Abstract
Background
Hepatocyte growth factor (HGF)-mediated mesenchymal-to-epithelial transition factor (MET) gene amplification is a common mechanism for acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs). MET gene amplification has also been associated with hepatic metastases in patients with lung cancer. The aim of this study was to investigate whether hepatic metastases are associated with decreased efficacy of erlotinib in patients with adenocarcinoma.
Material/Methods
A cohort of 329 patients with stage IV lung adenocarcinoma, known EGFR mutation status, and who received treatment with erlotinib in the 2nd or 3rd line setting were enrolled into this study over a period of 4 years between January 2011 and January 2015. The cohort was stratified based on the presence or absence of hepatic metastases and the efficacy of erlotinib was defined based on disease control rate (DCR) and progression-free survival (PFS).
Results
Hepatic metastases were present in 220 of the 329 enrolled lung adenocarcinoma patients. EGFR-activating mutations (exon 19 deletion or an exon 21 L858R mutation) were identified in 113 (34.3%) patients. The DCR was significantly lower in the hepatic metastases group than in patients without hepatic metastases (39.5% vs. 51.4% P=0.045). In patients with hepatic metastases, median PFS was 2.3 months in the EGFR mutation-positive group versus 1.4 months in the EGFR mutation-negative group (95% CI 1.3–3.3 vs. 1.3–1.5; P=0.055). Of note, erlotinib therapy in patients with hepatic metastases was complicated by elevated alanine transaminase (ALT) levels.
Conclusions
Hepatic metastasis in patients with lung adenocarcinoma predicts poor response to erlotinib as a 2nd/3rd line therapy. Combination therapy, for example with MET-TKI, may be a good choice for patients with liver metastases with poor prognosis.
MeSH Keywords: Adenocarcinoma; Genes, erbB-1; Neoplasm Metastasis
Background
Lung cancer has high mortality worldwide [1–3]. Unfortunately, most patients with non-small cell lung cancer (NSCLC) are diagnosed at an advanced stage [2]. Platinum-based doublet chemotherapy is the standard 1st line therapy for advanced NSCLC [4,5]. Single-agent chemotherapy is used in the treatment of advanced NSCLC in 2nd line therapy, while the response rate (RR) is low and all of these agents have different toxicity profiles [6,7]. Guidelines by the IASLC, CAP, and AMP recommend epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement genetic testing of NSCLCs with an adenocarcinoma histological type or even a component of adenocarcinoma as the standard of care. Tyrosine kinase inhibitor (TKI) therapy is indicated as the standard of care for patients with adenocarcinomas that harbor EGFR mutations. EGFR-TKIs, such as gefitinib, erlotinib, icotinib, and afatinib, have been widely used not only in 1st line therapy, but also in maintenance and 2nd/3rd line therapy in advanced NSCLC [8–11].
However, some patients with EGFR mutation do not respond well to EGFR-TKIs. Additionally, nearly all the patients initially responding to EGFR-TKIs inevitably develop acquired resistance. Hepatocyte growth factor (HGF)-mediated mesenchymal-to-epithelial transition factor (MET) amplification through activating ERBB3/PI3K/AKT signaling is an important mechanism for acquired resistance to EGFR-TKI, and also plays an important role in the process of hepatic metastases. The incidence of hepatic metastases in patients with lung cancer is high, with rates as high as 37–51% [12–15]. Therefore, we hypothesized that hepatic metastasis predicts poor efficacy of EGFR-TKI [16,17].
In this study, we compared the efficacy of erlotinib in the 2nd/3rd line setting in 329 pulmonary adenocarcinoma patients stratified by the presence or absence of hepatic metastasis.
