Abstract
The glucose transporter GLUT1 at the blood-brain barrier (BBB) mediates glucose transport into the brain. Alzheimer's disease is characterized by early reductions in glucose transport associated with diminished GLUT1 expression at the BBB. Whether GLUT1 reduction influences disease pathogenesis remains, however, elusive. Here, we show that GLUT1 deficiency in mice overexpressing amyloid β-petpide (Aβ) precursor protein leads to: 1) early cerebral microvascular degeneration, blood flow reductions and dysregulation, and BBB breakdown; and (2) accelerated amyloid β-peptide (Aβ) pathology, reduced Aβ clearance, diminished neuronal activity, behavioral deficits, and progressive neuronal loss and neurodegeneration that develop after initial cerebrovascular degenerative changes. We also show that GLUT1 deficiency in endothelium, but not in astrocytes, initiates the vascular phenotype as shown by BBB breakdown. Thus, reduced BBB GLUT1 expression worsens Alzheimer's disease cerebrovascular degeneration, neuropathology and cognitive function suggesting that GLUT1 may represent a novel therapeutic target for Alzheimer's disease vasculo-neuronal dysfunction and degeneration.
Introduction
The glucose transporter GLUT1, encoded by SLC2A1, mediates glucose transport into the brain1, 2. GLUT1 is expressed at the blood-brain barrier (BBB), but not in neurons1, 2. GLUT1 exists in two isoforms – a 55 kDa isoform in brain endothelial cells and a 45 kDa isoform in adjacent astrocyte end-foot processes3. The crystal structure of human GLUT1 has been recently reported4. Brain glucose uptake correlates with GLUT1 levels in cerebral microvessels5-7. GLUT1 deficiency is found in patients with epilepsy, movement disorders and cognitive impairment8. In mice, GLUT1 haploinsufficiency (Slc2a1+/−) diminishes glucose cerebrospinal fluid (CSF) levels and leads to microencephaly9, 10. In the zebrafish, GLUT1 is required for the formation of the BBB11, raising a possibility for its dual role similar to twofold role of the major facilitator family domain containing 2a (MFSD2A) transporter, which transports essential fatty acids across the BBB into the brain and regulates BBB integrity12-14.
Dementia due to Alzheimer's disease (AD) is characterized by progressive metabolic disturbances15, neurovascular dysfuction1, 2 and BBB breakdown16. Diminished glucose uptake in the hippocampus, parietotemporal cortex and/or posterior cingulate cortex in individuals at genetic risk for AD17,18, positive family history19, and/or mild or no cognitive impairment who develop AD20, 21 has been shown by 2-[18F] fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET). FDG-PET changes precede brain atrophy and neuronal dysfunction in humans18, 22 and transgenic AD models23, which may reflect reductions in BBB glucose transport24,25. Reduced GLUT1 levels in cerebral microvessels were also found in AD26-29. Whether GLUT1 reductions can lead to cerebrovascular damage contributing to and/or accelerating AD-like neurodegeneration remains, however, elusive.
To address this question, we crossed transgenic mice overexpressing human amyloid β-peptide (Aβ) precursor protein (APPSw/0)30 with GLUT1-deficient Slc2a1+/− mice9. We also utilized conditional Slc2a1 lox/lox mice31 to determine the effects of cell-specific GLUT1 deletions from endothelium and astrocytes on the BBB phenotype. We show that GLUT1 is necessary for the maintenance of proper brain angioarchitecture, cerebral blood flow (CBF) and BBB integrity, and that GLUT1 reductions in APPSw/0 mice accelerate Aβ accumulation and lead to progressive neuronal dysfunction, behavioral deficits, neuronal loss and neurodegeneration that develop after initial cerebrovascular changes. We also show that GLUT1 deficiency in endothelium, but not in astrocytes, initiates the BBB breakdown. Our data suggest that GLUT1 reductions at the BBB play an early pathogenic role in neuronal demise in an AD-like neurodegenerative process.
Results
Microvascular reductions and diminished cerebral blood flow and glucose uptake in GLUT1-deficient APPSw/0 mice
Immunofluorescent staining for endothelial-specific Lycopersicon esculentum lectin and GLUT1 (Supplementary Fig 1a-b) and immunoblotting of brain microvessels (Supplementary Fig 1c-d), show ~50% reduction in GLUT1 brain endothelial levels in 6 month-old Slc2a1+/− and Slc2a1+/−APPSw/0 mice compared to their respective age-matched littermate controls (Supplementary Fig 1a-d). There was no difference in GLUT1 levels between Slc2a1+/+APPSw/0 and Slc2a1+/+ mice and/or Slc2a1+/−APPSw/0 and Slc2a1+/− mice (Supplementary Fig 1a-d).
No alterations in blood glucose were detected in Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 or Slc2a1+/−APPSw/0 mice (Supplementary Fig 1e). In contrast, significant 57% and 70% reductions in CSF glucose levels and the CSF-to-blood glucose ratios were found in 6-month-old Slc2a1+/− and Slc2a1+/−APPSw/0 mice compared to Slc2a1+/+ littermates (Supplementary Fig 1e-f). CSF glucose levels were also reduced by ~25% in APPSw/0 mice despite unaltered GLUT1 BBB levels. Notably, microvascular and CBF reductions, as found in early disease stage in APP lines32-34 (see Fig 1 below), can both diminish glucose brain uptake independently of GLUT1 expression by reducing the capillary surface area available for glucose transport and the flow-dependent delivery of glucose to the brain1, 2, respectively.
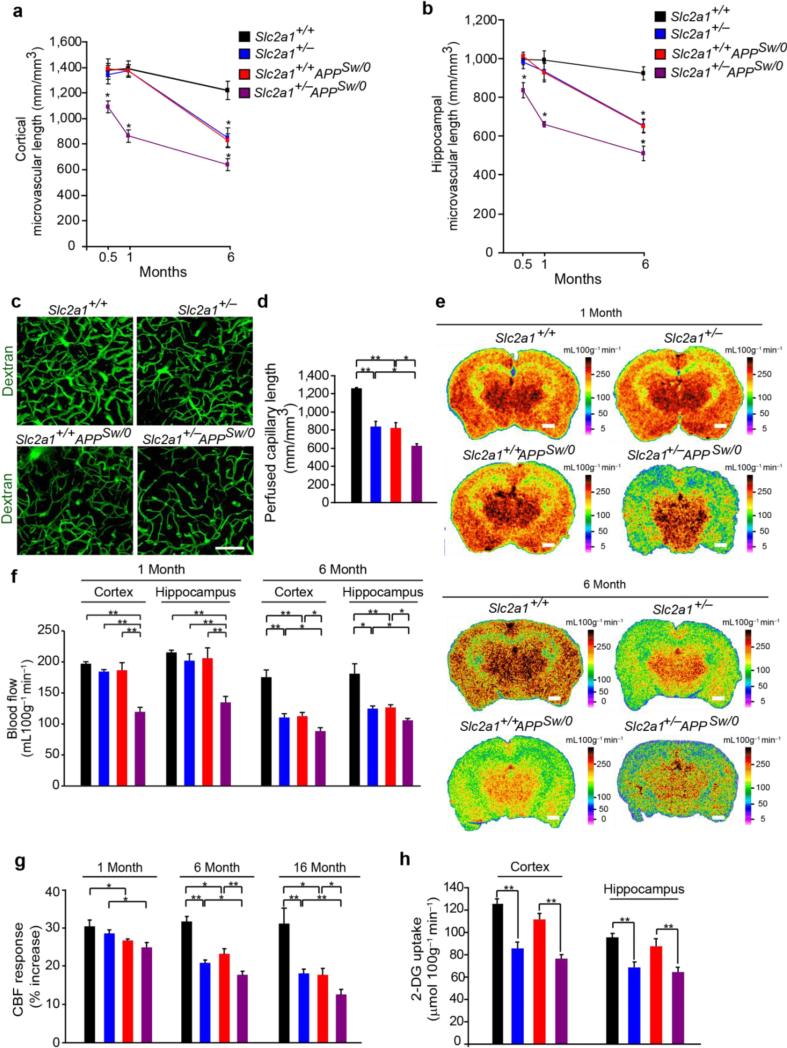
Figure 1. Microvascular and cerebral blood flow reductions and diminished glucose uptake in GLUT1-deficient APPSw/0 mice.
