Abstract
Low consumption of the omega-3 fatty acids, eicosapentaenoic (EPA) and docosahexaenonic acids (DHA), is linked to delayed brain development and, in late life, increased risk for Alzheimers Disease. The current review focuses on cognitive functioning during mid-life and summarizes available scientific evidence relevant to the hypothesis that adequate dietary consumption of the long-chain, omega-3 fatty acids is necessary for optimal cognitive performance. Taken together, the findings suggest that raising the currently low consumption among healthy adults may improve some aspects of cognitive performance. Nonetheless, evidence from randomized clinical trials is comparatively sparse and leaves unclear: a) whether such effects are clinically significant, b) whether effects of EPA and DHA differ, c) which dimensions of cognitive function are affected, d) the dose-response relationships, or e) the time course of the response. Clarification of these issues through both laboratory and clinical investigations is a priority given the broad implications for public health, as well as for military personnel and other positions of high performance demand and responsibility.
Keywords: omega-3 fatty acids, eicosapentaenoic (EPA) and docosahexaenonic acids (DHA), cognitive function, memory, executive function, psychomotor performance
INTRODUCTION
New discoveries are constantly expanding the known actions of the long-chain, omega-3 fatty acids, eicosapentaenoic and docosahexaenonic acids (EPA, DHA), from basic biological chemistry through human physiology and pathophysiology1-3. Particularly since 2000, many reports have shed light on the potential roles of these compounds in preventing and ameliorating diseases of the central nervous system4. EPA and DHA are essential nutrients, which can be synthesized in the human body from α-linolenic acid (ALA). However, ALA cannot be synthesized by humans; most persons in the US have low dietary intakes of these fatty acids with the main source being direct consumption of EPA and DHA from marine oils in seafood. As shown in lower mammals, eliminating EPA and DHA from the diet dramatically lowers brain concentration and produces a range of behavioral abnormalities5-7.
From the last trimester through the second year of life, the human brain undergoes very rapid growth and during this period is particularly susceptible to nutritional deficiencies. DHA is the most prevalent fatty acid in brain membrane phospholipids; its accretion is very rapid early in life and depends upon maternal delivery across the placenta and via breast milk5, 8. Several large cohort studies have shown that infants of mothers reporting low perinatal maternal fish consumption have low early childhood intelligence and increased risk of suboptimal outcomes for prosocial behavior, fine motor, communication, and social development scores9, 10. Randomized clinical trials (RCTs) suggest that supplementing the diets of either pregnant mothers or infants with DHA improves cognitive development11-15.
At the other end of our lifespan, Alzheimer’s Disease (AD) is an increasingly common and costly condition, and nutritional intervention is being proposed as a preventative or therapeutic modality. A series of cross-sectional, prospective and case-control studies implicates low consumption of fish or marine fats as a risk factor for AD16. Cell culture studies consistently show that DHA decreases beta amyloid and apoptosis while rodent models of AD are favorably affected by DHA supplementation16. Not surprisingly, this research has spurred funding of RCTs to test the efficacy of EPA and DHA as preventative or therapeutic agents for AD. While early, preliminary results have not demonstrated clear benefit16, several large trials are ongoing.
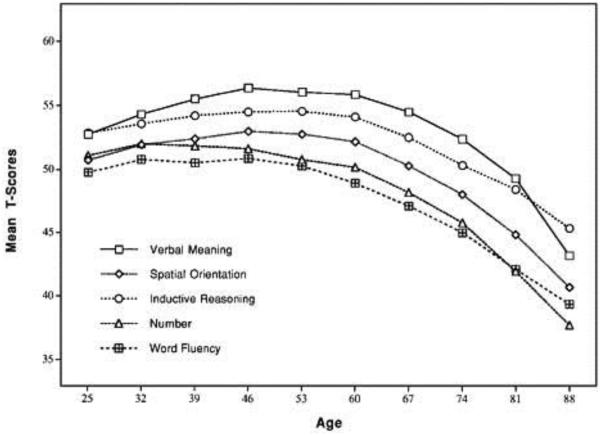
The apparent importance of the long-chain omga-3 fatty acids in early brain development and their potential utility in AD late in life begs the question of their role in adult brain health and in the “natural history” of cognitive performance across the lifespan (Figure 1). Most Americans and much of the developed world consume less than 200 mg per day of EPA and DHA17. Are such low quantities sufficient for optimal brain function and, in particular, cognitive performance? Cognitive abilities are the primary distinguishing feature of humans relative to non-human primates and lower mammals, and modern society both capitalizes upon and taxes those cognitive abilities. Does low dietary consumption of marine oils limit or constrain aspects of cognitive performance, under what circumstances, and if so, are these decrements ameliorated by relatively short periods of increased EPA and DHA intake?
FIGURE 1.
Cognitive functioning over the lifecourse. Graph displays mean T scores for markers of primary cognitive abilities, based on longitudinal estimates from 7-year within-subject data. Reproduced from the Seattle Longitudinal Study [1] with permission from the publisher.
The aim of this review is to summarize: a) laboratory and other preclinical evidence for the roles of EPA and DHA, particularly within the brain, b) observational data gathered from largely non-patient samples linking these fatty acids with normative cognitive functioning, and c) randomized clinical trials that attempt to experimentally document a causal relationship between EPA and DHA consumption and cognitive performance in generally healthy adults. Finally, this evidence is discussed in terms of its applicability to the general public and to military personnel.
PRECLINICAL RESEARCH AND POTENTIAL MECHANISMS
The polyunsaturated fatty acids (PUFA) are relatively unique among bioactive compounds in the breadth of their roles within cells and across bodily systems. Reviewed in greater detail elsewhere5, 16, 18-21, the multiple actions of the omega-3 fatty acids, EPA and DHA, in biological chemistry establishes their importance generally to health and disease and, therefore, the biological plausibility of various sequelae of nutritional deficiency. However, this same breadth of actions challenges any attempt to explicate the particular mechanism for any specific health or behavioral outcome.
ROLES IN BIOLOGICAL AND NEUROCHEMISTRY
Phospholipids are the basic building block of cell and organelle membranes, and a PUFA occupies the middle or sn-2 position of the glycerol backbone of most phospholipids. DHA and the n-6 fatty acid, arachidonic acid (AA), are highly concentrated in brain phospholipids, and DHA edges out AA as the most prevalent PUFA in human brain grey matter and synaptic membranes19, 22. As a highly-unsaturated fatty acid, DHA increases the fluidity of cell membranes relative to other PUFA. Fluidity is a physicochemical property of membranes that modulates the location and activity of membrane-bound proteins, including enzymes, ion transporters, and neurotransmitter receptors 23. In a psychiatric patient sample, 4 weeks of moderate-to-large doses [range] of EPA and DHA increased neural membrane fluidity, as measured by water proton transverse relaxation (T2)24.
From their repository site in membrane phospholipids, AA, EPA and DHA are released by phospholipases in response to various stimuli. The PUFA then participate in signal transduction via the phosphoinositol-3 and cyclic adenosine monophosphate pathways18. In addition, AA and EPA are precursors to eicosanoids -- families of prostaglandins, thromboxanes and leukotrienes regulating inflammation, vasomotion and hemostasis. Here, AA-derivatives are pro-inflammatory while EPA-derived eicosanoids are relatively anti-inflammatory. Although EPA exists in substantially lower concentrations than AA, increased EPA availability competes with, and reduces the production of, AA-derived eicosanoids. Additionally, EPA and DHA serve as precursors of resolvins which also serve to temper inflammation, vasoconstriction and thrombogenesis20. Most recently, DHA has been show to form nitro-fatty acids that are reactive electrophilic species which modulate cellular redox status, protein activity and electrophile-sensitive gene expression20, 25. This hypothesized role in oxidative stress receives corroboration from a study of schizophrenic patients randomized to 12 weeks of EPA supplementation or placebo. Using magnetic resonance spectroscopy to estimate metabolite levels, Berger and colleagues found that EPA increased glutathione in the temporal lobes bilaterally26. Cellular aging indexed by telomere length is predicted by omega-3 fatty acids. Namely, among 600 adults, Whooley and colleagues found an inverse relationship between baseline blood levels of marine omega-3 fatty acids and the rate of telomere shortening over 5 years27.
