Abstract
Recent evidence suggests that blockade of the hypocretin receptor 1 may act as a useful pharmacotherapy for cocaine abuse. Here we investigated the extent to which various doses of a hypocretin receptor 1 antagonist, SB-334867, affect cocaine self-administration at varying doses of cocaine and across a range of effort requirements, and tested if these SB-334867 doses produce sedative effects. First, we trained animals to self-administer one of three doses of cocaine on a progressive ratio schedule, and then tested the effects of three doses of SB-334867. Responding for cocaine was then analyzed to segregate features of relatively high and low effort requirements across the progressive ratio session. In another set of experiments we tested the sleep-promoting effects of the same doses of SB-334867. Our data indicate that blockade of hypocretin receptor 1 preferentially reduces high effort responding for cocaine at levels that do not promote sedation.
Keywords: SB-334867, EEG/EMG, progressive ratio, addiction, drug abuse, orexin
1.0 Introduction
Hypocretins (also known as orexins) are excitatory neuropeptides synthesized by a confined group of neurons located in the lateral hypothalamus and perifornical regions. These neurons project widely throughout the brain and interact with two G-protein coupled receptors, the hypocretin receptor 1 (HCRTr1) and hypocretin receptor 2 (HCRTr2) [1, 2]. Early investigation into the function of this system established its role in the regulation of arousal and arousal-related processes (for a review see; [3]). Later, a series of studies indicated that the hypocretin system may impact motivational processes via projections to the ventral tegmental area (VTA)[4–6]. Consistent with the anatomy, hypocretin peptides increase firing frequency of VTA dopamine (DA) neurons directly [7] and through enhancement of glutamatergic inputs to DA neurons [8, 9]. Moreover, hypocretin peptides enhance DA signaling in VTA target regions including the prefrontal cortex and the nucleus accumbens (NAc) [10–12], and increase the effects of cocaine on DA tone and stimulated DA release in the NAc core [12]. In accordance with these observations, blockade of HCRTr1 reduces DA neuron firing [13], however, the impact of HCRTr1 blockade on synaptic output of DA is more complex. While systemic blockade of HCRTr1 has no effect on DA tone in the NAc core [11, 14] or shell [15] as measured by in vivo micro dialysis, it does reduce phasic release of DA in the NAc core as measured by in vivo fast scan cyclic voltammetry [14]. Moreover, blockade of HCRTr1 reduces the effects of cocaine on DA tone and stimulated DA release in the NAc core [14]. These reports provide strong evidence that the hypocretin system participates in the regulation of reward and reinforcement processes dependent on DA signaling.
In addition to influencing DA signaling, the hypocretin system regulates aspects of cocaine self-administration behavior. Specifically, it has been suggested that hypocretin preferentially regulates appetitive behaviors that require high effort, but has little effect on consummatory behaviors associated with low effort [12, 14]. Classically, the modulation of appetitive behaviors has been tested using a progressive ratio (PR) schedule that increases response requirements, and therefore effort requirements, across a given session [16]. Under a PR schedule, hypocretin-1 peptide promotes responding for cocaine while blockade of HCRTr1 produces the opposite effects [9, 14]. In contrast, modulation of consummatory behaviors has classically been tested with fixed ratio (FR) schedules where lever press requirements, and therefore effort requirements, remain low and constant across a session. Under these conditions, hypocretin manipulations leave consummatory behaviors intact [12, 14, 17].
It should be noted, however, that the degree of effort an animal is required to expend within any self-administration paradigm is a function of both the number of responses required to obtain drug as well as the dose of drug provided [18], and the ability of pharmacological pretreatments to reduce cocaine self-administration appears to depend on this relationship [19]. The hypocretin antagonist studies outlined above used similar doses of cocaine (0.50 – 0.75 mg/kg) and analyzed responding solely at low or high response requirements. Therefore it remains unclear as to whether the effects of hypocretin manipulations on cocaine self-administration will vary as a function of dose provided and response requirement.
The hypocretins also participate in the regulation of arousal, and in particular sleep/wake behavior. This has raised concerns that some of the behavioral effects of HCRTr1 blockade may be mediated through gross deficits in arousal rather than more direct disruption of circuits implicated in motivation. Several studies have begun to disentangle the roles of hypocretin in the regulation of sleep/wake cycle and motivational processes, and it appears that the reward and reinforcement influences of hypocretin may be mediated primarily through the HCRTr1 receptor [12, 14, 17, 20], without associated changes in sleep/wake activity [21–23]. Nevertheless, there remains some debate over the sleep promoting / sedative effects of HCRTr1 blockade [see 24], and no study as of yet has monitored the effects of HCRTr1 blockade on motivation and sleep using identical hypocretin agents and dosing.
The current studies examined the extent to which the HCRTr1 antagonist SB-334867 affects responding for three unit doses of cocaine with varying reinforcing efficacies (0.375, 0.75 and 1.5 mg/kg), including the maximally reinforcing dose of cocaine for this schedule (1.5 mg/kg) [16]. Rats were trained to take cocaine on a PR schedule of reinforcement and were then tested with systemic treatment of 7.5, 15, or 30 mg/kg SB-334867, a HCRTr1 antagonist with 50 fold affinity for HCRTr1 over HCRTr2 [25]. Data were then analyzed in accordance to a two phase model that addresses features of relatively low effort and high effort behavior within the PR session. Finally, we tested the effects of 7.5, 15, and 30 mg/kg SB-334867 on sleep with electroencephalographic (EEG) and electromyographic (EMG) recordings in order to rule out sleep associated confounds.
2.0 Materials and Methods
2.1 Animals
Male Sprague-Dawley rats (340–440 g, Harlan, Frederick, MD) were given ad libitum access to food and water and kept on a reverse 12:12 hr light:dark cycle (lights on at 15:00 hr). All protocols and animal care procedures were maintained in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals: Eighth Edition (The National Academies Press, Washington, DC, 2011) and approved by the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
2.2 Chemicals and Dosing
SB-334867 is considered to be a relatively selective HCRTr1 antagonist with some off target interactions, but at least 30–100 fold higher selectivity for the HCRTr1 over HCRTr2 and other potential targets [25, 26]. SB-334867, was obtained as a free base (Tocris R & D, Minneapolis, MN), and was stored desiccated for no more than 3 months in a light impermeable bottle to minimize decomposition [27]. Drug was prepared as a suspension in 10% β-cyclodextran + 4% dimethyl sulfoxide in distilled H2O, and was administered 30 min prior to behavioral testing as a single 2 ml intraperitoneal (i.p.) dose. Selected doses were based on previous studies indicating changes in drug associated behavior and DA signaling [14, 17, 28–30].