(a-b) Quantification of lectin-positive microvascular profiles in cortex (b) and hippocampus (b) in 2-week, 1- and 6-month old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Mean ± SEM, n=3-6 mice per group; *p<0.05. (c-d) Representative in vivo multiphoton fluorescent angiograms obtained following intravascular injection of fluorescein-conjugated dextran (MW: 70 kDa) (c) and quantification of perfused microvascular length (d) in 6-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Scale bar, 50 μm. Mean ± SEM, n=3-4 mice per group; *p<0.05 or **p<0.01 (e-f) Representative C14-iodoantipyrine (C14-IAP) autoradiography (e) and quantification of C14-IAP autoradiograms measuring regional blood flow in the somatosensory cortex and CA1 hippocampal subfield (f) in 1- and 6-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Scale bar, 1 mm. Mean ± SEM, n=3-5 mice per group; *p<0.05 or **p<0.01. (g) Cerebral blood flow (CBF) responses to brain activation in the somatosensory cortex of 1-month, 6-month and 16-month old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. (h) Quantification of C14-2-deoxyglucose (2-DG) autoradiograms measuring regional glucose uptake in the somatosensory cortex and CA1 hippocampal subfield in 2-3 week-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Mean ± SEM, n=5-7 mice per group in a, b, d, f and g; and n=3 mice per group in h; *p<0.05 or **p<0.01.
To evaluate whether reduced BBB GLUT1 expression affects brain microvascular structure, we analyzed the length of capillary networks using lectin-positive profiles (as in Supplementary Fig 1a). We found significant 21% and 16% reductions in capillary length in 2 week-old Slc2a1+/−APPSw/0 mice in somatosensory cortex and hippocampus, respectively, when compared to other genotypes, which progress with age as shown by greater 37% and 33% and 54% and 49% reductions in capillary length in cortex and the CA1 hippocampal subfield in 1- and 6-month-old Slc2a1+/−APPSw/0 mice compared to Slc2a1+/+ littermate controls, respectively (Fig 1a-b). Slc2a1+/− and Slc2a1+/+APPSw/0 mice displayed less pronounced microvascular reductions only at a later stage at 6 months of age (Fig 1a-b).
To find out whether brain capillary reductions determined by ex vivo histologic quantification reflect a perfusion deficit in vivo, we utilized in vivo multiphoton microscopy following injection of a fluorescein-conjugated dextran vascular tracer (MW: 70,000 Da) to generate 3D 0.5 mm Z stack cortical angiograms. Analysis of angiograms revealed 32%, 26% and 48% reductions in perfused cortical capillary length in 6-month-old Slc2a1+/−, Slc2a1+/+APPSw/0 or Slc2a1+/−APPSw/0 mice compared to age-matched Slc2a1+/+ littermates, respectively (Fig 1c-d), suggesting that diminished BBB GLUT1 expression or APP overexpression is sufficient to reduce brain capillary density resulting in a perfusion deficit, but when acting together they exert strong synergistic effect causing early loss of brain capillaries at 2 weeks of age as shown in Slc2a1+/−APPSw/0 mice.
As regional brain capillary density closely correlates with regional CBF1, we next evaluated whether brain capillary reductions lead to diminished resting CBF. 14C-iodoantipyrine quantitative autoradiography revealed approximately 39% and 37% reductions in cortical and hippocampal blood flow in 1-month-old Slc2a1+/−APPSw/0 mice, respectively, when compared to other genotypes (Fig 1e-f). Slc2a1+/− and Slc2a1+/+APPSw/0 mice did not show reductions in cortical and hippocampal blood flow at 1 month of age. Within 6 months, however, both Slc2a1+/− and Slc2a1+/+APPSw/0 mice developed significant 36% and 31% and 36% and 30% reductions in cortical and hippocampal blood flow that were less pronounced compared to 50% and 45% reductions found in Slc2a1+/−APPSw/0 mice, respectively (Fig 1e-f). Hemodynamic and physiologic parameters which may influence CBF including mean arterial blood pressure, respiratory rate, heart rate, blood pH, arterial PCO2 and arterial PO2 did not significantly differ among Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 or Slc2a1+/−APPSw/0 mice on the same mixed genetic background (Supplementary Fig 2a-i).
Laser Doppler flowmetry studying functional hyperemic responses to whisker-barrel vibrassal stimulation revealed early and significantly greater age-dependent reductions in CBF responses in 1 month old Slc2a1+/−APPSw/0 mice compared to Slc2a1+/− and Slc2a1+/+APPSw/0 mice that both develop less pronounced changes beginning at 6 months of age (Fig 1g). No changes in CBF responses were observed in Slc2a1+/+ littermates. Collectively, these data show that both resting CBF and CBF responses are impaired early in animals with diminished GLUT1 function in the presence of the APP transgene, and are further worsened with age compared to either GLUT1-deficient mice or APP Sw/0 mice alone.
The autoradiography analysis with 2[14C]-deoxyglucose (2-DG) indicated diminished 2DG uptake by ~32-36% and 26-30% in the cortex and hippocampus (Fig 1h) and other brain regions (Supplementary Table 1) at an early stage in Slc2a1+/− and Slc2a1+/−APPSw/0 mice suggesting that reduced glucose BBB transport leads to reduced brain glucose levels.
GLUT1 deficiency leads to early blood-brain barrier breakdown
GLUT1 reductions result in BBB breakdown in the developing zebrafish11 and in vitro BBB models35. To examine whether similar relationship exists in the adult mammalian brain, we next sought to characterize BBB integrity through leakage of endogenous plasma proteins, such as fibrin and immunoglobulin G (IgG), in Slc2a1+/− mice. Young 2-week-old Slc2a1+/− mice demonstrated gross microvascular leakage and extravascular accumulation of circulating fibrin and immunoglobulin G (IgG) with 10-fold and 11-fold increases compared to control age-matched Slc2a1+/+ littermates, respectively (Fig 2a-c). A much smaller but statistically significant 1.5 and 2-fold increase in extravascular fibrin and IgG was observed in 2-week-old Slc2a1+/+APPSw/0 mice compared to age-matched Slc2a1+/+ controls (Fig 2a-c) consistent with previous findings demonstrating early cerebral microvascular changes and BBB breakdown in APPSw/0 mice prior to significant Aβ accumulation36-38. On the other hand, there was a substantial approximately 18- and 20-fold increase in parenchymal accumulates of plasma-derived fibrin and IgG in Slc2a1+/−APPSw/0 mice, respectively, when compared to Slc2a1+/+ littermates. These values were also 75% and 41% higher when compared to Slc2a1+/− littermates (Fig 2a-c). Western blot analysis of fibrin and IgG in vascular-depleted brain homogenates confirmed immunostaining results (Fig 2d-f). More progressive fibrin and IgG leakages were found in 6 month old mice compared to 2-week-old mice (Supplementary Fig 3), but the trend of changes observed between different genotypes remained the same as in 2 week old mice. These data suggest that GLUT1 is required for maintenance of BBB integrity in mice and that reduction in GLUT1 expression may accelerate and augment changes in vascular permeability seen with increased Aβ, such as APPsw/0 mice38.
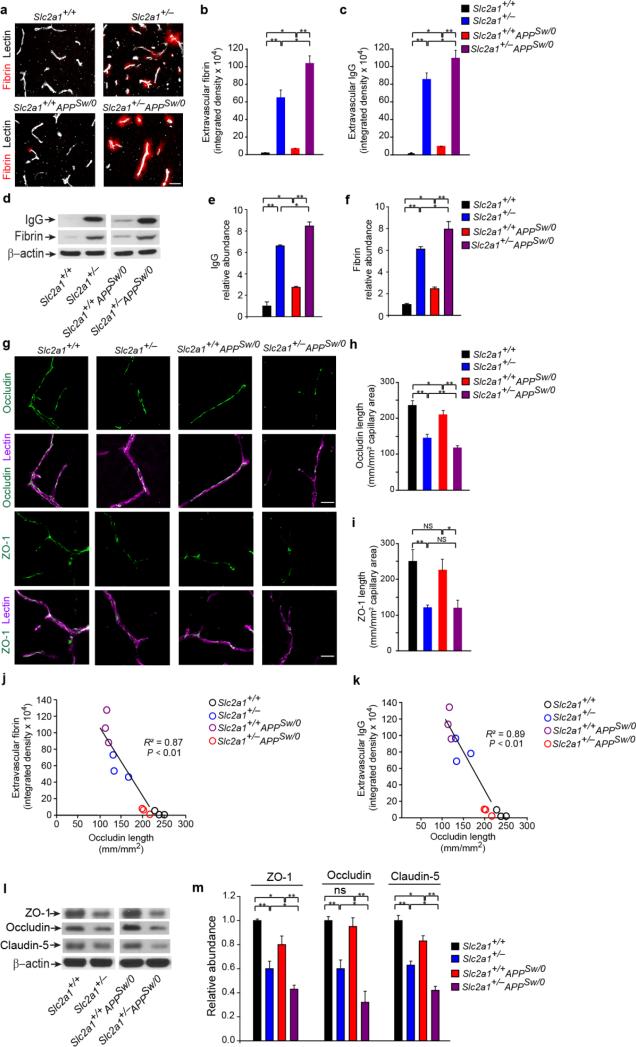
Figure 2. GLUT1 deficiency leads to early BBB breakdown in Slc2a1+/− and Slc2a1+/−APPSw/0 mice.