EFFECTS ON NEURAL TISSUE STRUCTURE AND FUNCTION
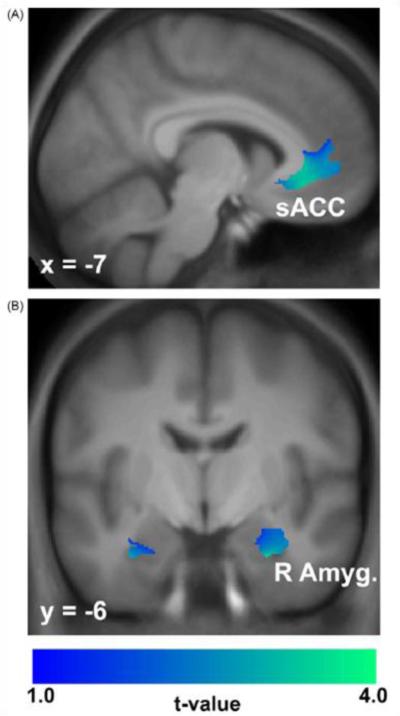
Acting through the preceding mechanisms and likely others yet undiscovered, the long-chain omega-3 PUFA exert neurotrophic effects. These include promotion of neurogenesis, dendritic arborization, selective pruning and myelination5. Elaboration of these effects have revealed changes in production of brain-derived neurotrophic factor and myelin proteins, activation of syntaxin-3, or the role of DHA in membrane growth5, 28-31. Our preliminary examination of brain morphology in relation to self-reported fish intake among healthy mid-life adults found that those with high consumption had greater gray matter volume in several corticolimic structures (Figure 2)32.
FIGURE 2.
Composite brain magnetic resonance images of 55 healthy adults. Profiled in color are areas of the subgenual anterior cingulate cortex (A) and right amygdala (B) where covariate-adjusted gray matter volume increased as a function of higher omega-3 dietary intake. Color-scaled t-values were derived from a linear regression model of voxel-wise gray matter volume that included age, sex and total gray matter volume as covariates. Reproduced from [2] with permission from the publisher.
In a complementary fashion, neural tissue degeneration may be slowed by neuroprotective or anti-apoptotic effects of DHA. This may occur via promotion of cell survival by neuroprotectin D1 (a project of DHA), or inhibition of caspase-316. Indirect evidence suggests that dietary EPA and DHA preserves neural tissue in humans. Among 2,300 older adults who completed a food frequency questionnaire as well as baseline and 5-year brain imaging, consumption of tuna and other baked or broiled fish was associated in covariate adjusted models with fewer sub-clinical infarcts and better white matter grade, but not with sulcal or ventricular grade33. A similar prospective study quantified circulating omega-3 fatty acid in plasma and found that higher omega-3 content was associated with lower prevalence of subclinical infarcts and better white matter grade, but not with incident subclinical infarcts or markers of brain atrophy34. Finally, Pottala and colleagues reported that among 1,111 post menopausal women higher red blood cell EPA+DHA, though unrelated to subclinical infarcts, corresponded to larger total brain and hippocampal volumes35.
Additionally, omega-3 fatty acids supply may moderate regional brain metabolism. Rats deprived of omega-3 PUFA manifest regional decreases in glucose utilization, possibly due to reduced production of the glucose transporter36, 37. Here again, preliminary data from humans find that cerebral glucose metabolism in limbic system structures correlates with plasma DHA in medication free depressed patients38.
The long-chain omega-3 fatty acids affect endothelial function39 and supplementation lowers resting blood pressure40. Thus, it may be postulated that these compounds may affect brain function via effects on regional cerebral blood flow. Here, a clinical study has found that 12 week supplementation with DHA, but not EPA, increased concentrations of oxygenated and total hemoglobin (measured with functional near IR spectroscopy) during cognitive tasks41.
Logically, the foregoing cellular and physiologic roles of PUFA may be expected to affect neurotransmission, either generally or selectively. Studies in rodents find that withholding omega-3 PUFA results in decreased serotonin turnover and dopaminergic neurotransmission30, 42. Some evidence from clinical studies also supports connections between the omega-3 PUFA and serotonergic and dopaminergic functioning4, 43-45, whereas a small fish oil supplementation experiment found no effect on striatal vesicular monoamine transporter type 246.
PSYCOPHYSIOLOGIC MECHANISMS
The autonomic nervous system has long been known to be involved in the orienting response (i.e., slowing of heart rate with exaggerated sinus arrhythmia, slowed respiration, and papillary dilation), which in turn facilitates attention and learning47. Such physiologic responses characterize parasympathetic activation and can be contrasted with responses to intense or threatening stimuli which activate the sympathetic nervous system causing cardiac acceleration, tachypnea and pupillary constriction. The balance between these modes of autonomic and neuroendocrine activation affects cognitive performance. Indeed, an inverted U-shaped relationship appears to exist between degree of psychological stress and memory performance, with some role possibly played by glucocorticoid-mediated effects on the hippocampus and prefrontal cortex48.
Accordingly, it is noteworthy that epidemiologic studies and randomized clinical trials indicate that raising dietary intake of the long chain, omega-3 fatty acids increases parasympathetic cardiac control, as reflected in slower heart rate and greater heart rate variability in both infants and adults47, 49-53. Fish oil supplementation may also moderate heart rate and blood pressure responses to psychological stress and exercise53-57. Preliminary evidence suggests that the omega-3 fatty acids may affect the adrenocortical axis56, 58. Thus, tempering autonomic and neuroendocrine stress responses may have salutary effects on cognitive functioning47.
Finally, mood or affect can influence cognitive abilities. For example, depressed mood and anxiety can reduce attention, working memory, episodic memory and executive function59. Therefore, any effects of omega-3 fatty acids on mood could, secondarily, change aspects of cognitive performance. A series of observational studies and randomized clinical trials of clinical depression generally suggest that, compared to low intake of EPA and DHA, high or raised consumption ameliorates depression60, 61. The clinical trial research finds that these mood effects specifically follow supplementation with EPA alone or in combination with DHA60. Additionally, individual differences in depressive symptoms among generally healthy samples of adolescents, mid-life adults and the elderly are associated with tissue or circulating levels of omega-3 fatty acids, particularly low EPA32, 62-64. This association may extend to neuroticism, the personality trait characterizing a person’s propensity to experience negative affect32, 63, 65. However, presently there is limited evidence from randomized trials that fish oil supplementation either reduces negative affect or increases positive mood states in non-patient samples66.
EFFECTS ON COMPONENT COGNITIVE PROCESSES
To the extent that the omega-3 PUFA affect cognitive performance, they may do so generally or, more likely perhaps, via relatively discrete aspects of cognitive functioning. Most cognitive tests (and daily life duties) require an individual to attend to or focus upon the task at hand. Therefore, general attentional skills, if lacking, can manifest as diffuse decrements in cognitive performance. Research with non-human primates points to effects of dietary omega-3 fatty acids on attention67. Clinically, the syndrome of attention deficit disorder is common in childhood and can persist throughout life. A number of randomized clinical trials have found that fish oil supplementation is efficacious in patients with attention deficit disorders68, 69 and we found that circulating levels of omega-3 PUFA are related to self-reported impulsivity in non-patient samples65, 70.
