Abstract
Liver fibrosis is an outcome of many chronic diseases, and often results in cirrhosis, liver failure, and portal hypertension. Liver transplantation is the only treatment available for patients with advanced stages of liver cirrhosis. Therefore, alternative methods are required to develop new strategies for anti-fibrotic therapy. Various kinds of hepatocyte injuries cause inflammatory reactions which lead to activation of hepatic stellate cells (HSCs). Continuous liver injuries maintain these activated HSCs, and they are called as myofibroblasts. Myofibroblasts proliferate in response to various kinds of cytokines and produce extracellular matrix proteins (ECMs). Myofibroblasts undergo apoptosis and inactivation when the underlying causative etiologies are cleared. Here we describe the current knowledge of targeting the activated HSCs as a therapeutic target for liver fibrosis.
Keywords: Hepatic stellate cell, hepatic fibrosis, liver cirrhosis, myofibroblast, fibrocyte, anti-fibrotic therapy
Introduction
Liver fibrosis is a wound-healing process of the liver in response to repeated and chronic liver injury with distinct etiologies, such as infectious diseases (e.g., viral hepatitis), metabolic derangements (non-alcoholic steatohepatitis), exposure to toxins (e.g., alcohol liver diseases), or autoimmune diseases (e.g., primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis). The identical morphology characteristics of liver fibrosis are the quantitative and qualitative deposition of extracellular matrix which is produced by myofibroblasts. Myofibroblasts are absent from the healthy liver, accumulate in the injured liver, and serve as the principle effector cells of fibrogenesis.
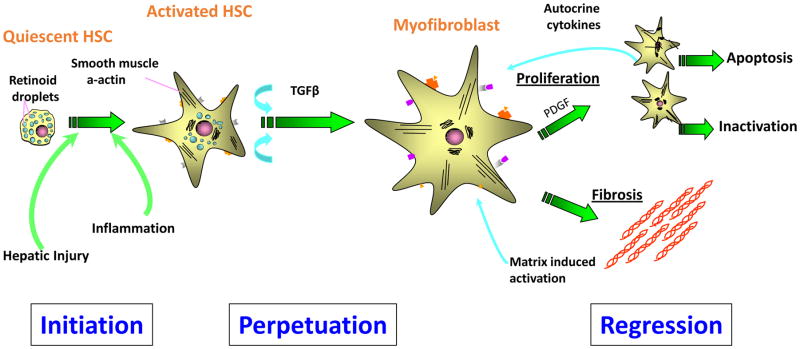
Several injury-triggered events are critical for pathogenesis of liver fibrosis and its resolution. Damage to hepatocytes cause inflammatory reactions which lead to activation of hepatic stellate cells (HSCs). Continuous liver injury causes perpetuation of activated HSCs in the liver, and they become myofibroblasts. Myofibroblasts proliferate in response to various cytokines and growth factors and produce extracellular matrix proteins (ECMs). Myofibroblasts undergo apoptosis and inactivation when the underlying causative etiologies are cleared (Fig. 1). Although control and clearance of the underlying causative etiology (e.g., virus suppression or alcohol absence) can slow down fibrosis progression and lead to fibrosis regression, our extensive knowledge on the mechanism leading to liver fibrosis through hepatocyte injury, inflammation, and activation of myofibroblasts to deposit extracellular matrix has not been translated into effective and potent reagents or therapies in human so far [1]. In this review we would like to summarize the current knowledge of targeting the possible ways to relieve liver fibrosis
Figure 1.
Hepatocyte damage causes an inflammatory reaction which leads to activation of hepatic stellate cells (HSCs). Continuous liver injuries causes perpetuation of activated HSCs in the liver and they become myofibroblasts. Myofibroblasts proliferate in response to various kinds of cytokines and produce extracellular matrix proteins (ECMs). Myofibroblasts undergo apoptosis or inactivation when the underlying causative etiologies are cleared.
Inhibition of hepatic injury
Liver injury is characterized by hepatocyte damage and death, increased inflammatory cells, and activated HSCs/myofibroblasts. Pharmacological inhibition of liver cell apoptosis may potentially attenuate liver injury, inflammation, and fibrosis by blocking hepatocyte death. Apoptosis is executed by a family of intracellular proteases referred to as caspases [2]. For example, a pan-caspase inhibitor IDN-6556 attenuated hepatic injury and fibrosis in mice [3].
