Abstract
Background
Native T1 mapping has emerged as a noninvasive non-contrast magnetic resonance imaging (MRI) method to assess for diffuse myocardial fibrosis. However, LV native T1 time in AF patients and its clinical relevance are unclear.
Methods
Fifty paroxysmal AF patients referred for PVI (60±8 years, 37 male) and 11 healthy control subjects (57±8 years, 10 male) were studied. All patients were in sinus rhythm during the MRI scan. Native T1 mapping images were acquired using a Modified Look-Locker imaging (MOLLI) sequence in 3 short-axis planes (basal, mid and apical slices) using an electrocardiogram triggered single-shot acquisition with a balanced steady-state free precession readout. Late gadolinium enhanced (LGE) MRI was acquired to evaluate for LV myocardial scar.
Results
LV ejection fraction was similar between groups (AF: 61±6%; controls: 60±6%, p=0.75). No LV myocardial scar was observed in any patient on LGE. Myocardial native T1 time was greater in AF patients (1099±52 vs 1042±20 msec, p<0.001). During a median follow-up period of 326 days, 18 of 50 (36%) patients experienced recurrence of AF. Multivariate Cox proportional hazard analysis identified elevated native T1 time as an independent predictor of recurrence of AF (HR: 6.53, 95% CI: 1.25–34.3, p=0.026).
Conclusions
There are differences in the native LV myocardial T1 time between AF patients with preserved LV function referred for PVI and normal controls. Native T1 time is an independent predictor of recurrence of AF after PVI in patients with paroxysmal AF.
Keywords: T1 mapping, magnetic resonance imaging, fibrosis, atrial fibrillation, cardiomyopathy, recurrence of atrial fibrillation
Background
Atrial fibrillation (AF) is the most common sustained arrhythmia, affecting 2% for those <65 years and 9% of those ≥65 years in the United States1. The symptoms of AF vary greatly, ranging from minimal to severe disability. AF is associated with a 5-fold increased risk of stroke2 and a 3-fold increased risk of heart failure3–5. In AF patients, diffuse myocardial interstitial fibrosis may occur due to tachycardia induced cardiomyopathy6. Previous studies demonstrated that tachycardia induced cardiomyopathy may progress to heart failure and lethal ventricular arrhythmia in AF patients7–9. Therefore, detection of underlying LV myocardial abnormalities in AF patients is essential for clinical management.
Native T1 mapping has been introduced as a robust magnetic resonance imaging (MRI) technique for the assessment of diffuse myocardial fibrosis in various cardiovascular disease. Recent studies has demonstrated that native T1 mapping detects left ventricular (LV) myocardial abnormalities in patients with hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM)10, cardiac amyloidosis11 and Anderson-Fabry disease11, 12. Native T1 mapping could be useful to assess the extent of myocardial injury by acute myocardial infarction13. To the best of our knowledge, no data are available regarding LV native T1 time in AF patients. We hypothesized that native T1 mapping could detect the subclinical LV myocardial abnormality in AF patients.
Therefore, we sought to compare native myocardial T1 time by using a modified Look-Locker inversion recovery (MOLLI) sequence in patients with AF and healthy control subjects; and to investigate whether native T1 time can predict the recurrence of AF after their first pulmonary vein isolation (PVI) in patients with paroxysmal AF.
Materials and Methods
Study subjects
Inclusion criteria of this prospective study included a history of paroxysmal AF patients referred for their first PVI. Exclusion criteria included patients in AF during MRI scan, reduced LV systolic function (LVEF<50%)14, cardiomyopathy (HCM, DCM15), cardiac sarcoidosis and amyloidosis, severe valvular heart disease, prior myocardial infarction and contraindication to MRI examination (claustrophobia, pacemaker implantation etc.). According to this criteria, we enrolled 50 paroxysmal AF patients. Eleven subjects without any history of cardiovascular diseases including AF were recruited as healthy controls.
Acquisition of MRI
All MRI data were acquired using a 1.5-T MRI system and a 32-channel cardiac phased array receiver coil (Achieva, Philips Healthcare, Best, The Netherlands). The study protocol was approved by our institutional review board, and written informed consent was obtained from all study subjects. Cine MRI, T1 mapping and 3 dimensional late gadolinium enhancement (LGE) MRI were imaged in all participants. T1 mapping of LV myocardium was performed using a MOLLI sequence16.
Cine MRI acquisition
Electrocardiogram (ECG) monitoring leads were positioned on supine patients. Vertical and horizontal long-axis cine MRI images of the LV were acquired using a steady-state free precession (SSFP) sequence. LV volumes and mass were calculated from an LV short-axis stack of cine images extending from the apex to the base (repetition time (TR), 3.3 ms; echo time (TE), 1.6 ms; flip angle (FA), 60°; field-of-view (FOV), 320×320 mm; acquisition matrix, 128×128; slice thickness, 8 mm; gap, 2 mm).
