Abstract
Allele-specific PCR based on subtype consensus sequences is a powerful technique for detecting low frequency drug resistant mutants in HIV-1 infected patients. However, this approach can be limited by genetic variation in the region complementary to the primers, leading to variability in allele detection. The goals of this study were to quantify this effect and then to improve assay performance
Keywords: HIV-1, allele–specific PCR, low frequency variants, subtype consensus sequence, patient specific HIV-1 consensus sequence
Infection with human immunodeficiency virus type 1 (HIV-1) remains a major global health challenge. Antiretroviral therapy (ART) has improved and lengthened the lives of those infected with HIV. Nevertheless, antiviral drug resistance continues to reduce the effectiveness of ART (Coffin, 1995; Martinez-Picado and Martinez, 2008). Nonnucleoside reverse transcriptase inhibitors (NNRTIs) are routinely used for the treatment and prevention of HIV-1 infection. NNRTIs target reverse transcriptase (RT), an enzyme essential for HIV-1 replication (De Clercq, 1994; Deeks, 2001). The single point mutations AAA to AAC or AAT in HIV-1 RT at codon 103 result in a lysine to asparagine substitution (K103N) which causes resistance to efavirenz and nevirapine (Harrigan et al., 2005; Wainberg, 2003). Detection of HIV-1 variants carrying these mutations is important for understanding the emergence of drug resistance and for designing optimal ART strategies. To this end, a sensitive real time allele-specific PCR (ASP) assay for quantifying low frequency HIV-1 variants containing NNRTI-resistant variants in patients infected with HIV-1 has been reported (Palmer et al., 2006a; Palmer et al., 2006b). Using this assay, detection of drug resistant variants over a broad range of frequencies (0.1 to 99.5%) is achievable providing insight into the emergence and persistence of drug resistance after the discontinuation of therapy (Palmer et al., 2006a) and after single-dose nevirapine treatment to prevent mother to child transmission (Palmer et al., 2006b). Other researchers have reported the detection of minor resistant HIV-1 variants using similar techniques (Halvas et al., 2006; Johnson et al., 2005; Loubser et al., 2006; Metzner et al., 2003). As for all PCR-based assays, ASP can be limited by genetic variation in the region complementary to the primers, leading to variability in detection efficiency. The accuracy of ASP may also be influenced by the technique employed and the method of data analysis. A recent report described improved ASP with the use of polymorphism-specific primers (Rowley et al., 2008). It is concluded that ASP is a powerful tool only when allele-specific primers perfectly match the patient HIV-1 sequence, requiring a bulk genotypic analysis of the region of interest for each patient plasma sample. However there is a method to correct for the effects of genetic variation on the quantitation of viral variants at a specific allele and to achieve acceptable assay performance without requiring genotypic analysis of every patient sample. Details of the technique and data analysis that enhance the detection and improve the accuracy of allele quantitation are described.
Viral RNA is extracted from patient samples infected with HIV-1 and a first round amplification is performed using quantitative real time PCR as published (Palmer et al., 2006a). Quantitation of the number of cDNA molecules synthesized and amplified in the first round RT-PCR is important to determine the level of sensitivity of subsequent ASP, without which the assay sensitivity can not be determined since variation in virus concentration, plasma components, and primer matches to target sequences can lead to variability in efficiency of RNA recovery, cDNA synthesis, and amplification.
Allele-specific primers that amplify selectively subtype specific HIV-1 mutant or wildtype (WT) alleles at codon 103 are then used in ASP to quantify the proportion of each allele in cDNA derived from patient plasma RNA (Table 1 A). Parallel reactions are performed using two primer sets: selective primers to quantify mutant or WT frequencies, and a non-selective primer to quantify total DNA.