Since reactive oxidative stress (ROS) mediates hepatocyte death, regulating ROS is a promising strategy of liver fibrosis therapy [4]. Peroxisome proliferator-activated receptor delta (PPARδ), a member of the nuclear receptor family, is emerging as a key metabolic regulator with pleiotropic actions on various tissues including fat, skeletal muscle, and liver. PPARδ agonist protects hepatocytes from cell death by reducing ROS generation of hepatocytes, leading to less liver fibrosis [5].
Nonalcoholic fatty liver disease (NAFLD) includes a spectrum of diseases ranging from isolated hepatic steatosis (HIS) to nonalcoholic steatohepatitis (NASH), the progressive form of the disease associated with inflammation and cellular injury, which can lead to cirrhosis. NAFLD has become the most common chronic liver disease in the United States. It is associated with obesity, type 2 diabetes, hyperlipidemia, insulin resistance, and the accumulation of triglycerides in hepatocytes. Although the pathogenesis of the hepatocytes damage in response to lipid accumulation is not fully elucidated, cellular membrane integrity seems to be important for regulating hepatocyte damages. Phosphatidylcholine (PC) is a major component of the cellular membrane, which is generated by a transmethylation reaction from phosphatidylethanolamine via a metabolic pathway that utilizes S-adenosylmethionine (SAMe) as a methyl donor. The PC/PE ratio may be a key regulator of cell membrane integrity and play a role in the progression of steatotsis to NASH. Animal studies show that chronic hepatic SAMe deficiency causes NASH and HCC. Furthermore, the formation of PC is reduced in various kinds of chronic liver diseases including intrahepatic cholestasis, cholestasis of pregnancy and alcoholic liver disease. There have been few randomized controlled trials to assess the efficacy of SAMe in chronic liver diseases. In these studies, SAMe treatment resulted in improvement in pruritus.
Inhibition of inflammation
Serum amyloid P (SAP) or pentraxin-2, a member of the pentraxin family, is a 27-kDa protein that is produced by the liver, secreted into the blood, and circulates as stable 135-kDa pentamers [6][7]. SAP binds to apoptotic cells and DNA and is cleared by macropharge-like cells through FcrRs [8]. SAP reduces neutrophil adhesion to ECM proteins, inhibits the differentiation of monocytes into fibrocytes, attenuates profibrotic macrophages, activates the complement pathway, and promotes phagocytosis of cell debris. SAP reduces bleomycin-induced lung fibrosis [9]. Injection of SAP into humans, mice and rats has no toxic effects.
Inhibiting perpetuation of hepatic stellate cells
After engulfment of apoptotic bodies, Kupffer cells are stimulated to produce TGF-β1 [10][11], which is a potent cytokine to activate hepatic stellate cells into myofibroblasts with increased expression of α–SMA, TGF-β, PDGF, CTGF, type I collagen, and tissue inhibitor of metalloproteinase 1 (TIMP1) [12]. Although TGF-β1 is one of the most potent stimuli of extracellular matrix synthesis, suppressing its expression remains a major challenge of antifibrotic therapy, since systemic blocking of TGF-β1 can provoke inflammation and increase the risk of neoplasia. Neutralization of TGF-β in animal models inhibits liver fibrosis and reduces the risk in developing cholangiocarcinoma [13][14]. Fresolimumab (GC1008) is a human anti-TGF-β1 monoclonal antibody that neutralizes all isoform of TGF-β. In patients with advanced malignant melanoma and renal cell carcinoma, fresolimumab demonstrated acceptable safety and preliminary evidence of antitumor activity [15–17]. Using radio-labeled (89)Zr-conjugated fresolimumab for PET to analyze TGF-β expression, GC1008 accumulated in primary tumors and metastases in a manner similar to IgG (89), and Zr-fresolimumab uptake is seen in sites of tumor ulceration and in scar tissue, where TGF-β is highly active [18]. Although there is a phase II clinical trial ongoing of fresolimumab, optimal strategies are still needed to restrict it to the fibrotic milieu. TGF-β transduces its signal to target genes through the ALK5 ser/thr kinase receptor. GW6604 (2-phenyl-4-(3-pyridin-2-yl-1H-pyrazol-4-yl) pyridine), an ALK5 inhibitor, inhibits the transcription and deposition of extracellular matrix and improves the deterioration of liver function in mice [19]. Yet, considering of the pleiotropic effects of TGF-β, treatment with an ALK5 inhibitor should be carefully examined to avoid unwanted effects [20].
