Overview
The prospective identification and therapeutic targeting of oncogenic tyrosine kinases with tyrosine kinase inhibitors (TKIs) has revolutionized the treatment of patients with non-small cell lung cancer (NSCLC). TKI therapy frequently induces dramatic clinical responses in molecularly defined cohorts of lung cancer patients, paving the way for the implementation of ‘precision medicine’. Unfortunately, acquired resistance, defined as tumor progression after initial response, seems to be an inevitable consequence of this treatment approach. This brief review will provide an overview of the complex and heterogeneous problem of acquired resistance to TKI therapy in NSCLC, with a focus on EGFR-mutant and ALK-rearranged NSCLC. In vitro models of TKI resistance as well as analysis of tumor biopsy samples at the time of disease progression have generated breakthroughs in our understanding of the spectrum of mechanisms by which a tumor can thwart TKI therapy and have provided important rational for development of novel approaches to delay or overcome resistance. Numerous on-going clinical trials implement strategies, including novel, more potent TKIs as well as rational combinations of targeted therapies, some of which have already proven to be effective in surmounting therapeutic resistance.
A. Molecular cohorts of Non-Small Cell Lung Cancer
Therapeutic targeting of oncogenes has emerged as a preeminent treatment paradigm for patients with NSCLC. Beginning in 2004 with the initial identification of EGFR mutations in a subset of lung adenocarcinomas (1-3), a decade later, molecular profiling of lung cancer, particularly lung adenocarcinoma, has evolved into a complex spectrum of clinically relevant and therapeutically actionable genomic alterations. These alterations occur at varying frequencies and at present, have varying levels of clinical evidence to support the use of targeted inhibitors in each setting. To date, the most well described molecular cohorts of NSCLC are those defined by the presence of EGFR mutations and ALK rearrangements. Treatment of patients with EGFR-mutant and ALK-rearranged NSCLC with specific tyrosine kinase inhibitors (TKIs) which target the EGFR and ALK tyrosine kinases, respectively, has led to remarkable clinical responses, including often dramatic tumor shrinkage and increased progression free survival (PFS) compared to standard cytotoxic chemotherapy (4-10).
Unfortunately, despite the exciting results, virtually every patient who receives TKI therapy and has an anti-tumor response will eventually develop disease progression. This tumor relapse while the patient is still receiving drug therapy is called ‘acquired resistance’ and typically occurs within 1-2 years after the initiation of the TKI (4-8, 11, 12). The development of drug resistance remains a major limitation to the successful treatment of patients with advanced NSCLC. Therefore, numerous pre-clinical and clinical studies have been directed at identifying and understanding on a mechanistic level the tumor specific factors which lead to acquired resistance in NSCLC. Given the complexity of the topic, in this review, we will specifically focus EGFR mutant and ALK-rearranged NSCLC as paradigms for the use of targeted therapies and the battle to overcome acquired TKI resistance in this disease.
B. EGFR mutant NSCLC
EGFR is the gene which encodes for the epidermal growth factor (EGF) receptor tyrosine kinase. EGFR propagates growth and survival signals through several downstream pathways within the cell, including the RAS-RAF-MEK-ERK (MAP kinase) and the PI3K-AKT-mTOR pathway. In NSCLC, EGFR mutations are typically detected in exons 18-21, which encode part of the EGFR tyrosine kinase domain. Approximately 90% of these mutations are small in-frame deletions in exon 19 or point mutations in exon 21 (L858R) (13). These mutations activate EGFR kinase activity and are typically detected in lung adenocarcinomas with a frequency of approximately 10% of patients with NSCLC in the United States and 35% in Asia(1-3).
EGFR mutations confer sensitivity to and are strong predictors of efficacy for the EGFR tyrosine kinase inhibitors (TKIs). Several classes of EGFR TKIs have been tested in tumors harboring activating EGFR mutations, including the “first-generation” drugs erlotinib and gefitinib and the “second-generation” drugs, afatinib, dacomitinib, and neratinib. Several randomized phase 3 studies have now demonstrated that patients with EGFR-mutant tumors (particularly the exon 19 deletion and L858R mutants) display an approximate 60-70% radiographic response rate (RR) and PFS of approximately 10-13 months with erlotinib, gefitinib, or afatinib therapy (4-8, 14, 15). These treatment outcomes are superior to those obtained with standard platinum based chemotherapy in the same patient population. Therefore, EGFR TKIs are now recommended as first line therapy for patients with EGFR mutant lung cancer. In the United States, erlotinib and afatinib are both approved by the Food and Drug Administration (FDA) as first line therapy for patients with EGFR mutant lung cancer.
C. Acquired Resistance to EGFR TKI therapy
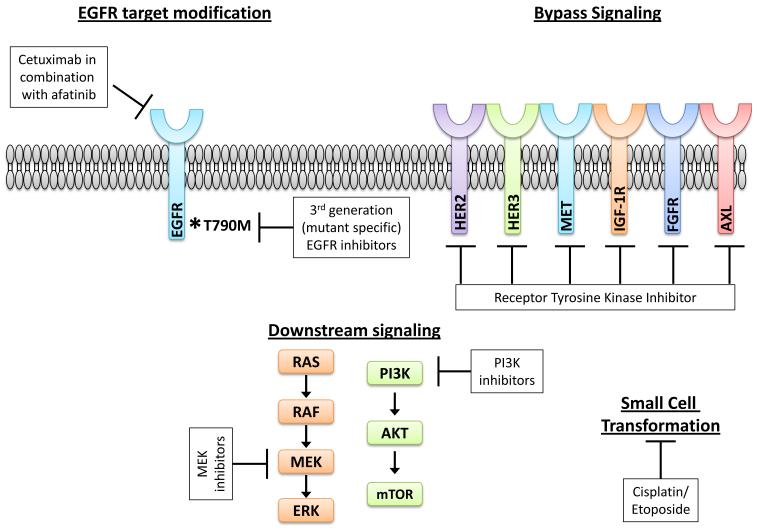
Acquired resistance to EGFR TKIs is a complex and heterogeneous phenomenon, with multiple potential mechanisms whereby the tumor evades the anti-EGFR directed therapy. However, the end result for each potential mechanism is sustained signaling through downstream pathways, such as the MAP kinase and PI3K-AKT-mTOR pathways, which propagate pro-growth and pro-survival signals within the tumor. Numerous in vitro studies modeling EGFR TKI resistance in EGFR mutant lung cancer cell lines as well as studies of actual patient tumor samples at the time of progressive disease on EGFR TKI therapy (16, 17) have yielded important insights into the underlying molecular pathogenesis of acquired resistance (Figure 1). These mechanisms include: (1) modification of the target oncogene, particularly the T790M ‘second site’ mutation, (2) upregulation of parallel signaling pathways, such as MET or HER2, to circumvent the inhibited EGFR, and (3) histological transformation, such as epithelial to mesenchymal transition or small cell transformation. Overall, a thorough understanding of the mechanistic basis for acquired resistance is paramount for developing strategies to delay or overcome resistance. Here, we will focus on clinically relevant mechanisms of acquired resistance and proposed strategies to treat progressive disease.
