Abstract
The class I phosphoinositide 3-kinase (PI3K) - mechanistic target of rapamycin complex 1 (mTORC1) signaling network directs cellular metabolism and growth. Activation of mTORC1, which is composed of mTOR, Raptor, mLST8, PRAS40, and DEPTOR, depends on the Rag and Rheb GTPases, and requires signals from amino acids, glucose, oxygen, energy (ATP), and growth factors (including cytokines and hormones such as insulin). Here we discuss the signal transduction mechanisms through which growth factor-responsive PI3K signaling activates mTORC1. We focus on how PI3K-dependent activation of Akt and spatial regulation of the TSC complex (composed of TSC1, TSC2, and TBC1D7) switches on Rheb at the lysosome, where mTORC1 is activated. Integration of PI3K- and amino acid-dependent signals upstream of mTORC1 at the lysosome is detailed in a working model. A coherent understanding of the PI3K-mTORC1 network is imperative as its dysregulation has been implicated in diverse pathologies including cancer, diabetes, autism, and aging.
Keywords: Insulin, TSC2, Rheb, Rag, Raptor, Lysosome
Introduction to PI3K and mTORC1 signaling
Signaling networks endow cells with the ability to sense their internal and external environment in an integrated manner and mount coordinated responses involving processes such as growth, proliferation, survival, and differentiation. Cells of multicellular organisms must simultaneously take into account intracellular levels of nutrients and stress, cell-cell contacts, and organismal metabolism and stress conditions. The relay of such information between cells and tissues is mediated in part by secreted ligands including growth factors, hormones, cytokines, and chemokines (referred to here as growth factors for simplicity) that bind receptor tyrosine kinases (RTKs) or G-protein coupled receptors (GPCRs) at the cell surface and thereby trigger intracellular signaling. One of the key signaling enzymes proximally activated by these receptors is the lipid kinase, class I phosphoinositide 3-kinase (PI3K) [1,2]. Here we exclusively discuss signaling downstream of class I and not class II or III PI3Ks, which are distinct in their structures, regulation, and functions [3]. PI3K, which functions as a heterodimer of catalytic and regulatory subunits, phosphorylates the inositol ring of the membrane phospholipid, phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2), to generate phosphatidylinositol-3,4,5-trisphosphate (PIP3) at the cytoplasmic face of the plasma membrane [3]. This activity of PI3K is counteracted by phosphatase and tensin homologue deleted on chromosome 10 (PTEN), which converts PIP3 back to PI-4,5-P2, and inositol-5-phosphatases including SH2-domain containing inositol phosphatase (SHIP), which convert PIP3 to PI-3,4-P2 [4]. Due to the activity of inositol-5-phosphatases, stimulation of PI3K can also result in the acute production of PI-3,4-P2. PI3K-generated PIP3 precipitates signaling cascades by recruiting a subset of pleckstrin homology (PH) domain-containing signaling proteins, such as the protein kinase Akt, to the plasma membrane through a specific interaction between PIP3 and the PH domain [4]. PI-3,4-P2 can also robustly bind a subset of PH-domain proteins including Akt and therefore can contribute to PI3K signaling in some contexts [4]. The various upstream events that can lead to activation of PI3K and its effectors are discussed in more detail in several recent reviews [1-4]. Through Akt and its other downstream effectors, PI3K functions as a major regulator of cellular metabolism, survival, growth, proliferation, and motility, and dysregulation of signaling downstream of PI3K has been implicated in numerous and diverse human pathologies [2,5].
One of the key regulators of metabolism and growth activated downstream of PI3K is the protein kinase complex, mechanistic target of rapamycin (mTOR) complex 1 (mTORC1). The kinase mTOR functions within two distinct multi-protein complexes designated mTORC1 and mTORC2, that differ in their subunit composition, upstream inputs, and downstream substrates and functions [5,6]. Here we focus on the regulation of mTORC1 which consists of the core subunits mTOR, regulatory-associated protein of mTOR (Raptor), and mammalian lethal with SEC13 protein 8 (mLST8), plus two endogenous inhibitors of the complex, 40kDa Proline-rich Akt substrate (PRAS40) and DEP domain-containing mTOR-interacting protein (DEPTOR) [5,6]. Through transcriptional, translational, and post-translational mechanisms mediated by its substrates, including ribosomal protein S6 kinase (S6K) and eukaryotic translation initiation factor 4E–binding protein (4E-BP), mTORC1 stimulates the biosynthesis of three major classes of macromolecules: proteins, lipids, and nucleic acids [7]. In addition, mTORC1 can promote the production of energy (ATP), reducing cofactors (NAPDH), and certain macromolecule precursors required for biosynthesis [7]. Finally, mTORC1 signaling inhibits the breakdown of lipids via lipolysis and β-oxidation, and the bulk degradation of cytoplasmic constituents via autophagy, presumably to prevent futile cycles of synthesis and degradation [8,9]. Cumulatively, this promotion of anabolic activity underlies the effects on cell, tissue, and organismal growth for which the pathway is best known. This central node of metabolic control is intimately linked to pathways that sense secreted growth factors as well as the cellular abundance of amino acids, glucose, oxygen, and energy which are concomitantly required for full activation of mTORC1 [10]. Growth factors and amino acids have an especially acute effect on mTORC1 activation, and it has come to be appreciated that these two inputs act through parallel, largely independent pathways [11-13]. We discuss the molecular events downstream of PI3K that lead to activation of mTORC1, with emphasis on how growth factor-stimulated PI3K signaling and amino acid signaling are integrated at the lysosome, where mTORC1 is activated.
The primary pathway from PI3K to mTORC1: switching on Rheb
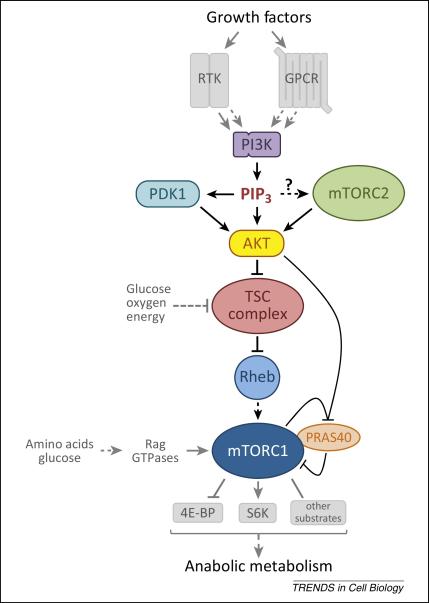
A little more than a decade ago, the PI3K-Akt pathway and mTOR pathway were both known to be important, growth factor-sensitive regulators of protein synthesis and cell growth, but whether they functioned within a linear pathway or in parallel remained an unresolved question [14]. Genetic and biochemical studies unified these pathways through identification of two missing links between Akt and mTORC1: the small GTPase Ras homolog enriched in brain (Rheb), and its negative regulator, the tuberous sclerosis complex (TSC) complex (the TSC complex) [15-32]. These, and subsequent studies, have shown that regulation of a switch involving Akt, the TSC complex, and Rheb, is the primary mechanism through which PI3K signaling activates mTORC1 (Figure 1).
Figure 1.
The primary pathway through which class I PI3K activates mTORC1. Growth factors (including hormones, cytokines, and chemokines) activate RTKs or GPCRs which, through a variety of mechanisms, activate PI3K. PI3K generates PIP3 which specifically binds Akt and PDK1 promoting the phosphorylation and activation of Akt by PDK1. Phosphorylation of Akt by mTORC2 boosts its activity several-fold and mTORC2 activation is at least partially PI3K-dependent. Akt inhibits the TSC complex, the specific GAP for the small GTPase Rheb, through multi-site phosphorylation of the TSC2 subunit. This relieves inhibition of Rheb, allowing it to become activate and stimulate mTORC1 kinase activity. Once mTORC1 is activated by Rheb, the simultaneous phosphorylation of its inhibitory subunit PRAS40 by Akt and mTORC1 itself causes PRAS40 to dissociate from mTORC1. This is thought to increase substrate access to the complex. Glucose, oxygen, and energy levels are also sensed upstream of the TSC complex. Amino acids (and glucose) are sensed upstream of mTORC1 via pathways that regulate the Rag GTPases, which do not directly activate mTORC1 but serve to bring it into proximity with Rheb in cells. mTORC1 directly phosphorylates numerous substrates including S6K and 4E-BP which mediate its control of anabolic metabolism, cellular growth, and proliferation.
