Abstract
Genetic manipulations, with or without lineage tracing for specific pancreatic cell types, are very powerful tools for studying diabetes, pancreatitis and pancreatic cancer. Nevertheless, the use of Cre/loxP systems to conditionally activate or inactivate the expression of genes in a cell type– and/or temporal-specific manner is not applicable to cell tracing and/or gene manipulations in more than one lineage at a time. Here we report a technique that allows efficient delivery of dyes for cell tagging into the mouse pancreas through the duct system, and that also delivers viruses carrying transgenes or siRNA under a specific promoter. When this technique is applied in genetically modified mice, it enables the investigator to perform either double lineage tracing or cell lineage tracing combined with gene manipulation in a second lineage. The technique requires <40 min.
INTRODUCTION
Diabetes mellitus has become a major health problem worldwide1. Pancreatic cancer is the fourth leading cause of cancer mortality, with a 5-year overall survival of only 6% (ref. 2). Thus, pancreatic research is of the utmost importance.
As Cre/loxP is a site-specific recombinase system of proven efficacy, it is frequently used in mouse studies of pancreatic development, diabetes, pancreatitis and pancreatic cancer. However, this system only allows conditional activation or inactivation of genes in a cell type– and/or temporal-specific manner in one lineage at a time. Although a combination of Cre/loxP system with other site-specific recombinase systems such as Flp/FRT or Dre/rox could theoretically provide manipulation of multiple lineages concurrently, the incurred complexity of mouse breeding and the stability of the genome present formidable challenges to achieving this3–5.
In this protocol, we present a technique based on previous publications6–12, which uses pancreatic intraductal infusion to target cell-tagging dyes, or viruses carrying transgenes or siRNA under a specific promoter, to specific pancreatic cell types13–16. When this approach is used in genetically modified mice, it can allow either double lineage tracing or lineage tracing of one lineage combined with gene manipulations in a second lineage.
Application and advantages over other methods
Intraductal infusion of a cell-tagging dye15, as well as an adeno-associated virus (AAV) carrying Cre recombinase under a Sox9 (duct-cell-specific) promoter, into an R26RTomato reporter mouse13,17 allows efficient and specific labeling of pancreatic duct cells. Specifically, visualization of the infused dyes in duct cells takes <1 h, and expression of the transgenes in AAV takes 12–24 h. This may be very useful for lineage-tracing studies of beta cell regeneration, diabetes, pancreatic cancer and so on. Similarly, infusion of a virus carrying the Cre recombinase under the control of another cell-specific promoter into a reporter mouse would allow specific labeling of other cell types in the pancreas. For example, infusion of an AAV carrying insulin-Cre into an R26RTomato reporter mouse results in specific labeling of beta cells; infusion of an AAV carrying glucagon-Cre into an R26RTomato reporter mouse results in specific labeling of alpha cells; and infusion of an AAV carrying elastase-Cre into an R26RTomato mouse results in specific labeling of acinar cells.
Intraductal infusion of a virus carrying a transgene or shRNA under specific promoters can be used to alter gene expression in specific type(s) of pancreatic cells, thus resulting in specific over-expression or knockdown of target genes. For example, we have recently used an AAV carrying a shRNA against vascular endothelial growth factor (VEGF) under the Sox9 promoter to specifically knock down VEGF production in pancreatic duct cells16. We have also recently used an AAV carrying a recombinant SMAD family member 7 (SMAD7) under the insulin promoter, to specifically overexpress SMAD7 in pancreatic beta cells14. Pancreatic duct infusion has also been successfully performed to induce biliary pancreatitis, for studying the onset of clinical biliary pancreatitis7.
This technology could potentially have clinical applications as well, as humans undergo pancreatic duct injections on a routine basis, without the need for surgery, through endoscopic retrograde cholangiopancreatography (ERCP). However, AAV delivery by ERCP may be difficult and is not currently used as a route of administration in patients with pancreatic cancer. Thus, delivery of therapeutic virus or drugs via ERCP may provide new options for treating some pancreatic diseases.