Native T1 mapping by MOLLI
T1 mapping images of LV myocardium were acquired in 3 short-axis planes (basal, mid and apical). Images were acquired during an end-expiration breath-hold using an ECG-triggered single-shot acquisition with a balanced SSFP readout (TR, 3.1 ms; TE, 1.5 ms; FA, 35°; FOV, 360×337 mm2; acquisition matrix, 188×135; voxel size, 1.9×2.5 mm2; slice thickness, 8 mm).
Late gadolinium enhancement
Fifteen minutes after the injection of 0.2 mmol/kg gadobenate dimeglumine (MultiHance; Bracco, Rome, Italy), LGE images were acquired using a 3 dimensional sequence17 (TR, 5.3 ms; TE, 2.1 ms; FA, 70°; FOV, 320×320×125 mm3; acquisition matrix, 224×224×23; spatial resolution,1.4×1.4×4 to 5mm; reconstruction resolution, 0.6×0.6×2 to 2.5mm).
Image analysis
Data were analyzed using a commercial workstation (Extend MR WorkSpace, version 2.3.6.3, Philips Healthcare). To determine LV mass, end-diastolic epi- and endocardial LV borders were manually traced on the short axis dataset. LV mass was calculated as the sum of the myocardial volume multiplied by the specific gravity (1.05 g/mL) of myocardial tissue18. Left atrial transverse dimensions were measured in the end-systolic phases using 4 chamber view. Short-axis slices of native T1 mapping images were analyzed using custom software (MediaCare, Boston, Massachusetts). After manually contouring endocardial and epicardial LV borders, the LV was divided into 6 segments for base and mid slices, 4 segments for apical slice using the anterior right ventricular-LV insertion point as reference. The 16 segment model was used to compare the native T1 time in each segment. Motion correction was performed using the Adaptive Registration of varying Contrast-weighted images for improved Tissue Characterization (ARCTIC) approach for both T1 mapping19. To evaluate inter-observer reproducibility, measurements of native T1 time from 10 AF patients and 10 normal subjects were independently taken by two observers. One of the two observers measured native T1 time twice to assess intra-observer reproducibility. Visual assessment was performed to evaluate presence or absence of LV myocardial scar on LGE MRI by two independent observers.
Procedure of PVI
The electrophysiology procedure was performed by a femoral venous approach. A decapolar catheter was positioned in the coronary sinus and a second catheter was placed in the right atrium. Transseptal punctures were performed to obtain left atrial access. Following transseptal puncture, patients received intravenous heparin to maintain a serum activated clotting time >250msec. Three-dimensional electroanatomic mapping of the left atrium and PV was performed using a non-irrigated 4 or 8 mm tip NaviStar™ catheter (Biosense Webster) and CARTO™ (Biosense Webster) and/or EnSite NavX™ (Endocardial Solutions) recording systems. Ablation was performed for 20–60 sec with a target temperature of 52°C. All PV were routinely isolated for all patients.
Clinical follow up
Follow up information was obtained from online medical record. Early AF recurrence was defined as AF occurring within 3 months after PVI. Late AF recurrence was defined as AF occurring between 3 months and 12 months after PVI20–22. AF recurrence was confirmed by either ECG or cardiac monitoring (remote implanted loop recorder). Complete follow-up was obtained from all patients.
Statistical analysis
Data were analyzed using SPSS software (version 17.0, SPSS, Inc., Chicago, IL, USA) and MedCalc for Windows (version 14.8.1, MedCalc Software, Ostend, Belgium). Continuous values are presented as means ± standard deviation (SD). Categorical values are expressed as number (%). Normality was determined by the Shapiro-Wilk test. Differences between AF patients and control subjects were evaluated using an unpaired t-test for normally distributed variables, and the Mann-Whitney U test for skewed variables. Bland and Altman plot23, coefficient of variation, intraclass correlation coefficient (ICC) were used to assess intra- and inter-observer reproducibility for measuring native T1 time. Repeatability coefficients were calculated as 1.96 times the SD of the differences on the Bland-Altman plots. The differences of native T1 time between 3 slices (base, mid and apex) were assessed using one way analysis of variance with Tukey’s correction. Elevated myocardial T1 time was defined as >1082msec, which was 2SD of native T1 time in healthy control subjects. We calculated the cumulative incidence of AF recurrence after PVI using the Kaplan-Meier method and compare the two groups with a Log-rank test (elevated LV T1 time group (n=31) vs non-elevated LV T1 time group (n=19)). Multivariate Cox proportional hazards model was used to estimate the hazard ratio (HR) for recurrence of AF and reported with 95% confidence interval (CI). A P value <0.05 was considered statistically significant.
Results
Patients’ characteristics
Table 1 summarizes the clinical characteristics of the AF patients and control subjects. AF patients were heavier (p=0.009) and with greater CHA2DS2-VASc score (p=0.011). Incidence of hypertension (p=0.046) and dyslipidemia (p=0.028) were higher in AF patients. There was no difference in age, gender, systolic blood pressure, diastolic blood pressure, heart rate or the incidence of diabetes mellitus or current smoking. The duration of AF was defined as the time between initial AF confirmed by an electrocardiogram and MRI acquisition date.
Table 1.