Figure 1. Mechanisms of acquired resistance to first- and second-generation EGFR TKIs in EGFR mutant NSCLC.
Resistance can be mediated by EGFR target modifications, most commonly the EGFR T790M second-site mutation, through bypass signaling pathways which circumvent the inhibited driver oncogene (EGFR), and through histological transformation (in this case, change in histology from NSCLC to SCLC). Potential strategies to overcome resistance are noted in the white boxes.
C.1. Strategies to overcome resistance mediated by EGFR target modification
Genomic alterations in the drug target, such as amplification and/or second site mutations, have been shown to occur as a common mechanism of resistance in many oncogene driven cancers treated with kinase inhibitor therapy. For example, second site mutations within the target oncogene have been described for BCR-ABL in CML (18), EGFR in NSCLC (19, 20), ALK in NSCLC (21, 22), and ROS1 in NSCLC (23). In the case of EGFR mutant NSCLC, the most common second site mutation involves substitution of a methionine in place of a threonine at position 790 (T790M) in the EGFR kinase domain. This T790M ‘gatekeeper’ mutation is the most common target-specific alteration identified in approximately 50% of patients with acquired resistance to the EGFR TKIs, erlotinib and gefitinib (19, 20). The T790M mutation is thought to confer TKI resistance through steric hindrance which interferes with drug binding and/or through alterations in the ATP affinity of the EGFR kinase (24). Several other second-site mutations within the EGFR kinase domain have also been to confer resistance to EGFR TKI therapy, though these mutations appear to occur at a much lower frequency (25).
In the case of T790M mediated resistance, one potential strategy to overcome resistance is through the development of novel EGFR inhibitors with increased potency. Erlotinib and gefitinib are ‘first-generation’ EGFR TKIs which were designed against wild-type EGFR and which reversibly bind to the EGFR kinase domain. ‘Second-generation’ inhibitors, such as afatinib, neratinib, and dacomitinib, irreversibly bind to the EGFR kinase domain and have activity against other EGFR (ErbB1) family members, including HER2 (ErbB2), HER2 (ErbB3), and/or HER4 (ErbB4). The initial hypothesis was that these ‘second-generation’ inhibitors would be able to overcome the T790M mutation. Indeed, pre-clinical data in cell line models did show that the irreversible inhibitors can overcome T790M in vitro (26-28).
While the ‘second-generation’ EGFR/HER2 TKI, afatinib, is FDA-approved for first-line therapy in EGFR mutant NSCLC (8), this agent has not yet proven to be a promising therapy in the setting of acquired resistance to ‘first-generation’ EGFR TKIs, such as erlotinib and afatinib, despite the in vitro studies suggesting that afatinib can overcome T790M. In the phase 3 LUX-lung 1 study, patients with advanced NSCLC, who had previously been treated with erlotinib or gefitinib for at least 12 weeks, were randomized to receive afatinib or placebo. There was no molecular selection for EGFR mutation required for entrance into the study, and the molecular mechanism(s) underlying the patient’s progressive disease on erlotinib or gefitinib were unknown. The RR and PFS were superior with afatinib, but the study did not meet its primary endpoint of improved overall survival in all study participants or in the subset of patients with known EGFR mutant lung cancer (29). Likewise, there were no responses in patients with known T790M in clinical trials of other ‘second-generation’ EGFR TKIs, including neratinib (30) and dacomitinib (31).
More recently, there has been tremendous excitement surrounding the clinical development of ‘third-generation’, mutant specific EGFR inhibitors. These ‘third-generation’ EGFR TKIs are irreversible inhibitors, analogous to the ‘second-generation’ EGFR TKIs, however, they have higher specificity for mutant EGFR (including T790M) compared to wild-type EGFR. There are several agents in this class, including AZD9291, rociletinib (CO-1686), HM61713, ASP8273, and EGF816. Of these agents, the mutant specific EGFR TKIs with the most clinical data reported to date include AZD9291 and rociletinib (CO-1686). Both AZD9291 and rociletinib have activity against EGFR activating (e.g. L858R, exon 19 deletion) and EGFR resistance (e.g. T790M) mutations, with little inhibition of wild-type EGFR (32, 33).
Results of a phase 1 study of AZD9291, which included both the dose escalation and dose expansion cohorts, were presented at ESMO 2014 (34). Among all evaluable patients, the overall RR in the T790M positive cohort was 61% (78/127 patients) with a PFS of 9.6 months. In the T790M negative cohort, the overall RR was 21% (13/61) with a PFS of 2.8 months. The most common adverse events (AEs) were diarrhea (39%), rash (36%) and nausea (18%), most of which were mild. Overall, AZD9291 appears to be well tolerated with dose reductions infrequently needed in the study population.
