Abstract
Estradiol rapidly activates, within minutes, various physiological functions and behaviors including cognition in rodents. This review describes rapid effects of estradiol on hippocampal dependent learning and memory tasks in rodents. Mechanisms underlying the memory enhancements including the activation of signaling molecules and the enhancement of dendritic spinogenesis are briefly reviewed. In addition, the role of estradiol in the cognitive resilience to chronic stress exhibited only in females is discussed including contributions of ovarian as well as intra-hippocampally derived estrogens to this sex difference. Finally, speculations on possible physiologic functions for rapid mnemonic changes mediated by estrogens are made. Overall, the emergence of a novel and powerful mechanism for regulation of cognition by estradiol is described.
Keywords: Recognition Memory, estradiol, hippocampus, dendritic spines, stress, sex differences
1. Introduction
Estradiol was first demonstrated to regulate learning and memory through alterations in neural morphology, physiology and chemistry approximately twenty years ago [see Luine, 1 for review]. Estrogen dependent increases in spine density and synapse number in the hippocampus, as well as increased activity of monoaminergic and cholinergic terminals, were similar to earlier descriptions of estrogen dependent modulations in preoptic-hypothalamic area neurons that regulate sexual behavior, ovulation and also food ingestion [2]. The mechanism for effects in both the hypothalamus and hippocampus depend on binding of estrogen to classical receptors (ERs) which subsequently act as ligand dependent transcription factors. Interactions of the receptor-ligand complexes at estrogen response elements (ERE) on DNA stimulate transcription of specific genes whose proteins then determine the unique physiological responses of estrogen target tissues such as the uterus, breasts, osteoclasts as well as the CNS. These genomic alterations by estradiol are delayed in onset (several hours to days) but result in long lasting and sustained effects on neural function and most likely underlie physiological, behavioral and cognitive changes that occur in females during the menstrual and estrous cycles, pregnancy, menopause and aging. For a description of the major neuronal systems altered and specific proteins changed by genomic actions of estrogens, see [2].
More recently, estradiol has been demonstrated to rapidly, within minutes, activate various physiological functions and behaviors in rodents, birds and possibly humans. These behaviors include female rodent sexual behavior [3], avian male sexual displays [4], nutrient ingestion [5], social learning [6] and cognition [7, 8]. The expression of these behaviors is dependent upon activation of different, but often, inter-related areas in the brain through estrogen’s interaction with membrane ERs. Rapid effects of estrogens were reported in the 70’s and 80’s (1–5), but it is only recently that they have been widely explored in behavioral paradigms; nonetheless, some consistent patterns of action are emerging.
This review will describe rapid effects of estradiol on hippocampal dependent learning and memory tasks in rodents, and the contribution of signaling molecules and dendritic spines in rapidly mediating memory enhancements will be briefly reviewed. The ecological advantages and usefulness for rapid mnemonic changes are currently unknown, but speculations are made. Finally, the contribution of this mechanism, combined with intra-hippocampal synthesis of estradiol, for mediating cognitive resilience to chronic stress demonstrated by females, but not males, is considered. Overall, the emergence of a novel and powerful mechanism for regulation of cognition by estradiol is described.
