Abstract
Improving the structural integrity of bone reduces fracture risk and development of osteoporosis later in life. Exercise can increase the mechanical properties of bone, and this increase is often attributed to the dynamic loading created during exercise. However, the increase in systemic PTH levels during exercise gives reason to hypothesize that PTH signaling also regulates bone adaptation in response to exercise. Therefore, the first aim of this study was to establish the impact PTH signaling has on bone adaptation during exercise by inhibiting PTH signaling with PTH(7-34) and the second aim was to determine if increasing PTH levels during exercise with PTH(1-34) can augment bone adaptation. Thirty minutes after a single bout of running on a treadmill, mice exhibited a two-fold increase in systemic PTH levels. Under the same exercise regimen, the influence of PTH signaling on bone adaptation during exercise was then evaluated in mice after 21 consecutive days of exercise and treatment with PTH(7-34), PTH(1-34), or vehicle. Exercise alone caused a significant increase in trabecular bone volume with adaptation to a more plate-like structure, which was inhibited with PTH(7-34) during exercise. Changes in structural and tissue-level mechanical properties during exercise occurred in the absence of significant changes to cortical bone geometry. Inhibition of PTH signaling during exercise attenuated the changes in structural-level mechanical properties, but not tissue-level properties. Enhanced PTH signaling during exercise with PTH(1-34) increased trabecular and cortical bone volume, but had little effect on the structural and tissue-level mechanical properties compared to exercise alone. Our study is the first to demonstrate that bone adaptation during exercise is not only a function of the dynamic loading, but also PTH release, and that PTH signaling contributes differently at the structural and tissue-levels.
Keywords: Exercise, PTH, Biomechanics, Analysis/Quantitation of Bone, Anabolics
INTRODUCTION
The prevalence of osteoporosis has grown over the years and this disease is estimated to have caused over 9 million fractures across the world, many of which cause permanent disabilities or complications resulting in death (1). Anabolic treatment strategies that enhance the mechanical properties of bone and/or offset the progression of bone loss are highly sought after, but not readily available (2). Current treatments attempt to improve the mechanical integrity of the skeleton by increasing the quantity of bone or improving its quality, as defined by its strength and toughness. Although exercise has been widely considered a potential treatment strategy to improve the mechanical properties of bone, the specific mechanisms through which mechanical properties are increased during exercise remain unclear (3). Insight into such mechanisms will contribute to developing treatments that utilize exercise to improve the integrity of bone or even prevent osteoporosis later in life.
Among humans, both treadmill running and swimming increase bone formation based on increased bone mineral density (BMD) (3), along with serum levels of bone-alkaline phosphatase (4,5). In rodents, both swimming and treadmill running increase trabecular bone volume and thickness (6,7), along with significant increases in both tissue and structural-level properties of cortical bone (8–12). The adaptation in structural-level properties of cortical bone is attributed to changes in geometry due to periosteal expansion (11,13). However, running can also modify the tissue-level properties of cortical bone without altering cross-sectional geometry (8,10,12). This adaptation in tissue-level properties, independent of geometric changes, has been associated with changes to the tissue composition, such as increases in collagen cross-linking and changes in the carbonate/phosphate ratio (10,14). Overall, literature supports the general concept that moderate exercise improves the mechanical function of bone by modifying either its structural-level or tissue-level mechanical properties.
The dynamic loading incurred during exercise is considered to have the dominant impact on bone adaptation, and can reach magnitudes of 2000 micro-strain in humans (15). Direct loading of the tibia or ulna of rodents under similar magnitudes of strain initiates osteocyte communication with osteoclasts and osteoblasts responsible for bone remodeling (16–18), along with the ensuing increases in bone strength (19–22). In addition to the influence dynamic loading has on bone adaptation, exercise also provides other stimuli that play a significant role in adaptation, such as the release of parathyroid hormone (PTH) (23). In both humans and animals, the transient increase in systemic PTH levels is dependent on the type, intensity and duration of exercise (4,5,9,23,24). Moderate running among human adults causes a significant increase in systemic levels of PTH at the onset of exercise that remains at high levels 1 to 2 hours after exercise has stopped, before returning to baseline (4,24). Transient increases in systemic PTH during exercise may be analogous to daily treatments of PTH, which over the course of several weeks increase trabecular bone volume along with cortical bone volume and modulus of elasticity (25). Given that PTH treatment can further increase cortical bone formation achieved under direct loading (25,26), PTH signaling during exercise has the potential to synergistically influence bone formation and the adaptation of its mechanical properties.
The purpose of this study therefore was to: 1) establish the impact PTH release has on bone adaptation during exercise by inhibiting the signaling mechanism with PTH(7-34) and 2) to augment bone adaptation by increasing the PTH levels during exercise with PTH(1-34). We hypothesized that inhibiting PTH signaling during exercise will inhibit trabecular bone formation along with inhibiting increases to the structural-level properties of cortical bone, while increasing the PTH levels with PTH(1-34) will increase bone formation and increase its mechanical properties at the structural-level.
METHODS
In-Vivo Protocols
Animal procedures were performed at the University of Michigan with University Committee on Use and Care of Animals (UCUCA) approval. Male C57Bl/6J mice (16 weeks old at the start of each experiment) purchased from Jackson Laboratories (ME), were individually housed throughout the entire study (because of their aggressive nature) with sufficient water, food, and items for enrichment.
Exercise Effects on Systemic PTH Levels
To establish the effect a single bout of exercise has on systemic PTH levels, 50 mice were first subjected to exercise that involved moderate running on a treadmill (Columbus Instruments, Ohio) with a 5° incline for 30-minutes/day and speeds increasing from 8 to 12 m/min over the course of 3 days. After 3 days of training, the mice were assigned to weight-matched groups: baseline or exercise. The exercise group was then divided into 5 sub-groups, each group representing a different time point at which blood samples were taken: 0, 1, 1.5, 3, and 24 hours after the onset of exercise. At each time point, blood samples were also taken from mice in the baseline group, which did not exercise on day 4. For each mouse, ~100 μl of blood was drawn from the submandibular vein into a centrifuge tube with an anti-coagulate and then immediately spun down at 1000 g at 8°C for 15 minutes. The plasma was immediately separated and stored at −80°C for later processing. Plasma samples were prepared and analyzed using the PTH ELISA Kit (Immutopics International) according to the manufacturer's protocol on a microplate reader (Varioskan Flash, Thermo Scientific).