Integrin αvβ6, which is another molecular absent in normal liver, promotes proliferation of cholangiocytes and plays functional roles in activating latent TGF-β1. Cholangiocytes exhibit marked increased expression of αvβ6 integrin in thioacetamide (TAA)- and BDL-induced fibrosis, and in human HCV fibrosis and end-stage cirrhosis [21][22]. Inhibition of αvβ6 could attenuate collagen deposition, improve liver function, and retard progression of biliary fibrosis in mouse orthotopic liver transplantation model [23]. Impressively, a single dose of integrin αvβ6 inhibitor exhibits antifibrogenic and profibrolytic effects in experimental models [22].
Lysophosphatidic acid (LPA) is a lipid mediator, which is produced mainly by activated platelets via hydrolysis of lysophosphatidylcholine by autotaxin (ATX). LPA is a bioactive lipid implicated in several functions, including proliferation, apoptosis, migration, and cancer cell invasion [24]. LPA and LPA1 receptor (LPA1R) are increased in many inflammatory states, including pulmonary fibrosis, liver fibrosis, and systemic sclerosis [25]. LPA exerts various physiological effects on the receptors of parenchymal cells and LPA1R antagonists showed anti-fibrotic effect on liver fibrosis, lung fibrosis and scleroderma model [25–27].
Inhibition of proliferation of HSCs
Inhibitors of receptor tyrosine kinase and Ser/Thr kinase also demonstrate some anti-fibrosis effects. Multitargeted receptor tyrosine kinase inhibitor Sorafenib, which has been approved for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma (HCC), and Sunitinib can improve experimental hepatic fibrosis, inflammation, and angiogenesis [28][29]. SiRNA of transient receptor potential melastatin 7 (TRPM7), a non-selective cation channel with protein serine/threonine kinase activity, attenuates TGF-β1-induced expression of myofibroblast markers, increases the ratio of MMPs/TIMPs, and decreases the phosphorylation of Smad2 and Smad3 associated collagen production [30][31]. Hepatic nuclear factor kappa B (NF-κB)-inducing kinase (NIK), a Ser/Thr kinase, which is increased in injured livers in both mice and humans, induces hepatocyte injury, activates bone marrow-derived macrophages, and leads to liver fibrosis, and this might serve as a candidate for liver fibrosis therapy [32].
The renin angiotensin pathway in hepatic stellate cells induces reactive oxygen species and accelerates hepatic fibrosis [33]. In response to sustained liver injury, the renin angiotensin system (RAS) locally accelerates inflammation, tissue repair and fibrogenesis by production of angiotensin II (Ang II), a vasoconstricting agonist implicated in the pathogenesis of liver fibrosis. RAS is described as a single cascade where renin converts angiotensinogen into angiotensin I (Ang I), which is converted to angiotensin II (Ang II) by angiotensin converting enzyme (ACE). Ang II mediates biological responses through two G-protein-coupled receptors, the Ang II receptor type 1 (AT1) and Ang II receptor type 2 (AT2). However, the fibrogenic actions of Ang II are mostly mediated by angiotensin receptor AT1. Stimulation of AT1 receptor by Ang II results in proliferation of HSCs and extracellular matrix deposition. Ang II also plays an important role in ROS formation by activating NADPH oxidase (NOX) in HSCs. In concordance, several experimental models of liver fibrosis in rodents have demonstrated that prolonged administration of angiotensin II directly causes HSC activation. Mice lacking AT1a receptors are protected from liver fibrosis. This makes RAS an attractive target for anti-fibrotic therapy. Although several small studies showed the usefulness of ACEi/ARB for liver fibrogenesis in patients with hepatitis C [34], the HALT-C cohort study did not show any anti-fibrogenic effects of ACEi/ARB for chronic hepatitis C patients [35].