2. Estradiol rapidly enhances recognition memory
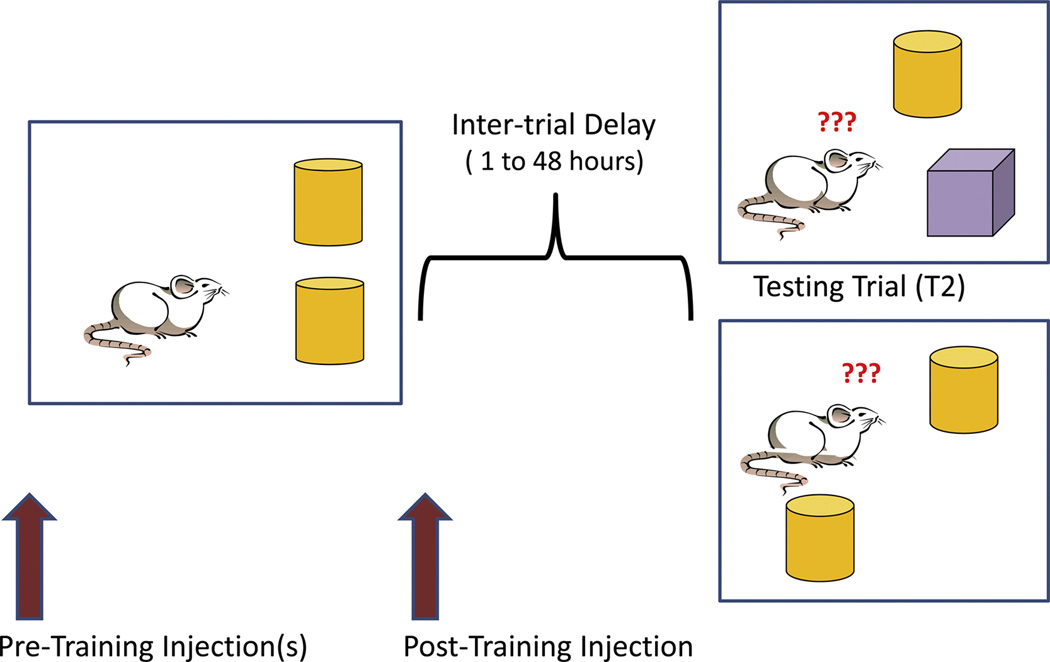
My laboratory and others have utilized a number of hippocampal dependent spatial memory tasks to show that chronic treatments with estradiol, several days to several weeks, enhance learning and memory. Ovariectomized (OVX) rats injected daily with estradiol benzoate (long acting form of estradiol) or implanted with Silastic capsules containing estradiol (release constant amounts of hormone for weeks) for 2–10 days show improved performance on the radial arm maze (RAM) [9], water maze [10] and novel object recognition and novel object placement [11,12,13]. For RAM and water maze studies, many trials over several days are required to learn the tasks, and these tasks are therefore ideal for assessing effects of chronic estradiol. However, for evaluating potential rapid effects of hormones or drugs, tasks with a shorter time course are necessary, and one-trial learning and memory tasks have recently been adopted [12,13]. Figure 1 depicts such tasks showing assessment of recognition memory using either a spatial configuration, novel object placement (NOP) or a non-spatial configuration, novel object recognition (NOR). As indicated, estradiol or other hormones/drugs can be given for days before the learning or sampling trial (T1) to assess chronic treatments, or minutes before T1 or immediately following T1 (known as post-training injections) in order to assess rapid hormonal effects. The retention trial (T2) is typically given from 1 to 48 h after T1. If a subject, rat or mouse, remembers the old object or the old location, then the new object or the object in the new location is explored more than the old object or object in old location because rats are exploratory and novelty seekers. Data is usually expressed as the exploration ratio (time exploring new object or location/total exploration time) where values over 0.5 indicate better than chance memory or the actual exploration times for old vs new can be compared.
Figure 1. Schematic of recognition memory task protocols.
On the left side of the figure, a rat is depicted during the Training or Sampling Trial (T1) where two identical objects are explored, typically for 3-5 min or until a set amount of exploration is obtained. An inter-trial delay is given, and then, shown on the right side, the retention or testing trial is given (usually for 3–5 min). If one identical object is replaced with a new object, this is the novel object recognition (NOR) protocol. If one of the objects is moved to a new location, this is the novel object placement (NOP) protocol, a spatial memory task. Hormones or drugs can be given pre-training (hours or days) to assess genomic changes or immediately post-training or up to two hours later in order to asses rapid changes. Post-training injections test effects of agents on memory consolidation.
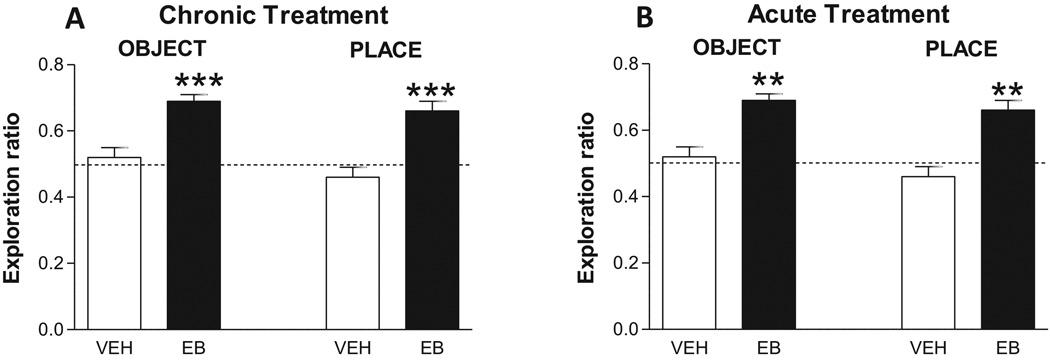
As shown in Figure 2A, chronic treatment with estradiol benzoate for two days results in significant enhancements in both NOR and NOP in the retention trial [11], a result similar to chronic treatment effects on the radial arm or water maze. These effects are mediated through classical ERs and genomic mechanisms and appear to involve enhancements of both learning and long term memory (12). Figure 2B shows effects of estradiol treatment on NOR and NOP when one estradiol injection is given immediately after the training trial. Significant enhancements are seen 4 h later in the retention trial, and further investigations of this effect by varying time of treatments after T1 indicate that estradiol is enhancing memory retention by increasing memory consolidation (See reference 13 for details). Further studies show that these effects are consistent with estradiol acting at membrane receptors [14]. Overall, Figure 2 illustrates that chronic (multiple treatments given over days) or acute (one treatment given for a few hours) estrogen treatments enhance recognition memory in rats, an effect reported in many studies [12].