Characteristics of study subjects
| AF patients, N=50 | Controls, N=11 | P-value | |
|---|---|---|---|
| Male | 37 (74%) | 10 (77%) | 0.83 |
| Age, years | 60 ± 8 | 57 ± 8 | 0.080 |
| BMI, kg/m2 | 27.9 ± 4.0 | 24.4 ± 3.0 | 0.009 |
| SBP, mmHg | 120 ± 15 | 108 ± 33 | 0.057 |
| DBP, mmHg | 75 ± 13 | 75 ± 14 | 0.84 |
| HR, beats per minutes | 63 ± 10 | 63 ± 9 | 0.70 |
| CAD risk factors | |||
| Hypertension | 14 (28%) | 0 (0%) | 0.046 |
| Dyslipidemia | 16 (32%) | 0 (0%) | 0.028 |
| Diabetes mellitus | 3 (6%) | 0 (0%) | 0.40 |
| Current smoker | 1 (2%) | 0 (0%) | 0.64 |
| Family history of CAD | 9 (18%) | 1 (8%) | 0.47 |
| Medication | |||
| Calcium channel blocker | 9 (18%) | 0 (0%) | 0.13 |
| ACE/ARB | 9 (18%) | 0 (0%) | 0.13 |
| Beta blocker | 23 (46%) | 0 (0%) | 0.004 |
| Anti-arrhythmic agent | 23 (46%) | 0 (0%) | 0.004 |
| Anticoagulant | 41 (82%) | 0 (0%) | < 0.001 |
| Statin | 18 (36%) | 0 (0%) | 0.017 |
| CHA2DS2-VASc score | 1.0 ± 1.1 | 0.1 ± 0.3 | 0.011 |
| Duration of AF, years | 3.9 ± 4.9 | - | - |
ACE; angiotensin converting enzyme inhibitors, AF; atrial fibrillation, ARB; angiotensin receptor blockers, BMI; body mass index, CAD; coronary artery disease, DBP; diastolic blood pressure, HR; heart rate, SBP; systolic blood pressure
Cardiac MRI findings
Table 2 summarizes cardiac MRI anatomic measures for the two groups. There were no significant difference in LV ejection fraction, end-diastolic volume, end-systolic volume or mass. Left atrial dimension was significantly higher in AF patients (p=0.018). No LV LGE was observed in any subjects.
Table 2.
Cardiac MRI measures
| AF patients, N=50 | Controls, N=11 | P-value | |
|---|---|---|---|
| Cine MRI parameters | |||
| LVEDV, mL | 158 ± 40 | 152 ± 39 | 0.64 |
| LVEDVI, mL/m2 | 77 ± 16 | 79 ± 14 | 0.79 |
| LVESV, mL | 63 ± 22 | 62 ± 19 | 0.75 |
| LVESVI, mL/m2 | 30 ± 9 | 32 ± 8 | 0.80 |
| LVSV, mL | 96 ± 24 | 90 ± 24 | 0.64 |
| LVSVI, mL/m2 | 47 ± 10 | 47 ± 8 | 0.83 |
| LVEF, % | 61 ± 6 | 60 ± 6 | 0.75 |
| LV mass, g | 97 ± 31 | 107 ± 41 | 0.36 |
| LV mass index, g/m2 | 47 ± 12 | 56 ± 16 | 0.59 |
| LA dimension, mm | 42 ± 11 | 34 ± 9 | 0.018 |
| LGE findings | |||
| Presence of LV LGE | 0 | 0 | - |
AF, atrial fibrillation; EDV, end-diastolic volume; EDVI, end-diastolic volume index; EF, ejection fraction; ESV, end-systolic volume; ESVI, end-systolic volume index; HR, heart rate; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricle; MRI, magnetic resonance imaging; RV, right ventricle; SV, stroke volume; SVI, stroke volume index
Native T1 time in AF patient and controls
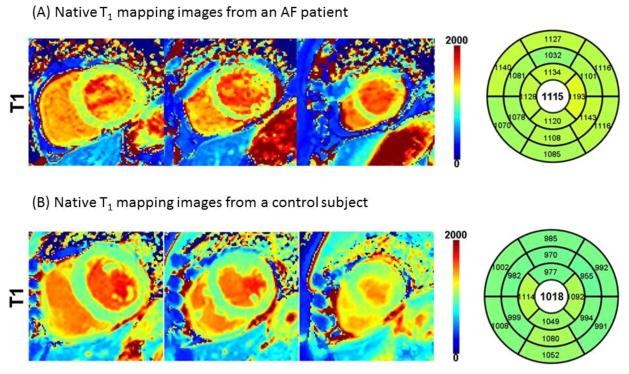
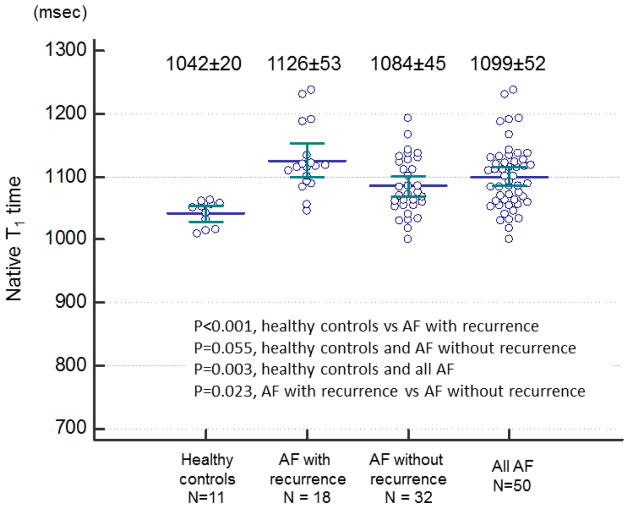
Example native T1 mapping images are shown in Figure 1. Figure 2 demonstrated comparison of individual native T1 time between AF patients and controls. Myocardial native T1 time was significantly elevated in patients with AF (1099±52 msec vs 1042±20 msec, p=0.003). Significant difference of LV native T1 time was found between healthy control and AF patients with recurrence (1042±20 msec vs 1126±53 msec, p<0.001), between AF patients with recurrence and AF patients without recurrence (1126±53 msec vs 1084±45 msec, p=0.023). However, no difference was found between healthy controls and AF without recurrence (1042±20 msec vs 1084±45 msec, p=0.055). Table 3 summarizes myocardial native T1 time for each slice. Native T1 time was elevated in all 3 slices in AF patients compared to normal subjects.
