Abstract
Emerging behavioral and neuroimaging studies in schizophrenia (SCZ) and major depressive disorder (MD) are mapping mechanisms of co-occurring and distinct affective disturbances across these disorders. This constitutes a critical goal towards developing rationally guided therapies for upstream neural pathways that contribute to comorbid symptoms across disorders. We highlight the current state of the art in our understanding of emotional dysregulation in SCZ versus MD by focusing on broad domains of behavioral function that can map onto underlying neural systems, namely deficits in hedonics, anticipatory behaviors, computations underlying value and effort, and effortful goal-directed behaviors needed to pursue rewarding outcomes. We highlight unique disturbances in each disorder that may involve dissociable neural systems, but also possible interactions between affect and cognition in MD versus SCZ. Finally, we review computational and translational approaches that offer mechanistic insight into how cellular-level disruptions can lead to complex affective disturbances, informing development of therapies across MD and SCZ.
Keywords: schizophrenia, major depression, affect, cognitive deficit, neuroimaging, cognitive neuroscience, computational modeling, translational mechanism
Abstract
Los nuevos estudios conductuales y de neuroimágenes en la esquizofrenia (EQZ) y en el trastorno depresivo mayor (TDM) permiten mapear las alteraciones afectivas concomitantes y específicas en estos trastornos. Esto constituye un objetivo fundamental para el desarrollo racional de terapias que actùen a nivel de las vías neurales ascendentes que contribuyen a los síntomas comórbidos en estos trastornos. Se pone de relieve el conocimiento actual del arte acerca del conocimiento de la desregulación emocional en la EQZ versus el TDM al centrarse en grandes areas del funcionamiento conductual que puedan mapear los sistemas neurales subyacentes, como los déficits en la hedónica, las conductas anticipatorias, los cálculos implícitos al valor y al esfuerzo, y las conductas dirigidas a un objetivo que requieren de un necesario esfuerzo para perseguir resultados gratificantes. Se destacan las alteraciones características en cada trastorno que pueden involucrar sistemas neurales disociables, como también posibles interacciones entre afecto y cognición en el TDM versus la EQZ. Por último se revisan enfoques computacionales y translacionales que ofrecen visiones mecanicistas de cómo las alteraciones a nivel celular pueden llevar a complejas alteraciones afectivas, que inspiren el desarrollo de terapias para el TDM y la EQZ.
Abstract
Les nouvelles études de comportement et de neuro-imagerie dans la schizophrénie (SCZ) et le trouble dépressif caractérisé (TDC) permettent de cartographier les perturbations affectives concomitantes ou particulières de ces maladies. C'est un enjeu essentiel du développement rationnel des traitements agissant sur les voies neuronales d'amont qui contribuent aux symptômes comorbides de ces troubles. Nous soulignons l'état actuel des techniques de compréhension du dérèglement émotionnel dans la SCZ versus le TDC en insistant sur les vastes domaines du fonctionnement comportemental qui peuvent être identifiés sur les cartographies des systèmes neuronaux sous-jacents, c'est-a-dire les déficits des comportements hédoniques et anticipatoires, les calculs sous-tendant la valeur et l'effort, ainsi que les comportements volontaires orientes vers un but, nécessaires a la poursuite de résultats gratifiants. Nous soulignons les perturbations particulières de chaque trouble, qui peuvent impliquer des systèmes neuronaux dissociables, mais aussi d'éventuelles interactions entre l'affect et la cognition dans le TDC versus la SCZ. Enfin, nous examinons les approches informatiques et translationnelles qui proposent une vision mécaniste de la façon dont les perturbations au niveau cellulaire peuvent induire des troubles affectifs complexes, influant sur le développement des traitements pour le TDC et la SCZ.
Introduction
Our current psychiatric nosology is not equipped to mechanistically map the relationships between psychiatric symptoms, neural systems, and cellular mechanisms. This problem is apparent in scenarios where clinicians are forced into dichotomous diagnostic decisions for patients who display overlapping symptoms across broad behavioral domains.1,2 A cardinal example is embodied in our conceptualization of emotional and cognitive deficits across schizophrenia (SCZ) and major depression (MD).3
SCZ is a complex neuropsychiatric syndrome characterized by a constellation of symptoms, such as hallucinations (hearing voices, seeing visions, etc), and delusions (fixed, false beliefs).4 However, deficits in cognitive function and motivated behavior are a key component of SCZ,5 often reflecting a patient's functional status.6 In turn, MD is conceptualized as primarily a mood disorder with deficits in motivation, and hedonics, as manifested by neurovegetative signs and symptoms. Clinicians often diagnose and consequently treat these psychiatric disorders as distinct entities, in line with formulations presented by the Diagnostic and Statistical Manual of Mental Disorders (DSM). 7,8 Yet, both conditions present with common signs and symptoms, including overlapping affective disruptions, such as anhedonia and amotivation.3 It is critical to appropriately conceptualize behavioral and neural dimensions underlying these behavioral disturbances, in order to improve diagnosis and targeted treatments of specific neural mechanisms giving rise to affective symptoms across MD and SCZ. This effort is captured by the current Research Domain Criteria (RDoC) Initiative, which posits that affective symptoms may cut across diagnoses.1,2
By leveraging cognitive neuroscience methods, clinical neuroscience research has begun to map neural correlates of affective deficits in SCZ and MD. There is now a growing emphasis on delineating psychological and neurobiological impairments leading to emotional deficits, such as amotivation and loss of goal-directed behavior in SCZ.9-11 Concurrently, studies have increasingly mapped the neural correlates of primary affective deficits in MD—in particular those linked to reward processing and anhedonia.12 Consequently, these two historically and behaviorally distinct areas of psychiatric research are poised for conceptual integration to define possible overlapping (or distinct) mechanistic pathways that give rise to observed symptoms. This effort is important for two broad reasons. First, considering dimensional perturbations across psychiatric disorders, in this case MD and SCZ, may help researchers reduce the massive search space and heterogeneity by considering neural computations that may cross diagnostic borders. Second, if there are indeed common (or distinct) neural mechanisms that govern affective symptom expression across SCZ and MD, then it is critical to pinpoint the specific neural mechanisms that map onto behavioral perturbations to guide treatment discovery.
This review considers such shared versus distinct mechanisms across MD and SCZ, covering deficits in hedonics, anticipatory behaviors, computations under lying value and effort and, finally, effortful goal-directed behaviors needed to pursue rewarding outcomes (for an in-depth review of this vast literature see Barch and colleagues3). This review builds on the neural and behavioral evidence to consider several additional perspectives: Emerging cellular-level hypotheses of SCZ and MD and how such neurobiological models can be mapped onto cognitive neuroscience studies in psychiatry. A particular example is discussed—namely “computational psychiatry”—a promising tool to link levels of analyses. In turn, treatment mechanisms are briefly discussed across MD and SCZ, examining how they may relate to the deficits in affective computations across the two conditions. A theoretical conceptualization is introduced detailing hypothesized interactions between affect and cognition in MD versus SCZ. Collectively, this piece considers emerging research that reaches across DSM diagnostic categories and highlights a need for a unified, neurobiologically grounded understanding of affective symptoms, independent of psychiatric categories. Such an understanding can inform either categorical or dimensional insights into symptom domains and offer a rational path for treatment refinement.
Behavioral and neuroimaging evidence for common versus distinct emotional dysregulation mechanisms in SCZ and MD
While affective disturbances present comorbidly across SCZ and MD, it is established that “affect” is not a unitary construct but rather constitutes a complex dimensional set of behaviors and neural computations involving dissociable neural systems.13,14 In turn, neuropsychiatric research has begun to delineate behavioral “dimensions” of affective perturbations that may be similar or distinct across both MD and SCZ.3 We focus on several such behavioral “domains” that have been studied across SCZ and MD: hedonics, anticipatory behaviors, computations underlying value and effort, and finally, effortful goal-directed behaviors needed to pursue rewarding outcomes. A comprehensive outline of this literature is beyond the scope of this focused review; for in-depth treatment we refer readers to recent work by Barch and colleagues.3 Here we summarize findings across these affective behaviors, pointing to common versus distinct perturbations in SCZ vs MD (Figure 1).
