, Megyesi J, Safirstein RL, Price PM. Cdk2-dependent phosphorylation of p21 regulates the role of Cdk2 in cisplatin cytotoxicity. American journal of physiology Renal physiology. 2011;300:F1171–1179. doi: 10.1152/ajprenal.00507.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley PJ, Bunz F. ATM and ATR: components of an integrated circuit. Cell cycle. 2007;6:414–417. doi: 10.4161/cc.6.4.3886. [DOI] [PubMed] [Google Scholar]
- Jaamaa S, et al. DNA damage recognition via activated ATM and p53 pathway in nonproliferating human prostate tissue. Cancer research. 2010;70:8630–8641. doi: 10.1158/0008-5472.CAN-10-0937. [DOI] [PubMed] [Google Scholar]
- Jackson PK. Nek8 couples renal ciliopathies to DNA damage and checkpoint control. Molecular cell. 2013;51:407–408. doi: 10.1016/j.molcel.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Jiang M, Dong Z. Regulation and pathological role of p53 in cisplatin nephrotoxicity. The Journal of pharmacology and experimental therapeutics. 2008;327:300–307. doi: 10.1124/jpet.108.139162. [DOI] [PubMed] [Google Scholar]
- Jiang M, et al. Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. The Journal of biological chemistry. 2007;282:2636–2645. doi: 10.1074/jbc.M606928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene. 2006;25:4056–4066. doi: 10.1038/sj.onc.1209440. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Kamath R, Jin S, Balasubramani M, Pandita TK, Rajasekaran B. Tip60-mediated acetylation activates transcription independent apoptotic activity of Abl. Molecular cancer. 2011;10:88. doi: 10.1186/1476-4598-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KP, et al. Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:814–822. doi: 10.1093/ndt/gfq528. [DOI] [PubMed] [Google Scholar]
- Kemp MG, Lindsey-Boltz LA, Sancar A. The DNA damage response kinases DNA-dependent protein kinase (DNA-PK) and ataxia telangiectasia mutated (ATM) Are stimulated by bulky adduct-containing DNA. The Journal of biological chemistry. 2011;286:19237–19246. doi: 10.1074/jbc.M111.235036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda S, et al. Activation of MEK kinase 1 by the c-Abl protein tyrosine kinase in response to DNA damage. Molecular and cellular biology. 2000;20:4979–4989. doi: 10.1128/mcb.20.14.4979-4989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Rajasekaran B, Brown KD. MLH1- and ATM-dependent MAPK signaling is activated through c-Abl in response to the alkylator N-methyl-N′-nitro-N′-nitrosoguanidine. The Journal of biological chemistry. 2007;282:32021–32031. doi: 10.1074/jbc.M701451200. [DOI] [PubMed] [Google Scholar]
- Koike M, Mashino M, Sugasawa J, Koike A. Histone H2AX phosphorylation independent of ATM after X-irradiation in mouse liver and kidney in situ. Journal of radiation research. 2008;49:445–449. doi: 10.1269/jrr.08010. [DOI] [PubMed] [Google Scholar]
- Kruger K, Thomale J, Stojanovic N, Osmak M, Henninger C, Bormann S, Fritz G. Platinum-induced kidney damage: Unraveling the DNA damage response (DDR) of renal tubular epithelial and glomerular endothelial cells following platinum injury. Biochimica et biophysica acta. 2015;1854:685–698. doi: 10.1016/j.bbamcr.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Kutuk O, Temel SG, Tolunay S, Basaga H. Aven blocks DNA damage-induced apoptosis by stabilising Bcl-xL. European journal of cancer. 2010;46:2494–2505. doi: 10.1016/j.ejca.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Lando DY, Chang CL, Fridman AS, Grigoryan IE, Galyuk EN, Hsueh YW, Hu CK. Comparative thermal and thermodynamic study of DNA chemically modified with antitumor drug cisplatin and its inactive analog transplatin. Journal of inorganic biochemistry. 2014;137:85–93. doi: 10.1016/j.jinorgbio.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Levy D, Adamovich Y, Reuven N, Shaul Y. The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell death and differentiation. 2007;14:743–751. doi: 10.1038/sj.cdd.4402063. [DOI] [PubMed] [Google Scholar]
- Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Molecular cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Li GM. Mechanisms and functions of DNA mismatch repair. Cell research. 2008;18:85–98. doi: 10.1038/cr.2007.115. [DOI] [PubMed] [Google Scholar]
- Livingston MJ, Dong Z. Autophagy in acute kidney injury. Seminars in nephrology. 2014;34:17–26. doi: 10.1016/j.semnephrol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy CA, Cortez D. Common mechanisms of PIKK regulation. DNA repair. 2009;8:1004–1008. doi: 10.1016/j.dnarep.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Wei Q, Dong G, Huo Y, Dong Z. DNA damage response in renal ischemia-reperfusion and ATP-depletion injury of renal tubular cells. Biochimica et biophysica acta. 2014;1842:1088–1096. doi: 10.1016/j.bbadis.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. The Journal of clinical investigation. 1998;101:777–782. doi: 10.1172/JCI1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins. 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji T, Kato A, Yasuda H, Fujigaki Y, Hishida A. Role of the increase in p21 in cisplatin-induced acute renal failure in rats. Journal of the American Society of Nephrology : JASN. 2001;12:900–908. doi: 10.1681/ASN.V125900. [DOI] [PubMed] [Google Scholar]