The acquisition, retention and retrieval of new, discrete information, sometimes referred to as “learning and memory” or “episodic memory” is another basic facet of cognitive functioning which has been examined in laboratory studies of omega-3 fatty acids. Generally, dietary deprivation results in poor learning ability and these decrements resolve with repletion of omega-3 fatty acids71, 72.
Other fundamental components of cognition performance include processing speed (including psychomotor speed and general mental efficiency) and executive functioning (consisting of working memory, response inhibition, problem-solving and planning). Here, the above discussed effects of EPA and DHA on brain chemistry, structure and function could well manifest in altered performance in these domains, but awaits closer examination with animal models7, 73, 74.
COGNITIVE PERFORMANCE AS A FUNCTION OF FISH AND OMEGA-3 INTAKE – OBSERVATIONAL STUDIES
Observational studies of moderate to large numbers of individuals provide reasonable data in which to test that hypothesized association between dietary consumption of long-chain, omega-3 fatty acids and cognitive functioning. Cognitive abilities increase with brain maturation until at age 20-30, plateau and then begin a gradual decline at age 40-50 (Figure 1). Thus, childhood/adolescence and late-life aging are periods of changing cognitive performance that lend themselves to prospective studies of performance change. During middle adulthood cognitive abilities are stable and prospective studies have little advantage over cross-sectional analyses. In all observational studies, there exists substantial risk of confounding by other health and sociodemographic factors.
Some observational studies use dietary questionnaires to quantify fish or EPA and DHA consumption, whereas others directly measure fatty acids in blood samples. The former approach has the widely recognized limitations of general imprecision along with tendencies toward biased recall. Measuring blood levels both provides a more directly quantified biomarker of dietary habits and also an index of supply of individual fatty acids to the brain. Even so, blood levels of EPA and DHA do not correlate highly with dietary measures, have not been well-validated against tissue levels in humans75, 76, are affected by non-dietary factors related to fatty acid metabolism and pharmacokinetics, and have uncertain stability over time.
As noted earlier, pre- and early post-natal brain and behavioral development appears to be improved by provision of long-chain fatty acids. In a study school children living in the US, polyunsaturated fatty acid intake was associated with better attention and working memory performance77, but findings germane to omega-3 fatty acids were not reported specifically. Observational studies of the role of EPA and DHA on cognitive development during childhood remain too limited to allow any conclusions78.
Brain maturation is being completed during the late teenage years, and a single, large study has examined during this stage of brain development the relationship between dietary omega-3 fatty acid intake and cognitive performance79. Approximately 4,000 Swedish males aged 15 years completed a dietary questionnaire in conjunction with their parents and then underwent military conscription IQ testing at age 18. Using as a referent fish intake less than once a week, a statistically strong and graded association was found between higher fish consumption at age 15 and global IQ, verbal and visuospatial scores at age 18. Attempts to control for parental education and SES did not substantially attenuate the findings.
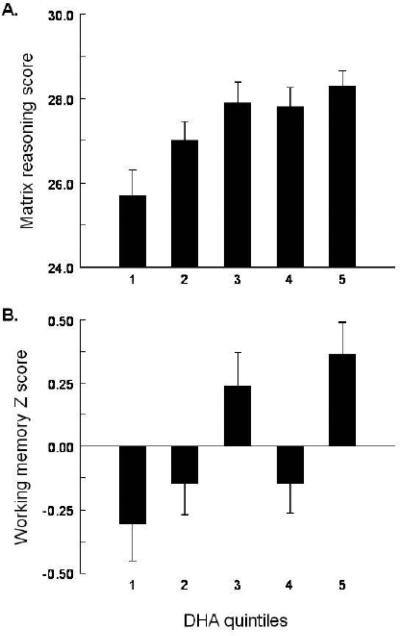
Several reports provide some data on polyunsaturated fatty acids in blood samples in relation to cognitive functioning in young and middle-aged adults. The first enrolled 54 women with a mean age of 30 years and administered four neuropsychological tests assessing verbal memory, response inhibition and general psychomotor speed on four occasions over five months80. EPA and DHA were unrelated to baseline performance indices, whereas performance improvement over 5 months on one test was inversely related to DHA levels. In contrast, in 280 healthy volunteers between 30 and 54 years of age phospholipid DHA was directly proportional to non-verbal reasoning, working memory, executive function and vocabulary81 [Figure 3]. Cognitive performance was not reliably related to EPA or to alpha-linolenic acid, the 18-carbon precursor of EPA and DHA. This latter study covaried age, gender, race and blood pressure, and findings relating DHA to non-verbal reasoning and working memory persisted with additional adjustment for education and vocabulary (markers of crystallized intelligence). Finally, among U.S. military servicemembers, red blood cell omega-3 fatty acid levels correlated positively with neurocognitive performance in the domains of cognitive flexibility and executive function but not processing speed, complex attention or reaction time82.
FIGURE 3.
Dose-response relationship between serum phospholipid DHA (mole %) and cognitive performance scores among healthy, mid-life adults. Panels A and B present Wechsler Abbreviated Intelligence Matrix Reasoning and Wechsler Memory Scale Working Memory results, respectively. Values are means, ± standard error, adjusted for age, sex and race, by DHA quintile (n=56 for each). Analysis of variance revealed a significant effect of DHA quintile on matrix reasoning (P=0.002) and working memory (P=0.001). Reproduced from [3] with permission from the publisher.
A larger set of studies has reported findings in the elderly, defined here as samples with a mean age exceeding 55 years. Three cross-sectional studies are available in which investigators tested associations between self-reported fish intake and cognitive performance assessed with multi-component neuropsychological batteries83-85. Each included adjustments for potentially confounding health and socioeconomic factors. Kalmijn and colleagues found evidence that fish intake is primarily related to psychomotor speed rather than episodic memory and mental flexibility83. Analyses of baseline data from the OPAL trial revealed broad associations between fish consumption and multiple dimensions of cognitive performance which were attenuated by additional adjustment for psychological symptoms84. The third such study also found associations with multiple aspects of cognitive function, and these relationships appeared to plateau as self-reported fish intake increased85.
Prospective studies based upon self-reported diet data have followed from 210 to nearly 4,000 elderly participants for three to six years86-88. After adjustment for age, gender, alcohol, education and baseline cognitive scores, each study found evidence for slower cognitive decline as a function of higher fish intake.
Among investigations utilizing blood sample analyses, Whalley and colleagues89 reported that even with adjustment for childhood IQ, total omega-3 fatty acids -- DHA in particular -- was associated with overall cognitive function and four year cognitive decline90. Other prospective studies find that the greater the sum of circulating long-chain omega-3 fatty acids the smaller the observed decline in cognitive performance91, with psychomotor speed and executive function most, and memory least , related to omega-3 levels in older persons92, 93. However, a recent large study of elderly women found no such associations94.
COGNITIVE PERFORMANCE AS A FUNCTION OF FISH AND OMEGA-3 INTAKE – RANDOMIZED CLINICAL TRIALS
RCTs can provide the final and most compelling evidence for cognitive benefits from the long-chain, omega-3 fatty acids. Here, participants meeting set enrollment criteria are randomly assigned to supplementation with a fixed dose of omega-3 fatty acids or a matching placebo. Objective tests of cognitive functioning are administered at baseline and again at the completion of the supplementation period. Changes in performance scores among supplemented subjects are then compared to those observed in the placebo-treated groups. Placebo-treated groups are critical since repeated test administration can result in improved scores simply due to practice effects and, alternatively, among older subjects performance scores can worsen over time due to age-related cognitive decline.