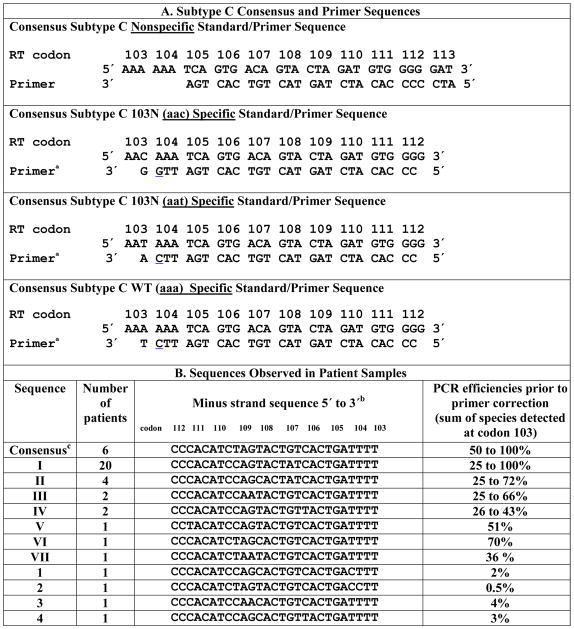
Table 1.
Relationship between sequence polymorphism around codon 103 and amplification efficiency.
Penultimate 3′ mismatch shown in
 is built in for discrimination between alleles.
is built in for discrimination between alleles.
Only the wild-type allele is shown. Patient polymorphisms are denoted in red
This sequence was common in the LANL database, sequence I was most common in the patient group.
The selective primers match a mutant (C or T) or wildtype base (A or G) at the 3′ end of codon 103, but mismatch all templates at the penultimate position, a modification that has little impact on amplification of sequences complimentary to the 3′ end of the primer but reduces markedly amplification (≥1000 fold) when the 3′ end of the primer is also mismatched (Cha et al., 1992; Hance et al., 2001; Palmer et al., 2006a). The specific nucleotide used for the intentional mismatch was given a great deal of attention and the best mismatched pair was determined empirically. For the allele specific 103n aac primer, the mispair of A-C template- primer at the penultimate position is not used because although it leads to efficient amplification when the aac mutation is present, the background amplification or presumably the mispriming of WT that occurs, ranges from 0.07% to 0.4% with a mean value of 0.2% of total WT copies amplified. Conversely, when the mispair of A-G (Table 1A) is used at the penultimate base the amplification of the mutant 103n aac retains it’s sensitivity down to 0.1% detection, (Halvas et al., 2006) and the WT background is decreased to a range of 0.006% to 0.1% with a mean value of 0.01% (Palmer et al., 2006a). Presumably, the three hydrogen bonds between the 3′ nucleotides C and G is strong enough to maintain sensitivity. The stringency of the reaction is considerably more valuable to maintain with the possibility of erring on the side of missing a 103n aac mutation rather thanto falsely identify an aac mutation at position 103. In the case of the allele specific primer for 103n aat, the more efficient A-C template-primer mismatch is used because the background amplification of WT is extremely low, between 0.001% and 0.01% with a mean of 0.003%. The allele specific primer for 103k aaa utilizes the A-C template primer mismatch as well, but curiously the background is not as low as the 103n aat assay, ranging from 0.05% to 0.2% with a mean of 0.1%. Thus the limit of detection of the WT ASP is only down to 0.5%. The difference between the 103n aat and 103k aaa assays is the position of the thymidine; the thymidine being in the template in the first and in the primer in the second. The A-G template-primer mismatch is is not used for these assays sbecause eventhough the background is decreased, the limit of detection was not acceptable presumably due to only two hydrogen bonds between the 3′ nucleotides of A and T template primer.
The non-selective primer initiates amplification four nucleotides 3′ of the target base and allows amplification of mutant or wildtype sequences. This independent quantitation of amplified copies enables the accurate determination of the percent variant at each allele and the detection of sequence polymorphism in the target region that may affect PCR efficiency. When the minimum of 1000 copies of CDNA/reaction is obtained after the first round of RT-PCR, the amplification curves plateau after 45 cycles and indicate that a 1:10,000 dilution of these first round amplification products will result in ~107 starting copies for the subsequent ASP when using the nonselective primer. Samples that do not yield ~107 starting copies are assigned a failing quality score and indicate possible polymorphism.
