Abstract
Hypertension caused by angiotensin II is dependent on vascular superoxide (O2·–) production. The nicotin-amide adenine dinucleotide phosphate (NAD[P]H) oxidase is a major source of vascular O2·– and is activated by angiotensin II in vitro. However, its role in angiotensin II–induced hypertension in vivo is less clear. In the present studies, we used mice deficient in p47phox, a cytosolic subunit of the NADPH oxidase, to study the role of this enzyme system in vivo. In vivo, angiotensin II infusion (0.7 mg/kg per day for 7 days) increased systolic blood pressure from 105±2 to 151±6 mm Hg and increased vascular O2·– formation 2- to 3-fold in wild-type (WT) mice. In contrast, in p47phox−/− mice the hypertensive response to angiotensin II infusion (122±4 mm Hg; P<0.05) was markedly blunted, and there was no increase of vascular O2·– production. In situ staining for O2·– using dihydroethidium revealed a marked increase of O2·–production in both endothelial and vascular smooth muscle cells of angiotensin II–treated WT mice, but not in those of p47phox−/− mice. To directly examine the role of the NAD(P)H oxidase in endothelial production of O2·–, endothelial cells from WT and p47phox−/− mice were cultured. Western blotting confirmed the absence of p47phox in p47phox−/− mice. Angiotensin II increased O2·– production in endothelial cells from WT mice, but not in those from p47phox−/− mice, as determined by electron spin resonance spectroscopy. These results suggest a pivotal role of the NAD(P)H oxidase and its subunit p47phox in the vascular oxidant stress and the blood pressure response to angiotensin II in vivo.
Keywords: oxidative stress; endothelium; angiotensin II; hypertension, experimental
Activation of the renin angiotensin system is critically involved in the pathogenesis of hypertension and atherosclerosis.1 The recently completed Heart Outcomes Prevention Evaluation (HOPE) trial demonstrated a remarkable decrease in cardiovascular morbidity and mortality by ACE inhibition in individuals at increased risk for cardiovascular events.2 A major mechanism whereby angiotensin II, the principal effector peptide of the renin-angiotensin system, may contribute to vascular pathology is stimulation of super-oxide (O2·–) formation in vascular cells.3,4 We and others have shown that treatment with liposome-encapsulated super-oxide dismutase (SOD) or the membrane-permeable SOD mimetic tempol markedly blunts the increase in blood pressure caused by angiotensin II administration, suggesting that stimulation of O2·– formation is critically involved in the blood pressure response to angiotensin II.5,6
One source of O2·– that is stimulated by angiotensin II in endothelial and vascular smooth muscle cells is the nicotin-amide adenine dinucleotide phosphate (NAD[P]H) oxidase.3,7,8 Several recent in vitro studies have suggested an important role of the p47phox subunit of the NAD(P)H oxidase for angiotensin II stimulated O2·– production in vascular smooth muscle cells.8,9 It is not known, however, whether activation of the NAD(P)H oxidase and its subunit p47phox are critical for angiotensin II–stimulated O2·– production in endothelial cells. To address this issue, we studied mice lacking p47phox, a cytosolic subunit of the NAD(P)H oxidase,10 and examined responses to angiotensin II administered chronically in vivo. We also cultured endothelial cells from these mice and examined the effect of angiotensin II on O2·– production in vitro.
Methods
Animals Studied
Male C57BL/6 mice were obtained from Jackson Laboratories (25 to 35 g; Bar Harbor, Me) and were used at the age of 6 to 8 months. Male mice lacking p47phox10 were backcrossed at least ×7 to the C57BL/6 background and were used at the age of 6 to 8 months. For implantation of osmotic minipumps, the mice were anesthetized with intraperitoneal Avertin 2.5% (0.3 mL per 25 g of body weight, IP). The intrascapular region was shaved, and an osmotic minipump (Alzet Model 2002; Alza Corp) that contained angiotensin II was inserted via a 1-cm incision to permit subcutaneous infusion of angiotensin II ([Val5]angiotensin II, infusion rate 0.7 mg/kg per day). Sham-operated animals underwent an identical surgical procedure, except that either no pump or an empty osmotic pump was implanted. On day 7 of angiotensin II infusion, the animals were killed by CO2 inhalation, and their aortas were harvested for study. The Emory University Institutional Animal Care and Use Committee approved all animal experiments.