Material and Methods
Patients
From January 2011 to January 2015, 220 lung adenocarcinoma patients with hepatic metastases were enrolled into the study, and 109 stage IV lung adenocarcinoma patients without hepatic metastases were recruited continuously from January 2011. Eligible patients had confirmed stage IV lung adenocarcinoma (Union for International Cancer Control classification version 7) with a confirmed activating mutation of EGFR (exon 19 deletion or an exon 21 L858R mutation). All patients received 2nd/3rd line chemotherapy treatment and had platinum-based doublet chemotherapy as 1st line therapy. They also had measurable disease according to Response Evaluation Criteria In Solid Tumors (RECIST version 1.1), an Eastern Cooperative Oncology Group performance status (PS) of 0–2, age ≥18, and adequate hematological, biochemical, and organ function. Patients with unstable systemic disease or uncontrolled brain metastases were excluded. This research was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University, and informed consent was obtained from all of the patients before enrollment.
Treatment
We performed history-taking, physical examination, hematologic and biochemical testing, and chest and abdomen computed tomographic scans before erlotinib treatment. Assessments of toxic effects and quality of life were obtained. Patients received erlotinib 150 mg daily. Assessment of toxicity was done according to National Cancer Institute Common Toxicity Criteria version 4.0. Patients were evaluated every 3 weeks, and hematology and blood chemistry analyses were done. Tumor size was assessed every 6 weeks [18–20].
DNA extraction and EGFR mutation analysis
All EGFR mutational analyses were performed using the Amplification Refractory Mutation System (ARMS) in Tongji University Medical School Cancer Institute (Shanghai, China). The details were described in our previous articles [21,22].
Statistical analysis
The chi-square test was used to analyze the association between hepatic metastases and clinical data and disease control rate (DCR). For the survival analysis, patients were censored at the last date at which they were known to be alive. All time-to-event outcomes, such as progression-free survival (PFS), were estimated using the Kaplan-Meier method and compared across groups with the log-rank test or the Cox proportional hazards model. The SPSS statistical package for Windows version 13.0 was used. All P values were 2-sided, and statistical significance was defined as p<0.05.
Results
Patient characteristics
We enrolled 329 stage IV lung adenocarcinoma patients with known EGFR mutation status. Table 1 shows the clinical characteristics of all the patients. Hepatic metastases was more common in patients younger than 65 years old (p=0.028), and the PS of these patients was significantly higher (p<0.001) (Table 1).