Two reports of randomized clinical trials in 6-10 year-old school children (one in well-nourished and one in “marginally nourished” participants) found no cognitive benefits from low dose supplementation – roughly 100 mg of EPA and DHA per day95, 96. A third trial conducted in children of low socioeconomic status found that giving 280 mg/day of EPA and DHA improved verbal memory scores97.
The principle features and findings of RCT enrolling mid-life adults are provided in Table 198-102 , whereas trials in elderly but non-demented adults are discussed separately below103-113. The goal here is to tabulate evidence from RCT of generally-healthy adults rather than persons with neuropsychiatric disorders, as the results of this findings are most informative regarding the effects of omega-3 fatty acids in the population at large. In Table 1, we have included a trial of individuals reporting depressive symptoms because such symptoms are common in the general population and, in this RCT, the enrolled subjects did not appear to have a major depressive disorder and none were on medication or behavioral therapy.
Table 1.
Randomized trials of cognitive effects of long-chain omega-3 fatty acid supplementation in non-elderly adults
| Study | Subject Criteria | Subjects | Duration, supplements |
Cognitive measures | Results | Comments |
|---|---|---|---|---|---|---|
|
Antypa et al.,
2009 [4] |
Dutch volunteers who reported eating fish ≤ 1x/wk No smoking, no MDD, no meds, ≤3 drinks/day |
N=54 18-27y/o 81% female |
4 weeks 1740mg EPA, 250mg DHA vs. olive oil |
5 neuropsychological tests measuring psychomotor speed, sustained attention response inhibition, episodic memory, and gambling |
Supplementation increased gambling to maximize gain and did not affect other scores. |
Found reduction in self- reported control/perfectionism, and cognitive reactivity. |
|
Fontani et al.,
2005 [5] |
Italian volunteers from non- competitive athletic associations |
N=49 22-51 y/o 65% female |
5 weeks 1600mg EPA, 800mg DHA vs. olive oil |
4 neuropsychological tests measuring psychomotor speed, sustained attention, response inhibition, and working memory |
Supplementation improved psychomotor speed and did not affect other scores. |
Reported findings may not have taken into account effects of placebo. Supplementation also improved positive and negative mood states. |
|
Rogers et al,
2008 [6] |
British general practice patients and community volunteers reporting: a) mild-to-moderate depressive symptoms, b) not taking psychotropic medication, and c) not consuming high quantities of EPA and DHA. |
N=218 18-70 y/o 77% female |
12 weeks 630mg EPA, 850mg DHA vs. olive oil |
5 neuropsychological tests measuring psychomotor speed, sustained attention, response inhibition, and working memory |
Supplementation marginally improved response inhibition and did not affect other scores. |
No effect on mood ratings. Blood EPA +DHA more than doubled in fish oil group. |
|
Stonehouse et
al, 2013 [7] |
New Zealand healthy adult volunteers reporting very low EPA+DHA consumption |
N=228 18-45 y/o 73% female |
26 weeks 1160mg DHA,160mg EPA vs. high-oleic acid sunflower oil |
9 neuropsychological tests measuring episodic memory, working memory, attention and processing speed |
Supplementation improved episodic memory reaction time, improved episodic memory performance in women only, improved working memory reaction time in men only. No effects on other scores. |
23% drop-out rate No moderation of treatment effects by APOE genotype. |
|
Jackson et al,
2012 [8] |
British university students or graduates reporting ≤1 portion oily fish/week |
N=159 18-35 y/o 67% female |
12 weeks 450mg DHA+90mg EPA vs. 300mg EPA+200mg DHA vs. olive oil |
15 neuropsychological tests (plus a “cognitive demand” battery) measuring episodic and working memory, psychomotor speed, attention, and executive function |
DHA-rich supplementation improved one test of reaction time. Both active supplementations worsened scores on one test of episodic memory. Other performance measures were unaffected. |
Analyses were based on 140 subjects. No effect on mood ratings. |
These five RCTs tested the potential cognitive effects of increased consumption of omega-3 fatty acids in generally healthy persons during middle-adulthood. Each was conducted in Western Europe or New Zealand, was small-to-moderate in size, and enrolled predominantly women. The supplementation dose was between 500 and 2400 mg per day of EPA and DHA, and treatment duration ranged from four to 26 weeks.
Each trial employed a neuropsychological test battery which assessed several dimensions of cognitive function. These studies variously found that EPA and DHA supplementation improved memory, psychomotor speed and prepotent response inhibition. However, significant intervention effects for specific performance domains were not consistently observed and the majority of statistical comparisons were null. The reports note sporadic auxiliary treatment benefits based on self-reported measures of cognitive processes and affect.
The trial by Antypa and colleagues100 uniquely examined gambling by asking subjects to chose between set, modest gains (or losses) versus a gamble that could provide larger gains (or losses) or result in no gain or loss. Fish oil supplementation increased the selection of the gambling option to maximize gains without affecting loss-related gambling, suggesting the induction of an optimistic view of potential gains without increasing gambling generally. In Roger et al99, subjects were all reporting depressive symptoms at the time of enrollment, and the omega-3 fatty acid supplementation did not affect mood ratings. The latter finding is at odds with apparent efficacy of fish oil as a supplementary treatment for major depression60, 61. EPA, in particular, may have mood elevating effects, suggesting that the comparatively low EPA dose of 630 mg in the Rogers trial may have been insufficient.
The two most recent trials101, 102 included the most comprehensive cognitive testing. Stonehouse et al101 found evidence of performance improvement on tests of episodic and working memory with a DHA supplement that varied as a function of gender, whereas Jackson and colleagues102 found no notable beneficial effects from either DHA-rich or EPA-rich supplementation.
A somewhat larger body of clinical trial evidence addresses the cognitive effects of EPA and DHA supplementation in non-demented, elderly individuals. These data are less relevant to optimizing cognitive functioning in mid-life and, therefore, are summarized here only briefly. Across the reviewed trials, the supplementation dose ranged from 80 to 2300 mg per day, and treatment durations were 13 to 104 weeks. Seven small trials (i.e., 10-70 participants per treatment group) enrolled either healthy elderly volunteers or those with mild cognitive impairment103-105, 109-112. All but one111 reported some evidence of cognitive benefit relative to placebo. Among four larger trials, one found improved performance on memory tests108, one found improved attention performance but only in men and apo E4 carriers106, and two found no treatment effects relative to placebo107, 113. In one of these latter two trials, one enrolled persons with high fish consumption at baseline107 and the other used the rather low dose of 400 mg/day of EPA+DHA113.
Collectively, these clinical trials can be said to provide preliminary evidence of salutary cognitive effects of raising dietary intake of EPA and DHA in adults. Nonetheless, most of the trials were small, and any benefit was only observed on a subset of administered cognitive tests. Memory, attention, psychomotor speed and response inhibition or impulsivity were variously reported to improve. Meta-analyses restricted to trials conducted with elderly participants have concluded that available evidence does not support the existence of robust benefits from omega-3 fatty acid supplementation114, 115.
Several design issues warrant serious consideration. If one accepts the model that omega-3 fatty acids supplementation may facilitate cognitive performance because these are essential nutrients for normal brain functioning, then it follows that trials should recruit individuals whose customary diet includes only low quantities of EPA and DHA. Many trials make not mention of such restrictions98, 103, 105, 109, or allowed EPA+ DHA consumption up to 800 mg per day, a very high intake by US standards106.
