Abstract
Background
The aim of this study was to investigate the efficacy of hyperbaric oxygen in secondary brain injury after trauma and its mechanism in a rat model.
Material/Methods
A rat model of TBI was constructed using the modified Feeney’s free-fall method, and 60 SD rats were randomly divided into three groups – the sham group, the untreated traumatic brain injury (TBI) group, and the hyperbaric oxygen-treated TBI group. The neurological function of the rats was evaluated 12 and 24 hours after TBI modeling; the expression levels of TLR4, IκB, p65, and cleaved caspase-3 in the peri-trauma cortex were determined by Western blot; levels of TNF-α, IL-6, and IL-1β were determined by ELISA; and apoptosis of the neurons was evaluated by TUNEL assay 24 hours after TBI modeling.
Results
Hyperbaric oxygen therapy significantly inhibited the activation of the TLR4/NF-κB signaling pathway, reduced the expression of cleaved caspase-3, TNF-α, IL-6 and IL-1β (P<0.05), reduced apoptosis of the neurons and improved the neurological function of the rats (P<0.05).
Conclusions
Hyperbaric oxygen therapy protects the neurons after traumatic injury, possibly through inhibition of the TLR4/NF-κB signaling pathway.
MeSH Keywords: Brain Injuries, Hyperbaric Oxygenation, Toll-Like Receptor 4
Background
With the rapid development of the economy and society, the incidence of TBI in China is rising year by year, and its mortality and morbidity rates remain high, making it a great burden for the patients’ families both mentally and economically [1]. It has been revealed that TBI can be divided into two phases: the initial injury caused by violence, which is inevitable, and the secondary injury within hours or days after initial injury, caused by the inflammatory response, oxidative stress, calcium overload, and a series of other pathological processes, which is the major target of current interventions [2]. Previous studies have demonstrated the effectiveness of hyperbaric oxygen therapy in treatments for secondary brain damage after trauma [3], but the mechanism has not been fully clarified. In this study, we investigated the efficacy of hyperbaric oxygen for secondary brain injury after trauma, with a focus on the TLR4/NF-κB signaling pathway, in an effort to clarify the mechanism of its protective effect and to provide guidance for the safer and more efficient clinical use of hyperbaric oxygen.
Material and Methods
Grouping of experimental animals
Sixty healthy adult male SD rats were randomly divided into 3 groups: the sham group, the untreated TBI group, and the hyperbaric oxygen-treated TBI group. A rat model of TBI was constructed using the modified Feeney’s free-fall method. The rats were anesthetized using chloral hydrate at 4 mg/kg and fixed on a bracket. After skin preparation, a 5 mm opening was made with an orthopedic drill at 3 mm to the right of the coronal suture and 3 mm behind the sagittal suture, keeping the dura intact. Then, a 40 g object was dropped from 15 cm high and vertically crashed into the exposed dura to make a 3 mm deep and 4 mm diameter hole. The sham group was only drilled but not injured by the falling object. All of the experimental program and operation procedures in this study were approved by the experimental animal ethics committee. All the rats were free to eat and to drink water.
Hyperbaric oxygen therapy
Hyperbaric oxygen therapy was performed 2 h after TBI, as previously reported [4]. The rats were placed into the animal chamber, which was purged with pure oxygen for 10 min to ensure that the oxygen fraction in the chamber was >95%. The pressure was then steadily increased to 0.12 MPa and maintained for 60 min. Next, the pressure was steadily decreased to normal pressure over 20 min. Hyperbaric oxygen therapy was performed twice with a 10 h interval. The rats’ behavior in the high-pressure chamber was closely monitored. The sham control group and untreated TBI group were also placed in the same chambers and were subjected to the same experimental procedures, only without the hyperbaric oxygen treatment.
Western blot analyses
At 24 h after injury, the rats were anesthetized, and 100 ml of normal saline was infused through the cardiac apex. The tissue around the trauma was resected and stored at −80°C for later use. Nuclear and cytoplasmic protein was extracted using a kit as instructed by the manufacturer. Protein concentration was determined by Bradford assay, and 1/4 volume of 5× loading buffer was added, followed by a boiling water bath for 20 min for denaturation. A sample consisting of 35 μg of protein was loaded, separated by electrophoresis, transferred to membrane, blocked, and incubated with antibodies to TLR4, IκB, P65, cleaved caspase-3, GAPDH, or H3 (1:200, purchased from Santa Cruz, USA) at 4°C on a shaker overnight, then washed, incubated with the corresponding HRP-conjugated secondary antibody, and washed again. Finally, ECL solution was added to reveal the bands, whose gray value was analyzed by Image J software.
ELISA to determine TNF-α, IL-6 and IL-1β concentration
ELISA assay kits for TNF-α, IL-6 and IL-1β were all purchased from Unitech Biotechnology, and experiments were conducted according to the manufacturer’s instructions.