Blood Pressure Measurement
Systolic blood pressures were measured by a computerized tail-cuff system (Visitech Systems).11 Before the osmotic pump was implanted, the mice were trained in the blood pressure device to accustom them to the procedure. On each day of blood pressure determination, 10 measurements were obtained and averaged for each mouse.
Measurements of Vascular Superoxide Production
Animals were euthanized by CO2 inhalation. The aortas were rapidly removed and placed into chilled modified Krebs/HEPES buffer (composition in mmol/L: NaCl 99.01, KCl 4.69, CaCl2 2.50, MgSO4 1.20, KH2PO4 1.03, NaHCO3 25.0, Na-HEPES 20.0, and glucose 5.6; pH 7.4), cleaned of excessive adventitial tissue, and cut into 4- to 5-mm ring segments with care taken not to injure the endothelium. Vascular O2·– production was determined using lucigenin-enhanced chemiluminescence as described before.12 This method has recently been validated for O2·– measurements in vascular tissue when low concentrations of lucigenin (5 μmol/L) are used.13,14
As a second approach to quantify vascular O2·– production, we employed dihydroethidium (HE) staining of intact vascular rings as described previously.15 Paired aortas from Angiotensin II-infused and sham animals were processed in parallel, and images were acquired with identical acquisition parameters. Reagents were purchased from Sigma-Aldrich.
Measurements of Superoxide Production in Cultured Endothelial Cells
Mouse aortic endothelial cells (MAECs) from wild-type and p47phox−/− mice were isolated using a matrigel culture as described previously and selected over vascular smooth muscle cells using heparin.16 Cells were maintained in DMEM (Gibco-BRL) containing 10% fetal calf serum (FCS, Hyclone Laboratories) supplemented with endothelial cell growth supplement (ECGS; 75 μg/mL; Sigma E-2759), heparin (10 U/mL), and antibiotics. Endothelial cells were used for studies at passage 3. On the day of confluence, the cell media was changed to DMEM with 5% serum overnight, and cells were studied the next day, one day post-confluency. Endothelial cell O2·– production was measured using electron-spin resonance spectroscopy (ESR) and the spin trap 1-hydroxy-3-carboxy-pyrrolidine (CP-H; Alexis Corporation). Cells were rinsed with ice-cold 50 mmol/L PBS buffer (pH 7.4) and removed from the plate by scrapping. After centrifugation at 800g (7 minutes), the cells were resuspended in 400 μL PBS buffer and kept on ice. To inhibit iron-catalyzed oxidation of the spin trap, DTPA (0.2 mmol/L) was added to all samples. ESR measurements were performed in 50-μL glass capillaries (Corning). The ESR spectra were recorded using a Bruker EMX spectrometer (Bruker Corporation) and a super-high Q microwave cavity. O2·– formation was determined by following the oxidation of CP-H to paramagnetic 3-carboxy-proxyl (CP·).17,18 The ESR instrumental settings were as follows: field sweep, 50 G; microwave frequency, 9.78 GHz; microwave power, 20 mW; modulation amplitude, 2 G; conversion time, 656 ms; time constant, 656 ms; 512 points resolution and receiver gain, 1×105 (74 dB). Time scans were recorded using 1312 ms conversion time, 5248 ms time constant, and monitoring the ESR amplitude of low-field component of ESR spectrum of carboxy-proxyl nitroxide for 300 s.
