Abstract
ALADIN is a component of the nuclear pore complex in higher eukaryotes. An Arabidopsis knockout line that had a T-DNA insertion in the ALADIN gene was defective in plant growth and thylakoid development and had reduced photosynthetic activity resulting from lower chlorophyll accumulation. The mutation appeared to decrease the level of chloroplast RuBisCO subunits and PSBA and PGL35 proteins. Unexpectedly, the T-DNA insertion in the ALADIN gene decreased the expression of the neighboring gene PSRP5, which functions in translation in chloroplasts. The mutant phenotype was rescued by expressing PSRP5, but not by expressing ALADIN. The abnormal phenotypes were also detected in an artificial microRNA (amiRNA)-mediated PSRPS5 knockdown, but not in an amiRNA-mediated ALADIN knockdown line. Thus, users of T-DNA insertions should be aware that a T-DNA insertion in one gene can have effects on the expression of neighboring genes.
Introduction
Since the completion of the Arabidopsis genome sequence, a key aim of the plant research community has been to identify the function of each gene. One widely-used reverse genetic technique is insertional mutagenesis mediated by Agrobacterium transformation and T-DNA insertion [1]. T-DNA flanked by specific 25-bp direct repeats is transferred from Agrobacterium into plants [2]. A piece of T-DNA (more than 5-kbp fragment) is inserted randomly into genome, resulting in causing significant effects on the gene function. Depending on the insertion site and the nature of T-DNA, it leads several effects, such as knockout, knockdown, and knockon. Over the past decade, phenotypes associated with T-DNA insertions have played a critical role in advancing plant research. Large collections of Arabidopsis T-DNA insertion lines have been, and continue to be, developed around the world [3–7]. Approximately 88% of all Arabidopsis genes are thought to have been disrupted at least once [1]. Recently, by using a next-generation sequencing method, flanking sequences of 146,740 insertions were also identified (http://www.arabidopsis.org). T-DNA mutagenesis has been used in functional genomic analysis of species other than Arabidopsis, such as rice [8] and Brachypodium [9].
Despite its utility, T-DNA mutagenesis has several limitations. One disadvantage is that T-DNA integrations are often complex and can lead to the deletion or chromosomal rearrangement of the surrounding genomic DNA [10, 11]. This significantly complicates subsequent molecular analysis in the mutant. Here, we describe a T-DNA-associated mutation that unexpectedly affected the expression level of a neighboring gene in Arabidopsis, identified during the course of reverse genetic analysis of a nucleoporin.
Materials and Methods
Plant materials
Arabidopsis thaliana (ecotype Wassilewskija) was used as wild type. A T-DNA insertion mutant (FLAG_453B04) in the Ws background was obtained from the Versailles Arabidopsis Stock Centre at the Jean-Pierre Bourgin Institute of the National Institute for Agricultural Research (INRA).
Transgenic plants
Genomic fragments containing either the ALADIN (At3g56900) gene or the ALADIN + PSRP5 (At3g56910) genes were generated using specific primers (S1 Table) and cloned into pENTR1A (Invitrogen, USA). The ALADIN genomic fragment contains a region from 2-kbp upstream to 0.3-kbp downstream of the ALADIN coding sequence. The ALADIN + PSRP5 genomic fragment contains a region form 0.13-kbp upstream of the PSRP5 coding sequence to 2-kbp upstream of the ALADIN coding sequence. Cloned DNA fragments were transferred from the entry clone to the pFASTG01 Gateway destination vector [12] by an in-vitro recombination attL × attR reaction. ALADIN and PSRP5-directed artificial microRNA (amiRNA) constructs were designed using a Web-based program (http://wmd2.weigelworld.org) [13, 14]. Corresponding fragments were generated using specific primers (S1 Table) and were cloned into pENTR1A (Invitrogen). Cloned DNA fragments were then transferred from the entry clone to the pFASTG02 Gateway destination vector [12] by an in-vitro recombination attL × attR reaction.
RT-PCR
Total RNA was isolated from 14-day-old plants using an RNeasy Plant Mini Kit (Qiagen, USA). Reverse transcription was performed using Ready-To-Go RT-PCR Beads (GE Healthcare, USA) with an oligo (dT)12-18 primer. Gene-specific primers are given in S1 Table. PCR products were visualized using agarose gel electrophoresis with EtBr.