Apoptotic hepatocytes activate HSCs through Nox/ROS signaling. Chronic liver injury induces ongoing hepatocyte injury and death increases ROS production and decreases antioxidant activity, which is one of the characteristics of chronic liver disease that triggers liver fibrogenesis. NOX produces ROS via transferring electron from nicotinamide adenine dinucleotide phosphate to molecular oxygen, which is different from other redox enzymes that produce superoxide as a byproduct. The mammalian NOX family is composed of seven isoforms: NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2, which are distinctively expressed in specific cell types in the liver. Hepatic stellate cells express three Nox isoforms, Nox1, Nox2, and Nox 4 [36][37]. HSCs from p47phox-deficient mice (without a regulatory component of NOX) fail to generate ROS in response to angiotensin II, platelet derived growth factor (PDGF), leptin, or apoptotic bodies, and p47phox-deficient mice demonstrate reduced liver fibrosis after BDL or the hepatotoxin CCl4 [38][39]. Since NOX1 and NOX4 are expressed in α-SMA positive activated HSCs, GKT137831, a potent dual NOX1/NOX4 inhibitor, attenuates ROS production and inhibits activation of HSCs [37]. Multicenter, long-term clinical trials are still needed to evaluate the role of antioxidants in NASH.
Promotion of apoptosis of activated HSCs
Daily cannabis use is an independent risk factor for increased liver fibrosis in HCV patients [40]. CB1 and CB2 receptors are increased in liver fibrosis. CB1 KO mice are resistant to liver fibrosis, while CB2 KO mice have increased liver fibrosis [41][42]. CB1 agonists activate hepatic stellate cells to myofibroblasts. CB1 receptor agonists, such as Rimonabant, inhibit and reverse experimental liver fibrosis [43][44]. A peripherally acting CB1 antagonist could treat liver fibrosis without inducing depression.
ECM degradation is mediated by matrix metalloproteinases (MMPs), a family of zinc-dependent enzymes grouped into collagenases, gelatinases, stromelysins, and membrane-type MMPs. MMP activity is regulated by tissue inhibitors of metalloproteinases (TIMPs 1–4), which bind in substrate- and tissue-specific manners to MMPs, blocking their proteolytic activity. In concordance, monoclonal Anti-TIMP1 Ab partially reverses established CCl4 induced fibrosis [45]. Furthermore, persistent expression of TIMP-1 in vivo was associated with persistence of activated HSCs, and during resolution of fibrosis, a decrease in TIMP-1 protein levels correlated with decreased numbers of HSCs [46].
Promotion of HSC inactivation
Clinical and experimental hepatic fibrosis is reversible. Regression of liver fibrosis is associated with resorption of fibrous scar and disappearance of collagen producing myofibroblasts. The fate of these myofibroblasts has been recently revealed: Some myofibroblasts undergo apoptosis during regression of fibrosis, while other myofibroblasts revert to a quiescent-like phenotype. Inactivation of myofibroblasts is a newly described phenomenon [47] which now requires mechanistic investigation. Inactivation of HSCs is associated with re-expression of lipogenic genes PPAR-γ, Insig1, and CREBP. PPAR-γ is reported to be important for maintaining and re-establishing the quiescent phenotype (qHSCs) [48].
Inhibition of deposition of type I collagen
In liver fibrosis, type I collagen is the most prominent increased components of the extracellular matrix. The cross-linking of type I collagen is also increased, which is modulated by the matrix enzyme lysyloxidase-like-2 (LOXL2). Although blocking collagens have unwanted off-target effects, inhibition of LOXL2 by a monoclonal antibody (AB0023) reduces the production of cytokines, attenuates TGF-β signaling, and inhibits the activate fibroblasts (Barry-Hamilton, 2010. Similar to AB0023, another humanized monoclonal LOXL2 antibody (GS-6624) is in randomized, double blind, phase II clinical trials to treat NASH and PSC [49].
Conclusion
The cellular and molecular mechanism of liver fibrosis has been extensively studied, and new therapies based on these understandings are currently in early-phase clinical development.
Acknowledgments
Grants: NIH 2 P50 AA011999, 5 P42 ES010337, 5 U01 AA021856
Footnotes
Note de titre: This article is part of the special issue “Alcohol, Virus and Steatosis evolving to cancer” featuring the conference papers of the 10th International Symposium organized by the Brazilian Society of Hepatology in São Paulo, Brazil, September 30th–October 1st, 2015.