Table 1.
Characteristics of all cases.
| Items | Total | Hepatic metastases | EGFR mutation | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | P | Negative | Positive | P | ||
| Sex, n (%) | |||||||
| Male | 168 (51.1%) | 53 (48.6%) | 115 (51.4%) | 0.559 | 127 (58.8%) | 41 (36.3%) | <0.001 |
| Female | 161 (48.9%) | 56 (52.3%) | 105 (47.7%) | 89 (41.2%) | 72 (63.7%) | ||
| Age, mean | 57 | 60 | 55 | 56 | 58 | ||
| <65; n (%) | 251 (76.3%) | 75 (68.8%) | 176 (80.0%) | 0.028 | 171 (79.2%) | 80 (70.8%) | 0.102 |
| ≥65; n (%) | 78 (23.7%) | 34 (31.2%) | 44 (20.0%) | 45(20.8%) | 33 (29.2%) | ||
| Smoking, n (%) | |||||||
| Smoker | 155 (47.1%) | 45 (41.3%) | 110 (50.0%) | 0.159 | 121 (56.0%) | 34 (30.1%) | <0.001 |
| Non-smoker | 174 (52.9%) | 64 (58.7%) | 110 (50.0%) | 95 (44.0%) | 79 (69.9%) | ||
| Performance status (ECOG), n (%) | |||||||
| 0–1 | 271 (82.3%) | 105 (96.3%) | 166 (75.5%) | <0.001 | 173 (80.1%) | 98 (86.7%) | 0.170 |
| 2 | 58 (17.6%) | 4 (3.7%) | 54 (24.5%) | 43 (19.9%) | 15 (13.3%) | ||
| T stage, n (%) | |||||||
| 1–2 | 80 (24.3%) | 31 (28.4%) | 49 (22.3%) | 0.223 | 48 (22.2%) | 32 (28.3%) | 0.226 |
| 3–4 | 249 (75.7%) | 78 (71.6%) | 171 (77.7%) | 168 (77.8%) | 81 (71.7%) | ||
| N stage, n (%) | |||||||
| 0–1 | 51 (15.5%) | 18 (16.5%) | 33 (15.0%) | 0.747 | 30 (13.9%) | 21 (18.6%) | 0.266 |
| 2–3 | 278 (84.5%) | 91 (83.5%) | 187 (85.0%) | 186 (86.1%) | 92 (81.4%) | ||
| Metastasis sites | |||||||
| Brain | 101 (30.7%) | 70 (69.3%) | 31 (30.7%) | 59 (58.4%) | 42 (41.6%) | ||
| Bone | 61 (18.5%) | 42 (68.9%) | 19 (31.1%) | 41 (67.2%) | 20 (32.8%) | ||
| Pleura | 89 (27.1%) | 67 (75.3%) | 22 (24.7%) | 55 (61.8%) | 34 (38.2%) | ||
| Liver | 220 (66.9%) | 0 (0.0%) | 220 (100%) | 153 (69.5%) | 67 (30.5%) | ||
| Lung | 92 (28.0%) | 62 (67.4%) | 30 (32.6%) | 65 (70.7%) | 27 (29.3%) | ||
| Other sites | 51 (15.5%) | 29 (56.9%) | 22 (43.1%) | 27 (52.9%) | 24 (47.1%) | ||
| Number of hepatic metastases, n (%) | |||||||
| ≤3 | 141 (64.1%) | 93 (61.2%) | 48 (70.6%) | 0.224 | |||
| >3 | 79 (35.9%) | 59 (38.8%) | 20 (29.4%) | ||||
| EGFR mutation, n (%) | |||||||
| Negative | 216 (65.7%) | 64 (58.7%) | 152 (69.1%) | 0.066 | |||
| Positive | 113 (34.3%) | 45 (41.3%) | 68 (30.9%) | ||||
| Exon 19 deletion | 69 (61.1%) | ||||||
| Exon 21 mutation | 44 (38.9%) | ||||||
Therapeutic effect
In the hepatic metastases group, the disease control rate was 39.5%, while in patients without hepatic metastases the disease control rate was 60.5% (P=0.045) (Table 2). In males (p<0.001), smokers (p=0.004), N2–3 (p=0.039), number of hepatic metastases >3 (p=0.004), and EGFR-positive (p<0.001) patients, the DCR rate was higher in patients without hepatic metastases than in patients with hepatic metastases.
Table 2.
Therapeutic effect in patients without hepatic metastases and with hepatic metastases.