Limitations
Intraductal infusion with a virus or a dye may cause pancreatitis. The use of a cell-tagging dye only labels duct cells. Among the viruses available for gene therapy, AAV appears to be the best choice for intraductal infusion, owing to its safety, long-term expression of the transgene and its apparent lower risk of inducing inflammation (compared with lentivirus and adenovirus)8,18–20. Intraductal infusion of an AAV entails a limited-sized construct, as the total insert for AAV vector should be <5 kb (refs. 8,19).
Experimental design
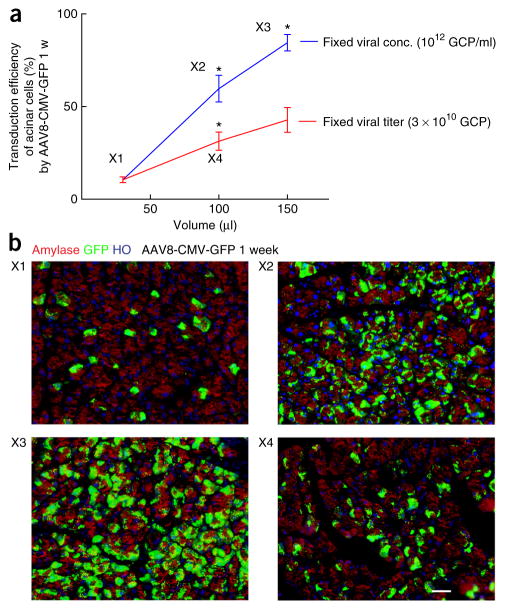
The details of this intraductal infusion system may need to be changed depending on the specific experimental goal. The volume of the infused reagents and the speed of the infusion both affect the outcome. When the virus is infused with a fixed viral concentration, the transduction efficiency increases with the infused viral volume. When the virus is infused with a fixed total viral amount, the transduction efficiency increases with the infused viral volume, suggesting that if virus availability is limited the transduction efficiency can be increased by diluting the virus in an increased volume (Fig. 1). If specific targeting of dyes or virus to pancreatic duct cells is needed, a lower infusion volume, but at a much slower infusion rate, is optimal. Targeting of the entire pancreas, or specifically the endocrine pancreas, requires a higher volume of infusate, but here the infusion can be at a higher rate. The infusion details, which have been worked out in our laboratory, are shown in Table 1. The transduction efficiency of major pancreatic cell types by various infusion methods has been summarized in Table 2. As the duration of surgery is ~40 min per mouse, which limits the number of mice that can be injected per day, the choice of the infusion time needs to be carefully justified because it can contribute markedly to the procedure time.
Figure 1.
The transduction efficiency in acinar cells 1 week after AAV8-CMV-GFP infusion at different viral volumes. (a) The changes in the transduction efficiency of acinar cells with the changes in the infused viral volume when either fixed viral concentration (conc.) or fixed viral titer. X1–X4 represent different infusion conditions. Error bars are s.e.m. n = 5. *P < 0.05 compared with a shown smaller volume. (b) Representative pancreatic images of amylase by staining with an anti-amylase antibody (red; Sigma), GFP by direct fluorescence (green) and HO (Hoechst 33342; blue, nuclei staining) at X1–X4 1 week after AAV8-CMV-GFP infusion. Scale bar, 20 μm.
TABLE 1.