Analogously, promising results were reported for the Phase 1/2 trial of rocelitinib (CO-1686). In the TIGER-X study, two formulations and multiple doses/schedules of rociletinib were evaluated (35). Data from 56 T790M-positive patients treated with rociletinib at the recommended phase 2 dose of 625mg twice a day (n=30) and the step-down dose of 500mg twice a day (n=26) indicated a 67% overall RR in the 27 patients with evaluable disease. Median PFS was 10.4 months. CO-1686 is well tolerated with hyperglycemia as a frequent adverse event (32% all grades and 14% grade 3/4).
Numerous other mutant specific EGFR inhibitors are being evaluated in clinical trials, including HM61713 (NCT01588145), ASP8273 (NCT02192697, NCT02113813), and EGF816 (NCT02108964). Overall, these mutant specific EGFR TKIs appear to be the most effective therapeutic strategy tested to date to overcome T790M-mediated acquired resistance to erlotinib, gefitinib, and afatinib. In fact, both AZD9291 and rociletinib received FDA breakthrough status in 2014.
Despite the excitement surrounding the show efficacy of mutant specific EGFR TKIs in T790M-positive tumors, there still remains a large cohort (40-50%) of patients with T790M-negative tumors who have developed acquired resistance to erlotinib, gefitinib, or afatinib. One potential strategy that has been postulated for this cohort includes a combination of the EGFR monoclonal antibody, cetuximab, combined with afatinib, in patients with acquired resistance. This combination, which dual targets EGFR, has been studied pre-clinically (36) and in phase 1 clinical trials (37). Amongst the 126 patients treated with this combination, the objective RR was 29% and was comparable in T790M-positive and T790M-negative tumors (32% vs. 25%; P = 0.341). Median PFS was 4.7 months. Adverse events included expected toxicities of EGFR inhibitors, such as rash, diarrhea, and fatigue. Therapy-related grade 3/4 adverse events occurred in 44%/2% of patients, respectively. Studies of afatinib and cetuximab in both the first-line setting and at the time of acquired resistance are being planned.
C.2. Strategies to overcome resistance mediated by bypass signaling pathways
The majority of focus and attention with respect to acquired resistance to EGFR TKIs has centered upon overcoming resistance mediated by the T790M mutation. However, several recent reports have described alternative ways in which a tumor may circumvent inhibited EGFR, specifically through activation of collateral signaling networks which can transmit the same downstream pro-growth and pro-survival effects within the tumor. Therapeutic strategies aimed at overcoming resistance mediated by this ‘bypass signaling’ are typically devised to provide continuous inhibition of the driver oncogene (e.g., EGFR) while also co-inhibiting the compensatory signaling loops which circumvent the inhibited driver. Activation of several different receptor tyrosine kinases have been documented at the time of acquired resistance to erlotinib and gefitinib, including MET (16, 17), HER2 (38), HER3 (39), IGF-1R (40), FGFR1 (41), and AXL (42). In addition, activation of the MAP kinase pathway can drive EGFR TKI resistance in vitro and in vivo (43). The MAP kinase pathway may be activated in this context by virtue of mutations in BRAF, a component of the MAP kinase signaling cascade, as well as through reduced expression of neurofibromin, a RAS GTPase-activating protein (GAP) encoded by the NF1 gene which functions as a negative regulator of RAS (44). Finally, activation of the PI3K-AKT-mTOR pathway, by virtue of PIK3ca mutations, can drive resistance to erlotinib and gefitinib (17).
Numerous clinical trials have been designed to test rational drug combinations which may overcome therapeutic resistance by targeting bypass signaling pathways. Unfortunately, results to date have been somewhat disappointing (For review see (45)).
C.3. Strategies to overcome resistance mediated by histological transformation
Changes in tumor histology, including epithelial to mesenchymal transformation (EMT) as well as transformation to small cell lung cancer (SCLC), have been documented at the time of acquired resistance to EGFR TKIs (16, 17). The molecular mechanisms initiating these phenotypic changes have not been clearly elucidated to date. In the fraction of patients with EGFR TKI resistance driven by SCLC transformation (3-14%), the original EGFR mutation is retained. These patients may benefit from treatment with standard platinum based chemotherapy regimens used in the standard management for SCLC (46, 47).
D. ALK-rearranged NSCLC
ALK is the gene which encodes for the anaplastic lymphoma kinase. ALK is a receptor tyrosine kinase that is normally expressed in the developing nervous system (48), however, genomic alterations in ALK – including ALK amplification, activating point mutations in the ALK kinase domain, and ALK chromosomal rearrangements - are found in a wide variety of malignancies (49). In NSCLC, ALK is activated through chromosomal rearrangements, most commonly EML4-ALK, which is a fusion between echinoderm microtubule-associated protein-like 4 (EML4) and ALK, both on chromosome 2. Analogous to EGFR, ALK fusion proteins signal downstream through the MAP kinase and the PI3K-AKT-mTOR pathways. ALK rearrangements are typically detected in lung adenocarcinoma and occur at a frequency of approximately 3-7% (11, 50).
Several large clinical trials have now shown that patients with advanced NSCLC harboring ALK rearrangements derive significant clinical benefits from treatment with ALK TKI therapy. Crizotinib is the first-in-class ALK TKI to be tested in this patient population. Of note, in addition to ALK, crizotinib also targets MET and ROS1. In the phase 1, first-in-man study of crizotinib in patients with advanced NSCLC harboring an ALK rearrangement (PROFILE 1005) , the objective RR was 60.8% (87/143 patients) and the median PFS was 9.7 months (11, 51). Based on the high RRs documented in this study, crizotinib was granted FDA approval in 2011 for the treatment of advanced, ALK-rearranged NSCLC. Crizotinib has also been studied in randomized phase 3 trials. In PROFILE 1007, crizotinib was compared with single agent chemotherapy (pemetrexed or docetaxel) in patients with ALK-rearranged NSCLC who had disease progression after first line platinum-based chemotherapy. Crizotinib therapy resulted in higher RRs (65% crizotinib vs. 20% chemotherapy) and significantly longer PFS (7.7 months crizotinib vs. 3.0 months chemotherapy) (9). There was no difference in OS between the groups (20.3 vs. 22.8 months for crizotinib vs. chemotherapy), likely due to significant crossover of patients from the chemotherapy arm at the time of disease progression. In the PROFILE 1014 study, crizotinib was evaluated in the first-line setting versus chemotherapy (cisplatin or carboplatin plus pemetrexed) in 343 patients with advanced, ALK-rearranged NSCLC (10). The objective RR was 74% with crizotinib versus 45% with chemotherapy, and PFS was significantly longer (10.9 months for crizotinib vs. 7.0 months for chemotherapy). Median overall survival was not reached in either group. Therefore, crizotinib is now recommended as first line therapy for patients with ALK-rearranged lung cancer.
