Abstract
BACKGROUND and PURPOSE
Degree of stent retriever engagement with target thrombi may be reflected by 1) immediate reperfusion (IR) upon first deployment, indicating displacement of clot toward the vessel wall, and 2) early loss of immediate reperfusion (ELOIR), indicating penetration of retriever struts through the thrombus. The relation of these early findings to final reperfusion and clinical outcomes has not been well delineated.
METHODS
We investigated IR and ELOIR in patients undergoing stent retriever mechanical thrombectomy at an academic medical center between March 2012 and June 2014.
RESULTS
Among 56 patients, IR itself was not associated with final successful reperfusion, which occurred in 66.7% of IR patients and 71.4% of non-IR patients (p=0.999). However, ELOIR was associated a higher rate of final successful reperfusion (92% vs 44%, p = 0.046). Patients with ELOIR had a higher nominal rate of final favorable outcome (42% vs 22%, p=0.64).
CONCLUSION
ELOIR during the embedding period after deployment of stent retrievers is associated with SFR, likely due to greater thrombus engagement with the stent retriever. ELOIR may be a useful finding to guide duration of embedding time in clinical practice and design of novel stent retrievers.
Keywords: acute stroke, stent, revascularization
INTRODUCTION
Recently, FIVE (MR CLEAN, SWIFT PRIME, RAVASCAT, ESCAP EXTEND IA) prospective randomized controlled trials1–5 demonstrated that endovascular recanalization therapies with the use of stent retrievers as a primary device improved the clinical outcome in patients with acute large vessel occlusions when compared to medical management. Consequently, stent retrievers will continue to serve as a primary thrombectomy device in the treatment of acute ischemic stroke. The dynamics of initial stent retriever interaction with target thrombi have not been closely studied in patients. Immediate reperfusion (IR) upon first deployment of the stent retriever at the occlusion site may indicate good physical engagement with the target occlusion and potential bridging support to threatened penumbral tissues to sustain until permanent reperfusion is achieved by subsequent clot retrieval. Early loss of immediate reperfusion (ELOIR) while the stent retriever remains in place in the target occlusion for the first 3 to 5 minutes after deployment may indicate greater penetration of stent struts into the thrombus. A more substantial “cheese grater” effect of stent struts passing into the thrombus with clot springing back to reocclude the lumen could indicate deeper engagement with the clot and greater traction for retrieval ELOIR therefore could be a useful sign for ideal embolus capture within the stent retriever. To our knowledge, this phenomenon, ELOIR has not been studied in the clinical cases. The present study aims to evaluate whether IR and ELOIR influence successful final reperfusion.
METHODS
Patients
From a prospectively maintained registry, we identified all patients with acute ischemic stroke treated with stent retrievers between March 2012 and June 2014 at a single academic comprehensive stroke center. Eligibility criteria for this study were: 1) Acute cerebral ischemia, 2) Treatment with a stent retriever (Solitaire, Covidien, Irvine, CA, USA and/or Trevo, Stryker Neurovascular, Mountain View, CA, USA), and 3) acquisition of a digital subtraction angiogram (DSA) upon stent retriever deployment permitting evaluation for presence or absence of IR. The patients who only had fluoroscopic assessment for IR after the stent retriever deployment were excluded. Demographic and clinical data collected on all patients are summarized in Table 1.
TABLE 1.