Direct activation of mTORC1 by Rheb
Rheb, which is essential for development in both flies and mice, is a potent activator of mTORC1 [25,33,34]. Two Rheb family members, Rheb1 and Rheb2 (also known as RhebL1) are found in mammals, and although Rheb1 is the essential isoform in mice and appears to be the dominant regulator of mTORC1, both isoforms are ubiquitously expressed [35,36] and can activate mTORC1 [37-39]. In contrast, Rheb does not stimulate mTORC2 kinase activity in vitro or signaling in vivo [39-41]. Active Rheb can indirectly inhibit PI3K and mTORC2 signaling by inducing various mTORC1-dependent negative feedback loops [1,42]. Like all GTPases, Rheb cycles between GTP- and GDP-bound states that differ in conformation and function. GTP-bound Rheb, but not GDP-bound Rheb, robustly stimulates mTORC1 activity [39,43] through what is likely a direct interaction with the kinase domain of mTOR, mLST8, and perhaps Raptor [39,43-45]. This role appears to be unique to Rheb amongst small GTPases [28,31]. If recombinant Rheb is purified from bacteria [39] or mammalian cells [43,45] and loaded with GTP, its subsequent addition to in vitro mTORC1 kinase assays is sufficient to stimulate mTORC1 activity towards its physiological substrates. Likewise mTORC1 only exhibits in vitro kinase activity if co-purified from cells with Rheb mutants that are highly GTP bound, but not those that are nucleotide deficient [44]. Because of the unique role played by Rheb, it is required for activation of mTORC1 in response to both amino acids and growth factors [13].
The evidence that Rheb directly activates mTORC1 is quite strong, but two indirect mechanisms have also been suggested. First, active Rheb has been proposed to competitively bind a putative endogenous inhibitor of mTORC1 known as FK506-binding protein 38 (FKBP38), thereby relieving an inhibitory interaction between FKBP38 and the FKBP12-Rapamycin Binding (FRB) domain of mTOR [45-47]. However, in multiple independent studies, FKBP38 knockdown or overexpression failed to have clear effects on mTORC1 signaling in cells, and its presence was not required for Rheb to stimulate mTORC1 kinase activity in vitro [39,48-50]. Alternatively, Rheb has been proposed to indirectly activate mTORC1 by acutely stimulating phospholipase D1 (PLD1) to produce the lipid, phosphatidic acid (PA) [51,52]. PA, a constituent of membranes and precursor for other lipids, has been argued to allosterically activate mTORC1 by interacting directly with its FRB domain [53] or by displacing FKBP38 from the FRB domain [54]. However, this intermediary role for PA is not supported by the ability of recombinant Rheb to activate purified mTORC1 in vitro [39,43]. Further, unlike mTOR, Raptor, and Rheb, PLD is not essential for development or fertility in flies [55] or mice [56-58]. Two other PA-producing enzymes, diacylglycerol kinase (DGK) and lysophosphatidic acid acyltransferase (LPAAT), have also been reported to influence mTORC1 signaling, but there is no evidence that these act downstream of Rheb [52]. Hence, while PA may play a role in regulating mTORC1, current data suggests that neither PA nor FKBP38 mediate Rheb's effects on mTORC1 and instead GTP-bound Rheb likely activates mTORC1 directly, albeit through a largely undefined mechanism.
Inhibition of Rheb by the TSC complex
The activation states of small GTPases, such as Rheb, are directly controlled by two classes of regulators: GTPase activating proteins (GAPs), which accelerate the hydrolysis of GTPase-bound GTP to GDP, and guanine nucleotide exchange factors (GEFs) which promote dissociation of GDP and reloading of GTP [59]. The only established, direct regulator of Rheb activation is its GAP, the TSC complex, which has no other known physiological substrates [27-29,31]. The specificity of this relationship is derived in part from unique aspects of the TSC2-Rheb interface and GAP mechanism [60-62]. Because Rheb exhibits low intrinsic GTPase activity that favors its active, GTP-bound state, the GAP activity of the TSC complex is critical for the acute conversion of Rheb to its inactive, GDP-bound form [62-64]. Intriguingly, there is evidence that the TSC complex interacts most strongly with Rheb when it is GDP-bound [65]. This raises the possibility that the TSC complex might be able to sequester Rheb-GDP so that it does not reload GTP and activate mTORC1, a capability that would be especially efficient if Rheb GTP loading is otherwise unregulated (see Rheb-GEF discussion below). The critical role the TSC complex plays in restraining Rheb activity is most apparent in TSC complex-deficient cells where Rheb GTP loading [29,66] and mTORC1 signaling [18,24,67] become constitutively elevated and insensitive to growth factors. Genetic ablation of key TSC complex subunits in flies and mice is lethal during development [17,18,68,69], and importantly this lethality can be rescued in flies by genetically or pharmalogically reducing mTORC1 signaling [70-72].
The TSC complex is composed of three subunits: tuberous sclerosis complex 1 (TSC1), TSC2, and Tre2-Bub2-Cdc16-1 domain family member 7 (TBC1D7) (note that “tuberous sclerosis complex” refers to the genetic disorder that results from inactivating mutations in TSC1 or TSC2 while “the TSC complex” refers to the protein complex) [73-75]. Though typically depicted as a simple heterotrimer, accumulating evidence indicates this complex actually has a large oligomeric structure consisting of multiple (perhaps five) copies of each subunit (Figure 2) [65,76,77]. Loss of any TSC complex subunit leads to growth factor-independent mTORC1 activation, but loss of TSC1 or TSC2 has a more pronounced effect than loss of TBC1D7 [75]. TSC2 contains the Rheb-GAP domain which is essential for inhibition of Rheb and mTORC1 [27], while TSC1 acts as a scaffold that binds and stabilizes both TSC2 and TBC1D7 [75,78]. In the absence of TSC1, TSC2 is ineffective at inhibiting mTORC1 signaling and cell growth, in part, because it is more prone to degradation in cells [18,79], but also because it appears to become intrinsically less efficient as a GAP for Rheb (due perhaps to an abnormal conformation) [27]. The function of TBC1D7 is not yet fully understood but, as a core subunit of the TSC complex, its loss leads to a decreased association between TSC1 and TSC2 resulting in decreased total cellular Rheb-GAP activity [75]. Although TBC1D7 has been reported to exhibit GAP activity towards the GTPase Rab17 in vitro [80], this may not be its primary conserved function since a specific Rab17 ortholog does not appear to exist in flies [81] while a Drosophila ortholog of TBC1D7 not only exists but associates with Drosophila TSC1 and TSC2 [82].
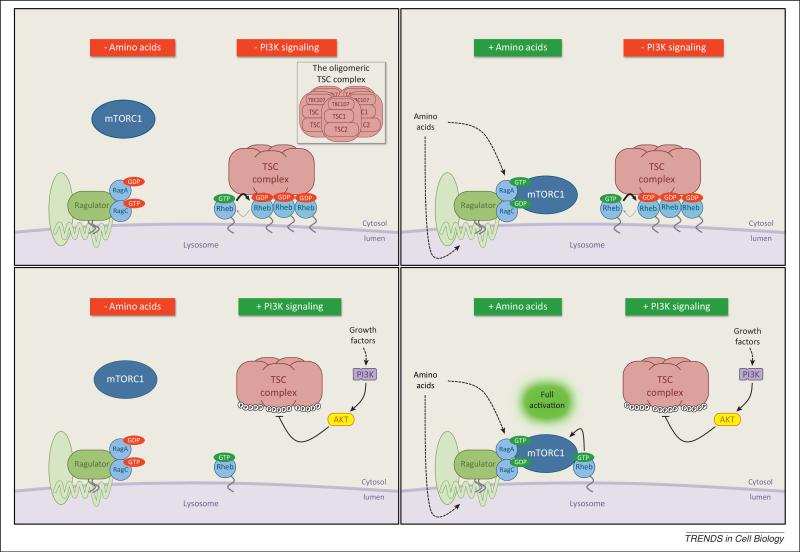
Figure 2.