E. Acquired Resistance to ALK TKI therapy
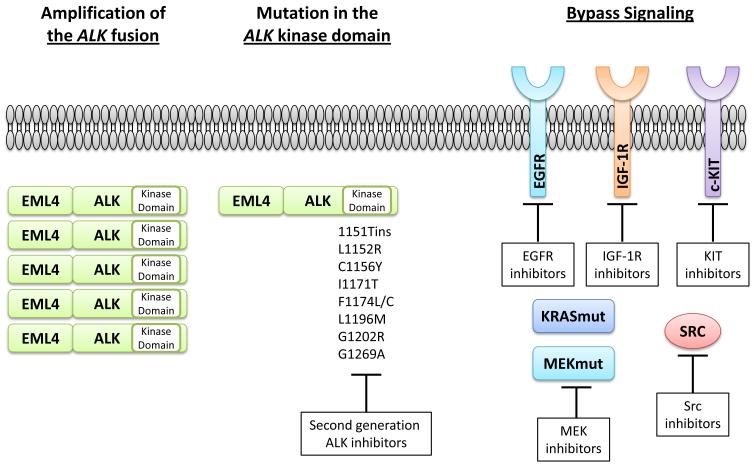
Analogous to EGFR mutant lung cancer treated with EGFR TKI therapy, acquired resistance to the ALK TKI therapy remains a barrier to the most effective management of this disease. Also analogous to EGFR, acquired resistance to ALK inhibition appears to be a complex and heterogeneous phenomenon which can be mediated through modification of the target oncogene and upregulation of parallel signaling pathways to circumvent the inhibited ALK fusion protein. Acquired resistance to ALK TKI therapy, particularly crizotinib, has been the focus of intense basic science and clinical research (Figure 2). Target gene modification, including ALK amplification and mutation within the ALK kinase domain, have been described in both pre-clinical models as well as in patient tumor samples at the time of disease progression on crizotinib therapy (21, 22, 52). Unlike EGFR where T790M is the predominant mutation found in 50-60% of patients with EGFR TKI resistance, only ~1/3rd or less of patients with crizotinib resistant tumors harbor an ALK kinase domain mutation and there are numerous mutations which can drive resistance. The two most common mutations described to date include L1196M, which is at the ‘gatekeeper’ position and is analogous to EGFR T790M, and G1269A, where G1269 is the residue immediately before the conserved DFG activation motif in the kinase domain (21, 22). Other ALK kinase domain mutations which can drive resistance include T1151ins, L1152R, C1156Y, I1171T, F1174L, G1202R, and S1206Y (21, 22, 53-55). Interestingly, these different mutations can confer a variable degree of crizotinib resistance in vitro (21). In addition, to underscore the heterogeneity of crizotinib resistance, the C1156Y and L1196M ALK mutations were both found at different tumor sites within the same patient at the time of crizotinib resistance (53).
Figure 2. Mechanisms of acquired resistance to crizotinib in ALK- rearranged NSCLC.
Resistance can be mediated by ALK target modifications, including ALK amplification and ALK kinase domain mutations and through bypass signaling pathways which circumvent the inhibited driver oncogene (the ALK fusion). Potential strategies to overcome resistance are noted in the white boxes.
Activation of alternative signaling pathways which can bypass the drug inhibited ALK fusion protein has also been described as a mechanism of crizotinib resistance. This bypass pathway activation can be driven by both genomic and non-genomic changes. For example, increased EGFR phosphorylation (without EGFR mutation or amplification) was observed in 4/9 tumor biopsy samples at the time of crizotinib resistance compared to the pre-crizotinib tumor sample (21). These clinical data are in accord with what has been described for cell line models of ALK inhibitor resistance (54, 56). In addition, increased IGF-1R phosphorylation was observed in cell culture models of ALK TKI resistance, and this result was mirrored in patient tumor biopsy samples taken at the time of progressive disease (57). Most recently, Src activation was also found to be a driver of crizotinib resistance (58). The end result of activation of each of these proteins is continued signaling through redundant downstream pathways, despite the presence of the ALK inhibitor.
Genomic mechanisms which can drive ALK TKI resistance include mutations in KRAS and MAP2K1. KRAS point mutation were found in 2/12 patients with ALK+/crizotinib resistant tumors (22). MAP2K1, which encodes the protein MEK, mutations were found in 1/9 patients with ALK+/crizotinib resistant tumors (58). Of note, both of these alterations activate the MAP kinase pathway. Furthermore, amplification of the cKIT receptor tyrosine kinase was detected in 2/6 patient samples with matched pre- and post-crizotinib tumor biopsies (21).
E.1. Strategies to overcome resistance mediated by ALK target modification
Several ‘second-generation’ ALK inhibitors are currently being developed clinically. In general, these ALK inhibitors have more on-target efficacy against ALK in vitro compared to crizotinib and can overcome some of the ALK kinase domain mutations associated with crizotinib resistance.