Figure 1. Native T1 mapping images from a 62 year-old male atrial fibrillation (AF) patient and a 64 year-old male control subject.
(A) AF patient. Native T1 time was elevated at 1115 msec. (B) Healthy control subject. Mean native T1 time was 1018 msec, and is within normal range.
Figure 2. Comparison of individual native T1 time in AF patients and healthy controls.
Significant difference of LV native T1 time was found between healthy control and AF patients with recurrence, between healthy controls and all AF patients, between AF patients with recurrence and AF patients without recurrence. However, no difference was found between healthy controls and AF without recurrence.
Table 3.
Native myocardial T1 time in each slice levels
| Base | Mid | Apex | +P value between slices | |
|---|---|---|---|---|
| AF patients, n=50 | 1093 ± 72 | 1100 ± 65 | 1109 ± 66 | 0.51 |
| Control subjects, n=11 | 1035 ± 25 | 1037 ± 32 | 1060 ± 25 | 0.075 |
| *P value (AF vs controls) | 0.010 | 0.003 | 0.018 |
Values are presented as mean ± SD.
P-values represent significance of difference between 3 slices (base, mid and apex) both in AF patients and controls (one way analysis of variance with Tukey’s correction).
P-values represent significance of difference between AF patients and controls in base, mid and apex.
AF, atrial fibrillation
Clinical follow-up
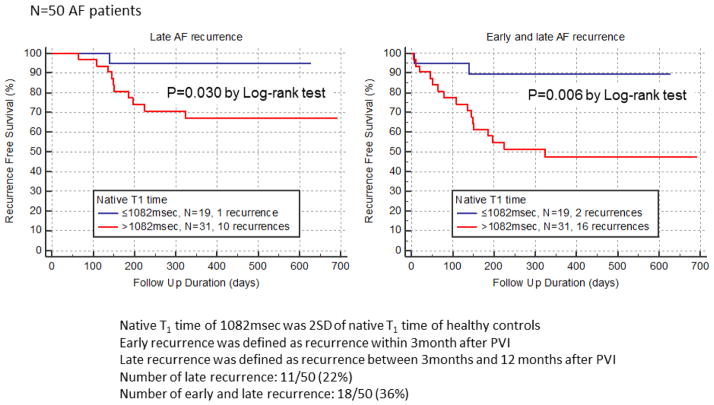
During a median follow-up duration of 326 days (range: 4–691 days), 18 of 50 (36%) AF patients experienced recurrence of AF after PVI (16 recurrences for patients with elevated LV T1 (>1082msec), 2 recurrences for patients with non-elevated LV T1 (≤1082msec)). Figure 3 illustrated Kaplan Meier event free survival curve for AF recurrence after PVI. Significant difference was observed between AF patients with elevated LV native T1 time and AF patients with non-elevated LV native T1 time both in late AF recurrence (p=0.030 by Log-rank test) and in early and late AF recurrence (p=0.006 by Log-rank test). Table 4 shows the results of multivariate Cox proportional hazard analysis for AF recurrence after PVI. Multivariate analysis demonstrated that native LV T1 time >1082msec is an independent and significant predictor of AF recurrence (hazard ratio: 6.53, 95%CI: 1.25–34.3, p=0.009)
Figure 3. Kaplan Meier event free survival curve for AF recurrence after pulmonary vein isolation.
AF recurrence rate was significantly higher in AF patients with native T1 time >1082msec than those with ≤1082msec both in late recurrence (p=0.030 by Log rank test) and in early and late recurrence (p=0.006).
Table 4.