Baseline Demographics and Clinical Characteristics
| immediate reperfuison | ||||||
|---|---|---|---|---|---|---|
| n | % | (+) | (−) | |||
| n | 56 | 42 | 14 | |||
| age (mean) | 69.6 | 70.1 | 68.2 | |||
| female | 33 | 58.9% | 24 | 57.1% | 9 | 64.3% |
| hypertension | 38 | 67.9% | 30 | 71.4% | 8 | 57.1% |
| diametes mellitus | 8 | 14.3% | 8 | 19.0% | 0 | 0.0% |
| hyperlipidemia | 22 | 39.3% | 19 | 45.2% | 3 | 21.4% |
| current smoker | 5 | 8.9% | 2 | 4.8% | 3 | 21.4% |
| chronic heart failure | 7 | 12.5% | 5 | 11.9% | 2 | 14.3% |
| atrial fibrillation | 24 | 42.9% | 17 | 40.5% | 7 | 50.0% |
| coronory artery disease | 8 | 14.3% | 7 | 16.7% | 1 | 7.1% |
| previous stroke | 10 | 17.9% | 8 | 19.0% | 2 | 14.3% |
| antiplatelet agent | 13 | 23.2% | 9 | 21.4% | 4 | 28.6% |
| anticoagulation agent | 12 | 21.4% | 11 | 26.2% | 1 | 7.1% |
| NIHSS on admission(mean) |
17.4 | 17.2 | 17.9 | |||
| M1 occlusion | 27 | 48.2% | 20 | 47.6% | 7 | 50.0% |
| M2 occlusion | 4 | 7.1% | 4 | 9.5% | 0 | 0.0% |
| ICA occlusion | 20 | 35.7% | 15 | 35.7% | 5 | 35.7% |
| posterior circulation | 5 | 8.9% | 3 | 7.1% | 2 | 14.3% |
| intravenous t-PA administration |
34 | 60.7% | 26 | 61.9% | 8 | 57.1% |
| general anesthesia | 12 | 21.4% | 10 | 23.8% | 2 | 14.3% |
| ballooon guiding catheter | 44 | 78.6% | 32 | 76.2% | 12 | 85.7% |
| early loss of immediate reperfusion |
12 | 57.1% | 12 | 28.6% | n.a | |
| rescue therapy | 9 | 16.1% | 5 | 11.9% | 4 | 28.6% |
| successful reperfusion(TICI≧2b) |
38 | 67.9% | 28 | 66.7% | 10 | 71.4% |
| favorable outcome(mRS≦ 2) |
21 | 37.5% | 15 | 35.7% | 6 | 42.9% |
| mortality | 9 | 16.1% | 8 | 19.0% | 1 | 7.1% |
Assessment of Immediate Reperfusion
Immediately after deployment of the stent retriever a DSA was performed to evaluate for the presence or absence of IR. Any visualization of the distal branches beyond the occlusive lesion immediately after the deployment of the stent retriever was defined as IR. After an embedding time of 5 minutes, a second control DSA was obtained. For patients with IR, the finding of the additional control DSA was classified into 3 groups; 1) complete loss of reperfusion without visualization of the distal branches (Fig. 1 A, B), 2) partial loss of reperfusion with diminished visualization of the distal branches (Fig. 1 C, D), 3) persistent reperfusion with no change compared to the first control angiogram performed immediately after the stent retriever deployment. In this small cohort study, patients with complete and with partial early loss of reperfusion were combined into single ELOIR group situations.
FIGURE 1.
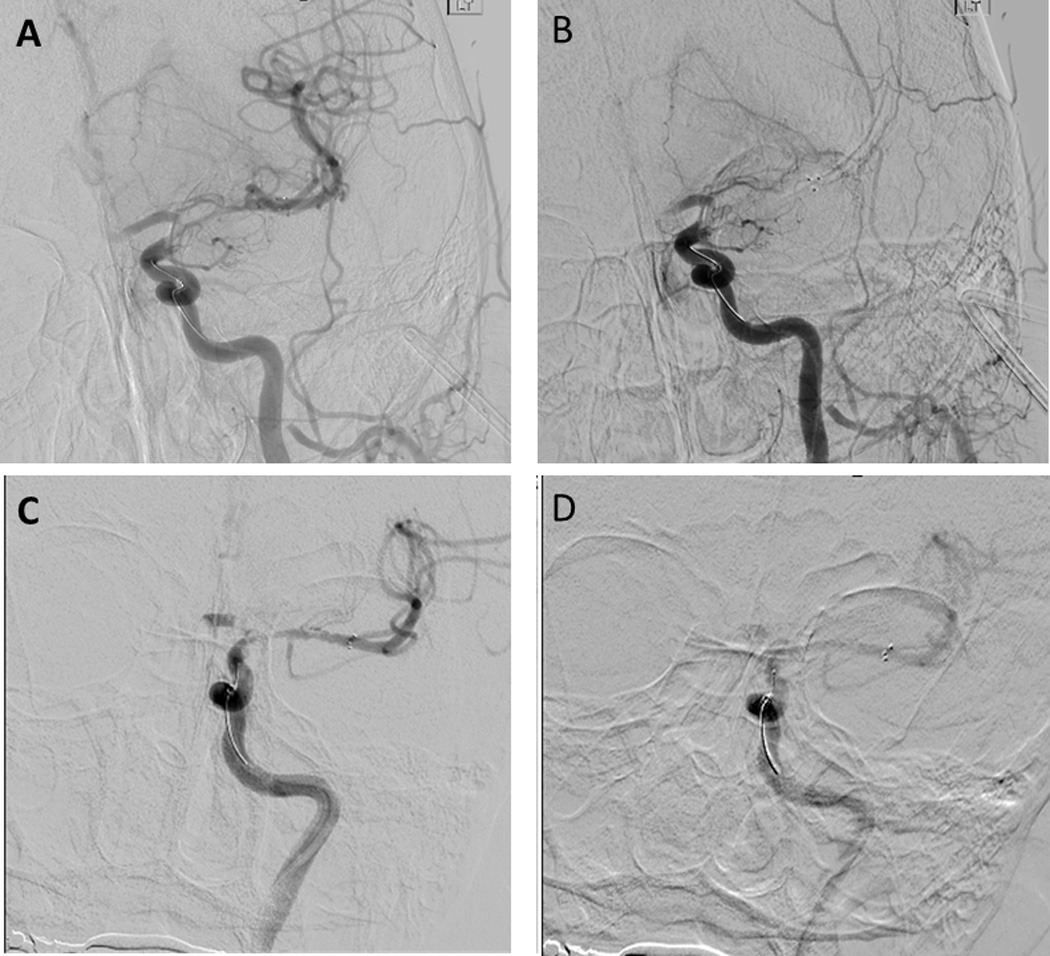
Group 1, complete flow stasis; This shows immediate flow restoration of MCA branches (A). After the embedding time the distal branches of MCA was not visualized at all (B).