A working model of mTORC1 activation by PI3K signaling and amino acids at the lysosome. Proper activation of mTORC1 requires both the Rag and Rheb GTPases, which are activated by amino acids and growth factors respectively. These signals are integrated at the cytoplasmic face of the lysosomal membrane where mTORC1 is activated. In the absence of amino acids, mTORC1 localizes away from the lysosome. Amino acid signaling activates the Rag GTPases which directly recruit mTORC1 to the lysosome but do not stimulate its kinase activity. The Rags, which function as heterodimers consisting of A or B with C or D isoforms, associate with the lysosome through their interaction with the membrane-tethered Ragulator complex. The Ragulator also associates with integral membrane proteins and complexes of the lysosome that participate in amino acid sensing (light green, unlabeled). A variety of other amino acid-sensitive regulators of mTORC1 have been identified but are not shown. In the absence of growth factors, the TSC complex, which is the GAP for Rheb, localizes to the lysosome in a Rheb-dependent manner and keeps Rheb inactive. Stimulation with growth factors leads to the PI3K-dependent activation of Akt with then phosphorylates TSC2 on multiple sites within the TSC complex. These phosphorylation events induce dissociation of the intact TSC complex from the lysosome and Rheb, allowing Rheb to become active and directly simulate mTORC1. Hence amino acid signaling to the Rags and growth factor-PI3K signaling to Rheb represent parallel, independent inputs that are each necessary but no sufficient for full activation of mTORC1. The TSC complex is a large oligomeric structure, composed of approximately five copies of each subunit (TSC2, TSC2, and TBC1D7) (inset box). In addition to functioning as a GAP for Rheb and converting it to its inactive GDP-bound state, the TSC complex might also prevent Rheb from activating mTORC1 by sequestering its GDP-bound form. Basal activation of either the Rags or Rheb may result in incomplete effects of amino acid or growth factor starvations on mTORC1 activation.
Despite intense study of mTORC1 regulation, a GEF for Rheb has yet to be established. The chaperone-like, translationally-controlled tumor suppressor protein (TCTP), has been proposed to act as a Rheb-GEF [83,84]. However, this role was not supported by independent studies that failed to detect Rheb-GEF activity for TCTP in vitro [85], and found mTORC1 signaling to be unimpaired in TCTP knockdown or knockout cells [49,85,86]. Two other proteins, Dedicator Of Cytokinesis 7 (DOCK7) [87] and Protein Associated with Myc (PAM) [88] have also been speculated to be Rheb GEFs but there is no indication that these have physiological Rheb-GEF activity. It remains possible that a Rheb GEF simply does not exist and that spontaneous reloading of GTP on Rheb is adequate for its acute activation. This is conceivable considering Rheb's low intrinsic GTPase activity [62-64], its intrinsic potential to release GDP [85,89], and the substantial excess of GTP over GDP in cells [90]. Interestingly, membrane association appears to influence Rheb's intrinsic nucleotide exchange rate [61,89]. Hence, much remains to be revealed about the life-cycle of Rheb but currently the TSC complex is the only firmly established direct regulator of Rheb activation.
Inhibition of the TSC complex by Akt
As the major regulator of Rheb, the TSC complex is the convergence point for many growth and stress signals that control mTORC1, including those indicating the abundance of growth factors, glucose, glutamine, oxygen, and energy (ATP) [10]. In general, the TSC complex inhibits Rheb and mTORC1 when growth factors or energy are limiting. Phosphorylation of TSC2 (and to a lesser extent, TSC1) by kinases that are responsive to these conditions constitutes the primary mode of TSC complex regulation [78]. For instance, phosphorylation of TSC2 by ERK and RSK downstream of the growth factor-responsive Ras-Raf-MEK pathway inhibits the TSC complex and leads to Rheb and mTORC1 activation, while phosphorylation of TSC2 by AMPK and GSK3 in response to energy stress (e.g. due to limiting levels of glucose) spurs the TSC complex to inhibit Rheb and mTORC1 [78]. Downstream of PI3K, Akt directly inhibits the TSC complex through phosphorylation of TSC2 on five residues (S939, S981, S1130, S1132, and T1462) that lie outside of the GAP domain [21-23,78]. Phosphorylation of all five sites is required for full stimulation of mTORC1 [22,91]. In flies, mutation of the Akt-targeted sites on TSC2 (corresponding to S939 and T1462) can block Akt-induced tissue growth [23] but mutation of these phospho-sites is tolerated during fly development even in the absence of PRAS40, another mTORC1 inhibitor regulated by Akt [92,93]. To fully understand the metabolic requirements for Akt-dependent regulation of the TSC complex and mTORC1 (e.g., in response to feeding and insulin production), additional in vivo studies will be required including the generation of mice expressing TSC2 lacking all Akt-targeted phospho-sites.. The direct inhibition of the TSC complex by Akt, is complimented by the ability of Akt to suppress AMPK activity and its positive effects on the TSC complex through phosphorylation of AMPK's catalytic subunit and stimulation of ATP production via glucose metabolism [94]. The mechanisms by which phosphorylation of the TSC complex affect its function remain incompletely understood, but it has recently become clear that spatial regulation of the complex underlies its inhibition by Akt.
Activation of Akt by PI3K
Akt is the primary link between PI3K and the TSC complex upstream of mTORC1. Indeed, inhibition of Akt can be sufficient to block PI3K-dependent activation of mTORC1 in response to insulin or in certain PTEN-deficient cell lines [65]. To become fully active in response to growth factors, all three Akt isoforms found in mammals must be phosphorylated on two conserved residues, T308 and S473 (Akt1 numbering), and this is facilitated by PI3K-generated PIP3 in several respects. Phosphorylation of T308 within the activation segment of the kinase domain is absolutely required for Akt activity and is catalyzed by 3-phosphoinositide-dependent kinase 1 (PDK1). PDK1, like Akt, contains a PH domain, and the binding of PIP3 at the plasma membrane concentrates the constitutively active PDK1 with Akt, but also induces a conformational change in Akt that exposes its activation segment for phosphorylation of T308 [95]. Maximal activation of Akt requires its additional phosphorylation on a carboxy-terminal site, S473, by mTORC2. Phosphorylation of S473 on its own is not sufficient to promote Akt activity, but boosts it up to ten-fold by promoting a conformational change that stabilizes the active conformation of the kinase domain and T308 phosphorylation [96]. Akt activity is also enhanced through phosphorylation of two other carboxy-terminal sites, S477 and T479, by mTORC2 in response to growth factors, or by cyclin-dependent kinases in a cell cycle-dependent manner [97]. In addition, mTORC2 is required for co-translational phosphorylation of the conserved residue, Akt-T450, which constitutively stabilizes Akt [98]. Hence, mTORC2-dependent phosphorylation of Akt plays a stabilizing role, and although it is not essential for all levels of Akt activity, numerous studies indicate that these phospho-sites are required for the full spectrum of physiological PI3K-Akt signaling to mTORC1 (Box 1). There is evidence that mTORC2 activity is stimulated in a PI3K-dependent manner, but the underlying mechanism is incompletely understood [41,99-102]. Because PDK1 is constitutively active and mTORC2 activity is at least partially PI3K-dependent, the primary growth factor-regulated event upstream of Akt activation is the production of PIP3 by PI3K. At the level of Akt, PI3K signaling is opposed by protein phosphatase 2A (PP2A) [103] and PH domain leucine-rich repeat protein phosphatase (PHLPP) [104] which dephosphorylate Akt-T308 and -S473 respectively, plus the inositol phosphate IP7 which inhibits Akt by binding its PH domain [105].
In summary, the Akt-TSC complex-Rheb axis serves as a PI3K-dependent switch for turning mTORC1 on and off in response to growth factors and constitutes the primary mechanism by which PI3K signaling controls mTORC1.