2.3 Self administration
2.3.1 Surgery
Rats used for self-administration experiments were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg), and implanted with an intravenous (i.v.) silastic catheter placed into the right jugular vein. Rats received post-surgical antibiotic (Neo-Predef, Pharmacia & Upjohn Company, New York, NY) and analgesic (5 mg/kg; Ketoprofen, Patterson Veterinary, Devens, MA) and recovered for 3 days prior to training.
2.3.2 Training and Testing
Rats were trained to self-administer cocaine on a FR schedule in which single lever presses result in single injections of cocaine. I.v. catheters were connected through a stainless steel spring to a counter balanced swivel (Instech Laboratories, Plymouth Meeting, PA, USA). Lever responses resulted in delivery of 0.75 mg/kg cocaine (in saline; National Institute on Drug Abuse) over an approximate 5 sec period followed by a 20 secinter-trialinterval. FR training sessions were terminated after 20 injections. Once stable patterns of cocaine self-administration were reached (~2–4 days) rats were separated into one of three groups, and switched to 0.375, 0.75 or 1.5 mg/kg cocaine dose on a PR schedule for additional training and SB-334867 testing.
Throughout the PR schedule rats were given access to a response lever at 10:00 hr, and single cocaine injections were contingent upon an increasing number of responses: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, and 603 [16]. When the required number of responses was made, a single 0.375, 0.75 or 1.5 mg/kg cocaine injection was delivered. The self-administration session was terminated after 6 hr. Following three days of stable baseline responding (less than 20% variance with no ascending or descending trends), rats were treated with vehicle or varying doses of SB-334867.Rats received i.p. vehicle or SB-334867 in the middle of their dark phase, which corresponded to 30 min before onset of the self-administration session (09:30 hr). Rats were treated with vehicle and each dose of SB-334867 based on a Latin-square design, with a minimum of 3 days between treatments. Individual rats were trained on the PR schedule at one dose of cocaine, and tested at each dose of SB-334867. All rats were tested during the dark/activity phase of the light/dark cycle.
2.3.3 Data Analysis
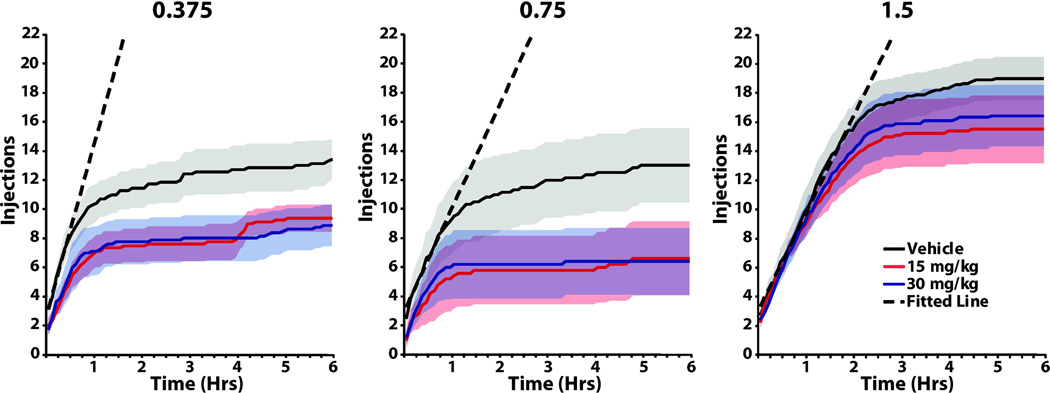
To examine the effect of SB-334867 on patterns of responding we averaged the cumulative number of injections across animals in to 5 min time bins across the duration of the PR session. Initial analysis of these plots indicates two distinct phases of responding (Figure 1). First, there is an initial linear phase, during which the average injection rate remains largely constant and is faster for low doses of cocaine (Table 1). These characteristics are similar to that observed in studies using low effort FR schedules [31, 32], and indicate that responding during this period may represent consumption responding. For these reasons we have labeled this as the consumption phase of responding. Second, there is a later, non-linear phase that occurs due to increased lever press requirements. Responding during this phase represents motivation for an animal to obtain a reward, thus we labeled this as the appetitive phase of responding. To quantitatively define the temporal profile of the two phases for each cocaine dose we fitted the cumulative injection data from the vehicle treatment group with multiple linear functions. Lines were fitted starting from the first 5 min time bin, and we selected the line that had an R2 greater than 0.99 and that encompassed the greatest amount of time (Table 1). The data encompassed by the line was defined as the consumption phase. The Supplementary Information available online contains a more detailed explanation for these analyses.
Figure 1.
SB-334867 differentially alters responding for multiple doses of cocaine on a progressive ratio schedule. Shown is the mean ± SEM number of injections taken across the 6 hr progressive ratio session for 0.375, 0.75, or 1.5 mg/kg unit doses of cocaine and following pre treatment with Vehicle, 15 or 30 mg/kg SB-334867. The dashed line represents the linear fit used to segregate phases of responding for each unit dose of cocaine. Analyses showed no significant differences for the 7.5 mg/kg dose of SB-334867 and thus this data was not included for clarity. Solid lines represent mean values while corresponding shaded regions represent SEM.
Table. 1.