Measurement of chlorophyll content and the maximum quantum efficiency of photosystem II
Chlorophyll content was determined in mature first and second leaves of 14-day-old plants as described previously [15]. The maximum quantum efficiency of photosystem II was quantified using a Mini-PAM chlorophyll fluorometer (Walz) as described previously [16]. The difference between maximum chlorophyll fluorescence (Fm) and minimum chlorophyll fluorescence at the open photosystem II center is defined as variable fluorescence (Fv). The maximum quantum efficiency of photosystem II is indicated by Fv/Fm.
Transmission electron microscopy
Mature leaves of 14-day-old plants were used for transmission electron microscopy. Samples were prepared as described previously [17]. Images were obtained using a transmission electron microscope (JEM-1200 EX; JEOL, Japan) at an acceleration voltage of 80 kV.
SDS-PAGE and immunoblot analysis
Protein extracts from plants were subjected to SDS-PAGE followed by either Coomassie Brilliant Blue (CBB) staining or immunoblot analysis. Immunoreactive signals were detected using an ECL detection system (GE Healthcare) with the following antibodies: anti-RBCL (1:1000 dilution), anti-RBCS (1:1000), anti-PSBA (1:5000), and anti-PGL35 (1:1000) (Agrisera, Sweden).
Statistics
Mean, standard deviation (S.D.), and two-tailed Student t-test calculations were performed using Microsoft Excel with StatPlus software.
Results and Discussion
An aladin knockout mutant is defective in photosynthesis
ALADIN (ALacrima Achalasia aDrenal Insufficiency Neurologic disorder) is an evolutionally conserved nucleoporin that is a member of the nuclear pore complex (NPC) in higher animals and plants [18, 19]. In humans, mutations in the ALADIN gene lead to a rare autosomal recessive disorder called the triple A syndrome [20, 21]. However, mice lacking a functional ALADIN gene do not exhibit this phenotype and are indistinguishable phenotype from wild-type mice [22, 23], suggesting a species-specific function for ALADIN. In this study, a single line knockout mutant from the FLAG T-DNA Versailles INRA collection was used to examine the physiological role of ALADIN in Arabidopsis (Fig 1A). Another ALADIN T-DNA knockout mutant (SALK_148848 line) was available, but any plants had no T-DNA insertion in ALADIN gene. Genomic sequencing of the T-DNA flanking region confirmed that the aladin-1 mutant (FLAG_453B04) had a T-DNA insertion in the middle of the ALADIN gene (S1 Fig). RT-PCR revealed that the mutant accumulated no full-length ALADIN transcript (Fig 1B) but a truncated transcript (S2 Fig).
Fig 1. Isolation of the aladin-1 mutant.

(A) Schematic representation of the ALADIN gene. A T-DNA insertion site in the FLAG_453B04 line is shown. The orientation of the left border sequence is indicated by an arrow. (B) RT-PCR analysis of ALADIN transcription in the aladin-1 mutant. ACTIN2 (ACT2) was used as a loading control. (C-D), Three-week- (C) and five-week- (D) old wild-type (WT) and aladin-1 plants.
The aladin-1 mutant exhibited pleiotropic growth defects at various developmental stages. Compared to the wild type, the mutant showed extremely stunted growth (Fig 1C), including dwarfism (Fig 1D), and a shorter root length in seedlings (Fig 2A). The shorter root phenotype was partially rescued by exogenous sucrose (Fig 2A and 2B), suggesting an impairment of carbon metabolism in the mutant. Mutant leaves were pale green in color and contained less chlorophyll than wild type (Fig 2C). These results indicated that the aladin-1 mutant was significantly impaired in chloroplast development and photosynthesis. Next, electron microscopic analysis was performed to examine the ultrastructure of the chloroplasts. Chloroplasts in epidermal cotyledon cells were smaller and had a more irregular shape in the mutant than in wild type (Fig 2D, upper panels). Furthermore, in mutant cells, thylakoid membranes were highly fragmented and vesicle-like structures were apparent (Fig 2D, lower panels). The maximum quantum efficiency of photosystem II was 55% lower in the mutant than in wild type (Fig 2E). These results indicated that the aladin-1 mutation hindered the normal development of the thylakoid membrane and led to a loss of photosynthetic activity.
Fig 2. The aladin-1 mutant exhibits low photosynthetic activity.