Secondary PI3K-dependent events in mTORC1 activation: phosphorylation of mTORC1 subunits
PRAS40 phosphorylation
In addition to activating mTORC1 by stimulating GTP loading on Rheb, PI3K signaling negatively regulates PRAS40, an inhibitory subunit of mTORC1. PRAS40, which binds and inhibits mTORC1 under conditions of low PI3K-mTORC1 signaling, is thought to compete with other substrates for their interaction with Raptor, the substrate-binding subunit of mTORC1 [43,106,107]. When PI3K-mTORC1 signaling is stimulated, PRAS40 dissociates from mTORC1. This requires simultaneous phosphorylation of PRAS40 on T246 by Akt, and on S183 and S221 by mTORC1 itself [108,109]. Because mTORC1 activity is required for PRAS40 dissociation, this event is inherently downstream of Rheb activation. But, under some conditions, mTORC1 can be robustly activated by Rheb without PRAS40 phosphorylation at T246 or dissociation from mTORC1. This can occur for instance in response to ERK signaling [110], in TSC complex-deficient cells [43], or upon overexpression of Rheb [109]. Rheb-GTP can also overcome inhibition of mTORC1 by PRAS40 in in vitro kinase assays [43]. Reflecting the distinct roles of the TSC complex-Rheb axis and PRAS40 in regulating mTORC1, genetic ablation of TSC1 or TSC2 is lethal during development in flies and mice while ablation of PRAS40 is tolerated [93,111]. Thus, Rheb activation and PRAS40 inhibition are not redundent mechanisms for activating mTORC1 but instead perform complimentary roles with Rheb activity being required for PRAS40 dissociation. Incidentally, the other inhibitory subunit of mTORC1, DEPTOR, is degraded following its mTORC1-dependent phosphorylation and therefore its role in activating mTORC1 also requires Rheb activity [112].
mTOR and Raptor phosphorylation
Several residues on mTOR and Raptor within mTORC1 undergo PI3K-dependent phosphorylation that has been reported to promote mTORC1 activity. On mTOR, S1261 is phosphorylated by an unknown Rheb-dependent kinase [113], while another site, S1415, is reportedly phosphorylated by IKKα downstream of Akt [114]. Non-phosphorylatable mutations at either site dampen mTORC1 activity. Although the first phospho-sites to be identified on mTOR are also PI3K-responsive, including the S6K-targeted sites T2446/S2448 [115] and mTOR autophosphorylation site S2481 [116], they have no effect on mTORC1 activity and no known function. It is important to note that all of these sites are known to be phosphorylated within both mTORC1 and mTORC2, except for S1415, which has yet to be analyzed in mTORC2 [113,116,117]. On Raptor, S863, is phosphorylated by mTOR and additional mTORC1-dependent phospho-sites lie within the same general region of Raptor [118,119]. Mutation of these sites has been found to diminish mTORC1 kinase activity in vitro and downstream signaling in cells [118,119]. But none of these acutely PI3K-dependent phospho-sites on mTOR or Raptor serve as an on/off switch for mTORC1. Instead, phosphorylation at most of these sites requires Rheb and mTORC1 activity. One potential function of these phospho-sites might be to either directly or indirectly enhance substrate access to mTORC1 once it is activated by Rheb.
The lysosome: key site of mTORC1 regulation by PI3K signaling
The Akt-TSC complex-Rheb axis is the primary link between PI3K and mTORC1. However, multiple mechanisms have been proposed to explain how phosphorylation of TSC2 by Akt prevents the TSC complex from inhibiting Rheb. Akt has been suggested to inhibit the TSC complex by inducing its degradation [120,121] or disassembly [22,23,122,123]. But analyses of endogenous TSC complexes from growth factor-stimulated cells have confirmed that the complex remains stable and intact under conditions where TSC2 is maximally phosphorylated and mTORC1 is robustly activated by PI3K-Akt signaling [21,65,92,124]. Importantly, PI3K-Akt signaling also does not turn off the GAP activity of the TSC complex as indicated by in vitro GAP assays using exogenous [28,122] or endogenous [65] TSC complexes. Instead, recent biochemical and immunolabeling data has revealed that Akt inhibits the TSC complex, without effects on its integrity or GAP activity, by triggering changes in its localization at an endomembrane compartment – the lysosome [65]. Cell fractionation experiments provided the first evidence that Rheb [63], mTOR [125], and TSC1/TSC2 [73] could associate with membranes and that PI3K-Akt signaling could alter the relationship of TSC1/TSC2 with membranes [122,124]. Ultimately, through efforts to understand mechanisms of amino acid sensing upstream of mTORC1, the cytoplasmic face of the lysosomal membrane was identified as a pivotal site for mTORC1 activation by both amino acids and growth factors [13].
A subpopulation of Rheb, which tethers to membranes through a farnesyl lipid modification, resides at the lysosome [65,126-128]. Farnesylation of Rheb provides a relatively weak membrane anchor but is essential for robust mTORC1 signaling [27]. At the lysosome, amino acid and growth factor signals activate mTORC1 by controlling mTORC1-Rheb colocalization and Rheb activation respectively. In the presence of amino acids, mTORC1 is recruited to the lysosome through an interaction with the Rag GTPases (Box 2) [11,13]. Rag binding does not directly activate mTORC1 but instead serves to bring it into proximity with lysosomally localized Rheb. A major pool of the TSC complex has also been identified at the lysosome and dynamic regulation of its localization here holds the key to Rheb activation [65,75,128,129]. In the absence of growth factors, the TSC complex accumulates at the lysosome [65,75]. But in response to growth factor-stimulated PI3K signaling, phosphorylation of TSC2 by Akt induces the TSC complex to abruptly dissociate from this compartment and move to an undefined cellular location [65,128]. Importantly, mutating the five Akt-targeted sites on TSC2 to non-phosphorylatable residues, or fusing a lysosomal targeting sequence to TSC2 to lock it at the lysosome, is sufficient to make TSC complex localization and mTORC1 signaling insensitive to growth factor-PI3K signaling [65]. As growth factors do not acutely alter the lysosomal localization of Rheb [65], the acute dissociation of the TSC complex from the lysosome takes it out of proximity with Rheb, allowing Rheb to load GTP and activate mTORC1, given that amino acids are also present to promote the lysosomal localization of mTORC1. In contrast to growth factor-PI3K signaling, at least two studies have found that amino acids do not acutely alter the lysosomal localization of the TSC complex [65,128]. However, whether amino acid signals can acutely influence TSC complex and Rheb function under some conditions is a question that is not yet completely resolved (Box 3).
How the TSC complex associates with the lysosome is not yet fully understood. Despite sequence-based predictions that TSC1 contains a transmembrane domain [130], structural studies do not support this [77] and biochemical evidence suggests neither TSC1 nor TSC2 are integral membrane proteins [131]. Fractionation studies have suggested that TSC1 mediates the association of TSC2 with membranes [122], but knockdowns of TSC1 and TBC1D7 had little effect on the lysosomal localization of TSC2 suggesting that TSC2 itself might mediate association of the TSC complex with membranes [75]. Somewhat surprisingly, Rheb appears to be required for proper localization of the TSC complex to the lysosome [65]. Knocking down Rheb or blocking its farnesylation so that it can no longer associate with membranes results in loss of the TSC complex, but not mTORC1, from lysosomes even in the absence of growth factor-PI3K signaling [65]. Rheb may directly tether the TSC complex to the lysosome as recombinant Rheb (especially when GDP-loaded) binds robustly to endogenous TSC complexes in a cell-free assay but does so with weakened affinity if TSC complexes are isolated from insulin-stimulated cells, an effect blocked by Akt inhibition [65]. Additional studies will be required to clarify the mechanisms through which the TSC complex is spatially regulated at the lysosome and to identify other membrane-associated proteins or lipids that might be required for this dynamic process.
A model of mTORC1 activation by PI3K signaling and amino acids at the lysosome
Insights into mTORC1 regulation continue to emerge at a rapid pace and several recent findings have yielded as many questions as answers. Keeping this in mind, we summarize a working model of mTORC1 activation by PI3K signaling at the lysosome that builds directly off of the established model of amino acid sensing by mTORC1 at this compartment and draws on the groundbreaking work of many groups in delineating the components and wiring of the pathway [1-4,6,11-13](Figure 2).