Choice of infusion method (infusion volume, rate).
| Volume (based on body weight)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Body weight | Target | Concentration | 15–20 g | 20–30 g | 30–45 g | 45–60 g | Infusion rate | Infusion time (20–30 g) |
| CFDA-SE | Duct cells | 10 μM | 25 μl | 30 μl | 35 μl | 35 μl | 1 μl/min | 30 min |
| DDAO-SE | Duct cells | 10 μM | 25 μl | 30 μl | 35 μl | 35 μl | 1 μl/min | 30 min |
| AAV6-Sox9P-X | Duct cells | 1012 GCPs/ml | 25 μl | 30 μl | 35 μl | 35 μl | 1 μl/min | 30 min |
| AAV8-CMV-X | All pancreatic cells | 1012 GCPs/ml | 120 μl | 150 μl | 165 μl | 180 μl | 6 μl/min | 25 min |
| AAV8-RIP-X | Beta cells | 1012 GCPs/ml | 120 μl | 150 μl | 165 μl | 180 μl | 6 μl/min | 25 min |
GCPs, genome copy particles.
TABLE 2.
Transduction efficiency of AAV infusion.
| Transduction efficiency (%) | Acinar cells | Duct cells | Beta cells | Alpha cells |
|---|---|---|---|---|
| AAV8-CMV-GFP | 84.5 ± 7.9 | 1.2 ± 0.4 | 71.5 ± 9.2 | 65.9 ± 8.2 |
| AAV6-CMV-GFP | 55.1 ± 7.3 | 44.2 ± 6.4 | 23.9 ± 5.5 | 18.6 ± 4.5 |
| AAV8-RIP-GFP | <0.1% | <0.1% | 75.6 ± 7.6 | <0.1% |
The infusion was performed on 20–30-g mice, and thus 150 μl of 1012 GCPs/ml AAV was infused at a rate of 6 μl/min. Quantification was performed from five mice in each condition, and was presented as mean ± s.e.m. For duct labeling efficiency and specificity by CFDA-SE, or by AAV6-Sox9p-Cre to R26RRTomato mice, or by AAV6-Sox9p-shVEGFa, please refer to previous publications13,15,16. GCPs, genome copy particles.
Controls
Control mice should be included and treated in the exact same way as the experimental mice. To control for infusion of dyes, a control infusate consists of the same volume of DMSO-PBS, the vehicle for the carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE) and N,N-dimethyldodecylamine-N-oxide succinimidyl (DDAO-SE). In the case of infusion of transgene/ siRNA viruses, viruses carrying empty vectors or reporter only (e.g., GFP) are used as controls.
MATERIALS
REAGENTS
We have used both male and female wild-type and transgenic mice, all with a C57BL/6 background. Ages ranged from 3 weeks to 1 year, and the mice weighed 10–60 g. We achieved satisfactory results and survival of all the treated animals ! CAUTION Experiments involving rodents must conform to all relevant institutional and governmental regulations.
Hoechst 33342 (Cell Signaling Technology)
Isoflurane (2–2.5% (wt/vol), Butler Schein, cat. no. 11695-6776-2)
Nair hair remover
PBS (Life Technologies, cat. no. 14190-144)
CellTrace far red DDAO-SE (Invitrogen, cat. no. C34553)
Vybrant CFDA-SE cell tracer kit (Life Technologies, cat. no. V12883)
AAVs (serotype 6 and serotype 8, with various transgene/siRNA under various promoters; virus production is beyond the scope of this protocol and can be referred to in previous publications21–24)
Ketoprofen (Fort Dodge Animal Health)
EQUIPMENT
Syringe (for loading virus or dyes; NORM-JECT, cat. no. 4010-200V0)
Gauze (4 inch × 4 inch; Fisher Scientific, cat. no. 19-160-277)
Infusion syringe pump (New Era Pump Systems, model ME-300)
Infusion catheter (World Precision Instruments, cat. no. CMF31G; the type of material for catheters is a combination of plastic and fused silica (no metal components are used))
Sutures: 6-0 suture (Ethicon, cat. no. K802H) is used to close the hole in the duodenum and 4-0 suture (Ethicon, cat. no. VCP214H) is used to close the abdomen of the mice
Cotton applicator (Q-tips, Puritan Medical Products Company LLC, cat. no. 806-WC)
Surgical instruments: microclamp (Roboz, cat. no. RS-7439); needle holder (Roboz, cat. no. RS-7882); micro-dissecting forceps (Roboz, cat. no. RS-5163); Hudson forceps (Roboz, cat. no. RS-5237); and micro-dissecting scissors (Roboz, cat. no. RS-5982)
Dissecting microscope (Olympus SZX-ILLK100, Olympus)
REAGENT SETUP
Virus solution
Prepare a certain volume of 1012 genome copy particle (GCP) ssAAV in PBS. A biosafety cabinet should be used for AAV manipulation. Viruses can be stored at −70 °C for more than 2 years.