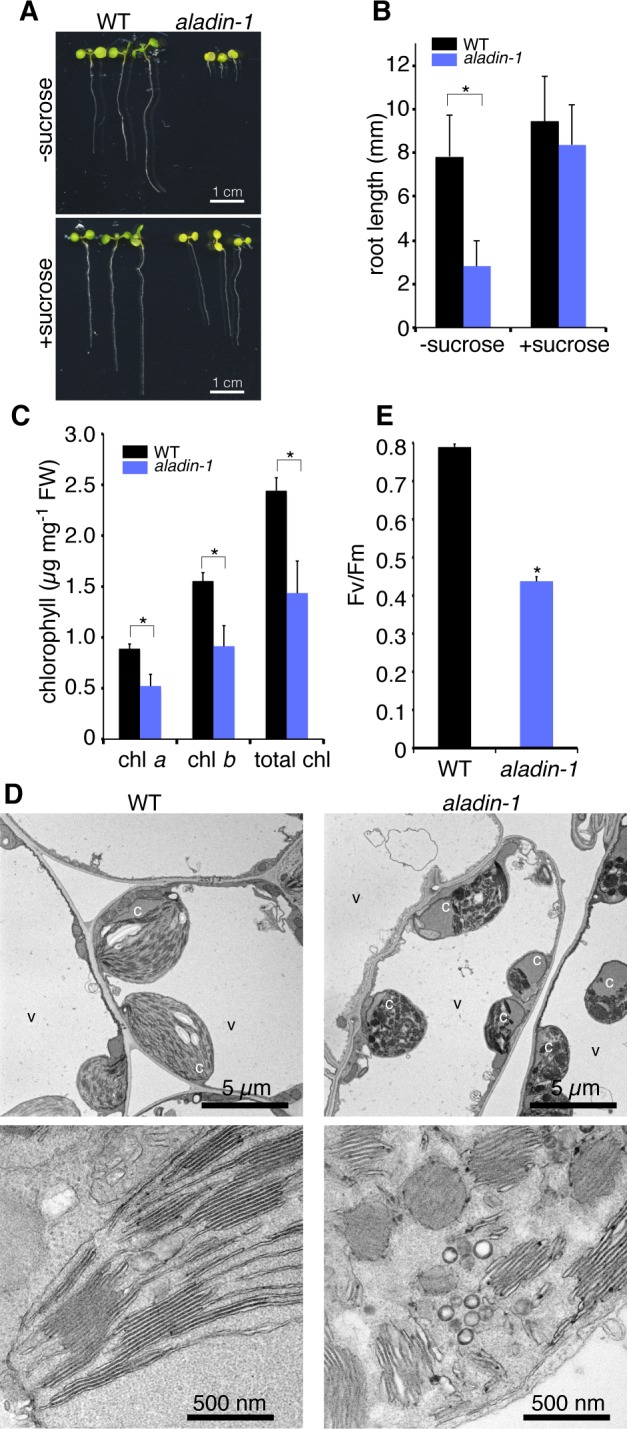
(A) Seven-day-old wild-type (WT) and aladin-1 seedlings grown on MS plates with (+ sucrose) or without (- sucrose) sucrose. (B) Root length of 10-day-old WT and aladin-1 seedlings grown on MS plates with (+ sucrose) or without (- sucrose) sucrose. Mean ± standard deviation for n > 20 (Student’s t-test, *P < 0.001). (C) Quantification of chlorophyll a (chl a), chlorophyll b (chl b), and total chlorophyll (total chl) in mature WT and aladin-1 leaves. Mean ± standard deviation for n > 3 (Student’s t-test, *P < 0.001). (D) Electron micrographs of mesophyll cells (upper) and chloroplasts (lower) in mature leaves from 14-day-old WT and aladin-1 plants. c, chloroplast; v, vacuole. (E) Maximum quantum yield of photosysytem II (Fv/Fm).
Accumulation of chloroplast proteins was investigated by SDS-PAGE and immunoblot of lysates from mature leaves. Accumulation of RuBisCO subunits and PSBA (photosystem II reaction center protein A) was significantly lower in the mutant than in wild type (Fig 3A and 3B). By contrast, another nuclear-encoded chloroplast protein, PGL35 (plastoglobulin 35 kDa), was present at similar levels in wild type and the mutant (Fig 3B). Transcription levels of the genes encoding the RuBisCO subunits and PSBA were indistinguishable between the wild type and the mutant (Fig 3C). These results suggested that aladin-1 was impaired in the accumulation of some chloroplast proteins needed for proper chloroplast function.