Multivariate Cox proportional hazard analysis for AF recurrence after pulmonary vein isolation
| HR | 95% CI | P value | |
|---|---|---|---|
| Age, per years | 0.97 | 0.85 – 1.10 | 0.65 |
| Gender, male | 1.97 | 0.29 – 13.7 | 0.49 |
| Hypertension, yes | 0.23 | 0.033 – 1.64 | 0.14 |
| Dyslipidemia, yes | 1.67 | 0.29 – 9.57 | 0.57 |
| Obesity (BMI>25 kg/m2) | 0.79 | 0.16 – 3.81 | 0.76 |
| Family history of CAD, yes | 0.43 | 0.062 – 2.95 | 0.39 |
| LVEDVI, per mL/m2 | 1.12 | 0.77 – 1.64 | 0.56 |
| LVESVI, per mL/m2 | 0.89 | 0.52 – 1.51 | 0.66 |
| LVSVI, per ml/m2 | 0.89 | 0.60 – 1.30 | 0.54 |
| LVEF, per % | 1.06 | 0.75 – 1.49 | 0.76 |
| LV mass index, per g/m2 | 1.02 | 0.96 – 1.09 | 0.48 |
| LA dimension, per mm | 1.01 | 0.95 – 1.07 | 0.83 |
| CHA2DS2-VASc score, per 1 score | 1.67 | 0.87 – 3.19 | 0.12 |
| AF duration, per year | 1.04 | 0.91 – 1.18 | 0.58 |
| Native T1 time, >1082msec | 6.53 | 1.25 – 34.3 | 0.026 |
AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; EDVI, end-diastolic volume index; EF, ejection fraction; ESVI, end-systolic volume index; HR, hazard ratio; LA, left atrial; LV, left ventricle; OR, odds ratio
Reproducibility for measurement of native T1 time
The results of reproducibility for native T1 time measurements were summarized in Table 5. Both in AF patients and control subjects, high reproducibility of native T1 time measurements were observed for intra-observer reproducibility and inter-observer reproducibility.
Table 5.
Reproducibility of native T1 measurement in AF patients and healthy controls
| 10 healthy controls | 10 AF subjects | |||
|---|---|---|---|---|
| Intra-observer reproducibility | Inter-observer reproducibility | Intra-observer reproducibility | Inter-observer reproducibility | |
| Repeatability coefficient | 25msec | 27msec | 40msec | 46msec |
| Intra class correlation coefficient | 0.96 (95%CI: 0.85–0.99, p<0.05) | 0.95 (95%CI: 0.80–0.99, p<0.05) | 0.92 (95%CI: 0.87–0.95, p<0.05) | 0.91 (95%CI: 0.86–0.94, p<0.05) |
| Coefficient of Variation | 0.8% | 1.1% | 1.4% | 1.6% |
AF, atrial fibrillation; CI, confidence interval
Repeatability coefficients were calculated as 1.96 times the standard deviation of the differences on the Bland-Altman plots.
Discussion
In this prospective study of consecutive AF patients referred for MRI prior to PVI, we identified elevated native T1 time in AF patients as compared with control subjects. To the best of our knowledge, this is the first investigation to examine native T1 time in AF patients by using the MOLLI sequence. Furthermore, we found the significant difference of AF recurrence after PVI between patients with elevated LV T1 time and patients with non-elevated LV T1 time, suggesting that native LV T1 time might be useful for the risk stratification for AF recurrence after PVI in paroxysmal AF patients.
Diffuse left ventricular myocardial abnormality in AF patients detected by post-contrast and native T1 mapping
Several studies have demonstrated the clinical utility of post-contrast T1 mapping for the detection of diffuse myocardial abnormality in AF patients. Ling et al. showed that post-contrast T1 mapping can identify diffuse LV fibrosis in AF patients24. This report provided new insight into association between AF and adverse LV remodeling. Neilan et al. revealed that extra cellular volume quantification of LV myocardium is an independent predictor of AF recurrence in hypertensive AF patients who underwent PVI20. McLellan et al. demonstrated a shorter post-contrast T1 time of LV myocardium in patients with recurrent AF as compared to those without AF recurrence; post-contrast T1 time is an independent predictor of AF recurrence25. These reports showed the pathophysiological link between LV myocardial fibrosis detected by post contrast T1 mapping and atrial fibrillation. In the current study, we measured the LV native (non-contrast) myocardial T1 time in AF patients undergoing PVI and found a significant difference between AF patients and control subjects. Although the difference of native T1 time between AF patients and healthy controls was statistically significant, comparison of individual native T1 time showed substantial overlap between AF patients and healthy controls (13 of 50 (26%) AF patients were less than native T1 time of 1062msec, which is maximum native T1 time of healthy subjects), suggesting that native T1 time might not be able to differentiate AF patients from healthy controls in about one fourth of the AF population. However, overlap of native T1 time was small between healthy control and AF patients with recurrence (only 2 of 18 patients, Figure 2). Important finding of this study was that the elevated native T1 time was an independent and significant predictor of AF recurrence after PVI. These results indicated that LV native T1 time might be useful as a possible surrogate marker to detect high-risk AF patients who are going to develop AF recurrence after PVI in near future, rather than an isolated marker to differentiate abnormal LV myocardium of AF patient and healthy LV myocardium of normal subjects.
