Abstract
Purpose
Gestational diabetes mellitus (GDM) continues to be a significant health disorder triggering harmful complications in pregnant women and fetuses. Our knowledge of GDM epidemiology in Yemen is largely based on very limited data. The aim of this study was, therefore, to determine the prevalence and risk factors of GDM among pregnant women in Dhamar governorate, Yemen.
Patients and methods
A total of 311 subjects were randomly selected for this cross sectional survey. Health history data and blood samples were collected using a pretested questionnaire. To determine the prevalence of GDM, the fasting and random blood glucose techniques were applied according to the recommendations of the American Diabetes Association, using alternative methods that are more convenient to the targeted population. Poisson’s regression model incorporating robust sandwich variance was utilized to assess the association of potential risk factors in developing GDM.
Results
The prevalence of GDM was found to be 5.1% among the study population. Multivariate analysis confirmed age ≥30 years, previous GDM, family history of diabetes, and history of polycystic ovary syndrome as independent risk factors for GDM prevalence. However, body mass index ≥30 kg/m2 and previous macrosomic baby were found to be dependent risk factors.
Conclusion
This study reports new epidemiological information about the prevalence and risk factors of GDM in Yemen. Introduction of proper maternal and neonatal medical care and health education are important in order to save the mother and the baby.
Keywords: gestational diabetes mellitus, alternative diagnostic criteria, prevalence risk, Yemen
Introduction
Gestational diabetes mellitus (GDM) is defined as “carbohydrate intolerance resulting in hyperglycemia of variable severity with onset or first recognition during pregnancy”.1 Maternal hyperglycemia causes fetal hyperinsulinemia.2 Many maternal and fetal adverse effects are associated with this carbohydrate disorder, such as fetal macrosomia, perinatal mortality, cesarean delivery, and preeclampsia.3,4 Later in life, this affected community tends to suffer from more complications, such as type 2 diabetes mellitus and obesity, however.5–7 Early diagnosis of GDM is, therefore, imperative to avoid such health problems.
Published reports show variations in the prevalence rates of GDM in most countries of the Arabian Peninsula, which comprises two distinct economies: the high-economy Gulf Cooperation Countries (GCC), and the poor, neglected Yemen. GDM was reported to vary between 4.2% and 24.9% in the GCC countries of Oman, Qatar, Bahrain, Kuwait, Saudi Arabia, and the United Arab Emirates.8–15 In Yemen, however, there are no epidemiological results reported in the literature, PubMed and Google Scholar databases, about the prevalence and risk factors of GDM.
Several health factors increase the risk of developing GDM: for example, older age, previous GDM, body mass index (BMI) >30 kg/m2, family history of diabetes, previous macrosomic baby weighing ≥4.5 kg, and ethnicity of high prevalence, particularly South Asian, black Caribbean, and Middle Eastern.16 In addition, history of polycystic ovary syndrome (PCOS), glycosuria in current pregnancy, history of chronic hypertension, and previous stillbirth were indicated as significant predictors.17–19 Other risk factors were also reported; for a detailed review, see the clinical guideline of National Collaborating Centre for Women’s and Children’s Health.6
Although the 75 g oral glucose tolerance test (OGTT) is the most reliable diagnostic technique for confirming GDM, using this method has been confirmed to be time consuming and expensive and to induce discomfort in the patients and the health providers.20 Recently, the American Diabetes Association (ADA) recommended alternative procedures that are more adaptable. The ADA suggested that GDM can be confirmed without an OGTT when on 2 consecutive days a pregnant woman’s fasting blood glucose (FBG) level is >126 mg/dL or random blood glucose (RBG) level is >200 mg/dL.21 More details on this topic can be found in the Handbook of Clinical Laboratory Testing During Pregnancy.20
However, the early recognition of GDM prevalence is essential for considering the size of this health problem, taking the curative measures, and increasing the awareness of vulnerable women regarding the risk factors that would be important to prevent or at least decrease the risk of adverse outcome. Therefore, the present study aimed to determine the prevalence of GDM using the FBG and RBG techniques and to investigate the potential risk factors among pregnant women in Dhamar governorate, Yemen.