(a) Representative confocal microscopy analysis of fibrin (red) and lectin-positive capillaries (white) in 2-week-old Slc2a1+/+,Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Scale bar, 25 μm. (b-c) Quantification of extravascular fibrin (b) and immunoglobulin G (IgG) (c) deposits in 2-week old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Mean ± SEM, n=3-5 mice per group; *p<0.05 or **p<0.01. (d-f) Representative immunoblotting of fibrin and IgG levels in capillary-depleted brain tissue (d) and quantification of IgG (e) and fibrin (f) relative abundance in capillary-depleted brains in 2-week-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. β-actin was used as a loading control. Mean ± SEM, n=3-5 mice per group; **p<0.01. (g-i) The tight junction proteins occludin or zonula occludens-1 (ZO-1) (green) and lectin-positive capillary profiles (red) (g) and quantification of endothelial occludin (h) and ZO-1 (i) length in the microvessels in 2-week-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mouse hippocampus. Scale bar, 25 μm. Mean ± SEM, n=3-5 mice per group; *p<0.05 or **p<0.01. (j-k) Negative correlation between the extracellular fibrin (j) or IgG deposits (k) and decreased occludin length in the microvessels in 2-week-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mouse hippocampus. (l) Representative immunoblotting analysis of microvascular ZO-1, occludin and claudin-5 (l) and their respective protein abundance in microvessels (m) in a 2-week-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mouse. β-actin was used as loading control. Mean ± SEM, n=3-5 mice per group; *p<0.05 or **p<0.01. Full length blots are presented in Supplementary Figure 11.
We next studied whether the expression of the BBB tight junction proteins underlying the barrier properties of brain endothelium is altered by GLUT1 deficiency in the mammalian brain as it is in the lower vertebrate brain lacking GLUT111. Immunostaining for occludin and lectin and ZO-1 and lectin indicated early and significant reductions in both tight junction proteins by approximately 40% and 50%, respectively, in Slc2a1+/− mice when compared to age-matched Slc2a1+/+ littermates (Fig 2g-h). We also found a moderate ~20% reduction in occludin length, but not ZO-1, in Slc2a1+/+APPSw/0 mice compared to age-matched Slc2a1+/+ controls (Fig 2g-h), which may explain the increased BBB leakage in APPSw/0 mice (Fig 2a-f). Slc2a1+/−APPSw/0 mice had significantly greater losses of occludin and ZO-1 when compared to Slc2a1+/+APPSw/0 mice, suggesting that reductions in GLUT1 in the setting of an abundance of Aβ results in greater tight junctional reductions and permeability deficits. The reductions in tight junction protein expression in different mouse lines correlated well with increased BBB permeability, as illustrated by negative correlations between the extravascular fibrin and IgG deposits and the occludin capillary length (Fig. 2j-k), respectively, when compared to Slc2a1+/+ littermate controls. Immunoblotting of isolated microvessels confirmed greatest reductions in occludin, ZO-1 and claudin-5 in 2 week old Slc2a1+/−APPSw/0 mice compared to other age-matched genotypes (Fig. 2l-m).
GLUT1 deficiency leads to accelerated cerebral β-amyloidosis
The BBB is a critical site of transport exchanges regulating brain Aβ1, 2. To investigate whether diminished BBB GLUT1 expression influences progression of CNS β-amyloidosis, we analyzed Aβ pathology. Aβ40 and Aβ42 levels were increased by 28% and 95% and 73% and 48% increases in the cortex, and 51% and 80% and 89% and 53% in the hippocampus of 6- and 16-month-old Slc2a1+/−APPSw/0 mice compared to their respective age-matched littermate controls (Fig 3a-b). At 1 month of age, however, Aβ levels in the cortex and hippocampus of Slc2a1+/−APPSw/0 mice were not elevated (Fig. 3a-b), suggesting that early reductions in capillary density in these mice (Fig. 1a-b) are not related to Aβ, but could be due to either early postnatal and/or developmental synergistic effects of GLUT1 deficiency and APP overexpression. Aβ immunostaining confirmed 53% and 62% increases in cortical and hippocampal Aβ load in 16-month-old Slc2a1+/−APPSw/0 compared to Slc2a1+/+APPSw/0 mice, respectively (Fig 3c-d), suggesting that GLUT1 deficiency accelerates and exacerbates cerebral β-amyloidosis in APPSw/0 mice.
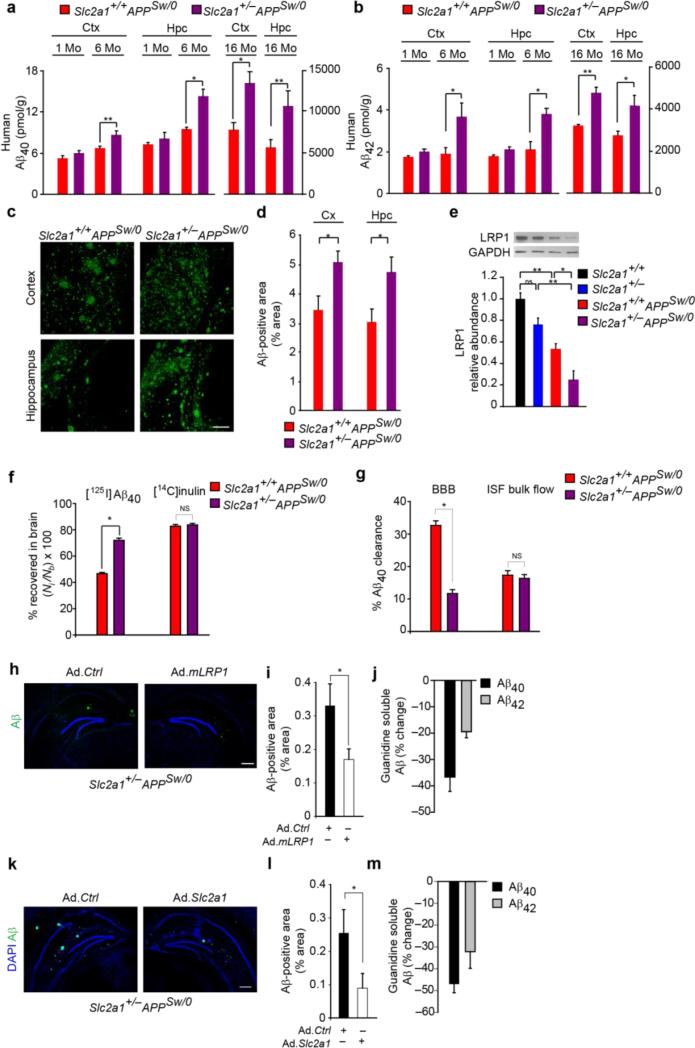
Figure 3. Aβ pathology in GLUT1-deficient APPSw/0 mice and reversal by LRP1 and GLUT1 re-expression.