First, mTORC1 must be recruited to its site of activation, the lysosome. This is mediated by Rag GTPase heterodimers that are tethered to the lysosome through their association with the Ragulator complex and that are switched to an active conformation in response to amino acids. In their active state, Rag heterodimers directly bind mTORC1 and localize it to the lysosome without directly stimulating its kinase activity [12,13]. The lysosomal localization of mTORC1 is not acutely sensitive to growth factors and does not depend on Rheb [65,132,133]. Second, Rheb must be activated and then activate mTORC1. Rheb is tethered directly to the lysosome and this localization is not acutely sensitive to amino acids and growth factors [65,127]. The TSC complex localizes to the lysosome in a Rheb-dependent manner when growth factors are absent, keeping Rheb “inactive” [65]. In response to growth factor-stimulated PI3K signaling, the intact TSC complex dissociates from Rheb at the lysosome allowing Rheb to become active and directly activate mTORC1 [65,128]. The lysosomal localization of the TSC complex is not acutely sensitive to amino acids [65,128]. These first two steps are largely independent of one another and so do not necessarily occur in a particular order. Third, phosphorylation events on mTORC1 subunits increase substrate access to the active complex. For instance, phosphorylation of PRAS40 by Akt and mTORC1 induces its dissociation from mTORC1, allowing increased Raptor binding by other substrates [108,109]. Since this third step requires mTORC1 kinase activity, it follows the first two Rag and Rheb dependent steps, which principally promote mTORC1 activation.
In this model, the Rags and Rheb, constitute parallel, independent inputs that are each necessary but not individually sufficient for mTORC1 activation in cells. Since, in this model, amino acids primarily activate the Rags and growth factor-PI3K signaling primarily activates Rheb, these stimuli likewise represent largely independent signals that are necessary but not individually sufficient to fully activate mTORC1. Under some conditions, amino acids may appear to be sufficient for mTORC1 activation even when exogenous growth factors are absent. But in such cases, Rheb may be basally active, for instance, due to culture conditions, autocrine signaling, mutations within upstream pathways, or perhaps because a subpopulation of Rheb may be inaccessible to the TSC complex at an one time. Mutations in the amino acid sensing machinery (e.g. in the RagA/B GAP complex, GATOR1) can also occur and result in constitutive, amino acid-independent activation of mTORC1 that makes growth factors sufficient to activate mTORC1 [134]. However, spontaneous activating mutations in amino acid-sensing pathways upstream of the Rags appear to be less common than activating mutations in growth factor signaling pathways upstream of Rheb [134], and this may be one reason why amino acid withdrawal more consistently leads to a near complete inactivation of mTORC1 in cell culture, compared to growth factor withdrawal.
The above model emphasizes the acute effects of amino acids and growth factor-PI3K signaling that lead to the rapid activation of mTORC1 at the lysosome. However, the broad effects of growth factors on cells and the diverse roles amino acids play in metabolism ensure that these stimuli will exert more complex secondary effects on mTORC1-regulating events at the lysosome, especially at longer time scales. There are also reports of mTORC1 regulation at other membranous compartments and it will be important to clarify the roles of these sites relative to that of the lysosome (Box 4). Finally, one should keep in mind that the dynamics of growth factor and nutrient levels, Rheb and Rag activation, and other steps of mTORC1 regulation are likely to vary between physiological states and tissues, and therefore the limiting input upstream of mTORC1 may be context-dependent in vivo [93,135].
Concluding Remarks
Understanding at a fine scale how signals are transduced through the PI3K-mTORC1 pathway can shed light on physiological processes such as insulin-regulated glucose metabolism, and pathological conditions such as cancer and diabetes. Here we have focused on how growth factor-stimulated PI3K signaling activates Rheb and is integrated with nutrient signaling at the lysosome to activate mTORC1. We do not yet completely understand how key pathway components traffic to the lysosome, how the TSC complex associates with the lysosomal membrane, where mTORC1 or the TSC complex reside when dissociated from the lysosome, or whether other signals such as energy stress control TSC complex localization. Continuing to probe the location of signaling events will be key to further advances in our understanding of this signaling network.
Box 1. Does mTORC2 actually function upstream of mTORC1?
Questions have been raised concerning the role of mTORC2 upstream of mTORC1 since in some mTORC2-deficient settings, Akt-T308 phosphorylation and mTORC1 signaling can be maintained at near-normal levels [136,137]. Certainly, the initial phosphorylation of T308 does not require prior S473 phosphorylation, and the activity of Akt phosphorylated on T308 alone may be sufficient for a subset of its physiological roles. However, numerous complimentary studies support a role for Akt-S473 phosphorylation in increasing or maintaining T308 phosphorylation, Akt activity, and mTORC1 signaling. Supportive data from these studies includes depletions of the core mTORC2 component Rictor [99,100,138], acute inhibition of mTORC2 with mTOR catalytic inhibitors [112,139,140], knockdowns of the S473 phosphatase PHLPP [104], mutation of S473 [141], and structural studies of Akt [142]. Under some conditions, the effects of mTORC2 loss on Akt activity and signaling may be obscured by compensatory mechanisms. For instance, in mTORC2-deficient cells, Akt can be stabilized by the chaperone Hsp90 [143] which can block Akt degradation and protect T308 from dephosphorylation [144]. Also, kinases closely related to Akt may redundantly phosphorylate shared substrates [96]. Similarly, ERK-RSK signaling, which can activate mTORC1 independently of Akt, could contribute to mTORC1 signaling in mTORC2-deficient cells and tissues [78]. Although mTORC2 is not the most critical link in the PI3K-mTORC1 pathway, the available data in aggregate suggests that mTORC2-dependent phosphorylation of Akt is required for the full range of PI3K-dependent signaling to mTORC1.
Box 2. Amino acid signaling, the Rag GTPases, and mTORC1 activation at the lysosome.
A sufficient supply of free cellular amino acids is essential for full activation of mTORC1. While discussed briefly here, the complex amino acid sensing mechanisms upstream of mTORC1 are covered in more detail in several recent reviews [11-13,145]. When amino acids are depleted from cells, mTORC1 is largely inhibited and reintroduction of amino acids rapidly activates mTORC1. Underlying this response are acute changes in mTORC1 localization at the lysosome, a key site of mTORC1 activation [13]. In the absence of amino acids, mTORC1 assumes a diffuse localization pattern that is cytoplasmic but otherwise undefined. The addition of amino acids induces a rapid accumulation of mTORC1 at the cytoplasmic surface of the lysosome [127,132]. In contrast, growth factors do not have a major, acute effect on mTORC1 localization [65,127]. These amino acid-sensitive changes in mTORC1 localization are mediated through Rheb-independent mechanisms involving a second family of small GTPases, the Rags [72,127]. Of note, glucose sensing upstream of mTORC1 also appears to be partially Rag-dependent [146]. The Rag family includes two types of isoforms (A/B and C/D) that function as a heterodimer (e.g. between A and C isoforms). These Rag heterodimers bind a multi-subunit complex known as the Ragulator which is constitutively tethered to the lysosome through lipid modifications on one of its subunits [132,147]. In response to amino acids, the Rag heterodimers undergo a switch in their nucleotide binding states and assume an “active” conformation that specifically binds mTORC1 through Raptor [72,127]. This interaction, which is essential for robust mTORC1 activation in cells, recruits mTORC1 to the lysosomal surface but does not directly stimulate its kinase activity [127]. Instead, mTORC1 is directly activated by Rheb, a subpopulation of which localizes to the lysosome [65,126-128] in an manner that has been reported to be insensitive to acute amino acid and growth factor treatments [65,127]. Changes in the activation state of Rheb or its depletion from the lysosome does not have a major effect on the lysosomal localization of mTORC1 [65,132,133]. Therefore, amino acid signaling ultimately activates the Rag GTPases which bring mTORC1 into proximity with its activator Rheb at the cytoplasmic surface of the lysosomal membrane. A variety of proteins and complexes have been found to participate in regulation of the Rag heterodimers and mTORC1 in response to amino acids and several of these also localize to the lysosome [13,145]. In addition, other small GTPases continue to emerge as mTORC1 regulators though their roles are currently less well established [145,148]. In general, mounting evidence suggests that multiple amino acid sensors and pathways may function upstream of the Rags and mTORC1 [145].
Box 3. Is the TSC complex-Rheb axis influenced by amino acids?
