Abstract
The phytohormone abscisic acid (ABA) plays important roles during seed germination and early seedling development. Here, we characterized the function of the Arabidopsis WRKY6 transcription factor in ABA signaling. The transcript of WRKY6 was repressed during seed germination and early seedling development, and induced by exogenous ABA. The wrky6-1 and wrky6-2 mutants were ABA insensitive, whereas WRKY6-overexpressing lines showed ABA-hypersensitive phenotypes during seed germination and early seedling development. The expression of RAV1 was suppressed in the WRKY6-overexpressing lines and elevated in the wrky6 mutants, and the expression of ABI3, ABI4, and ABI5, which was directly down-regulated by RAV1, was enhanced in the WRKY6-overexpressing lines and repressed in the wrky6 mutants. Electrophoretic mobility shift and chromatin immunoprecipitation assays showed that WRKY6 could bind to the RAV1 promoter in vitro and in vivo. Overexpression of RAV1 in WRKY6-overexpressing lines abolished their ABA-hypersensitive phenotypes, and the rav1 wrky6-2 double mutant showed an ABA-hypersensitive phenotype, similar to rav1 mutant. Together, the results demonstrated that the Arabidopsis WRKY6 transcription factor played important roles in ABA signaling by directly down-regulating RAV1 expression.
Author Summary
The WRKY6 protein is a WRKY transcription factor which plays important roles in plant pathogen defense, phosphate translocation, and arsenate resistance. This study demonstrated that the expression of WRKY6 was obviously repressed during seed germination and significantly induced by exogenous ABA. In the presence of exogenous ABA, the two wrky6 mutants showed ABA-insensitive phenotypes, whereas the WRKY6-overexpressing lines were hypersensitive to ABA. The WRKY6 transcription factor repressed RAV1 expression and enhanced the expression of ABI3, ABI4 and ABI5, which was down-regulated by RAV1. The WRKY6 protein could bind to the W-box motif within the RAV1 promoter, indicating that WRKY6 directly regulated RAV1 expression. Overexpression of RAV1 abolished the ABA-sensitivity of WRKY6-overexpressing lines, and repression of RAV1 impaired the ABA-insensitivity of wrky6 mutants. Our results reveal the important roles of WRKY6 in ABA signaling during seed germination and early seedling development.
Introduction
Abscisic acid (ABA) is a key phytohormone that plays important roles in plant responses to stresses and plant development [1–2]. ABA is accumulated in the developing embryo, and modulates seed development and storage product accumulation [1]. In addition, ABA prevents premature seed germination and controls seed dormancy to ensure that seeds germinate under favorable conditions [1]. After germination, ABA content declines rapidly [3–4], and exogenous ABA inhibits seed germination [5–6].
ABA functions through complex signaling networks, and some components of these networks are identified. The ABA receptors PYR/PYL/RCAR are identified in Arabidopsis thaliana [7–8]. Molecular genetics studies in Arabidopsis identify a number of genes involved in ABA signaling. The snrk2.2 srnk2.3 double mutant shows strong ABA-insensitive phenotypes in seed germination and root growth inhibition, and the two protein kinases SnRK2.2 and SnRK2.3 are demonstrated to mediate a major part of ABA signaling during seed germination [9]. The abi3, abi4, and abi5 mutants also show ABA-insensitive phenotypes during seed germination and early seedling development [10–12], and the ABI3, ABI4, and ABI5 genes encode B3-type, APETALA2 domain and basic Leucine zipper (bZIP)-type transcription factors, respectively [10–13]. Three other bZIP-type transcription factors, AREB1/ABF2, AREB2/ABF4, and ABF3, are also involved in ABA signaling. During seed germination, none of the areb1, areb2 and abf3 mutants show ABA-sensitive phenotypes compared with wild-type plants, and during the vegetative growth stage, AREB1/ABF2, AREB2/ABF4, and ABF3 are key regulators of ABA signaling in response to osmotic stress [14–16].
The WRKY family is one of the largest transcription factor families in plants [17]. The WRKY proteins contain the conserved WRKY domain and zinc finger motif [18]. The conservation of the WRKY domain is mirrored by a remarkable conservation of the binding site, the W box (T)(T)TGAC(C/T) [18–19]. WRKY proteins act as repressors as well as activators by binding to their target genes’ promoters. Several WRKY transcription factors have been reported to be involved in the ABA signaling network. Three evolutionarily related WRKY transcription factors (AtWRKY18, AtWRKY40 and AtWRKY60) are negative regulators in ABA signaling, and AtWRKY40 directly represses the expression of ABI4 and ABI5 by binding to the promoters of ABI4 and ABI5 [20]. The knockout mutant of AtWRKY63, the abo3 mutant, is hypersensitive to exogenous ABA during seed germination and the vegetative growth stage [21], and the Arabidopsis wrky2 mutant has similar phenotypes to the abo3 mutant except that AtWRKY2 has no effect on stomatal closure [22]. Recently, the AtWRKY41 protein is reported to control seed dormancy via direct regulation of ABI3 expression [23], and AtWRKY8 functions in the TMV-cg defense response by mediating ABA and ethylene signaling [24].
In this study, we find that the Arabidopsis WRKY6 is a positive regulator in ABA signaling during seed germination and early seedling development. The knockout of WRKY6 enhances plant ABA insensitivity during seed germination and early seeding growth, and WRKY6-overexpressing lines show ABA-hypersensitive phenotypes. The WRKY6 transcription factor represses RAV1 expression and enhances the expression of ABI3, ABI4 and ABI5, which are down-regulated by RAV1. The WRKY6 protein can bind to the W-box motif within the RAV1 promoter, indicating that WRKY6 directly regulates RAV1 expression. Overexpression of RAV1 abolishes the ABA-sensitivity of WRKY6-overexpressing lines, and repression of RAV1 impairs the ABA-insensitivity of wrky6 mutants, demonstrating that RAV1 is genetically epistatic to WRKY6.
Results
Disruption of WRKY6 reduces, and overexpression of WRKY6 enhances, ABA sensitivity during seed germination and early seedling development
Arabidopsis WRKY6 (WRKY transcription factor 6, At1g62300) is a WRKY transcription factor [25] and, from public microarray data, we found that WRKY6 expression was relatively high in dry seeds and reduced after imbibition. Then we examined the expression of WRKY6 during seed germination and early seedling development. The transcript level of WRKY6 was markedly repressed during seed germination (Fig 1A), indicating that WRKY6 may be involved in seed germination and early seedling development. When germinated and grown on Murashige and Skoog (MS) medium containing 0.5 μM ABA (MS+ABA), WRKY6 expression was significantly induced (Fig 1B). The transcript level of WRKY6 was further tested in seedlings treated with exogenous ABA. The 7-d-old wild-type seedlings were transferred to MS solution with or without 100 μM ABA for 3 h, and then harvested for qRT-PCR assay. The qRT-PCR results showed that the transcript level of WRKY6 was significantly induced by exogenous ABA (Fig 1C).
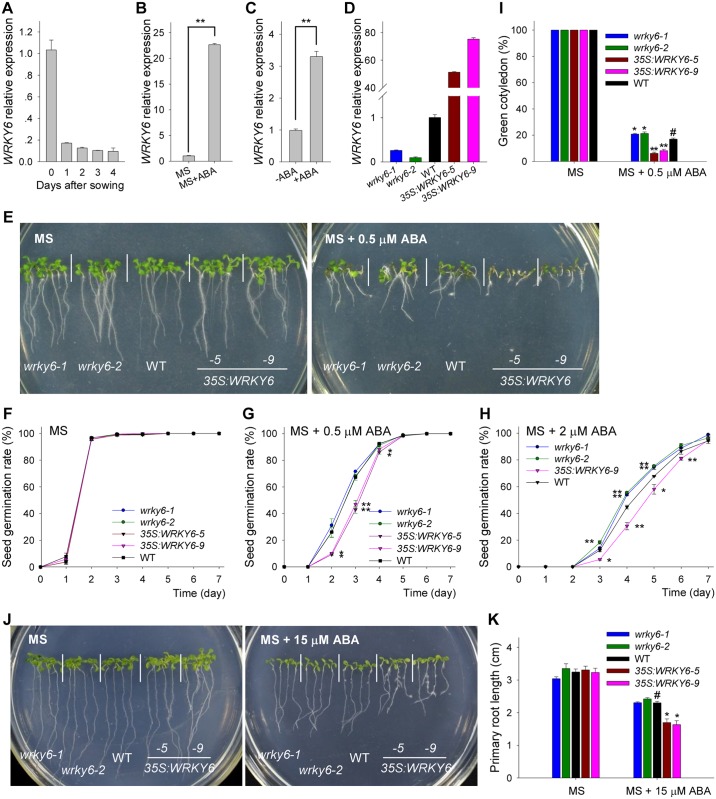
Fig 1. ABA-sensitivity of wrky6 mutants and WRKY6-overexpressing lines.