(a-b) Cortical and hippocampal Aβ40 (a) and Aβ42 (b) levels in 1-, 6- and 16-month old Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Mean ± SEM, n=3-6 mice per group; *p<0.05 or **p<0.01. (c-d) Aβ-immunodetection (green) in the somatosensory cortex and CA1 hippocampal subfield (c) and Aβ load (d) in 16-month old Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Scale bar, 200 μm. Mean ± SEM, n=4-5 mice per group; *p<0.01. (e) Immunoblotting for the low-density lipoprotein receptor-related protein 1 (LRP1) in isolated microvessels (upper panel) and quantification (lower panel) in 16-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Mean ± SEM, n=4 mice per group; *p<0.05 or **p<0.01. (f) Clearance of [125I]-labeled human synthetic Aβ40 from CA1 hippocampal subfield in 16-month-old Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. [14C]inulin was used to assess brain interstitial fluid (ISF) bulk flow. Time, 30 minutes post-injection. Mean ± SEM, n=3-5 mice per group; *p<0.05. (g) [125I]-Aβ40 clearance via BBB and ISF bulk flow (from the data shown in f). (h-i) Aβ-immunodetection (green) in CA1 hippocampal subfield (h) and quantification of Aβ-positive area (i) in 10-month-old Slc2a1+/−APPSw/0 mouse injected with empty adenovirus (Ad.Ctrl) and adenovirus carrying LRP1 minigene (Ad.mLRP1). Black, Ad.Ctrl hippocampus; White, Ad.mLRP1 hippocampus. Scale bar, 300 μm. Mean ± SEM, n=3 mice; **p<0.01. (j) CA1 hippocampal guanidine soluble Aβ40 (black) and Aβ42 (grey) levels in 10-month old Slc2a1+/−APPSw/0 mice injected with Ad.Ctrl and Ad.mLRP1. Graph - percent change of Ad.mLRP1 hippocampus to Ad.Ctrl hippocampus. Mean ± SEM, n=3 mice per group; *p<0.05. (k-m) Aβ-immunodetection (green) in CA1 hippocampal subfield (k) and quantification of Aβ-positive area (l) in 8-10-month-old Slc2a1+/−APPSw/0 mouse injected with empty adenovirus (Ad.Ctrl) and adenovirus carrying Slc2a1 gene (Ad.Slc2a1). Black, Ad.Ctrl hippocampus; White, Ad.Slc2a1 hippocampus. Scale bar, 300 μm. Mean ± SEM, n=3 mice; *p<0.05. (m) CA1 hippocampal guanidine soluble Aβ40 (black) and Aβ42 (grey) levels in 8-10-month-old Slc2a1+/−APPSw/0 mice injected with Ad.Ctrl and Ad.Slc2a1. Graph - percent change of Ad.Slc2a1 hippocampus to Ad.Ctrl hippocampus. Mean ± SEM, n=3 mice; *p<0.05. Full length blots are presented in Supplementary Figure 11.
To investigate the mechanism(s) by which GLUT1 BBB transporter may influence Aβ homeostasis, we studied whether GLUT1 deficiency alters brain capillary levels of low-density lipoprotein receptor-related protein 1 (LRP1), a key Aβ clearance transporter at the BBB1,2. Immunoblotting revealed a significant 47% reduction in brain capillary LRP1 in 16-month-old Slc2a1+/+APPSw/0 mice compared to age-matched Slc2a1+/+ littermates, consistent with a previous report39, and a more severe 75% reduction in 16-month-old Slc2a1+/−APPSw/0 mice compared to age-matched Slc2a1+/+ littermates (Fig 3e). Surprisingly, brain capillary LRP1 levels were also reduced by 26% in 16-month-old Slc2a1+/− mice compared to Slc2a1+/+ age-matched littermates (Fig 3e). In agreement with these findings, in vivo hippocampal clearance assay for human Aβ40 indicated increased Aβ40 retention by 59% in Slc2a1+/−APPSw/0 mice when compared to Slc2a1+/+APPSw/0 mice in the absence of altered clearance of the metabolically inert molecule inulin (Fig 3f). In agreement with the observed LRP1 BBB reductions and increased Aβ40 retention, we found ~67% reduction in BBB clearance of Aβ without changes in Aβ interstitial fluid bulk flow (Fig 3g). Notably, APP, soluble APP-β, β-secretase, neprilysin and insulin degrading enzyme levels were not altered in 16-month-old Slc2a1+/−APPSw/0 mice compared to Slc2a1+/+APPSw/0 littermates indicating that enhanced APP production and/or processing or reduced Aβ enzymatic degradation do not contribute to exacerbations in β-amyloidosis in Slc2a1+/−APPSw/0 mice (Supplementary Fig 4a-g). Together, these data are consistent with a faulty LRP1-dependent Aβ clearance at the BBB.
To confirm that diminished LRP1-dependent Aβ clearance is critical for Aβ accumulation in Slc2a1+/−APPSw/0 mice, we re-expressed LRP1 minigene (mLRP1)40 in the hippocampus of Slc2a1+/−APPSw/0 mice using adenoviral-mediated transfer (Ad.mLRP1). This led to robust expression of the LRP1 minigene in the hippocampus, particularly within blood vessels of Slc2a1+/−APPSw/0 mice (Supplementary Fig 5a-c) resulting in significant reductions in Aβ load and Aβ40 and Aβ42 levels by 45%, 35% and 22% compared to the contralateral control hippocampus injected with the control empty vector, respectively (Fig 3h-j). These data indicate that reduced microvascular LRP1 levels contribute to diminished Aβ clearance in GLUT1-deficient APPsw/0 mice in addition to reductions in brain capillary surface area and resting CBF.
To confirm that GLUT1 regulates LRP1-dependent Aβ clearance at the BBB in vivo, we performed a rescue experiment with GLUT1, and re-expressed GLUT1 in the hippocampus of Slc2a1+/−APPSw/0 mice using adenoviral-mediated Ad.Slca1 transfer. Compared to the control hippocampus injected with the control empty vector, Ad.Slca1 increased the expression of both GLUT1 and LRP1 in brain microvessels (Supplementary Fig 5d-g), which in turn reduced the Aβ load, Aβ40 and Aβ42 levels by 37%, 45% and 33%, respectively (Fig 3k-m). Collectively, these data implicate that re-expression of GLUT1 in Slc2a1+/−APPSw/0 mice slows down development of Aβ pathology by increasing cerebrovascular LRP1 levels.
To better understand the link between GLUT1 and LRP1, we next performed a series of experiments using primary murine brain endothelial cells (BEC). Consistent with a recent report showing that GLUT1 levels in brain microvessels in different mouse strains correlate closely with LRP1 levels, i.e., the lower the GLUT1 levels, the lower the LRP1 levels41, and our findings of reduced LRP1 levels in brain microvessels of Slca1+/− mice (Fig 3e), we found that GLUT1 inhibition with si.Slca1 leads to transcriptional LRP1 suppression in Slc2a1+/+ BEC, whereas Ad.Slca1 adenoviral-mediated re-expression of GLUT1 in Slca1+/− BEC increases LRP1 levels (Supplementary Fig 6a-c). Silencing LRP1 failed to decrease GLUT1 levels (Supplementary Fig 6d) suggesting that changes in GLUT1 expression influence LRP1 expression.
In search of a possible molecular mechanism, we studied whether the sterol regulatory element binding protein 2 (SREBP2) – the only known transcriptional suppressor of LRP142,43 previously shown to inhibit LRP1 in brain vascular cells in AD and AD models42 – is involved in mediating GLUT1 effects. si.Slca1-mediated GLUT1 inhibition increased SREBP2 levels and suppressed LRP1 levels in Slc2a1+/+ BEC, whereas inhibition of GLUT1 in the presence of silenced SREBP2 (si.Srebp2) failed to suppress LRP1 (Supplementary Fig 6e-h). SREBP2 levels were increased in Slca1+/− BEC when compared to Slc2a1+/+ BEC, which correlated with LRP1 reduction (Supplementary Fig. 6i-k). Together, these findings indicate that SREBP2 is critical for LRP1 suppression in BEC.