Consumption phase characteristics for animals self-administering 0.375, 0.75 and 1.5 mg/kg cocaine and treated with vehicle. Tabulated are the R2 values for the lines used to segregate phases of responding, the duration of the consumption phase, the mean ± SEM number of injections taken during the consumption phase, and the mean ± SEM injection rate for each dose of cocaine tested.
| 0.375 | 0.75 | 1.5 | |
| R2 | 0.9907 | 0.9912 | 0.9915 |
| Time (min) | 30 | 50 | 125 |
| Injections | 7.85 ± 1.03 | 8.5 ± 2.04 | 15.89 ± 1.57 |
| Rate (inj/hr) | 11.80 ± 2.04 | 8.09 ± 1.89 | 6.71 ± 0.74 |
To examine the effects of SB-334867 on consumption responding we measured the total number of injections taken and the injection rate across the consumption phase. To examine the effect of SB-334867 on appetitive responding we measured lever presses across the entire session as well as ‘breakpoints’ which are defined as the total number of injections an animal received in a given testing session. For statistical analysis, all measures were expressed as a percentage change relative to the previous 3 days of baseline responding, and the effect of vehicle relative to baseline responding was examined using paired t-tests. The effects of hypocretin antagonists on self-administration were not normally distributed, and thus were assessed using Kruskal-Wallace one-way analysis of variance (vehicle and each dose of antagonist). When statistical significance was obtained, Dunnett’s post hoc tests, using vehicle as the control, were conducted. Post hoc tests were conducted using one-tailed tests given that multiple previous studies have clearly designated the directionality of SB-334867 effects on cocaine self-administration [9, 14].
2.4 Sleep
2.4.1 Surgery
Animals were anesthetized with 5% isoflurane, and then placed in a stereotaxic apparatus where anesthesia was maintained with 1.5% isoflurane. Two stainless steel screws were implanted for EEG recording; one placed above neocortex (Bregma +1 mm A/P, +3.0 mm M/L) and the other above the hippocampus (Bregma −2.5 mm A/P, +3.2 mm M/L). Two stainless steel electrodes (Cooner Wire, Chatsworth, CA) were implanted into the neck muscle for recording EMG activity. Rats received post-surgical antibiotic (Neo-Predef, Pharmacia & Upjohn Company, New York, NY) and analgesic (5 mg/kg; Ketoprofen, Patterson Veterinary, Devens, MA), and were allowed to recover for 3 days before testing.
2.3.2 Testing
Following recovery, animals were placed into anacrylic recording chamber (14 × 14 × 20 inches), with ad libitum access to food and water. Electrodes were connected to a Power lab 4/35 system (AD Instruments, Colorado Springs, CO) using a commutator (Crist Instrument, Hagerstown, MD). Animals were then given approximately 12 hr to acclimate to the testing environment, during which no signals were recorded. After the initial 12 hr acclimation period, baseline EEG (0.3–100.0 Hz band pass) and EMG signals (1.0–50.0 Hz band pass) were amplified, filtered, and recorded using Labchart 7 (AD Instruments) for a period of 24 hr. Following the baseline recording, animals were treated with vehicle or SB-334467 at one of three doses (7.5, 15, or 30 mg/kg) using a repeated measures, counterbalanced design, such that all rats were tested twice, once with vehicle and once with one of three doses of SB-334867 with 72 hrs between treatments. Compounds were administered i.p. in a single 2 mL injection, given in the middle of the animal’s dark phase (09:30 hr). EEG/EMG activity was recorded for the 24 hrs following vehicle or SB-334867 treatment after which rats were removed from the sleep chamber and returned to their home cage to await further sleep/wake testing. The second recording session followed the identical experimental procedures, with 12 hrs of acclimation, a 24 hr baseline recording, and a 24 hr post-treatment sleep/wake recording.
2.4.3 Data analysis
EEG and EMG signals were analyzed to determine the relative percentage of time spent in waking (Wake), non-rapid eye movement (NREM), and rapid eye movement (REM) sleep. EEG signals were sorted into frequency bands (Delta = 0.3–4Hz; Theta = 6–10Hz; Alpha = 9–13Hz; Gamma = 30–50Hz), and the relative prevalence of these bands was used to determine the sleep state of the animals as follows: 1) NREM sleep was defined as high-voltage EEG consisting of greater than 50% delta and low-voltage EMG; 2) REM sleep was defined as low-voltage EEG consisting of greater than 50% theta, combined with EMG activity of approximately 50% lower amplitude than that observed in NREM sleep; 3) Wake was defined as low-voltage EEG consisting of less than 40% delta and less than 20% theta with EMG activity of an average amplitude twice that observed in NREM. To be scored as a distinct epoch, the appropriate EEG and EMG activity patterns were required to persist for a minimum of 30 sec. Time spent in each state was scored and totaled for each drug condition in a cumulative fashion at 2, 4, and 6 hrs post-injection. Sleep data was normally distributed, and thus were assessed using a 3-way ANOVA with Treatment (vehicle or SB-334867) and Time (2, 4, or 6 hr) as repeated measures variables and Dose (7.5, 15, 30 mg/kg) as the between subjects variable. When significance was obtained, a two-way ANOVA was conducted on the respective groups. These analyses were conducted independently for Wake, NREM and REM sleep.