Each ASP assay has its own set of reaction conditions and is performed under stringent MgCl2 concentrations as well as annealing conditions. The annealing temperatures are performed at > 5 degrees above the Tm of each selective primer. The number of target templates in the sample is determined by comparing the threshold cycle of amplification with each primer against a standard curve prepared from known amounts of mutant and wild type DNA sequences. The percent mutant or WT is calculated by dividing the quantity of mutant or WT sequences by the quantity of total sequences and multiplying by 100, i.e., AAn/Total × 100 where “AAn” is the measured number of copies with the selective primer and “Total” is the number of copies measured with the non selective primers in each assay. Values are then normalized to the sum of the alleles detected. Preliminary experiments using 50 patient samples with perfectly matched sequences in the primer target region, as determined by standard genotyping, showed variability in the sum of the alleles detected ranging from 20 to 110% with 95% of assays resulting in a quality score of >25%. Although infrequent, when the sum of alleles at codon 103 exceeds 100%, the limiting acceptable value is 400%. Thus if the sum of the alleles detected is less than 25% or greater than 400% the assay results are reported as “indeterminate” at that codon, i.e., (%AAG + %AAA + %AAC + %AAT) < 25% or >400%. Such a designation indicates that both WT and mutant alleles are amplified inefficiently. To verify that the quantitation of variants in patient samples is correct, this method has been compared to the technique of single genome sequencing (SGS). Using the same set of samples for the detection of variants in patients infected with HIV-1 revealed concordance between these two methods (Ambrose et al., 2007; Halvas E.K et al., 2009).
In the present study, polymorphism in the sequence adjacent to codon 103 is a major cause of inefficient amplification. Assay performance is improved by modifying the assay primers and standards to match changes corresponding to the HIV-1 consensus sequence in the patient’s sample, while maintaining the penultimate mismatch. In all cases analyzed, use of patient consensus-specific primers and standards increased the efficiency of amplification from less than 5% to nearly 100%.
In recent studies of NNRTI resistance in women infected with HIV-1 (subtype C) treated with single-dose nevirapine to prevent mother to child transmission, 4 of 41 patients yielded indeterminate results from ASP for K103N (Palmer et al., 2006b; Palmer, 2007.). Therefore, pretherapy consensus viral sequences obtained by bulk RT-PCR of the HIV-1 RT gene in samples from each of the 41 patients was examined (Table 1 B). This analysis revealed that single and double nucleotide variation located in the middle or 5′ end of the targeted region was tolerated by the specific primer without substantially reducing amplification efficiency (sequences I–VII in Table 1 B). However, other changes in the primer target sequence substantially reduced amplification efficiency (sequences 1–4 in Table 1 B).
The HIV-1 variant in patient 1 had three mismatches in the primer target region; two near its center at codons 108 and 109; and one near the 3′ end of the primer in codon 104 (sequence 1 in Table 1). Using primers and standards redesigned to match the exact target sequences, the efficiency of amplification increased from 1–2% to 90–100% of all alleles present at codon 103 (Figure 1 – – 1st data set for patient 1). To test the role of variation at the different positions on assay sensitivity, primers and DNA standards were changed to match only the variant at codon 104. Samples from patient 1 were reanalyzed and amplification efficiency increased to nearly 100 % (Figure 1–2nd data set for patient 1). These results indicate that the internal mismatches at codons 108 and 109 had little effect on PCR efficiency and that the mismatch at codon 104, three nucleotides from the 3′terminus, was solely responsible for the marked reduction in PCR efficiency (Kwok et al., 1990). This result is consistent with the acceptable efficiency of amplification of sequence II (Table 1 B), which contains the same two internal mismatches.