Disclosure
No potential conflict of interest relevant to this article was reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hauff P, Gottwald U, Ocker M. Early to Phase II drugs currently under investigation for the treatment of liver fibrosis. Expert Opin Investig Drugs. 2015;24:309–27. doi: 10.1517/13543784.2015.997874. [DOI] [PubMed] [Google Scholar]
- 2.Thornberry NA. Caspases: key mediators of apoptosis. Chem Biol. 1998;5:R97–103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- 3.Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–6. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- 4.Koyama Y, Taura K, Hatano E, Tanabe K, Yamamoto G, Nakamura K, et al. Effects of oral intake of hydrogen water on liver fibrogenesis in mice. Hepatol Res. 2014;44:663–77. doi: 10.1111/hepr.12165. [DOI] [PubMed] [Google Scholar]
- 5.Iwaisako K, Haimerl M, Paik YH, Taura K, Kodama Y, Sirlin C, et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor delta agonist. Proc Natl Acad Sci U S A. 2012;109:E1369–76. doi: 10.1073/pnas.1202464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–46. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox N, Pilling D, Gomer RH. Serum amyloid P: a systemic regulator of the innate immune response. J Leukoc Biol. 2014;96:739–43. doi: 10.1189/jlb.1MR0114-068R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford JR, Pilling D, Gomer RH. FcgammaRI mediates serum amyloid P inhibition of fibrocyte differentiation. J Leukoc Biol. 2012;92:699–711. doi: 10.1189/jlb.0112033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–44. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadok VA, McDonald PP, Bratton DL, Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998;26:653–6. doi: 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- 11.Szondy Z, Sarang Z, Molnar P, Nemeth T, Piacentini M, Mastroberardino PG, et al. Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci U S A. 2003;100:7812–7. doi: 10.1073/pnas.0832466100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meindl-Beinker NM, Matsuzaki K, Dooley S. TGF-beta signaling in onset and progression of hepatocellular carcinoma. Dig Dis. 2012;30:514–23. doi: 10.1159/000341704. [DOI] [PubMed] [Google Scholar]
- 13.Fan X, Zhang Q, Li S, Lv Y, Su H, Jiang H, et al. Attenuation of CCl4-induced hepatic fibrosis in mice by vaccinating against TGF-beta1. PLoS One. 2013;8:e82190. doi: 10.1371/journal.pone.0082190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S, et al. Transforming growth factor beta neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One. 2013;8:e54499. doi: 10.1371/journal.pone.0054499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacouture ME, Morris JC, Lawrence DP, Tan AR, Olencki TE, Shapiro GI, et al. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor beta by the monoclonal antibody fresolimumab (GC1008) Cancer Immunol Immunother. 2015;64:437–46. doi: 10.1007/s00262-015-1653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One. 2014;9:e90353. doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–43. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oude Munnink TH, Arjaans ME, Timmer-Bosscha H, Schroder CP, Hesselink JW, Vedelaar SR, et al. PET with the 89Zr-labeled transforming growth factor-beta antibody fresolimumab in tumor models. J Nucl Med. 2011;52:2001–8. doi: 10.2967/jnumed.111.092809. [DOI] [PubMed] [Google Scholar]
- 19.de Gouville AC, Boullay V, Krysa G, Pilot J, Brusq JM, Loriolle F, et al. Inhibition of TGF-beta signaling by an ALK5 inhibitor protects rats from dimethylnitrosamine-induced liver fibrosis. Br J Pharmacol. 2005;145:166–77. doi: 10.1038/sj.bjp.0706172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Gouville AC, Huet S. Inhibition of ALK5 as a new approach to treat liver fibrotic diseases. Drug News Perspect. 2006;19:85–90. doi: 10.1358/dnp.2006.19.2.977444. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–12. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popov Y, Patsenker E, Stickel F, Zaks J, Bhaskar KR, Niedobitek G, et al. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol. 2008;48:453–64. doi: 10.1016/j.jhep.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Zhang L, Chen L, Wang H, Zhang Y, Bie P. Role of integrin alphavbeta6 in the pathogenesis of ischemia-related biliary fibrosis after liver transplantation. Transplantation. 2013;95:1092–9. doi: 10.1097/TP.0b013e3182884866. [DOI] [PubMed] [Google Scholar]