3.0 Results
3.1 HCRTr1 blockade reduces consumption responding for a low dose of cocaine
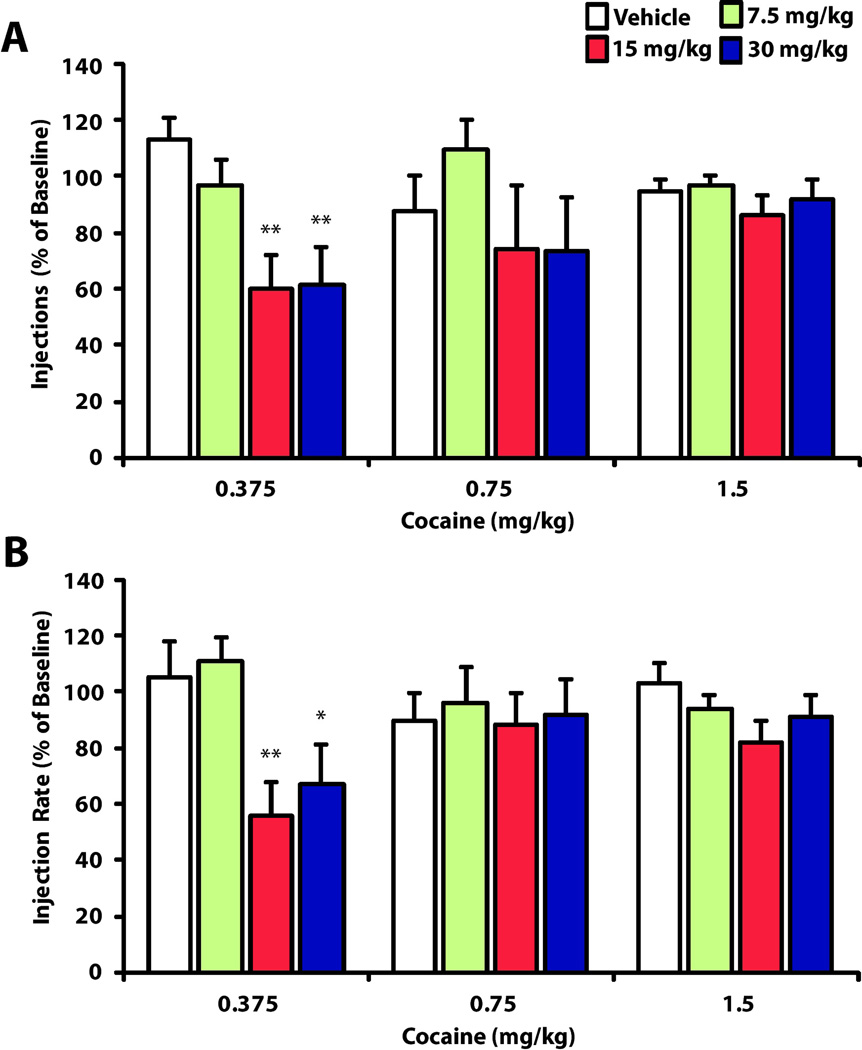
To examine whether HCRTr1 blockade alters consumption of varying doses of cocaine (0.375, 7.5, and 1.5 mg/kg), animals were treated with i.p. vehicle (0.375, n=7; 0.75, n=6; 1.5 n=10), 7.5 mg/kg SB-334867 (0.375, n=10; 0.75, n=6; 1.5 n=12), 15 mg/kg SB-334867 0.375, n=9; 0.75, n=6; 1.5 n=9), or 30 mg/kg SB-334867 (0.375, n=8; 0.75, n=6; 1.5 n=10). We used the number of injections received and the rate of injection during the consumption phase to define features of responding for cocaine. In general, vehicle had no effect on either measure of consumption responding (Injections: t(21) = 0.876, p = 0.391; Rate: t(21) = 0.201, p = 0.843). Similarly, SB-334867 did not alter the number of injections taken or the rate of intake at either the 0.75 (Injections: H(3) = 1.178, P = 0.758; Rate: H(3) = 0.135, P = 0.987) or 1.5 (Injections: H(3) = 1.007, P = 0.799; Rate: H(3) = 4.782 P = 0.188) mg/kg doses of cocaine. In contrast, at the 0.375 mg/kg dose of cocaine, SB-334867 significantly reduced both number of injections (H(3) = 13.297, P = 0.004) and rate of injection (H(3) = 13.887, P = 0.003), and post hoc analysis demonstrated that these effects were dose dependent, reaching significance at the 15 and 30 mg/kg SB-334867 doses (p < 0.05). The effects of SB-334867 on indices of consumption behavior are illustrated in Figure 2.
Figure 2.
SB-334867 decreases consumption responding for low unit doses of cocaine. (A) Shown are the total number of injections received by the end of the consumption phase as a mean percent of baseline ± SEM after pre treatment with Vehicle, 7.5, 15 and 30 mg/kg SB-334867. (B) Shown is the injection rate as a mean percent of baseline ± SEM after pre treatment with Vehicle, 7.5, 15, and 30 mg/kg SB-334867. *P < 0.05 and **P < 0.01.
3.2 HCRTr1 blockade reduces appetitive responding for multiple doses of cocaine
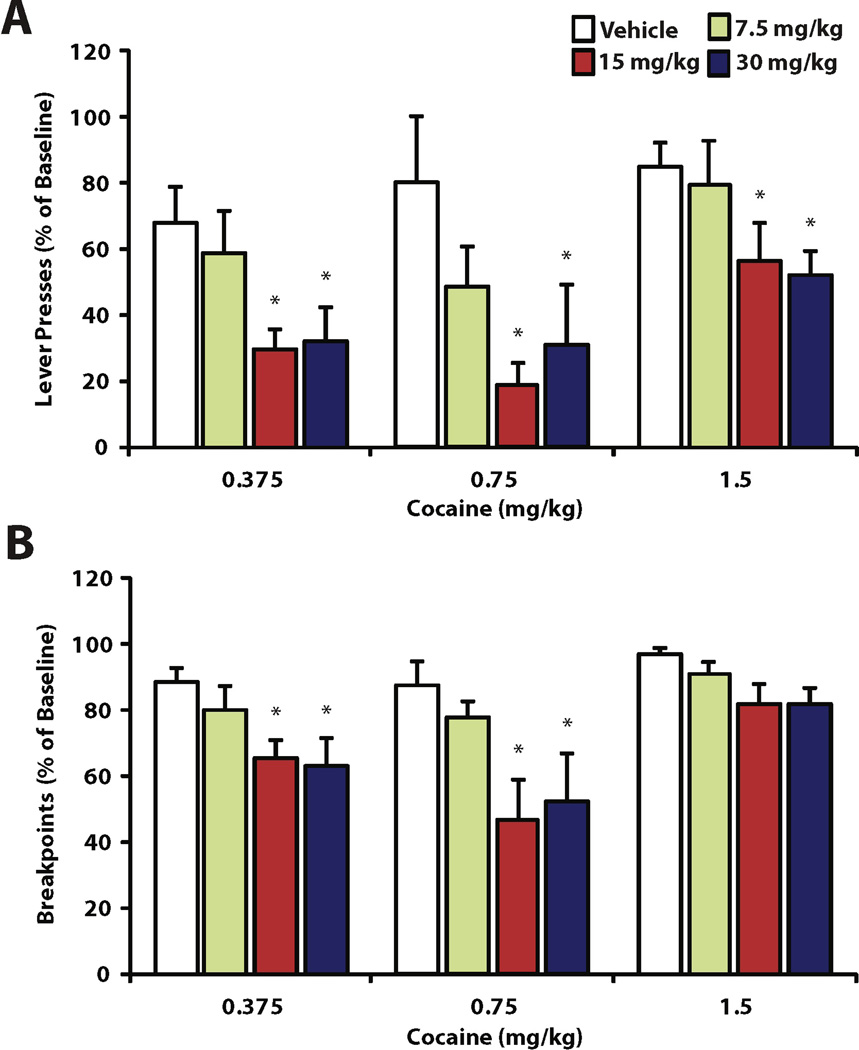
To examine whether HCRTr1 blockade alters appetitive responding for varying doses of cocaine (0.375, 7.5, and 1.5 mg/kg), animals were treated with i.p. vehicle or SB-334867 (7.5, 15 or 30 mg/kg; subject numbers are as indicated in section 3.1). We used break points and total lever presses across the entire session to define features of the appetitive phase of responding. In general, vehicle had no effect on either measure of high effort responding (lever presses: t(21) = 0.491, p = 0.602; breakpoints: t(21) = 0.713, p = 0.783). In contrast, SB-334867 significantly reduced total lever presses for all doses of cocaine (0.375 mg/kg; H(3) = 9.588, p = 0.022; 0.75 mg/kg; H(3) = 8.469, p = 0.037; 1.5 mg/kg; H(3) = 8.116, p = 0.044). Moreover, SB-334867 reduced breakpoints for all doses of cocaine, reaching significance at 0.375 and 0.75 mg/kg (0.375 mg/kg; H(3) = 9.467, p = 0.024; 0.75 mg/kg; H(3) = 8.342, p = 0.017) but not for 1.5 mg/kg (H(3) = 7.675, p = 0.053). The effects of SB-334867 were dose dependent for all cases where significance was obtained, with both 15 and 30 mg/kg SB-334867 producing significant decreases in responding for cocaine (p < 0.05). The effects of SB-334867 on indices of appetitive behavior are illustrated in Figure 3.