Efficacy of PTH(7-34) for Inhibiting PTH/PTH-Related Protein Receptor
The efficacy of PTH(7-34) to inhibit activation of the PTH/PTH-related protein receptor (PPR) was established based on its ability to inhibit changes in c-Fos gene expression regulated downstream of the PPR. Given that PTH(1-34) activation of the PPR increases c-Fos expression among osteoblasts and osteocytes (27), mice were first treated with PTH(7-34) and then PTH(1-34) either 1 hour or 3 hours later. A total of 35 mice were divided into 5 groups: Vehicle, PTH(7-34), PTH(1-34), PTH(7-34) + PTH(1-34)1hr, and PTH(7-34) + PTH(1-34)3hr. Subcutaneous injections were given to administer 50μl of saline solution (0.9% NaCl) as a vehicle, 60μg/kg (body weight) of (d-Try12, Try34)-bPTH(7-34) (Bachem, CA) in saline solution, or 40μg/kg of hPTH(1-34) (Bachem, CA) in saline solution. After the final injection, mice were euthanized via CO2 asphyxiation, and the tibia extracted, cleaned of excess soft tissue, and then homogenized to extract the mRNA using a Trizol method. The mRNA was purified before generating cDNA (Taqman cDNA synthesis kit, Applied Biosystems). The qRT-PCR was carried out with an Applied BioSystems 7500 RealTime PCR machine along with primers for c-Fos (Mm00487425, Taqman) and GAPDH as an internal control (Mm99999915, Taqman). The standard curve method was used to determine gene expression for each sample relative to GAPDH levels, and then reported as a fold change compared to vehicle treated mice.
Exercise Effects on Bone Adaptation
To determine the influence PTH signaling during exercise has on bone adaptation, 105 male mice were assigned to 1 of 7 weight-matched groups: 3 sedentary groups, 3 exercise groups, and a baseline group. The exercise groups were subjected to treadmill running at 12 m/min on a 5° incline for 30 minutes each day over 21 consecutive days. Each exercise group received daily subcutaneous injections of either 50μl of saline solution (0.9% NaCl) as a vehicle, 60μg/kg (body weight) of (d-Try12, Try34)-bPTH(7-34) (Bachem, CA) in saline solution, or 40μg/kg of hPTH(1-34) (Bachem, CA) in saline solution. Both vehicle and PTH(7-34) treatments were administered 3-hours prior to exercise, while PTH(1-34) was administered 15 minutes prior to exercise. Each sedentary group was also treated with either vehicle, PTH(7-34), or PTH(1-34) over the course of 21 days.
To quantify bone formation histomorphometrically, each mouse was also given an intraperitoneal (IP) injection of Alizerin Red (25mg/kg of body weight) on day 3, and an IP injection of Xylene Orange (90mg/kg body weight) on day 15. Each mouse was then sacrificed on day 22, and both tibiae were extracted and stored at −20°C wrapped in gauze soaked with 1X PBS (phosphate buffered saline) + 57mg/L of calcium. One tibia from each mouse was selected for μCT analysis and mechanical testing, while the contra-lateral tibia was used for histomorphometry.
Micro-CT
Tibiae were scanned prior to mechanical testing in a Scanco microCT system (μCT100 Scanco Medical, Bassersdorf, Switzerland) with the following settings: 12 μm voxel size, medium resolution, 70 kVp, 114 μA, 0.5 mm AL filter, 500ms integration time. Image slices were processed with a greyscale threshold optimized between 0–1000 across a population of samples. For each sample, a 500 μm thick volume of interest, 50 μm below the proximal growth plate, was selected for three-dimensional analysis of the trabecular bone structure. Based on the direct 3-dimensional method provided in the manufacturer's software, the following trabecular parameters were calculated: bone volume fraction (BV/TV), tissue mineral density (TMD), trabecular number (Tb.N), spacing (Tb.Sp), thickness (Tb.Th), and structural model index (SMI). To determine the degree of anisotropy (directional dependence) of the trabeculae, eigenvalues of the fabric tensor were calculated based on the mean intercept length technique, and normalized to obtain H1, H2, and H3 along with the angle between the principal vector and the tibia's longitudinal axis (theta) (28). The degree of anisotropy (DA) of the trabeculae was defined by the ratio between the largest and smallest vectors of the normalized eigenvalues.
Once each tibia was scanned and mechanically tested (see following section), images of each tibia were first rotated to match their position during mechanical testing. The cortical bone thickness (Ct.Th), cross-sectional area (Ct.Ar), moment of inertia about the anterior-posterior axis (Iap) and moment of inertia about the medial-lateral axis (Iml), distance from the most lateral surface to the neutral axis (DN-A), mineral content (BMC), and mineral density (BMD) were determined across a 180 μm thick section at the fracture site and a standard site. The fracture site was defined where failure was initiated during mechanical testing, while the standard site was defined midway between the loading points.
Mechanical Testing
Structural-level and tissue-level properties of each tibia scanned for μCT analysis were determined under four-point bending using an Admet eXpert 450 Universal Testing Machine (11). The base support span was 9mm with a loading span of 3mm. The tibia was positioned in the loading device such that the lateral surface was in compression by placing the most distal portion of the tibia and fibula junction (TFJ) directly over the left-most support point. Each tibia was loaded at a rate of 0.01 mm/s until failure, while the load and displacement were recorded, which were then used to calculate the tissue-level properties based on beam-bending theory.
Histomorphometry
Contralateral tibiae from the 21 day experiment were first dehydrated in graded ethanol concentrations (70%, 80%, 100%) and then embedded in methyl methacrylate (Koldmount Cold Mount kit, Mager Scientific, MI). Sections 1 to 2mm thick were cut at the mid-diaphysis with a diamond wafering blade (Mager Scientific, MI) on a low-speed sectioning saw (South Bay Technology, Model 650, CA) and then polished to a final thickness between 50 and 70 μm on wet silicon carbide abrasive disks. A fluorescence microscope was used to identify tissue surfaces labeled red (Alizerin Red) and orange (xylene). The distance between adjacent labels was quantified to determine the mineralizing surfaces (MS), mineral apposition rate (MAR), and bone formation rate (BFR) along the endosteum and periosteum according to standardized histomorphometric analysis (29).