- Myers K, Gagou ME, Zuazua-Villar P, Rodriguez R, Meuth M. ATR and Chk1 suppress a caspase-3-dependent apoptotic response following DNA replication stress. PLoS genetics. 2009;5:e1000324. doi: 10.1371/journal.pgen.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Molecular cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Nehme A, Baskaran R, Nebel S, Fink D, Howell SB, Wang JY, Christen RD. Induction of JNK and c-Abl signalling by cisplatin and oxaliplatin in mismatch repair-proficient and -deficient cells. British journal of cancer. 1999;79:1104–1110. doi: 10.1038/sj.bjc.6690176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh GS, Kim HJ, Shen A, Lee SB, Khadka D, Pandit A, So HS. Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolyte & blood pressure : E & BP. 2014;12:55–65. doi: 10.5049/EBP.2014.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Bhatt K, Dong Z. Checkpoint kinase 1 (Chk1)-short is a splice variant and endogenous inhibitor of Chk1 that regulates cell cycle and DNA damage checkpoints. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:197–202. doi: 10.1073/pnas.1104767109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z. Inhibition of PKCdelta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. The Journal of clinical investigation. 2011a;121:2709–2722. doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney international. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Pabla N, Dong Z. Sibling rivalry in checkpoint control of cell cycle and DNA damage response. Cell cycle. 2012;11:1866–1867. doi: 10.4161/cc.20416. [DOI] [PubMed] [Google Scholar]
- Pabla N, Huang S, Mi QS, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. The Journal of biological chemistry. 2008;283:6572–6583. doi: 10.1074/jbc.M707568200. [DOI] [PubMed] [Google Scholar]
- Pabla N, Ma Z, McIlhatton MA, Fishel R, Dong Z. hMSH2 recruits ATR to DNA damage sites for activation during DNA damage-induced apoptosis. The Journal of biological chemistry. 2011b;286:10411–10418. doi: 10.1074/jbc.M110.210989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M, Pabla N, Ding HF, Dong Z. Nek1 interacts with Ku80 to assist chromatin loading of replication factors and S-phase progression. Cell cycle. 2013a;12:2608–2616. doi: 10.4161/cc.25624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M, Pabla N, Dong Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cellular and molecular life sciences : CMLS. 2013b;70:4009–4021. doi: 10.1007/s00018-013-1307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Lee JH. The Mre11/Rad50/Nbs1 complex and its role as a DNA double-strand break sensor for ATM. Cell cycle. 2005;4:737–740. doi: 10.4161/cc.4.6.1715. [DOI] [PubMed] [Google Scholar]
- Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney international. 2008;74:631–640. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- Preyer M, Shu CW, Wang JY. Delayed activation of Bax by DNA damage in embryonic stem cells with knock-in mutations of the Abl nuclear localization signals. Cell death and differentiation. 2007;14:1139–1148. doi: 10.1038/sj.cdd.4402119. [DOI] [PubMed] [Google Scholar]
- Price PM, Megyesi J, Saf Irstein RL. Cell cycle regulation: repair and regeneration in acute renal failure. Kidney international. 2004a;66:509–514. doi: 10.1111/j.1523-1755.2004.761_8.x. [DOI] [PubMed] [Google Scholar]
- Price PM, Safirstein RL, Megyesi J. Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. American journal of physiology Renal physiology. 2004b;286:F378–384. doi: 10.1152/ajprenal.00192.2003. [DOI] [PubMed] [Google Scholar]
- Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney international. 2009;76:604–613. doi: 10.1038/ki.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PM, Yu F, Kaldis P, Aleem E, Nowak G, Safirstein RL, Megyesi J. Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. Journal of the American Society of Nephrology : JASN. 2006;17:2434–2442. doi: 10.1681/ASN.2006020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer letters. 2013;332:237–248. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Sahu BD, Kuncha M, Sindhura GJ, Sistla R. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2013;20:453–460. doi: 10.1016/j.phymed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prieto R, Sanchez-Arevalo VJ, Servitja JM, Gutkind JS. Regulation of p73 by c-Abl through the p38 MAP kinase pathway. Oncogene. 2002;21:974–979. doi: 10.1038/sj.onc.1205134. [DOI] [PubMed] [Google Scholar]
- Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. The Journal of biological chemistry. 2005;280:31230–31239. doi: 10.1074/jbc.M503305200. [DOI] [PubMed] [Google Scholar]