In TSC complex-deficient cells, growth factor withdrawal fails to inhibit mTORC1 while amino acid withdrawal remains effective at suppressing mTORC1 signaling [24,26,66,132,149,150]. This indicates that mTORC1 responds to amino acids through TSC complex-independent mechanisms, and we now know these involve the Rag GTPases and other amino acid sensing machinery acting in parallel to Rheb [12,13,145]. However, the sensitivity of mTORC1 signaling to amino acid withdrawal is variable in TSC complex-deficient cells compared to wild-type cells. This may simply reflect the increased likelihood in TSC complex-deficient cells (even those starved of amino acids) that mTORC1 will randomly encounter Rheb-GTP. Indeed, Rheb overexpression, which also increases chance encounters between mTORC1 and activated Rheb, is sufficient to activate mTORC1 in amino acid starved cells [25-28].
However, recently it has been argued that the TSC complex-Rheb axis participates specifically in amino acid sensing, along with the Rags, upstream of mTORC1 [129]. In this model, in the presence of amino acids, active Rag heterodimers and active Rheb both tether mTORC1 to the lysosome, while Rheb stimulates mTORC1 activity. When amino acids are withdrawn, inactive Rag heterodimers disengage mTORC1 and then recruit and tether the TSC complex to the lysosome to inactivate Rheb (even if growth factors are present). Inactive Rheb then disengages mTORC1 completing its release from the lysosome. Hence, in this model, the TSC complex-Rheb axis plays a key role in amino acid sensing upstream of mTORC1, and the Rags and Rheb do not represent independent inputs into mTORC1 because they are simultaneously regulated by amino acids.
In contrast, other studies have found that amino acid treatments (and accompanying changes in Rag activity) do not acutely affect lysosomal localization of the TSC complex [65,128]. Also, in other studies, altering Rheb activity, blocking Rheb farnesylation, or depleting Rheb from cells did not significantly affect mTORC1 localization at the lysosome [65,127,132,133]. Data from these studies suggests that the TSC complex-Rheb axis does not play a major role in acutely responding to amino acids, that Rheb does not tether mTORC1 to the lysosome, and that the Rags and Rheb represent independent inputs upstream of mTORC1. Recently it was also proposed that amino acid signaling regulates Rheb localization to the lysosome [128]. Again, this contrasts with other data that the lysosomal localization of Rheb is insensitive to acute amino acid and growth factor treatments [65,127]. Molecular connections between the growth factor and amino acid sensing pathways upstream of mTORC1 may certainly exist, but further studies are required to clarify the effects of amino acids on the TSC complex-Rheb axis. In these studies, it will be important to monitor known phosphorylation events on the TSC complex that might affect its localization and function. For instance, some experimental paradigms used for starvation and readdition of amino acids can have unexpected effects on PI3K-Akt signaling perhaps as part of a non-specific stress response [151].
Box 4. Is mTORC1 activated at sites other than the lysosome?
In addition to the lysosome, the peroxisome and golgi complex, have been proposed to be sites of signaling to mTORC1. In one study, the TSC complex and Rheb were reported to predominantly localize to the peroxisome with very little TSC complex detected at the lysosome [123]. At the peroxisome, PI3K-Akt signaling was proposed to inhibit the TSC complex by inducing the dissociation of TSC2 from TSC1 at the peroxisomal surface. This would allow Rheb to become active at the peroxisome and active mTORC1 at an undefined compartment (mTORC1 was not reported to localize to peroxisomes). Conversely, reactive oxygen species from the peroxisomal matrix were proposed to activate the TSC complex and inhibit Rheb. This contrasts with studies that have found little if any TSC2 at the peroxisome [65] and a major, growth factor-regulated pool of the TSC complex at the lysosome [65,75,128]. It is also not immediately clear how to reconcile the finding that most Rheb localizes to and is regulated at the peroxisome, with evidence that Rheb activates mTORC1 at the lysosome [13]. To clarify the roles of the peroxisome and lysosome in mTORC1 signaling it will be interesting to determine whether mTORC1 and any amino acid-sensing machinery also localizes to the peroxisome, or whether the TSC complex-Rheb axis regulated at the peroxisome controls mTORC1 activation at the lysosome.
In another study, the primary site of mTORC1 activation was proposed to be the golgi complex [152]. Here, the GTPase Rab1A was proposed to load GTP and then recruit mTORC1 to the membrane in response to amino acids for activation by golgi-associated Rheb. Colocalization of mTORC1 with Rheb was shown to occur largely at the golgi with very little Rheb at the lysosome. Again, this contrasts with data that the lysosome is a major site of mTORC1 activation by both amino acids and growth factors [13]. The effect of growth factors on golgi-associated Rheb was not explored, but in another study very little TSC2 was detected at the golgi suggesting it is not a major site for growth factor regulation of Rheb and mTORC1 [65].
Although it was argued that Rab1A and the Rags operate in parallel and regulate independent pools of mTORC1, Rab1A knockdowns severely inhibited Rag-induced mTORC1 signaling and vice versa [152]. Therefore, whether Rab1A and the Rags regulate the same pool of mTORC1 warrants a more thorough exploration. Many lysosomal constituents traverse the endoplasmic reticulum (ER) and golgi complex [153], and Rheb is known to undergo post-farnesylation processing on the ER [154]. Considering Rab1 regulates trafficking from the ER to the golgi and perhaps other endomembranes [155], one possibility is that Rab1A might be required for the trafficking of certain proteins to or from the lysosome that are required for proper mTORC1 signaling. Therefore, the relationship between Rab1A and the Rags, and between the golgi complex and the lysosome in mTORC1 signaling requires further clarification.
Highlights.
mTORC1 activity requires the Rag and Rheb GTPases and signals from amino acids and growth factors
Growth factor-stimulated PI3K-Akt signaling activates Rheb and mTORC1 at the lysosome
Amino acid signaling promotes mTORC1-Rheb colocalization at the lysosome
Akt activates Rheb by inducing dissociation of its GAP, the TSC complex, from the lysosome
Acknowledgements
We thank Alex Toker, Brendan Manning, Kristin Brown, and Evan Lien for helpful comments. This work was supported by National Institutes of Health grants K99-CA194314 (C.C.D.) and R01-GM041890 (L.C.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke JE, Williams RL. Synergy in activating class I PI3Ks. Trends Biochem Sci. 2015;40:88–100. doi: 10.1016/j.tibs.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Thorpe LM, et al. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanhaesebroeck B, et al. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 5.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howell JJ, et al. A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans. 2013;41:906–912. doi: 10.1042/BST20130041. [DOI] [PubMed] [Google Scholar]
- 8.Ricoult SJH, Manning BD. The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep. 2013;14:242–251. doi: 10.1038/embor.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlop EA, Tee AR. mTOR and autophagy: A dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.08.006. DOI: 10.1016/j.semcdb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SG, et al. Nutrient regulation of the mTOR complex. 1 signaling pathway. Mol. Cells. 2013;35:463–473. doi: 10.1007/s10059-013-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jewell JL, et al. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014 doi: 10.1016/j.tcb.2014.03.003. DOI: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Adv Cancer Res. 2002;86:1–39. doi: 10.1016/s0065-230x(02)86001-8. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapon N, et al. The Drosophila Tuberous Sclerosis Complex Gene Homologs Restrict Cell Growth and Cell Proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 17.Potter CJ, et al. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–368. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 18.Kwiatkowski DJ, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 19.Goncharova EA, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 20.Tee AR, et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning BD, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 22.Inoki K, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 23.Potter CJ, et al. Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat. Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 25.Stocker H, et al. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat. Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 26.Saucedo LJ, et al. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 27.Tee AR, et al. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 28.Inoki K, et al. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garami A, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 30.Castro AF, et al. Rheb Binds Tuberous Sclerosis Complex 2 (TSC2) and Promotes S6 Kinase Activation in a Rapamycin- and Farnesylation-dependent Manner. J Biol Chem. 2003;278:32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 32.Patel PH, et al. Drosophila Rheb GTPase is required for cell cycle progression and cell growth. J Cell Sci. 2003;116:3601–3610. doi: 10.1242/jcs.00661. [DOI] [PubMed] [Google Scholar]