CFDA-SE or DDAO-SE solution
The CFDA-SE (green fluorescence) or DDAO-SE (far-red fluorescence) cell tracer kits provide a versatile and well-retained cell-tracing reagent in a convenient and easy-to-use form. Small-scale experiments can be performed without preparing excess quantities of perishable CFDA-SE or DDAO-SE stock solution, according to the manufacturer’s instructions. The injection solution should be freshly prepared.
EQUIPMENT SETUP
Infusion equipment
First load the desired volume of dyes or viruses into the syringe mounted to the infusion catheter (Table 1), and then set it firmly into the infusion syringe pump. The proper infusion rate should be adjusted and then the infusion stream should be tested to ensure that it is free of obstruction. ▲ CRITICAL Avoiding air bubble formation inside the syringe and the catheter is crucial for achieving satisfactory infusion results.
Surgery apparatus
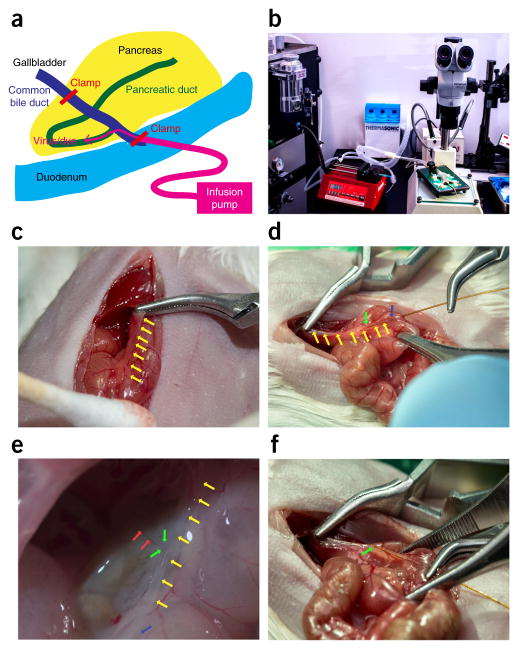
The surgery is performed on a prewarmed plate, with the isoflurane anesthetic machine equipped nearby to supply the mouse via inhalation. After a laparotomy and exposure of the duodenum and pancreas, a 31-gauge blunt-ended catheter is placed into the duct through the sphincter of Oddi in the duodenum, and it is connected to a microinfusion apparatus to allow slow and stable infusion of reagents (see below for details; Fig. 2a,b).
Figure 2.
Intraductal infusion surgery. (a) Schematic of the organization of the infusion. (b) Actual organization of the equipment. (c) A microclamp is placed on the bile duct near the liver to prevent perfusion of the liver. (d) A 30-gauge needle is used to make a small duodenal hole opposite to the sphincter of Oddi. Next, a 31-gauge blunt-ended catheter is carefully placed into the biliary-pancreatic duct through the sphincter of Oddi in the duodenum. (e) The tip of the catheter should be positioned at the origin of the pancreas branch in the biliary-pancreatic duct. (f) The catheter can be then secured with the second microclamp at the sphincter of Oddi to prevent backflow into the duodenum. Yellow arrows point to the biliary-pancreatic duct, green arrows point to the tip of the catheter in the biliary-pancreatic duct, blue arrows point to the sphincter of Oddi and red arrows point to the pancreatic duct branch in the biliary-pancreatic duct.