TUNEL assay to determine cell apoptosis
Paraffin-embedded brain tissue was cut into 4 μm sections and analyzed by the TUNEL assay to determine apoptosis of the neurons. The TUNEL assay kit was purchased from Roche, USA, and the experiment was conducted strictly according to the manufacturer’s instructions. The TUNEL assay result for the tissue
around the trauma was observed under an optical microscope, and 10 random visual sections under 400× magnification were evaluated for the percentage of TUNEL-positive cells.
Neurological function evaluation of the rats after TBI
The neurological function of the rats was evaluated by the Neurological Severity Scores (NSS) [5], including motor function, sensory function, balance ability, physiological reflex defect, and abnormal movement. For each of the 18 projects, the inability to complete a task or lack of corresponding response was scored 1 point. Cases with scores of 13–18 points in total are regarded as severe injury, 7–12 points as moderate injury, and 1–6 points as mild injury.
Statistical analysis
The SPSS 15.0 software was used for statistical analysis, and the data are presented as means ± standard deviation (x±S). The neurological function scores were compared by the Kruskal-Wallis test, and comparisons among multiple groups were performed using one-way analysis of variation.
Results
The effect of hyperbaric oxygen on the expression of TLR4, IκB, P65, cleaved caspase-3
Compared to the sham group (Figures 1, 2; Table 1), the TLR4, p65, and cleaved caspase-3 levels in peri-trauma tissue in the untreated TBI group were significantly elevated (P<0.05), while hyperbaric oxygen significantly inhibited the expression of TLR4, p65, and cleaved caspase-3 (P<0.05); in addition, hyperbaric oxygen significantly inhibited the degradation of IκB after TBI (P<0.05).
Figure 1.

Levels of TLR4, IκB, and cleaved caspase-3. (Representative Figure: 1, sham group; 2, untreated TBI group; 3, hyperbaric oxygen-treated TBI group)
Figure 2.
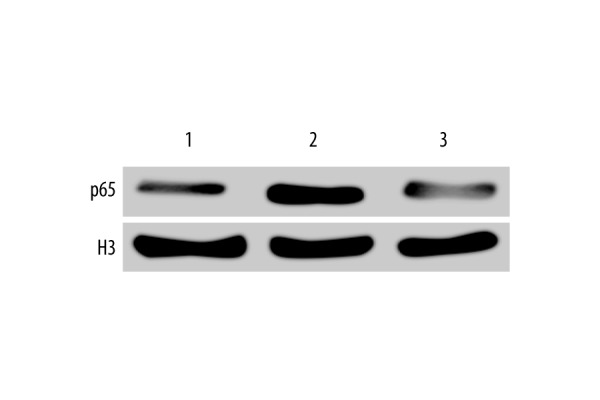
P65 level. (Representative Figure: 1, sham group; 2, untreated TBI group; 3, hyperbaric oxygen-treated TBI group).
Table 1.
Levels of TLR4, IκB, and cleaved caspase-3.
| Groups | Sample size | TLR4 | IκB | Cleavage caspase-3 | P65 |
|---|---|---|---|---|---|
| Sham | 10 | 0.02±0.01 | 0.89±0.37 | 0.09±0.01 | 0.02±0.01 |
| Untreated TBI | 10 | 1.02±0.45* | 0.19±0.11* | 0.63±0.25* | 0.84±0.45* |
| Hyperbaric oxygen-treated TBI | 10 | 0.21±0.15# | 0.69±0.31# | 0.12±0.08# | 0.22±0.12# |
P<0.05 compared with the sham group;
P<0.05 compared with the untreated TBI group.
The effect of hyperbaric oxygen on the expression of TNF-α, IL-6 and IL-1β
Compared to the sham group, the levels of TNF-α, IL-6 and IL-1β in the untreated TBI group were significantly increased (Table 2, P<0.05), while hyperbaric oxygen significantly inhibited expression of TNF-α, IL-6 and IL-1β (Table 2, P<0.05).
Table 2.
Levels of TNF-α, IL-6 and IL-1β, and apoptosis index of the three groups.
| Groups | Sample size | TNF-α (pg/mg) | IL-1β (pg/mg) | IL-6 (ng/mg) | Apoptotic index (%) |
|---|---|---|---|---|---|
| Sham | 10 | 40.2±6.24 | 19.7±2.01 | 3.9±0.02 | 1.2±0.1 |
| Untreated TBI | 10 | 100.5±8.79* | 34.2±4.23* | 10.6±1.63* | 29±6.3* |
| Hyperbaric oxygen-treated TBI | 10 | 72.6±7.23# | 26.9±3.01# | 6.9±1.15# | 15±4.2# |
P<0.05 compared with the sham group;
P<0.05 compared with the untreated TBI group.