A, Expression of WRKY6 was analyzed by qRT-PCR in wild-type plants (WT) during seed germination and early seedling development. The wild-type imbibed seeds were germinated and grown on MS medium, and then the plants were harvested at the indicated time. Data are shown as mean ± SE (n = 3). B, qRT-PCR analysis of WRKY6 expression in response to exogenous ABA. Wild-type imbibed seeds were germinated on MS medium (MS) or MS medium with 0.5 M ABA (MSABA) for 1 d, and then the seeds were harvested. Data are shown as mean ± SE (n = 3). C, qRT-PCR analysis of WRKY6 expression in 7-d-old wild-type seedlings treated with or without 100 M ABA for 3 h. Data are shown as mean ± SE (n = 3). D, Expression of WRKY6 was analyzed by qRT-PCR in the wrky6 mutants (wrky6-1 and wrky6-2) and WRKY6-overexpressing lines (35S:WRKY6-5 and 35S:WRKY6-9). Data are shown as mean ± SE (n = 3). E, Phenotypic comparison. Imbibed seeds were transferred to MS or MS 0.5 μM ABA medium and grown for 10 d. F-H, Seed germination assay. Imbibed seeds were transferred to MS (F), MS medium containing 0.5 M ABA (G) or 2 M ABA (H), and then the seed germination rates were calculated at the indicated time. Data are shown as mean ± SE (n = 3). More than 300 seeds were measured in each replicate. I, Cotyledon-greening analysis. Imbibed seeds were transferred to MS or MS 0.5 μM ABA medium for 7 d before determining cotyledon-greening percentages. Data are shown as mean ± SE (n = 3). More than 300 seeds were measured in each replicate. J-K, Primary root length measurement with and without ABA addition. The 4-d-old seedlings were transferred to MS or MS 15 μM ABA medium for 7 d, and then the photos were taken and the primary root length was measured. Asterisks in G, H, I and K indicate statistically significant differences compared with wild-type plants: *, P 0.05; **, P 0.01. Wild-type plant (WT) was used as a control (#).
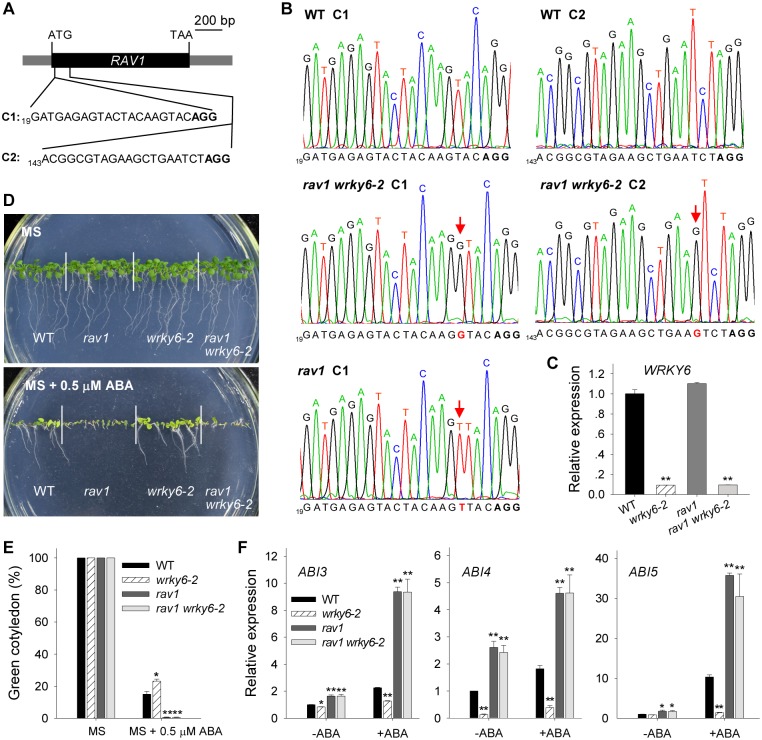
WRKY6-overexpressing lines and wrky6 mutants were used to study the physiological function of WRKY6 in seed germination. The WRKY6-overexpressing lines (35S:WRKY6-5 and 35S:WRKY6-9) and the wrky6-1 mutant were provided by Dr. Somissich [26]. A WRKY6 T-DNA insertion line (Salk_012997), named wrky6-2, was ordered from the ABRC (Arabidopsis Biological Resource Center). The qRT-PCR results showed that WRKY6 expression was significantly repressed in the wrky6-1 and wrky6-2 mutants, and elevated in 35S:WRKY6-5 and 35S:WRKY6-9, compared with wild-type plants (Fig 1D). When germinated and grown on MS medium, all plants showed no obvious difference in their phenotypes (Fig 1E, left panel). When grown on MS medium containing 0.5 μM ABA (MS + 0.5 μM ABA), the wrky6-1 and wrky6-2 mutants were more insensitive to ABA than wild-type plants, whereas 35S:WRKY6-5 and 35S:WRKY6-9 showed ABA hyper-sensitive phenotypes (Fig 1E, right panel). When grown on MS medium containing 0.5 μM ABA, the wrky6-1 and wrky6-2 mutants were less ABA insensitive than the abi4 and abi5 mutants (S1 Fig). Seed germination was further tested, and in the absence of ABA (MS), the seed germination percentages of different genotypes were similar (Fig 1F). When germinated and grown on MS medium containing 0.5 μM ABA (MS + 0.5 μM ABA), the two WRKY6-overexpressing lines (35S:WRKY6-5 and 35S:WRKY6-9) showed significantly reduced seed germination percentages, and the seed germination percentages of the two wrky6 mutants were similar to wild-type plants (Fig 1G). When germinated and grown on MS medium containing 2 μM ABA (MS + 2 μM ABA), the two WRKY6 mutants (wrky6-1 and wrky6-2) showed significantly increased seed germination percentages compared with wild-type plants, and the WRKY6-overexpressing line (35S:WRKY6-9) showed reduced seed germination percentage relative to wild-type plants (Fig 1H). The cotyledon-greening percentages were also measured, and in the absence of ABA (MS), they were similar among different genotypes (Fig 1I). When germinated and grown on MS medium containing 0.5 μM ABA (MS + 0.5 μM ABA), the WRKY6-overexpressing lines (35S:WRKY6-5 and 35S:WRKY6-9) had lower, whereas the wrky6 mutants (wrky6-1 and wrky6-2) had higher, cotyledon-greening percentages than wild-type plants (Fig 1I).
To further test whether WRKY6 was involved in ABA mediated root growth inhibition, the 4-d-old wrky6 mutants, WRKY6-overexpressing lines and wild-type seedlings were transferred to MS medium with or without 15 μM ABA for 7 d. When grown on MS medium, the primary root length was similar among different genotypes (Fig 1J and 1K). When grown on MS medium containing 15 μM ABA, the WRKY6-overexpressing lines (35S:WRKY6-5 and 35S:WRKY6-9) showed shorter primary root compared with wild-type seedlings, and the primary root lengths of wrky6-1 and wrky6-2 were similar to that of wild-type plants (Fig 1J and 1K). Together, these data indicate that WRKY6 plays important roles in ABA signaling during seed germination and seedling development.
Disruption and overexpression of WRKY6 alter expression of a set of ABA-responsive genes
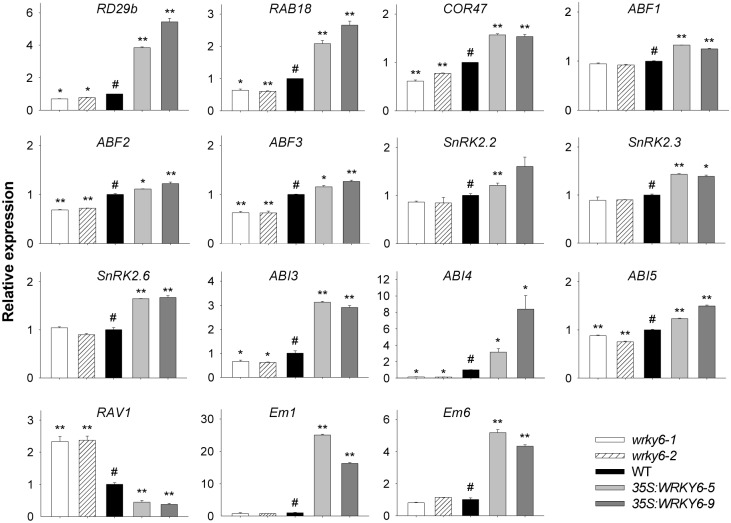
As WRKY6 is a WRKY transcription factor involved in ABA signaling (Fig 1), the expression of ABA inducible genes, such as RD29b, RAB18 and COR47, was tested in the WRKY6-overexpressing lines and wrky6 mutants. The transcript levels of RD29b, RAB18, and COR47 were elevated in the WRKY6-overexpressing lines and repressed in the wrky6 mutants (Fig 2). Then the expression of the following ABA-responsive genes was tested: ABFs (ABF1, ABF2/AREB1 and ABF3) [27], SnRK2s [28–29], ABI3 [10], ABI4 [11], ABI5 [12], RAV1 [30], Em1 [31] and Em6 [31]. The qRT-PCR results showed that the transcript levels of these ABA-responsive genes were elevated in the WRKY6-overexpressing lines, and the expression of most of these genes was repressed in the wrky6 mutants (Fig 2). It is notable that the expression of RAV1 was significantly repressed in the WRKY6-overexpressing lines and upregulated in the wrky6-1 and wrky6-2 mutants (Fig 2). It is also notable that the expression of ABI3 and ABI4 was elevated in the WRKY6-overexpressing lines and suppressed in the wrky6 mutants (Fig 2).
Fig 2. Expression of ABA-responsive genes in wrky6 mutants and WRKY6-overexpressing lines.