Statistical Analysis
Paired t-tests were used to determine significant changes in body weight from day 1 to day 21 of the exercise program, with a p-value less than 0.05 denoting significance. All other outcomes were tested for statistical differences between groups in SPSS using a two-way ANOVA for effects of exercise and PTH, with a p-value less than 0.05 indicating a significant difference. A Student-Newman Keuls post hoc test was used to test specific interactions between all three sedentary groups, or all three exercise groups, as well as interactions between exercise and sedentary groups of each individual treatment.
RESULTS
Exercise Increases Systemic PTH Levels
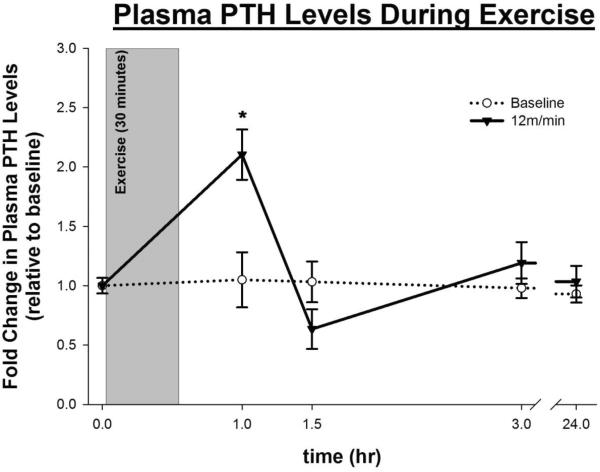
When subjected to a single bout of exercise, mice exhibited a 2-fold increase in systemic PTH levels compared to basal-levels within 1 hour after the onset of exercise (333 ± 49 pg/ml vs. 175 ± 18 pg/ml, p-value = 0.017) (Fig 1). The systemic PTH levels returned to basal-levels at 1.5 hours after the onset of exercise and remained at basal-levels thereafter.
Figure 1.
Figure 1
PTH(7-34) Inhibits Cellular Response to PTH in Bone
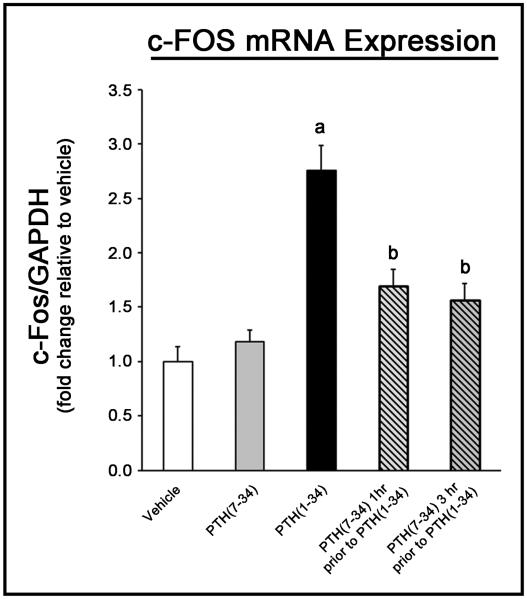
The ability to inhibit the cellular response to PTH within bone using PTH(7-34) was then established based on c-Fos mRNA expression in response to PTH(1-34). Treatment with PTH(1-34) alone caused a significant increase in c-Fos expression (Fig 2), similar to other studies (27). Pre-treating mice with PTH(7-34) 1 hour or 3 hours prior to treatment with PTH(1-34) significantly inhibited the change in c-Fos expression by ~75%, while treatment alone with PTH(7-34) had no significant effect. As a result, pre-treatment with PTH(7-34) prior to exercise was considered sufficient to inhibit the cellular response to PTH during exercise.
Figure 2.
Figure 2
Exercise Causes Weight Loss
Mice in the exercise groups treated with vehicle, PTH(7-34) or PTH(1-34) had significant weight loss that averaged −1.34 ± 0.16g (p-value < 1×10−6), −1.65 ± 0.26g (p-value < 1×10−7), and −1.22 ± 0.20g (p-value < 7×10−6) respectively over the 21 day course of the experiment. Sedentary mice treated with PTH(7-34) also exhibited significant weight loss, which averaged −0.46 ± 0.22g (p-value = 0.044). Sedentary mice treated with vehicle or PTH(1-34) had no significant change in body weight over the 3 week experiment, averaging −0.20 ± 0.24g (p-value = 0.4) and 0.247 ± 0.27g (p-value = 0.3), respectively.
Exercise Increases Trabecular Bone Formation and Decreases Directionality of Trabeculae
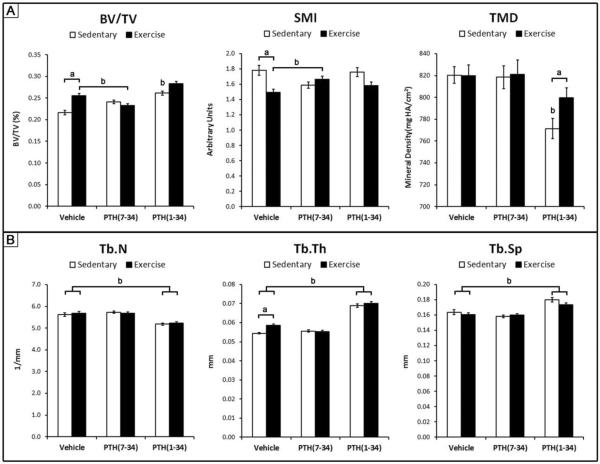
In response to 3 weeks of exercise alone, there was a significant increase in trabecular BV/TV and a significant decrease in SMI compared to sedentary controls (Fig 3). The decrease in SMI indicates that the trabecular structure is shifted towards a more plate-like structure, as opposed to a rod-like structure. The Tb.Th following exercise was significantly larger compared to sedentary controls, while Tb.Sp, Tb.N, and TMD were unaffected.
Figure 3.
Figure 3
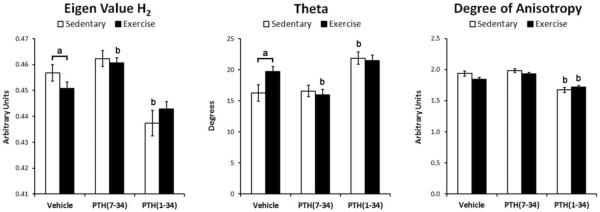
All three eigenvalues of the fabric tensor were unequal, indicating an orthotropic material with the material symmetry predominately aligned with the long axis, represented by H2. Compared to sedentary controls, exercise caused a significant decrease in the eigenvalue H2 and significantly increased its angle (theta) with the anatomical long axis of the tibia (Fig 4). The significant increase in theta of H2 demonstrates less alignment of the trabeculae with the longitudinal axis of the tibia following exercise.