PROCEDURE
Anesthesia ● TIMING 1 min
-
1|
Anesthetize mice with 2–2.5% (wt/vol) isoflurane by inhalation.
▲ CRITICAL STEP The inhalation should be closely adjusted, according to the breathing and heart rate of the mice during surgery. A typical infusion surgery takes 30–40 min. Unnecessary delays in the surgery should be avoided.
Preparation of the mouse for surgery ● TIMING 1 min
-
2|
Put the mouse onto the 37 °C warming pad that has been cleaned three times with alternate changes of Betadine and ethanol (or 3× chlorhexidine/ethanol). Immobilize the mouse on the plexiglass using surgical tape and use Nair to remove hair from the lower chest and abdomen. Clean and disinfect the now hairless area with gauze soaked in 70% (vol/vol) ethanol.
Laparotomy ● TIMING 1 min
-
3|
Use scissors to incise the skin at the midline of the abdomen. Next, cut the abdominal muscle along the linea alba to create a midline upper abdominal laparotomy incision of 1.5 cm.
▲ CRITICAL STEP Avoid injuring any underlying organs when performing the laparotomy.
Exposure of the pancreatic duct ● TIMING 0.5 min
-
4|
Use Q-tips to gently pull out the stomach. Rotate and stretch the duodenum to expose the biliary-pancreatic duct and its junction with the duodenum (the sphincter of Oddi), which appears pale (Fig. 2c).
▲ CRITICAL STEP The stomach, duodenum and pancreas should be handled mainly with Q-tips. Avoiding pinches with steel instruments may substantially reduce tissue trauma, edema and bleeding, and it will be crucial to achieving reliable experimental results and good recovery of the mouse. If the procedure is performed properly, pancreatitis will be extremely infrequent. Aside from cotton applicators, carefully handled steel surgical instruments such as ring forceps can also be used to manipulate the duodenum without causing tissue trauma. Care must also be taken to avoid overstretching the duodenum, which may cause injury to the duodenum, and may even distort the biliary-pancreatic duct. Intestine outside the abdominal cavity should be covered with moistened gauze (with warm saline) to avoid substantial heat loss and drying of the tissues.
? TROUBLESHOOTING
Cannulation of the duct ● TIMING 0.5–3 min
-
5|
Perform a slight backward rotation of the duodenum to expose the distal biliary-pancreatic duct. With a view of the back side of the duodenum and the duodenal portion of the pancreas, the region of the papilla should also be visible (Fig. 2c).
▲ CRITICAL STEP Surgical exposure is crucial, as a microclamp needs to be precisely placed on the common bile duct above the branch-point of the pancreatic duct, to prevent infusion into the liver (Fig. 2c).
? TROUBLESHOOTING
-
6|
To prevent perfusion of the liver, place a microclamp on the bile duct above the branching of the pancreatic duct. When the first clamp is placed (Fig. 2c), clamping of the portal vein located immediately below the common bile duct should be avoided.
-
7|
Use a 30-gauge needle to make a small hole opposite to the sphincter of Oddi, 1–2 mm away from the ampulla of Vater. Next, pass a 31-gauge blunt-ended cannula through the small hole that has been created by the 30-gauge needle in the duodenal wall and then the sphincter of Oddi (Fig. 2d). The tip of the catheter should be positioned at the origin of the pancreatic duct branch in the biliary-pancreatic duct (Fig. 2e). If the anatomy is unclear, the tip of the catheter can be placed just past the sphincter of Oddi.
▲ CRITICAL STEP Cannulation is the most important and most difficult part of the protocol, and it requires stable hands and careful manipulation. A blunt-ended catheter helps reduce the risk of damage to the duct.