The imbibed seeds were germinated and grown on MS medium for 7 d, and then the seedlings were harvested for qRT-PCR. Data are shown as mean ± SE (n = 3). Asterisks indicate statistically significant differences compared with wild-type plants: *, P 0.05; **, P 0.01. Wild-type plant (WT) was used as a control (#).
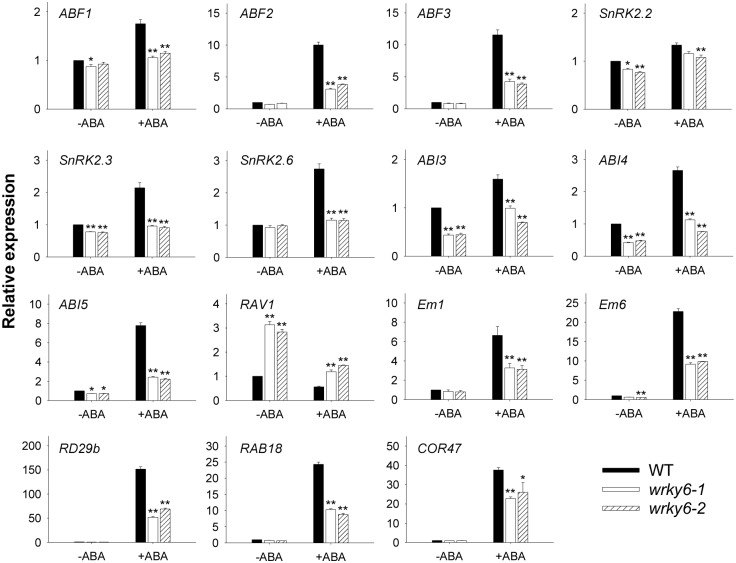
The expression of these ABA-responsive genes was also tested in the wrky6 mutants and wild-type plants under exogenous ABA treatment. After the seedlings were treated with 100 μM ABA for 3 h, the expression of these genes, except RAV1, was induced in the wild-type seedlings, and this inducement by exogenous ABA was obviously repressed in the wrky6-1 and wrky6-2 mutants (Fig 3). The RAV1 was repressed by exogenous ABA in the wild-type seedlings, and the transcript levels of RAV1 in wrky6-1 and wrky6-2 mutants were much higher than that in wild-type seedlings with or without ABA treatment (Fig 3). These data demonstrate that disruption and overexpression of WRKY6 alter the expression of the ABA-responsive genes. The expression of these genes was still ABA inducible in the wrky6 mutants, indicating that besides WRKY6, there were other transcription factors regulating these genes expression.
Fig 3. Expression of ABA-responsive genes in wrky6 mutants and wild-type seedlings treated with exogenous ABA.
The 7-d-old wrky6 mutants and wild-type seedlings were transferred to MS solution with or without 100 μM ABA for 3 h, and then the seedlings were harvested for qRT-PCR. Data are shown as mean ± SE (n = 3). Asterisks indicate statistically significant differences compared with relevant wild-type plants (WT): *, P 0.05; **, P 0.01.
WRKY6 directly regulates RAV1 expression
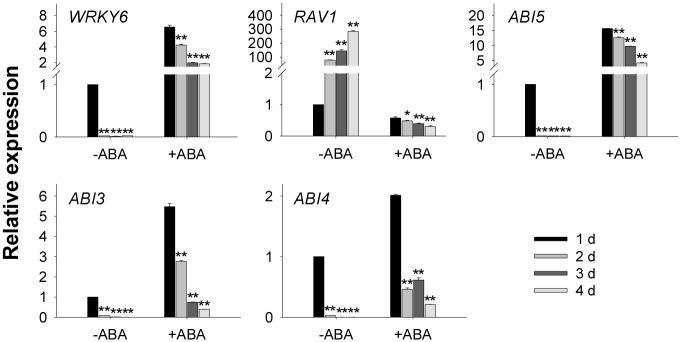
As our previous work showed that Arabidopsis RAV1 directly down-regulated the expression of ABI3, ABI4, and ABI5 [30]—and the RAV1 expression was lower, whereas the expression of ABI3, ABI4 and ABI5 was elevated in WRKY6-overexpressing lines (Figs 2 and 3)—we hypothesized that WRKY6 directly regulated RAV1 expression. Then the expression of ABI3, ABI4 and ABI5 was further tested during the seed germination with or without exogenous ABA. During the seed germination, the transcript level of WRKY6 was obviously repressed, and the RAV1 expression was obviously induced (Fig 4). The transcript levels of ABI3, ABI4 and ABI5, which directly down-regulated by RAV1, were obviously suppressed during seed germination (Fig 4). And the transcript levels of WRKY6, ABI3, ABI4 and ABI5 were obviously induced, and the RAV1 expression was repressed, by exogenous ABA (Fig 4). These data imply that WRKY6 may directly regulate the RAV1 expression.
Fig 4. Expression of WRKY6, RAV1 and ABIs in wild-type plants during seed germination and early seedling development.
The imbibed wild-type seeds were transferred to the MS medium with or without 0.5 μM ABA, and then the plants were harvested at the indicated time for qRT-PCR. Data are shown as mean ± SE (n = 3). Asterisks indicate statistically significant differences compared with relevant wild-type plants (WT): *, P 0.05; **, P 0.01.
WRKY proteins act as regulators by binding to W-box(es) within their target genes promoters. First the RAV1 promoter sequence was analyzed and the results showed that there were two W-box motifs within the RAV1 promoter (Fig 5A). To further test the function of WRKY6 on regulation of RAV1 expression, a transient expression experiment in tobacco leaves was performed—WRKY6 repressed RAV1 promoter activity (Fig 5B).
Fig 5. WRKY6 directly represses RAV1 expression.
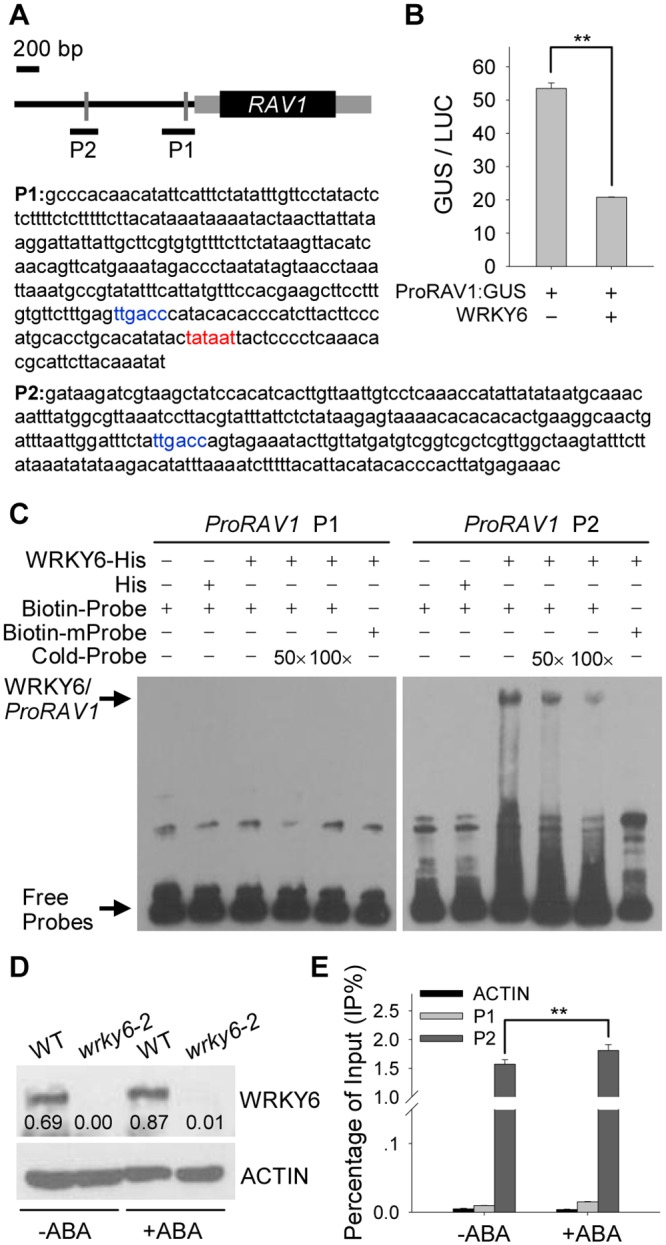
A, Schematic representation of RAV1 locus. RAV1 putative promoter is indicated by black line showing relative positions of W-box motifs (gray lines), and transcribed sequence by black box (exon) and gray boxes (untranslated regions). Relative positions and sizes of different PCR-amplified fragments are indicated by black lines under the W-boxes. The sequence of W-box is shown in blue and the TATA box is shown in red. B, Transient overexpression of WRKY6 fused to ProRAV1:GUS in Nicotiana benthamiana leaves. Data are shown as mean ± SE (n = 4). Asterisks indicate statistically significant differences: **, P 0.01. C, EMSA of WRKY6 binding to RAV1 promoter in vitro. Each biotin-labeled DNA probe was incubated with WRKY6-His protein. The mutation probes of P1 and P2 have the mutated W-box (TTGACC was replaced by TACGTC). D, Immunoblot analysis of WRKY6 protein. The 7-d-old wrky6-2 mutant and wild-type seedlings were transferred to MS solution with or without 100 μM ABA for 3 h, and then the seedlings were harvested for immunoblot analysis using anti-WRKY6 antibody. The relative band intensities of WRKY6, normalized relative to the intensity with the value of ACTIN (as 100%), are indicated by numbers below the bands. E, ChIP-qPCR assay of WRKY6 binding to RAV1 promoter in vivo. The 7-d-old wild-type seedlings were transferred to MS solution with or without 100 μM ABA for 3 h, and then the seedlings were harvested for ChIP-qPCR assay using anti-WRKY6 antibody. Data are shown as mean ± SE (n = 3). Asterisks indicate statistically significant differences: **, P 0.01.