Figure 4.
Figure 4
PTH Signaling During Exercise Contributes To Trabecular Bone Adaptation
As hypothesized, PTH(7-34) treatment during exercise resulted in a significantly lower trabecular BV/TV compared to exercise alone, as well as a significantly greater SMI (Fig. 3). The normalized eigenvector H2 was also significantly greater in magnitude with a smaller angle theta compared to vehicle treatment during exercise (Fig 4).
PTH(1-34) treatment superposed with sedentary and exercise conditions caused a significantly greater Tb.Th, Tb.Sp, and significantly smaller Tb.N compared to the respective vehicle treated controls (Fig 3). The trabecular BV/TV was statistically greater in sedentary mice treated with PTH(1-34) compared to vehicle treatment, but was not statistically different between exercise groups treated with vehicle or PTH(1-34). Superimposing PTH(1-34) treatment with exercise significantly increased the trabecular TMD compared to sedentary mice treated with PTH(1-34). The degree of anisotropy was significantly reduced by PTH(1-34) treatment in both sedentary and exercise conditions compared to the respective vehicle treated controls (Fig. 3). PTH(1-34) treatment of sedentary mice also significantly decreased the eigenvalue H2 and increased its angle theta. Given the inhibitory and additive effect that PTH(7-34) and PTH(1-34) had during exercise respectively, trabecular bone adaptation during exercise is attributed, at least in part, to PTH signaling.
Cortical Bone Formation and Geometry are Increased by PTH(1-34), but not Exercise
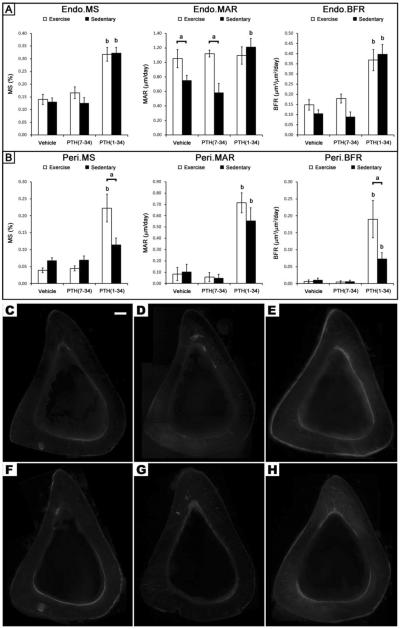
Despite the changes in trabecular bone, exercise had no effect on cortical bone formation rate at either the endosteal or periosteal surface (Fig 5). The only significant effect exercise had on bone histomorphometric indices was a reduction in the mineralization rate along the endosteum (Endo.MAR), but this decrease was not large enough to augment the bone formation rate (Endo.BFR) (Fig 5). PTH(1-34) treatment led to a significantly greater Endo.BFR, due to an increase in Endo.MS, while the significant increase in Perio.BFR was due to an increase in Perio.MS and Perio.MAR. A similar response to PTH(1-34) was observed in the exercise group; however, the Endo.MS was not significantly greater than in the vehicle treated exercise controls.
Figure 5.
Figure 5
At the standard site, exercise had no significant impact on Ct.Ar, Ct.Th, Dn-a, MOI, BMC, and BMD compared to sedentary controls (Table 1). Treatment with PTH(7-34) during exercise or sedentary conditions had no significant impact on cortical bone geometry, BMC, or BMD compared to controls (Table 1). Similar findings were observed at the fracture site (data not shown).
Table 1.
Micro-CT analysis of cortical bone at the standard site.
|
|
|
|||||
|---|---|---|---|---|---|---|
| Sedentary | Exercise | |||||
|
|
|
|||||
| Vehicle Ave. (sem) | PTH(7-34) Ave. (sem) | PTH(1-34) Ave. (sem) | Vehicle Ave. (sem) | PTH(7-34) Ave. (sem) | PTH(1-34) Ave. (sem) | |
|
|
|
|||||
| Tibia Length (cm) | 17.5 (0.06) | 17.4 (0.06) | 17.4 (0.07) | 17.4 (0.06) | 17.3 (0.05) | 17.4 (0.05) |
| Ct.Ar (mm2) | 0.746 (0.016) | 0.743 (0.017) | 0.795 (0.014)a | 0.728 (0.019) | 0.726 (0.009) | 0.789 (0.010)b |
| Ct.Th (mm) | 0.205 (0.004) | 0.208 (0.003) | 0.224 (0.003)a | 0.207 (0.003) | 0.208 (0.003) | 0.220 (0.002)b |
| DN-A (mm) | 0.604 (0.007) | 0.600 (0.009) | 0.608 (0.010) | 0.606 (0.011) | 0.589 (0.010) | 0.605 (0.006) |
| Iap (mm4) | 0.093 (0.004) | 0.089 (0.004) | 0.094 (0.004) | 0.085 (0.005) | 0.082 (0.002) | 0.094 (0.002) |
| Iml (mm4) | 0.172 (0.008) | 0.167 (0.008) | 0.181 (0.006) | 0.162 (0.010) | 0.159 (0.005) | 0.184 (0.006) |
| BMD (mg HA/cm2) | 1200.8 (6.2) | 1210.9 (5.2) | 1202.3 (8.2) | 1204.2 (5.9) | 1215.7 (5.4) | 1202.0 (3.5) |
| BMC (mg HA) | 150.5 (3.6) | 151.2 (3.6) | 160.7 (3.3)a | 147.3 (3.7) | 148.3 (2.2) | 159.3 (2.1)b |
indicates p-value < 0.05 compared to Sedentary + Vehicle and Sedentary + PTH(7-34)
indicates p-value < 0.05 compared to Exercise + Vehicle and Exercise + PTH(7-34)
Among sedentary mice, PTH(1-34) treatment increased Ct.Ar, Ct.Th and BMC at the standard site compared to vehicle or PTH(7-34) treated mice (Table 1). The addition of PTH(1-34) treatment during exercise had a similar effect at the standard site, but also caused a significant increase in Ct.Ar, Ct.Th and BMC at the fracture site compared to vehicle treatment during exercise (data not shown).