Figure 3.
SB-334867 decreases appetitive responding for cocaine. Shown are the total number of (A) lever presses and (B) breakpoints as a mean percent of baseline ± SEM after pretreatment with Vehicle, 7.5, 15 and 30 mg/kg SB-334867. *P < 0.05
3.3 HCRTr1 blockade does not alter sleep
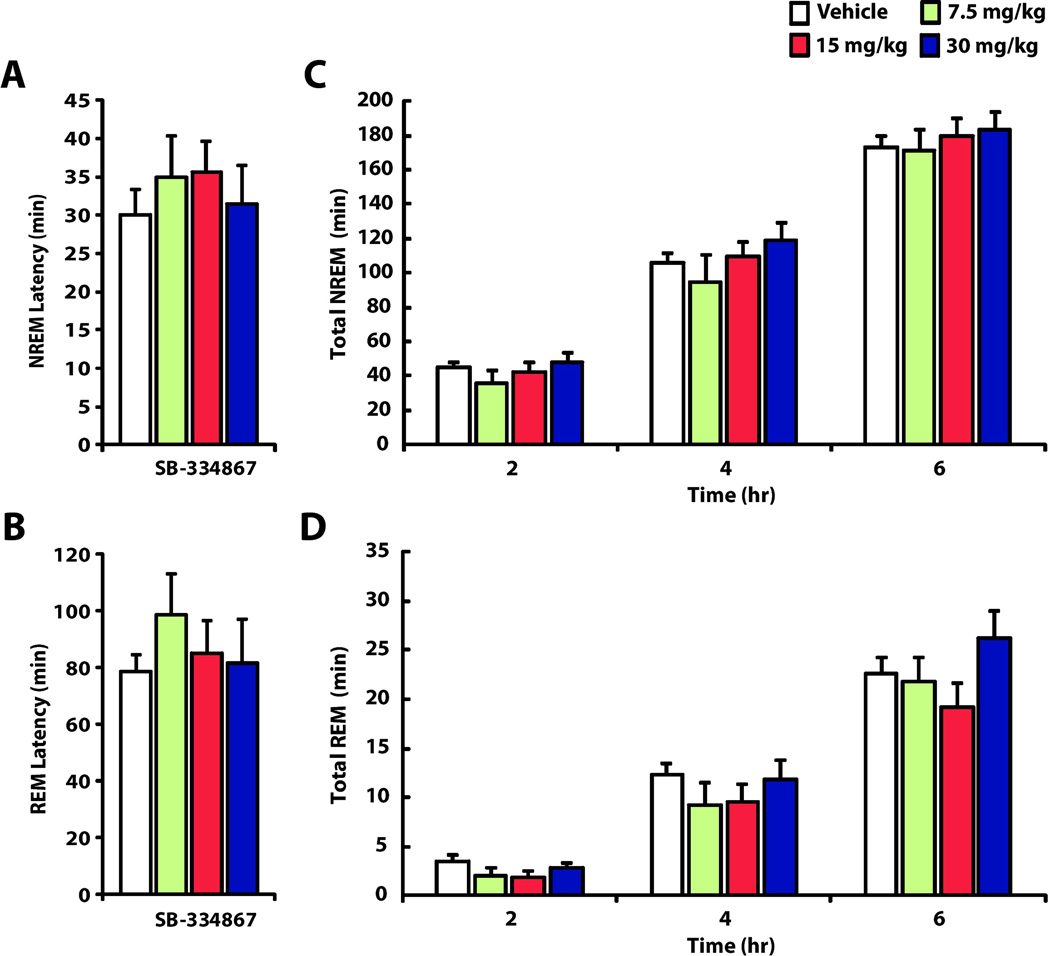
To determine if doses of SB-334867 that decrease self-administration also promote sleep, we recorded EEG/EMG activity in animals after administration of SB-334867. All animals (n=18) were treated with i.p. vehicle and one of three doses of SB-334867 (7.5, 15 or 30 mg/kg) in a counterbalanced design. As shown in figure4a and 4b, we observed that SB-334867 did not affect the latency to enter NREM sleep (F(3, 34) = 0.290, p = 0.831), or REM sleep (F(3, 34) = 0.622, p = 0.606) which is similar to what has previously been described [33]. Furthermore, a 3-way ANOVA indicated no significant effects of Treatment (Vehicle or SB-334867) for Wake (F(1, 14) = 0.73, p= 0.79), NREM (F(1, 14) = 0.0004, p= 0.98), or REM (F(1, 14) = 0.84, p= 0.374) and no significant effect of Dose (7.5, 15, or 30 mg/kg) for Wake (F(2, 14) = 1.03, p = 0.38), NREM (F(2, 14) = 1.28, p = 0.31), or REM (F(2, 14) = 1.34, p = 0.29). As expected, we did observe a significant effect of Time for Wake (F(2, 28) = 364.3, p< 0.001), NREM (F(2, 28) = 396.8, p< 0.001), and REM (F(2, 28) = 107.8, p< 0.001), indicating a cumulative increase in time spent in each of the sleep measures across the 2, 4, and 6 hr post-injection intervals. Importantly, however, there were no interactions between Time and Treatment or Time and SB-334867 Dose.