-
8|
Clamp the papilla around the catheter with another microclamp at the sphincter of Oddi to prevent backflow or leaking during the viral infusion (Fig. 2f). The microclamp is of low pressure to avoid injury of the duodenum. The back end of the catheter is connected to a microinfusion apparatus.
Infusion ● TIMING 25–30 min
-
9|
Turn on the pump and infuse the predetermined amount of solution, at the flow rate determined as outlined in Table 1.
? TROUBLESHOOTING
Removal of the cannula and microclamps ● TIMING 0.5 min
-
10|
After the infusion is completed, remove the microclamp on the sphincter of Oddi and withdraw the catheter gently from the duct. Next, remove the microclamp on the biliary-pancreatic duct.
Closure ● TIMING 2 min
-
11|
After infusion, close the hole in the duodenum created by the catheter with a 6-0 suture. This suture does not seem to affect the experimental results, nor does it seem to affect survival of the mice.
-
12|
Return the intestines to the abdomen and suture the abdominal muscles with a 4-0 suture using a running stitch.
Postoperative care ● TIMING 10 min
-
13|
Return the mouse to its cage on a heated pad (37 °C) until it fully recovers. Give ketoprofen (Sigma, cat. no. k1751) at a dose of 5 mg/kg s.c. once per day continuously for 3 d after surgery for analgesia.
? TROUBLESHOOTING
Step 4: successful exposure with Q-tips
All efforts need to be made to expose the duodenum quickly but without damage. The use of Q-tips is important. Sufficient mouse dissection practice will help to most efficiently and promptly locate and position the duodenum. In addition, Q-tips are especially important for safely separating organs when there are preexisting adhesions due to the use of tamoxifen and other i.p. injected drugs.
Step 5: cannulation of the duct
The cannulation is not challenging for experienced surgeons, but practice is required. If the portal vein is clamped, the intestine becomes dark owing to venous congestion. If the papilla is not properly cannulated, instead of entering the catheter into the common biliary-pancreatic duct, direct injection of the exocrine pancreas parenchyma may occur. This event may be less likely with our modified infusion protocol, as we use a blunt-ended catheter. The use of the blunt-ended catheter also allows us to gently move the tip of the catheter in order to help determine whether the catheter is properly placed in the biliary-pancreatic duct. As bleeding may affect the interpretation of the data, it should be avoided. Seldom did we find substantial bleeding in the procedure, and in such situations the animal was excluded from the experiment.
Step 9: infusion
As mentioned previously, the avoidance of air bubbles in the infusion system is very important in order to avoid pancreatic damage. In addition, the catheter can become obstructed before and during the infusion. It is crucial to clean the catheter after infusion with distilled water, rather than with PBS, as PBS salts may precipitate after drying out. Proper care of the catheter will allow it to be used indefinitely.
The speed of the infusion should be strictly controlled, according to guidelines in Table 1, because the infusion rate can markedly affect the results. Too fast a speed may result in only partial perfusion of the pancreas, leaving the tail pancreas unperfused. In addition, for infusion with dyes to label duct cells, too rapid an infusion may increase the pressure inside the duct and may result in leakage of the dye outside of the duct system, and thus nonspecific labeling, as discussed previously15. On the basis of our previous experience, a properly performed infusion should not cause marked pancreatitis13–16.