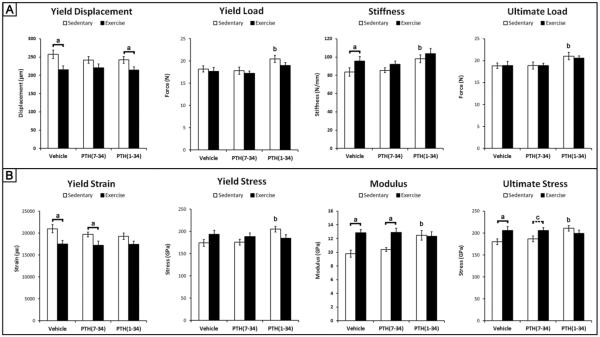
Exercise Alters Structural-Level Properties through PTH Signaling
The structural properties of the tibia that exercise had the largest influence on were yield displacement and stiffness (Fig 6A). The significant increase in stiffness in response to exercise was a result of a significant decrease in the yield displacement, since the yield load was not significantly different compared to sedentary controls. Comparisons across all three exercise and all three sedentary groups demonstrated that exercise had a main effect on work, specifically a significant reduction in pre-yield work (2.42 ± 0.1 mJ sedentary, 2.13 ± 0.1 mJ exercise, p-value = 0.042) and a significant increase in post-yield work (2.14 ± 0.2 sedentary, 2.85 ± 0.3 exercise, p-value = 0.039).
Figure 6.
Figure 6
PTH(7-34) treatment reduced the impact of exercise on both yield displacement and stiffness, demonstrating that PTH signaling contributes to the changes in structural-level properties of cortical bone during exercise (Fig 6A). The addition of PTH(1-34) during exercise did not significantly change any of the structural-level properties compared to exercise controls treated with vehicle, but exhibited a significant decrease in yield displacement compared to sedentary controls treated with PTH(1-34). PTH(1-34) treated sedentary mice had significantly higher stiffness due to an increase in yield load compared to vehicle treated sedentary mice. Despite the main effect of exercise on pre-yield and post-yield work, no significant interactions were found between the individual groups.
Exercise Modifies Tissue-level Properties Independent of PTH Signaling
Despite the absence of changes in cross-sectional geometry or mineral density following exercise, at the tissue-level, there was a significant increase in ultimate stress and modulus with a significant decrease in yield strain compared to sedentary controls (Fig 6B). Unlike the structural-level properties, PTH(7-34) treatment during exercise did not inhibit the significant changes in tissue-level properties, indicating that PTH signaling during exercise does not contribute to the changes in tissue-level properties.
Similar to the structural-level properties, the addition of PTH(1-34) during exercise had no significant influence on tissue-level properties compared to exercise mice treated with vehicle or sedentary controls treated with PTH(1-34) (Fig 6B). Sedentary mice treated with PTH(1-34) did exhibit a significant increase in ultimate stress, yield stress and modulus compared to sedentary mice treated with vehicle.
The average post-yield toughness of all three exercise groups was 2.3 ± 0.2 MPa and significantly greater than the combined average for all three sedentary groups 1.7 ± 0.2 MPa (p-value = 0.035). This main effect of exercise on the post-yield toughness was driven by a significant interaction between exercise and sedentary mice treated with the vehicle (2.39 ± 0.3 MPa exercise, 1.46 ± 0.1 MPa sedentary, p-value = 0.045). No significant interactions were found between groups treated with either PTH(7-34) or PTH(1-34) for pre-yield or post-yield toughness.
DISCUSSION
Based on our findings, the systemic release of PTH during exercise provides a key signaling mechanism for both trabecular and cortical bone adaptation. Although bone adaptation in response to exercise is commonly attributed to dynamic loading, inhibition of PTH signaling during exercise via PTH(7-34) treatment revealed its key role in regulating bone adaptation. Following three weeks of exercise, PTH signaling contributed to an increase in trabecular bone volume that was characterized as a more plate-like structure with thicker trabeculae (Fig. 3). In addition, PTH signaling also contributed to cortical bone adaptation that resulted in increased structural-level properties of the tibia demonstrated by an increase in ultimate load and stiffness without the expense of decreased yield load (Fig 6A). In contrast, the gain in tissue-level properties following three weeks of exercise was independent of PTH signaling (Fig. 6B) and is mediated by another mechanism. Overall this is the first study to demonstrate that PTH signaling during exercise contributes to trabecular and cortical bone adaptation, and that PTH signaling influences the adaptation of cortical bone at the structural and tissue-levels differently.
The dynamic loading and transient increase in PTH levels during exercise provide a unique combination of stimuli, which together regulate bone adaptation. Other exercise regimens that include running and swimming have exhibited a similar increase in trabecular bone formation (6,7). Based on our results, the shift towards a more `plate-like' structure during exercise was not only a function of PTH, but likely due to a combination of factors given the negligible impact PTH(1-34) alone had. This shift to a more `plate-like' structure along with the change in eigenvalues is counter to the shift towards a more `rod-like' structure observed with aging and onset of osteoporosis (30,31). However, the mechanical advantage gained by this adaptation remains unclear despite a more plate-like architecture being associated with increased toughness and resistance to microdamage (32,33).
Similar to previous studies, exercise caused a significant increase in ultimate stress and toughness without significant changes in cortical area, mineral density, or moment of inertia (Fig 6 and Table 1) (10,12). In contrast, the increase in cortical area and BMC following PTH(1-34) treatment corresponded with an increase in structural and tissue-level mechanical properties, such as stiffness and modulus (Fig 6), similar to previous studies (34,35). Although exercise didn't change the quantity of tissue being formed (Fig 5 and Table 1), mechanical loading and PTH can enhance osteoblast expression of collagenous and non-collagenous proteins that have distinct influences over the ensuing mechanical properties (36,37). Exercise can modify the intrinsic tissue properties of cortical bone by altering the carbonate to phosphate ratio, mineral to matrix ratio, crystallinity, as well as the ratio between mature and immature collagen cross-linking (10,14,38,39). Thus, we suspect that the matrix formed during exercise is being modified, and it would prove useful for future studies to identify the physiological stimuli that mediate this response.