| Without hepatic metastases | With hepatic metastases | P | ||
|---|---|---|---|---|
| Total, n (%) | SD+PR+CR | 56 (51.4%) | 87 (39.5%) | 0.045 |
| PD | 53 (48.6%) | 133 (60.5%) | ||
| Sex, n (%) | ||||
| Male | SD+PR+CR | 28 (52.8%) | 25 (21.7%) | <0.001 |
| PD | 25 (47.2%) | 90 (78.3%) | ||
| Female | SD+PR+CR | 28 (50.0%) | 62 (59.0%) | 0.318 |
| PD | 28 (50.0%) | 43 (41.0%) | ||
| Age, n (%) | ||||
| <65 | SD+PR+CR | 35 (46.7%) | 63 (35.8%) | 0.121 |
| PD | 40 (53.3%) | 113 (64.2%) | ||
| ≥65 | SD+PR+CR | 21 (61.8%) | 24 (54.5%) | 0.645 |
| PD | 13 (38.%) | 20 (45.5%) | ||
| Smoking, n (%) | ||||
| Smoker | SD+PR+CR | 22 (51.4%) | 26 (39.5%) | 0.004 |
| PD | 23 (48.6%) | 84 (60.5%) | ||
| Non-smoker | SD+PR+CR | 34 (53.1%) | 61 (55.5%) | 0.875 |
| PD | 30 (46.9%) | 49 (44.5%) | ||
| Performance status (ECOG), n (%) | ||||
| 0–1 | SD+PR+CR | 53 (50.5%) | 65 (39.2%) | 0.079 |
| PD | 52 (49.5%) | 101 (60.8%) | ||
| 2 | SD+PR+CR | 3 (75.0%) | 22 (40.7%) | 0.305 |
| PD | 1 (25.0%) | 32 (59.3%) | ||
| T stage, n (%) | ||||
| 1–2 | SD+PR+CR | 20 (64.5%) | 20 (40.8%) | 0.066 |
| PD | 11 (35.5%) | 29 (59.2%) | ||
| 3–4 | SD+PR+CR | 36 (46.2%) | 67 (39.2%) | 0.333 |
| PD | 42 (53.8%) | 104 (60.8%) | ||
| N stage, n (%) | ||||
| 0–1 | SD+PR+CR | 10 (55.6%) | 17 (51.5%) | 1.000 |
| PD | 8 (44.4%) | 16 (48.5%) | ||
| 2–3 | SD+PR+CR | 46 (50.5%) | 70 (37.4%) | 0.039 |
| PD | 45 (49.5%) | 117 (62.6%) | ||
| Number of hepatic metastases, n (%) | ||||
| ≤3 | SD+PR+CR | 66 (46.8%) | 0.004 | |
| PD | 75 (53.2%) | |||
| >3 | SD+PR+CR | 21 (26.6%) | ||
| PD | 58 (73.4%) | |||
| EGFR mutation, n (%) | ||||
| Negative | SD+PR+CR | 17 (26.6%) | 52 (34.2%) | 0.338 |
| PD | 47 (73.4%) | 100 (65.8%) | ||
| Positive | SD+PR+CR | 39 (86.7%) | 35 (51.5%) | <0.001 |
| PD | 6 (13.3%) | 33 (48.5%) | ||
Survival analysis
Median PFS in EGFR mutation-positive patients was 4.4 months and it was 1.4 months in EGFR mutation-negative patients (95% CI 2.799–6.001 vs. 1.329–1.471; P<0.001).
In patients with hepatic metastases, median PFS was 2.3 months in the EGFR mutation-positive group and 1.4 months in the EGFR mutation-negative (95% CI 1.314–3.286 vs. 1.325–1.475; P=0.055) (Figure 1).
Figure 1.
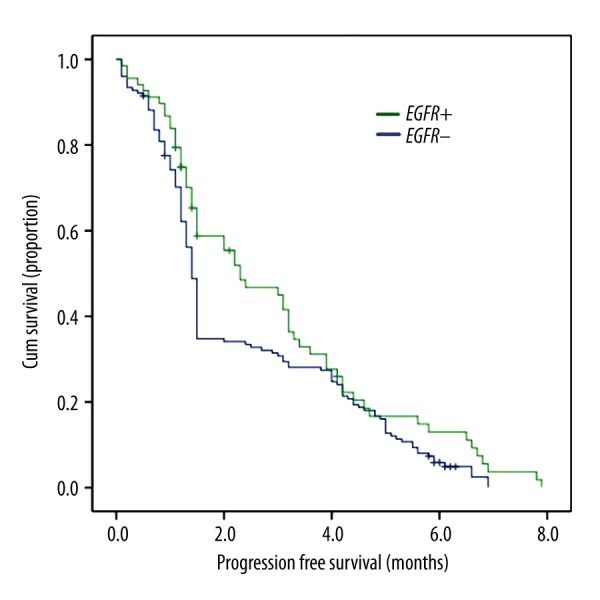
Association of EGFR Mutation and PFS in patients with hepatic metastases.