Neuronal dysfunction and cognitive impairment in Slc2a1+/−APPSw/0 mice
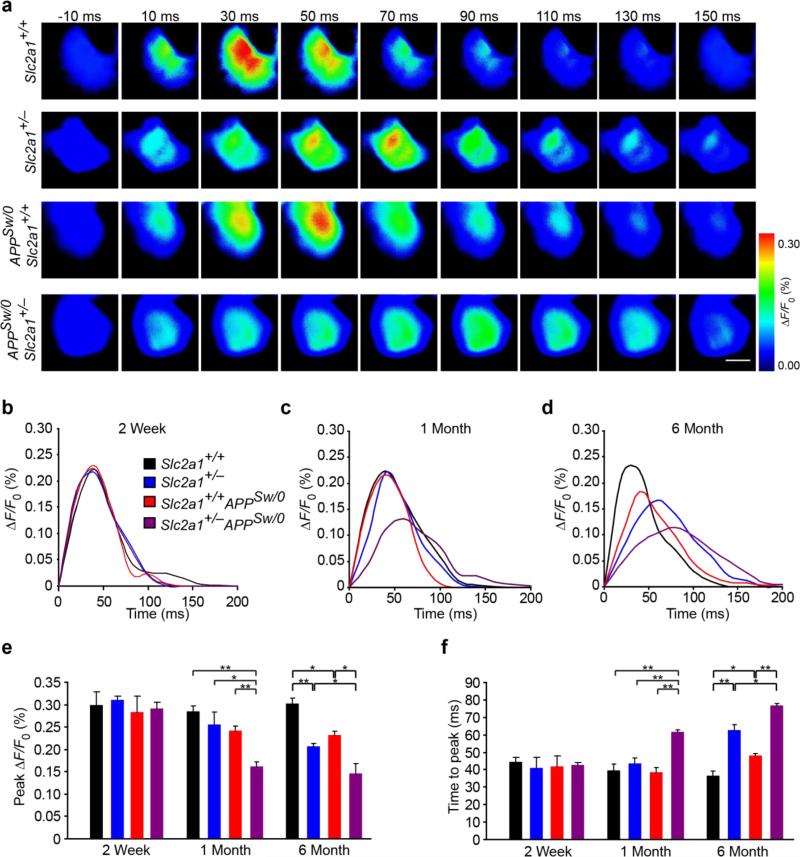
We utilized in vivo voltage sensitive dye (VSD) imaging to determine evoked membrane potential responses with millisecond temporal resolution immediately following hindlimb mechanical stimulation44. In Slc2a1+/+ mice, hindlimb stimulation evoked a robust, transient depolarization (<100 ms) originating in the contralateral hindlimb somatosensory cortex which subsequently propagated to adjacent motor and forelimb somatosensory cortices prior to returning to baseline values (Fig 4a). At 2 weeks of age, evoked cortical depolarization displayed no abnormalities in peak amplitude of response, as measured by peak change in fluorescence normalized to baseline fluorescence (peak ΔF/Fo) and response latency in Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 or Slc2a1+/−APPSw/0 mice (Fig 4b, e, f). Conversely, reductions in evoked hindlimb cortical response amplitude and prolongation of response latency were noted in Slc2a1+/−APPSw/0, but not in Slc2a1+/− and Slc2a1+/+APPSw/0 mice, beginning at 1 month of age, respectively (Fig 4a,c-f). By 6-months, cortical responses in Slc2a1+/−APPSw/0 mice were substantially reduced with severely prolonged response latency (Fig 4a,d-f). We also found more moderate reductions in cortical activity in Slc2a1+/− and Slc2a1+/+APPSw/0 mice beginning at 6 month of age (Fig 4a, d-f). These data demonstrate that vascular changes readily detectable at 2 weeks of age in Slc2a1+/−APPSw/0 mice (Fig 1a,b; Fig 2) precede neuronal dysfunction. Similar, BBB breakdown observed early in Slc2a1+/− and Slc2a1+/+APPSw/0 mice also precedes diminished evoked membrane potential responses in these mice although they develop slower than in Slc2a1+/−APPSw/0 mice.
Figure 4. Neuronal dysfunction in GLUT1-deficient APPSw/0 mice.
(a) Representative time-lapse-imaging analysis of voltage sensitive dye (VSD) signal response to hind-limb somatosensory cortex stimulation in 6-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Scale bar, 500 μm. (b-d) Quantitative time-lapse-imaging profile analysis of VSD response to hind-limb somatosensory cortex stimulation in 2-week-old (b), 1-month-old (c) and 6-month-old (d) Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. ΔF/F0 indicates the percentage change in fluorescence from the baseline fluorescent signal following stimulation. (e-f) Quantification of peak fluorescent VSD signal amplitude (e) and time-to-peak latency (f) in 2-week-old, 1-month-old and 6-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Mean ± SEM, n=3-4 mice per group; *p<0.05 or **p<0.01.
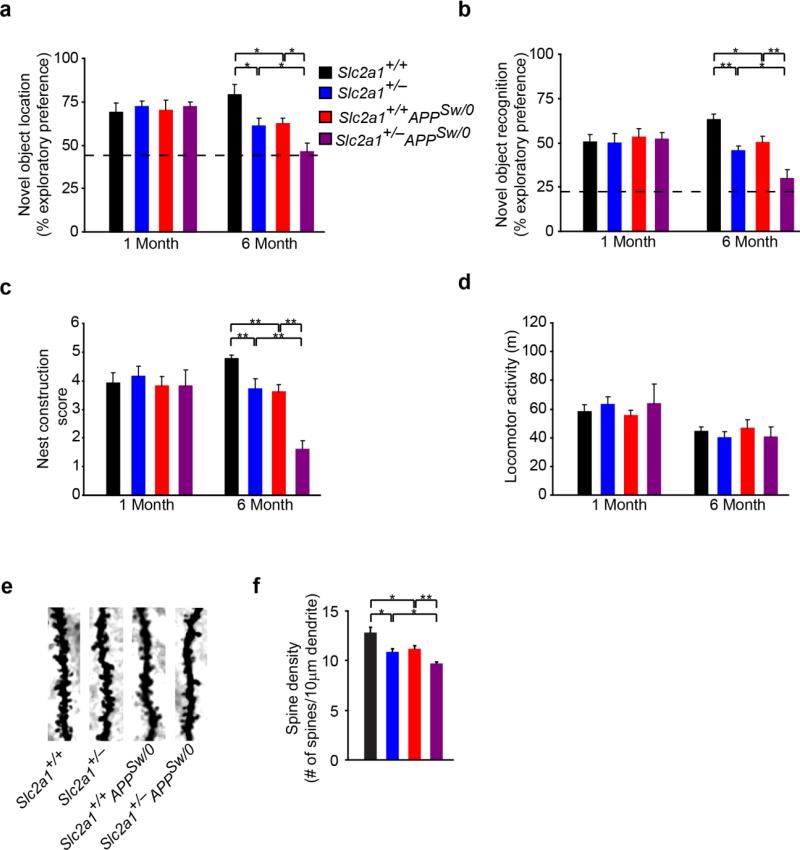
Next, we sought to evaluate function of neuronal circuitries underlying hippocampal learning and spatial memory through the assessment of novel object location (Fig 5a) and novel object recognition memory (Fig 5b). At 1-month of age, no impairment in novel object location or recognition memory was detected in 1-month-old Slc2a1+/−APPSw/0 mice (Fig 5a-b). At 6 months of age, however, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice all showed significant impairment in novel object location and recognition memory (Fig 5a-b). As observed with VSD imaging, novel object location and recognition memory was most severely affected in Slc2a1+/−APPSw/0 mice (Fig 5a-b). Assessment of nest construction confirmed the onset of behavioral impairment in 6-month-old Slc2a1+/−, Slc2a1+/+APPSw/0 and the more severely affected Slc2a1+/−APPSw/0 mice, but not at 1-month of age (Fig 5c). Behavioral deficits were not the result of reduced locomotor activity (Fig 5d). Golgi-Cox histological analysis revealed graded dendritic spine reductions in the dentate gyrus in 6-month-old Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice (Fig 5e-f) which closely mimicked genotype-dependent impairments in hippocampal function.
Figure 5. Accelerated cognitive impairment in GLUT1-deficient APPSw/0 mice.
(a-c) Quantification of exploratory preference measuring hippocampal-dependent novel object location memory (a), novel object recognition memory (b), nest construction score (c) and mean locomotor activity (d) in 1- and 6-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Mean ± SEM, n=9-15 mice per group; *p<0.05 or **p<0.01. (e) Representative high-magnification bright-field microscopy microscopy analysis of Golgi-Cox staining showing dendritic spine density in the CA1 hippocampal region of 6-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. (f) Quantification of dendritic spine density in 6-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Mean ± SEM, n=3-5 mice per group; *p<0.05 or **p<0.01.