The effect of hyperbaric oxygen on apoptosis of rat neurons after TBI
Apoptotic neurons with condensed nuclei were stained brown in the TUNEL assay (Figure 3), while normal cells were large, round, and not stained. In the sham group, few apoptotic neurons were observed; in the untreated TBI group, significantly more apoptotic neurons were observed (Figure 3, Table 2, P<0.05); and after hyperbaric therapy, the number of peri-trauma apoptotic neurons was significantly reduced (P<0.05).
Figure 3.
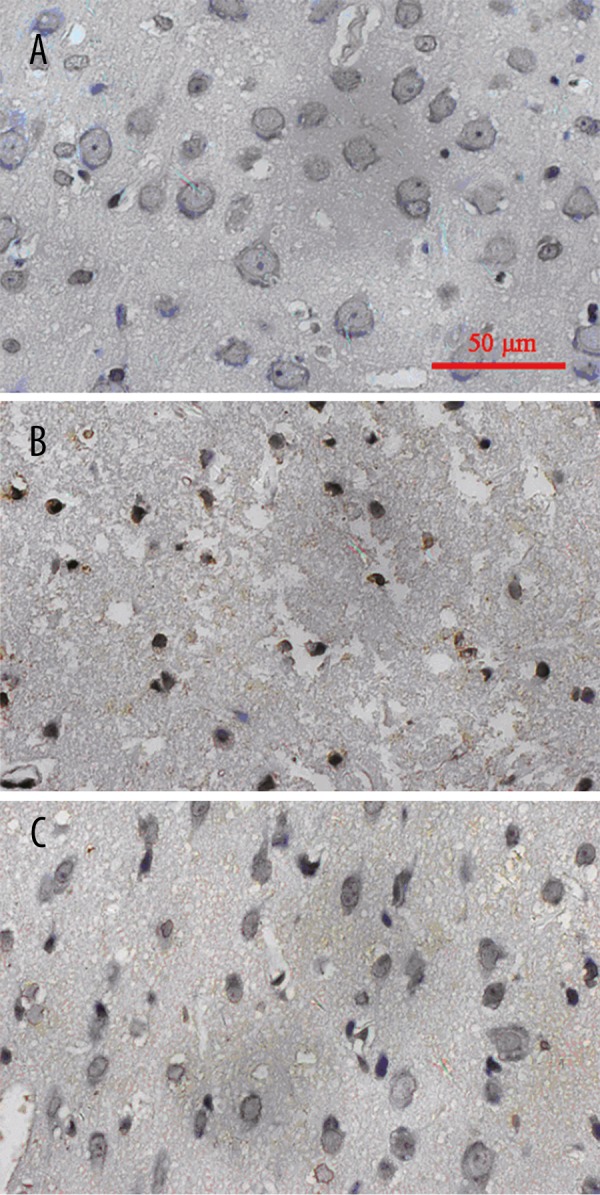
TUNEL assay results of the three groups. (Representative figure: (A) Sham group; (B) Untreated TBI group; (C) Hyperbaric oxygen-treated TBI group).
The effect of hyperbaric oxygen on neurological function of rats with TBI
Compared with the sham group, the neurological function of rats of the TBI group decreased significantly (Table 3, P<0.05), whereas hyperbaric oxygen significantly improved the neurological function of the rats after TBI (Table 3, P<0.05).
Table 3.
Neurological function score of the three groups of rats 12 and 24 hours after TBI.
| Groups | Sample size | 12 h | 24 h |
|---|---|---|---|
| Sham | 20 | 1.1±0.02 | 1.3±0.01 |
| Untreated TBI | 20 | 14.65±3.26 | 15.62±2.34* |
| Hyperbaric oxygen-treated TBI | 20 | 14.01±2.25 | 13.41±1.64# |
P<0.05 compared with the sham group;
P<0.05 compared with the untreated TBI group.
Discussion
Numerous studies have shown that hyperbaric oxygen can increase oxygen concentration, increase oxygen diffusion distance, and induce a variety of mechanisms to correct the acidosis neuroprotective effect. This study showed that hyperbaric oxygen can significantly reduce the rate of neuronal apoptosis after traumatic brain injury, significantly improving neurological function in rats, which is consistent with previous studies. Recent studies have also found that hyperbaric oxygen could suppress the inflammatory response after traumatic brain injury to exert neuroprotective effects. This study focused on the inflammatory response mediated by the TLR4/NF-κB signaling pathway. TLR4 is an important member of the TLR family, a group of Type I transmembrane molecules, consisting of an extracellular segment, a transmembrane segment and an intracellular TIR segment. It plays an important role in hypoxic-ischemic brain injury, cerebral hemorrhage, spinal cord injury, and other acute injury of the central nervous system [6–8]. High expression of TLR4 has been observed in the tissue around brain trauma in both animal models and clinical studies [9], while TLR4 knockout mice showed significantly alleviated secondary brain injury after brain trauma [10,11], and a specific TLR4 inhibitor also significantly alleviated brain damage, suggesting that therapies targeting TLR4 have great potential in improving the prognosis of TBI. In this study, TLR4 protein expression significantly increased after TBI, and hyperbaric oxygen therapy significantly inhibited TLR4 expression, reduced apoptosis of neurons after TBI and improved the neurological function of the rats, suggesting that down-regulation of TLR4 is an important part of the neuron-protective effect of hyperbaric oxygen therapy.