In EGFR mutation-positive patients, median progression-free survival (PFS) was significantly longer in patients without hepatic metastases than in those with hepatic metastases (9.1 [95% CI 8.023–10.177] vs. 2.3 [1.314–3.286] months; P<0.001) (Figure 2).
Figure 2.
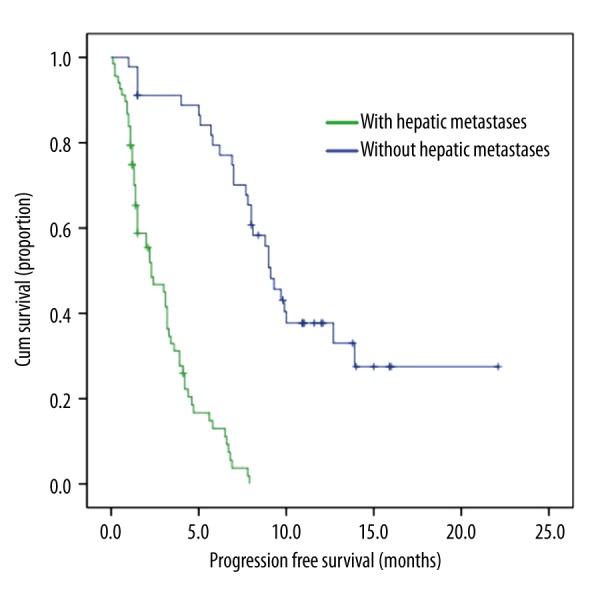
Association of hepatic metastases and PFS in patients with EGFR mutation.
Survival analysis in the whole population was performed (Table 3). The progression-free survival benefit seemed to be consistent across all clinical subgroups irrespective of sex, age, performance status, smoking status, T stage, N stage, number of hepatic metastases, or hepatic metastases status, suggesting that smoking status, EGFR mutations, and hepatic metastases are the most important factor in the PFS benefit in the whole population survival analysis.
Table 3.
Survival analysis in the whole population.
| HR | P | 95%CI | |
|---|---|---|---|
| Sex (Male vs. Female) | 1.087 | 0.703 | 0.709–1.665 |
| Age (<65 vs. ≥65) | 0.799 | 0.112 | 0.602–1.061 |
| ECOG PS (0–1 vs. 2–3) | 0.802 | 0.182 | 0.581–1.109 |
| Smoking status (Yes vs. No) | 0.605 | 0.029 | 0.385–0.949 |
| T stage (1–2 vs. 3–4) | 1.331 | 0.065 | 0.983–1.802 |
| N stage (0–1 vs. 2–3) | 0.807 | 0.220 | 0.572–1.137 |
| Number of hepatic metastases (≤3 vs. >3) | 0.860 | 0.359 | 0.622–1.188 |
| EGFR mutation (No vs. Yes) | 0.420 | <0.001 | 0.311–0.566 |
| Hepatic metastases (No vs. Yes) | 1.830 | <0.001 | 1.331–2.515 |
Treatment-related adverse effects
The most frequent drug-related adverse effects were mild-to-moderate skin toxicity (56.1%) and diarrhea (55.3%) (Table 4). Liver toxicity was observed in more than 20% of patients without hepatic metastases. Dose reduction to 100 mg/d was necessary in 21 patients with hepatic metastases, due to increased alanine transaminase (ALT).
Table 4.