Trials typically used placebo capsules of a seed or plant oil to assist with blinding. However, depending on the placebo oil chosen, some physiologic or behavioral effects are possible. All five reviewed trials of non-elderly adults (Table 1) used olive oil or oleic acid for a placebo intervention whereas such has been criticized as possibly favorably affecting some neurobehavioral outcomes116. Fish oil capsules cause “fishy belching” in some individuals, particularly with higher doses, very possibly disrupting participant blinding. Two trials attempted to mask this aftertaste with citrus flavoring99, 100; of these, one99 reported confirmation of treatment concealment.
Dose size is also an open question. In the U.S., dietary intake of the long-chain omega-3 PUFAs averages 110 mg per day17, and supplementing 400 mg daily increases circulating levels by 50%106. Nonetheless, no investigation of cognitive effects has defined either the dose threshold for effect detection or the shape of the dose-response curve. Two dose-ranging studies of affective disorders found benefit from 1,000 mg per day of EPA per day with no incremental benefit from high doses117, 118. If the long-chain omega-3 fatty acids are viewed as essential micronutrients, one might expect a graded physiologic response to low-range doses, with response flattening as the nutritional requirement is met. Across the RCTs of cognitive outcomes reviewed here, doses ranged from 80 to 2400 mg per day. Studies employing doses under 800mg per day generally found no treatment effects102, 105, 107, 109, 113. The single study which compared doses found no substantial difference between 400 mg and 1400 mg per day of EPA and DHA106.
Most trials gave a combination of EPA and DHA, and whether any effects are attributable one or another is unknown. Based on laboratory investigations and observational studies discussed above, one might postulate that that DHA is the active ingredient, and some of the strongest trial evidence of cognitive benefit from omega-3 fatty acid supplementation was observed with relatively large doses of DHA108. Interestingly, trials in depression have found mood effects from EPA, but not DHA60.
Finally, the time course of any cognitive effects has not been directly studied. Accretion of supplemented fatty acids into tissue membranes extends over several months119, 120. Nonetheless, effects on cognitive and psychiatric symptoms have been reported in just 4-6 weeks66, 121 and, in the RCTs summarized here, detection of cognitive effects bears no obvious relationship to treatment duration.
SUMMARY
A well-developed literature of pre-clinical experiments has discovered a myriad of roles and actions of the long-chain omega-3 fatty acids which, when considered in conjunction with epidemiological and observational study evidence, make highly plausible the essentiality of dietary intake of EPA and DHA for normal brain growth and function. Using newer imaging techniques, advances in our understanding of the basic biological chemistry of EPA and DHA appear to translate to measurable phenomena within, and detectable changes to, the adult human brain.
In particular, the findings of these varied investigations indicate collectively that low to nil dietary intake is not conducive to optimal cognitive functioning in mid-life adults, nor perhaps during any stage in the human lifespan. At the same time, the fundamental roles and pleiotropic effects of the long-chain omega-3 fatty acids make difficult – if not preclude – the assignment of particular “pharmacodynamic” actions to specific behavioral or health outcomes. Again in particular, we know little about which aspect of their biological chemistry might be responsible for apparent effects on cognitive functioning, or even whether teasing apart their actions in this context is feasible or necessarily illuminating.
Ultimately, RCTs are needed to provide direct evidence of changes in cognitive performance following “correction” of low dietary intake of long-chain omega-3 fatty acids. To date, RCT findings reveal neither robust benefits nor a clear lack of efficacy. While it is tempting to believe that consuming more long chain omega-3 fatty acids will ameliorate widespread yet mild decrements in aspects of cognitive performance, RCT evidence is still weak and preliminary. As enumerated above, multiple methodological issues have been incompletely addressed. These uncertainties could well result in mixed results from future trials, and methodological differences between trials may help discern in what circumstances increased omega-3 consumption improves cognitive functioning.
With respect to military applications, the role of dietary intake of omega-3 fatty acids on job performance among war fighters has not been directly studied. Nonetheless, available research does have potential implications for the military. Soldiers are required to execute their duties under circumstances that can involve unpredictable environmental stressors, significant physical demands, and threats of personal injury or death. Such varied and sometimes severe stressors challenge one’s ability to make rapid and appropriate decisions and effectively execute orders. To the extent that fish oil supplementation can temper autonomic and neuroendocrine activation to physical and/or psychological stress, attention and cognitive performance may be enhanced. Similarly, raised intake may mitigate either depressive symptoms or impulsive decision-making, and similarly enhance performance among military personnel. Therefore, given the typically low consumption of omega-3 fatty acids among U.S. civilians and suggestive randomized trial evidence to date, it is quite plausible that increasing dietary consumption of omega-3 PUFAs will improve soldier performance. Military studies of diet manipulation with measurement of technical proficiency or simulated task execution could be conducted to provide direct evidence.
ACKNOWLEDGEMENTS
No acknowledgements
Footnotes
FINANCIAL DISCLOSURE
None of the authors currently have, or had during the time of data collection, any financial or personal relationship with the funding organization (US National Institutes of Health). Drs. Conklin, Yao, and Muldoon report receipt of a $9,000 research grant in 2008 from Isodi Natura to support serum fatty acid determinations used a separate study. These authors report no other biomedical financial interests or potential conflicts of interest. Dr. Ryan and Manuck report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Connor WE. Importance of n-3 fatty acids in health and disease. The American journal of clinical nutrition. 2000;71(1 Suppl):171S–175S. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- 2.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. Journal of the American Dietetic Association. 2009;109(4):668–679. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Yashodhara BM, Umakanth S, Pappachan JM, Bhat SK, Kamath R, Choo BH. Omega-3 fatty acids: a comprehensive review of their role in health and disease. Postgraduate medical journal. 2009;85(1000):84–90. doi: 10.1136/pgmj.2008.073338. [DOI] [PubMed] [Google Scholar]
- 4.Conklin SM, Reddy RD, Muldoon MF, Yao JK. Fatty acids and psychiatric disorders. In: Chow CK, editor. Fatty Acids in Foods and Their Health Implications, 1229-1256. Third CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- 5.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain research. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 6.Brenna JT, Diau GY. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77(5-6):247–250. doi: 10.1016/j.plefa.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedorova I, Salem N., Jr. Omega-3 fatty acids and rodent behavior. Prostaglandins, leukotrienes, and essential fatty acids. 2006;75(4-5):271–289. doi: 10.1016/j.plefa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral-cortex phospholipid fatty-acid composition and diet. Lancet. 1992;340(8823):810–813. doi: 10.1016/0140-6736(92)92684-8. [DOI] [PubMed] [Google Scholar]
- 9.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369(9561):578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 10.Oken E, Osterdal ML, Gillman MW, Knudsen VK, Halldorsson TI, Strom M, et al. Associations of maternal fish intake during pregnancy and breastfeeding duration with attainment of developmental milestones in early childhood: a study from the Danish National Birth Cohort. The American journal of clinical nutrition. 2008;88(3):789–796. doi: 10.1093/ajcn/88.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111(1):e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 12.McCann JC, Ames BN. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. American Journal of Clinical Nutrition. 2005;82(2):281–295. doi: 10.1093/ajcn.82.2.281. [DOI] [PubMed] [Google Scholar]
- 13.Judge MP, Harel O, Lammi-Keefe CJ. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. The American journal of clinical nutrition. 2007;85(6):1572–1577. doi: 10.1093/ajcn/85.6.1572. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen C, Haugholt K, Lindgren M, Aurvag AK, Ronnestad A, Gronn M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121(6):1137–1145. doi: 10.1542/peds.2007-1511. [DOI] [PubMed] [Google Scholar]
- 15.Agostoni C, Zuccotti GV, Radaelli G, Besana R, Podesta A, Sterpa A, et al. Docosahexaenoic acid supplementation and time at achievement of gross motor milestones in healthy infants: a randomized, prospective, double-blind, placebo-controlled trial. The American journal of clinical nutrition. 2009;89(1):64–70. doi: 10.3945/ajcn.2008.26590. [DOI] [PubMed] [Google Scholar]
- 16.Boudrault C, Bazinet RP, Ma DW. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer's disease. The Journal of nutritional biochemistry. 2009;20(1):1–10. doi: 10.1016/j.jnutbio.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Agriculture, Agricultural Research Service Nutrient Intakes from Food: Mean Amounts Consumed per Individual, One Day, 2005-2006. 2008 Available at: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0506/Table_1_NIF_05.pdf; Accessed March 21, 2014.