Immunoblot Analysis of p47phox and Angiotensin II Type 1–Receptor Expression in Endothelial Cells
Protein samples were prepared from mouse aortic endothelial cells and lysed directly in SDS sample buffer. Protein from endothelial cell lysates (20 μg) was separated by SDS-PAGE, transferred to membranes, and probed with anti-p47phox antibody (BD Transduction Laboratories) or angiotensin II type 1 (AT1)-receptor antibody (Santa Cruz Biotechnology). For the p47phox immunoblot analysis, mouse macrophage lysates (10 μg) were loaded as positive controls. Protein was visualized by chemiluminescence.
Data Analysis
All data are expressed as mean±SEM. Comparisons between groups of animals or treatments were made by one-way ANOVA, followed by Student-Newman-Keuls test. Values of P<0.05 were considered statistically significant.
Results
Effects of Angiotensin II Treatment on Vascular Superoxide Production in Wild-Type (C57/BL6) and p47phox−/− Mice
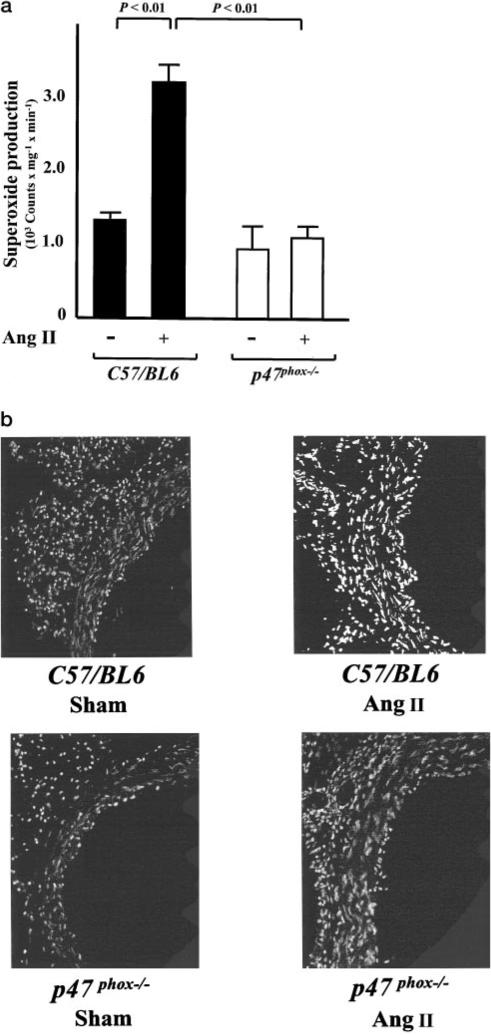
Angiotensin II infusion caused a 2- to 3-fold increase in vascular O2·– production in wild-type (C57/BL6) mice (Figure 1a). In contrast, in p47phox−/− mice, no increase in vascular O2·– formation was observed after treatment with angiotensin II (Figure 1a). To estimate O2·– production in mouse aortas in situ, we used HE staining. Conversion of HE by O2·– to ethidium results in nuclear fluorescence. Aortas from angiotensin II–treated wild-type mice consistently showed a markedly increased fluorescence, both in the endothelium and in vascular smooth muscle, indicating increased O2·– production (Figure 1b). In p47phox−/− mice, however, no increase in HE-detectable O2·– production was observed after angiotensin II infusion (Figure 1b).
Figure 1.
Effect of angiotensin II (Ang II) infusion on vascular superoxide production in mouse aortas from wild-type (C57/BL6) and p47phox−/− mice. a, Superoxide production in sham and Ang II–treated mice as determined with lucigenin-enhanced chemiluminescence (5 μmol/L; n=5 to 9). b, In situ detection of superoxide production with dihydroethidium (HE) in sham and Ang II–treated mice. Data are representative of 3 separate experiments.
Effect of Angiotensin II Treatment on Blood Pressure in Wild-Type and p47phox−/− Mice
In wild-type mice, angiotensin II infusion caused an increase in blood pressure from 105±2 to 151±6 mm Hg (Figure 2). Importantly, in p47phox−/− mice, the blood pressure response to angiotensin II was markedly blunted (Figure 2).