The most well studied ‘second-generation’ ALK inhibitor to date is ceritinib (LDK-378). This agent potently inhibits ALK in vitro, in addition to IGF-1R and insulin receptor. Pre-clinical studies have shown that ceritinib can overcome the L1196M and G1269A mutations, the mutations which appear to be most common with crizotinib resistance (59). In a phase 1 study of ceritinib, which included an expansion cohort at the maximum tolerated dose (750mg daily), the overall RR was 58% and the median PFS was 7.0 months amongst the 114 patients who received at least 400mg daily (60). Amongst the 80 patients who had previously been treated with crizotinib, the RR was 56%, and responses were observed in tumors both with and without crizotinib resistance mutations detected. The most common adverse events reported were elevated liver enzymes and GI toxicity (nausea, diarrhea). Based on this study, ceritinib was granted accelerated approval by the US FDA in April 2014. Finally, ceritinib has documented efficacy against CNS disease.
Alectinib (CH5424802) is also a ‘second-generation’ ALK TKI with a distinct chemical structure that can overcome some of the crizotinib resistance mutations, such as the L1196M gatekeeper (61). In a Phase 1/2 study of this agent in Japan, 43/46 patients with ALK inhibitor naïve disease who were treated with the recommended phase 2 dose achieved an objective response (62). Alectinib has also been tested in a cohort of patients who progressed on or were intolerant of crizotinib (63). Of 44 evaluable patients, the objective RR was 55% (24/44). Crizotinib resistance mechanisms were not reported. The CNS RR in patients with CNS metastases at baseline was 52% (11/21). The most common adverse events reported were fatigue, myalgia, and peripheral edema. Alectinib received breakthrough therapy designation from the US FDA in 2013. A randomized phase 3 study (ALEX) comparing alectinib to crizotinib in treatment-naïve patients with ALK+ lung cancer is on-going (NCT02075840).
Several other next-generation ALK inhibitors are being studied. Like ceritinib and alectinib, X-396 is more potent against ALK in vitro and can overcome crizotinib resistance mutations (64). A phase 1/2 trial of this agent is on-going (NCT01625234); preliminary results indicate that 59% (10/17) of patients achieve a partial response, including patients who had received prior crizotinib (65). Adverse events were mild and included rash, nausea, vomiting, fatigue, and edema. AP26113, which targets both ALK and also has activity against the EGFR T790M mutation in vitro, has been tested in a phase 1/2 study (NCT01449461). The objective RR was 72% with median PFS of 56 weeks in the 72 patients with ALK+ NSCLC at the time of data cut-off (66). In the 65 of patients who had received prior crizotinib, the RR was 69% and median PFS 47.3 weeks. CNS responses were documented. Finally, PF-06463922 is a derivative of crizotinib designed to be a more potent ALK/ROS1 inhibitor. This agent was also optimized to overcome some of the pharmacokinetic limitations of crizotinib (67). A phase 1/2 trial is currently ongoing (NCT01970865).
E.2. Strategies to overcome resistance mediated by bypass signaling pathways
Such strategies to combat ALK TKI resistance mediated by bypass signaling are only beginning to emerge. Possible rational combination therapies which have been postulated include ALK inhibitor plus either EGFR inhibitor, IGF-1R inhibitor, Src inhibitor, or MEK inhibitor, based on the studies described above. It is also worth noting that ALK inhibitors have been tested in combination with Heat Shock 90 (HSP90) inhibitors. ALK fusions are known HSP90 client proteins and in vitro studies demonstrate the efficacy of HSP90 inhibitors in both ALK TKI sensitive and ALK TKI resistant models (68). Furthermore, clinical responses have been documented in patients with ALK+ lung cancer, both in the crizotinib -naïve and -resistant states (68, 69). Combination ALK plus HSP90 inhibition is currently being evaluated in clinical trials (NCT01579994, NCT01712217, NCT01772797).
F. Concluding Remarks
The identification and prospective targeting of oncogenic driver mutations has revolutionized the care of patients with NSCLC. However, therapeutic resistance remains a significant barrier to the successful management of this disease. Using the two most well established molecular cohorts of NSCLC, those defined by EGFR mutations and ALK rearrangements, we have reviewed the molecular mechanisms of acquired resistance as well as the strategies to overcome resistance. The development of more potent and more selective second- and third-generation oncogenic kinase inhibitors appears to be the strategy with the most momentum and most documented clinical success to date in both of these cohorts of lung cancer patients. However, there are several challenges to address and opportunities to explore moving forward, including:
1. How do we effectively address the heterogeneity of resistance mechanisms between different individuals and even in one individual patient? Will multiple biopsies at different sites of disease be necessary? feasible? Will blood based markers, such as circulating free DNA, become a way for clinicians to adequately assess resistance mechanisms?
2. How do we design and implement novel clinical trials that take into consideration the rapid pace of scientific discovery and the demand to bring more effective therapies into the forefront of care? Furthermore, how do we design these trials to encompass systematic analysis of biomarkers which are found in increasingly smaller percentages of patients? Cooperative group trials, such as the NCI ALK Master Protocol, which is currently being developed, will certainly assist in achieving this goal.
3. What other effective combination therapies can be devised to tackle the problem of TKI resistance? Will co-treatment with different classes of drugs, such as TKIs and immune checkpoint inhibitors, be effective in overcoming resistance? How should these agents be dosed – simultaneously? sequentially?
4. Finally, how can cutting-edge studies of TKI resistance in NSCLC be translated to other malignancies? Many of the current and emerging therapeutic targets in NSCLC are also found in other malignancies, such as ALK, ROS1, and RET. In order to more broadly tackle and surmount the problem of acquired resistance, studies in NSCLC can hopefully be used to inform management in distinct tumor types harboring these same targets.
Overall, a thorough understanding of the mechanistic basis for acquired resistance and the development of innovative therapeutic strategies to overcome resistance is paramount to most effectively combat resistance and therefore to improve the care of patients with lung cancer.
Key Points.
The identification of clinically relevant molecular cohorts of patients with non-small cell lung cancer (NSCLC) defined by the presence of actionable genomic alterations has revolutionized the care of patients with this disease.