- 18.Simopoulos AP. Omega-6/omega-3 essential fatty acids: biological effects. World review of nutrition and dietetics. 2009;99:1–16. doi: 10.1159/000192755. [DOI] [PubMed] [Google Scholar]
- 19.Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia Pacific journal of clinical nutrition. 2008;17(Suppl 1):220–228. [PubMed] [Google Scholar]
- 20.Massaro M, Scoditti E, Carluccio MA, De Caterina R. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins, leukotrienes, and essential fatty acids. 2008;79(3-5):109–115. doi: 10.1016/j.plefa.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Progress in lipid research. 2008;47(2):147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Wurtman RJ. Synapse formation and cognitive brain development: effect of docosahexaenoic acid and other dietary constituents. Metabolism: clinical and experimental. 2008;57(Suppl 2):S6–10. doi: 10.1016/j.metabol.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapkin RS, McMurray DN, Davidson LA, Patil BS, Fan YY, Lupton JR. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. The British journal of nutrition. 2008;100(6):1152–1157. doi: 10.1017/S0007114508992576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirashima F, Parow AM, Stoll AL, Demopulos CM, Damico KE, Rohan ML, et al. Omega-3 fatty acid treatment and T-2 whole brain relaxation times in bipolar disorder. American Journal of Psychiatry. 2004;161(10):1922–1924. doi: 10.1176/ajp.161.10.1922. [DOI] [PubMed] [Google Scholar]
- 25.Groeger Alison L., Cole Marsha P., Woodcock Steven R., Bonacci Gustavo, Rudolph Tanja K., Rudolph Volker, Freeman Bruce A., Francisco J. Schopfer: Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nature Chem Biol. doi: 10.1038/nchembio.367. CC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger GE, Wood SJ, Wellard RM, Proffitt TM, McConchie M, Amminger GP, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology. 2008;33(10):2467–2473. doi: 10.1038/sj.npp.1301628. [DOI] [PubMed] [Google Scholar]
- 27.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. Jama. 303(3):250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvati S, Natali F, Attorri L, Di Benedetto R, Leonardi F, Di Biase A, et al. Eicosapentaenoic acid stimulates the expression of myelin proteins in rat brain. Journal of neuroscience research. 2008;86(4):776–784. doi: 10.1002/jnr.21537. [DOI] [PubMed] [Google Scholar]
- 29.Cunnane SC PM, Vandal M, Freemantle E, Tremblay-Mercier J, Begin M. Linking low docosahexaenoic acid intake to Alzheimers disease: caution recommended. OCL. 2007;14:1–5. [Google Scholar]
- 30.Levant B, Ozias MK, Davis PF, Winter M, Russell KL, Carlson SE, et al. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: Interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33(9):1279–1292. doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440(7085):813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- 32.Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neuroscience letters. 2007;421(3):209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 33.Virtanen JK, Siscovick DS, Longstreth WT, Jr., Kuller LH, Mozaffarian D. Fish consumption and risk of subclinical brain abnormalities on MRI in older adults. Neurology. 2008;71(6):439–446. doi: 10.1212/01.wnl.0000324414.12665.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Virtanen JK, Siscovick DS, Lemaitre RN, Longstreth WT, Spiegelman D, Rimm EB, et al. Circulating omega-3 polyunsaturated fatty acids and subclinical brain abnormalities on MRI in older adults: the Cardiovascular Health Study. Journal of the American Heart Association. 2013;2(5):e000305. doi: 10.1161/JAHA.113.000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI Study. Neurology. 2014 doi: 10.1212/WNL.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ximenes da Silva A, Lavialle F, Gendrot G, Guesnet P, Alessandri JM, Lavialle M. Glucose transport and utilization are altered in the brain of rats deficient in n-3 polyunsaturated fatty acids. Journal of neurochemistry. 2002;81(6):1328–1337. doi: 10.1046/j.1471-4159.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 37.Pifferi F, Roux F, Langelier B, Alessandri JM, Vancassel S, Jouin M, et al. (n-3) polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. The Journal of nutrition. 2005;135(9):2241–2246. doi: 10.1093/jn/135.9.2241. [DOI] [PubMed] [Google Scholar]
- 38.Sublette ME, et al. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2009;80(1):57–64. doi: 10.1016/j.plefa.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egert S, Stehle P. Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Current opinion in clinical nutrition and metabolic care. 2011;14(2):121–131. doi: 10.1097/MCO.0b013e3283439622. [DOI] [PubMed] [Google Scholar]
- 40.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. Journal of hypertension. 2002;20(8):1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Jackson PA, Reay JL, Scholey AB, Kennedy DO. DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: a near IR spectroscopy pilot study. The British journal of nutrition. 2012;107(8):1093–1098. doi: 10.1017/S0007114511004041. [DOI] [PubMed] [Google Scholar]
- 42.Zimmer L, Delion-Vancassel S, Durand G, Guilloteau D, Bodard S, Besnard JC, et al. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. Journal of lipid research. 2000;41(1):32–40. [PubMed] [Google Scholar]
- 43.Yao JK, van Kammen DP, Moss HB, Sokulski DE. Decreased serotonergic responsivity in platelets of drug-free patients with schizophrenia. Psychiatry research. 1996;63(2-3):123–132. doi: 10.1016/0165-1781(96)02862-4. [DOI] [PubMed] [Google Scholar]
- 44.Hibbeln JR, Salem N., Jr. Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62(1):1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Hibbeln JR, Linnoila M, Umhau JC, Rawlings R, George DT, Salem N., Jr. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biological psychiatry. 1998;44(4):235–242. doi: 10.1016/s0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 46.Narendran R, Frankle WG, Mason NS, Muldoon MF, Moghaddam B. Improved working memory but no effect on striatal vesicular monoamine transporter type 2 after omega-3 polyunsaturated fatty acid supplementation. PloS one. 2012;7(10):e46832. doi: 10.1371/journal.pone.0046832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gustafson KM, Colombo J, Carlson SE. Docosahexaenoic acid and cognitive function: Is the link mediated by the autonomic nervous system? Prostaglandins, leukotrienes, and essential fatty acids. 2008;79(3-5):135–140. doi: 10.1016/j.plefa.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luethi M, Meier B, Sandi C. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Frontiers in behavioral neuroscience. 2008;2:5. doi: 10.3389/neuro.08.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christensen JH, Skou HA, Fog L, Hansen V, Vesterlund T, Dyerberg J, et al. Marine n-3 fatty acids, wine intake, and heart rate variability in patients referred for coronary angiography. Circulation. 2001;103(5):651–657. doi: 10.1161/01.cir.103.5.651. [DOI] [PubMed] [Google Scholar]
- 50.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117(9):1130–1137. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 51.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112(13):1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 52.Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. The American journal of clinical nutrition. 1999;70(3):331–337. doi: 10.1093/ajcn/70.3.331. [DOI] [PubMed] [Google Scholar]
- 53.Ninio DM, Hill AM, Howe PR, Buckley JD, Saint DA. Docosahexaenoic acid-rich fish oil improves heart rate variability and heart rate responses to exercise in overweight adults. The British journal of nutrition. 2008;100(5):1097–1103. doi: 10.1017/S0007114508959225. [DOI] [PubMed] [Google Scholar]