Figure 1. Conceptual schematic of reward representations deficits in schizophrenia (SCZ). This figure outlines a conceptual overview of several processes thought to be involved in translating reward information into goal-directed behaviors, which may be compromised in SCZ and major depression (MD). The specific aspects of reward processing involve: (a) basic reward processing or “liking”; (b) reward-related learning and “wanting”; (c) reward information integration; (d) effort computation; and (e) reward representations over time. Breakdowns in the underlying computations across one or more of these processes may be involved in SCZ or MD. GABA, γ-aminobutyric acid; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex. Adapted from ref 176: Anticevic A, Dowd EC, Barch DM. Cognitive and motivational neuroscience of psychotic disorders. In: Charney D, Nestler EJ, Sklar P, Buxbaum J, eds. Neurobiology of Mental Illness. 4th ed. Oxford, UK: Oxford University Press; 2014. Copyright © Oxford University Press 2014.

Hedonic responses to primary and secondary rewards—“liking”
There is a large body of literature, comprised of single experiments,15-39 conceptual reviews,9,40 and meta-analyses,41 suggesting that individuals with SCZ exhibit expected patterns of valence and arousal (eg, in-the-moment liking) in their self-reported emotional responses to emotion-eliciting stimuli. The complementary neuroimaging literature also suggests that SCZ is associated with relatively intact responses to primary and secondary rewards. For instance, studies examining striatal response to monetary reward have shown relatively intact activation patterns in SCZ.42-45 Other studies found relatively intact responses following simple visual presentations of pleasant stimuli. Dowd and colleagues reported similar patterns of brain activation in response to both negative and positive stimuli across brain regions associated with the perception and experience of emotion in SCZ compared with healthy controls.46 This work also reported activity reductions across ventral and dorsal striatum to positive stimuli, which correlated with the magnitude of self-reported anhedonia, suggesting a link between reduced striatal signaling and negative symptoms. However, other work has reported reduced striatal responses to the receipt of juice, with the magnitude of this reduction associated with the severity of anhedonia scores,47 as well as reduced striatal responses to food cues.48 This is consistent with the notion of anhedonia being related to dysfunctional striatal signaling. Complicating this is the reported evidence of reduced striatal responses to loss avoidance in SCZ,49 perhaps reflecting dissociations in ”positive“ vs ”negative“ valence dimensions in SCZ. A more complex picture has emerged when examining responses in PFC circuits in response to receiving rewards,49 suggesting that there may exist some abnormalities in reward-related receipt, possibly related to attentional/cognitive factors, discussed below. Collectively, the self-report literature in SCZ provides relatively consistent evidence for intact self-reports of ”liking.“ However, there is evidence that higher self-reported anhedonia or negative symptoms are associated with less ”liking.“ .32,34,46,50 This may be the critical aspect that links the disruptions across MD and SCZ, namely the link between striatal responses to rewarding stimuli and anhedonia among individuals with SCZ,46,47 which may appear cross-diagnostically.
However, an important difference between SCZ and MD may lie in there being more robust evidence for altered “in-the-moment” responses to receipt of reward in MD. MD studies typically report reduced self-reported and physiological marker responses to positive stimuli.51 There is neuroimaging evidence consistent with this view,52,53 associating reduced striatal responsiveness with levels of anhedonia (but not overall symptom severity). Related to this idea, an interesting report found a relationship between ventral striatum reactivity and anhedonia in response to stress,54 a key clinical feature of MD. Thus, the relationship between disrupted striatal computations and anhedonia may exist “transdiagnostically” whereby SCZ is associated with a milder impairment compared with MD in this particular dimension. Barch and colleagues conceptualized this aspect of disrupted behavior in SCZ and MD as linked to the RDoC dimension of “reduced initial responsiveness to reward.”3
Finally, another computation relevant to “in-the-moment” processing involves the immediate representation of expected value (EV).This issue is thoroughly reviewed by Waltz and Gold55: in SCZ there seem to be deficits in immediate EV computations that relate to cognitive capacity—another important dimension on which SCZ and MD may differ.
Collectively, the self-report and neuroimaging literature in SCZ and MD point towards somewhat dissociable deficits in primary reward processing. However, a key question remains whether deficits in experiencing rewards are independent of anhedonia in SCZ. Level of observed reward disruption across MD and SCZ may be a matter of “degree” rather than reflecting a qualitatively distinct mechanism. This dichotomy is elegantly discussed by Barch and colleagues,3 who suggested that deficits in “liking” may represent an important neurobiological dissociation across the two disorders. It may be possible that the degree of self-reported anhedonia correlates with reward responses and that this can be found across either MD or SCZ patients. Simply put, do individuals with SCZ who report high anhedonia process reward differently from those individuals with SCZ who do not report any anhedonia (ie, is it a “primary” SCZ disturbance or a continuum of disturbance that always maps onto anhedonia irrespective of diagnosis)? Future studies should explicitly compare, in the same experiment, the degree to which the two disorders map onto a “dimensional” picture of primary reward disruptions in relation to behavioral symptoms (ie, anhedonia).
Anticipating future rewards—“wanting”
In contrast to “liking” the concept of “wanting” is typically linked to the ability to learn and anticipate rewards. Again, complete treatment of this topic is beyond the scope of this focused clinical review (for a more detailed review please see ref 56). Briefly, leading neuroscientists and theorists in this area posit that:
“... “wanting” emerged early in evolution as an elementary form of stimulus-guided goal direction, to mediate pursuit, of a few innate food or sex unconditioned stimuli. Subsequently extended to learned “wants,” incentive salience might have been preserved separately from ‛liking’ to facilitate comparison and choice among competing rewards that have incommensurate ‛likes’ (eg, food, sex, and shelter).”56
Neural mechanisms that underlie “wanting” may help the organism learn and represent values that they may “like”—a process thought to be mediated by reward prediction error and dopaminergic signaling via mesolimbic pathways. Such “wanting” is crucial for conducting motivated behaviors. Critically, this computation is distinct from the “in-the-moment” initial response to a rewarding stimulus (discussed above). Therefore, this related but distinct computation governing motivated behavior may be associated with distinct neural disruptions in SCZ and MD. A highly related computational mechanism involves reinforcement learning and the ability to appropriately learn cue-outcome associations by linking stimuli with future rewarding outcomes. While learning deficits are likely separable from “wanting” deficits in SCZ, here we combine these two concepts for parsimony. For a more comprehensive treatment of these distinct mechanisms we refer the readers to recent work on this topic.3
To date, most SCZ studies have examined this question in terms of disrupted anticipation of rewards, signaled via reward prediction mechanisms. A frequently used, and, influential, paradigm is the “monetary incentive delay” (MID) task developed by Knutson and colleagues,57 and based on an existing nonhuman primate protocol. These types of paradigms examine neural responses to reward-predicting cues, rather than to the rewards themselves. Several studies have shown that SCZ is associated with attenuated ventral striatum responses to cues predicting rewards. This has been reported in unmedicated patients49,58-60 and in patients receiving typical antipsychotics, but not in individuals treated with atypicals,43,44,61,62 nor in prodromal individuals.63 Other studies reported an improvement in ventral striatal responses to anticipation cues in antipsychotic-naive patients with SCZ following treatment.60,64 This suggests that at least some aspects of reward anticipation may be attenuated after treatment and may emerge in association with full-blown illness.
Studies have also reported a significant association between severity in negative symptoms and anticipatory ventral striatal activity, suggesting that both “liking” and “wanting” deficits may jointly contribute to the final symptom profile.65 For instance, studies found that the severity of apathy was negatively associated with magnitude of striatal signaling.43 Another study showed a relationship between negative symptom severity and ventral striatal activation during anticipated gains.66 Finally, Barch and colleagues studied reward prediction using a Pavlovian task examining implicit reward learning in SCZ. They reported reduced striatal activation in response to reward-predictive cues, which was associated with greater anhedonia scores in SCZ.45 Collectively, this literature highlights links between alterations in “anticipatory” reward processing and negative symptoms in SCZ.