Fig 3. Translation of chloroplast proteins is significantly impaired in aladin-1.
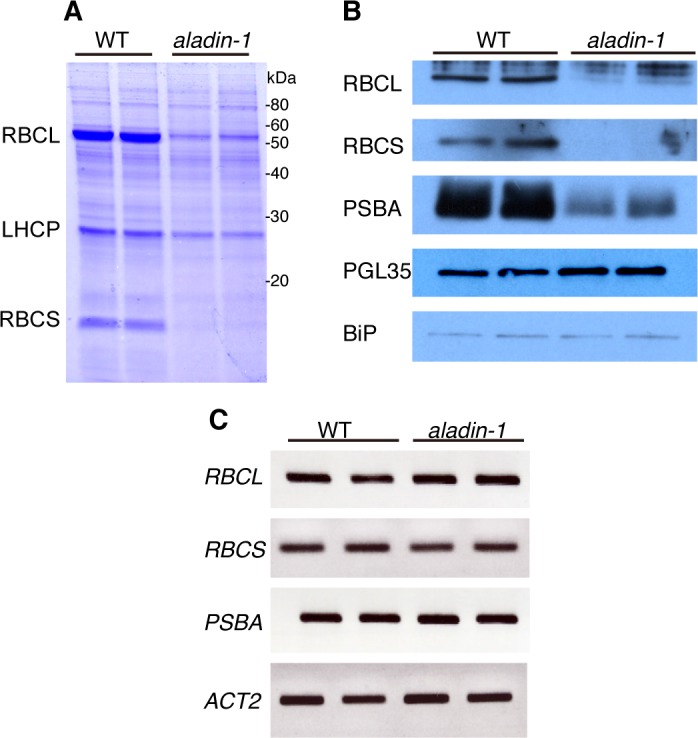
(A-B) Protein extracts from 7-day-old wild-type (WT) and aladin-1 seedlings were subjected to SDS-PAGE followed by either Coomassie Brilliant Blue staining (A) or immunoblotting (B) with anti-RuBisCO large subunit (RBCL), RuBisCO small subunit (RBCS), light-harvesting chlorophyll a/b binding protein (LHCP), photosystem II reaction center protein A (PSBA), plastoglobulin 35 kDa (PGL35), or binding protein (BiP) antibodies. Molecular masses are indicated on Fig 3A (kDa). Technical duplicate was done: two independent extractions of protein (A) and mRNA (B) were subjected to two lanes on each panel. (C) RT-PCR analysis of RBCL, RBCS, PSBA, and ACT2 transcription in WT and aladin-1. Technical duplicate was done: two independent extractions of mRNA were subjected to two lanes on each panel.
The gene responsible for the aladin-1 phenotype is PLASTID-SPECIFIC 50S RIBOSOMAL PROTEIN 5, which is located next to the ALADIN gene
To determine whether defects in ALADIN were responsible for the aladin-1 phenotype, the mutant was transformed with a genomic fragment containing wild-type ALADIN. However, the transformants exhibited similar RuBisCO accumulation (Fig 4C) and growth defects (Fig 4D) as aladin-1, indicating that the genomic fragment of ALADIN was unable to rescue the aladin-1 phenotype. Transgenic plants were generated that stably expressed artificial microRNA (amiRNA) for ALADIN in a wild-type background (ALADIN KD plants). ALADIN expression was significantly lower in ALADIN KD plants than in untransformed wild-type plants (S3 Fig). However, ALADIN KD plants accumulated RuBisCO proteins normally (Fig 4C) and had a wild-type growth pattern (Fig 4D). These results indicated that ALADIN defects are not responsible for the mutant phenotype in aladin-1.
Fig 4. PLASTID-SPECIFIC RIBOSOMAL PROTEIN 5 (PSRP5) is responsible for the aladin-1 mutant phenotype.