Patients and methods
Study area and subjects
The study was conducted in Dhamar governorate. It is located ~100 km south of Sana’a, the capital city of Yemen. The area is situated at an altitude of ~1,600–3,200 m with average temperature ranging from −1°C to 19°C. The economy is mostly agricultural. Dhamar governorate comprises 12 rural districts, and Dhamar city houses the main health facilities. The health centers associated with antenatal care clinics that were included in this study are Dhamar General Hospital, Maternal and Child Health Center, Mehrass Dispensary for Gynecology and Obstetrics, and Dar Al Shifa Hospital.
A total of 311 nondiabetic pregnant women participated in this study. They were aged 15–49 years with a gestational age ranging from 24 to 40 weeks. Before commencing data collection, informative meetings were held with the subjects in order to give a clear description of the aim of the study. All study subjects gave verbal consent. They were informed that their involvement was completely voluntary and they could decline to contribute at any point during the survey. The protocol of the study had been approved by the ethics committee of the faculty, Thamar University Medical Ethics Committee.
Study design
This was a cross sectional study carried out between August 2013 and March 2014. The sample size was calculated based on the middle prevalence value of GDM in the Arabian Peninsula (10%) with a 95% confidence level and a ±3.4 degree of precision. The study subjects were randomly selected from the antenatal care clinics using a systematic sampling method. Several visits were made to the antenatal care clinics, where demographic and anthropometric data and blood samples were collected from the participants by trained health staff. To determine the prevalence of GDM, the methods of FBG and RBG were used for diagnosing the blood samples according to the ADA criteria.20,21 A pregnant woman with GDM was confirmed if on 2 consecutive days, the level of her FBG or RBG was >126 or 200 mg/dL, respectively. As stated in the “Introduction” section, many health history factors increase the risk of developing GDM. Hence, the differences between the pregnant women groups (women with risk factors and women with no risk factors) were calculated with reference to the prevalence of GDM.
Questionnaire
The pregnant women selected for the study were interviewed, in their antenatal care clinics settings, using a pretested questionnaire constructed in Arabic, the native language of the participants. The questions were designed to gather information on their health history. Information on the following potential risk factors was collected during this survey: age ≥35 years, BMI ≥30 kg/m2, previous GDM, family history of diabetes, previous macrosomic baby, previous stillbirth, and history of PCOS.
Anthropometric assessment
Height and weight of all the participants were measured and recorded. The height was measured to the nearest 0.1 cm without shoes or any other tampered material with the participant standing on a flat surface. Pregravid weight was recorded to the nearest 0.1 kg. The BMI (kg/m2) was then calculated.
Biochemical screening
The collected blood samples were centrifuged, and blood glucose was immediately examined in the antenatal care clinic settings. Two analytical procedures were used, and the mean was calculated: glucose oxidase method (Lab Kit, Madrid, Spain) using a spectrophotometer RT-9200 semiautomatic chemistry analyzer and hexokinase method (Roche Diagnostics, GmbH, Mannheim, Germany) using a COBAS/INTEGRA 400/400 plus system.22,23 The operational descriptions of the FBG and RBG samples were as the following, respectively: no caloric intake for at least 8 hours; and any time of the day without regard to the time since the last meal.
Data analysis
The present study used the IBM SPSS (Version 22.0) statistical software for analysis of the data. General characteristics of the study participants were presented as a mean ± standard error of the mean (SEM) or percentage (%). For the data analysis, all the study variables were dichotomously evaluated. They were GDM prevalence (FBG ≤126 mg/dL or RBG ≤200 mg/dL =0 and FBG >126 mg/dL or RBG >200 mg/dL =1), age (<35 years =0 and ≥35 years =1), BMI (<30 kg/m2 =0 and ≥30 kg/m2 =1), previous GDM (no =0 and yes =1), family history of diabetes (no =0 and yes =1), previous macrosomic baby (no =0 and yes =1), previous stillbirth (no =0 and yes =1), and history of PCOS (no =0 and yes =1). Univariate analysis was used to examine the association between GDM as the dependent variable and the health history as the explanatory variables. Variables that showed an association with GDM at a P-value of ≤0.2 were used to adjust the multivariate regression model in order to evaluate their independent effect in developing GDM.24,25 According to the recent literature of regression models that are most appropriate for the cross sectional studies, Poisson’s regression model incorporating the robust sandwich variance was used to estimate the prevalence risk (PR).26,27 As a result, the estimated risk (ER) of GDM was then calculated (ER = PR −1). P≤0.05 was considered as the level of significance.