In MD the literature has also documented alterations in reward anticipation. This is perhaps best summarized by a recent meta-analysis, which reported evidence for reduced activity across a reward network that included subcortical and limbic regions.67 The meta-analysis also revealed reductions in striatal signals following anticipation of monetary rewards. However, as highlighted by the authors of this meta-analytic work, this literature is associated with massive heterogeneity in the tasks used to probe associated deficits in reward processing, as well as the clinical status of the individual MD samples (ie, differing severity of mood states). Therefore, more consensus is needed to fully establish the presence and the nature of anticipatory reward processing deficits in MD with evidence pointing to presence of such deficits.68 As noted, a related computation to facilitate appropriately representing future reward involves the ability to learn or associate cues with future pleasurable (or aversive outcomes). Thus, the extent to which these “wanting” deficits in MD are more strongly related to “liking” deficits versus impairment in learning mechanisms needs to be fully established. There is evidence for such deficits in MD,12,69-71 which was also found to be associated with greater reported anhedonia.72 However, unlike SCZ, individuals with MD seem to show little evidence for impairments when there is explicit instruction or feedback, as summarized by Barch and colleagues.3,73 Barch and colleagues intriguingly posit that this may reflect higher levels of cognitive control impairment in SCZ relative to MD, resulting in limited capacity to learn from explicit instruction.3 This highlights how deficits in computations underlying affect and cognition may differentially interact in SCZ relative to MD.13 In fact, one of the core symptoms in SCZ relates inability to represent and maintain information over time (ie, deficits in working memory, WM), which may interact with affective computations and collectively contribute to motivational deficits.13
Representing reward value versus effort
As stated, another major aspect of motivated behavior involves representing how hard one has to “work” for a given reward or “effort” computation. Even if “liking” and “wanting” computations remain intact, motivated behavior could be disrupted if representations of “effort” or “cost” associated with pursuing a reward are altered. Emerging research suggests that the dorsal anterior cingulate cortex (dACC) may be important for effort evaluation associated with different action plans, with contributions from dopaminergic signaling in the nucleus accumbens.74-77 Several studies demonstrated that depleting dopamine in the nucleus accumbens or lesions to the dACC cause animals to select lower reward choices that are associated with lower effort over higher reward options that require more relative effort.74,75,78-81 This literature is still emerging in human studies across both SCZ and MD. Limited studies in SCZ on this topic support the notion that SCZ is associated with reduced error-related ACC responses,82-89 perhaps reflecting deficits in effort-related neural computations. That said, it is not clear whether these deficits directly map onto lower effort-related computations in the context of reward tasks specifically. Studies on a related topic have not found evidence of reduced cognitive effort in SCZ,90 possibly calling into question that dACC abnormalities are indeed related to ”effort“ per se in the context of reward processing.
In MD studies reported reduced effort allocation, suggesting that MD patients exhibit a lower tendency to engage more effort for proportionately greater rewards.91,92 However, this effect may at least in part relate to severity of depressive symptomatology.93 One possibility, summarized by Barch and colleagues,3 is that while both SCZ and MD exhibit effort computation deficits, the mechanisms governing these behavioral outcomes may be quite distinct. In MD there may be “primary” deficits in the mesolimbic pathways and dopamine projections, whereas in SCZ the effort computation deficits may be linked to “primary” alterations in cognitive control capacity. Future cross-diagnostic studies on this topic will help address these hypotheses by mapping behavioral and neural patterns in response to reward-related effort computations.
Goal-directed actions: representing rewards over time
One additional cross-diagnostic component of reward-related processing relates to the ability to internally generate, represent, and ultimately execute goal-directed action in the service of desired outcomes. This process is closely related to “cognitive control” and the ability to accurately represent information over time. There is unequivocal evidence that SCZ is associated with behavioral and neural deficits in cognitive control capacity.94,95 Typically, frontoparietal circuits are implicated in cognitive control deficits in SCZ and lateral PFC in particular, which is required to represent information over time,96-98 Furthermore, lateral PFC activity can mediate “motivated” cognitive control enhancements that occur in association with rewarding outcomes in both basic99-102 and human research.103-106 Intact cognitive control circuits may be critically involved in maintaining information related to value of rewards to form coherent goal representations so that specific action plans can be guided to achieve the desired rewarding outcome. Thus, an important question is whether some of the motivational impairments observed in SCZ reflect, at least in part, problems in translating reward information into goal representations that can be maintained in frontoparietal control circuits and utilized to guide goal-directed motivated behavior.
To date, there is still little direct experimental work on this topic. One approach is to quantify how motivational incentives impact cognitive performance, potentially via modulation of activity across cognitive control circuits. Evidence suggests that SCZ is associated with reduced ability to improve performance on cognitive control tasks when rewarded.107-110 Other studies, however, suggest some improvement in performance following receipt of reward in SCZ.111-113 That said, this work has not explicitly manipulated cognitive control or employed more challenging “executive” tasks. Also, there is little neuroimaging evidence that would suggest that rewards could alter neural activity during cognitive paradigms in SCZ. This question was indirectly addressed by a study by Ursu and colleagues.114 They presented participants with affective or neutral pictures, followed by a delay during which subjects “maintained” the affective state, Following the delay, all participants provided ratings of their emotional experience, Interestingly, during the initial stimulus presentation phase individuals with SCZ and healthy comparison subjects showed little difference in neural activity, consistent with intact “in-the-moment” response to affective stimuli. However, when required to “maintain” the affective content over time, individuals with SCZ exhibited reductions in blood oxygen level-dependent signal across regions previously linked to cognitive control, which correlated with negative symptom severity. This effect is congruent with the hypothesis that individuals with SCZ may have difficulty representing information about rewards and incentives that can be used to drive goal-directed behavior.115
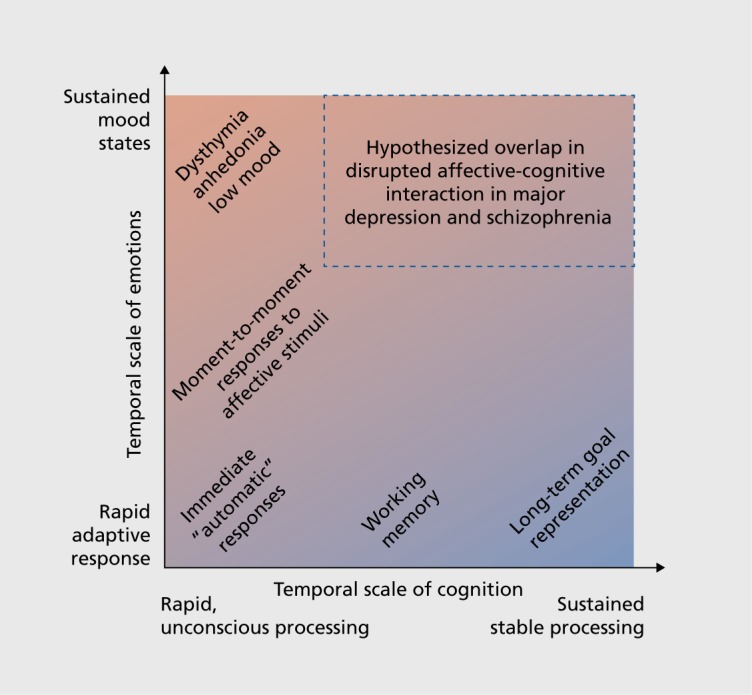
In MD the picture is more mixed and the severity of cognitive impairment may not be as severe as that associated with SCZ.116 Studies do suggest, however, that MD is associated with alterations in neural activity during paradigms requiring emotional regulation.117,118 Thus, while MD may be associated with deficits in cognitive control, these deficits may emerge more selectively in the context of affective regulation demand as opposed to a “primary” deficit in cognitive control. One possibility is that there are important diagnostic differences in situations where rewards and goals require continuous representation over time. For instance, Sheline and colleagues reported that individuals with MD fail to activate brain regions associated with self-referential computations while reappraising negative images,118 also observed by other groups.119 These suppression deficits have been linked to negative rumination in MD.120 This is in contrast to SCZ, where lack of task-induced suppression of “self-referential” neural regions has been reported most consistently during cognitive demand. Therefore, in MD there may exist a failure to suppress certain areas that are typically deactivated during cognitive tasks (in particular medial prefrontal cortex in MD). In turn, in MD this may result in primarily overactive representations of negative internal thought, which in turn affect the ability to engage in executive processes. Conversely, in SCZ there may exist a “primary” inability to engage executive resources.121 If one considers the interplay between affective and executive areas in the context of dynamical systems, the resulting regime in MD may be associated with primary hyperactivity of affective regions and secondary reductions of control-related regions. Such an outcome in MD may nevertheless have detrimental effects on cognitive performance and perhaps the ability to represent goals over time, In turn, mapping whether primary deficits in cognition “drive” motivational disturbances in SCZ (but not MD) will be crucial to rationally inform treatment targets for specific symptoms that may emerge as secondary consequences (Figure 2).