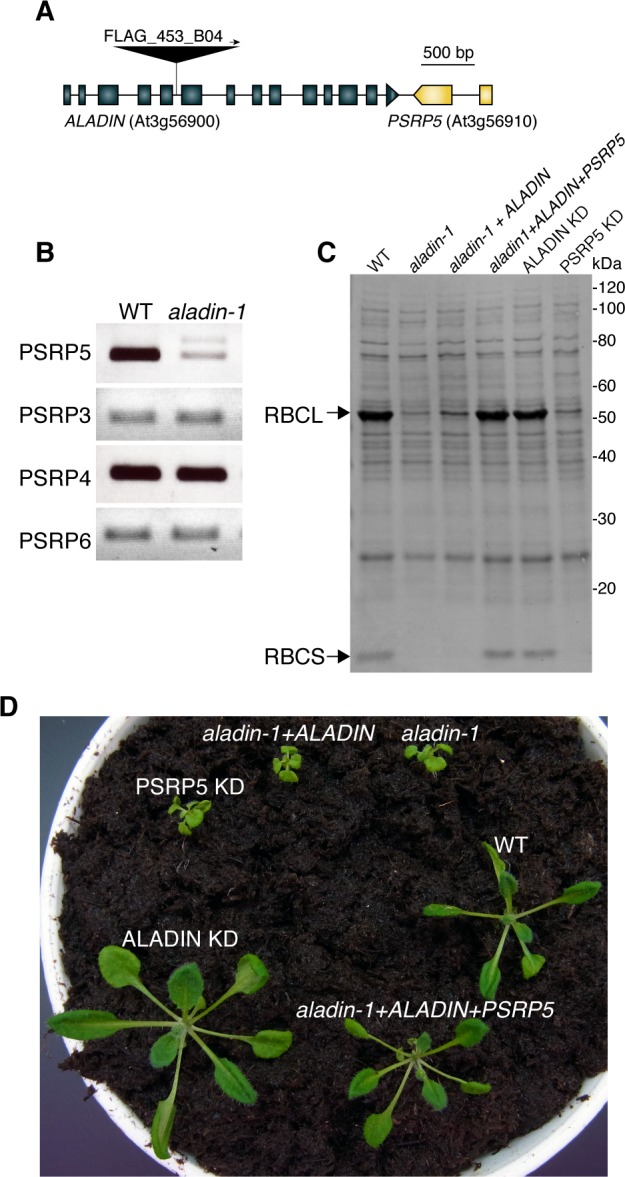
(A) Schematic representation of the ALADIN (At4g33200) and PSRP5 (At3g56910) genes. The position of the T-DNA insertion in aladin-1 is indicated by a triangle. Closed boxes and solid lines indicate exons and introns, respectively. (B) RT-PCR analysis of PSRP3, PSRP4, PSRP5, and PSRP6 transcription in wild type (WT) and aladin-1. (C) Extracts of 7-day-old seedlings of WT, aladin-1, aladin-1 complemented with a genomic fragment containing ALADIN (aladin-1 + ALADIN), aladin-1 complemented with a genomic fragment containing both ALADIN and PSRP5 (aladin-1 + ALADIN + PSRP5), aladin knockdown (ALADIN KD), and PSRP5 knockdown (PSRP5 KD) subjected to SDS-PAGE followed by Coomassie Brilliant Blue staining. Arrows indicate the positions of RBCL (upper) and RBCS (lower). (D) Three-week-old WT, aladin-1, aladin-1 + ALADIN, aladin-1 + ALADIN + PSRP5, ALADIN KD, and PSRP5 KD plants.
Examination of the Arabidopsis genome database revealed that PLASTID-SPECIFIC RIBOSOMAL PROTEIN 5 (PSRP5) was located next to ALADIN on chromosome 3 (Fig 4A). PSRP5, which has chloroplast targeting peptide at its N-terminal, is a component of the plastid ribosomal protein (PRP) complex [24, 25]. Accumulation of PSRP5 transcripts was significantly lower in the aladin-1 mutant than in wild type (Fig 4B). By contrast, transcripts encoding other components of the PRP complex (PSRP3, PSRP4, and PSRP6) were detected at similar levels in the mutant and wild type. A genomic fragment containing PSRP5 was able to rescue the aladin-1 mutant defects in RuBisCO accumulation (Fig 4C) and growth (Fig 4D). Moreover, knockdown of PSRP5 with an amiRNA construct (PSRP5 KD) (S4 Fig) phenocopied the aladin-1 mutant defects in chloroplast proteins (Fig 4C) and growth (Fig 4D). These results indicated that PSRP5, rather than ALADIN, was responsible for the aladin-1 phenotype.