GLUT1 deficiency results in neurodegenerative changes in APPSw/0 mice
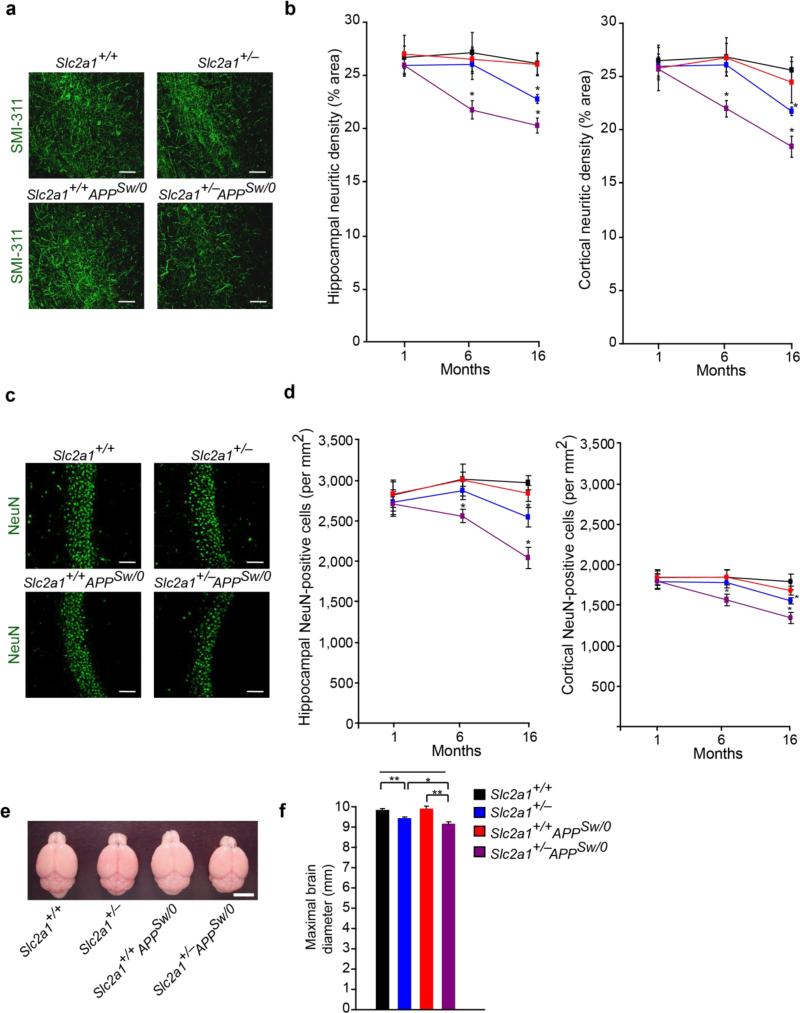
Quantification of SMI-311 positive non-phosphorylated neurofilaments in the hindlimb somatosensory cortex and CA1 hippocampal subfield44 revealed no significant differences in 1-month-old Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice compared to age-matched control Slc2a1+/+ littermates confirming that vascular dysfunction precedes detectable neuronal structural changes (Fig 6a-b). At later time points at 6 months of age, significant 18% and 22% reductions in hippocampal and cortical SMI-311 positive neurofilaments were noted in Slc2a1+/−APPSw/0 mice, respectively, compared to other genotypes (Fig 6a-b). Significant 15% and 13% reductions in neurofilament density were detected in the cortex and hippocampus in 16-month-old Slc2a1+/− mice, but not in Slc2a1+/+APPSw/0 or age-matched littermate controls, that compares to more pronounced 23% and 28% respective reductions in age-matched Slc2a1+/−APPSw/0 mice (Fig 6a-b).
Figure 6. Accelerated neurodegenerative changes in GLUT1-deficient APPSw/0 mice.
(a) SMI-311 immunodetection showing neurites (green) in the CA1 hippocampal region of 16-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Scale bar, 50 μm. (b) Quantification of SMI-311-positive neuritic density in somatosensory cortex and CA1 hippocampal subfield in 1-, 6- and 16-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Scale bar, 50 μm. Mean ± SEM, n=3-4 mice per group; *p<0.05 or **p<0.01. (c) NeuN-positive neurons (green) in the CA1 hippocampal subfield of 16-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. (d) Quantification of the number of NeuN-positive neurons in somatosensory cortex and CA1 hippocampal subfield in 1-, 6- and 16-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Mean ± SEM, n=3-6 mice per group; *p<0.05 or **p<0.01. (e-f) Representative low-magnification bright-field microscopy of mouse brains (e) and quantification of maximal brain diameter (f) in 6-month-old Slc2a1+/+, Slc2a1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. Scale bar, 5 mm. Mean ± SEM, n=5-6 mice per group; *p<0.05 or **p<0.01.
To evaluate neuronal loss, we next quantified neuron-specific nuclear protein A60 (NeuN) positive cells in the cortex and hippocampus of different mice. This quantification revealed significant 15% and 16% and 25% and 31% reductions in hippocampal and cortical NeuN-positive cells in 6- and 16-month-old Slc2a1+/−APPSw/0 mice, respectively (Fig 6c-d). No changes were observed in other genotypes within 6 months of age. Significant 13% and 14% reductions in NeuN-positive cells were detected, however, in the CA1 hippocampal subfield and somatosensory cortex, respectively, at a later stage in 16-month-old Slc2a1+/− mice (Fig 6c-d). Consistent with a previous report45, we did not find a significant neuronal loss in Slc2a1+/+APPSw/0 mice at all-time points analyzed (Fig 6c-d).
We also analyzed whether there was gross alterations in brain architecture through measurement of mean cortical diameter, an index of brain size. This analysis demonstrated significant 4% and more severe 7% reductions in 6-month-old Slc2a1+/− and Slc2a1+/−APPSw/0 mice, respectively, but no significant alterations in brain size in Slc2a1+/+APPSw/0 mice (Fig 6e-f).
GLUT1 deficiency in endothelium initiates vascular BBB changes
Brain microvessels from Slc2a1+/+ mice express 55 kDa GLUT1 endothelial isoform3, whereas capillary-depleted brain homogenates containing astrocytes and neurons, but not microvessels (as shown by multiple cell-specific markers on immunoblots), express 45 kDa GLUT1 astrocytic isoform3 at very low levels (Supplementary Fig. 7a-c). When compared to the 55 kDa isoform, the levels of the 45 kDa GLUT1 isoform were approximately 26-fold lower. In Slca1+/− mice compared to Slc2a1+/+ controls, we found >50% and 40% losses of the 55 and 45 kDa GLUT1, respectively (Supplementary Fig 7d-e). Moreover, in the capillary-depleted brains of Slca1+/− mice compared to Slc2a1+/+ mice, we detected a ~2-fold increase in GLUT2 levels, a glucose transporter that is expressed in astrocytes3,46, and a 2.6-fold increase in GLUT3 levels, a glucose transporter that is expressed in neurons46,47, suggesting metabolic adaptations in different cell types in the presence of GLUT1 deficiency (Supplementary Fig 7f-g).
Using primary murine BEC and astrocytes from Slc2a1+/+ mice, we confirmed that the 55 kDa GLUT1 in BEC is expressed at significantly higher levels by ~20-fold compared to the 45 kDa GLUT1 in astrocytes (Supplementary Fig. 7h) and that GLUT1 haploinsuficiency leads to ~60% loss of GLUT1 from BEC and >40% loss of GLUT1 from astrocytes, and a 3.5-fold increase in GLUT2 levels in astrocytes (Supplementary Fig 7i-j). Thus, global GLUT1 deficiency leads to a critical loss of glucose transporters from endothelium that likely has a major effect on the vascular phenotype in Slca1+/− mice. In contrast, astrocytes express substantially lower levels of GLUT1 at baseline, and during GLUT1 global deficiency maintain less significant loss of glucose transporters, as indicated by relatively smaller loss of GLUT1 and a compensatory increase in GLUT2.
Then, we took advantage of conditional Slc2a1 lox/lox mice31 and generated Slc2a1 lox/+; Tie2-Cre+/0 and Slc2a1 lox/+; GFAP-Cre+/0 mice with partial GLUT1 deletions from endothelium and astrocytes, respectively, to determine their respective effects on the BBB integrity that we show is compromised early in Slca1+/− mice at 2 weeks of age (Fig. 2). As expected, we found diminished levels of GLUT1 in endothelium in Slc2a1 lox/+; Tie2-Cre+/0 mice by double immunostaining for GLUT1 and lectin-positive endothelial profiles (>40%) and immunoblotting for 55 kDa endothelial isoform in isolated brain microvessels (~45%), but no change in the astrocyte-associated 45 kDa isoform in capillary-depleted brains (Supplementary Fig. 8a-d). In contrast, Slc2a1 lox/+; GFAP-Cre+/0 mice did not have loss of GLUT1 from endothelium, confirming that GLUT1 deletion from astrocytes does not affect function of endothelial GLUT1. In Slc2a1 lox/+; GFAP-Cre+/0 mice, GLUT1 was undetectable in brain parenchyma by immunostaining as reported3, but there was >50% loss of 45 kDa isoform in the astrocyte-containing capillary-depleted brains (Supplementary Fig 8d).