Then an electrophoretic mobility shift assay (EMSA) was conducted to test whether WRKY6 bound to the RAV1 promoter in vitro. The recombinant WRKY6-His protein and His protein alone were expressed in Escherichia coli and purified. The WRKY6-His fusion protein can bind to the P2 fragment of the RAV1 promoter, and this binding was effectively reduced by adding increasing amounts of unlabeled competitor with the same P2 sequence (Fig 5C). When the W-box motif in the P2 fragment was mutated from TTGACC to TACGTC, the binding complex was not detected (Fig 5C). No super-shifted WRKY6-P1 complexes were detected in EMSA (Fig 5C). These data indicate that WRKY6 protein can bind to the P2 fragment of RAV1 promoter in vitro.
Furthermore, a chromatin immunoprecipitation (ChIP) assay was conducted to determine whether WRKY6 bound to the RAV1 promoter in vivo. The anti-WRKY6 antibody (AS111778; Agrisera) was tested in the wrky6-2 mutant and wild-type seedlings, and the anti-WRKY6 antibody can specifically recognize the WRKY6 protein (Fig 5D). For the WRKY6 expression was induced by exogenous ABA (Fig 1C), the protein level of WRKY6 was also tested under ABA treatment. After treated with 100 μM ABA for 3 h, the WRKY6 protein was elevated in the wild-type seedlings and still not detected in the wrky6-2 mutant (Fig 5D). Then the ChIP assay was conducted with anti-WRKY6 antibody. The chromatin immunoprecipitated with the anti-WRKY6 antibody was enriched in the P2 fragment of the RAV1 promoter, and the enrichment was enhanced under ABA treatment (+ABA) (Fig 5E). In contrast, fragments from the P1 fragment of the RAV1 promoter and the exon region of the Actin gene (ACTIN) did not show any detectable binding by WRKY6 with or without ABA treatment (Fig 5E). These results demonstrate that WRKY6 directly regulates RAV1 expression.
Overexpression of RAV1 abolishes ABA-sensitivity of the WRKY6-overexpressing line
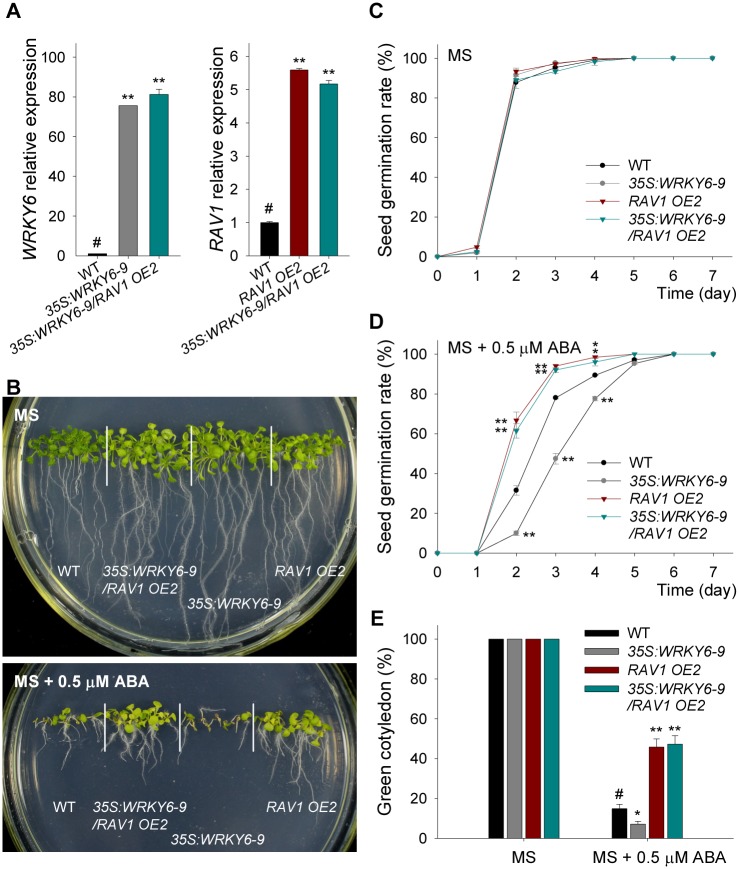
The 35S:WRKY6-9 was crossed with RAV1-overexpressing line (RAV1 OE2; [30]), and the 35S:WRKY6-9/RAV1 OE2 double overexpressing line was obtained (Fig 6A). When germinated and grown on MS medium, there were no obvious phenotype differences among all genotypes (Fig 6B, top panel), and their seed germination rates were similar (Fig 6C). In the presence of 0.5 μM ABA (MS + 0.5μM ABA), the 35S:WRKY6-9/RAV1 OE2 double overexpressing line displayed ABA-insensitive phenotypes, similar to RAV1 OE2 (Fig 6B, bottom panel); and both 35S:WRKY6-9/RAV1 OE2 and RAV1 OE2 had similar higher seed germination compared with wild-type plants, whereas the seed germination of 35S:WRKY6-9 was significantly reduced relative to wild-type (Fig 6D). The cotyledon-greening percentage was also measured. In the absence of ABA, the different genotypes had similar cotyledon-greening percentages (Fig 6E). In the presence of 0.5 μM ABA, both 35S:WRKY6-9/RAV1 OE2 and RAV1 OE2 had higher, whereas the 35S:WRKY6-9 had lower, cotyledon-greening percentages than wild-type plants (Fig 6E).
Fig 6. Overexpression of RAV1 impairs the ABA-sensitive phenotypes of WRKY6-overexpressing line.
A, The expression of WRKY6 and RAV1 was tested by qRT-PCR in 35S:WRKY6-9, RAV1 OE2, 35S:WRKY6-9/RAV1 OE2 and wild-type plants (WT). Data are shown as mean ± SE (n = 3). B, Phenotypic comparison. Imbibed seeds were germinated and grown on MS medium (MS) or MS medium containing 0.5 μM ABA (MS + 0.5 μM ABA) for 10 d. C-D, Seed germination assay. Imbibed seeds were transferred to MS medium (C) or MS + 0.5 μM ABA medium (D), and then the seed germination rates were calculated at the indicated time. Data are shown as mean ± SE (n = 3). More than 300 seeds were measured in each replicate. E, Cotyledon-greening analysis. Imbibed seeds were germinated and grown on MS or MS + 0.5 μM ABA medium for 7 d before determining cotyledon-greening percentage. Data are shown as mean ± SE (n = 3). More than 300 seeds were measured in each replicate. Asterisks in D and E indicate statistically significant differences compared with wild-type plants: *, P < 0.05; **, P < 0.01. Wild-type plant (WT) was used as a control (#).
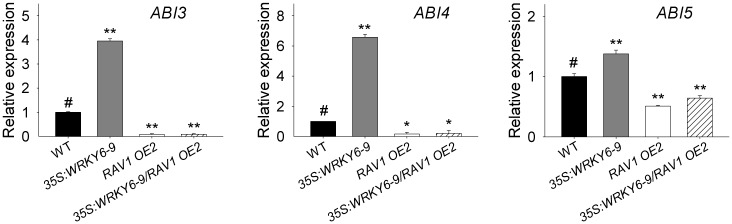
Expression of RAV1 target genes, ABI3, ABI4 and ABI5, was also tested by qRT-PCR and all were elevated in the WRKY6-overexpressing line (35S:WRKY6-9), but repressed in the 35S:WRKY6-9/RAV1 OE2 lines, similar to RAV1 OE2, compared with wild-type plants (Fig 7). These data together with phenotype tests indicated that RAV1 overexpression abolished the ABA-sensitivity of WRKY6-overexpressing line.
Fig 7. Expression of ABI3, ABI4 and ABI5 in 35S:WRKY6-9, RAV1 OE2, 35S:WRKY6-9/RAV1 OE2 and wild-type plants.
The imbibed seeds were germinated and grown on MS medium for 5 d, and then the seedlings were harvested for qRT-PCR. Data are shown as mean ± SE (n = 3). Asterisks indicate statistically significant differences compared with wild-type plants: *, P 0.05; **, P 0.01. Wild-type plant (WT) was used as a control (#).