- 54.O'Keefe JH, Jr., Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. The American journal of cardiology. 2006;97(8):1127–1130. doi: 10.1016/j.amjcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 55.Peoples GE, McLennan PL, Howe PR, Groeller H. Fish oil reduces heart rate and oxygen consumption during exercise. Journal of cardiovascular pharmacology. 2008;52(6):540–547. doi: 10.1097/FJC.0b013e3181911913. [DOI] [PubMed] [Google Scholar]
- 56.Delarue J, Matzinger O, Binnert C, Schneiter P, Chiolero R, Tappy L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes & metabolism. 2003;29(3):289–295. doi: 10.1016/s1262-3636(07)70039-3. [DOI] [PubMed] [Google Scholar]
- 57.Sesek J GS, Symoniak M, Conklin S. Acute low-dose supplementation of long-chain n-3 fatty acids is associated with reduced cardiovascular reactivity to mental stress. Psychosom Med. 2009;71:A-1. [Google Scholar]
- 58.Hibbeln JR, Bissette G, Umhau JC, George DT. Omega-3 status and cerebrospinal fluid corticotrophin releasing hormone in perpetrators of domestic violence. Biological psychiatry. 2004;56(11):895–897. doi: 10.1016/j.biopsych.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 59.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. Journal of affective disorders. 2009;119(1-3):1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 60.Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. The American journal of psychiatry. 2006;163(6):969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- 61.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. The Journal of clinical psychiatry. 2006;67(12):1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 62.Mamalakis G, Jansen E, Cremers H, Kiriakakis M, Tsibinos G, Kafatos A. Depression and adipose and serum cholesteryl ester polyunsaturated fatty acids in the survivors of the seven countries study population of Crete. European journal of clinical nutrition. 2006;60(8):1016–1023. doi: 10.1038/sj.ejcn.1602413. [DOI] [PubMed] [Google Scholar]
- 63.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom Med. 2007;69(9):932–934. doi: 10.1097/PSY.0b013e31815aaa42. [DOI] [PubMed] [Google Scholar]
- 64.Feart C, Peuchant E, Letenneur L, Samieri C, Montagnier D, Fourrier-Reglat A, et al. Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City Study. The American journal of clinical nutrition. 2008;87(5):1156–1162. doi: 10.1093/ajcn/87.5.1156. [DOI] [PubMed] [Google Scholar]
- 65.Muldoon MF, Yao JK, Flory JD, Manuck SB. Omega-3 fatty acids, negative affect, hostility and impulsivity. Psychosom Med. 2010;71:A-1. CS. [Google Scholar]
- 66.Fontani G, Corradeschi F, Felici A, Alfatti F, Bugarini R, Fiaschi AI, et al. Blood profiles, body fat and mood state in healthy subjects on different diets supplemented with Omega-3 polyunsaturated fatty acids. European Journal of Clinical Investigation. 2005;35(8):499–507. doi: 10.1111/j.1365-2362.2005.01540.x. [DOI] [PubMed] [Google Scholar]
- 67.Reisbick S, Neuringer M, Gohl E, Wald R, Anderson GJ. Visual attention in infant monkeys: effects of dietary fatty acids and age. DevPsychol. 1997;33(3):387–395. doi: 10.1037//0012-1649.33.3.387. [DOI] [PubMed] [Google Scholar]
- 68.Sinn N. Nutritional and dietary influences on attention deficit hyperactivity disorder. Nutrition reviews. 2008;66(10):558–568. doi: 10.1111/j.1753-4887.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- 69.Vaisman N, Kaysar N, Zaruk-Adasha Y, Pelled D, Brichon G, Zwingelstein G, et al. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: effect of dietary n-3 fatty acids containing phospholipids. The American journal of clinical nutrition. 2008;87(5):1170–1180. doi: 10.1093/ajcn/87.5.1170. [DOI] [PubMed] [Google Scholar]
- 70.Conklin SM, Harris JI, Manuck SB, Yao JK, Hibbeln JR, Muldoon MF. Serum omega-3 fatty acids are associated with variation in mood, personality and behavior in hypercholesterolemic community volunteers. Psychiatry research. 2007;152(1):1–10. doi: 10.1016/j.psychres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 71.Umezawa M, Ohta A, Tojo H, Yagi H, Hosokawa M, Takeda T. Dietary alpha-linolenate/linoleate balance influences learning and memory in the senescence-accelerated mouse (SAM) Brain Res. 1995;669(2):225–233. doi: 10.1016/0006-8993(94)01250-l. [DOI] [PubMed] [Google Scholar]
- 72.Song C, Horrobin D. Omega-3 fatty acid ethyl -eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1 beta administration. Journal of lipid research. 2004;45(6):1112–1121. doi: 10.1194/jlr.M300526-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Fedorova I, Hussein N, Baumann MH, Di Martino C, Salem N., Jr. An n-3 fatty acid deficiency impairs rat spatial learning in the Barnes maze. Behavioral neuroscience. 2009;123(1):196–205. doi: 10.1037/a0013801. [DOI] [PubMed] [Google Scholar]
- 74.Chung WL, Chen JJ, Su HM. Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. The Journal of nutrition. 2008;138(6):1165–1171. doi: 10.1093/jn/138.6.1165. [DOI] [PubMed] [Google Scholar]
- 75.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain research bulletin. 2001;56(2):79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 76.Yao J, Stanley JA, Reddy RD, Keshavan MS, Pettegrew JW. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. BiolPsychiatry. 2002;52(8):823–830. doi: 10.1016/s0006-3223(02)01397-5. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Hebert JR, Muldoon MF. Dietary fat intake is associated with psychosocial and cognitive functioning of school-aged children in the United States. The Journal of nutrition. 2005;135(8):1967–1973. doi: 10.1093/jn/135.8.1967. [DOI] [PubMed] [Google Scholar]
- 78.Eilander A, Hundscheid DC, Osendarp SJ, Transler C, Zock PL. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: a review of human studies. Prostaglandins, leukotrienes, and essential fatty acids. 2007;76(4):189–203. doi: 10.1016/j.plefa.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Aberg MA, Aberg N, Brisman J, Sundberg R, Winkvist A, Toren K. Fish intake of Swedish male adolescents is a predictor of cognitive performance. Acta Paediatr. 2009;98(3):555–560. doi: 10.1111/j.1651-2227.2008.01103.x. [DOI] [PubMed] [Google Scholar]
- 80.de Groot RH, Hornstra G, Jolles J. Exploratory study into the relation between plasma phospholipid fatty acid status and cognitive performance. Prostaglandins, leukotrienes, and essential fatty acids. 2007;76(3):165–172. doi: 10.1016/j.plefa.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 81.Muldoon MF RC, Sheu L, Yao JK, Conklin SM, Manuck SB. Serum phospholipid docosahexaenonic acid is associated with cognitive functioning during middle-adulthood. Journal of Nutrition. 2010;140:848–853. doi: 10.3945/jn.109.119578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnston DT, Deuster PA, Harris WS, Macrae H, Dretsch MN. Red blood cell omega-3 fatty acid levels and neurocognitive performance in deployed U.S. Servicemembers. Nutritional neuroscience. 2013;16(1):30–38. doi: 10.1179/1476830512Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 83.Kalmijn S, van Boxtel MPJ, Ocke M, Verschuren WMM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62(2):275–280. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- 84.Dangour AD, Allen E, Elbourne D, Fletcher A, Richards M, Uauy R. Fish consumption and cognitive function among older people in the UK: baseline data from the OPAL study. The journal of nutrition, health & aging. 2009;13(3):198–202. doi: 10.1007/s12603-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 85.Nurk E, Drevon CA, Refsum H, Solvoll K, Vollset SE, Nygard O, et al. Cognitive performance among the elderly and dietary fish intake: the Hordaland Health Study. The American journal of clinical nutrition. 2007;86(5):1470–1478. doi: 10.1093/ajcn/86.5.1470. [DOI] [PubMed] [Google Scholar]