- 33.Goorden SMI, et al. Rheb is essential for murine development. Mol Cell Biol. 2011;31:1672–1678. doi: 10.1128/MCB.00985-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou J, et al. Rheb1 is required for mTORC1 and myelination in postnatal brain development. Dev Cell. 2011;20:97–108. doi: 10.1016/j.devcel.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gromov PS, et al. A novel approach for expression cloning of small GTPases: identification, tissue distribution and chromosome mapping of the human homolog of rheb. FEBS Lett. 1995;377:221–226. doi: 10.1016/0014-5793(95)01349-0. [DOI] [PubMed] [Google Scholar]
- 36.Yuan J, et al. Identification and characterization of RHEBL1, a novel member of Ras family, which activates transcriptional activities of NF-kappa B. Mol Biol Rep. 2005;32:205–214. doi: 10.1007/s11033-005-0984-x. [DOI] [PubMed] [Google Scholar]
- 37.Tabancay AP, et al. Identification of dominant negative mutants of Rheb GTPase and their use to implicate the involvement of human Rheb in the activation of p70S6K. J Biol Chem. 2003;278:39921–39930. doi: 10.1074/jbc.M306553200. [DOI] [PubMed] [Google Scholar]
- 38.Tee AR, et al. Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1. FEBS Lett. 2005;579:4763–4768. doi: 10.1016/j.febslet.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 39.Sato T, et al. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009;284:12783–12791. doi: 10.1074/jbc.M809207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q, et al. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci USA. 2006;103:6811–6816. doi: 10.1073/pnas.0602282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang J, et al. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sancak Y, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Long X, et al. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 45.Dunlop EA, et al. Mammalian target of rapamycin complex 1-mediated phosphorylation of eukaryotic initiation factor 4E-binding protein. 1 requires multiple protein-protein interactions for substrate recognition. Cell Signal. 2009;21:1073–1084. doi: 10.1016/j.cellsig.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 46.Bai X, et al. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 47.Ma D, et al. The switch I region of Rheb is critical for its interaction with FKBP38. J Biol Chem. 2008;283:25963–25970. doi: 10.1074/jbc.M802356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maehama T, et al. RalA functions as an indispensable signal mediator for the nutrient-sensing system. J Biol Chem. 2008;283:35053–35059. doi: 10.1074/jbc.M805822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, et al. Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem. 2008;283:30482–30492. doi: 10.1074/jbc.M803348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uhlenbrock K, et al. Reassessment of the role of FKBP38 in the Rheb/mTORC1 pathway. FEBS Lett. 2009;583:965–970. doi: 10.1016/j.febslet.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, et al. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci USA. 2008;105:8286–8291. doi: 10.1073/pnas.0712268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foster DA, et al. Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR). Journal of Biological Chemistry. 2014;289:22583–22588. doi: 10.1074/jbc.R114.566091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang Y, et al. Phosphatidic Acid-Mediated Mitogenic Activation of mTOR Signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 54.Yoon M-S, et al. Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect. Journal of Biological Chemistry. 2011;286:29568–29574. doi: 10.1074/jbc.M111.262816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaLonde M, et al. A role for Phospholipase D in Drosophila embryonic cellularization. BMC Dev. Biol. 2006;6:60. doi: 10.1186/1471-213X-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elvers M, et al. Impaired alpha(IIb)beta(3) integrin activation and shear-dependent thrombus formation in mice lacking phospholipase D1. Science signaling. 2010;3:ra1. doi: 10.1126/scisignal.2000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norton LJ, et al. PLD1 rather than PLD2 regulates phorbol-ester-, adhesion-dependent and Fc{gamma}-receptor-stimulated ROS production in neutrophils. J Cell Sci. 2011;124:1973–1983. doi: 10.1242/jcs.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thielmann I, et al. Redundant functions of phospholipases D1 and D2 in platelet α-granule release. J. Thromb. Haemost. 2012;10:2361–2372. doi: 10.1111/j.1538-7836.2012.04924.x. [DOI] [PubMed] [Google Scholar]
- 59.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 60.Daumke O, et al. The GTPase-activating protein Rap1GAP uses a catalytic asparagine. Nature. 2004;429:197–201. doi: 10.1038/nature02505. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, et al. Biochemical and Functional Characterizations of Small GTPase Rheb and TSC2 GAP Activity. Mol Cell Biol. 2004;24:7965–7975. doi: 10.1128/MCB.24.18.7965-7975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazhab-Jafari MT, et al. An autoinhibited noncanonical mechanism of GTP hydrolysis by Rheb maintains mTORC1 homeostasis. Structure. 2012;20:1528–1539. doi: 10.1016/j.str.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Clark GJ, et al. The Ras-related Protein Rheb Is Farnesylated and Antagonizes Ras Signaling and Transformation. J Biol Chem. 1997;272:10608–10615. doi: 10.1074/jbc.272.16.10608. [DOI] [PubMed] [Google Scholar]
- 64.Yu Y, et al. Structural basis for the unique biological function of small GTPase RHEB. J Biol Chem. 2005;280:17093–17100. doi: 10.1074/jbc.M501253200. [DOI] [PubMed] [Google Scholar]
- 65.Menon S, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roccio M, et al. Regulation of the small GTPase Rheb by amino acids. Oncogene. 2006;25:657–664. doi: 10.1038/sj.onc.1209106. [DOI] [PubMed] [Google Scholar]
- 67.Jaeschke A, et al. Tuberous sclerosis complex tumor suppressor–mediated S6 kinase inhibition by phosphatidylinositide-3-OH kinase is mTOR independent. J Cell Biol. 2002;159:217–224. doi: 10.1083/jcb.jcb.200206108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ito N, Rubin GM. gigas, a Drosophila homolog of tuberous sclerosis gene product-2, regulates the cell cycle. Cell. 1999;96:529–539. doi: 10.1016/s0092-8674(00)80657-1. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi T, et al. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 1999;59:1206–1211. [PubMed] [Google Scholar]
- 70.Radimerski T, et al. Lethality of Drosophila lacking TSC tumor suppressor function rescued by reducing dS6K signaling. Genes Dev. 2002;16:2627–2632. doi: 10.1101/gad.239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, et al. Drosophila target of rapamycin kinase functions as a multimer. Genetics. 2006;172:355–362. doi: 10.1534/genetics.105.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim E, et al. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Plank TL, et al. Hamartin, the product of the tuberous sclerosis 1 (TSC1) gene, interacts with tuberin and appears to be localized to cytoplasmic vesicles. Cancer Res. 1998;58:4766–4770. [PubMed] [Google Scholar]
- 74.van Slegtenhorst M, et al. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet. 1998;7:1053–1057. doi: 10.1093/hmg/7.6.1053. [DOI] [PubMed] [Google Scholar]
- 75.Dibble CC, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012;47:535–546. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoogeveen-Westerveld M, et al. The TSC1-TSC2 complex consists of multiple TSC1 and TSC2 subunits. BMC Biochem. 2012;13:18. doi: 10.1186/1471-2091-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun W, et al. Crystal structure of the yeast TSC1 core domain and implications for tuberous sclerosis pathological mutations. Nat Commun. 2013;4 doi: 10.1038/ncomms3135. [DOI] [PubMed] [Google Scholar]
- 78.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benvenuto G, et al. The tuberous sclerosis-1 (TSC1) gene product hamartin suppresses cell growth and augments the expression of the TSC2 product tuberin by inhibiting its ubiquitination. Oncogene. 2000;19:6306–6316. doi: 10.1038/sj.onc.1204009. [DOI] [PubMed] [Google Scholar]
- 80.Yoshimura S-I, et al. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- 82.Glatter T, et al. Modularity and hormone sensitivity of the Drosophila melanogaster insulin receptor/target of rapamycin interaction proteome. Mol Syst Biol. 2011;7:547. doi: 10.1038/msb.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsu Y-C, et al. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature. 2007;445:785–788. doi: 10.1038/nature05528. [DOI] [PubMed] [Google Scholar]
- 84.Dong X, et al. Molecular basis of the acceleration of the GDP-GTP exchange of human ras homolog enriched in brain by human translationally controlled tumor protein. J Biol Chem. 2009;284:23754–23764. doi: 10.1074/jbc.M109.012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rehmann H, et al. Biochemical characterisation of TCTP questions its function as a guanine nucleotide exchange factor for Rheb. FEBS Lett. 2008;582:3005–3010. doi: 10.1016/j.febslet.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 86.Chen SH, et al. A knockout mouse approach reveals that TCTP functions as an essential factor for cell proliferation and survival in a tissue-or cell type-specific manner. Mol Biol Cell. 2007;18:2525–2532. doi: 10.1091/mbc.E07-02-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nellist M, et al. Phosphorylation and binding partner analysis of the TSC1-TSC2 complex. Biochem Biophys Res Commun. 2005;333:818–826. doi: 10.1016/j.bbrc.2005.05.175. [DOI] [PubMed] [Google Scholar]