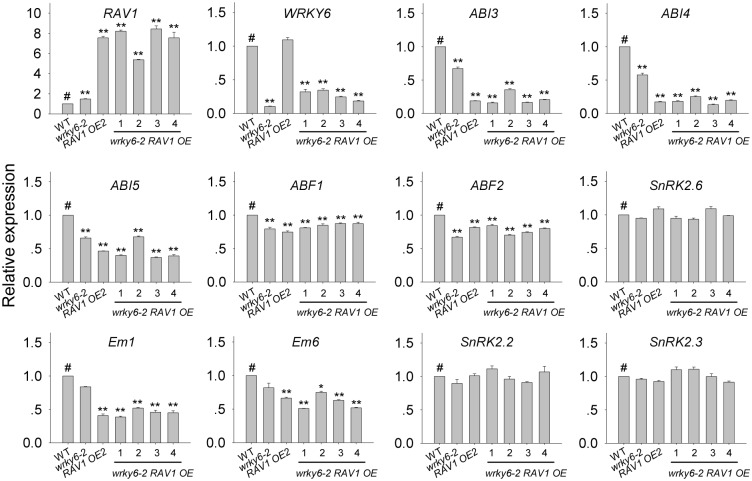
We also introduced Super:RAV1 [30] to the wrky6-2 mutant, and got four wrky6-2 RAV1OE (T1) transgenic lines (Fig 8). The four wrky6-2 RAV1OE lines had the higher RAV1 expression and much lower WRKY6 expression than wild-type plants (Fig 8). The transcript levels of ABI3, ABI4 and ABI5 in wrky6-2 RAV1 OE lines were lower than those in wild-type plants, even lower than those in the wrky6-2 mutant, similar to those in RAV1 OE2 (Fig 8). The transcript levels of Em1 and Em6 in wrky6-2 RAV1OE lines were also lower than those in wild-type and wrky6-2 mutant, similar to those in RAV1 OE2 (Fig 8). These data indicate that overexpression of RAV1 represses the expression of ABI3, ABI4 and ABI5, and WRKY6 modulates the expression of ABI3, ABI4 and ABI5 through down-regulating the RAV1 expression. The expression of ABFs and SnRK2s was also tested. The transcript levels of ABF1 and ABF2 in the wrky6-2 mutant, RAV1 OE2 and wrky6-2 RAV1OE lines were similar, and slightly lower than those in wild-type plants (Fig 8). And the transcript levels of SnRK2s were similar among RAV1 OE2, wrky6-2 RAV1 OE lines and wild-type plants (Fig 8).
Fig 8. Expression of ABFs, Ems and SnRK2s in the wrky6-2 mutant, RAV1 OE2, wrky6-2 RAV1 OE transgenic lines and wild-type plants.
The genes expression was tested by qRT-PCR in the wrky6-2 mutant, RAV1 OE2, wrky6-2 RAV1 OE transgenic lines (T1) and wild-type plants (WT). Three technical replicates were performed. Asterisks indicate statistically significant differences compared with wild-type plants: *, P 0.05; **, P 0.01. Wild-type plant (WT) was used as a control (#).
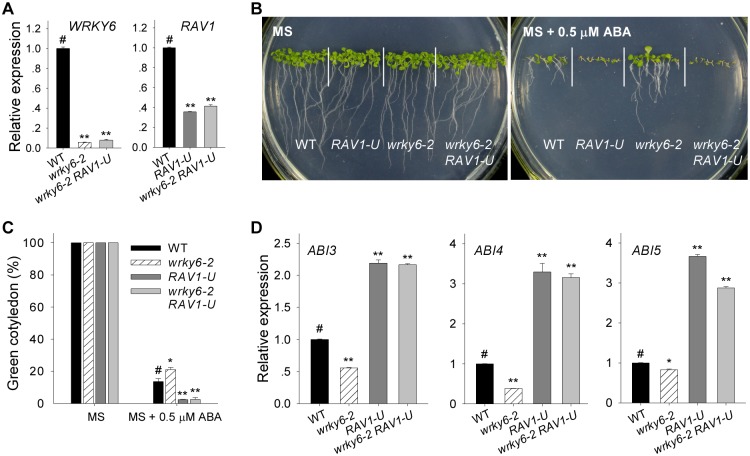
Disruption of RAV1 abolishes ABA-insensitivity of the wrky6 mutant
The RAV1-underexpressing line (RAV1-U) is an antisense transgenic line, which has relatively lower RAV1 expression [30, 32]. When grown on MS medium containing 0.5 μM ABA, RAV1-U shows ABA hyper-sensitive phenotypes [30]. The genetic relationship between WRKY6 and RAV1 was analyzed by crossing wrky6-2 with RAV1-U to produce the wrky6-2 RAV1-U double mutant (Fig 9A). In the absence of ABA (MS), all lines showed similar phenotypes (Fig 9B, left panel). When germinated and grown on MS medium containing 0.5 μM ABA (MS + 0.5 μM ABA), the wrky6-2 mutant displayed an ABA-insensitive phenotype, whereas the wrky6-2 RAV1-U double mutant showed an ABA-sensitive phenotype, similar to RAV1-U (Fig 9B, right panel). The cotyledon-greening percentages were also tested and, in the absence of ABA (MS), were similar for the different genotypes (Fig 9C). When germinated and grown on MS medium containing 0.5 μM ABA (MS + 0.5 μM ABA), the wrky6-2 RAV1-U double mutant (similar to RAV1-U) had a much lower, and the wrky6-2 mutant had a higher, cotyledon-greening percentage than wild-type plants (Fig 9C).
Fig 9. The ABA-insensitivity of the wrky6 mutant is abolished by suppression of RAV1.
A, The transcript levels of WRKY6 and RAV1 were tested by qRT-PCR. Data are shown as mean ± SE (n = 3). B, Phenotypic comparison. Imbibed seeds were transferred to MS medium (MS) or MS medium containing 0.5 μM ABA (MS + 0.5 μM ABA) for 10 d. C, Cotyledon-greening analysis. Imbibed seeds were transferred to MS or MS + 0.5 μM ABA medium for 7 d before determining cotyledon-greening percentage. Data are shown as mean ± SE (n = 3). More than 300 seeds were measured in each replicate. D, Expression of ABI3, ABI4, and ABI5 was tested by qRT-PCR in the wrky6-2 mutant, RAV1-U, wrky6-2 RAV1-U double mutant and wild-type plants. Each data represents the mean ± SE (n = 3). Asterisks indicate statistically significant differences compared with wild-type plants: *, P < 0.05; **, P < 0.01. Wild-type plants (WT) were used as a control (#).
Expression of ABI3, ABI4 and ABI5 was tested by qRT-PCR and showed clearly elevated transcript levels in the wrky6-2 RAV1-U double mutant, similar to that in RAV1-U, and repressed in the wrky6-2 mutant (Fig 9D).
Further, we used the CRISPR/Cas9 technology to generate rav1 mutant and rav1 wrky6-2 double mutant. A pair of closely located sgRNA targets in the RAV1 gene were selected (Fig 10A). The CRISPR construct was transformed into wild-type Arabidopsis and the wrky6-2 mutant, and the homozygous rav1 mutant and rav1 wrky6-2 double mutant were obtained. The rav1 wrky6-2 double mutant contained a nucleotide insertion in C1 and C2 sites, separately (Fig 10B), and the rav1 mutant had a nucleotide insertion in C1 site (Fig 10B). These insertions lead to frameshift mutation. The qRT-PCR results showed that the transcript level of WRKY6 was significantly repressed in the rav1 wrky6-2 double mutant, similar to that in wrky6-2 mutant (Fig 10C). These data indicate that we obtain the rav1 mutant and rav1 wrky6-2 double mutant.
Fig 10. Disruption of RAV1 abolishes the ABA-insensitivity of the wrky6 mutant.
A, Diagram of RAV1 showing two target sites (C1 and C2) for CRISPR/Cas9 technology. PAM motifs are marked with bold letters. The coding sequence (CDS) and untranslated regions (UTR) of RAV1 are indicated by black box and gray boxes, separately. B, The rav1 mutant and rav1 wrky6-2 double mutant were generated by CRISPR/Cas9 technology. The mutation in the RAV1 gene is evaluated by sequencing, and the mutant sites in RAV1 are indicated by red letters and arrows. C, qRT-PCR analysis of WRKY6 expression in the wrky6-2 mutant, rav1 mutant, rav1 wrky6-2 double mutant and wild-type plants (WT). Data are shown as mean ± SE (n = 3). D, Phenotypic comparison. Imbibed seeds were transferred to MS or MS + 0.5 μM ABA medium for 10 d. E, Cotyledon-greening analysis. Imbibed seeds were transferred to MS or MS + 0.5 μM ABA medium for 7 d before determining cotyledon-greening percentage. Data are shown as mean ± SE (n = 3). F, qRT-PCR analysis of ABIs in the wrky6-2 mutant, rav1 mutant, rav1 wrky6-2 double mutant and wild-type plants (WT) treated with or without exogenous ABA treatment. The 7-d-old seedlings were transferred to MS solution with or without 100 μM ABA for 3 h, and then the seedlings were harvested for qRT-PCR. Data are shown as mean ± SE (n = 3). Asterisks indicate statistically significant differences compared with relevant wild-type plants (WT): *, P 0.05; **, P 0.01.
When grown on MS medium containing 0.5 μM ABA, the rav1 wrky6-2 double mutant showed ABA hyper-sensitive phenotypes, similar to the rav1 mutant (Fig 10D), and both rav1 mutant and rav1 wrky6-2 double mutant had much lower cotyledon-greening percentages compared with wild-type plants (Fig 10E). The expression of ABIs was also tested, and the qRT-PCR results showed that the transcript levels of ABI3, ABI4, and ABI5 were elevated in the rav1 wrky6-2 double mutant, similar to that in the rav1 mutant, typically under ABA treatment (Fig 10F).