A unique finding from this study was the attenuation in periosteal bone formation during exercise despite the addition of PTH(1-34) (Fig 5H). Under sedentary conditions, treatment with PTH(1-34) caused an increase in periosteal BFR from an increase in mineralizing surface and apposition rate, similar to previous studies (34,35,40,41). Although the addition of PTH(1-34) during exercise significantly increased the periosteal BFR compared to exercise alone, it was significantly less compared to PTH(1-34) treatment of sedentary mice due to a significant decrease in the mineralizing surface (Fig 5H). The typical increase in mineralizing surface by PTH(1-34) at the periosteum could be counteracted during exercise by a reduction in calcium availability due to an increased demand associated with muscle activity or development along the periosteum (42,43). In the case that muscle demand for calcium is a limiting factor for mineralization at the periosteum, increasing the available calcium may enhance this response. Although, calcium supplementation alongside exercise can increase the bone mineral density at the femoral neck (44), its impact on mineralization at the periosteum is unclear. In addition, the use of calcium supplements must be carefully assessed to ensure they do not impair the systemic release of PTH during exercise, which occurs in response to a decrease in calcium reabsorption through the kidney (24,45).
Increasing the levels of PTH during exercise via PTH(1-34) treatment superimposed the effects of PTH(1-34) onto those of exercise to regulate the adaptation of trabecular architecture. The increase in Tb.Th from PTH(1-34) treatment corresponded with an increase in Tb.Sp, similar to other studies that have attributed this type of response to the fusion of adjacent trabeculae as they grow thicker, which in turn explains the decrease in Tb.N (46,47). Although a moderate increase in trabecular BV/TV was achieved with the addition of PTH(1-34) treatment during exercise (Fig. 3), it was not significantly greater compared to exercise or PTH(1-34) treatment alone. Similarly, PTH(1-34) treatment during exercise did not enhance the changes in structural or tissue-level mechanical properties normally gained in response to exercise or PTH(1-34) individually (Fig. 6). In contrast, treating with PTH(1-34) 30–45 minutes prior to dynamic loading has an additive effect on trabecular and cortical bone formation that is greater than the individual effect of PTH(1-34) or dynamic loading (25,26). As a result, the lack of significance or additive effect from PTH(1-34) observed in our study is considered a function of timing in PTH(1-34) treatment. Initiating the cellular response to PTH 30 or 45 minutes prior to exercise has the potential to increase BV/TV or enhance the mechanical properties beyond what we observed by treating only 15 minutes prior to exercise (25,26). Overall, PTH(1-34) treatment 15 minutes prior to exercise provided a means to increase bone quantity, which can have long term benefits, while additional studies are needed to establish the appropriate timing of PTH(1-34) that will optimize its impact on bone adaptation. In addition, the lack of change in cortical area and BFR during exercise indicates that the primary action of PTH released during exercise is not the same as PTH(1-34).
At the whole bone level, exercise caused significant changes in both structural and tissue-level mechanical properties (Fig 6), without significant changes in cortical bone geometry, mineral density, or BFR (Fig 5). Under the same exercise regimen, different age mice undergo similar changes in yield-deformation and ultimate stress (10–12), while younger mice exhibit additional changes in yield-load, cortical area, and mineral density (11,14). With increasing age, bone adaptation in response to exercise is significantly diminished (48). In addition to age, the intensity of exercise can also have a significant effect on bone adaptation (49). As a result, it may prove useful to investigate if increasing the intensity of exercise would increase cortical area in addition to providing the changes in mechanical properties observed in our study.
One of the limitations in this study was the inability to distinguish which source of PTH was mediating the adaptation in cortical and trabecular bone during exercise. PTH secreted by the parathyroid gland, along with the PTH related protein (PTH-rP) secreted within bone or other tissues have the ability to activate the PTH/PTH-rP receptor (PPR) (50). The transient increase in systemic levels of PTH measured during a single bout of exercise (Fig. 1) represents the 84 amino acid sequence of intact PTH secreted by the parathyroid gland. At the cellular level, PTH and PTH-rP have the same affinities for the PPR due to structural similarities within the 1–13 and 29–34 amino acid sequences (50). As a result, the available PTH in bone during exercise is under-represented by the systemic measurements of PTH conducted here. Despite this limitation, we demonstrated that bone adaptation in response to exercise is influenced by PTH or PTH-rP mediated signaling. The use of PTH(7-34) to inhibit the cellular response to PTH or PTH-rP in bone has been widely used and demonstrated to not alter systemic PTH levels or urinary secretion of calcium, phosphorus, and adenosine 3',5' – cyclic monophosphate (51–54).
In conclusion, exercise subjects bone to increased dynamic loading and PTH levels, both of which are key stimulants for bone adaptation. In this study we demonstrated that PTH signaling during exercise increases trabecular bone formation with a plate-like structure and improves structural-level mechanical properties of cortical bone, while tissue-level properties are modified independent of PTH signaling during exercise. In addition, we found that PTH(1-34) treatment during exercise increases trabecular bone formation, along with persiosteal and endosteal cortical bone formation beyond what exercise alone provides. Overall, the distinctive increase in PTH levels during exercise plays a key role in bone adaptation, specifically trabecular bone formation and the structural-level properties of cortical bone, while PTH(1-34) can be used to improve cortical bone formation with superior structural and tissue-level mechanical properties.
ACKNOWLEDGEMENTS
This work was funded by the following sources: NIH/NIDCR DE07057 (DHK), NIH/NIAMS AR056657 (DHK), and NIH/NIAMS AR064668 (JDG).
Funding Sources: NIH DE07057 (DHK), AR056657 (DHK), AR064668 (JDG)
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
DISCLOSURES: The authors state that they have no conflicts of interest.