Results
General characteristics of the participants
A total of 311 pregnant women aged 15–49 years participated in this survey. The mean (±SEM) of age and BMI were 25.14 years (±0.37) and 24.61 kg/m2 (±0.19), respectively. Table 1 shows the general health characteristics of the subjects according to the frequency (%) of the potential risk factors of GDM. The most frequent variables were previous stillbirth (29.6%) and family history of diabetes (23.2%). Accordingly, 12.5% of the pregnant women were ≥35 years, 12.2% had previous macrosomic baby, and 10% reported history of PCOS, whereas 5.8% women were obese (BMI ≥30 kg/m2) and 2.9% reported previous GDM.
Table 1.
General health characteristics of the pregnant women participated in the study (N=311)
| Characteristics | Values |
|---|---|
| Physical characteristicsa | |
| Age (years) | 25.14±(0.37) |
| Weight (kg) | 60.66±(0.50) |
| Height (cm) | 156.92±(0.42) |
| BMI (kg/m2) | 24.61±(0.19) |
| Frequency of the potential risk factors of GDMb | |
| Age ≥35 years | 39 (12.5) |
| BMI ≥30 kg/m2 | 18 (5.8) |
| Previous GDM | 9 (2.9) |
| Family history of diabetes | 72 (23.2) |
| Previous macrosomic baby | 38 (12.2) |
| Previous stillbirth | 92 (29.6) |
| History of PCOS | 31 (10.0) |
Notes:
Values are in mean ± (SEM).
Values are in number (%).
Abbreviations: BMI, body mass index; GDM, gestational diabetes mellitus; PCOS, polycystic ovary syndrome; SEM, standard error of the mean.
Prevalence of GDM
Figure 1 shows the prevalence of GDM among the study participants in Dhamar governorate, Yemen. GDM was diagnosed in 16 (5.1%) women, based on the ADA alternative methods of FBG and RBG.
Figure 1.
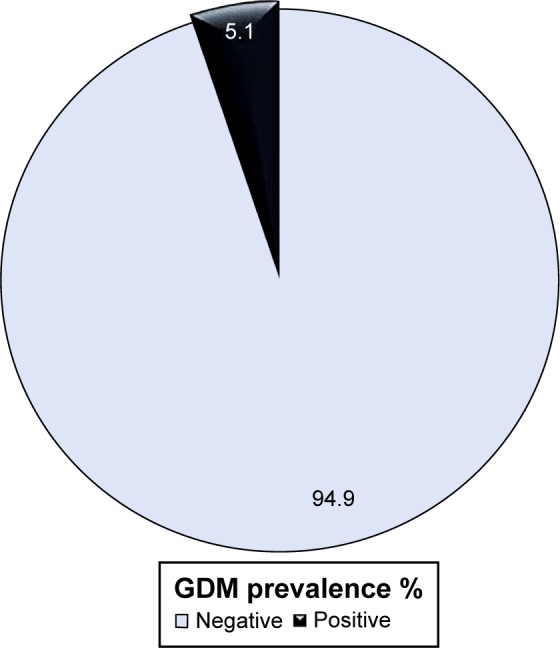
Prevalence (%) of GDM among the pregnant women in Dhamar governorate, Yemen (N=311).
Abbreviation: GDM, gestational diabetes mellitus.
Association of the potential risk factors with GDM
Dependent effect of the potential risk factors
The univariate regression analysis revealed a significant association between GDM and age ≥35 years, BMI ≥30 kg/m2, previous GDM, family history of diabetes, previous macrosomic baby, and history of PCOS (Table 2). Previous stillbirth, however, emerged as an insignificant risk factor for GDM. In the light of this analysis, the PR of GDM increases by 8.97 times by age ≥35 years (GDM =23.1 vs 2.6; P<0.001); by as much as 3.76 times among the BMI group ≥30 kg/m2 (GDM =16.7 vs 4.4; P=0.026); by 26.10 times in pregnant women who reported previous GDM (GDM =77.8 vs 3.0; P<0.001); and by 7.30, 4.31, and 3.01 times in those with a family history of diabetes (GDM =15.3 vs 2.1; P<0.001), a previous macrosomic baby (GDM =15.8 vs 3.7; P=0.003), and a history of PCOS (GDM =12.9 vs 4.3; P=0.043), respectively. Because all the health variables, including previous stillbirth, showed P-values of ≤0.2, they were used to develop the multivariate regression model.
Table 2.