- Shimodaira H, Yoshioka-Yamashita A, Kolodner RD, Wang JY. Interaction of mismatch repair protein PMS2 and the p53-related transcription factor p73 in apoptosis response to cisplatin. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2420–2425. doi: 10.1073/pnas.0438031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva BA, Stambaugh JR, Yokomori K, Shah JV, Berns MW. DNA damage to a single chromosome end delays anaphase onset. The Journal of biological chemistry. 2014;289:22771–22784. doi: 10.1074/jbc.M113.535955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridevi P, Nhiayi MK, Wang JY. Genetic disruption of Abl nuclear import reduces renal apoptosis in a mouse model of cisplatin-induced nephrotoxicity. Cell death and differentiation. 2013;20:953–962. doi: 10.1038/cdd.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojic L, Brun R, Jiricny J. Mismatch repair and DNA damage signalling. DNA repair. 2004;3:1091–1101. doi: 10.1016/j.dnarep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Tang J, Wang JY, Parker LL. Detection of early Abl kinase activation after ionizing radiation by using a peptide biosensor. Chembiochem : a European journal of chemical biology. 2012;13:665–673. doi: 10.1002/cbic.201100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasova D, Anders HJ. Cell cycle control in the kidney. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014 doi: 10.1093/ndt/gfu395. [DOI] [PubMed] [Google Scholar]
- Toh WH, Siddique MM, Boominathan L, Lin KW, Sabapathy K. c-Jun regulates the stability and activity of the p53 homologue, p73. The Journal of biological chemistry. 2004;279:44713–44722. doi: 10.1074/jbc.M407672200. [DOI] [PubMed] [Google Scholar]
- Topping RP, Wilkinson JC, Scarpinato KD. Mismatch repair protein deficiency compromises cisplatin-induced apoptotic signaling. The Journal of biological chemistry. 2009;284:14029–14039. doi: 10.1074/jbc.M809303200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruya K, et al. Involvement of p53-transactivated Puma in cisplatin-induced renal tubular cell death. Life sciences. 2008;83:550–556. doi: 10.1016/j.lfs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Vasquez KM. Targeting and processing of site-specific DNA interstrand crosslinks. Environmental and molecular mutagenesis. 2010;51:527–539. doi: 10.1002/em.20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nature reviews Drug discovery. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Repression of ATR pathway by miR-185 enhances radiation-induced apoptosis and proliferation inhibition. Cell death & disease. 2013;4:e699. doi: 10.1038/cddis.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Pabla N, Wang CY, Wang W, Schoenlein PV, Dong Z. Caspase-mediated cleavage of ATM during cisplatin-induced tubular cell apoptosis: inactivation of its kinase activity toward p53. American journal of physiology Renal physiology. 2006;291:F1300–1307. doi: 10.1152/ajprenal.00509.2005. [DOI] [PubMed] [Google Scholar]
- Wang JY. Regulation of cell death by the Abl tyrosine kinase. Oncogene. 2000;19:5643–5650. doi: 10.1038/sj.onc.1203878. [DOI] [PubMed] [Google Scholar]
- Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. American journal of physiology Renal physiology. 2007;293:F1282–1291. doi: 10.1152/ajprenal.00230.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D, Turchi JJ. Chemotherapy induced DNA damage response: convergence of drugs and pathways. Cancer biology & therapy. 2013;14:379–389. doi: 10.4161/cbt.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K. Nuclear trafficking of pro-apoptotic kinases in response to DNA damage. Trends in molecular medicine. 2008;14:305–313. doi: 10.1016/j.molmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Miki Y. Enabling death by the Abl tyrosine kinase: mechanisms for nuclear shuttling of c-Abl in response to DNA damage. Cell cycle. 2005;4:777–779. doi: 10.4161/cc.4.6.1746. [DOI] [PubMed] [Google Scholar]
- Yuan M, Luong P, Hudson C, Gudmundsdottir K, Basu S. c-Abl phosphorylation of DeltaNp63alpha is critical for cell viability. Cell death & disease. 2010;1:e16. doi: 10.1038/cddis.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannini L, Delia D, Buscemi G. CHK2 kinase in the DNA damage response and beyond. Journal of molecular cell biology. 2014;6:442–457. doi: 10.1093/jmcb/mju045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. Tubular p53 regulates multiple genes to mediate AKI. Journal of the American Society of Nephrology : JASN. 2014;25:2278–2289. doi: 10.1681/ASN.2013080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YX, et al. Cisplatin upregulates MSH2 expression by reducing miR-21 to inhibit A549 cell growth. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2013;67:97–102. doi: 10.1016/j.biopha.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, et al. The induction of cell cycle regulatory and DNA repair proteins in cisplatin-induced acute renal failure. Toxicology and applied pharmacology. 2004;200:111–120. doi: 10.1016/j.taap.2004.04.003. [DOI] [PubMed] [Google Scholar]