Figure 2. Hypothesized interaction between affect and cognition in major depression (MD) and schizophrenia (SCZ). This schematic highlights the potential interplay between emotional and cognitive computations that may be affected in MD and SCZ. It selves to highlight the intuition that affective and cognitive processing operates at multiple temporal scales and interacts in different ways across these scales. Future studies that attempt to parse the nature of cross-diagnostic affective deficits in SCZ and MD may consider a broader perspective of affect and cognition as integrally linked computations sub-serving linked behavioral dimensions,14 which can “dysinteract” in complex ways across diagnostic categories.13 .
Translating deficits in SCZ and MD across levels of analyses
Limitations of cognitive neuroscience approaches—mapping circuit mechanisms across levels analysis
We discussed the complex cognitive and motivational processes affected in SCZ and MD from the “cognitive neuroscience” perspective, mainly due to the functional resolution of noninvasive human neuroimaging. Such approaches have reduced our search space. However, when used in isolation, these methods face barriers for identifying underlying cellular mechanisms, which is crucial to identity pharmacological therapies for cognitive and motivational impairments that cut cross-diagnostically. Thus, it will be critical to close these gaps in our understanding of emotion and cognition in SCZ and MD across levels of explanation: from synaptic signaling at the microcircuit level, to system-level disruptions and ultimately abnormal behavior.
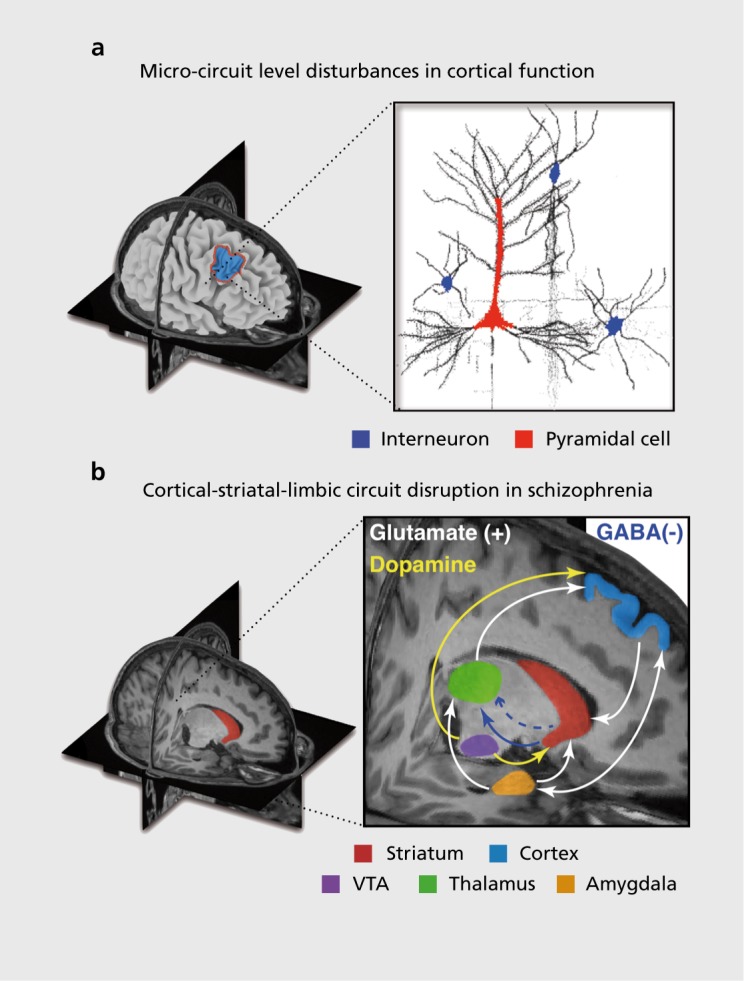
A comprehensive review of synaptic hypotheses in SCZ and MD is beyond the scope of this review (see refs 122,133). For parsimony, we briefly highlight how evolving cellular-level hypotheses in SCZ offer a foundation for understanding higher-order emergent neural system and behavioral deficits that warrant mechanistic explanations. A number of studies to date have implicated alterations in structure and function across distributed cortico-striatal-thalamo-cortical circuits in SCZ, which may relate to the complex cognitive and motivational alterations46,95,134-141 (Figure 3). These areas form interacting cortico-subcortical functional loops, which function in concert to produce motivated behavior and are regulated by multiple neuromodulatory mechanisms142,143 (Figure 3). Disruptions across interacting neurotransmitter systems, including dopamine (DA), γ-aminobutyric acid (GABA) and glutamate have been implicated in SCZ.124,126,144,145 For example, there is mounting evidence for DA signaling disruptions at the level of the dorsal striatum in SCZ140,146 (for review see ref 125). SCZ patients may also exhibit disruptions involving glutamateric NMDARs,124 as well as disruptions in GABA synthesis and signaling from interneurons onto pyramidal cells.123,126,147-149 The field is still equivocal regarding which of these alterations may be upstream of symptoms.122 It is likely that considering the dynamical interactions across these neurotransmitter systems will be needed to yield a more complete understanding of the illness and in turn the complexity of emerging symptom profiles in SCZ.150
Figure 3. Conceptual illustration of neural circuitry across levels of computation that may be involved in affective and cognitive disturbances in schizophrenia (SCZ). The figure highlights how, in order to explain deficits at the phenomenological/behavioral level, we need to bridge observations across multiple levels of analysis in SCZ. (a) At the regional level there is clear evidence for both structural and functional abnormalities in cortical134-138 and striatal/thalamic circuits in SCZ.46,95,134-136,139-141 Based on emerging findings from basic animal,148,155 post-mortem126,147 and pharmacological studies,124 there is an increasing understanding of microcircuit abnormalities that may be at play in SCZ, contributing to regional abnormalities.127 One possibility is that that abnormalities in the balance of excitation (E)/inhibition (I) in cortical micro-circuitry (E/I balance) contribute to downstream system-level disturbances that encompass distributed circuits and neurotransmitter systems in SCZ. One leading hypothesis postulates an imbalance between cortical excitation and inhibition between pyramidal cells (red) and interneurons (blue), producing a state of ”disinhibition,“ which may in turn affect regional and neural system-level function in schizophrenia.155 (b) Less is known about how some of these regional deficits and microcircuit alterations manifest in possible system-level disruptions in functional connections between prefrontal, striatal, limbic, and thalamic nodes in SCZ.151 Deficits in these interacting functional systems need to be considered when interpreting abnormalities between affective and cognitive operations in SCZ and also in major depression. Considering effects across all of these levels will be critical to mechanistically understand complex schizophrenia phenomenology. GABA; γ-aminobutyric acid; VTA, ventral tegmental area. Adapted from ref 176: Anticevic A, Dowd EC, Barch DM. Cognitive and motivational neuroscience of psychotic disorders. In: Charney D, Nestler EJ, Sklar P, Buxbaum J, eds. Neurobiology of Mental Illness. 4th ed. Oxford, UK: Oxford University Press; 2014. Copyright © Oxford University Press 2014.