Treatment-related adverse effects.
| Toxicity, n (%) | Without hepatic metastases | With hepatic metastases | Total | |||
|---|---|---|---|---|---|---|
| Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | |
| Neutropenia | 3 (2.8%) | 0 (0.0%) | 17 (7.7%) | 0 (0.0%) | 20 (6.1%) | 0 (0.0%) |
| Thrombocytopenia | 2 (1.8%) | 0 (0.0%) | 15 (6.8%) | 0 (0.0%) | 17 (5.2%) | 0 (0.0%) |
| Anemia | 6 (5.5%) | 0 (0.0%) | 12 (5.5%) | 5 (2.3%) | 18 (5.5%) | 5 (1.5%) |
| Infection | 4 (3.7%) | 0 (0.0%) | 9 (4.1%) | 0 (0.0%) | 13 (4.0%) | 0 (0.0%) |
| Skin rash | 62 (56.9%) | 2 (1.8%) | 120 (54.5%) | 4 (1.8%) | 182 (56.1%) | 6 (1.8%) |
| Diarrhea | 32 (28.4%) | 0 (0.0%) | 62 (28.2%) | 0 (0.0%) | 94 (55.3%) | 0 (0.0%) |
| Stomatitis | 21 (29.4%) | 0 (0.0%) | 33 (15.0%) | 0 (0.0%) | 54 (17.7%) | 0 (0.0%) |
| Paronychia | 17 (15.6%) | 0 (0.0%) | 35 (15.0%) | 0 (0.0%) | 52 (16.4%) | 0 (0.0%) |
| Vomiting or nausea | 9 (8.3%) | 0 (0.0%) | 23 (10.5%) | 0 (0.0%) | 32 (9.7%) | 0 (0.0%) |
| Increased ALT | 25 (22.9%) | 0 (0.0%) | 77 (35.0%) | 21 (9.5%) | 102 (31.0%) | 21 (6.4%) |
| Fatigue | 15 (13.8%) | 0 (0.0%) | 37 (16.8%) | 2 (1.0%) | 52 (15.8%) | 2 (0.06%) |
Discussion
The aim of our study was to investigate efficacy of erlotinib as 2nd/3rd line treatment in Chinese lung adenocarcinoma patients with hepatic metastases. We found that hepatic metastasis is a poor predictive marker for erlotinib in 2nd/3rd line treatment in patients with lung adenocarcinoma.
In advanced NSCLC, docetaxel or pemetrexed as the 2nd line chemotherapy can prolong survival after 1st line chemotherapy for NSCLC [6,7]. A study showed that in patients treated with docetaxel as 3rd line chemotherapy, survival was identical to that of patients treated with supportive care, but time to progression was longer for docetaxel patients than for best supportive care patients [6]. Erlotinib and gefitinib have been widely studied [23–37]. In phase II clinical trials of gefitinib [35,36], 10–20% of patients who received gefitinib after the 1st line therapy responded to gefitinib, and in a phase II clinical trial of erlotinib in previously treated NSCLC patients, the response rate was 12.3% [37]. These response rates are higher than with chemotherapy [6,7]. Clinical trials also demonstrated that erlotinib can prolong survival in previously treated NSCLC patients [11].
In our study, hepatic metastases were more common in the patients younger than 65 years old, and the PS of these patients were significantly higher. The DCR was 60.5% in patients without hepatic metastases, which is similar to results of a previous study [11]. However, the DCR and PFS in patients with hepatic metastases were lower than in patients without hepatic metastases. In males and smokers, N2–3, number of hepatic metastases >3, number of EGFR-positive patients, and DCR rate were significant higher in patients without hepatic metastases than in patients with hepatic metastases. In patients with hepatic metastases, PFS was not significantly different between the EGFR mutation-positive group and the EGFR mutation-negative group.