Figure 2.
Effect of Ang II infusion on systolic blood pressure in wild-type (C57/BL6) and p47phox−/− mice (n=5 to 9). *P<0.05 (ang II-infused wild-type vs ang II-infused p47phox−/− mice).
Effect of Angiotensin II on Superoxide Production in Cultured Endothelial Cells from Wild-Type and p47phox−/− Mice
To study the role of p47phox in angiotensin II–induced O2·– formation in endothelial cells independent of changes in blood pressure we measured O2·– formation in cultured endothelial cells from wild-type and p47phox−/− mice. Whereas in wild-type endothelial cells, angiotensin II administration (10–6 mol/L) caused a substantial increase in CP-H oxidation, there was no change in CP-H oxidation in p47phox-deficient endothelial cells in response to angiotensin II (Figures 3a and 3b). The increase of CP-H oxidation caused by angiotensin II in wild-type endothelial cells was completely inhibited by superoxide dismutase (50 U PEG-SOD), indicating that CP-H oxidation was caused by O2·– (Figure 3a).
Figure 3.
Determination of superoxide formation in cultured aortic endothelial cells from wild-type and p47phox−/− mice using ESR spectroscopy. a, Increase of CP-H oxidation by endothelial cells in response to Ang II; effect of superoxide dismutase (50 U PEG-SOD). b, Representative “time scan” of CP-H oxidation in wild-type and p47phox-deficient endothelial cells stimulated with Ang II. c, Western blot analysis of p47phox expression in endothelial cells from wild-type (WT) and p47phox−/− mice. Protein extracted from mouse macrophage lysates was used as a positive control. d, Western blot analysis of AT1-receptor expression in endothelial cells from WT and p47phox−/−mice. Data are representative of 3 separate experiments.
Immunoblot Analysis of p47phox and AT1-Receptor Expression in Endothelial Cells
The p47phox subunit of the NAD(P)H oxidase was expressed in aortic endothelial cells from wild-type mice but not in endothelial cells cultured from p47phox-deficient mice (Figure 3c). The expression of the AT1 receptor was similar in p47phox-deficient and wild-type endothelial cells (Figure 3d).
Discussion
Our present results demonstrate a critical role of the NAD(P)H oxidase and its subunit p47phox for the vascular oxidant stress response to angiotensin II. The increase in vascular O2·– production in response to angiotensin II administration was diminished in p47phox−/− mice and the amount of hypertension caused by angiotensin II was reduced in these animals compared with wild-type mice. Angiotensin II stimulated O2·– production in cultured aortic endothelial cells from wild-type mice but not in p47phox-deficient endothelial cells.
We have previously shown that angiotensin II administration in rats causes a substantial increase in vascular O2·– formation and impairs endothelium-dependent vasodilation.4 Treatment with liposome-encapsulated superoxide dismutase (SOD) or the membrane-permeable SOD mimetic tempol blunts the increase in blood pressure caused by angiotensin II and preserves the bioavailability of nitric oxide, suggesting that the increase in vascular O2·– formation is critical for the blood response to the octapeptide.5,6 An important source of O2·– in endothelial and vascular smooth muscle cells is the NAD(P)H oxidase,3,19 which is stimulated by angiotensin II in both cell types.3,7–9 In addition, increased vascular NAD(P)H oxidase activity and increased expression of its subunit p22phox was observed in angiotensin II–treated rats in vivo,4,20 raising the question whether activation of this enzyme complex is critical for the oxidant stress and blood pressure response to angiotensin II. In the present studies, we used mice deficient in p47phox,10 a cytosolic subunit of the NAD(P)H oxidase, to address this question. Whereas angiotensin II administration caused a 2- to 3-fold increase of vascular O2·– production in wild-type mice, no such increase was observed in p47phox-deficient mice after angiotensin II treatment. Importantly, the blood pressure response to angiotensin II was markedly blunted in mice lacking p47phox compared with wild-type mice, suggesting a pivotal role of NAD(P)H oxidase activation for the blood pressure increase caused by angiotensin II.