Group 2, diminished visualization; the distal branches of MCA diminishes blood flow visualization (D), compared to the angiogram before embedding(C).
Reperfusion
Reperfusion was measured according to Thrombolysis in Cerebral Infarction (TICI) reperfusion parameters6. Successful reperfusion was defined as achieving a TICI score of ≥ 2b), as documented on the angiogram at the end of the stent retriever procedure prior to the use of any rescue therapy.
Statistical analysis
Statistical analyzes were performed using the Statistical Package for the Social Sciences (SPSS), version 22.0 (SPSS Inc, Chicago, IL). Fisher exact or χ2 tests (two-sided) were used for categorical variables and the Mann-Whitney U test for continuous variables. A P value of less than .05 was considered significant.
RESULTS
During the study period, 205 patients underwent revascularization treatment for acute ischemic stroke, among whom 56 had an immediate digital subtraction angiography was performed upon stent deployment, meeting eligibility criteria for this study. Demographic and baseline characteristics for these 56 patients are shown in Table 1. Mean age was 69.6 years (SD +/− 15.2), 33 (59%) were female, and mean NIH Stroke Scale score was 18 (SD +/− 8.7). IQR 13.0–22.3). Intravenous t-PA was administrated before endovascular mechanical revascularization procedure in 34 patients (60.7%). The site of the target occlusion was the intracranial internal carotid artery in 20 (35.7%), M1 MCA in 27 (48.2%), M2 MCA in 4 (7.1%), and posterior circulation in 5 (8.9%).
Solitaire stent retrievers only were used in 78.6% of patients, Trevo only in 14.3%, and both in 7.1%. In this series, the TICI ≥2b revascularization rate after stent retriever interventions was 67.9%. Rescue therapies after stent retriever such as intraarterial t-PA and/or thrombus aspiration were used in 16.1%. Average stent retriever attempts in this cohort were 2.1 times.
IR was observed in 42 patients (75.0%) after the deployment of stent retriever. Demographic and clinical characteristics did not substantially differ between patients with and without IR (Table 1). The frequency of final successful reperfusion did not differ between IR patients (28/42 (66.7%) and non-IR patients, 10/14 (71.4%), p = 0.999)(Table 2). Control digital subtraction angiography to identify the persistence of IR or ELOIR after 5 minutes embedding time was performed in 21 patients (Table 2). ELOIR was found in 12 patients (57%), including complete loss in 6 and partial loss in 6 patients. Patients with ELOIR had a higher rate of successful final reperfusion (11/12, 92%) than patients without ELOIR ((4/9, 44%), p = 0.046) (Table 2).
TABLE 2.