Lung cancer with EGFR-activating mutations responds favorably to the EGFR tyrosine kinase inhibitors gefitinib and erlotinib. However, many patients with EGFR-activating mutations show intrinsic resistance. In our research, DCR and PFS were lower in patients with hepatic metastases, perhaps because HGF, a ligand of MET oncoprotein, induces EGFR TKI resistance of lung adenocarcinoma cells with EGFR-activating mutations by restoring the phosphatidylinositol 3-kinase/Akt signaling pathway via phosphorylation of MET, but not EGFR or ErbB3 [14]. MET amplification activates ERBB3/PI3K/AKT signaling in EGFR mutant lung cancers, and causes resistance to EGFR kinase inhibitors. MET activation by its ligand, HGF, also induces drug resistance, but through GAB1 signaling [15]. We should also consider the possibility that the actual status of EGFR genes in hepatic metastases could be different from the status of the tumor sample analyzed. The samples used for EGFR gene analysis in this study were derived from lung tumors before initiation of chemotherapy, not from liver tumors, and all the patients had received 1 or 2 prior chemotherapy regimens. Previous research suggests that the EGFR mutation status changes after chemotherapy [39]. All the above-mentioned factors may influence the efficacy of erlotinib and may cause poor curative effect of erlotinib in the liver.
Erlotinib was well tolerated in our study. The most common toxicities are rash and diarrhea in patents without hepatic metastases. Patient response was primarily correlated with the grade of rash, consistent with results of several other trials [11]. Patients who had hepatic metastases had much higher ALT levels after taking EGFR-TKIs. Hepatic function should receive more attention in patients with hepatic metastases.
Our study has some limitations which should be taken into consideration. Firstly, this was a retrospective study. Secondly, the patients in our study came from a single center.
Conclusions
We found that hepatic metastasis is a poor predictive marker for erlotinib as 2nd/3rd line treatment in patients with lung adenocarcinoma. Combination therapy, for example with MET-TKI, may be a good choice for patients with liver metastases with poor prognosis. Further research is needed to explore the overall survival between patients with hepatic metastases and patients without hepatic metastases.
Footnotes
Conflict of interest statement
There are no conflicts of interest.
Source of support: This study was supported in part by a grant from the Young Scientist Program of Shanghai National Health and Family Planning Commission and a grant from the Chronic Disease Comprehensive Prevention and Control Project of the Shanghai Shenkang Hospital Development Center (No. SHDC12012313)
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Murray T, Ward E, et al. Cancer statistics 2005. Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22:330–53. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 5.D’Addario G, Pintilie M, Leighl NB, et al. Platinum-based versus non-platinumbased chemotherapy in advanced non-small-cell lung cancer: A meta-analysis of the published literature. J Clin Oncol. 2005;23:2926–36. doi: 10.1200/JCO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd FA, Dancey J, Ramlau R, et al. A prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 7.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [Erratum: J Clin Oncol, 2004;22:209. [DOI] [PubMed] [Google Scholar]
- 8.Giaccone G, Gallegos Ruiz M, Le Chevalier T, et al. Erlotinib for frontline treatment of advanced non-small cell lung cancer: a phase II study. Clin Cancer Res. 2006;12:6049–55. doi: 10.1158/1078-0432.CCR-06-0260. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, openlabel, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 10.Lee JK, Kim TM, Koh Y, et al. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer. 2012;77:460–63. doi: 10.1016/j.lungcan.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 12.Stenbygaard LE, Sørensen JB, Larsen H, Dombernowsky P. Metastatic pattern in non-resectable non-small cell lung cancer. Acta Oncol. 1999;38:993–98. doi: 10.1080/028418699432248. [DOI] [PubMed] [Google Scholar]
- 13.Tas F, Aydiner A, Topuz E, et al. Factors influencing the distribution of metastases and survival in extensive disease small cell lung cancer. Acta Oncol. 1999;38:1011–15. doi: 10.1080/028418699432275. [DOI] [PubMed] [Google Scholar]