One organizational principle that could unify these interactive systems across levels of analysis is to consider how they may be jointly impacted by cortical microcircuit alterations126,127,151 That is, perhaps if we were to start from cellular-level models, we may ultimately be able to better understand complex dynamics that emerge at higher levels of observation involving neural systems and behavior.151-153 Consider that optimal cortical function depends on the balanced interaction of excitatory (E) and inhibitory (I) neurons154 (ie, E/I balance). Disruptions in E/I balance can have drastic behavioral consequences, with implications for a number of neuropsychiatry conditions.127,155 In SCZ specifically there may be a functional deficit in the interaction between excitatory and inhibitory cortical neurons.126,127,147,148,156,157 Such E/I imbalance may arise from multiple factors affecting cortical inhibition. One mechanism may involve reduced inhibitory drive via GABA interneurons onto pyramidal cells, which causes elevated E/I balance or disinhibition. 126,127 Postmortem studies analyzing brain tissue of SCZ patients consistently reveal reduced levels of the mRNA for the 67-kilodalton isoform of glutamic acid decarboxylase (GAD67, encoded by GAD1). This is a key mechanism that contributes to optimal GABA levels in cortical circuits and may be disrupted in cortical circuits in SCZ.147 GABAergic interneurons function by exerting lateral inhibition and synchronizing persistent firing of pyramidal cells in cortical circuits,158 thus providing one potential mechanism for the tuning of representations across cortex. Disruptions in E/I balance may be one crucial pathophysiological mechanism operating in SCZ,127 relevant to the patterns of neural and behavioral responses that we discuss presently. At present, it is unknown how these cellular disruptions in E/I balance may manifest at the level of neural systems and ultimately diverse motivational impairments in SCZ.155
Integration of multidisciplinary methods to map cross-diagnostic symptom mechanisms
The ultimate goal is to close the gaps between circuit mechanisms and symptoms. State-of-the-art clinical neuroscience research offers multiple paths forward. One approach that could help unify levels of analysis may involve the emerging field of “computational psychiatry” which aims to mathematically formalize neural and behavioral deficits across diagnostic categories and symptoms.159,161 Particularly relevant are models rooted in neurophysiologic data and that build on assumptions based on molecular and systems neuroscience162-164—namely biophysically based models. Progress has been made in leveraging such computational tools in the context of WM deficits following N-methyl D-aspartate receptor (NMDAR) antagonism.165,166 Low levels of NMDAR conductance disruption from I onto E cells (ie, inhibitory connections) can profoundly affect behavior and neural activation during WM performance1,66,167 suggesting one putative mechanism for observations reported in SCZ, with cross-diagnostic relevance for MD.121 More recently, such microcircuit models have been extended to neural systems.168-170 Building on these approaches, future “computational psychiatry” studies are positioned to generate testable and neurobiologically grounded predictions for mechanisms that may operate cross-diagnostically. In turn, a complementary approach involves examining hypotheses regarding neural dysfunction via safe and transient pharmacological challenge that can be administered inside the MR scanner to healthy adults.171 These and other complementary neuroscientific approaches will be critical to close the explanatory gaps between synaptic hypotheses and symptoms that co-occur across both SCZ and MD.
Considering the role of glutamate across SCZ and MD
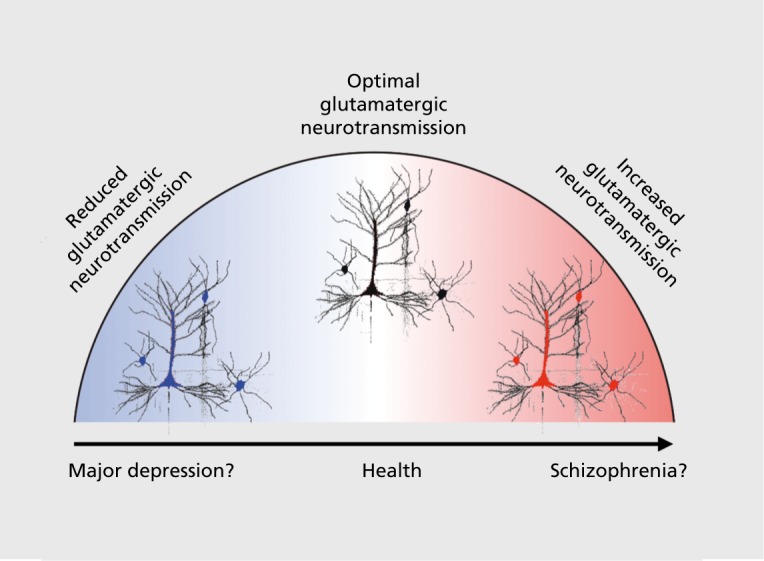
One final consideration that may help unify the pathophysiological mechanisms underlying SCZ and MD symptoms involves glutamatergic pharmacotherapies for SCZ and MD132,172 Here it is useful to consider the complex effects of NMDAR antagonists such as ketamine, which when administered at a low dose acutely to psychotic individuals tend to exacerbate symptoms.173 Conversely, when administered to individuals with MD, ketamine exhibits rapid-acting antidepressant properties, albeit after a brief period.130,132,174 Thus, deficits in glutamatergic signaling may represent, at least in part, a common neurobiological theme across these clinical conditions. Figure 4 highlights the possibility that in MD altering glutamatergic signaling, via administration of an NMDAR antagonist, may exert therapeutic benefits by increasing proliferation of dendritic spines due to increased glutamatergic neurotransmission.175 However, in the context of a possibly preexisting disinhibited microcircuit, as is hypothesized in SCZ,123,127,147 NMDAR antagonist administration may exacerbate psychotic symptoms and cognitive deficits, at least transiently, but without concomitant antidepressant effects. Forthcoming pharmacological neuroimaging and cross-diagnostic studies will be needed to understand the paradoxically dissociable effects of the same pharmacological manipulation across neuropsychiatric conditions.
Figure 4. Simplified hypothesized cellular-level mechanisms in schizophrenia (SCZ) and major depression (MD): central role of glutamatergic neurotransmission. This simple “inverted U” model illustrates the role of glutamate across clinical conditions such as MD and SCZ. This conceptual example is based on evidence that glutamatergic therapies involving acute N-methyl D-aspartate receptor antagonism act therapeutically on mood in the context of MD,174 but result in exacerbation of psychotic symptoms in individuals diagnosed with a psychotic disorder.177 This conceptual illustration highlights the idea that it may be important to understand the role of glutamatergic deficits across psychiatric conditions and role of pharmacotherapies targeting this complex system.124 .
Concluding remarks and future directions
We posit that, in order to develop effective treatments for impairing affective symptoms across MD and SCZ, we may need to adopt a “translational” neuroscience framework. We briefly articulated the use of a “computational psychiatry” framework to understand the potential role of NMDA receptor dysfunction and excitatory/inhibitory circuit deficits in SCZ. Regardless of the specific mechanisms tested, the use of such a framework, combined with experimental tools, will help inform treatments for cognitive and motivational impairment across diagnoses. Ultimately, more research is needed that directly compares cognitive and motivation deficits across typical diagnostic boundaries, with a focus on understanding whether similar deficits at the behavioral level are linked to similar deficits at the neural level. Despite existing challenges, the field of clinical neuroscience is at an exciting junction where progress can be accelerated by combining emerging neuroimaging approaches with translational techniques that can reveal mechanisms.