Analysis of Immediate reperfusion and its early loss
| N | Final successful reperfusion | Favorable outcome | Mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (+) | (−) | OR(95% CI) |
P value | (+) | (−) | OR(95% CI) |
P value | (+) | (−) | OR(95%CI ) |
P value | |||
| Immediate Reperfusion |
(+) | 42 | 28 | 14 | 0.80(0.21 –3.01) |
0.999 | 15 | 27 | 0.74(0.22 –2.54) |
0.752 | 8 | 34 | 1.76(0.187– 16.47) |
0.999 |
| (−) | 14 | 10 | 4 | 6 | 8 | 1 | 13 | |||||||
| Early loss of Immediate Reperfusion |
(+) | 12 | 11 | 1 | 2.50(0.36 –17.5) |
0.046 | 5 | 7 | 2.50(0.36 –17.5) |
0.642 | 2 | 10 | 1.60(0.12–2 0.99) |
0.999 |
| (−) | 9 | 4 | 5 | 2 | 7 | 3 | 6 | |||||||
DISCUSSION
IR upon device deployment and ELOIR are unique effects associated with use of the current stent retrievers. We found that ELOIR was associated with a higher rate of final successful reperfusion, but the presence of IR itself had no correlation with final successful reperfusion. Without a proper understanding of the clot capturing mechanism of stent retrievers, it may be counterintuitive that ELOIR, including complete reocclusion, is a good sign for ultimate success of the endovascular thrombectomy procedure. We propose that ELOIR occurs when the stent retriever successfully carves into the thrombus in the target occlusive lesion and the thrombus is captured in the stent cavity. The extensive engagement of the thrombus in the stent retriever indicated by ELOIR reduces the possibility of slippage and/or fragmentation of the captured thrombus and leads to the higher chance of successful reperfusion.
Composition of the thrombus is one factor that can determine the level of thrombus engagement. Liebeskind et al observed marked heterogeneity both in pathology and composition of the retrieved thrombi 7. This heterogeneity may explain why 3 discrete ELOIR groups were observed in this study (Fig. 1). Stent retrievers are unlikely to expand deep into relatively firm occlusive lesions and as a result IR will be sustained in these patients (Group 3: No ELOIR). In contrast, stent retrievers are likely to expand deeply through and to more fully capture soft thrombi within the stent cavity, with ELOIR (Group 1: Comlete ELOIR). When stent retrievers are placed within intermediate firmness thrombi, the ELOIR effect is intermediate in degree as a result of partial engagement of the clot (Group 2: Partial ELOIR).
Studies to date have not systematically varied the embedding time in acute ischemic stroke patients and it is uncertain how long an embedding time after deployment is optimal. The best embedding time is likely to vary with patient-specific and device-specific factors, the radial force and structure of the stent retriever, curvature and diameter of the occlusive lesion, and composition of the thrombus. In our series, more than half (56%) of Group 3 (No ELOIR) did not accomplish successful reperfusion. It is possible that a longer embedding time in those cases would have helped with better thrombus engagement. However, the downside of longer embedding time is that it extends the total procedure time and may delay in achieving the final effective recanalization. Since the large prospective stent retriever trials showed substantial clinical benefit with 5 minutes embedding time, the results of the present study are exploratory and do not directly support a procedural strategy to wait much longer than 5 minutes until ELOIR is observed. This observation may be useful in order to optimize the embedding time particularly when a new stent retriever is introduced to the clinical practice. Stent retriever itself would be important. Wenger et al compared the relation between the stent design and the thrombus capture with the artificial models8. They documented the difference of the thrombus capture caused by the stent design. Therefore ELOIR may be a useful finding to design of novel stent retrievers.
The number of this study was relatively small, and thus larger prospective studies are required to confirm this phenomenon.
CONCLUSION
This study found that ELOIR after the 5 minute embedding period following deployment of stent retrievers was associated with successful reperfusion. ELOIR may be an angiographic sign of adequate thrombus engagement with the stent retriever.
Acknowledgments
None
Source of funding
None
Footnotes
DISCLOSURE
Dr. Duckwiler is a Scientific Advisor for Blockade Medical, Asahi Intec, and Medtronic.
Dr. Tateshima is a Scientific Advisor for Medtronic, Stryker, PulsarVascular, Lazarus Effect, Blockade Medical, and Century Medical Inc.
Dr. Jahan is a Scientific Advisor for Medtronic, and Medina Medical.
Dr Liebeskind is a consultant for Stryker, and Covidien.
Saver is a scientific advisor for Medtronic/Covidien, Stry,er, and Neuravi.
REFERENCE
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 6.Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol. 2008;29:582–587. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenger KJ, Berkefeld J, Wagner M. Flat panel detector computed tomography for the interaction between contrast-enhanced thrombi and stent retrievers in stroke therapy: a pilot study. Clin Neuroradiol. 2014;24:251–254. doi: 10.1007/s00062-013-0246-6. [DOI] [PubMed] [Google Scholar]