Treatment of patients whose lung tumors harbor specific oncogenic mutations often results in dramatic response to targeted therapies, such as tyrosine kinase inhibitors (TKIs). This is best exemplified by EGFR-mutant and ALK-rearranged NSCLCs treated with EGFR TKIs and ALK TKIs, respectively.
Resistance to TKI therapy appears to be an inevitable consequence of this treatment approach. Resistance can be either primary (de novo) or acquired. Specifically, acquired resistance is defined by tumor growth after initial tumor regression while the patient is still receiving the TKI therapy.
Mechanisms of acquired resistance include drug target gene modification (amplification, ‘second-site’ mutations), activation of ‘bypass tracks’ which serve as compensatory signaling loops, and/or histological transformation.
Rebiopsy at the time of acquired resistance is essential for understanding the specific mechanism(s) of resistance at play in the tumor and for directing the patient to the most appropriate line of therapy. Several strategies to overcome acquired resistance, including novel, more potent inhibitors as well as rational combinations of targeted inhibitors, have already proven successful in clinical trials.
Acknowledgements
C.M. Lovly was supported by the NIH under award numbers R01CA121210 and P01CA129243.
Footnotes
Disclosure of Potential Conflicts of Interest: C.M. Lovly reports receiving a commercial research grant from AstraZeneca and Novartis; speaker’s bureau honoraria from Abbott Molecular, Harrison and Star, and Qiagen; and is a consultant/advisory board member for Pfizer and Novartis.
References
- 1.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–11. doi: 10.1073/pnas.0405220101. Epub 2004/08/27. 0405220101 [pii]. PubMed PMID: 15329413; PubMed Central PMCID: PMC516528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. Epub 2004/05/01. NEJMoa040938 [pii]. PubMed PMID: 15118073. [DOI] [PubMed] [Google Scholar]
- 3.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. Epub 2004/05/01. 1099314 [pii]. PubMed PMID: 15118125. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatinpaclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. doi: 10.1056/NEJMoa0810699. Epub 2009/08/21. doi: NEJMoa0810699 [pii] PubMed PMID: 19692680. [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. doi: 10.1056/NEJMoa0909530. Epub 2010/06/25. doi: 362/25/2380 [pii] PubMed PMID: 20573926. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8. doi: 10.1016/S1470-2045(09)70364-X. Epub 2009/12/22. doi: S1470-2045(09)70364-X [pii] PubMed PMID: 20022809. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X. PubMed PMID: 22285168. [DOI] [PubMed] [Google Scholar]
- 8.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34. doi: 10.1200/JCO.2012.44.2806. PubMed PMID: 23816960. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. doi: 10.1056/NEJMoa1214886. PubMed PMID: 23724913. [DOI] [PubMed] [Google Scholar]
- 10.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77. doi: 10.1056/NEJMoa1408440. PubMed PMID: 25470694. [DOI] [PubMed] [Google Scholar]
- 11.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. doi: 10.1056/NEJMoa1006448. Epub 2010/10/29. PubMed PMID: 20979469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(12)70344-3. Epub 2012/09/08. doi: S1470-2045(12)70344-3 [pii] PubMed PMID: 22954507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Mod Pathol. 2008;21(Suppl 2):S16–22. doi: 10.1038/modpathol.3801018. Epub 2008/06/24. doi: 3801018 [pii] PubMed PMID: 18437168. [DOI] [PubMed] [Google Scholar]
- 14.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker Analyses and Final Overall Survival Results From a Phase III, Randomized, Open-Label, First-Line Study of Gefitinib Versus Carboplatin/Paclitaxel in Clinically Selected Patients With Advanced Non-Small-Cell Lung Cancer in Asia (IPASS) J Clin Oncol. 2011;29(21):2866–74. doi: 10.1200/JCO.2010.33.4235. Epub 2011/06/15. doi: JCO.2010.33.4235 [pii] PubMed PMID: 21670455. [DOI] [PubMed] [Google Scholar]
- 15.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. doi: 10.1016/S1470-2045(11)70184-X. PubMed PMID: 21783417. [DOI] [PubMed] [Google Scholar]
- 16.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–7. doi: 10.1158/1078-0432.CCR-12-2246. PubMed PMID: 23470965; PubMed Central PMCID: PMC3630270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. Epub 2011/03/25. doi: 3/75/75ra26 [pii] PubMed PMID: 21430269; PubMed Central PMCID: PMC3132801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208–15. doi: 10.1182/blood-2010-12-326405. PubMed PMID: 21562040. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. doi: 10.1056/NEJMoa044238. Epub 2005/02/25. doi: 352/8/786 [pii] PubMed PMID: 15728811. [DOI] [PubMed] [Google Scholar]
- 20.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. Epub 2005/03/02. doi: 05-PLME-RA-0027R1 [pii] PubMed PMID: 15737014; PubMed Central PMCID: PMC549606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med. 2012;4(120):120ra17. doi: 10.1126/scitranslmed.3003316. Epub 2012/01/27. doi: scitranslmed.3003316 [pii] PubMed PMID: 22277784; PubMed Central PMCID: PMC3385512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doebele RC, Pilling AB, Aisner D, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of Resistance to Crizotinib in Patients with ALK Gene Rearranged Non-Small Cell Lung Cancer. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-2906. Epub 2012/01/12. doi: 1078-0432.CCR-11-2906 [pii] PubMed PMID: 22235099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awad MM, Katayama R, McTigue M, Liu W, Deng YL, Brooun A, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;368(25):2395–401. doi: 10.1056/NEJMoa1215530. PubMed PMID: 23724914; PubMed Central PMCID: PMC3878821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11(3):217–27. doi: 10.1016/j.ccr.2006.12.017. Epub 2007/03/14. doi: S1535-6108(07)00028-1 [pii] PubMed PMID: 17349580; PubMed Central PMCID: PMC1939942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232–41. doi: 10.1158/1078-0432.CCR-06-0658. PubMed PMID: 17189394. [DOI] [PubMed] [Google Scholar]