Figure 4.
SB-334867 does not promote sleep. Shown are the mean latency to enter (A) NREM and (B) REM sleep ± SEM as well as time spent in (C) NREM and (D) REM at 2, 4, and 6 hr after pretreatment with Vehicle, 7.5, 15 and 30 mg/kg SB334867.
Follow-up analyses comparing Treatment effects (Vehicle vs SB-334867) were conducted using 2-way ANOVAs independently for the 2, 4, and 6 hr intervals. We found thatSB-334867 did not affect the average time spent in Wake, NREM or REM when compared to vehicle, and this was consistent for the 2 hr (Wake F(1, 14) = 0.429, p = 0.53;NREM F(1, 14) = 1.0, p = 0.33; REM F(1, 14) = 2.65, p = 0.126), 4 hr (Wake F(1, 14) = 0.18, p = 0.68;NREM F(1, 14) = 0.4, p = 0.85; REM F(1, 14) = 0.20, p = 0.661), and 6 hr (Wake F(1, 14) = 0.06, p = 0.81; NREM F(1, 14) = 0.08, p = 0.79; REM F(1, 14) = 0.01, p = 0.91) post-injection intervals (Figure 4 c and d).
4.0 Discussion
4.1 Summary
We have demonstrated that the ability of SB-334867 to reduce two aspects of self-administration responding for cocaine is dependent on the dose of cocaine. For doses of cocaine with low reinforcing efficacy, SB-334867 exerts potent, dose-dependent attenuation of both consumption and appetitive responding. For unit doses with moderate or maximal reinforcing efficacy, SB-334867 treatment leaves consumption phase responding intact, yet reduces appetitive phase responding in a dose-dependent manner. Studies examining the effects of SB-334867 on sleep/wake activity indicate that doses that reduce the motivation to take cocaine do not produce sedation or alter sleep/wake patterns, there by confirming that the effects of SB-334867 on reinforcement behavior is not mediated by gross alterations to arousal.
4.2 Segregation of appetitive and consumption behavior
Appetitive drug seeking and consummatory drug taking behavior can be independently modeled in rodents by providing access to drug at varying unit prices [34], with unit price defined as the ratio of response requirements to the dose of cocaine provided (responses/mg drug) [18]. Cocaine self-administration at high unit prices represents appetitive behavior, while self-administration at low unit prices represents consummatory behavior [34]. Importantly, it appears that the mechanisms underlying these appetitive and consummatory behaviors are dissociable [35, 36], and drugs that modulate cocaine self-administration can have distinct effects on each of these aspects of self-administration behavior [37]. These observations emphasize the need to measure how candidate addiction pharmacotherapies alter self-administration across varying doses of cocaine and response requirements.
In the context of HCRTr1 regulation of cocaine self-administration, we have previously shown that blockade of HCRTr1 reducesPmax, the behavioral economic index of price, but does not alter cocaine consumption [14]. This finding raises the possibility that the HCRT system may influence appetitive drug seeking and consummatory drug taking independently. Although the results from those studies are informative in that regard, unit prices were manipulated by decreasing cocaine dose across the session while maintaining a constant response requirement [37]. In contrast, in the present studies we manipulated unit price by varying response requirements across cocaine doses. While behavioral economic theory might suggest that unit prices derived through these different approaches are equivalent, several neurochemical studies indicate that phasic DA signals in the NAc encode information on the magnitude of rewards but not response cost in well trained animals [36, 38, 39]. These observations suggest that drug pretreatments that alter responding for cocaine through modulation of DA systems may exert different effects depending on how unit prices were manipulated. For these reasons we used a strategy for measuring drug pre treatment effects on appetitive and consumption responding under conditions of increasing response requirement across multiple doses of cocaine.
The present two-phase analysis parses PR self-administration into consumption and appetitive phases. During the initial phase of the PR schedule, rats receive injections with relatively few lever presses, and we suspect that during this phase rats readily titrate to preferred blood levels of cocaine. Two features of our analysis support this. First, rate of intake is higher for lower doses of cocaine, and second the rate of intake remains constant across the consumption phase. These features are consistent with the observation that under conditions with relatively low response requirements rats display higher injection rates of low doses of cocaine in order to sufficiently achieve and maintain preferred blood levels [40]. This manner of responding is reminiscent of what is observed when examining self-administration behavior under a low effort FR schedule [31] that has been used to test changes in consummatory behavior [34]. Together these observations suggest that responding during the initial phase of a PR schedule requires low effort relative to the later phase, allows rats to effectively titrate to preferred blood levels of cocaine, and can be analyzed to represent comsummatory behaviors. Total responding across the PR schedule has been well established as a measure of appetitive drives [34, 41], thus our analysis provides a mean for measuring both consumption and appetitive behaviors within a single PR session.
4.3 SB-334867 modulates high effort responding for cocaine
In the context of this study, low unit prices correspond with low effort access to cocaine while high unit prices correspond with relatively high effort access to cocaine. We examined the effects of SB-334867 on self-administration at multiple unit prices by manipulation of both unit dose of cocaine and lever response requirements. The unit doses of cocaine used herein were 0.375, 0.75 and 1.5 mg/kg, which represent relatively high, medium, and low unit price respectively. Lever response requirements to obtain these unit doses increased across the PR session such that the early consumption phase of the session represents a period of relatively low unit prices while the later appetitive phase represents a period of relatively high unit prices.