- 24.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–91. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 25.Mazzocca A, Dituri F, Lupo L, Quaranta M, Antonaci S, Giannelli G. Tumor-secreted lysophostatidic acid accelerates hepatocellular carcinoma progression by promoting differentiation of peritumoral fibroblasts in myofibroblasts. Hepatology. 2011;54:920–30. doi: 10.1002/hep.24485. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi T, Yamamoto T. Anti-fibrotic effect of lysophosphatidic acid receptors LPA and LPA antagonist on experimental murine scleroderma induced by bleomycin. Exp Dermatol. 2015 doi: 10.1111/exd.12752. [DOI] [PubMed] [Google Scholar]
- 27.Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC, et al. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol. 2010;160:1699–713. doi: 10.1111/j.1476-5381.2010.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–56. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 29.Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–26. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 30.Fang L, Zhan S, Huang C, Cheng X, Lv X, Si H, et al. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicol Appl Pharmacol. 2013;272:713–25. doi: 10.1016/j.taap.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Fang L, Huang C, Meng X, Wu B, Ma T, Liu X, et al. TGF-beta1-elevated TRPM7 channel regulates collagen expression in hepatic stellate cells via TGF-beta1/Smad pathway. Toxicol Appl Pharmacol. 2014;280:335–44. doi: 10.1016/j.taap.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Shen H, Sheng L, Chen Z, Jiang L, Su H, Yin L, et al. Mouse hepatocyte overexpression of NF-kappaB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis. Hepatology. 2014;60:2065–76. doi: 10.1002/hep.27348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kisseleva T, Brenner DA. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:305–17. doi: 10.1016/j.bpg.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corey KE, Shah N, Misdraji J, Abu Dayyeh BK, Zheng H, Bhan AK, et al. The effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Liver Int. 2009;29:748–53. doi: 10.1111/j.1478-3231.2009.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abu Dayyeh BK, Yang M, Dienstag JL, Chung RT. The effects of angiotensin blocking agents on the progression of liver fibrosis in the HALT-C Trial cohort. Dig Dis Sci. 2011;56:564–8. doi: 10.1007/s10620-010-1507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paik YH, Iwaisako K, Seki E, Inokuchi S, Schnabl B, Osterreicher CH, et al. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730–41. doi: 10.1002/hep.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paik YH, Kim J, Aoyama T, De Minicis S, Bataller R, Brenner DA. Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal. 2014;20:2854–72. doi: 10.1089/ars.2013.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys. 2007;462:266–72. doi: 10.1016/j.abb.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Minicis S, Seki E, Paik YH, Osterreicher CH, Kodama Y, Kluwe J, et al. Role and cellular source of nicotinamide adenine dinucleotide phosphate oxidase in hepatic fibrosis. Hepatology. 2010;52:1420–30. doi: 10.1002/hep.23804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patsenker E, Sachse P, Chicca A, Gachet MS, Schneider V, Mattsson J, et al. Elevated levels of endocannabinoids in chronic hepatitis C may modulate cellular immune response and hepatic stellate cell activation. Int J Mol Sci. 2015;16:7057–76. doi: 10.3390/ijms16047057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–55. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Pacher P, Gao B. Endocannabinoids and liver disease. III Endocannabinoid effects on immune cells: implications for inflammatory liver diseases. Am J Physiol Gastrointest Liver Physiol. 2008;294:G850–4. doi: 10.1152/ajpgi.00523.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai E, Zhang J, Zhang D, Yang L, Wang Y, Jiang X, et al. Rimonabant inhibits proliferation, collagen secretion and induces apoptosis in hepatic stellate cells. Hepatogastroenterology. 2014;61:2052–61. [PubMed] [Google Scholar]
- 44.Baldassarre M, Giannone FA, Napoli L, Tovoli A, Ricci CS, Tufoni M, et al. The endocannabinoid system in advanced liver cirrhosis: pathophysiological implication and future perspectives. Liver Int. 2013;33:1298–308. doi: 10.1111/liv.12263. [DOI] [PubMed] [Google Scholar]
- 45.Parsons CJ, Bradford BU, Pan CQ, Cheung E, Schauer M, Knorr A, et al. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106–15. doi: 10.1002/hep.20425. [DOI] [PubMed] [Google Scholar]
- 46.Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, et al. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–76. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 47.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–53. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Xu J, Brenner DA, Kisseleva T. Reversibility of Liver Fibrosis and Inactivation of Fibrogenic Myofibroblasts. Curr Pathobiol Rep. 2013;1:209–14. doi: 10.1007/s40139-013-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]