Taken together, these results demonstrate that disruption of RAV1 abolishes the ABA-insensitivity of the wrky6-2 mutant.
WRKY6 can not directly regulate the expression of ABI3, ABI4 and ABI5
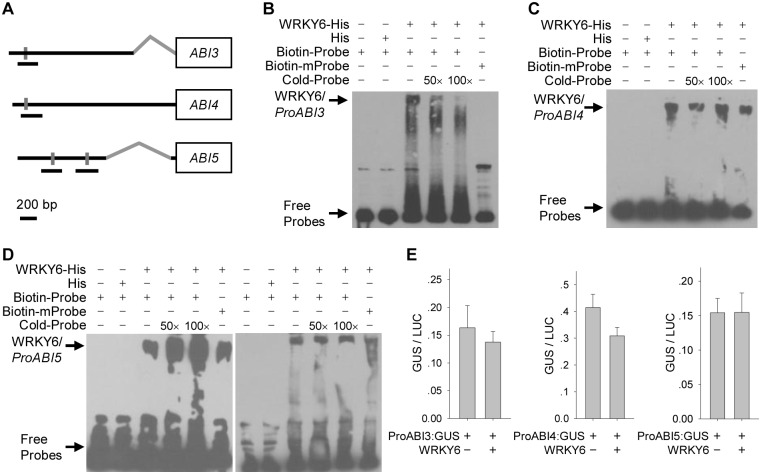
There were one or two W boxes within the promoters of ABI3, ABI4 and ABI5 (Fig 11A), and the expression of ABI3, ABI4 and ABI5 was elevated in the WRKY6-overexpressing lines and repressed in the wrky6 mutants (Fig 2). It is hypothesized that WRKY6 directly regulates the expression of ABI3, ABI4 and ABI5. Then the EMSA experiment was conducted, and the results showed that WRKY6 can bind to the W-box within the ABI3 promoter in vitro (Fig 11B). Although the super-shifted WRKY6-ProABI4 and WRKY6-ProABI5 complexes were detected, these bindings were not reduced by adding the unlabeled competitors, or not missing with the mutation probe with the mutated W-box (TTGACC was changed to TACGTC) (Fig 11C and 11D), indicating that WRKY6 can not bind to the promoters of ABI4 and ABI5 in vitro. To further test the function of WRKY6 in regulation of ABI3, ABI4, and ABI5 expression, the transient expression experiment in tobacco leaves was performed. Although WRKY6 can bind to the ABI3 promoter in vitro, WRKY6 can not regulate ABI3 expression in tobacco leaves (Fig 11E). And WRKY6 can not regulate the expression of ABI4 and ABI5 in tobacco leaves (Fig 11E). All these data indicate that WRKY6 can not directly regulate the expression of ABI3, ABI4, and ABI5.
Fig 11. WRKY6 can not directly regulate the expression of ABI3, ABI4 and ABI5.
A, Diagrams of ABI3, ABI4 and ABI5 promoters showing relative positions of W-box motifs (gray lines). Relative positions and sizes of different PCR-amplified fragments are indicated by black lines under the W boxes. The gray bent lines indicate the introns within the 5’UTR of ABI3 and ABI5. B-D, EMSA of WRKY6 binding to promoters of ABI3, ABI4 and ABI5 in vitro. Each biotin-labeled DNA probe was incubated with WRKY6-His protein. The mutation probes have the mutated W-box (TTGACC was replaced by TACGTC). E, Transient overexpression of WRKY6 fused to ProABIs:GUS in Nicotiana benthamiana leaves. Data are shown as mean ± SE (n = 6).
Discussion
WRKY6 is an important regulator in ABA signaling during seed germination and early seedling development
Arabidopsis WRKY6 is a WRKY transcription factor [25]. In this study, we demonstrated that Arabidopsis WRKY6 played important roles in ABA signaling during seed germination and early seedling development. When germinated and grown on MS medium containing ABA, the wrky6 mutants were ABA-insensitive while WRKY6-overexpressing lines were ABA-hypersensitive compared with wild-type plants (Fig 1). As a WRKY transcription factor, WRKY6 is localized in the nucleus and has a DNA-binding domain (WRKY domain) [25]. One reason for the ABA-response phenotypes is that WRKY6 regulated the expression of ABA-response genes. The AREB/ABFs are bZIP-type transcription factors, which recognize the ABA-responsive elements (ABRE) in the promoters of ABA-inducible genes [27], and the expression of AREB1/ABF2, AREB2/ABF4 and ABF3 is induced by dehydration, high salinity and ABA treatment in vegetative tissues [33]. The expression of ABF2 and ABF3 was induced by exogenous ABA, and this inducement was obviously repressed in the wrky6 mutants (Fig 3), and the cotyledon-greening percentages of wrky6-1 and wrky6-2 were much higher than wild-type plants (Fig 1I), indicating that WRKY6 played a role in response to ABA signaling during post-germination growth partially by regulating expression of ABF2 and ABF3. Further promoter sequence analysis results showed that there was no W box (TTGACC/T) within the 2-kb promoters of ABF1, ABF2 and ABF3, indicating that WRKY6 can not directly regulate the expression of ABF1, ABF2 and ABF3.
The transcription factors ABI3, ABI4, and ABI5 are well known positive regulators of ABA signaling during seed germination [10–12]. The qRT-PCR results showed that the transcript levels of ABI3, ABI4, and ABI5—typically the expression of ABI3 and ABI4—were repressed in wrky6 mutants (wrky6-1 and wrky6-2) and elevated in WRKY6-overexpressing lines (Figs 2 and 3), suggesting that WRKY6 modulated the expression of ABI3, ABI4, and ABI5. The qRT-PCR results also showed that RAV1 expression was significantly induced in wrky6 mutants (wrky6-1 and wrky6-2) and repressed in WRKY6-overexpressing lines (35S:WRKY6-5 and 35S:WRKY6-9) (Figs 2 and 3). Our previous work showed that the Arabidopsis RAV1 transcription factor negatively regulated the expression of ABI3, ABI4, and ABI5 [30], suggesting that WRKY6 modulates the expression of ABI3, ABI4, and ABI5 by negatively regulating RAV1 expression. The EMSA and ChIP analyses showed that WRKY6 could bind to the RAV1 promoter in vitro and in vivo (Fig 5), demonstrating that WRKY6 negatively regulated RAV1 expression by binding to the RAV1 promoter.
Usually, WRKY transcription factors contain the conserved WRKY domain and bind to the W box(es) within their target genes’ promoters [18–19]. Interestingly, the genes ABI3, ABI4, and ABI5 contain several W boxes in their promoters [20, 23], and the expression of ABI3, ABI4, and ABI5 was enhanced in WRKY6-overexpressing lines and repressed in wrky6 mutants (Figs 2 and 3). Previous reports showed that WRKY40 directly represses ABI5 expression [20], and WRKY41 directly regulates ABI3 expression [23]. Thus we investigated whether WRKY6 directly regulated the expression of ABI3, ABI4, and ABI5, and whether the ABA-response phenotypes of 35S:WRKY6 and wrky6 mutants were due to the direct regulation of WRKY6 on ABI3, ABI4, and ABI5. The phenotype of 35S:WRKY6-9/RAV1 OE2 was first tested. When grown on MS medium containing ABA, the WRKY6-overexpressing line (35S:WRKY6-9) showed an ABA-hypersensitive phenotype, whereas overexpression of RAV1 in 35S:WRKY6-9 (35S:WRKY6-9/RAV1 OE2) repressed the ABA-hypersensitivity of 35S:WRKY6-9 (Fig 6), indicating that its ABA-hypersensitivity was mainly due to repression of RAV1 by WRKY6. And the transcript levels of ABI3, ABI4 and ABI5 in wrky6-2 RAV1 OE were lower than those in wrky6-2 mutant, similar to those in RAV1 OE2 (Fig 8), indicating that the expression of ABI3, ABI4 and ABI5 was regulated by RAV1, not by WRKY6. Then the wrky6 RAV1-U and rav1 wrky6-2 double mutant was generated. When grown on MS medium containing ABA, the wrky6-2 mutant showed an ABA-insensitive phenotype, and the repression or disruption of RAV1 in the wrky6-2 mutant (i.e. wrky6-2 RAV1-U and rav1 wrky6-2) abolished the ABA-insensitivity of wrky6-2 (Figs 9 and 10), indicating that the ABA-insensitivity of wrky6-2 was mainly due to the disruption of the regulation by WRKY6 of RAV1, and RAV1 was epistatic to WRKY6. The EMSA results showed that WRKY6 could not bind to the promoters of ABI4 (Fig 11C) and ABI5 (Fig 11D), indicating that WRKY6 could not directly regulate the expression of ABI4 and ABI5. WRKY6 can bind to the ABI3 promoter in vitro (Fig 11B), whereas WRKY6 can not modulate the ABI3 expression in plants (Fig 11E), indicating that WRKY6 also can not directly regulate ABI3 expression. These data demonstrate that WRKY6 acted as a positive regulator mainly via direct regulation of RAV1 expression.