- 14.Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–87. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 15.Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martelli O, Coppola L, De Quarto AL, et al. Fulminant hepatic failure caused by diffuse intrasinusoidal metastatic liver disease: a case report. Tumori. 2000;86:424–27. doi: 10.1177/030089160008600512. [DOI] [PubMed] [Google Scholar]
- 17.Ihara N, Yashiro N, Kinoshita T, et al. Diffuse intrasinusoidal liver metastasis of small cell lung cancer causing fulminant hepatic failure: CT findings – a case report. Radiat Med. 2001;19:275–77. [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Lilenbaum RC, Herndon JE, II, List MA, et al. Singleagent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730) J Clin Oncol. 2005;23:190–96. doi: 10.1200/JCO.2005.07.172. [DOI] [PubMed] [Google Scholar]
- 20.Georgoulias V, Ardavanis A, Agelidou A, et al. Docetaxel versus docetaxel plus cisplatin as front-line treatment of patients with advanced non-small-cell lung cancer: A randomized, multicenter phase III trial. J Clin Oncol. 2004;22:2602–9. doi: 10.1200/JCO.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Ren S, Kuang P, Zheng L, et al. Analysis of driver mutations in female non-smoker Asian patients with pulmonary adenocarcinoma. Cell Biochem Biophys. 2012;64:155–60. doi: 10.1007/s12013-012-9384-8. [DOI] [PubMed] [Google Scholar]
- 22.He Y, Li S, Ren S, et al. Impact of family history of cancer on the incidence of mutation in epidermal growth factor receptor gene in non-small cell lung cancer patients. Lung Cancer. 2013;81:162–66. doi: 10.1016/j.lungcan.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone DP, Ding K, Roder H, et al. Prognostic and predictive role of the VeriStrat plasma test in patients with advanced non-small-cell lung cancer treated with erlotinib or Placebo in the NCIC Clinical Trials Group BR.21 Trial. J Thorac Oncol. 2012;7:1653–60. doi: 10.1097/JTO.0b013e31826c1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, Ren S, Yan L, et al. Clinical efficacy of erlotinib in patients previously treated for advanced non-small cell lung cancer. Respirology. 2009;14:709–15. doi: 10.1111/j.1440-1843.2009.01564.x. [DOI] [PubMed] [Google Scholar]
- 26.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 27.Bronte G, Rizzo S, La Paglia L, et al. Driver mutations and differential sensitivity to targeted therapies: a new approach to the treatment of lung adenocarcinoma. Cancer Treat Rev. 2010;36(Suppl 3):S21–29. doi: 10.1016/S0305-7372(10)70016-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhu CQ, da Cunha Santos G, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR 21. J Clin Oncol. 2008;26:4268–75. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 29.Sone T, Kasahara K, Kimura H, et al. Epidermal growth factor receptor mutations and gene amplification in nonsmall cell lung cancer: molecular analysis of IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005;23:8081–92. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- 30.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 31.Mak RH, Doran E, Muzikansky A, et al. Outcomes after combined modality therapy for EGFR-mutant and wild-type locally advanced NSCLC. Oncologist. 2011;16:886–95. doi: 10.1634/theoncologist.2011-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto K, Ichinose Y, Ohe Y, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol. 2012;7:115–21. doi: 10.1097/JTO.0b013e3182307f98. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:3238–47. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 34.Sridhar SS, Seymour L, Shepherd FA. Inhibitors of epidermal-growth-factor receptors: A review of clinical research with a focus on non-small-cell lung cancer. Lancet Oncol. 2003;4:397–406. doi: 10.1016/s1470-2045(03)01137-9. [DOI] [PubMed] [Google Scholar]
- 35.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL Trial) J Clin Oncol. 2003;21:2237–46. doi: 10.1200/JCO.2003.10.038. [Erratum: J Clin Oncol, 2004; 22: 4811] [DOI] [PubMed] [Google Scholar]
- 36.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA. 2003;290:2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:3238–47. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 38.Massarelli E, Andre F, Liu DD, et al. A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer. 2003;39:55–61. doi: 10.1016/s0169-5002(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 39.Bai H, Wang Z, Chen K, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:3077–83. doi: 10.1200/JCO.2011.39.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]