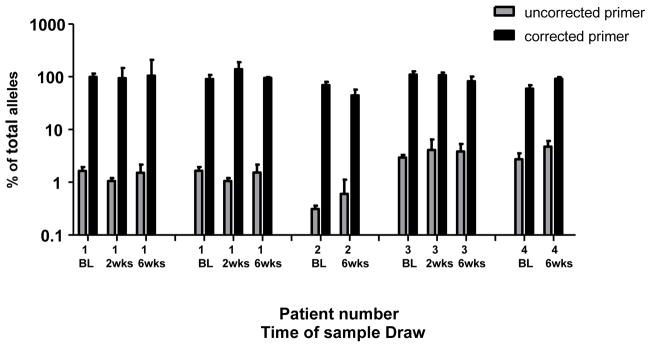
Figure 1.
Comparison of allele-specific PCR efficiency of patient samples between primers based on HIV-1 subtype C consensus sequences and primers based on patient-specific HIV-1 consensus sequences at codon 103. The total amplification efficiency of patient samples 1–4 (Table 1) was calculated as the sum of the efficiencies of detection of the wild-type and mutant alleles. Shown are results using the uncorrected consensus primers (grey) and the corrected primers (black) with error bars representing standard deviations of triplicate observations for samples taken immediately before single-dose nevirapine (BL) and 2 and/or 6 weeks following treatment. Patient 1 has 2 data sets; the 1st are samples amplified with primers that match the HIV-1 sequence infecting patient 1, the 2nd are samples amplified with primers only corrected to match the sequence at codon 104 in patient 1.
For patient 2, the consensus HIV sequences had mismatches at two consecutive residues in codon 104 (sequence 2 in Table 1 B). Using redesigned primers and standards, the percent of total alleles detected at codon 103 increased from <0.5 to between 40 and 70% (Figure 1). Although the efficiency of PCR was improved by 100 fold, the sum of all the alleles detected remained lower than 100%, perhaps due to minor viral populations with additional polymorphism in the primer regions not detected by bulk sequence analysis.
The viral variant found in patient 3 contained mismatches from the consensus sequence at codons 108 and 109, as in patient 1; however, this viral variant had two nucleotide mismatches in codon 109 (Table 1). As described above, the use of redesigned primers and standards to match the patient specific viral sequence greatly increased the detection of all species at residue 103, from 3% to 100% (Figure 1). Thus, the addition of a third internal mismatch to those at residues 108 and 109 had a significant effect on allele amplification.
Sequence analysis of the virus in patient 4 showed mismatches at three positions; codons 106, 108 and 109. When plasma samples from patient 4 were analyzed with redesigned primers and standards, the sum of the alleles at codon 103 increased from 3% to between 60 and 80%. The addition of the third mismatch at position 106 to those at 108 and 109 resulted in poor amplification efficiency. It is worth noting that in all cases described the amplification efficiencies were not influenced by the presence of the WT or mutant at codon 103. Thus, even though the overall efficiency varied with certain primer combinations, its ability to detect and quanitate low frequency alleles was not affected by the specific primer used.
After identifying these sequence variations around the 103 site that affected adversely amplification efficiency, a larger data set was explored to predict how frequently these primers are likely to amplify inefficiently. Of the ~390 HIV-1 subtype C sequences in the Los Alamos National Library Database, the frequency of polymorphic sites in the primer target region with >2 mismatches is 1%; however, at codon 104, the frequency of polymorphic sites is 6.4%. Notably, 98.5% of these polymorphic sites would be corrected with one of the primers described. In a current study (data not shown) using samples from different geographical regions from sub-Saharan Africa, the frequency of polymorphism at codon 104 was much greater and present in 23% of the patients infected with HIV. Because the target region of the nonselective primer does not include the 104 codon, every patient sample with a polymorphism at codon 104 which was tested for minority variants at codon 103, resulted in a marked reduction in PCR efficiency and quality scores of <5%. This provided a marker for a polymorphism at position 104 and indicated the need to use selective primers and standards encompassing a 104 polymorphism. As a general rule, polymorphism in the middle of the 5′ end of the primer which adversely affected amplification efficiency resulted in quality scores of >5% but <25%. The library of primers and standards described performed well and acceptable assay performance was achieved in 94% of patient samples with only 6 samples out of 94 resulting in indeterminate results.