REFERENCES
- 1.Cuthbert BN., Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36(6):1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuthbert BN., Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barch DM., Pagliaccio D., Luking K. Mechanisms underlying motivational deficits in psychopathology: similarities and differences in depression and schizophrenia. [Epub ahead of print] Curr Topics Behav Neurosci. 2015 doi: 10.1007/7854_2015_376. [DOI] [PubMed] [Google Scholar]
- 4.Walker E., Kestler L., Bollini A., Hochman KM. Schizophrenia: etiology and course. Annu Rev Psychol. 2004;55:401–430. doi: 10.1146/annurev.psych.55.090902.141950. [DOI] [PubMed] [Google Scholar]
- 5.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(10):e12. [PubMed] [Google Scholar]
- 6.Ventura J., Hellemann GS., Thames AD., Koellner V., Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res. 2009;113(2):189–199. doi: 10.1016/j.schres.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupfer DJ., Kuhl EA., Regier DA. DSM-5-the future arrived. JAMA. 2013;309(16):1691–1692. doi: 10.1001/jama.2013.2298. [DOI] [PubMed] [Google Scholar]
- 8.Regier DA., Kuhl EA., Kupfer DJ. The DSM-5: Classification and criteria changes. World Psychiatry. 2013;12(2):92–98. doi: 10.1002/wps.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kring AM., Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold JM., Waltz JA., Prentice KJ., Morris SE., Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barch DM., Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizzagalli DA., Holmes AJ., Dillon DG., et al Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anticevic A., Corlett PR. Cognition-emotion dysinteraction in schizophrenia. Front Psychol. 2012;3:392. doi: 10.3389/fpsyg.2012.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braver TS., Krug MK., Chiew KS., et al Mechanisms of motivation-cognition interaction: challenges and opportunities. Cogn Affect Behav Neurosci. 2014;14(2):443–472. doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor SF., Liberzon I., Decker LR., Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophr Res. 2002;58(2-3):159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 16.Mathews JR., Barch DM. Episodic memory for emotional and nonemotional words in schizophrenia. Cogn Ernot. 2004;18(6):721–740. [Google Scholar]
- 17.Kring AM., Kerr SL., Smith DA., Neale JM. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. J Abnorm Psychol. 1993;102(4):507–517. doi: 10.1037//0021-843x.102.4.507. [DOI] [PubMed] [Google Scholar]
- 18.Kring AM., Neale JM. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? J Abnorm Psychol. 1996;105(2):249–257. doi: 10.1037//0021-843x.105.2.249. [DOI] [PubMed] [Google Scholar]
- 19.Kring AM., Kerr SL., Earnst KS. Schizophrenic patients show facial reactions to emotional facial expressions. Psychophysiology. 1999;36:186–192. [PubMed] [Google Scholar]
- 20.Earnst KS., Kring AM., Kadar MA., Salem JE., Shepard DA., Loosen PT. Facial expression in schizophrenia. Biol Psychiatry. 1996;40:556–558. doi: 10.1016/0006-3223(96)00171-0. [DOI] [PubMed] [Google Scholar]
- 21.Earnst KS., Kring AM. Emotional responding in deficit and non-deficit schizophrenia. Psychiatry Res. 1999;88(3):191–207. doi: 10.1016/s0165-1781(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 22.Flack WF., Laird JD., Cavallaro LA. Emotional expression and feeling in schizophrenia: Effects of specific expressive behaviors on emotional experiences. J Clin Psychol. 1999;55(1):1–20. doi: 10.1002/(sici)1097-4679(199901)55:1<1::aid-jclp1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 23.Berenbaum H., Oltmanns TF. Emotional experience and expression in schizophrenia and depression. J Abnorm Psychol. 1992;101(1):37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crespo-Facorro B., Paradiso S., Andreasen NC., et al Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286(4):427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- 25.Curtis CE., Lebow B., Lake DS., Katsanis J., lacono WG. Acoustic startle reflex in schizophrenic patients and their first-degree relatives: Evidence of normal emotional modulation. Psychophysiology. 1999;36:469–475. doi: 10.1017/s0048577299980757. [DOI] [PubMed] [Google Scholar]
- 26.Quirk SW., Strauss ME., Sloan DM. Emotional response as a function of symptoms in schizophrenia. Schizophr Res. 1998;32(1):31–39. doi: 10.1016/s0920-9964(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 27.Quirk SW., Strauss ME. Visual exploration of emotion eliciting images by patients with schizophrenia. J Nerv Ment Dis. 2001;189(11):757–765. doi: 10.1097/00005053-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Taylor JG., Fragopanagos NF. The interaction of attention and emotion. Neural Netw. 2005;18(4):353–369. doi: 10.1016/j.neunet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Moberg PJ., Arnold SE., Doty RL., et al Impairment of odor hedonics in men with schizophrenia. Am J Psychiatry. 2003;160(10):1784–1789. doi: 10.1176/appi.ajp.160.10.1784. [DOI] [PubMed] [Google Scholar]
- 30.Schlenker R., Cohen R., Hopmann G. Affective modulation of the startle reflex in schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1995;245:309–318. doi: 10.1007/BF02191873. [DOI] [PubMed] [Google Scholar]
- 31.Rupp CI., Fleischhacker WW., Kemmler G., Et al Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr Res. 2005;74(2-3):149–161. doi: 10.1016/j.schres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Herbener ES., Rosen C., Khine T., Sweeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. J Abnorm Psychol. 2007;116(1:43–55. doi: 10.1037/0021-843X.116.1.43. [DOI] [PubMed] [Google Scholar]
- 33.Herbener ES., Song W., Khine TT., Sweeney JA. What aspects of emotional functioning are impaired in schizophrenia? Schizophr Res. 2008;98(13):239–246. doi: 10.1016/j.schres.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burbridge JA., Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol. 2007;116:30–42. doi: 10.1037/0021-843X.116.1.30. [DOI] [PubMed] [Google Scholar]
- 35.Doop ML., Park S. On knowing and judging smells: identification and hedonic judgment of odors in schizophrenia. Schizophr Res. 2006;81(23):317–319. doi: 10.1016/j.schres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Schneider F., Habel U., Reske M., Toni I., Falkai P., Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Res. 2007;155(2):103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Aghevli MA., Blanchard JJ., Horan WP. The expression and experience of emotion in schizophrenia: a study of social interactions. Psychiatry Res. 2003;119(3):261–270. doi: 10.1016/s0165-1781(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 38.Tremeau F., Antonius D., Cacioppo JT., et al In support of Bleuler: objective evidence for increased affective ambivalence in schizophrenia based upon evocative testing. Schizophr Res. 2009;107(2-3):223–231. doi: 10.1016/j.schres.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Dowd E., Barch DM. Subjective emotional experience in schizophrenia: Neural and behavioral markers. Biol Psychiatry. 2010;67(10):902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen AS., Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anticevic A., Van Snellenberg JX., Cohen RE., Repovs G., Dowd EC., Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull. 2012;38(3):608–621. doi: 10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirsch P., Ronshausen S., Mier D., Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40(5):196–198. doi: 10.1055/s-2007-984463. [DOI] [PubMed] [Google Scholar]
- 43.Simon JJ., Biller A., Walther S., et al Neural correlates of reward processing in schizophrenia - Relationship to apathy and depression. Schizophr Res. 2010;118(1-3):154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Walter H., Kammerer H., Frasch K., Spitzer M., Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berl). 2009;206(1):121–132. doi: 10.1007/s00213-009-1586-4. [DOI] [PubMed] [Google Scholar]
- 45.Dowd EC., Barch DM. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS ONE. 2012;7(5):e35622. doi: 10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dowd EC., Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67(10):902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waltz JA., Schweitzer JB., Gold JM., et al Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2009;34(6):1567–1577. doi: 10.1038/npp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grimm O., Vollstadt-Klein S., Krebs L., Zink M., Smolka MN. Reduced striatal activation during reward anticipation due to appetite-provoking cues in chronic schizophrenia: a fMRI study. Schizophr Res. 2012;134(23):151–157. doi: 10.1016/j.schres.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 49.Schlagenhauf F., Sterzer P., Schmack K., et al Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009;65(12):1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Blanchard JJ., Bellack AS., Mueser KT. Affective and social-behavioral correlates of physical and social anhedonia in schizophrenia. J Abnorm Psychol. 1994;103(4):719–728. doi: 10.1037//0021-843x.103.4.719. [DOI] [PubMed] [Google Scholar]
- 51.Bylsma LM., Morris BH., Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 52.McCabe C., Cowen PJ., Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl). 2009;205(4):667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smoski MJ., Rittenberg A., Dichter GS. Major depressive disorder is characterized by greater reward network activation to monetary than pleasant image rewards. Psychiatry Res. 2011;194(3):263–270. doi: 10.1016/j.pscychresns.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corral-Frías NS., Nikolova YS., Michalski LJ., Baranger DA., Hariri AR., Bogdan R. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med. 2015;45(12):2605–2617. doi: 10.1017/S0033291715000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waltz JA., Gold JM. Motivational deficits in schizophrenia and the representation of expected value. Curr Topics Behav Neurosci. [Epub ahead of print] 2015 doi: 10.1007/7854_2015_385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berridge KC., Robinson TE. Parsing reward. Trends Neurosci. 2003;269:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 57.Knutson B., Adams CM., Fong GW., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wrase J., Schlagenhauf F., Kienast T., et al Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 59.Esslinger C., Englisch S., Inta D., et al Ventral striatal activation during attribution of stimulus saliency and reward anticipation is correlated in unmedicated first episode schizophrenia patients. Schizophr Res. 2012;140(1-3):114–121. doi: 10.1016/j.schres.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 60.Nielsen MO., Rostrup E., Wulff S., et al Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. 2012;69(12):1–10. doi: 10.1001/archgenpsychiatry.2012.847. [DOI] [PubMed] [Google Scholar]