● TIMING
Step 1, anesthesia: 1 min
Step 2, preparation of the mouse for surgery: 1 min
Step 3, laparotomy: 1 min
Step 4, exposure of the pancreatic duct: 0.5 min
Steps 5–8, cannulation of the duct: 0.5–3 min
Step 9, infusion: 25–30 min
Step 10, removal of the cannula and microclamps: 0.5 min
Steps 11 and 12, closure: 2 min
Step 13, postoperative care: 10 min
ANTICIPATED RESULTS
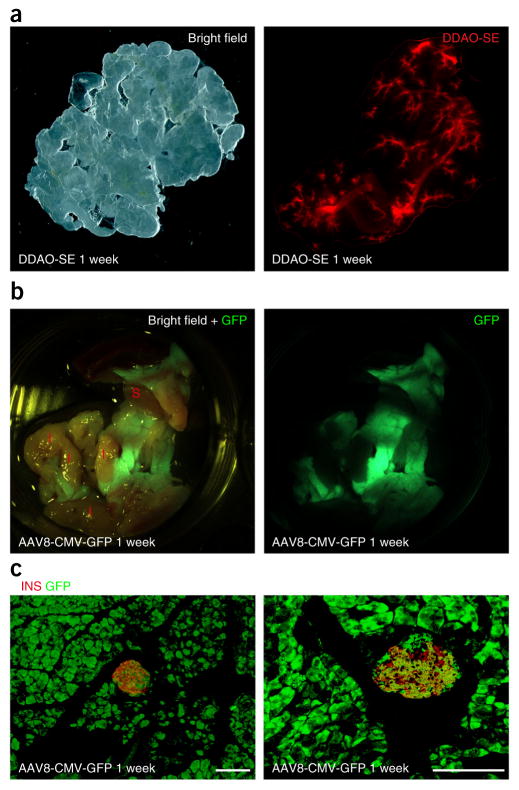
When a cell-tagging dye is infused, the duct system can be specifically and efficiently labeled within 1 h (Fig. 3a; see also Xiao et al.15). When an AAV carrying Cre recombinase under a Sox9 (duct cell–specific) promoter is infused into an R26RTomato reporter mouse, duct cells can be specifically and efficiently labeled (see Guo et al.13). When an AAV carrying shRNA against VEGF under the Sox9 promoter is infused, it can specifically and efficiently knock down VEGF production in pancreatic duct cells (see Xiao et al.16). When an AAV carrying a recombinant SMAD7 under the insulin promoter is infused, it results in specific overexpression of SMAD7 in pancreatic beta cells (see Xiao et al.14). When an AAV carrying a GFP reporter under a cytomegalovirus (CMV) promoter is infused, it results in nearly complete expression of GFP throughout the pancreas (Fig. 3b,c). We found that infusion with AAV resulted in the expression of the transgenes in 12–24 h.
Figure 3.
Representative infusion images. Infusion conditions were determined according to Table 1. (a) Representative gross images of the pancreas 1 week after DDAO-SE infusion. (b,c) Representative gross (b) and immunohistochemical images (c) of the pancreas 1 week after AAV8-CMV-GFP infusion. GFP, direct green fluorescence (green); I, intestine; INS, insulin staining (red); S, spleen. Scale bars, 50 μm.
Acknowledgments
This work was supported, in whole or in part, by the Cochrane-Weber endowed Fund in Diabetes Research (X.X., NO19831) and the US National Institutes of Health (G.K.G., R01 DK098196).