In addition to smooth muscle cells, a major source of vascular O2·– stimulated by angiotensin II in vivo appears to be the endothelium. In wild-type, but not in p47phox-deficient mice, there was a marked increase of endothelial O2·–production evoked by angiotensin II as detected by HE fluorescent staining. Lavigne et al have recently demonstrated the requirement of p47phox for angiotensin II–stimulated O2·–production in vascular smooth muscle cells in vitro.8 In the present study, we therefore analyzed the role of p47phox in angiotensin II–induced oxidant stress in endothelial cells. In cultured endothelial cells from wild-type mice, but not in p47phox-deficient cells, there was a marked increase of O2·–formation after exposure to angiotensin II as revealed by ESR spectroscopy, suggesting that activation of the NAD(P)H oxidase within the endothelium is dependent on p47phox. One possible explanation for there being less O2·– formation in response to angiotensin II in p47phox-deficient endothelial cells could be a reduced AT1-receptor gene expression. Our Western blot analysis, however, showed similar expression of the AT1 receptor in p47phox-deficient and wild-type endothelial cells.
Basal O2·– production was similar in endothelial cells from wild-type and p47phox-deficient mice, suggesting that p47phox is not essential for basal O2·– formation in endothelial cells. There are other sources of O2·– (ie, xanthine oxidase, cytochrome p450, mitochondria), which could also account for basal O2·– formation in p47phox-deficient cells. Our results are compatible with the recent findings of Li et al21 in cultured coronary microvascular endothelial cells from p47phox-deficient mice, who found no reduction in basal O2·–production, but a reduced response to TNF-α and phorbol ester in p47phox-deficient microvascular endothelial cells.21
In the present study, we observed expression of p47phox in aortic endothelial cells from wild-type, but not from p47phox-deficient mice. The expression of the NAD(P)H oxidase subunit p47phox has been demonstrated in other endothelial cell lines, ie, human umbilical vein endothelial cells and murine microvascular endothelial cells.21,22 In neutrophils the importance of p47phox for activation of the NAD(P)H oxidase is well documented because its phosphorylation appears to be the limiting step required for assembly of the active enzyme complex.23 Furthermore, mutations of p47phox are a cause of chronic granulomatous disease, an immune deficiency resulting from impaired phagocyte activity.23 The results of the present studies suggest that p47phox is equally vital to the activation of the NAD(P)H oxidase by angiotensin II within the endothelium.
Perspectives
In summary, the present study indicates that the NAD(P)H oxidase and its cytosolic subunit p47phox play a pivotal role in mediating the hypertension caused by angiotensin II. Our results are in keeping with a recent study by Rey et al who demonstrated that treatment with a chimeric peptide designed to inhibit the association of p47phox with the NAD(P)H oxidase membrane components diminished the blood pressure response to angiotensin II.24 Our experiments also show that p47phox and the NAD(P)H oxidase are essential for angiotensin II–stimulated O2· production in cultured aortic endothelial cells. Increased endothelial O2·– production in response to angiotensin II may also be important for the proinflammatory and proatherogenic effects of the octapeptide. Stokes et al25 have recently demonstrated that p47phox deficiency diminished the hypercholesterolemia-induced leukocyte-endothelial cell adhesion, and we have previously shown that activation of the vascular NAD(P)H oxidase in hypercholesterolemia is dependent on angiotensin II.26 Furthermore, when crossed with p47phox−/− mice, apolipoprotein E(Apo[e])-deficient mice have a rather marked reduction in atherosclerosis in the descending aorta compared with mice lacking only Apo(e).27 Given these now very clear crucial roles in vascular disease, the NAD(P)H oxidase and p47phox represent important targets for therapeutic intervention, and their specific inhibitors would very likely be useful in treatment of common disorders such as hypertension and atherosclerosis.