- 61.Juckel G., Schlagenhauf F., Koslowski M., et al Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 62.Schlagenhauf F., Juckel G., Koslowski M., et al Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl). 2008;196(4):673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- 63.Juckel G., Friedel E., Koslowski M., et al Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66(1):50–56. doi: 10.1159/000337130. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen MO., Rostrup E., Wulff S., et al Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry. 2012;71(10):898–905. doi: 10.1016/j.biopsych.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Juckel G., Schlagenhauf F., Koslowski M., et al Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl). 2006;187(2):222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- 66.Waltz JA., Schweitzer JB., Ross TJ., et al Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. 2010;35(12:2427–2439. doi: 10.1038/npp.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W-N., Chang S-H., Guo L-Y., Zhang K-L., Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;1512:531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 68.McFarland BR., Klein DN. Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress Anxiety. 2009;26(2):117–122. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- 69.Henriques JB., Glowacki JM., Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol. 1994;103(3):460. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- 70.Vrieze E., Pizzagalli DA., Demyttenaere K., et al Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73(7):639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pechtel P., Dutra SJ., Goetz EL., Pizzagalli DA. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 2013;47(12):1864–1869. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liverant Gl., Sloan DM., Pizzagalli DA., et al Associations among smoking, anhedonia, and reward learning in depression. Behav Ther. 2014;45(5):651–663. doi: 10.1016/j.beth.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barch DM., Treadway MT., Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014;123(2):387–397. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salamone JD., Correa M., Farrar A., Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl). 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 75.Salamone JD. Functions of mesolimbic dopamine: changing concepts and shifting paradigms. Psychopharmacology (Berl). 2007;191(3):389. doi: 10.1007/s00213-006-0623-9. [DOI] [PubMed] [Google Scholar]
- 76.Croxson PL., Walton ME., O'Reilly JX., Behrens TE., Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29(14):4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Botvinick MM., Huffstetler S., McGuire JT. Effort discounting in human nucleus accumbens. Cogn Affect Behav Neurosci. 2009;9(1):16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rudebeck PH., Walton ME., Smyth AN., Bannerman DM., Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9(9):1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 79.Rudebeck PH., Buckley MJ., Walton ME., Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313(5791):1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 80.Walton ME., Rudebeck PH., Bannerman DM., Rushworth MF. Calculating the cost of acting in frontal cortex. Ann N Y Acad Sci. 2007;1104:340–356. doi: 10.1196/annals.1390.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudebeck PH., Walton ME., Millette BH., Shirley E., Rushworth MF., Bannerman DM. Distinct contributions of frontal areas to emotion and social behaviour in the rat. Eur J Neurosci. 2007;26(8):2315–2326. doi: 10.1111/j.1460-9568.2007.05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alain C., McNeely HE., Yu H., Christensen BK., West R. Neurophysiological evidence of error monitoring deficits in patients with schizophrenia. Cereb Cortex. 2002;12:840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- 83.Kerns JG., Cohen JD., MacDonald AW, 3rd., et al Decreased conflict and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 84.Kopp B., Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J Abnorm Psychol. 1999;108(2):337–346. doi: 10.1037//0021-843x.108.2.337. [DOI] [PubMed] [Google Scholar]
- 85.Mathalon DH., Fedor M., Faustman WO., Gray M., Askari N., Ford JM. Response-monitoring dysfunction in schizophrenia: An event-related brain potential study. J Abnorm Psychol. 2002;111(1):22–41. [PubMed] [Google Scholar]
- 86.Morris SE., Heerey EA., Gold JM., Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr Res. 2008;99(1-3):274–285. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris SE., Yee CM., Nuechterlein KH. Electrophysiological analysis of error monitoring in schizophrenia. J Abnorm Psychol. 2006;115(2):239–250. doi: 10.1037/0021-843X.115.2.239. [DOI] [PubMed] [Google Scholar]
- 88.Laurens KR., Ngan ET., Bates AT., Kiehl KA., Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- 89.Polli FE., Barton JJ., Thakkar KN., et al Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131(Pt 4):971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 90.Gold JM., Kool W., Botvinick MM., Hubzin L., August S., Waltz JA. Cognitive effort avoidance and detection in people with schizophrenia. Cogn Affect Behav Neurosci. 2014;15(1):145–154. doi: 10.3758/s13415-014-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Treadway MT., Bossaller NA., Shelton RC., Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121(3):553. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Treadway MT., Peterman JS., Zald DH., Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161(2):382–385. doi: 10.1016/j.schres.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang X-h., Huang J., Zhu C-Y., et al Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first episode and remitted depression patients. Psychiatry Res. 2014;220(3):874–882. doi: 10.1016/j.psychres.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 94.Barch DM., Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reichenberg A., Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133(5):833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 96.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 97.Miller EK., Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 98.Braver TS., Cohen JD. Dopamine, cognitive control, and schizophrenia: The gating model. Prog Brain Res. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- 99.Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- 100.Sakagami M., Watanabe M. Integration of cognitive and motivational information in the primate lateral prefrontal cortex. Ann N Y Acad Sci. 2007;1104:89–107. doi: 10.1196/annals.1390.010. [DOI] [PubMed] [Google Scholar]
- 101.Krawczyk DC., Gazzaley A., D'Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi S., Nomoto K., Watanabe M., Hikosaka O., Schultz W., Sakagami M. Influences of rewarding and aversive outcomes on activity in macaque lateral prefrontal cortex. Neuron. 2006;51(6):861–870. doi: 10.1016/j.neuron.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 103.Beck SM., Locke HS., Savine AC., Jimura K., Braver TS. Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PLoS ONE. 2010;5(2):e9251. doi: 10.1371/journal.pone.0009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jimura K., Locke HS., Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc Natl Acad Sci USA. 2010;107(19):8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Savine AC., Braver TS. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. J Neurosci. 2010;30(31):10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Tsujimoto S., Sawaguchi T. Context-dependent representation of response-outcome in monkey prefrontal neurons. Cereb Cortex. 2005;15(7):888–898. doi: 10.1093/cercor/bhh188. [DOI] [PubMed] [Google Scholar]
- 107.Green MF., Satz P., Ganzell S., Vaclav JF. Wisconsin Card Sorting Test performance in schizophrenia: remediation of a stubborn deficit. Am J Psychiatry. 1992;149(1):62–67. doi: 10.1176/ajp.149.1.62. [DOI] [PubMed] [Google Scholar]
- 108.Hellman SG., Kern RS., Neilson LM., Green MF. Monetary reinforcement and Wisconsin Card Sorting performance in schizophrenia: why show me the money? Schizophr Res. 1998;34(1-2):67–75. doi: 10.1016/s0920-9964(98)00088-7. [DOI] [PubMed] [Google Scholar]
- 109.Vollema MG., Geurtsen GJ., van Voorst AJ. Durable improvements in Wisconsin Card Sorting Test performance in schizophrenic patients. Schizophr Res. 1995;16(3):209–215. doi: 10.1016/0920-9964(94)00079-n. [DOI] [PubMed] [Google Scholar]
- 110.Roiser JP., Stephan KE., den Ouden HE., Barnes TR., Friston KJ., Joyce EM. Do patients with schizophrenia exhibit aberrant salience? Psychol Med. 2009;39(2):199–209. doi: 10.1017/S0033291708003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Penn DL., Combs D. Modification of affect perception deficits in schizophrenia. Schizophr Res. 2000;46(2-3):217–229. doi: 10.1016/s0920-9964(00)00005-0. [DOI] [PubMed] [Google Scholar]
- 112.Kern RS., Green MF., Goldstein MJ. Modification of performance on the span of apprehension, a putative marker of vulnerability to schizophrenia. J Abnorm Psychol. 1995;104(2):385–389. doi: 10.1037//0021-843x.104.2.385. [DOI] [PubMed] [Google Scholar]
- 113.Rassovsky Y., Green MF., Nuechterlein KH., Breitmeyer B., Mintz J. Modulation of attention during visual masking in schizophrenia. Am J Psychiatry. 2005;162(8):1533–1535. doi: 10.1176/appi.ajp.162.8.1533. [DOI] [PubMed] [Google Scholar]