The 2-kb promoter sequences of SnRK2s and Ems were also analyzed, and the results showed that there was no W box in SnRK2.2 promoter, one in SnRK2.6 and Em1 promoters, two in SnRK2.3 promoter and three in Em6 promoter. And the transcript levels of the SnRK2s and Ems were elevated in the WRKY6-overexpressing lines (Fig 2) and repressed in the wrky6 mutants under ABA treatment (Fig 3), indicating that WRKY6 transcription factor may directly regulate the expression of SnRK2.3, SnRK2.6, Em1 and Em6.
In summary, our data show that the Arabidopsis WRKY6 transcription factor plays important roles in ABA signaling (Fig 12). The WRKY6 expression is repressed during seed germination and early seedling development, and induced by exogenous ABA. WRKY6 transcription factor acts in the ABA signal transduction pathway predominantly by directly down-regulating RAV1 expression; RAV1 mediates seed germination and early seedling development by directly down-regulating expression of ABI3, ABI4 and ABI5.
Fig 12. Hypothetical model of WRKY6/RAV1/ABIs-regulatory pathway in plant responses to ABA signaling during seed germination and early seedling development.
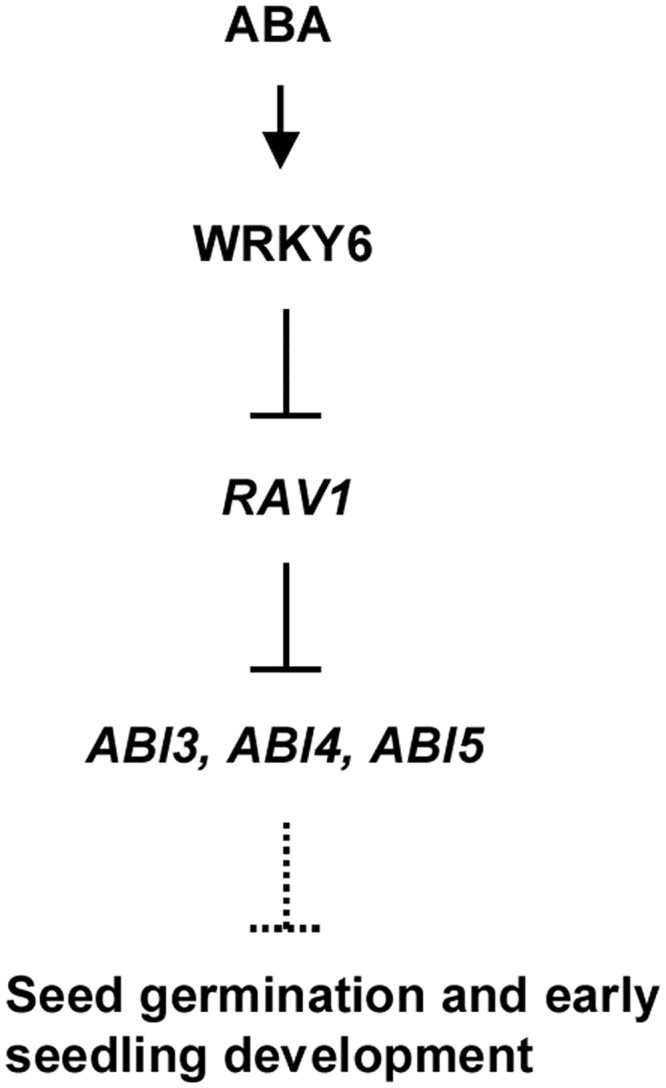
ABA induces the activity of WRKY6, and WRKY6 binds to the RAV1 promoter to repress RAV1 expression. RAV1 directly represses the expression of ABI3, ABI4 and ABI5, which promote seed germination and early seedling development.
WRKY6 plays important roles in plant development and stress response
The WRKY6 gene, encoding a WRKY transcription factor, is expressed in all tissues [25], suggesting that WRKY6 plays widespread roles during different phases of plant development. The WRKY6 transcript is present in roots, shoots, flowers, siliques and senescent leaves, with the highest transcript level of WRKY6 in senescent leaves [25]. Overexpression of WRKY6 results in dwarfed Arabidopsis with partly necrotic leaves, early flowering and a reduction in their apical dominance [26]. Interestingly, overexpressing RAV1 caused a retardation of rosette leaf development, and underexpression of RAV1 caused an earlier flowering phenotype [32]. Recently, Arabidopsis RAV1 was reported to positively regulate leaf senescence, and overexpression of RAV1 caused premature leaf senescence [34]. The data in the present study showed that WRKY6 directly repressed RAV1 expression. The data suggested that the WRKY6-RAV1 regulatory pathway was involved in leaf senescence and flowering.
In addition to modulating leaf senescence and flowering, the expression of WRKY6 was repressed during seed germination and early seedling development, and obviously induced by exogenous ABA (Fig 1A–1C). When grown on MS medium with ABA, the wrky6 mutants showed ABA-insensitive phenotypes while the WRKY6-overexpressing lines were ABA-hypersensitive (Fig 1E). WRKY6 could bind to the RAV1 promoter to repress RAV1 expression (Fig 5). Further genetic results showed that RAV1 was the main target gene of WRKY6 during seed germination and early seedling development (Figs 6–10). These data provide evidence of the major role of WRKY6 during seed germination and early seedling development.
WRKY6 is also involved in controlling processes related to pathogen defense [25–26]. WRKY6 positively influences the promoter activity of the pathogen defense-associated PR1 gene, most likely involving NPR1 function [26]. In addition to abiotic stress, WRKY6 has also been reported to be involved in biotic stress responses. WRKY6 is a negative regulator in phosphate translocation [35]. When grown on inorganic phosphorus (Pi)-sufficient condition, WRKY6 represses PHO1 expression and reduces Pi translocation from roots to shoots. During Pi starvation, the WRKY6 protein is degraded and the repression of PHO1 by WRKY6 is abolished [35]. The expression of WRKY6 is induced by boron (B) deficiency, and wrky6 mutants showed growth defects compared with wild-type plants under B deficient condition [36]. Recently, WRKY6 was reported to be induced by arsenate stress, and the WRKY6 mediated the expression of a phosphate transporter gene and restricted arsenate-induced transposon activation [37]. Taken together, the WRKY6 transcription factor plays important roles in plant development and biotic and abiotic stress responses.
Materials and Methods
Plant materials and growth conditions
The wild-type plant used in this study was A. thaliana Col-0. The WRKY6-overexpressing lines (35S:WRKY6-5 and 35S:WRKY6-9), the wrky6-1 mutant, the RAV1-overexpressing line (RAV1 OE2) and the RAV1-underexpressing line (RAV1-U) were described previously [26, 30, 32]. The WRKY6 T-DNA insertion line Salk_012997, named wrky6-2 in the present study, was ordered from the ABRC. For the seed germination assay, seeds were surfaced sterilized and kept at 4°C for 72 h in darkness before germination. About 300 seeds of each genotype were sown on the same plate containing MS medium [with 3% (w/v) sucrose] with 0, 0.5 and 2 μM ABA, and were kept at 22°C under constant illumination of 60 μmol·m−2·s−1. Germination was defined as an obvious emergence of the radicle through the seed coat. The seed germination percentages were evaluated daily during the germination test.
qRT-PCR assay
For qRT-PCR analysis, total RNA of seedlings and seeds was extracted with Trizol reagent (Invitrogen) and RNeasy Plant Mini kit (Bioteke), separately. The total RNA (8 μg) was treated with DNase I (RNase Free) (Takara) to eliminate genomic DNA contamination. Then the cDNA was synthesized from the treated total RNA (4 μg) by SuperScript II Reverse Transcriptase (Invitrogen) using Radom Hexamer Primers (Promega). 40 ng cDNA (except RAB18, with 80 ng cDNA) and 50 nM each primer were used for each quantitative PCR reaction, which was performed by using the Power SYBR Green PCR Master Mix (Life Technologies) on a 7500 Real Time PCR System machine (Life Technologies) following the manufacturer’s protocols. The thermal treatment was 10 min at 95°C, then 40 cycles of 15 s at 95°C, 1 min at 60°C. Amplification was followed by a melt curve analysis. The 2-ΔΔCt method was used for relative quantification [38]. Actin2/8 expression was used as an internal control. The statistical significance was evaluated by Paired t-test analysis. The primers used are listed in S1 Table.
Transient expression assay in Nicotiana benthamiana
The transient GUS expression assay was performed as described previously [35]. The ProRAV1:GUS and Super:WRKY6 constructs were described previously [30, 35]. To construct RroABI3, ProABI4 and ProABI5, the ∼2kb promoters of ABI3, ABI4 and ABI5 were cloned into the pCAMBIA1381 vector. The primer sequences used are listed in S1 Table. For each infiltration sample, Super:LUC was added as an internal control. The GUS and LUC activities of the infiltrated leaves were quantitatively determined, and the GUS/LUC ratio was used to quantify the promoter activity.
Protein expression and EMSA experiment
The coding sequence of WRKY6 was amplified and cloned into the pET30a vector. The primer sequences used are listed in S1 Table. The recombinant plasmid was introduced to E. coli strain BL21. E. coli cells were induced with 0.2 mM IPTG overnight at 18°C and collected by centrifugation. The WRKY6-His protein was purified using Ni-Sepharose 6 Fast Flow (GE Healthcare), and the protein concentration was determined by Bio-Rad protein assay. The pET30a vector was also introduced into E. coli strain BL21, and a protein with His tag was purified. This purified protein was named His protein, and used as a control in EMSA experiment.