We found that 15 and 30 mg/kg of SB-334867 similarly reduced consumption phase responding when unit prices are high (0.375 mg/kg), but did not alter consumption phase responding when unit prices are lower (0.75 and 1.5 mg/kg). Likewise, we found that SB-334867 (15 and 30 mg/kg) substantially reduced appetitive phase responding when unit prices were high (0.375 and 0.75 mg/kg), but sub-optimally reduced appetitive phase responding when unit prices were low (1.5 mg/kg). Together, our results indicate that blockade of HCRTr1 more efficiently reduces self-administration responding when unit prices are high (Figure 5). This, in turn, supports the hypothesis that hypocretin actions at HCRTr1 influences behaviors that require high effort, but leaves behaviors requiring low effort largely intact.
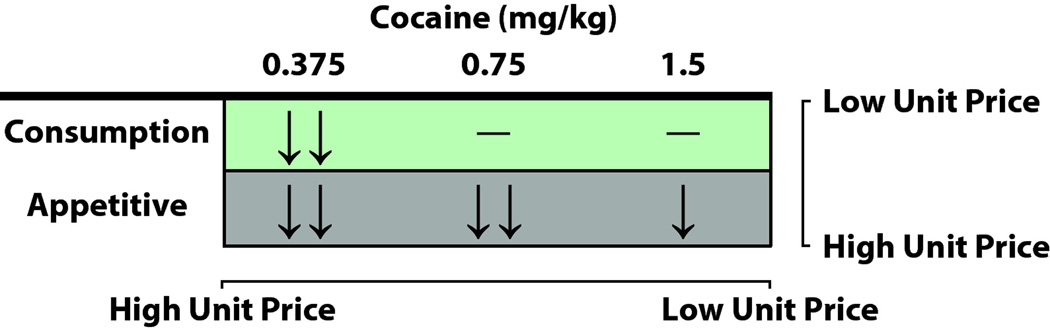
Figure 5.
HCRTr1 blockade reduces self-administration at high unit prices, but not at low unit prices. SB-334867 effectively reduces consumption and appetitive phase responding when unit prices are high due to low dose availability for animals in the 0.375 mg/kg group. When unit prices are reduced by providing a higher dose of cocaine in the 0.75 mg/kg group, SB-334867 only reduces appetitive phase responding when unit prices are high due to increased response requirements. When unit prices are further reduced by providing and even higher dose of cocaine in the 1.5 mg/kg group, SB-334867 efficacy was reduced even during the appetitive phase when response requirements were high. Arrows indicate changes in individual metrics of self-administration. For the consumption phase, double arrows indicate significant reductions in number of injections and injection rate. For the appetitive phase, double arrows indicate significant reductions in breakpoints and lever presses, while a single arrow indicates a reduction in only lever presses. Dashes indicate no changes in self-administration.
4.4 Neural correlates of Hcrt1 blockade in the modulation of motivation for cocaine
The dissociable effects of HCRTr1 blockade on low versus high effort self-administration are consistent with the observation that pharmacological treatments can differentially affect cocaine self-administration under conditions requiring low effort or high effort [37], yet the neural correlates of these processes remain unresolved. One possible mechanism for the development and maintenance of cocaine self-administration centers on phasic DA responses as important signals that participate in determining energy cost expenditures. Phasic DA signals in the NAc drive cocaine seeking behaviors [42] and have been shown to track reward magnitude, with stronger DA responses correlated to preferred rewards [36, 38]. These observations have led to the suggestion that, in well-trained rats, phasic DA signaling within the NAc incorporates information on reward value to provide a threshold for worthwhile energy expenditures [36]. In agreement with this, it has been shown that pharmacological inhibition of DA signals decreases responding for preferred rewards requiring higher effort responding [43]. In this context, one would expect that self-administration of cocaine at high doses would be associated with large amplitude phasic DA events [42], while low doses of cocaine would be associated with smaller amplitude phasic DA responses that less effectively drive motivation to lever press for cocaine. We have previously demonstrated that blockade of HCRTr1 via SB-334867 attenuates electrically-evoked phasic DA signal strength and decreases willingness to expend effort for cocaine [14]. Thus, it is possible that the behavioral effects of HCRTr1 antagonists are mediated by altering the relationship between the phasic DA events and the unit dose of rewards. Current efforts are devoted to defining the interactions between HCRTr1 manipulations, dose, and task-related phasic DA signals.
4.5 HCRTr1 blockade on sleep/wake activity
The possible interaction between the sleep/wake effects and the motivational effects of the hypocretin system have consistently raised concerns that decreases in drug self-administration following HCRTr1 blockade may be attributed to gross deficits in arousal. Indeed, there is ample evidence that the hypocretins modulate sleep/wake and loco motor activity [44–48]. For example, optogenetic stimulation of hypocretin containing neurons, or treatment with hypocretin peptides promotes transitions from sleep to waking [44, 47, 49] while optogenetic silencing of hypocretin neurons promotes sleep [50]. Despite these and many other observations, a debate remains as to what extent the HCRTr1 and/or HCRTr2 participate in governing sleep/wake activity.
Several studies using transgenic mice have begun to separate the apparently distinct roles of these two receptors. HCRTr2 knockout (KO) mice display sleep/wake disturbances similar to hypocretin peptide KO mice [51] while HCRTr1 KO mice display only a mild sleep disorder [52]. Dual HCRTr1/HCRTr2 KO mice, however, display more severe sleep/wake disturbances than KO of either receptor alone [51, 53]. This suggests that both hypocretin receptors participate in sleep processing, but that role of HCRTr1 is far less significant than the role of HCRTr2. Consistent with this,HCRTr2 KO mice show reduced wake-promoting effects of the hypocretin-1 peptide, while HCRTr1 KOs display only mild decreases in the wake-promoting effects of hypocretin-1 [53].
Pharmacological manipulation of hypocretin receptors similarly suggest a minimal role for the HCRTr1 in sleep/wake processing. Smith and colleagues were the first to demonstrate that SB-334867 failed to increase sleep when rats were tested during their sleep phase [33]. In contrast, one recent publication reports modest increases in NREM and REM sleep following SB-334867[24]. Despite this discrepancy, our current findings are in agreement with the majority of research investigating HCRTr1 involvement in sleep/wake processing. Indeed, neither SB-408124, ACT-335827, nor GSK1059865, three different HCRTr1 selective antagonists, had an impact on sleep indices when tested in rats [21, 23, 54], and GSK 109865 did not increase NREM, REM, or total sleep in rodents [22]. Therefore our current findings globally confirm that HCRTr1 antagonism does not produce gross deficits to arousal, which further indicates that the effects of HCRTr1 blockade on cocaine self-administration was are not appreciably influenced by changes in sleep/wake activity.