REFERENCES
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis International. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Marie PJ, Kassem M. Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. European Journal of Endocrinology. 2011;165(1):1–10. doi: 10.1530/EJE-11-0132. [DOI] [PubMed] [Google Scholar]
- 3.Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcified Tissue International. 2000;67(1):10–18. doi: 10.1007/s00223001089. [DOI] [PubMed] [Google Scholar]
- 4.Scott JPR, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. Journal of Applied Physiology. 2011;110(2):423–432. doi: 10.1152/japplphysiol.00764.2010. [DOI] [PubMed] [Google Scholar]
- 5.Maimoun L, Simar D, Caillaud C, Coste O, Barbotte E, Peruchon E, Rossi M, Mariano-Goulart D. Response of calciotropic hormones and bone turnover to brisk walking according to age and fitness level. Journal of Science and Medicine in Sport. 2009;12(4):463–467. doi: 10.1016/j.jsams.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Joo Y, Sone T, Fukunaga M, Onodera S. Effects of endurance treadmill exercise on bone mineral density and bone microarchitecture in the young growing rat: Microcomputed tomography and dual energy x-ray absorptiometry study. Osteoporosis International. 2000;11:44–45. [Google Scholar]
- 7.Warner SE, Shea JE, Miller SC, Shaw JM. Adaptations in cortical and trabecular bone in response to mechanical loading with and without weight bearing. Calcified Tissue International. 2006;79(6):395–403. doi: 10.1007/s00223-005-0293-3. [DOI] [PubMed] [Google Scholar]
- 8.Huang TH, Lin SC, Chang FL, Hsieh SS, Liu SH, Yang RS. Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. Journal of Applied Physiology. 2003;95(1):300–307. doi: 10.1152/japplphysiol.01076.2002. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto J, Shimamura C, Takeda T, Abe H, Ichimura S, Sato Y, Toyama Y. Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats. Journal of Bone and Mineral Metabolism. 2004;22(1):26–31. doi: 10.1007/s00774-003-0443-5. [DOI] [PubMed] [Google Scholar]
- 10.Kohn DH, Sahar ND, Wallace JM, Golcuk K, Morris MD. Exercise Alters Mineral and Matrix Composition in the Absence of Adding New Bone. Cells Tissues Organs. 2009;189(1–4):33–37. doi: 10.1159/000151452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace JM, Rajachar RM, Allen MR, Bloomfield SA, Robey PG, Young MF, Kohn DH. Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone. 2007;40(4):1120–1127. doi: 10.1016/j.bone.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace JM, Ron MS, Kohn DH. Short-Term Exercise in Mice Increases Tibial Post-Yield Mechanical Properties While Two Weeks of Latency Following Exercise Increases Tissue-Level Strength. Calcified Tissue International. 2009;84(4):297–304. doi: 10.1007/s00223-009-9228-8. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto J, Yeh JK, Aloia JF. Differential effect of treadmill exercise on three cancellous bone sites in the young growing rat. Bone. 1999;24(3):163–169. doi: 10.1016/s8756-3282(98)00189-6. [DOI] [PubMed] [Google Scholar]
- 14.Wallace JM, Golcuk K, Morris MD, Kohn DH. Inbred Strain-Specific Effects of Exercise in Wild Type and Biglycan Deficient Mice. Annals of Biomedical Engineering. 2010;38(4):1607–1617. doi: 10.1007/s10439-009-9881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405–410. doi: 10.1016/8756-3282(96)00028-2. [DOI] [PubMed] [Google Scholar]
- 16.Brodt MD, Silva MJ. Aged Mice Have Enhanced Endocortical Response and Normal Periosteal Response Compared With Young-Adult Mice Following 1 Week of Axial Tibial Compression. Journal of Bone and Mineral Research. 2010;25(9):2006–2015. doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosley JR, Lanyon LE. Strain rate as a controlling influence on adaptive modeling in response to dynamic loading of the ulna in growing male rats. Bone. 1998;23(4):313–318. doi: 10.1016/s8756-3282(98)00113-6. [DOI] [PubMed] [Google Scholar]
- 18.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annual Review of Biomedical Engineering. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 19.Chen XY, Zhang XZ, Guo Y, Li RX, Lin JJ, Wei Y. The establishment of a mechanobiology model of bone and functional adaptation in response to mechanical loading. Clinical Biomechanics. 2008;23:S88–S95. doi: 10.1016/j.clinbiomech.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 20.De Souza RL, Matsuura M, Eckstein F, Rawlinson SCF, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: A new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37(6):810–818. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Holguin N, Brodt MD, Sanchez ME, Kotiya AA, Silva MJ. Adaptation of Tibial Structure and Strength to Axial Compression Depends on Loading History in Both C57BL/6 and BALB/c Mice. Calcified Tissue International. 2013;93(3):211–221. doi: 10.1007/s00223-013-9744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. Journal of Bone and Mineral Research. 2002;17(8):1545–1554. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 23.Maimoun L, Sultan C. Effect of Physical Activity on Calcium Homeostasis and Calciotropic Hormones: A Review. Calcified Tissue International. 2009;85(4):277–286. doi: 10.1007/s00223-009-9277-z. [DOI] [PubMed] [Google Scholar]
- 24.Brahm H, PiehlAulin K, Ljunghall S. Bone metabolism during exercise and recovery: The influence of plasma volume and physical fitness. Calcified Tissue International. 1997;61(3):192–198. doi: 10.1007/s002239900322. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone. 2008;43(2):238–248. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Chow JWM, Fox S, Jagger CJ, Chambers TJ. Role for parathyroid hormone in mechanical responsiveness of rat bone. American Journal of Physiology-Endocrinology and Metabolism. 1998;274(1):E146–E154. doi: 10.1152/ajpendo.1998.274.1.E146. [DOI] [PubMed] [Google Scholar]
- 27.McCauley LK, Koh AJ, Beecher CA, Rosol TJ. Proto-oncogene c-fos is transcriptionally regulated by parathyroid hormone (PTH) and PTH-related protein in a cyclic adenosine monophosphate-dependent manner in osteoblastic cells. Endocrinology. 1997;138(12):5427–5433. doi: 10.1210/endo.138.12.5587. [DOI] [PubMed] [Google Scholar]
- 28.Cowin SC. The Relationship between the Elasticity Tensor and the Fabric Tensor. Mechanics of Materials. 1985;4(2):137–147. doi: 10.1016/j.mechmat.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone and Mineral Research. 2013;28(1):1–16. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding M, Hvid I. Quantification of age-related changes in the structure model type and trabecular thickness of human tibial cancellous bone. Bone. 2000;26(3):291–295. doi: 10.1016/s8756-3282(99)00281-1. [DOI] [PubMed] [Google Scholar]