For EMSA assays, the fragment of the promoters were obtained by PCR using biotin-labeled or -unlabeled primers (see S1 Table). Biotin-unlabeled fragments of the same sequences were used as competitors. The reaction mixture (20 μL) for EMSA contained 0.5 μg purified protein, 1 μL 50 μg/mL biotin-labeled annealed oligonucleotide, 2 μL 10×binding buffer (100 mM Tris, 500 mM KCl, and 10 mM DTT, pH 7.5), 1 μL 1% Nonidet P-40, 0.5 μL 1 mg/mL poly (dI-dC), and ultrapure water. The reactions were incubated at 22°C for 30 min. The reactions were fractionated on a 5% native polyacrylamide gel in 0.5 ×TBE buffer. The detection of biotin-labeled DNA by chemiluminescence was performed using a LightShift Chemiluminescent EMSA Kit (Pierce) following the manufacturer’s protocol.
ChIP-qPCR assay
The ChIP experiment was performed as described previously [30, 35]. For the ChIP assay, 1 g of 7-d-old seedlings grown on MS medium was transferred to MS solution with or without 100 μM ABA for 3 h, then harvested and cross-linked by 1% formaldehyde for 10 min, and then the purified cross-linked nuclei were resuspended in 4 mL lysis buffer. Following sonication, 1 mL lysis buffer with nuclei was used for each immunoprecipitation (IP). The anti-WRKY6 antibody (AS111778; Agrisera, http://www.agrisera.com/) was used to immunoprecipitate DNA/protein complexes from the chromatin preparation. IP DNA was dissolved in 25 μL TE buffer, and 1 μL IP DNA was analyzed by qPCR using the primers listed in S1 Table. As a control, ‘input’ DNA was isolated from 50 μL lysis buffer with nuclei without the IP step. The input DNA was suspended in 25 μL TE buffer and 1 μL input DNA was analyzed by qPCR. The ratio of IP DNA over the input was presented as the percentage of input (IP %). An Actin fragment (ACTIN) was amplified as control. At least three independent experiments were performed with similar results. Data are mean values of three replicates ± standard error (SE) from one experiment.
Generation of rav1 mutant and rav1 wrky6-2 double mutant using CRISPR/Cas9 technology
A pair of closely located sgRNA targets (C1: GATGAGAGTACTACAAGTAC and C2: ACGGCGTAGAAGCTGAATCT) in RAV1 gene was selected and cloned into the pHEE2A-TRI vector as described [39]. Then the CRISPR construct was transformed into wild-type Arabidopsis and the wrky6-2 mutant to obtain rav1 mutant and rav1 wrky6-2 double mutant, separately. The homozygous rav1 mutant and rav1 wrky6-2 double mutant were identified by sequencing.
Accession numbers
Sequence data for the Arabidopsis genes described in this study can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers, At1g62300 for WRKY6, At1g49720 for ABF1, At1g45249 for ABF2, At4g34000 for ABF3, At3g50500 for SnRK2.2, At5g66880 for SnRK2.3, At4g33950 for SnRK2.6, At3g24650 for ABI3, At2g40220 for ABI4, At2g36270 for ABI5, At1g13260 for RAV1, At3g51810 for Em1, and At2g40170 for Em6.
Supporting Information
(PDF)
(PDF)
Acknowledgments
We thank Imre E. Somssich (Max-Planck-Institut für Züchtungsforschung, Abteilung Biochemie, Germany) for kindly providing AtWRKY6 overexpression lines 35S:WRKY6-5 and 35S:WRKY6-9 and the AtWRKY6 knockout mutant (wrky6-1). We thank Dr. Qijun Chen (China Agricultural University, China) for providing pHEE2A-TRI vector and the assistance of CRISPR/Cas9 technology.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the ‘973’ Project (2011CB100305 to YFC; 2012CB114203 to WHW), the National Natural Science Foundation of China (Nos. 30970220 and 31170248 to YFC; Nos. 30380013 and 31121002 to WHW) and the ‘111’ Project (No. B06003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Verslues PE, Zhu JK. New developments in abscisic acid perception and metabolism. Curr Opin Plant Biol. 2007;27: 7781–7790. [DOI] [PubMed] [Google Scholar]
- 2.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol. 2010;61: 651–679. 10.1146/annurev-arplant-042809-112122 [DOI] [PubMed] [Google Scholar]
- 3.Ali-Rachedi S, Bouinot D, Wagner MH, Bonnet M, Sotta B, Grappin P, et al. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta. 2004;219: 479–488. [DOI] [PubMed] [Google Scholar]
- 4.Gubler F, Millar AA, Jacobsen JV. Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol. 2005;8: 183–187. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein RR, Lynch TJ. Abscisic acid inhibition of radicle emergence but not seedling growth is suppressed by sugars. Plant Physiol. 2000; 122: 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14: S15–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324: 1064–1068. 10.1126/science.1172408 [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324: 1068–1071. 10.1126/science.1173041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii H, Verslues PE, Zhu JK. Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabisopsis. Plant Cell. 2007;19: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992;4: 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell. 1998;10: 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein RR, Lynch TJ. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 2000;12: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Molina L, Chua NH. A null mutation in a bZIP factor confers ABA-insensitivity in Arabidopsis thaliana. Plant Cell Physiol. 2000;41: 541–547. [DOI] [PubMed] [Google Scholar]
- 14.Kang JY, Choi HI, Im MY, Kim SY. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell. 2002;14: 343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Kang JY, Cho DI, Park JH, Kim SY. ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J. 2004;40: 75–87. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, et al. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010;61: 672–685. 10.1111/j.1365-313X.2009.04092.x [DOI] [PubMed] [Google Scholar]
- 17.Ulker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7: 491–498. [DOI] [PubMed] [Google Scholar]
- 18.Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5: 199–206. [DOI] [PubMed] [Google Scholar]
- 19.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15: 247–258. 10.1016/j.tplants.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 20.Shang Y, Yan L, Liu ZQ, Cao Z, Mei C, Xin Q, et al. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell. 2010;22: 1909–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren X, Chen Z, Liu Y, Zhang H, Zhang M, Liu Q, et al. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J. 2010;63: 417–429. 10.1111/j.1365-313X.2010.04248.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang W, Yu D. Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biol. 2009;9: 96 10.1186/1471-2229-9-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding ZJ, Yan JY, Li GX, Wu ZC, Zhang SQ, Zheng SJ. WRKY41 controls Arabidopsis seed dormancy via direct regulation of ABI3 transcript levels not downstream of ABA. Plant J. 2014;79: 810–823. 10.1111/tpj.12597 [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Zhang L, Li D, Wang F, Yu D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA. 2014;110: E1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robatzek S, Somssich IE. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 2001;28: 123–133. [DOI] [PubMed] [Google Scholar]
- 26.Robatzek S, Somssich IE. Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 2002;16: 1139−1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid—dependent signal transduction pathway under drought and high-salinity. Proc Natl Acad Sci USA. 2000;97: 11632–11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, et al. Type 2C protein phosphatases directly regulate abscisic acid activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA. 2009;106: 17588–17592. 10.1073/pnas.0907095106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujii H, Verslues PE, Zhu JK. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc Nat Acad Sci USA. 2011;108: 1717–1722. 10.1073/pnas.1018367108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng CZ, Chen Y, Wang C, Kong YH, Wu WH, Chen YF. Arabidopsis RAV1 transcription factor, phosphorylated by SnRK2 kinases, regulates the expression of ABI3, ABI4, and ABI5 during seed germination and early seedling development. Plant J. 2014;80: 654–668. 10.1111/tpj.12670 [DOI] [PubMed] [Google Scholar]
- 31.Gaubier P, Raynal M, Hull G, Huestis GM, Grellet F, Arenas C, et al. Two different Em-like genes are expressed in Arabidopsis thaliana seeds during maturation. Mol Gen Genet. 1993;238: 409–418. [DOI] [PubMed] [Google Scholar]
- 32.Hu YX, Wang YH, Liu XF, Li JY. Arabidopsis RAV1 is down-regulated by brassinosteroid and may act as a negative regulator during plant development. Cell Res. 2004;14: 8–15. [DOI] [PubMed] [Google Scholar]
- 33.Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, et al. AREB1 is a transcription activator of novel ABRE dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 2005;17: 3470–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo HR, Kim JH, Kim J, Kim J, Lee U, Song IJ, et al. The RAV1 transcription factor positively regulates leaf senescence in Arabidopsis. J Exp Bot. 2014;61: 3947–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell. 2009;21: 3554–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasajima I, Ide Y, Yokota Hirai M, Fujiwara T. WRKY6 is involved in the response to boron deficiency in Arabidopsis thaliana. Physiol Plant. 2010;139: 80–92. 10.1111/j.1399-3054.2010.01349.x [DOI] [PubMed] [Google Scholar]
- 37.Castrillo G, Sánchez-Bermejo E, de Lorenzo L, Crevillén P, Fraile-Escanciano A, Tc M, et al. WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell. 2013;25: 2944–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25: 402–408. [DOI] [PubMed] [Google Scholar]
- 39.Wang ZP, Xing HL, Dong L, Zhang HY, Han CY, Wang XC, Chen QJ. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015;16:144 10.1186/s13059-015-0715-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.