Univariate analysis for the potential risk factors of GDM among the pregnant women in Dhamar area, Yemen (N=311)
| Potential risk factors | GDM prevalence, category (%) | PR | P-valuea | |
|---|---|---|---|---|
| Age ≥35 years | ≥35 (23.1) | <35 (2.6) | 8.97 | <0.001 |
| BMI ≥30 kg/m2 | ≥30 (16.7) | <30 (4.4) | 3.76 | 0.026 |
| Previous GDM | Yes (77.8) | No (3.0) | 26.10 | <0.001 |
| Family history of diabetes | Yes (15.3) | No (2.1) | 7.30 | <0.001 |
| Previous macrosomic baby | Yes (15.8) | No (3.7) | 4.31 | 0.003 |
| Previous stillbirth | Yes (7.6) | No (4.1) | 1.85 | 0.207 |
| History of PCOS | Yes (12.9) | No (4.3) | 3.01 | 0.043 |
Note:
Level of significance was determined by Poisson’s regression univariate model incorporating the RSV.
Abbreviations: BMI, body mass index; GDM, gestational diabetes mellitus; PCOS, polycystic ovary syndrome; PR, prevalence risk; RSV, robust sandwich variance.
Independent effect of the potential risk factors
The independent effect of each potential risk factor on the prevalence of GDM is shown in Table 3. The multivariate analysis confirmed age (PR =4.29; P=0.005), previous GDM (PR =10.18; P<0.001), family history of diabetes (PR =3.48; P=0.049), and history of PCOS (PR =2.66; P=0.046) as independent risk factors for developing GDM among the study participants. BMI ≥30 kg/m2 and a previous macrosomic baby, however, emerged as dependent risk factors (P>0.05).
Table 3.
Multivariate analysis for the potential risk factors of GDM among the pregnant women in Dhamar, Yemen (N=311)
| Potential risk factors | Adjusted PR | 95% CI | P-valuea |
|---|---|---|---|
| Age ≥35 years | 4.29 | 1.54–11.92 | 0.005 |
| BMI ≥30 kg/m2 | 1.26 | 0.27–5.81 | 0.771 |
| Previous GDM | 10.18 | 4.26–24.32 | <0.001 |
| Family history of diabetes | 3.48 | 1.01–12.06 | 0.049 |
| Previous macrosomic baby | 1.38 | 0.56–3.37 | 0.481 |
| Previous stillbirth | 1.03 | 0.44–2.40 | 0.950 |
| History of PCOS | 2.66 | 1.02–6.93 | 0.046 |
Note:
Level of significance was determined by Poisson’s regression multivariate model incorporating the RSV adjusted by age, BMI, previous GDM, family history of diabetes, previous macrosomic baby, previous stillbirth, and history of PCOS.
Abbreviations: BMI, body mass index; CI, confidence interval; GDM, gestational diabetes mellitus; PCOS, polycystic ovary syndrome; PR, prevalence risk; RSV, robust sandwich variance.
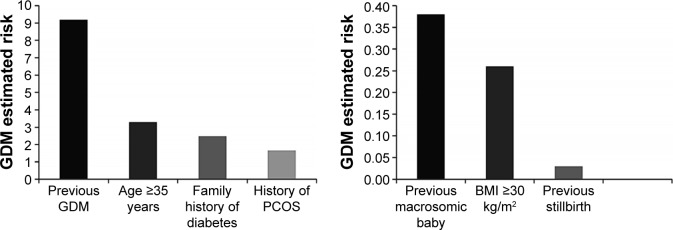
GDM estimated risk
Figure 2 shows the adjusted ER of developing GDM among Yemeni pregnant women in Dhamar governorate, Yemen. Previous GDM showed the strongest association (ER =9.18), followed by age ≥35 years (ER =3.29), family history of diabetes (ER =2.48), and history of PCOS (ER =1.66). Accordingly, a lower degree of GDM risk was shown by previous macrosomic baby (ER =0.38), BMI ≥30 kg/m2 (ER =0.26), and previous stillbirth (ER =0.03).
Figure 2.
Estimated risk for developing GDM among the pregnant women in Dhamar governorate, Yemen (N=311).
Abbreviations: BMI, body mass index; GDM, gestational diabetes mellitus; PCOS, polycystic ovary syndrome.