- 26.Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102(21):7665–70. doi: 10.1073/pnas.0502860102. Epub 2005/05/18. doi: 0502860102 [pii] PubMed PMID: 15897464; PubMed Central PMCID: PMC1129023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67(24):11924–32. doi: 10.1158/0008-5472.CAN-07-1885. Epub 2007/12/20. doi: 67/24/11924 [pii] PubMed PMID: 18089823. [DOI] [PubMed] [Google Scholar]
- 28.Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–11. doi: 10.1038/onc.2008.109. PubMed PMID: 18408761; PubMed Central PMCID: PMC2748240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13(5):528–38. doi: 10.1016/S1470-2045(12)70087-6. Epub 2012/03/29. doi: S1470-2045(12)70087-6 [pii] PubMed PMID: 22452896. [DOI] [PubMed] [Google Scholar]
- 30.Sequist LV, Besse B, Lynch TJ, Miller VA, Wong KK, Gitlitz B, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(18):3076–83. doi: 10.1200/JCO.2009.27.9414. Epub 2010/05/19. doi: JCO.2009.27.9414 [pii] PubMed PMID: 20479403. [DOI] [PubMed] [Google Scholar]
- 31.Reckamp KL, Giaccone G, Camidge DR, Gadgeel SM, Khuri FR, Engelman JA, et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer. 2014;120(8):1145–54. doi: 10.1002/cncr.28561. PubMed PMID: 24501009; PubMed Central PMCID: PMC4164026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–61. doi: 10.1158/2159-8290.CD-14-0337. PubMed PMID: 24893891; PubMed Central PMCID: PMC4315625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter AO, Tjin R, Haringsma H, et al. CO-1686, an orally available, mutant-selective inhibitor of the epidermal growth factor receptor (EGFR), causes tumor shrinkage in non-small cell lung cancer (NSCLC) with T790M mutations. Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics: AACR; Mol Cancer Ther. 2011;10(11 Suppl) Abstract C189. [Google Scholar]
- 34.Yang J, Kim D, Planchard D, Ohe Y, Ramalingam SS, Ahn M, Kim S, Su W, Horn L, Haggstrom D, Felip E, Kim J, Frewer P, Cantarini M, Ghiorghiu S, Ranson M, Janne PA. Updated safety and efficacy from a phase I study of AZD9291 in patients (pts) with EGFR-TKI-resistant non-small cell lung cancer (NSCLC) Annals of Oncology. 2014;25(suppl_4):iv146–iv64. [Google Scholar]
- 35.Soria JC, Sequist L, Goldman J, Wakelee H, Gadgeel S, Varga A, Yu H, Solomon B, Ou SH, Papadimitrakopoulou V, Oxnard G, Horn L, Dziadziuszko R, Chao B, Spira A, Liu S, Mekhail T, Matheny S, Litten J, Camidge DR. Interim phase 2 results of study CO-1686-008: A phase 1/2 study of the irreversible, mutant selective, EGFR inhibitor rociletinib (CO-1686) in patients with advanced non small cell lung cancer. 2014 EORTC, NCI, AACR Meeting; 2014; Abstract LBA 10. [Google Scholar]
- 36.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119(10):3000–10. doi: 10.1172/JCI38746. Epub 2009/09/18. 38746 [pii]. PubMed PMID: 19759520; PubMed Central PMCID: PMC2752070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janjigian YY, Smit EF, Groen HJ, Horn L, Gettinger S, Camidge DR, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4(9):1036–45. doi: 10.1158/2159-8290.CD-14-0326. PubMed PMID: 25074459; PubMed Central PMCID: PMC4155006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2(10):922–33. doi: 10.1158/2159-8290.CD-12-0108. PubMed PMID: 22956644; PubMed Central PMCID: PMC3473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sequist L, Modiano MR, Rixe O, Jackman DM, Andreas K, Pearlberg J, Moyo VM, Harb WA. A Study of MM-121 Combination Therapy in Patients With Advanced Non-Small Cell Lung Cancer. J Clin Oncol. 2012;30(suppl) abstr 7556. [Google Scholar]
- 40.Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118(7):2609–19. doi: 10.1172/JCI34588. Epub 2008/06/24. PubMed PMID: 18568074; PubMed Central PMCID: PMC2430495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terai H, Soejima K, Yasuda H, Nakayama S, Hamamoto J, Arai D, et al. Activation of the FGF2-FGFR1 autocrine pathway: a novel mechanism of acquired resistance to gefitinib in NSCLC. Mol Cancer Res. 2013;11(7):759–67. doi: 10.1158/1541-7786.MCR-12-0652. PubMed PMID: 23536707. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44(8):852–60. doi: 10.1038/ng.2330. PubMed PMID: 22751098; PubMed Central PMCID: PMC3408577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A. 2012;109(31):E2127–33. doi: 10.1073/pnas.1203530109. PubMed PMID: 22773810; PubMed Central PMCID: PMC3411967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bruin EC, Cowell C, Warne PH, Jiang M, Saunders RE, Melnick MA, et al. Reduced NF1 Expression Confers Resistance to EGFR Inhibition in Lung Cancer. Cancer Discov. 2014;4(5):606–19. doi: 10.1158/2159-8290.CD-13-0741. PubMed PMID: 24535670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu HA, Riely GJ, Lovly CM. Therapeutic strategies utilized in the setting of acquired resistance to EGFR tyrosine kinase inhibitors. Clin Cancer Res. 2014;20(23):5898–907. doi: 10.1158/1078-0432.CCR-13-2437. PubMed PMID: 25303979; PubMed Central PMCID: PMC4253858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zakowski MF, Ladanyi M, Kris MG. EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med. 2006;355(2):213–5. doi: 10.1056/NEJMc053610. Epub 2006/07/14. doi: 355/2/213 [pii] PubMed PMID: 16837691. [DOI] [PubMed] [Google Scholar]