When bound by its receptor during TBI, TLR4 interacts with downstream myeloid differentiation factor 88 and activates IKK, which phosphorylates IκB and leads to the degradation of IκB. Then, NF-κB p65 protein is released from IκB and translocates from the cytoplasm to the nucleus to interact with the promoters of its target genes, regulating the expression of a series of inflammatory cytokines. NF-κB is the master regulator of a series of inflammatory cytokines and is significantly elevated in the tissue surrounding brain trauma, and specific NF-κB inhibitors significantly reduced the expression of inflammatory cytokines after TBI and relieved secondary brain injury [12]. In addition, recent studies have shown that hyperbaric oxygen therapy significantly inhibited microglia activation and inflammatory cytokine production in the central nervous system [13]. In this study, we found that p65 expression was significantly increased in peri-trauma tissue after TBI, which is consistent with previous studies, and hyperbaric oxygen therapy significantly inhibited p65 expression in the nucleus. Taken together, it is speculated that the inhibition of NF-κB signaling may be the basic mechanism in the neuroprotective effect of hyperbaric oxygen therapy.
Conclusions
Hyperbaric oxygen therapy significantly reduces apoptosis in peri-trauma tissue after TBI, inhibits the expression of inflammatory cytokines, and significantly improves the neurological function of rats after TBI. These effects are closely related to the inhibition of the TLR4/NF-κB signaling pathway.
Footnotes
Source of support: Departmental sources
References
- 1.Zhu BF, Chen JR, Wang F. Epidemiology analysis of 2079 traffic inju ry patients. Clinical Emergency. 2010;11:204–6. [Google Scholar]
- 2.Abdul-Muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2015;51(3):966–79. doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boussi-Gross R, Golan H, Fishlev G, et al. Hyperbaric o xygen therapy can improve post concussion syndrome years after mild traumatic brain in jury – randomized prospective trial. PLoS One. 2013;8:79995. doi: 10.1371/journal.pone.0079995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang GH, Zhang XG, Jiang ZL, et al. Neuroprotective effects of hyperbaric o xygen treatment on traumatic brain in jury in the rat. J Neurotrauma. 2010;27:1733–43. doi: 10.1089/neu.2009.1175. [DOI] [PubMed] [Google Scholar]
- 5.Si D, Li J, Liu J, et al. Progesterone protects blood-brain barrier function and improves neurological outcome following traumatic brain in jury in rats. Exp Ther Med. 2014;8:1010–14. doi: 10.3892/etm.2014.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Ge P, Zhu Y. TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediators Inflamm. 2013;2013:124614. doi: 10.1155/2013/124614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang H, Wang PF, Zhou Y, et al. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and in jury. J Neuroinflammation. 2013;10:27. doi: 10.1186/1742-2094-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao L, Kan EM, Lu J, et al. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain fo llowing hypoxia: ro le of TLR4 in hypo xic microglia. J Neuroinflammation. 2013;10:23. doi: 10.1186/1742-2094-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, Li H, Li T, et al. TLR4 inhibitor resatorvid provides neuroprotection in e xpe rimental traumatic brain injury: implication in the treatment of human brain injury. Neurochem Int. 2014;75:11–18. doi: 10.1016/j.neuint.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad A, Crupi R, Campolo M, et al. Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice. PLoS One. 2013;8:e57208. doi: 10.1371/journal.pone.0057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Yin H, Zhao M, Lu Q. TLR2 and TLR4 in autoimmune diseas es: a comprehensive review. Clin Rev Allergy Immunol. 2014;47:136–47. doi: 10.1007/s12016-013-8402-y. [DOI] [PubMed] [Google Scholar]
- 12.Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–54. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim SW, Wang CC, Wang YH, et al. Microglial activation induced by traumatic brain injury is suppressed by postinjury treatment with hyperbaric o xygen therapy. J Surg Res. 2013;184:1076–84. doi: 10.1016/j.jss.2013.04.070. [DOI] [PubMed] [Google Scholar]