PRPs are composed of a large 50S subunit and a small 30S subunit [25]. While the majority of PRP proteins are orthologous to bacterial proteins, plant plastids contain a small set of unique proteins termed plastid-specific ribosomal proteins (PSRPs). Several groups reported that deficiency of some PRPs and PSRPs led to pleiotropic phenotypes such as albinism, reduction of photosynthetic activity, and strongly impaired growth in Arabidopsis [24, 25] and rice [26]. They also reported that the T-DNA insertion line (SALK_051891) of PSRP5 was the most-severely-affected mutant among psrp mutants of Arabidopsis [24, 25]. These results suggested that protein translation in plastids had a fundamental role in plant growth. The aladin-1 mutant was severely impaired in chloroplast protein accumulation yet exhibited normal transcription (Fig 3). It is therefore possible that knockdown of PSRP5, which lies next to ALADIN on chromosome 3, is the cause of the aladin-1 mutant phenotype.
It remains to be determined how the T-DNA insertion within ALADIN influences the neighboring PSRP5 gene. Previous research found that T-DNA insertion lines harbored unexpectedly high frequencies of interchromosomal rearrangements [27]. In those cases, mutants contained single T-DNA inserts and segregated normally, but sequences from loci unlinked to the insertion site were found to flank the T-DNA border. This was not the case for aladin-1, in which the genomic sequence between the T-DNA border and PSRP5 was identical to that of the corresponding wild-type sequence (S1 Fig). It is, therefore, unlikely that T-DNA integration in aladin-1 caused interchromosomal rearrangements affecting PSRP5 expression. However, we cannot exclude the possibility that genetic alterations at different loci from the region we sequenced affect PSRP5 expression. Alternatively, despite the fact that the insertion occurred 2.2 kbp downstream of PSRP5, the T-DNA may have disrupted a cis-regulatory element controlling PSRP5 expression (Fig 4A). Isolated regulatory regions located from their target genes have been described previously, but were mainly found in animals [28, 29]. One plant example is the Arabidopsis GL1 gene, in which an enhancer region essential for GL1 function is located approximately 1 kbp from the 3′ end of the coding region [30].
The nucleoporin ALADIN is thought to be an outer-ring component of the Nup107–160 nucleoporin complex, which is the largest subcomplex of the NPC [19]. The human Nup107–160 complex plays a critical role in NPC formation and scaffolding [31]. Arabidopsis mutations were reported in several genes encoding Nup107–160 subcomplex proteins. The nup160 [32], nup96 [32], hos1 [33], and gle1 [34] mutants exhibited severely impaired growth. However, in this study, knockdown of ALADIN produced no visibly defective phenotype (Fig 4D). These results indicated that Arabidopsis ALADIN was not required for NPC function under normal growth conditions. On the contrary, in human cultured cells, a point mutation in ALADIN leads to hypersensitivity to oxidative stress and subsequent accumulation of damaged DNA, resulting in cell death [35]. It is possible to expect that Arabidopsis ALADIN is involved in response to stress conditions in the cells. The ALADIN knockdown generated in this study will be a useful resource for determining the molecular role of ALADIN in the NPC.
In this study, we found that a non-target gene was unexpectedly affected by T-DNA knockdown of a neighboring gene. This emphasizes the importance of analyzing multiple mutant alleles to minimize the risk of phenotypic misinterpretation. However, where mutations are tightly linked, backcrossing with multiple alleles cannot always ensure successful genetic separation. In addition, cis-element regions of neighboring gene(s) can sometimes be included in genomic rescue constructs, and care must be taken when interpreting the results of complementation experiments that use such constructs. When unexpected phenotypes are observed, ascertaining the transcription levels of neighboring genes may be necessary to conclusively ascribe a mutant phenotype to loss-of-function of the gene of interest.
Supporting Information
Red, left border; Blue, ALADIN; Green, PSRP5.
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
Acknowledgments
We are grateful to Versailles Arabidopsis Stock Centre at the Jean-Pierre Bourgin Institute of the National Institute for Agricultural Research for the seeds of a T-DNA insertion mutant (FLAG_453B04).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Grants-in-Aid for Scientific Research to K.T. [nos. 15K14545 (https://kaken.nii.ac.jp/d/p/15K14545.en.html) and 26711017 (https://kaken.nii.ac.jp/d/p/26711017.en.html)] and to I.H.N. [no. 15H05776 (https://kaken.nii.ac.jp/d/p/15H05776.en.html)], and a Grant-in-Aid for Specially Promoted Research to I.H.N. [no. 22000014 (https://kaken.nii.ac.jp/d/p/22000014.en.html)] from the Japan Society for the Promotion of Science (JSPS).