Acknowledgments
We gratefully acknowledge excellent technical support by Graciela Gamez. This work was supported by National Institutes of Health (NIH) grants HL390006 (D.G.H.) and HL59248 (D.G.H.), NIH Program Project Grant HL58000 (D.G.H.), and a Department of Veterans Affairs merit grant (D.G.H.). U.L. was supported by a grant from the German Cardiac Society and the Feodor Lynen Grant of the Alexander von Humboldt Foundation.
References
- 1.Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis. I. Oxidative stress and atherogenesis. Circulation. 2002;105:393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 3.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NAD(P)H oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II–mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NAD(P)H oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II–induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 6.Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to tempol in angiotensin II–infused hypertensive rats. Hypertension. 2001;37:77–83. doi: 10.1161/01.hyp.37.1.77. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Schmeisser A, Garlichs CD, Plotze K, Damme U, Mugge A, Daniel WG. Angiotensin II–induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res. 1999;44:215–222. doi: 10.1016/s0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 8.Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001;104:79–84. doi: 10.1161/01.cir.104.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Schieffer B, Luchtefeld M, Braun S, Hilfiker A, Hilfiker-Kleiner D, Drexler H. Role of NAD(P)H oxidase in angiotensin II–induced JAK/STAT signaling and cytokine induction. Circ Res. 2000;87:1195–1201. doi: 10.1161/01.res.87.12.1195. [DOI] [PubMed] [Google Scholar]
- 10.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hyper-tension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 12.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 13.Skatchkov MP, Sperling D, Hink U, Mulsch A, Harrison DG, Sindermann I, Meinertz T, Munzel T. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun. 1999;254:319–324. doi: 10.1006/bbrc.1998.9942. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- 15.Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- 16.Suh SH, Vennekens R, Manolopoulos VG, Freichel M, Schweig U, Prenen J, Flockerzi V, Droogmans G, Nilius B. Characterization of explanted endothelial cells from mouse aorta: electrophysiology and Ca2[H11001] signaling. Pflugers Arch. 1999;438:612–620. doi: 10.1007/s004249900085. [DOI] [PubMed] [Google Scholar]
- 17.Dikalov S, Skatchkov M, Bassenge E. Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochem Biophys Res Commun. 1997;231:701–704. doi: 10.1006/bbrc.1997.6174. [DOI] [PubMed] [Google Scholar]
- 18.Dikalov S, Fink B, Skatchkov M, Bassenge E. Comparison of glyceryl trinitrate-induced with pentaerythrityl tetranitrate-induced in vivo formation of superoxide radicals: effect of vitamin C. Free Radic Biol Med. 1999;27:170–176. doi: 10.1016/s0891-5849(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 19.Somers MJ, Burchfield JS, Harrison DG. Evidence for a NADH/ NAD(P)H oxidase in human umbilical vein endothelial cells using electron spin resonance. Antioxid Redox Signal. 2000;2:779–787. doi: 10.1089/ars.2000.2.4-779. [DOI] [PubMed] [Google Scholar]
- 20.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Qt, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NAD(P)H oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM. Essential role of the NAD(P)H oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90:143–150. doi: 10.1161/hh0202.103615. [DOI] [PubMed] [Google Scholar]
- 22.Jones SA, O'Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NAD(P)H oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 23.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 24.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 25.Stokes KY, Clanton EC, Russell JM, Ross CR, Granger DN. NAD(P)H oxidase-derived superoxide mediates hypercholesterolemia-induced leukocyte-endothelial cell adhesion. Circ Res. 2001;88:499–505. doi: 10.1161/01.res.88.5.499. [DOI] [PubMed] [Google Scholar]
- 26.Warnholtz A, Nickenig G, Schulz E, Macharzina R, Brasen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Bohm M, Meinertz T, Munzel T. Increased NADH-oxidase–mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 27.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]