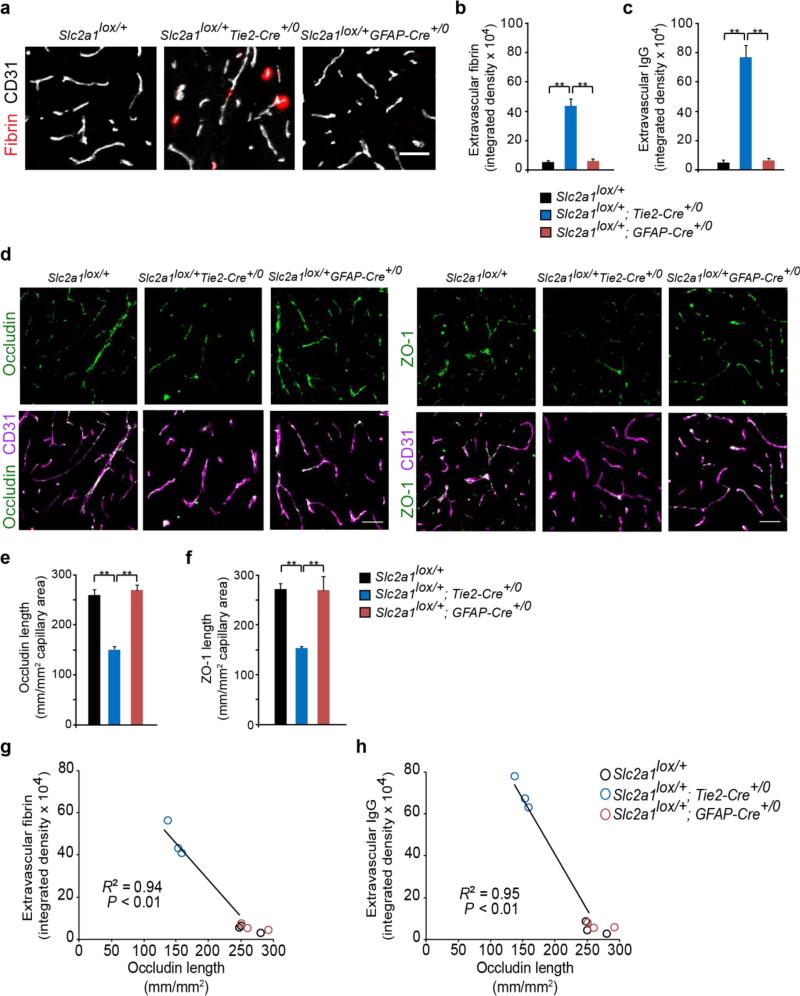
We next found the BBB breakdown in endothelial GLUT1-deficient Slc2a1 lox/+; Tie2-Cre+/0 mice, but not in astrocyte GLUT1-deficient Slc2a1 lox/+; GFAP-Cre+/0 mice, as shown by 10- and 15-fold greater extravascular accumulation of fibrin and IgG in brain parenchyma (Fig 7a-c), and 45% and 47% reductions in the lengh of the BBB tight junction proteins occludin and ZO-1, respectively (Fig 7d-f), in Slc2a1 lox/+; Tie2-Cre+/0 mice compared to Slc2a1 lox/+ littermate controls. In contrast, GLUT1-deficiency in astrocytes in Slc2a1 lox/+; GFAP-Cre+/0 mice did not result in BBB breakdown. A decrease in the occludin length in Slc2a1 lox/+; Tie2-Cre+/0 mice correlated with increased BBB breakdown to fibrin and IgG (Fig 7g-h), when compared to Slc2a1 lox/+; GFAP-Cre+/0 mice and Slc2a1 lox/+ littermate controls. Collectively, these data indicate that GLUT1 deficiency in endothelium, but not astrocytes, initiates the vascular phenotype with BBB breakdown.
Figure 7. GLUT1 deficiency in endothelial cells (Slc2a1lox/+Tie2-Cre+/0 mice), but not astrocytes (Slc2a1lox/+GFAP-Cre+/0 mice) leads to early BBB breakdown.
(a) Representative confocal microscopy analysis of plasma-derived fibrin (red) and CD31-positive capillaries (white) in 2-week-old Slc2a1lox/+, Slc2a1lox/+Tie2-Cre+/0, Slc2a1lox/+GFAP-Cre+/0 mice. Scale bar, 25 μm. (b-c) Quantification of extravascular fibrin (b) and IgG (c) positive deposits in 2-week old Slc2a1lox/+, Slc2a1lox/+Tie2-Cre+/0, Slc2a1lox/+GFAP-Cre+/0 mice. Mean ± SEM, n=3-5 mice per group; *p<0.05 or **p<0.01. (d) Representative confocal microscopy analysis of the tight junction proteins occludin or zonula occludens-1 (ZO-1) (green) and CD31-positive capillary profiles. (e-f) Quantification of endothelial occludin (e) and ZO-1 (f) tight junctional length in the microvessels in 2-week-old Slc2a1lox/+, Slc2a1lox/+Tie2-Cre+/0, Slc2a1lox/+GFAP-Cre+/0 mice. Scale bar, 25 μm. Mean ± SEM, n=3-5 mice per group; *p<0.05 or **p<0.01. (g-h) Negative correlation between extracellular fibrin (g) or IgG deposits (h) and decreased occludin length in the microvessels in 2-week-old Slc2a1lox/+, Slc2a1lox/+Tie2-Cre+/0, Slc2a1lox/+GFAP-Cre+/0 mice.
GLUT1 deficiency in red blood cells does not contribute to central effects
GLUT1 is present in red blood cells (RBC)46. Our data show that Slc2a1 haplosufficiency does not affect RBC indices and hemoglobin oxygen saturation and dissociation in Slca1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice. We found moderate 23% reduction in GLUT1 membrane protein levels in RBC's from Slc2a1+/− mice likely due to compensatory upregulation (Supplementary Fig 9a). RBCs in Slc2a1+/+, Slca1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice had normal shape (Supplementary Fig 9b) and normal mechanical properties under shear stress conditions (1.5-50 Pascal) (Supplementary Fig 9c). Slc2a1+/+, Slca1+/−, Slc2a1+/+APPSw/0 and Slc2a1+/−APPSw/0 mice all had normal hematological parameters such as the amount of hemoglobin, average RBC size, the amount of hemoglobin per RBC, the amount of hemoglobin relative to the size of the cell, and red cell distribution width, as well as normal saturation level of oxygen in hemoglobin and P50 value of oxygen dissociation (Supplementary Fig. 9d-l). These data suggest that peripheral changes in RBCs do not make significant contributions to the observed effects within the brain.
Discussion
We show that GLUT1 in addition to its well known role in transporting glucose into the brain1,2 is necessary for the maintenance of proper brain capillary networks, blood flow and BBB integrity, as well as neuronal function and structure (Supplementary Fig 10). Capillary degeneration and BBB breakdown occur early in AD mice with GLUT1 deficiency (Slc2a1+/−APPSw/0) at 2 weeks of age, and are associated with reduced brain perfusion and diminished glucose uptake into the brain, whereas neuronal dysfunction, behavioral deficits, elevated Aβ levels and behavioral and neurodegenerative changes develop later within 6 months of age.
GLUT1 deficiency in endothelium, but not astrocytes, initiates the vascular phenotype with BBB breakdown. Besides reductions in the tight junction proteins, early metabolic changes in the endothelium due to reduced glucose uptake might contribute to endothelial cell injury and BBB breakdown. Loss of GLUT1 from brain endothelium generates multiple parallel pathogenic mechanisms in the cerebral microcirculation, which can contribute to the observed neuronal dysfunction and neurodegeneration. Each of these vascular insults alone, i.e., the BBB breakdown, diminished BBB glucose transport, CBF reductions, capillary degeneration, neurovascular uncoupling and impaired Aβ BBB clearance, may be independently neuronal toxic, but their synergism likely amplifies the overall pathogenic effects of GLUT1 deficiency.
Metabolic stress due to chronically reduced glucose levels in brain, if not compensated, may lead to neuronal and glial hypometabolism and oxidative stress, which can contribute to neurotoxicity48. Additionally, shifts in metabolism can take place in different cell types within the neurovascular unit, and at different stages during limited glucose availability. The metabolic pathways through which BBB GLUT1 reductions influence brain metabolism, including endothelial glucose metabolism, the astrocyte lactate shuttle and/or oxidative metabolism, and neuronal supply, are presently unknown and likely represent an important focus for future studies.
When combined with APP overexpression, GLUT1 reduction results in early neurodegeneration marked by the retraction of neurites, neuronal loss and substantial behavioral deficits at 6 months of age that are not seen in APPSw/0 mice as shown in the present and earlier reports45,49. GLUT1 deficiency leads to reductions in Aβ-clearance and accelerates Aβ pathology via reduced expression of LRP1 in the microvasculature. Importantly, these changes are reversible as demonstrated by the GLUT1 and LRP1 rescue experiments in the hippocampus. Therefore, neuronal loss in Slc2a1+/−APPSw/0 mice, likely reflects synergistic effects of GLUT1-mediated vascular injury, diminished neuronal glucose delivery, and greater accumulation of Aβ neurotoxic species at the neuronal interface.