- 31.Brouwers JEM, Lambers FM, Gasser JA, van Rietbergen B, Huiskes R. Bone degeneration and recovery after early and late bisphosphonate treatment of ovariectomized wistar rats assessed by in vivo micro-computed tomography. Calcified Tissue International. 2008;82(3):202–211. doi: 10.1007/s00223-007-9084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrison JG, Slaboch CL, Niebur GL. Density and architecture have greater effects on the toughness of trabecular bone than damage. Bone. 2009;44(5):924–929. doi: 10.1016/j.bone.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teo JCM, Si-Hoe KM, Keh JEL, Teoh SH. Relationship between CT intensity, micro-architecture and mechanical properties of porcine vertebral cancellous bone. Clinical Biomechanics. 2006;21(3):235–244. doi: 10.1016/j.clinbiomech.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Ejersted C, Andreassen TT, Nilsson MHL, Oxlund H. Human Parathyroid Hormone(1-34) Increases Bone-Formation and Strength of Cortical Bone in Aged Rats. European Journal of Endocrinology. 1994;130(2):201–207. doi: 10.1530/eje.0.1300201. [DOI] [PubMed] [Google Scholar]
- 35.Hirano T, Burr DB, Turner CH, Sato M, Cain RL, Hock JM. Anabolic effects of human biosynthetic parathyroid hormone fragment (1-34), LY333334, on remodeling and mechanical properties of cortical bone in rabbits. Journal of Bone and Mineral Research. 1999;14(4):536–545. doi: 10.1359/jbmr.1999.14.4.536. [DOI] [PubMed] [Google Scholar]
- 36.Swarthout JT, D'Alonzo RC, Selvamurugan N, Partridge NC. Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene. 2002;282(1–2):1–17. doi: 10.1016/s0378-1119(01)00798-3. [DOI] [PubMed] [Google Scholar]
- 37.Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends in Molecular Medicine. 2009;15(5):208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Follet H, Boivin G, Rumelhart C, Meunier PJ. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone. 2004;34(5):783–789. doi: 10.1016/j.bone.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Morris MD, Mandair GS. Raman Assessment of Bone Quality. Clinical Orthopaedics and Related Research. 2011;469(8):2160–2169. doi: 10.1007/s11999-010-1692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iida-Klein A, Lu SS, Cosman F, Lindsay R, Dempster DW. Effects of cyclic vs. daily treatment with human parathyroid hormone (1-34) on murine bone structure and cellular activity. Bone. 2007;40(2):391–398. doi: 10.1016/j.bone.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Zhou H, Iida-Klein A, Lu SS, Ducayen-Knowles M, Levine LR, Dempster DW, Lindsay R. Anabolic action of parathyroid hormone on cortical and cancellous bone differs between axial and appendicular skeletal sites in mice. Bone. 2003;32(5):513–520. doi: 10.1016/s8756-3282(03)00057-7. [DOI] [PubMed] [Google Scholar]
- 42.Frost HM, Schonau E. The “muscle-bone unit” in children and adolescents: A 2000 overview. Journal of Pediatric Endocrinology & Metabolism. 2000;13(6):571–590. doi: 10.1515/jpem.2000.13.6.571. [DOI] [PubMed] [Google Scholar]
- 43.Wassner SJ, Li JB, Sperduto A, Norman ME. Vitamin-D Deficiency, Hypocalcemia, and Increased Skeletal-Muscle Degradation in Rats. Journal of Clinical Investigation. 1983;72(1):102–112. doi: 10.1172/JCI110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prince R, Devine A, Dick I, Criddle A, Kerr D, Kent N, Price R, Randell A. The Effects of Calcium Supplementation (Milk Powder or Tablets) and Exercise on Bone-Density in Postmenopausal Women. Journal of Bone and Mineral Research. 1995;10(7):1068–1075. doi: 10.1002/jbmr.5650100711. [DOI] [PubMed] [Google Scholar]
- 45.Ashizawa N, Fujimura R, Tokuyama K, Suzuki M. A bout of resistance exercise increases urinary calcium independently of osteoclastic activation in men. Journal of Applied Physiology. 1997;83(4):1159–1163. doi: 10.1152/jappl.1997.83.4.1159. [DOI] [PubMed] [Google Scholar]
- 46.Lynch MA, Brodt MD, Stephens AL, Civitelli R, Silva MJ. Low-Magnitude Whole-Body Vibration Does Not Enhance the Anabolic Skeletal Effects of Intermittent PTH in Adult Mice. Journal of Orthopaedic Research. 2011;29(4):465–472. doi: 10.1002/jor.21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheehan S, Muthusamy A, Paul E, Sikes RA, Gomes RR. Short-term intermittent PTH 1-34 administration enhances bone formation in SCID/Beige mice. Endocrine Journal. 2010;57(5):373–382. doi: 10.1507/endocrj.k09e-349. [DOI] [PubMed] [Google Scholar]
- 48.Hoshi A, Watanabe H, Chiba M, Inaba Y. Effects of exercise at different ages on bone density and mechanical properties of femoral bone of aged mice. Tohoku Journal of Experimental Medicine. 1998;185(1):15–24. doi: 10.1620/tjem.185.15. [DOI] [PubMed] [Google Scholar]
- 49.Peng ZQ, Vaananen HK, Tuukkanen J. Ovariectomy-induced bone loss can be affected by different intensities of treadmill running exercise in rats. Calcified Tissue International. 1997;60(5):441–448. doi: 10.1007/s002239900260. [DOI] [PubMed] [Google Scholar]
- 50.Esbrit P, Alcaraz MJ. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochemical Pharmacology. 2013;85(10):1417–1423. doi: 10.1016/j.bcp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Rosen HN, Lim M, Garber J, Moreau S, Bhargava HN, Pallotta J, Spark R, Greenspan S, Rosenblatt M, Chorev M. The effect of PTH antagonist BIM-44002 on serum calcium and PTH levels in hypercalcemic hyperparathyroid patients. Calcified Tissue International. 1997;61(6):455–459. doi: 10.1007/s002239900367. [DOI] [PubMed] [Google Scholar]
- 52.Caulfield MP, Rosenblatt M. Parathyroid-Hormone Receptor Interactions. Trends in Endocrinology and Metabolism. 1990;1(3):164–168. doi: 10.1016/1043-2760(90)90030-7. [DOI] [PubMed] [Google Scholar]
- 53.Goldman ME, Mckee RL, Caulfield MP, Reagan JE, Levy JJ, Gay CT, Dehaven PA, Rosenblatt M, Chorev M. A New Highly Potent Parathyroid-Hormone Antagonist - [D-Trp12, Tyr34]Bpth-(7--34)Nh2. Endocrinology. 1988;123(5):2597–2599. doi: 10.1210/endo-123-5-2597. [DOI] [PubMed] [Google Scholar]
- 54.Horiuchi N, Hongo T, Clemens TL. A 7-34 Analog of the Parathyroid Hormone-Related Protein Has Potent Antagonist and Partial Agonist Activity Invivo. Bone and Mineral. 1991;12(3):181–188. doi: 10.1016/0169-6009(91)90031-t. [DOI] [PubMed] [Google Scholar]