Discussion
The main objectives of this survey were to determine the prevalence of GDM and predict the potential risk factors among the pregnant women in Yemen. The present study revealed that the prevalence rate of GDM is 5.1% among the study population in Dhamar governorate. This result is both in conflict and in agreement with some studies conducted in the neighboring countries of Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates. In general, the prevalence rate observed in this survey is related to the universal range varying from 3% to 14% among all pregnancies in different populations.28 Although not all the potential risk factors showed independent and/or significant associations with GDM, the effect of these risks could be influenced by moderating or confounding variables.
Although the OGTT was not performed, this work is the first step toward enhancing our knowledge of GDM prevalence and its risk factors in Yemen. The study presented a lower rate of GDM prevalence than those reported in most of the neighboring GCC countries, ranging from 10.1% to 24.9%.10–15 However, it has a number of similarities with the results of the studies by Barakat et al (4.2%) in Oman and Al-Kuwari and Al-Kubaisi (6.4%) in Qatar.8,9 A recent review study examined the factors associated with GDM care in these countries,29 which showed that the reported variations in the prevalence rates of GDM could be attributed to multiple factors, such as the use of different criteria of diagnosis, increase in the economic migration of multiethnic population, increase in the rates of obesity, and limitation of the conducted surveys. The circumstances in Yemen are completely different and cannot be compared to those of the high-income GCC countries. Food insecurity and malnutrition are chronic stress factors in Yemen. According to the United Nations World Food Program, Yemen is on the edge of famine, with almost half of the Yemeni people being food insecure.30 Yemen also has one of the highest rates of malnutrition in the world.31 Moreover, at the time of writing, Yemeni people are facing a destructive regional war and global terrorism, which is pushing the economy and health services to serious collapse.
In agreement with previous reports around the world, the following health variables have been found to be significant risk factors for GDM during the univariate analysis: age ≥35 years, BMI ≥30 kg/m2, previous GDM, family history of diabetes, previous macrosomic baby, and history of PCOS.16–18 Although the outcome of the adjusted multivariate model showed BMI ≥30 kg/m2 and previous macrosomic baby as dependent risks for GDM, the probability that these variables had regulated the independent association of the other variables is statistically accepted. Kew et al concluded that a prior pregnancy that resulted in a macrosomic baby is not necessarily presumptive evidence of undiagnosed GDM but may be a result of the influence of other predictors, such as obesity.32 A previous study from Iran reported stillbirth during previous pregnancy as a significant predictor for GDM.19 But this association was not revealed in this survey, which showed similar outcomes as those of a multicenter, randomized, controlled trial that was conducted on 440 pregnant women (220 GDM and 220 controls) in Saudi Arabia.33
The results of the present study revealed previous GDM, age ≥35 years, family history of diabetes, and history of PCOS as the strongest predictors for developing GDM. Evidences from earlier surveys indicated that previous GDM and age ≥35 years are more associated with GDM than the other risk factors.10,34 Family history of diabetes was concluded in several cross sectional and prospective studies as a highly significant risk for developing GDM.35,36 Wang et al found that 54.9% of the incidence of GDM was significantly found among pregnant women with PCOS compared to 14.3% of those in the control group.18 Therefore, recognizing such risk factors among pregnant women in Yemen is important and should be done by the medical staff in order to prevent the adverse effects of GDM. However, women with no risk factors are also at risk of developing GDM. Low sensitivities and specificities had been produced when risk factors were used alone as a screening test.37 A study by Moses et al detected GDM in 39.2% of the pregnant women with no risk factors.38 The present study gives information about the risks of GDM that can help improve primary health care measures. However, all pregnant women should be examined to see whether they carry the risk factors.
Conclusion
To the best of our knowledge, this is the first report showing the prevalence of GDM in Yemen. GDM is seen to affect ~5% of the population. The results indicated previous GDM, age ≥35 years, family history of diabetes, and history of PCOS as independent risk factors. BMI ≥30 kg/m2 and previous macrosomic baby increased the risk of GDM but in a dependent manner. However, previous stillbirth was an insignificant variable. The results of the present study highlight the importance of early screening, diagnosis, and treatment of this maternal health concern. Introduction of proper educational programs about the risk factors of GDM is an essential preventive measure to decrease the risk of adverse perinatal outcome. Further studies in different regions of Yemen are recommended to reveal the general prevalence of GDM in the country.