Footnotes
AUTHOR CONTRIBUTIONS The concept of the study was designed by X.X. Surgery was developed and performed by X.X., P.G. and G.K.G. Viruses were produced by P.G. Surgery photographs were taken by K.P. Data were collected by X.X., P.G., K.P., C.S., L.P., S.F., I.G., Y.E.G., S.Z.H. and J.W. X.X., S.Z.H. and G.K.G. wrote the article.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Weir GC, Bonner-Weir S. Islet beta cell mass in diabetes and how it relates to function, birth, and death. Ann NY Acad Sci. 2013;1281:92–105. doi: 10.1111/nyas.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werner J, et al. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol. 2013;10:323–333. doi: 10.1038/nrclinonc.2013.66. [DOI] [PubMed] [Google Scholar]
- 3.Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013;18:9–20. doi: 10.1016/j.cmet.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao X, et al. No evidence for beta cell neogenesis in murine adult pancreas. J Clin Invest. 2013;123:2207–2217. doi: 10.1172/JCI66323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Laukkarinen JM, Van Acker GJ, Weiss ER, Steer ML, Perides G. A mouse model of acute biliary pancreatitis induced by retrograde pancreatic duct infusion of Na-taurocholate. Gut. 2007;56:1590–1598. doi: 10.1136/gut.2007.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perides G, van Acker GJ, Laukkarinen JM, Steer ML. Experimental acute biliary pancreatitis induced by retrograde infusion of bile acids into the mouse pancreatic duct. Nat Protoc. 2010;5:335–341. doi: 10.1038/nprot.2009.243. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez V, et al. In vivo genetic engineering of murine pancreatic beta cells mediated by single-stranded adeno-associated viral vectors of serotypes 6, 8 and 9. Diabetologia. 2011;54:1075–1086. doi: 10.1007/s00125-011-2070-3. [DOI] [PubMed] [Google Scholar]
- 9.Husain SZ, et al. Ryanodine receptors contribute to bile acid-induced pathological calcium signaling and pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1423–G1433. doi: 10.1152/ajpgi.00546.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittel UA, et al. Taurocholate-induced pancreatitis: a model of severe necrotizing pancreatitis in mice. Pancreas. 2008;36:e9–e21. doi: 10.1097/MPA.0b013e3181575103. [DOI] [PubMed] [Google Scholar]
- 11.Jose A, et al. Intraductal delivery of adenoviruses targets pancreatic tumors in transgenic Ela-myc mice and orthotopic xenografts. Oncotarget. 2013;4:94–105. doi: 10.18632/oncotarget.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loiler SA, et al. Localized gene expression following administration of adeno-associated viral vectors via pancreatic ducts. Mol Ther. 2005;12:519–527. doi: 10.1016/j.ymthe.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Guo P, et al. Specific transduction and labeling of pancreatic ducts by targeted recombinant viral infusion into mouse pancreatic ducts. Lab Invest. 2013;93:1241–1253. doi: 10.1038/labinvest.2013.113. [DOI] [PubMed] [Google Scholar]
- 14.Xiao X, et al. M2 macrophages promote beta-cell proliferation by upregulation of SMAD7. Proc Natl Acad Sci USA. 2014;111:E1211–E1220. doi: 10.1073/pnas.1321347111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao X, et al. Neurogenin3 activation is not sufficient to direct duct-to-beta cell transdifferentiation in the adult pancreas. J Biol Chem. 2013;288:25297–25308. doi: 10.1074/jbc.M113.484022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao X, et al. Pancreatic duct cells as a source of VEGF in mice. Diabetologia. 2014;57:991–1000. doi: 10.1007/s00125-014-3179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, et al. Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes. Diabetes. 2006;55:875–884. doi: 10.2337/diabetes.55.04.06.db05-0927. [DOI] [PubMed] [Google Scholar]
- 18.Guo P, et al. A simplified purification method for AAV variant by polyethylene glycol aqueous two-phase partitioning. Bioengineered. 2012;4:103–106. doi: 10.4161/bioe.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo P, et al. Rapid and simplified purification of recombinant adeno-associated virus. J Virol Methods. 2012;183:139–146. doi: 10.1016/j.jviromet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haurigot V, et al. Whole-body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest. 2013;123:3254–3271. doi: 10.1172/JCI66778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan IF, Hirata RK, Russell DW. AAV-mediated gene targeting methods for human cells. Nat Protoc. 2011;6:482–501. doi: 10.1038/nprot.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajitou A, et al. Design and construction of targeted AAVP vectors for mammalian cell transduction. Nat Protoc. 2007;2:523–531. doi: 10.1038/nprot.2007.51. [DOI] [PubMed] [Google Scholar]
- 23.Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- 24.Koerber JT, Maheshri N, Kaspar BK, Schaffer DV. Construction of diverse adeno-associated viral libraries for directed evolution of enhanced gene delivery vehicles. Nat Protoc. 2006;1:701–706. doi: 10.1038/nprot.2006.93. [DOI] [PubMed] [Google Scholar]