A number of conclusions can be drawn from these results. First, although most single or double mismatches near the middle or 5′ end of the primer have little effect on amplification efficiency, three or more differences in the middle of the primer or single differences within three nucleotides of its 3′ terminus significantly reduce PCR efficiency and lead to indeterminate results. Second, the use of standards and primers that were designed to correct for the most common polymorphism found at codon 104 will provide accurate ASP results. Third, use of a nonspecific amplification step in ASP allows detection of sequence variation that affects amplification efficiency. Fourth, although standard genotyping can be used for each patient sample in order to make an informed decision regarding the best primers to use for ASP, it is considerably cheaper and faster to perform ASP on all the samples and then sequence only the samples that produce an indeterminate result. Sequence specific primers and standards can then be used for the samples containing significant variations in the target region as described. Finally, the effects of viral sequence polymorphisms on the performance of ASP can be corrected most of the time by using a library of primers and standards developed to adjust for common variations in the target sequence. Thus, in contrast to other reports (Rowley et al., 2008), acceptable assay performance can be achieved without sequence analysis of each sample. This approach readily detected suboptimal assay performance and improved it by using primers and standards that were based on patient-specific HIV-1 consensus sequences.
Acknowledgments
We wish to thank Mary Kearney and Ann Wiegand for valuable scientific discussion and Sarah Cohen for sample management. This work was supported in part, by grants to John Mellors from the National Cancer Institute Science Applications International Corporation (SAIC) contract 20Xs190A, and by the Intramural Research Program of the NIH, the National Institute of Allergy and Infectious Disease (Virology Support Subcontract of the Adult AIDS Clinical Trials central Group U01AI38858). John Coffin was a Research Professor of the American Cancer Society with support from the George Kirby Foundation. The parent studies were funded by the National Department of Health of South Africa and Boehringer Ingelheim ZA, Johannesburg, South Africa. All participants in these studies have provided written informed consent for viral resistance testing.
Footnotes
The authors declare no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrose Z, Palmer S, Boltz VF, Kearney M, Larsen K, Polacino P, Flanary L, Oswald K, Piatak M, Jr, Smedley J, Shao W, Bischofberger N, Maldarelli F, Kimata JT, Mellors JW, Hu SL, Coffin JM, Lifson JD, KewalRamani VN. Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy. J Virol. 2007;81:12145–55. doi: 10.1128/JVI.01301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha RS, Zarbl H, Keohavong P, Thilly WG. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 1992;2:14–20. doi: 10.1101/gr.2.1.14. [DOI] [PubMed] [Google Scholar]
- Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–9. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- De Clercq E. HIV resistance to reverse transcriptase inhibitors. Biochem Pharmacol. 1994;47:155–69. doi: 10.1016/0006-2952(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Deeks SG. International perspectives on antiretroviral resistance. Nonnucleoside reverse transcriptase inhibitor resistance. J Acquir Immune Defic Syndr. 2001;26(Suppl 1):S25–33. doi: 10.1097/00042560-200103011-00004. [DOI] [PubMed] [Google Scholar]