Acknowledgments
The authors would like to thank the following fourth-year students of the Medical Laboratories Sciences (academic year 2012/2013) for their help in the collection of the study data: M Al-Mutahher, M AL-Halani, G AL-Mohalla, M Al-Madwamy, S AL-Manarri, S Saleh, A Mana, M Al-Marhabi, H Zyad, S Hassan, A Daban, B Al-Mogad, E Naser, A AL-Athraay, and Y Al-Haj. The authors would also like to acknowledge the staff of Dhamar public and private hospitals and health centers for their generous support and cooperation in the collection and analysis of the study data. The efforts of the pregnant women for their participation in this study are highly appreciated. This work was financially supported by the Faculty of Medicine and Health Sciences, Thamar University.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954;16(4):330–342. doi: 10.1530/acta.0.0160330. [DOI] [PubMed] [Google Scholar]
- 3.Crowther CA, Hiller JE, Moss JR, et al. For ACHOIS Trial Group Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 4.Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Aust N Z J Obstet Gynaecol. 2007;47(4):307–312. doi: 10.1111/j.1479-828X.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 5.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long term effects of the intrauterine environment. Northwestern university diabetes in pregnancy center. Diabetes Care. 1998;21(suppl 2):B142–B149. [PubMed] [Google Scholar]
- 6.National Collaborating Centre for Women’s and Children’s Health . Diabetes in Pregnancy: Management of Diabetes and Its Complications from Preconception to the Postnatal Period. London: RCOG Press; 2008. [PubMed] [Google Scholar]
- 7.Lee H, Jang HC, Park HK, Metzger BE, Cho NH. Prevalence of type 2 diabetes among women with a previous history of gestational diabetes mellitus. Diabetes Res Clin Pract. 2008;81(1):124–129. doi: 10.1016/j.diabres.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Barakat MN, Youssef RM, Al-Lawati JA. Pregnancy outcomes of diabetic women: charting Oman’s progress towards the goal of the Saint Vincent declaration. Ann Saudi Med. 2010;30(4):265–270. doi: 10.4103/0256-4947.65253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Kuwari MG, Al-Kubaisi SB. Prevalence and predictors of gestational diabetes in Qatar. Diabetol Croat. 2011;40:65–70. [Google Scholar]
- 10.Bener A, Saleh NM, Al-Hamaq A. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast-developing community: global comparisons. Int J Womens Health. 2011;3:367–373. doi: 10.2147/ijwh.s26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajab KE, Issa AA, Hasan ZA, Rajab E, Jaradat AA. Incidence of gestational diabetes mellitus in Bahrain from 2002–2012. Int J Gynaecol Obstet. 2012;117(1):74–77. doi: 10.1016/j.ijgo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Sultan F, Anan G, Ahmed S. Clinical epidemiology of gestational diabetes in Kuwait. Kuwait Med J. 2004;36:195–198. [Google Scholar]
- 13.Ardawi MS, Nasrat HA, Jamal HS, Al-Saqaaf HM, Mustafa BE. Screening for gestational diabetes mellitus in pregnant females. Saudi Med J. 2000;21(2):155–160. [PubMed] [Google Scholar]
- 14.Wahabi HA, Fayed AA, Alzeidan RA, Mandil AA. The independent effects of maternal obesity and gestational diabetes on the pregnancy outcomes. BMC Endocr Disord. 2014;14:47. doi: 10.1186/1472-6823-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal MM, Dhatt GS, Shah SM. Gestational diabetes mellitus: simplifying the international association of diabetes and pregnancy diagnostic algorithm using fasting plasma glucose. Diabetes Care. 2010;33(9):2018–2020. doi: 10.2337/dc10-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association Gestational diabetes mellitus. Diabetes Care. 2000;23:77–79. [Google Scholar]
- 17.Ashrafi M, Sheikhan F, Arabipoor A, Hosseini R, Nourbakhsh F, Zolfaghari Z. Gestational diabetes mellitus risk factors in women with polycystic ovary syndrome (PCOS) Eur J Obstet Gynecol Reprod Biol. 2014;181:195–199. doi: 10.1016/j.ejogrb.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhao X, Zhao H, et al. Risks for gestational diabetes mellitus and pregnancy-induced hypertension are increased in polycystic ovary syndrome. Biomed Res Int. 2013;2013:182582. doi: 10.1155/2013/182582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soheilykhah S, Mogibian M, Rahimi-Saghand S, Rashidi M, Soheilykhah S, Piroz M. Incidence of gestational diabetes mellitus in pregnant women. Iran J Reprod Med. 2010;8(1):24–28. [Google Scholar]