- 114.Ursu S., Kring AM., Gard MG., et al Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. Am J Psychiatry. 2011;168(3):276–285. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heerey EA., Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- 116.Barch DM., Sheline Yl., Csernansky JG., Snyder AZ. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53(5):376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- 117.Johnstone T., van Reekum CM., Urry HL., Kalin NH., Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheline Yl., Barch DM., Price JL., et al The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hamilton JP., Furman DJ., Chang C., Thomason ME., Dennis E., Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lemogne C., Delaveau P., Freton M., Guionnet S., Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012;136(12):e1–e11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 121.Anticevic A., Cole MW., Murray JD., Corlett PR., Wang XJ., Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4-6):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 123.Gonzalez-Burgos G., Lewis DA. NMDA receptor hypofunction, parval-bumin-positive neurons and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38(5):950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krystal JH., D'Souza DC., Mathalon D., Perry E., Belger A., Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4):215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 125.Laruelle M., Kegeles LS., Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- 126.Lewis DA., Curley AA., Glausier JR., Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 128.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;405:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 129.Moghaddam B., Javitt D. From Revolution to Evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.ladarola ND., Niciu MJ., Richards EM., et al Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krystal JH., Sanacora G., Duman RS. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol Psychiatry. 2013;73(12):1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanacora G., Zarate CA., Krystal JH., Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zarate CA., Singh J., Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry. 2006;59(11):1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 134.Csernansky JG., Gillespie SK., Dierker DL., et al Symmetric abnormalities in sulcal patterning in schizophrenia. Neurolmage. 2008;43(3):440–446. doi: 10.1016/j.neuroimage.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Csernansky JG., Joshi S., Wang L., et al Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci U S A. 1998;95(19):11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Csernansky JG., Schindler MK., Splinter NR., et al Abnormalities of thalamic volume and shape in schizophrenia. Am J Psychiatry. 2004;161(5):896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- 137.Harms MP., Wang L., Csernansky JG., Barch DM. Structure-function relationship of working memory activity with hippocampal and prefrontal cortex volumes. Brain Struct Funct. 2013;218(1):173–186. doi: 10.1007/s00429-012-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harms MP., Wang L., Campanella C., et al Structural abnormalities in gyri of the prefrontal cortex in individuals with schizophrenia and their unaffected siblings. Br J Psychiatry. 2010;196(2):150–157. doi: 10.1192/bjp.bp.109.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harms MP., Wang L., Mamah D., Barch DM., Thompson PA., Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci. 2007;27(50):13835–13842. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Laruelle M., Abi-Dargham A., Gil R., Kegeles L., Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46(1):56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 141.Mamah D., Conturo TE., Harms MP., et al Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry Res. 2010;183(2):144–150. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carlsson A., Waters N., Holm-Waters S., Tedroff J., Nilsson M., Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 143.Carlsson M., Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia - implications for schizophrenia and Parkinson's disease. Trends Neurosci. 1990;13(7):272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- 144.Abi-Dargham A., Mawlawi O., Lombardo I., et al Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kegeles LS., Abi-Dargham A., Zea-Ponce Y., et al Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48(7):627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- 146.Howes OD., Kambeitz J., Kim E., et al The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch Gen Psychiatry. 2012;69(8):776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lewis DA., Hashimoto T., Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 148.Lewis DA., Volk DW., Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl). 2004;174(1):143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 149.Nakazawa K., Zsiros V., Jiang Z., et al GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marsman A., van den Heuvel MP., Klomp DWJ., Kahn RS., Luijten PR., Hulshoff Pol HE. Glutamate in schizophrenia: A focused review and meta-analysis of 1H-MRS studies. Schizophr Bull. 2013;39(1):120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012;22(3):537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Loh M., Rolls ET., Deco G. A dynamical systems hypothesis of schizophrenia. PLoS Comput Biol. 2007;3(11):e228. doi: 10.1371/journal.pcbi.0030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rolls ET., Deco G. A computational neuroscience approach to schizophrenia and its onset. Neurosci Biobehav Rev. 2011;35(8):1644–1653. doi: 10.1016/j.neubiorev.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 154.Shadlen MN., Newsome WT. Noise, neural codes and cortical organization. Curr Opin Neurobiol. 1994;4:569–579. doi: 10.1016/0959-4388(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 155.Yizhar O., Fenno LE., Prigge M., et al Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Benes FM., McSparren J., Bird ED., SanGiovanni JP., Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48(11):996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 157.Lewis DA., Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63(10):1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 158.Rao SG., Williams GV., Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20(1):485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Anticevic A., Murray JD., Barch DM. Bridging levels of understanding in schizophrenia through computational modeling. Clin Psychol Sci. 2015;3(3):433–459. doi: 10.1177/2167702614562041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X-J., Krystal JH. Computational psychiatry. Neuron. 2014;84(3):638–654. doi: 10.1016/j.neuron.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Anticevic A., Cole MW., Repovs G., et al Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. 2013;4:169. doi: 10.3389/fpsyt.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang X-J. Toward a prefrontal microcircuit model for cognitive deficits in schizophrenia. Pharmacopsychiatry. 2006;39(suppl 1):S80–S87. doi: 10.1055/s-2006-931501. [DOI] [PubMed] [Google Scholar]
- 163.Wang X-J. Decision making in recurrent neuronal circuits. Neuron. 2008;60:215–234. doi: 10.1016/j.neuron.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang X-J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90(3):1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Anticevic A., Gancsos M., Murray JD., et al NMDA receptor function in large-scale anti-correlated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012;109(41):16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Murray JD., Anticevic A., Corlett PR., Gancsos M., Krystal JH., Wang X-J. Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb Cortex. 2014;24(4):859–782. doi: 10.1093/cercor/bhs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Anticevic A., Corlett PR., Cole MW., et al NMDA receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry. 2015;77(6):569–580. doi: 10.1016/j.biopsych.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 168.Yang GJ., Murray JD., Repovs G., et al Altered global brain signal in schizophrenia. Proc Natl Acad Sci U S A. 2014;111(20):7438–7443. doi: 10.1073/pnas.1405289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Deco G., Ponce-Alvarez A., Mantini D., Romani GL., Hagmann P., Corbetta M. Resting-state functional connectivity emerges from structurally and dynamically shaped slow linear fluctuations. J Neurosci. 2013;33(27):11239–11252. doi: 10.1523/JNEUROSCI.1091-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Anticevic A., Hu X., Xiao Y., et al First-episode schizophrenia exhibits elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35(1):267–286. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Corlett PR., Honey GD., Fletcher PC. From prediction error to psychosis: ketamine as a pharmacological model of delusions. J Psychopharmacol. 2007;(213):238–252. doi: 10.1177/0269881107077716. [DOI] [PubMed] [Google Scholar]
- 172.Udupa K., Chen R. The mechanisms of action of deep brain stimulation and ideas for the future development. Prog Neurobiol. 2015;133:27–49. doi: 10.1016/j.pneurobio.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 173.Lahti AC., Holcomb HH., Medoff DR., Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6(6):869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 174.Abdallah CG., Sanacora G., Duman RS., Krystal JH. Ketamine and rapidacting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Duman RS. Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci. 2014;16(1):11–27. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Anticevic A., Dowd EC., Barch DM. Cognitive and motivational neuroscience of psychotic disorders. In: Charney D, Nestler EJ, Sklar P, Buxbaum J, eds. Neurobiology of Mental Illness. 4th ed. Oxford, UK: Oxford University Press. 2014;16(1) [Google Scholar]
- 177.Lahti AC., Koffel B., LaPorte D., Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]