- Halvas EKWA, Boltz VF, Kearney M, Nissley DWM, Hammer SM, Palmer SE, Vaida F, Coffin JM, MJW Low Frequency NNRTI-Resistant Variants Contribute to Failure of Efavirenz-Containing Regimens in Treatment-Experienced Patients. J Infect Dis. 2009 doi: 10.1086/650542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvas EK, Aldrovandi GM, Balfe P, Beck IA, Boltz VF, Coffin JM, Frenkel LM, Hazelwood JD, Johnson VA, Kearney M, Kovacs A, Kuritzkes DR, Metzner KJ, Nissley DV, Nowicki M, Palmer S, Ziermann R, Zhao RY, Jennings CL, Bremer J, Brambilla D, Mellors JW. Blinded, multicenter comparison of methods to detect a drug-resistant mutant of human immunodeficiency virus type 1 at low frequency. J Clin Microbiol. 2006;44:2612–4. doi: 10.1128/JCM.00449-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance AJ, Lemiale V, Izopet J, Lecossier D, Joly V, Massip P, Mammano F, Descamps D, Brun-Vezinet F, Clavel F. Changes in human immunodeficiency virus type 1 populations after treatment interruption in patients failing antiretroviral therapy. J Virol. 2001;75:6410–7. doi: 10.1128/JVI.75.14.6410-6417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan PR, Mo T, Wynhoven B, Hirsch J, Brumme Z, McKenna P, Pattery T, Vingerhoets J, Bacheler LT. Rare mutations at codon 103 of HIV-1 reverse transcriptase can confer resistance to non-nucleoside reverse transcriptase inhibitors. Aids. 2005;19:549–54. doi: 10.1097/01.aids.0000163930.68907.37. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Li JF, Morris L, Martinson N, Gray G, McIntyre J, Heneine W. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192:16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- Kwok S, Kellogg DE, McKinney N, Spasic D, Goda L, Levenson C, Sninsky JJ. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990;18:999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubser S, Balfe P, Sherman G, Hammer S, Kuhn L, Morris L. Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission. Aids. 2006;20:995–1002. doi: 10.1097/01.aids.0000222071.60620.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Picado J, Martinez M. HIV-1 reverse transcriptase inhibitor resistance mutations and fitness: a view from the clinic and ex vivo. Virus Research. 2008 doi: 10.1016/j.virusres.2007.12.021. doi:10,1016/j.virusres.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Metzner KJ, Bonhoeffer S, Fischer M, Karanicolas R, Allers K, Joos B, Weber R, Hirschel B, Kostrikis LG, Gunthard HF. Emergence of minor populations of human immunodeficiency virus type 1 carrying the M184V and L90M mutations in subjects undergoing structured treatment interruptions. J Infect Dis. 2003;188:1433–43. doi: 10.1086/379215. [DOI] [PubMed] [Google Scholar]
- Palmer S, Boltz V, Maldarelli F, Kearney M, Halvas EK, Rock D, Falloon J, Davey RT, Jr, Dewar RL, Metcalf JA, Mellors JW, Coffin JM. Selection and persistence of non-nucleoside reverse transcriptase inhibitor-resistant HIV-1 in patients starting and stopping non-nucleoside therapy. Aids. 2006a;20:701–10. doi: 10.1097/01.aids.0000216370.69066.7f. [DOI] [PubMed] [Google Scholar]
- Palmer S, Boltz V, Martinson N, Maldarelli F, Gray G, McIntyre J, Mellors J, Morris L, Coffin J. Persistence of nevirapine-resistant HIV-1 in women after single-dose nevirapine therapy for prevention of maternal-to-fetal HIV-1 transmission. Proc Natl Acad Sci U S A. 2006b;103:7094–9. doi: 10.1073/pnas.0602033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Boltz V, Maldarelli F, Martinson N, McIntyre J, Gray G, Hopley M, Hall D, Coffin J, Mellors J. Addition of short-course Combivir to single-dose Nevirapine reduces the selection of NVP-resistant HIV-1 with infrequent emergence of 3TC-resistant variants. 14th Conference on Retroviruses and Opportunistic Infections.2007. [Google Scholar]
- Rowley CF, Boutwell CL, Lockman S, Essex M. Improvement in allele-specific PCR assay with the use of polymorphism-specific primers for the analysis of minor variant drug resistance in HIV-1 subtype C. J Virol Methods. 2008;149:69–75. doi: 10.1016/j.jviromet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg MA. HIV resistance to nevirapine and other non-nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):S2–7. doi: 10.1097/00126334-200309011-00002. [DOI] [PubMed] [Google Scholar]