- 86.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. American journal of epidemiology. 1997;145(1):33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- 87.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. The American journal of clinical nutrition. 2007;85(4):1142–1147. doi: 10.1093/ajcn/85.4.1142. [DOI] [PubMed] [Google Scholar]
- 88.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Archives of neurology. 2005;62(12):1849–1853. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- 89.Whalley LJ, Fox HC, Wahle KW, Starr JM, Deary IJ. Cognitive aging, childhood intelligence, and the use of food supplements: possible involvement of n-3 fatty acids. American Journal of Clinical Nutrition. 2004;80(6):1650–1657. doi: 10.1093/ajcn/80.6.1650. [DOI] [PubMed] [Google Scholar]
- 90.Whalley LJ, Deary IJ, Starr JM, Wahle KW, Rance KA, Bourne VJ, et al. n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. The American journal of clinical nutrition. 2008;87(2):449–454. doi: 10.1093/ajcn/87.2.449. [DOI] [PubMed] [Google Scholar]
- 91.Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes - The EVA Study. American Journal of Clinical Nutrition. 2003;77(4):803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 92.Dullemeijer C, Durga J, Brouwer IA, van de Rest O, Kok FJ, Brummer RJ, et al. n 3 fatty acid proportions in plasma and cognitive performance in older adults. The American journal of clinical nutrition. 2007;86(5):1479–1485. doi: 10.1093/ajcn/86.5.1479. [DOI] [PubMed] [Google Scholar]
- 93.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. The American journal of clinical nutrition. 2007;85(4):1103–1111. doi: 10.1093/ajcn/85.4.1103. [DOI] [PubMed] [Google Scholar]
- 94.Ammann EM, Pottala JV, Harris WS, Espeland MA, Wallace R, Denburg NL, et al. omega-3 fatty acids and domain-specific cognitive aging: secondary analyses of data from WHISCA. Neurology. 2013;81(17):1484–1491. doi: 10.1212/WNL.0b013e3182a9584c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, et al. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. The American journal of clinical nutrition. 2007;86(4):1082–1093. doi: 10.1093/ajcn/86.4.1082. [DOI] [PubMed] [Google Scholar]
- 96.Muthayya S, Eilander A, Transler C, Thomas T, van der Knaap HC, Srinivasan K, et al. Effect of fortification with multiple micronutrients and n-3 fatty acids on growth and cognitive performance in Indian schoolchildren: the CHAMPION (Children's Health and Mental Performance Influenced by Optimal Nutrition) Study. The American journal of clinical nutrition. 2009;89(6):1766–1775. doi: 10.3945/ajcn.2008.26993. [DOI] [PubMed] [Google Scholar]
- 97.Dalton A, Wolmarans P, Witthuhn RC, van Stuijvenberg ME, Swanevelder SA, Smuts CM. A randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostaglandins, leukotrienes, and essential fatty acids. 2009;80(2-3):143–149. doi: 10.1016/j.plefa.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Fontani G, Corradeschi F, Felici A, Alfatti F, Migliorini S, Lodi L. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. European Journal of Clinical Investigation. 2005;35(11):691–699. doi: 10.1111/j.1365-2362.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 99.Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. The British journal of nutrition. 2008;99(2):421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 100.Antypa N, Van der Does AJ, Smelt AH, Rogers RD. Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. Journal of psychopharmacology (Oxford, England) 2009;23(7):831–840. doi: 10.1177/0269881108092120. [DOI] [PubMed] [Google Scholar]
- 101.Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C, et al. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. The American journal of clinical nutrition. 2013;97(5):1134–1143. doi: 10.3945/ajcn.112.053371. [DOI] [PubMed] [Google Scholar]
- 102.Jackson PA, Deary ME, Reay JL, Scholey AB, Kennedy DO. No effect of 12 weeks' supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18-35 years. The British journal of nutrition. 2012;107(8):1232–1243. doi: 10.1017/S000711451100403X. [DOI] [PubMed] [Google Scholar]
- 103.Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, et al. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32(6):1538–1544. doi: 10.1016/j.pnpbp.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 104.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Archives of neurology. 2006;63(10):1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 105.Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, et al. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neuroscience research. 2006;56(2):159–164. doi: 10.1016/j.neures.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 106.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 107.Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. The American journal of clinical nutrition. 2010;91(6):1725–1732. doi: 10.3945/ajcn.2009.29121. [DOI] [PubMed] [Google Scholar]
- 108.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010 doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 109.Vakhapova V, Cohen T, Richter Y, Herzog Y, Korczyn AD. Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo-controlled trial. Dementia and geriatric cognitive disorders. 2010;29(5):467–474. doi: 10.1159/000310330. [DOI] [PubMed] [Google Scholar]
- 110.Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, et al. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. The British journal of nutrition. 2012;107(11):1682–1693. doi: 10.1017/S0007114511004788. [DOI] [PubMed] [Google Scholar]
- 111.Stough C, Downey L, Silber B, Lloyd J, Kure C, Wesnes K, et al. The effects of 90-day supplementation with the omega-3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiology of aging. 2012;33(4):824 e821–823. doi: 10.1016/j.neurobiolaging.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 112.Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, et al. Long-Chain Omega-3 Fatty Acids Improve Brain Function and Structure in Older Adults. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht163. [DOI] [PubMed] [Google Scholar]
- 113.Geleijnse JM, Giltay EJ, Kromhout D. Effects of n-3 fatty acids on cognitive decline: a randomized, double-blind, placebo-controlled trial in stable myocardial infarction patients. Alzheimers Dement. 2012;8(4):278–287. doi: 10.1016/j.jalz.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 114.Mazereeuw G, Lanctot KL, Chau SA, Swardfager W, Herrmann N. Effects of omega-3 fatty acids on cognitive performance: a meta-analysis. Neurobiology of aging. 2012;33(7):1482 e1417–1429. doi: 10.1016/j.neurobiolaging.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 115.Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. The Cochrane database of systematic reviews. 2012;6:CD005379. doi: 10.1002/14651858.CD005379.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial - comments by Zhang and Li. British Journal of Nutrition. 2008;100:1347–1378. doi: 10.1017/S0007114508975644. [DOI] [PubMed] [Google Scholar]
- 117.Horrobin D, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biological psychiatry. 2001;49(37S) [Google Scholar]
- 118.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 119.Salem N, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36(9):945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 120.Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. The American journal of clinical nutrition. 2007;86(6):1621–1625. doi: 10.1093/ajcn/86.5.1621. [DOI] [PubMed] [Google Scholar]
- 121.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. The American journal of psychiatry. 2002;159(3):477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 122.Schaie KW. The course of adult intellectual development. Am Psychol. 1994;49(4):304–13. doi: 10.1037//0003-066x.49.4.304. [DOI] [PubMed] [Google Scholar]