- 47.Popat S, Wotherspoon A, Nutting CM, Gonzalez D, Nicholson AG, O’Brien M. Transformation to “high grade” neuroendocrine carcinoma as an acquired drug resistance mechanism in EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;80(1):1–4. doi: 10.1016/j.lungcan.2012.12.019. PubMed PMID: 23312887. [DOI] [PubMed] [Google Scholar]
- 48.Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, et al. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14(18):2175–88. doi: 10.1038/sj.onc.1201062. Epub 1997/05/08. PubMed PMID: 9174053. [DOI] [PubMed] [Google Scholar]
- 49.Roskoski R., Jr. Anaplastic lymphoma kinase (ALK): structure, oncogenic activation, and pharmacological inhibition. Pharmacological research : the official journal of the Italian Pharmacological Society. 2013;68(1):68–94. doi: 10.1016/j.phrs.2012.11.007. PubMed PMID: 23201355. [DOI] [PubMed] [Google Scholar]
- 50.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–83. doi: 10.1158/1078-0432.CCR-08-0168. Epub 2008/07/03. doi: 14/13/4275 [pii] PubMed PMID: 18594010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–9. doi: 10.1016/S1470-2045(12)70344-3. PubMed PMID: 22954507; PubMed Central PMCID: PMC3936578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108(18):7535–40. doi: 10.1073/pnas.1019559108. Epub 2011/04/20. doi: 1019559108 [pii] PubMed PMID: 21502504; PubMed Central PMCID: PMC3088626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 363(18):1734–9. doi: 10.1056/NEJMoa1007478. Epub 2010/10/29. PubMed PMID: 20979473. [DOI] [PubMed] [Google Scholar]
- 54.Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71(18):6051–60. doi: 10.1158/0008-5472.CAN-11-1340. Epub 2011/07/28. doi: 0008-5472.CAN-11-1340 [pii] PubMed PMID: 21791641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki T, Okuda K, Zheng W, Butrynski J, Capelletti M, Wang L, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010;70(24):10038–43. doi: 10.1158/0008-5472.CAN-10-2956. Epub 2010/10/30. doi: 0008-5472.CAN-10-2956 [pii] PubMed PMID: 21030459; PubMed Central PMCID: PMC3045808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanizaki J, Okamoto I, Okabe T, Sakai K, Tanaka K, Hayashi H, et al. Activation of HER family signaling as a mechanism of acquired resistance to ALK inhibitors in EML4-ALK-positive non-small cell lung cancer. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-0392. Epub 2012/07/31. doi: 1078-0432.CCR-12-0392 [pii] PubMed PMID: 22843788. [DOI] [PubMed] [Google Scholar]
- 57.Lovly CM, McDonald NT, Chen H, Ortiz-Cuaran S, Heukamp LC, Yan Y, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med. 2014;20(9):1027–34. doi: 10.1038/nm.3667. PubMed PMID: 25173427; PubMed Central PMCID: PMC4159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crystal AS, Shaw AT, Sequist LV, Friboulet L, Niederst MJ, Lockerman EL, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346(6216):1480–6. doi: 10.1126/science.1254721. PubMed PMID: 25394791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friboulet L, Li N, Katayama R, Lee CC, Gainor JF, Crystal AS, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662–73. doi: 10.1158/2159-8290.CD-13-0846. PubMed PMID: 24675041; PubMed Central PMCID: PMC4068971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–97. doi: 10.1056/NEJMoa1311107. PubMed PMID: 24670165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19(5):679–90. doi: 10.1016/j.ccr.2011.04.004. PubMed PMID: 21575866. [DOI] [PubMed] [Google Scholar]
- 62.Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol. 2013;14(7):590–8. doi: 10.1016/S1470-2045(13)70142-6. PubMed PMID: 23639470. [DOI] [PubMed] [Google Scholar]
- 63.Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, Azada MC, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–28. doi: 10.1016/S1470-2045(14)70362-6. PubMed PMID: 25153538. [DOI] [PubMed] [Google Scholar]
- 64.Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, et al. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res. 2011;71(14):4920–31. doi: 10.1158/0008-5472.CAN-10-3879. Epub 2011/05/27. 0008-5472.CAN-10-3879 [pii]. PubMed PMID: 21613408; PubMed Central PMCID: PMC3138877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horn LIJ, Blumenshcein G, Wakelee H, Arkenau H-T, Dukart G, Liang C, Harrow K, Gibbons J, Lovly CM, Pao W. A phase I trial of X-396, a novel ALK inhibitor, in patients with advanced solid tumors. Journal of Clinical Oncology. 2014 abstract 8030. [Google Scholar]
- 66.Gettinger SNBL, Salgia R, Langer CJ, Gold K, Rosell R, Shaw AT, Weiss GJ, Narasimhan NI, DOrer DJ, Rivera V, Clackson T, Haluska FG, Camidge DR. ALK Inhibitor AP26113 in Patients With Advanced Malignancies, Including ALK+ Non-Small Cell Lung Cancer (NSCLC): Updated Efficacy and Safety Data. 39th Annual Congress of the European Society for Medical Oncology; 2014. [Google Scholar]
- 67.Johnson TW, Richardson PF, Bailey S, Brooun A, Burke BJ, Collins MR, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–44. doi: 10.1021/jm500261q. PubMed PMID: 24819116. [DOI] [PubMed] [Google Scholar]
- 68.Sang J, Acquaviva J, Friedland JC, Smith DL, Sequeira M, Zhang C, et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 2013;3(4):430–43. doi: 10.1158/2159-8290.CD-12-0440. PubMed PMID: 23533265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sequist LV, Gettinger S, Senzer NN, Martins RG, Janne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28(33):4953–60. doi: 10.1200/JCO.2010.30.8338. Epub 2010/10/14. doi: JCO.2010.30.8338 [pii] PubMed PMID: 20940188. [DOI] [PMC free article] [PubMed] [Google Scholar]