Our data using GLUT1 deficient mice with an early embryonic loss of GLUT1 suggest that GLUT1 may fulfill an important regulatory role in the microcirculation and Aβ clearance in the adult and aging brain. Future studies using murine models with inducible GLUT1 loss from endothelium might address whether there is a difference in the CNS response to an early embryonic GLUT1 loss compared to a later GLUT1 loss from brain. Interestingly, adult patients with GLUT1-deficiency syndrome50 do not show clinical evidence for AD. Whether GLUT1-deficiency can accelerate cognitive decline during aging in humans remains, however, to be seen. The present data suggest that GLUT1 deficiency can contribute to a disease process acting in tandem with Aβ to initiate and/or amplify vascular damage and Aβ accumulation.
The two-hit vascular hypothesis of Alzheimer's disease states that early vascular injury leads to BBB compromise and perfusion stress which can initiate Aβ-independent and Aβ-dependent mechanisms of neurotoxicity2. Here, we show that GLUT1 transporter at the BBB influences both Aβ-independent and Aβ-dependent neuronal injury. The molecular mechanisms governing GLUT1 reductions in AD and whether these may be pharmacologically targeted to restore GLUT1 expression remain unknown. Nevertheless, our data suggest that GLUT1 is an important therapeutic target for AD-related neurovascular dysfunction and neurodegeneration.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Health grants AG039452, AG023084 and NS034467 to B.V.Z. and U01 HL087947 and R01DK092065 to E.D.A. We thank Dr. Elyse Schauwecker for most helpful discussion.
Footnotes
Authors’ contribution: E.A.W. contributed to manuscript preparation, experimental design/analysis and conducted experiments. Y.N. and A.P.S. contributed to experimental design, data analysis and interpretation, and conducted experiments. S.R., R.D.B., J.D.S., S.H., J.S.S., P.K., A.R.N., R.B.V., J.M., E.Z. and Z.Z. conducted and analyzed experiments. H.J.M. contributed to hematological analysis and data interpretation. E.D.A. provided Slc2a1lox/lox mice and contributed to project design. J.S. generated Slc2a1lox/+Tie2-Cre mice. D.C.D. provided Slc2a1+/− mice and contributed to project design. B.V.Z. supervised and designed all experiments and analysis and wrote the manuscript.
Conflict of Interest
None of the authors has conflict of interest.
References
- 1.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng D, et al. Crystal structure of the human glucose transporter GLUT1. Nature. 2014;510:121–125. doi: 10.1038/nature13306. [DOI] [PubMed] [Google Scholar]
- 5.Allen A, Messier C. Plastic changes in the astrocyte GLUT1 glucose transporter and beta-tubulin microtubule protein following voluntary exercise in mice. Behav. Brain Res. 2013;240:95–102. doi: 10.1016/j.bbr.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Choeiri C, Staines W, Miki T, Seino S, Messier C. Glucose transporter plasticity during memory processing. Neuroscience. 2005;130:591–600. doi: 10.1016/j.neuroscience.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Zeller K, Rahner-Welsch S, Kuschinsky W. Distribution of Glut1 glucose transporters in different brain structures compared to glucose utilization and capillary density of adult rat brains. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1997;17:204–209. doi: 10.1097/00004647-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Pearson TS, Akman C, Hinton VJ, Engelstad K, De Vivo DC. Phenotypic spectrum of glucose transporter type 1 deficiency syndrome (Glut1 DS). Curr. Neurol. Neurosci. Rep. 2013;13:342. doi: 10.1007/s11910-013-0342-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, et al. A mouse model for Glut-1 haploinsufficiency. Hum. Mol. Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- 10.Ullner PM, et al. Murine Glut-1 transporter haploinsufficiency: postnatal deceleration of brain weight and reactive astrocytosis. Neurobiol. Dis. 2009;36:60–69. doi: 10.1016/j.nbd.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng P-P, et al. Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Ann. Neurol. 2010;68:835–844. doi: 10.1002/ana.22318. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen LN, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Zvi A, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Z, Zlokovic BV. Blood-brain barrier: a dual life of MFSD2A? Neuron. 2014;82:728–730. doi: 10.1016/j.neuron.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landau SM, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann. Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer's disease? Brain Pathol. Zurich Switz. 2014;24:371–386. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ossenkoppele R, et al. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology. 2013;80:359–365. doi: 10.1212/WNL.0b013e31827f0889. [DOI] [PubMed] [Google Scholar]
- 18.Protas HD, et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 2013;70:320–325. doi: 10.1001/2013.jamaneurol.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosconi L, et al. Amyloid and metabolic positron emission tomography imaging of cognitively normal adults with Alzheimer's parents. Neurobiol. Aging. 2013;34:22–34. doi: 10.1016/j.neurobiolaging.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landau SM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosconi L, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosconi L, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2006;47:1778–1786. [PubMed] [Google Scholar]
- 23.Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol. Dis. 2002;9:61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- 24.Jagust WJ, et al. Diminished glucose transport in Alzheimer's disease: dynamic PET studies. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1991;11:323–330. doi: 10.1038/jcbfm.1991.65. [DOI] [PubMed] [Google Scholar]
- 25.Piert M, Koeppe RA, Giordani B, Berent S, Kuhl DE. Diminished glucose transport and phosphorylation in Alzheimer's disease determined by dynamic FDG-PET. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1996;37:201–208. [PubMed] [Google Scholar]
- 26.Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Ann. Neurol. 1994;35:546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- 27.Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer's disease. Neurobiol. Aging. 1997;18:469–474. doi: 10.1016/s0197-4580(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 28.Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J. Neurochem. 1989;53:1083–1088. doi: 10.1111/j.1471-4159.1989.tb07399.x. [DOI] [PubMed] [Google Scholar]
- 29.Horwood N, Davies DC. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer's disease. Virchows Arch. Int. J. Pathol. 1994;425:69–72. doi: 10.1007/BF00193951. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 31.Young CD, et al. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PloS One. 2011;6:e23205. doi: 10.1371/journal.pone.0023205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deane R, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 33.Paris D, et al. Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci. Lett. 2004;366:80–85. doi: 10.1016/j.neulet.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Paris D, et al. Inhibition of angiogenesis by Abeta peptides. Angiogenesis. 2004;7:75–85. doi: 10.1023/B:AGEN.0000037335.17717.bf. [DOI] [PubMed] [Google Scholar]
- 35.Abdul Muneer PM, Alikunju S, Szlachetka AM, Murrin LC, Haorah J. Impairment of brain endothelial glucose transporter by methamphetamine causes blood-brain barrier dysfunction. Mol. Neurodegener. 2011;6:23. doi: 10.1186/1750-1326-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iadecola C, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat. Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 37.Niwa K, et al. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J. Exp. Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deane R, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Obermoeller-McCormick LM, et al. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J. Cell Sci. 2001;114:899–908. doi: 10.1242/jcs.114.5.899. [DOI] [PubMed] [Google Scholar]
- 41.Uchida Y, et al. A study protocol for quantitative targeted absolute proteomics (QTAP) by LC-MS/MS: application for inter-strain differences in protein expression levels of transporters, receptors, claudin-5, and marker proteins at the blood-brain barrier in ddY, FVB, and C57BL/6J mice. Fluids Barriers CNS. 2013;10:21. doi: 10.1186/2045-8118-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell RD, et al. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat. Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llorente-Cortés V, Costales P, Bernués J, Camino-Lopez S, Badimon L. Sterol regulatory element-binding protein-2 negatively regulates low density lipoprotein receptor-related protein transcription. J. Mol. Biol. 2006;359:950–960. doi: 10.1016/j.jmb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Bell RD, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J. Neuropathol. Exp. Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Benarroch EE. Brain glucose transporters: implications for neurologic disease. Neurology. 2014;82:1374–1379. doi: 10.1212/WNL.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 47.Vannucci SJ. Developmental expression of GLUT1 and GLUT3 glucose transporters in rat brain. J. Neurochem. 1994;62:240–246. doi: 10.1046/j.1471-4159.1994.62010240.x. [DOI] [PubMed] [Google Scholar]
- 48.Bolaños JP, Delgado-Esteban M, Herrero-Mendez A, Fernandez-Fernandez S, Almeida A. Regulation of glycolysis and pentose-phosphate pathway by nitric oxide: impact on neuronal survival. Biochim. Biophys. Acta. 2008;1777:789–793. doi: 10.1016/j.bbabio.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Sagare AP, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Seidner G, et al. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat. Genet. 1998;18:188–191. doi: 10.1038/ng0298-188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.