Possible mechanism of elevated native T1 time in AF patients
AF is known to be associated with various pathophysiologic disorders of cardiovascular system, including endothelial dysfunction26–30, inflammation and oxidative stress31–33, and atherosclerosis34–36. Freestone et al. demonstrated that endothelial dysfunction evaluated by flow-mediated dilatation is present in AF patients26. Lim et al. showed that catheter ablation and successful maintenance of sinus rhythm leads to a decrease in platelet activation and an improvement in endothelial function30. In addition, Li et al. revealed that inflammatory biomarkers such as Interleukin (IL)-6, IL-8, IL-10, tumor necrosis factors-alpha, monocyte chemoattractant protein 1, vascular endothelial growth factor, and N-terminal pro-brain natriuretic peptide were elevated in AF patients31. Another possible mechanism for elevated T1 time is myocardial edema. Previous studies showed that T1 time is substantially increased in acute myocardial edema in animal37 and human studies38. Ferreira et al. showed that native T1 mapping has a higher sensitivity compared with T2 weighted image and LGE image for detecting acute myocarditis38–40. Elevated native T1 time might be explained by myocardial edema, which could be related to high inflammatory activity in AF patients41. Although the evidence which clearly explains a mechanism of diffuse LV myocardial abnormality in AF patients is lacking, these cardiovascular pathophysiologic abnormalities may be associated with elevation of native T1 time observed in the current study.
Clinical Implication
Our AF population did not have LV systolic dysfunction on cine MRI and myocardial enhancement on LGE MRI in any study subjects. However, native T1 time was significantly elevated in AF patients and elevated native T1 time could predict future AF recurrence after PVI. This result was similar to the results of previous studies using post contrast T1 mapping images. However, the strong point of this study was that non-contrast (native) T1 mapping could assess myocardial T1 time even for AF patients with renal dysfunction at a high risk of nephrogenic systemic fibrosis42. Recent studies demonstrated the clinical utility of cardiac MRI and CT for AF ablation. Ang R. et al showed that pulmonary vein measurements on pre-procedural CT/MR imaging can predict difficult PVI and phrenic nerve injury during cryoballoon ablation43. Kim et al. reported that pericardial fat volume is associated with clinical recurrence after catheter ablation for persistent atrial fibrillation, but not paroxysmal atrial fibrillation44. Another study by Costa FM et al. revealed that left atrial volume is more important than the type of atrial fibrillation in predicting the long-term success of catheter ablation45. In the current study, the follow up duration for AF recurrence was relatively short, therefore, further study is necessary to investigate whether LV native T1 time is useful to predict AF occurrence in long term follow up period after PVI.
Study limitation
Our study has several limitations. First, this is single center study of a relatively small population of AF patients all referred for PVI. Second, we did not performed endomyocardial biopsy to confirm the presence of myocardial fibrosis. Third, although LGE MRI showed no myocardial infarction, we did not perform X-ray coronary angiography or computed tomography angiography to exclude coronary artery disease, which is common in this population. Forth, not all the AF patients and healthy control had implantable loop recorders. Therefore, asymptomatic AF recurrence might be missed.
Conclusion
Native LV myocardial T1 time is increased in AF patients with preserved LV function referred for PVI. Native T1 time is an independent predictor of recurrence of AF after PVI in patients with paroxysmal AF. These result suggested that LV native T1 time might be useful for the risk stratification to detect high risk AF patients for AF recurrence after PVI.