5.0 Conclusion
We found that systemic blockade of HCRTr1 reduces high effort responding for cocaine while leaving low effort responding and sleep/wake activity intact. Our results support the use of HCRTr1 antagonists as pharmacotherapies for the treatment of cocaine addiction. This possibility is encouraged by the clinical successes of baclofen, a GABAB agonist with a similar self-administration modification profile [55]. The wide-spread use of baclofen, however, has been severely limited by its off target effects including sedation and reduced loco motor activity [56]. Our results suggest that, while HCRTr1 blockade may be similarly effective at reducing cocaine self-administration as baclofen, it does not significantly alter arousal or loco motor activity [57], and thus may overcome some of the shortcomings seen with the use of baclofen as a treatment for substance abuse disorders. The possibility of clinical application of HCRTr1 antagonists is further encouraged by the recent FDA approval of the dual hypocretin receptor antagonist suvorexant, which signifies the acceptance of hypocretin receptor-based pharmacotherapies.
Supplementary Material
Highlights.
Blockade of HCRTr1 reduces cocaine self-administration with high effort requirements
Blockade of HCRTr1 does not alter cocaine self-administration with low effort requirements
HCRTr1 antagonism does not promote sleep at levels that alter cocaine self-administration
Acknowledgements
This research was supported by grant funding from the United states National Institute on Drug Abuse (Grant no. R01DA025279).
Abbreviations
- DA
Dopamine
- EEG
Electroencephalogram
- EMG
Electromyogram
- FR
Fixed ratio
- I.P.
Intraperitoneal
- I.V.
Intravenous
- HCRTr1
Hypocretin receptor 1
- HCRTr2
Hypocretin receptor 2
- NAc
Nucleus accumbens
- PR
Progressive ratio
- VTA
Ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.de Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep-to-wake transitions. Frontiers in pharmacology. 2014;5:16. doi: 10.3389/fphar.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 6.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. The Journal of comparative neurology. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 7.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- 12.España RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. The European journal of neuroscience. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C. The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization. Neurochem Int. 2010;56:11–15. doi: 10.1016/j.neuint.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 17.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. The European journal of neuroscience. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life sciences. 1990;47:1501–1510. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- 19.Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology. 1999;143:209–214. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- 20.Plaza-Zabala A, Maldonado R, Berrendero F. The hypocretin/orexin system: implications for drug reward and relapse. Molecular neurobiology. 2012;45:424–439. doi: 10.1007/s12035-012-8255-z. [DOI] [PubMed] [Google Scholar]
- 21.Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. The Journal of pharmacology and experimental therapeutics. 2009;330:142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 22.Gozzi A, Turrini G, Piccoli L, Massagrande M, Amantini D, Antolini M, et al. Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PloS one. 2011;6:e16406. doi: 10.1371/journal.pone.0016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dugovic C, Shelton JE, Yun S, Bonaventure P, Shireman BT, Lovenberg TW. Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Frontiers in neuroscience. 2014;8:28. doi: 10.3389/fnins.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morairty SR, Revel FG, Malherbe P, Moreau JL, Valladao D, Wettstein JG, et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PloS one. 2012;7:e39131. doi: 10.1371/journal.pone.0039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, et al. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorganic & medicinal chemistry letters. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- 26.Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacological reviews. 2012;64:389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- 27.McElhinny CJ, Lewin AH, Mascarella SW, Runyon S, Brieaddy L, Carroll FI. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorganic & medicinal chemistry letters. 2012;22:6661–6664. doi: 10.1016/j.bmcl.2012.08.109. [DOI] [PubMed] [Google Scholar]
- 28.Prince CD, Rau AR, Yorgason JT, Espana RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS chemical neuroscience. 2015;6:138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. The European journal of neuroscience. 2015;41:1149–1156. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. The Journal of pharmacology and experimental therapeutics. 1968;161:122–129. [PubMed] [Google Scholar]
- 32.Brebner K, Phelan R, Roberts DC. Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology. 2000;148:314–321. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- 33.Smith MI, Piper DC, Duxon MS, Upton N. Evidence implicating a role for orexin-1 receptor modulation of paradoxical sleep in the rat. Neuroscience letters. 2003;341:256–258. doi: 10.1016/s0304-3940(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 34.Roberts DC, Gabriele A, Zimmer BA. Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations. Neuroscience & Biobehavioral Reviews. 2013;37:2026–2036. doi: 10.1016/j.neubiorev.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:5526–5537. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan JO, Walton ME, Phillips PE. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nature neuroscience. 2010;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oleson EB, Richardson JM, Roberts DC. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology. 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biological psychiatry. 2010;68:306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wanat MJ, Kuhnen CM, Phillips PE. Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors. The Journal of Neuroscience. 2010;30:12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- 41.Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1996;15:417–423. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- 42.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 43.Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- 44.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10911–1096. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. The European journal of neuroscience. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 47.España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 48.España RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain research. 2002;943:224–236. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- 49.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsunematsu T, Kilduff TS, Boyden ES, Takahashi S, Tominaga M, Yamanaka A. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 52.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annual review of neuroscience. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 53.Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and-2 in the regulation of non-REM and REM sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steiner MA, Gatfield J, Brisbare-Roch C, Dietrich H, Treiber A, Jenck F, et al. Discovery and characterization of ACT-335827, an orally available, brain penetrant orexin receptor type 1 selective antagonist. Chem Med Chem. 2013;8:898–903. doi: 10.1002/cmdc.201300003. [DOI] [PubMed] [Google Scholar]
- 55.Roberts DC. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiology & behavior. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Bowery NG. GABAB receptor: a site of therapeutic benefit. Current opinion in pharmacology. 2006;6:37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Calipari ES, España RA. Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Frontiers in behavioral neuroscience. 2012;6:54. doi: 10.3389/fnbeh.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.