- 88.Maeurer C, et al. Sphingosine-1-phosphate induced mTOR-activation is mediated by the E3-ubiquitin ligase PAM. Cell Signal. 2009;21:293–300. doi: 10.1016/j.cellsig.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 89.Mazhab-Jafari MT, et al. Membrane-dependent modulation of the mTOR activator Rheb: NMR observations of a GTPase tethered to a lipid-bilayer nanodisc. J. Am. Chem. Soc. 2013;135:3367–3370. doi: 10.1021/ja312508w. [DOI] [PubMed] [Google Scholar]
- 90.Traut TW. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 91.Zhang HH, et al. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS ONE. 2009;4:e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong J, Pan D. Tsc2 is not a critical target of Akt during normal Drosophila development. Genes Dev. 2004;18:2479–2484. doi: 10.1101/gad.1240504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pallares-Cartes C, et al. Tissue-specific coupling between insulin/IGF and TORC1 signaling via PRAS40 in Drosophila. Dev Cell. 2012;22:172–182. doi: 10.1016/j.devcel.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 94.Hawley SA, et al. Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells. Biochem J. 2014;459:275–287. doi: 10.1042/BJ20131344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mora A, et al. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 96.Pearce LR, et al. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 97.Liu P, et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. 2014;508:541–545. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oh WJ, Jacinto E. mTOR complex. 2 signaling and functions. Cell Cycle. 2011;10:2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sarbassov DD, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 100.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 101.Gan X, et al. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. Journal of Biological Chemistry. 2011;286:10998–11002. doi: 10.1074/jbc.M110.195016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shanmugasundaram K, et al. PI3K regulation of the SKP-2/p27 axis through mTORC2. Oncogene. 2013;32:2027–2036. doi: 10.1038/onc.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andjelkovic M, et al. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brognard J, et al. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 105.Chakraborty A, et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vander Haar E, et al. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 107.Wang L, et al. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 108.Wang L, et al. Regulation of proline-rich Akt substrate of. 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008;283:15619–15627. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rapley J, et al. The mechanism of insulin-stimulated 4E-BP protein binding to mammalian target of rapamycin (mTOR) complex. 1 and its contribution to mTOR complex. 1 signaling. Journal of Biological Chemistry. 2011;286:38043–38053. doi: 10.1074/jbc.M111.245449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fonseca BD, et al. The binding of PRAS40 to 14-3-3 proteins is not required for activation of mTORC1 signalling by phorbol esters/ERK. Biochem J. 2008;411:141–149. doi: 10.1042/BJ20071001. [DOI] [PubMed] [Google Scholar]
- 111.Xiong X, et al. PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiol Dis. 2014;66:43–52. doi: 10.1016/j.nbd.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peterson TR, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Acosta-Jaquez HA, et al. Site-specific mTOR phosphorylation promotes mTORC1-mediated signaling and cell growth. Mol Cell Biol. 2009;29:4308–4324. doi: 10.1128/MCB.01665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dan HC, et al. Akt-dependent activation of mTORC1 complex involves phosphorylation of mTOR (mammalian target of rapamycin) by IκB kinase α (IKKα). Journal of Biological Chemistry. 2014;289:25227–25240. doi: 10.1074/jbc.M114.554881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holz MK, Blenis J. Identification of S6 Kinase. 1 as a Novel Mammalian Target of Rapamycin (mTOR)-phosphorylating Kinase. J Biol Chem. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- 116.Soliman GA, et al. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. Journal of Biological Chemistry. 2010;285:7866–7879. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosner M, et al. mTOR phosphorylated at S2448 binds to raptor and rictor. Amino Acids. 2010;38:223–228. doi: 10.1007/s00726-008-0230-7. [DOI] [PubMed] [Google Scholar]
- 118.Wang L, et al. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J Biol Chem. 2009;284:14693–14697. doi: 10.1074/jbc.C109.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Foster KG, et al. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. Journal of Biological Chemistry. 2010;285:80–94. doi: 10.1074/jbc.M109.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dan HC, et al. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–35370. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 121.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278:12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 122.Cai S-L, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang J, et al. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nat. Cell Biol. 2013;15:1186–1196. doi: 10.1038/ncb2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miyazaki M, et al. Insulin like growth factor-1-induced phosphorylation and altered distribution of tuberous sclerosis complex (TSC)1/TSC2 in C2C12 myotubes. FEBS J. 2010;277:2180–2191. doi: 10.1111/j.1742-4658.2010.07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sabatini DM, et al. Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science. 1999;284:1161–1164. doi: 10.1126/science.284.5417.1161. [DOI] [PubMed] [Google Scholar]
- 126.Saito K, et al. Novel Role of the Small GTPase Rheb: Its Implication in Endocytic Pathway Independent of the Activation of Mammalian Target of Rapamycin. J Biochem. 2005;137:423–430. doi: 10.1093/jb/mvi046. [DOI] [PubMed] [Google Scholar]
- 127.Sancak Y, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fawal M-A, et al. MCRS1 Binds and Couples Rheb to Amino Acid-Dependent mTORC1 Activation. Dev Cell. 2015 doi: 10.1016/j.devcel.2015.02.010. DOI: 10.1016/j.devcel.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 129.Demetriades C, et al. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell. 2014;156:786–799. doi: 10.1016/j.cell.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Slegtenhorst M, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 131.Yamamoto Y, et al. Multicompartmental distribution of the tuberous sclerosis gene products, hamartin and tuberin. Arch Biochem Biophys. 2002;404:210–217. doi: 10.1016/s0003-9861(02)00300-4. [DOI] [PubMed] [Google Scholar]
- 132.Sancak Y, et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Groenewoud MJ, et al. Mammalian Target of Rapamycin Complex I (mTORC1) Activity in Ras Homologue Enriched in Brain (Rheb)-Deficient Mouse Embryonic Fibroblasts. PLoS ONE. 2013;8:e81649–21. doi: 10.1371/journal.pone.0081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bar-Peled L, et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wolff NC, et al. Cell-Type-Dependent Regulation of mTORC1 by REDD1 and the Tumor Suppressors TSC1/TSC2 and LKB1 in Response to Hypoxia. Mol Cell Biol. 2011;31:1870–1884. doi: 10.1128/MCB.01393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jacinto E, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 137.Guertin DA, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 138.Guertin DA, et al. mTOR complex. 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Feldman ME, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.García-Martínez JM, et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 142.Yang J, et al. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 143.Facchinetti V, et al. The mammalian target of rapamycin complex. 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sato S, et al. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Abraham RT. Making sense of amino acid sensing. Science. 2015;347:128–129. doi: 10.1126/science.aaa4570. [DOI] [PubMed] [Google Scholar]
- 146.Efeyan A, et al. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bar-Peled L, et al. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Durán RV, Hall MN. Regulation of TOR by small GTPases. EMBO Rep. 2012;13:121–128. doi: 10.1038/embor.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smith EM, et al. The Tuberous Sclerosis Protein TSC2 Is Not Required for the Regulation of the Mammalian Target of Rapamycin by Amino Acids and Certain Cellular Stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 150.Nobukuni T, et al. Amino acids mediate mTOR/raptor signaling through activation of class. 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tato I, et al. Amino acids activate mammalian target of rapamycin complex 2 (mTORC2) via PI3K/Akt signaling. Journal of Biological Chemistry. 2011;286:6128–6142. doi: 10.1074/jbc.M110.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thomas JD, et al. Rab1A Is an mTORC1 Activator and a Colorectal Oncogene. Cancer Cell. 2014;26:754–769. doi: 10.1016/j.ccell.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 154.Winter-Vann AM, Casey PJ. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer. 2005;5:405–412. doi: 10.1038/nrc1612. [DOI] [PubMed] [Google Scholar]
- 155.Sandoval CO, Simmen T. Rab proteins of the endoplasmic reticulum: functions and interactors. Biochem Soc Trans. 2012;40:1426–1432. doi: 10.1042/BST20120158. [DOI] [PubMed] [Google Scholar]