References
- 1.O'Malley RC, Ecker JR. Linking genotype to phenotype using the Arabidopsis unimutant collection. Plant J. 2010; 61(6):928–40. 10.1111/j.1365-313X.2010.04119.x [DOI] [PubMed] [Google Scholar]
- 2.Azpiroz-Leehan R, Feldmann KA. T-DNA insertion mutagenesis in Arabidopsis: going back and forth. Trends Genet. 1997; 13(4):152–6. [DOI] [PubMed] [Google Scholar]
- 3.Samson F, Brunaud V, Balzergue S, Dubreucq B, Lepiniec L, Pelletier G, et al. FLAGdb/FST: a database of mapped flanking insertion sites (FSTs) of Arabidopsis thaliana T-DNA transformants. Nucleic Acids Res. 2002; 30(1):94–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, et al. A high-throughput Arabidopsis reverse genetics system. Plant Cell. 2002; 14(12):2985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003; 301(5633):653–7. [DOI] [PubMed] [Google Scholar]
- 6.Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B. An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol. 2003; 53(1–2):247–59. [DOI] [PubMed] [Google Scholar]
- 7.Woody ST, Austin-Phillips S, Amasino RM, Krysan PJ. The WiscDsLox T-DNA collection: an arabidopsis community resource generated by using an improved high-throughput T-DNA sequencing pipeline. J Plant Res. 2007; 120(1):157–65. [DOI] [PubMed] [Google Scholar]
- 8.Wang N, Long T, Yao W, Xiong L, Zhang Q, Wu C. Mutant resources for the functional analysis of the rice genome. Mol Plant. 2013; 6(3):596–604. 10.1093/mp/sss142 [DOI] [PubMed] [Google Scholar]
- 9.Thole V, Peraldi A, Worland B, Nicholson P, Doonan JH, Vain P. T-DNA mutagenesis in Brachypodium distachyon. J Exp Bot. 2012; 63(2):567–76. 10.1093/jxb/err333 [DOI] [PubMed] [Google Scholar]
- 10.Gheysen G, Herman L, Breyne P, Gielen J, Van Montagu M, Depicker A. Cloning and sequence analysis of truncated T-DNA inserts from Nicotiana tabacum. Gene. 1990; 94(2):155–63. [DOI] [PubMed] [Google Scholar]
- 11.Nacry P, Camilleri C, Courtial B, Caboche M, Bouchez D. Major chromosomal rearrangements induced by T-DNA transformation in Arabidopsis. Genetics. 1998; 149(2):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimada TL, Shimada T, Hara-Nishimura I. A rapid and non-destructive screenable marker, FAST, for identifying transformed seeds of Arabidopsis thaliana. Plant J. 2010; 61(3):519–28. 10.1111/j.1365-313X.2009.04060.x [DOI] [PubMed] [Google Scholar]
- 13.Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006; 18(5):1121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ossowski S, Schwab R, Weigel D. Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J. 2008; 53(4):674–90. 10.1111/j.1365-313X.2007.03328.x [DOI] [PubMed] [Google Scholar]
- 15.Argyros RD, Mathews DE, Chiang Y- H, Palmer CM, Thibault DM, Etheridge N, et al. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell. 2008; 20(8):2102–16. 10.1105/tpc.108.059584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shikanai T, Munekage Y, Shimizu K, Endo T, Hashimoto T. Identification and characterization of Arabidopsis mutants with reduced quenching of chlorophyll fluorescence. Plant Cell Physiol. 1999; 40(11):1134–42. [DOI] [PubMed] [Google Scholar]
- 17.Goto C, Tamura K, Fukao Y, Shimada T, Hara-Nishimura I. The Novel Nuclear Envelope Protein KAKU4 Modulates Nuclear Morphology in Arabidopsis. Plant Cell. 2014; 26(5):2143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I. Identification and characterization of nuclear pore complex components in Arabidopsis thaliana. Plant Cell. 2010; 22(12):4084–97. 10.1105/tpc.110.079947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura K, Hara-Nishimura I. The molecular architecture of the plant nuclear pore complex. J Exp Bot. 2013; 64(4):823–32. 10.1093/jxb/ers258 [DOI] [PubMed] [Google Scholar]
- 20.Tullio-Pelet A, Salomon R, Hadj-Rabia S, Mugnier C, de Laet MH, Chaouachi B, et al. Mutant WD-repeat protein in triple-A syndrome. Nat Genet. 2000; 26(3):332–5. [DOI] [PubMed] [Google Scholar]