Footnotes
Disclosure information
Shingo Kato, MD receives scholarship from Banyu Life Science Foundation International Reza Nezafat, PhD receives grant support from NIH R01EB008743, 1R21HL127650, 1R01HL129185, AHA 15EIA22710040 and Samsung Electronics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 4.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the Manitoba Follow-up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 6.Avitall B, Bi J, Mykytsey A, Chicos A. Atrial and ventricular fibrosis induced by atrial fibrillation: Evidence to support early rhythm control. Heart Rhythm. 2008;5:839–845. doi: 10.1016/j.hrthm.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Nerheim P, Birger-Botkin S, Piracha L, Olshansky B. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004;110:247–252. doi: 10.1161/01.CIR.0000135472.28234.CC. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, Okamura K, Chinushi M, Furushima H, Tanabe Y, Kodama M, Aizawa Y. Clinical characteristics, treatment, and outcome of tachycardia induced cardiomyopathy. Int Heart J. 2008;49:39–47. doi: 10.1536/ihj.49.39. [DOI] [PubMed] [Google Scholar]
- 9.Lemola K, Khan R, Nattel S, Talajic M, Roy D, Guerra PG, Lemola S, Dubuc M, Thibault B, Macle L, Khairy P. Ventricular proarrhythmic effects of atrial fibrillation are modulated by depolarization and repolarization anomalies in patients with left ventricular dysfunction. Pacing Clin Electrophysiol. 2009;32:99–105. doi: 10.1111/j.1540-8159.2009.02182.x. [DOI] [PubMed] [Google Scholar]
- 10.Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:475–484. doi: 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK, Pica S, Castelletti S, Piechnik SK, Robson MD, Gilbertson JA, Rowczenio D, Hutt DF, Lachmann HJ, Wechalekar AD, Whelan CJ, Gillmore JD, Hawkins PN, Moon JC. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:157–165. doi: 10.1016/j.jcmg.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G, Fontana M, Maestrini V, Flett AS, Robson MD, Lachmann RH, Murphy E, Mehta A, Hughes D, Neubauer S, Elliott PM, Moon JC. Identification and assessment of anderson-fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging. 2013;6:392–398. doi: 10.1161/CIRCIMAGING.112.000070. [DOI] [PubMed] [Google Scholar]
- 13.Dall’Armellina E, Piechnik SK, Ferreira VM, Si QL, Robson MD, Francis JM, Cuculi F, Kharbanda RK, Banning AP, Choudhury RP, Karamitsos TD, Neubauer S. Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction. J Cardiovasc Magn Reson. 2012;14:15. doi: 10.1186/1532-429X-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng AC, Auger D, Delgado V, van Elderen SG, Bertini M, Siebelink HM, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A, Smit JW, Leung DY, Bax JJ, Lamb HJ. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T1 mapping and subclinical myocardial dysfunction in diabetic patients: A pilot study. Circ Cardiovasc Imaging. 2012;5:51–59. doi: 10.1161/CIRCIMAGING.111.965608. [DOI] [PubMed] [Google Scholar]
- 15.Doesch C, Dierks DM, Haghi D, Schimpf R, Kuschyk J, Suselbeck T, Schoenberg SO, Borggrefe M, Papavassiliu T. Right ventricular dysfunction, late gadolinium enhancement, and female gender predict poor outcome in patients with dilated cardiomyopathy. Int J Cardiol. 2014;177:429–435. doi: 10.1016/j.ijcard.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Roujol S, Weingartner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: A head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272:683–689. doi: 10.1148/radiol.14140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akcakaya M, Rayatzadeh H, Basha TA, Hong SN, Chan RH, Kissinger KV, Hauser TH, Josephson ME, Manning WJ, Nezafat R. Accelerated late gadolinium enhancement cardiac MR imaging with isotropic spatial resolution using compressed sensing: Initial experience. Radiology. 2012;264:691–699. doi: 10.1148/radiol.12112489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semelka RC, Tomei E, Wagner S, Mayo J, Kondo C, Suzuki J, Caputo GR, Higgins CB. Normal left ventricular dimensions and function: Interstudy reproducibility of measurements with cine MR imaging. Radiology. 1990;174:763–768. doi: 10.1148/radiology.174.3.2305059. [DOI] [PubMed] [Google Scholar]
- 19.Roujol S, Foppa M, Weingartner S, Manning WJ, Nezafat R. Adaptive registration of varying contrast-weighted images for improved tissue characterization (ARCTIC): Application to T1 mapping. Magn Reson Med. 2014;74:1469–1482. doi: 10.1002/mrm.25270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neilan TG, Mongeon FP, Shah RV, Coelho-Filho O, Abbasi SA, Dodson JA, McMullan CJ, Heydari B, Michaud GF, John RM, Blankstein R, Jerosch-Herold M, Kwong RY. Myocardial extracellular volume expansion and the risk of recurrent atrial fibrillation after pulmonary vein isolation. JACC Cardiovasc Imaging. 2014;7:1–11. doi: 10.1016/j.jcmg.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade JG, Khairy P, Macle L, Packer DL, Lehmann JW, Holcomb RG, Ruskin JN, Dubuc M. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: Insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) trial. Circ Arrhythm Electrophysiol. 2014;7:69–75. doi: 10.1161/CIRCEP.113.000586. [DOI] [PubMed] [Google Scholar]