- 20.Dukes WJ, Chen AC, Jovanovic L. Diabetes in pregnancy. In: Gronowski AM, editor. Handbook of Clinical Laboratory Testing During Pregnancy. Totowa, NJ: Humana Press Inc; 2004. pp. 359–390. [Google Scholar]
- 21.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2002;25(suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 22.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor. Ann Clin Biochem. 1969;6:24–27. [Google Scholar]
- 23.Kaplan LA. Glucose. In: Pesce AJ, Kaplan LA, Pesce AJ, editors. Clinical Chemistry: Theory, Analysis, and Correlation. St Louis, MO: Mosby; 1984. pp. 1032–1036. [Google Scholar]
- 24.Bendel RB, Afifi AA. Comparison of stopping rules in forward regression. J Am Stat Assoc. 1977;72(357):46–53. [Google Scholar]
- 25.Groenwold RHH, Klungel OH, Grobbee DE, Hoes AW. Selection of confounding variables should not be based on observed associations with exposure. Eur J Epidemiol. 2011;26(8):589–593. doi: 10.1007/s10654-011-9606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Tan CS, Chia KS. A practical guide for multivariate analysis of dichotomous outcomes. Ann Acad Med Singapore. 2009;38(8):714–719. [PubMed] [Google Scholar]
- 27.Coutinho LM, Scazufca M, Menezes PR. Methods for estimating prevalence ratios in cross sectional studies. Rev Saude Publica. 2008;42(6):992–998. [PubMed] [Google Scholar]
- 28.Benhalima K, Crombrugge PV, Hanssens M, Devlieger R, Verhaeghe J, Mathieu C. Gestational diabetes: overview of the new consensus screening strategy and diagnostic criteria. Acta Clin Belg. 2012;67(4):255–261. doi: 10.2143/ACB.67.4.2062669. [DOI] [PubMed] [Google Scholar]
- 29.Okunoye G, Konje J, Lindow S, Perva S. Gestational diabetes in the gulf region: streamlining care to optimise outcome. J Local Global Health Sci. 2015;2015:2. [Google Scholar]
- 30.United Nations World Food Programme (WFP) [webpage on the internet] Yemen Emergency. [Accessed September 27, 2015]. Available from: http://m.wfp.org/emergencies/yemen.
- 31.United Nations World Food Programme (WFP) [webpage on the internet] Yemen Comprehensive Food Security Survey. Sana’a. WFP; [Accessed December 17, 2015]. Available from: http://documents.wfp.org/stellent/groups/public/documents/ena/wfp219039.pdf. [Google Scholar]
- 32.Kew S, Ye C, Sermer M, et al. Postpartum metabolic function in women delivering a macrosomic infant in the absence of gestational diabetes mellitus. Diabetes Care. 2011;34(12):2608–2613. doi: 10.2337/dc11-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasim T. Gestational diabetes mellitus: maternal and perinatal outcomes in 220 Saudi women. Oman Med J. 2012;27(2):140–144. doi: 10.5001/omj.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostlund I, Hanson U. Occurrence of gestational diabetes mellitus and the value of different screening indicators for the oral glucose tolerance test. Acta Obstet Gynecol Scand. 2003;82(2):103–108. doi: 10.1034/j.1600-0412.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 35.Hossein-Nezhad A, Maghbooli Z, Vassigh AR, Larijani B. Prevalence of gestational diabetes mellitus and pregnancy outcomes in Iranian women. Taiwan J Obstet Gynecol. 2007;46(6):236–241. doi: 10.1016/S1028-4559(08)60026-1. [DOI] [PubMed] [Google Scholar]
- 36.Bhat M, Ramesha KN, Sarma SP, Menon S, Sowmini CV, Ganesh KS. Determinants of gestational diabetes mellitus: a case control study in a district tertiary care hospital in south India. Int J Diabetes Dev Ctries. 2010;30(2):91–96. doi: 10.4103/0973-3930.62599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott A, Loveman E, McIntyre L, Waugh N. Screening for gestational diabetes: a systematic review and economic evaluation. Health Technol Assess. 2002;6(11):1–161. doi: 10.3310/hta6110. [DOI] [PubMed] [Google Scholar]
- 38.Moses R, Griffiths R, Davis W. Gestational diabetes: do all women need to be tested? Aust N Z J Obstet Gynaecol. 1995;35(4):387–389. doi: 10.1111/j.1479-828x.1995.tb02148.x. [DOI] [PubMed] [Google Scholar]