- 21.Handschug K, Sperling S, Yoon SJ, Hennig S, Clark AJ, Huebner A. Triple A syndrome is caused by mutations in AAAS, a new WD-repeat protein gene. Hum Mol Genet. 2001; 10(3):283–90. [DOI] [PubMed] [Google Scholar]
- 22.Huebner A, Kaindl AM, Knobeloch KP, Petzold H, Mann P, Koehler K. The triple A syndrome is due to mutations in ALADIN, a novel member of the nuclear pore complex. Endocr Res. 2004; 30(4):891–9. [DOI] [PubMed] [Google Scholar]
- 23.Huebner A, Mann P, Rohde E, Kaindl AM, Witt M, Verkade P, et al. Mice lacking the nuclear pore complex protein ALADIN show female infertility but fail to develop a phenotype resembling human triple A syndrome. Mol Cell Biol. 2006; 26(5):1879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romani I, Tadini L, Rossi F, Masiero S, Pribil M, Jahns P, et al. Versatile roles of Arabidopsis plastid ribosomal proteins in plant growth and development. Plant J. 2012; 72(6):922–34. 10.1111/tpj.12000 [DOI] [PubMed] [Google Scholar]
- 25.Tiller N, Weingartner M, Thiele W, Maximova E, Schottler MA, Bock R. The plastid-specific ribosomal proteins of Arabidopsis thaliana can be divided into non-essential proteins and genuine ribosomal proteins. Plant J. 2012; 69(2):302–16. 10.1111/j.1365-313X.2011.04791.x [DOI] [PubMed] [Google Scholar]
- 26.Lin D, Jiang Q, Zheng K, Chen S, Zhou H, Gong X, et al. Mutation of the rice ASL2 gene encoding plastid ribosomal protein L21 causes chloroplast developmental defects and seedling death. Plant Biol (Stuttg). 2015; 17(3):599–607. [DOI] [PubMed] [Google Scholar]
- 27.Tax FE, Vernon DM. T-DNA-associated duplication/translocations in Arabidopsis. Implications for mutant analysis and functional genomics. Plant Physiol. 2001; 126(4):1527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barton MC, Madani N, Emerson BM. Distal enhancer regulation by promoter derepression in topologically constrained DNA in vitro. Proc Natl Acad Sci U S A. 1997; 94(14):7257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagga R, Michalowski S, Sabnis R, Griffith JD, Emerson BM. HMG I/Y regulates long-range enhancer-dependent transcription on DNA and chromatin by changes in DNA topology. Nucleic Acids Res. 2000; 28(13):2541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larkin JC, Oppenheimer DG, Pollock S, Marks MD. Arabidopsis GLABROUS1 Gene Requires Downstream Sequences for Function. Plant Cell. 1993; 5(12):1739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walther TC, Alves A, Pickersgill H, Loiodice I, Hetzer M, Galy V, et al. The conserved Nup107-160 complex is critical for nuclear pore complex assembly. Cell. 2003; 113(2):195–206. [DOI] [PubMed] [Google Scholar]
- 32.Parry G, Ward S, Cernac A, Dharmasiri S, Estelle M. The Arabidopsis SUPPRESSOR OF AUXIN RESISTANCE proteins are nucleoporins with an important role in hormone signaling and development. Plant Cell. 2006; 18(7):1590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macgregor DR, Gould P, Foreman J, Griffiths J, Bird S, Page R, et al. HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES1 Is Required for Circadian Periodicity through the Promotion of Nucleo-Cytoplasmic mRNA Export in Arabidopsis. Plant Cell. 2013; 25(11):4391–404. 10.1105/tpc.113.114959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braud C, Zheng W, Xiao W. LONO1 encoding a nucleoporin is required for embryogenesis and seed viability in Arabidopsis. Plant Physiol. 2012; 160(2):823–36. 10.1104/pp.112.202192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirano M, Furiya Y, Asai H, Yasui A, Ueno S. ALADINI482S causes selective failure of nuclear protein import and hypersensitivity to oxidative stress in triple A syndrome. Proc Natl Acad Sci U S A. 2006; 103(7):2298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Red, left border; Blue, ALADIN; Green, PSRP5.
(EPS)
(EPS)
(EPS)
(EPS)
(EPS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