- 22.Yarmohammadi H, Shenoy C. Cardiovascular magnetic resonance imaging before catheter ablation for atrial fibrillation: Much more than left atrial and pulmonary venous anatomy. Int J Cardiol. 2015;179:461–464. doi: 10.1016/j.ijcard.2014.11.085. [DOI] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 24.Ling LH, Kistler PM, Ellims AH, Iles LM, Lee G, Hughes GL, Kalman JM, Kaye DM, Taylor AJ. Diffuse ventricular fibrosis in atrial fibrillation: Noninvasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol. 2012;60:2402–2408. doi: 10.1016/j.jacc.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 25.McLellan AJ, Ling LH, Azzopardi S, Ellims AH, Iles LM, Sellenger MA, Morton JB, Kalman JM, Taylor AJ, Kistler PM. Diffuse ventricular fibrosis measured by T1 mapping on cardiac MRI predicts success of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:834–840. doi: 10.1161/CIRCEP.114.001479. [DOI] [PubMed] [Google Scholar]
- 26.Freestone B, Chong AY, Nuttall S, Lip GY. Impaired flow mediated dilatation as evidence of endothelial dysfunction in chronic atrial fibrillation: Relationship to plasma von Willebrand factor and soluble E-selectin levels. Thromb Res. 2008;122:85–90. doi: 10.1016/j.thromres.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Guazzi M, Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart. 2009;95:102–106. doi: 10.1136/hrt.2007.135277. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich JR, Kaluzny M, Baumann S, Lehmann R, Hohnloser SH. Biomarkers of structural remodelling and endothelial dysfunction for prediction of cardiovascular events or death in patients with atrial fibrillation. Clin Res Cardiol. 2011;100:1029–1036. doi: 10.1007/s00392-011-0337-9. [DOI] [PubMed] [Google Scholar]
- 29.Akar JG, Jeske W, Wilber DJ. Acute onset human atrial fibrillation is associated with local cardiac platelet activation and endothelial dysfunction. J Am Coll Cardiol. 2008;51:1790–1793. doi: 10.1016/j.jacc.2007.11.083. [DOI] [PubMed] [Google Scholar]
- 30.Lim HS, Willoughby SR, Schultz C, Chakrabarty A, Alasady M, Lau DH, Roberts-Thomson KC, Worthley MI, Young GD, Sanders P. Successful catheter ablation decreases platelet activation and improves endothelial function in patients with atrial fibrillation. Heart Rhythm. 2014;11:1912–1918. doi: 10.1016/j.hrthm.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, Darbar D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. doi: 10.1016/j.hrthm.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oral H. Post-operative atrial fibrillation and oxidative stress: A novel causal mechanism or another biochemical epiphenomenon? J Am Coll Cardiol. 2008;51:75–76. doi: 10.1016/j.jacc.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Yang KC, Dudley SC., Jr Oxidative stress and atrial fibrillation: Finding a missing piece to the puzzle. Circulation. 2013;128:1724–1726. doi: 10.1161/CIRCULATIONAHA.113.005837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willeit K, Pechlaner R, Egger G, Weger S, Oberhollenzer M, Willeit J, Kiechl S. Carotid atherosclerosis and incident atrial fibrillation. Arterioscler Thromb Vasc Biol. 2013;33:2660–2665. doi: 10.1161/ATVBAHA.113.302272. [DOI] [PubMed] [Google Scholar]
- 35.Agmon Y, Khandheria BK, Meissner I, Schwartz GL, Petterson TM, O’Fallon WM, Gentile F, Spittell PC, Whisnant JP, Wiebers DO, Covalt JL, Seward JB. Association of atrial fibrillation and aortic atherosclerosis: A population-based study. Mayo Clin Proc. 2001;76:252–259. doi: 10.4065/76.3.252. [DOI] [PubMed] [Google Scholar]
- 36.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Rooij FJ, Lip GY, Witteman JC. Subclinical atherosclerosis and risk of atrial fibrillation: The rotterdam study. Arch Intern Med. 2007;167:382–387. doi: 10.1001/archinte.167.4.382. [DOI] [PubMed] [Google Scholar]
- 37.Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596–603. doi: 10.1016/j.jcmg.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: A comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:42. doi: 10.1186/1532-429X-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S. T1 mapping for the diagnosis of acute myocarditis using CMR: Comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging. 2013;6:1048–1058. doi: 10.1016/j.jcmg.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Biesbroek PS, Beek AM, Germans T, Niessen HW, van Rossum AC. Diagnosis of myocarditis: Current state and future perspectives. Int J Cardiol. 2015;191:211–219. doi: 10.1016/j.ijcard.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015 doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 42.Thomsen HS. Esur guideline: Gadolinium-based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17:2692–2696. doi: 10.1007/s00330-007-0744-5. [DOI] [PubMed] [Google Scholar]
- 43.Ang R, Hunter RJ, Baker V, Richmond L, Dhinoja M, Sporton S, Schilling RJ, Pugliese F, Davies C, Earley M. Pulmonary vein measurements on pre-procedural ct/mr imaging can predict difficult pulmonary vein isolation and phrenic nerve injury during cryoballoon ablation for paroxysmal atrial fibrillation. Int J Cardiol. 2015;195:253–258. doi: 10.1016/j.ijcard.2015.05.089. [DOI] [PubMed] [Google Scholar]
- 44.Kim TH, Park J, Park JK, Uhm JS, Joung B, Lee MH, Pak HN. Pericardial fat volume is associated with clinical recurrence after catheter ablation for persistent atrial fibrillation, but not paroxysmal atrial fibrillation: An analysis of over 600-patients. Int J Cardiol. 2014;176:841–846. doi: 10.1016/j.ijcard.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Costa FM, Ferreira AM, Oliveira S, Santos PG, Durazzo A, Carmo P, Santos KR, Cavaco D, Parreira L, Morgado F, Adragao P. Left atrial volume is more important than the type of atrial fibrillation in predicting the long-term success of catheter ablation. Int J Cardiol. 2015;184:56–61. doi: 10.1016/j.ijcard.2015.01.060. [DOI] [PubMed] [Google Scholar]