Figure 2. Effects of chronic and acute estrogen treatment on NOR and NOP.
A. Chronic estradiol treatment – Ovx rats received two days of S.C. injections with vehicle or 50 ug/kg of estradiol benzoate (EB), and NOR and NOP was tested separately 48 h after the last dose. Entries are ratios (new/old + new) of time spent exploring each object (NOR) or objects in each location (NOP) for vehicle- and EB-treated subjects. Dotted line at 0.5 indicates spending the same amount of time exploring new and old objects or locations. ** P < 0.01 *** P < 0.001 by Mann-Whitney U-tests. Redrawn from Jacome et al [11].
B. Acute Estradiol treatment – Ovx rats received vehicle or 5 ug/kg (NOR) or 20 ug/kg (NOP) of estradiol immediately after a Training Trial. 4 h later NOR and NOP were separately tested. Data presented as in A. ** P < 0.01. Redrawn from Inagaki et al [14].
3. Mechanisms for rapid enhancements of memory
3.1. Activation of cell signaling
Estrogens exert rapid effects on neural function by activating numerous cell-signaling cascades and epigenetic processes within 5–30 min of treatment in the hippocampus [8, 16] and also in the prefrontal cortex, although less evidence is extant for cortical areas [17]. These actions form the bases for estrogen’s ability to enhance the consolidation of hippocampal memories. The initial event responsible for memory consolidation appears to be the activation of glutamate receptors (mGluR), primarily MGluR1. These events trigger long term potentiation (LTP) induction, spine formation and memory formation [See Sweat, 18, for review]. Estradiol rapidly activates mGLuR signaling in vivo in both the PFC and the hippocampus, and ERs are found on membranes of dendrites and spines, in presynaptic terminals and near post-synaptic receptors where estrogen binding to the ERs initiates rapid activation of intracellular signaling cascades and immediately early genes which are critical for memory consolidation [8, 16].
3.2. Enhancement of spinogenesis
Increased dendritic spine density in hypothalamic and hippocampal as well as cortical areas is a well-described action of estradiol after either chronic or acute treatments [7, 19]. More spines and increased spine/synapse size involve estrogen dependent increases in phosphorylation of a number of signaling pathways which converge in the rapid formation of new spines and development of existing immature spines. Important pathways which are activated include the Ras/Raf/Mitogen activated protein kinase (MAP)/extracellular signal regulated kinase cascade(ERK) and the phosphatidylinositol-3-inase/Akt (P13K) pathway which in turn increase phosphorylation of the transcription factor cAMP response element-binding protein (CREB) [20]. CREB decreases transcription of cofillin, which allows for increased polymerization of actin polymers for assembly of spines and for increased transcription of actin and RhoA which underlie filopodial extension and spine formation [21]. Phosphorylation of P13K also leads to increases in Protein Kinase B (Akt) and 4E-BP1 which enhance the level of post synaptic density-95 (PSD-95) protein, an integral framework for synapse formation [22]. Enhanced ERK/mitogen activated protein kinase (MAPK) signaling by estradiol may also enhance memory consolidation by activation of epigenetic processes such as histone acetylation and DNA methylation that enhance the expression of genes and synthesis of proteins [23]. Interactions with brain derived neurotrophic factor (BDNF) may also contribute to estradiol’s ability to increase spines since BDNF also increases spines, and estradiol increases BDNF levels [19].
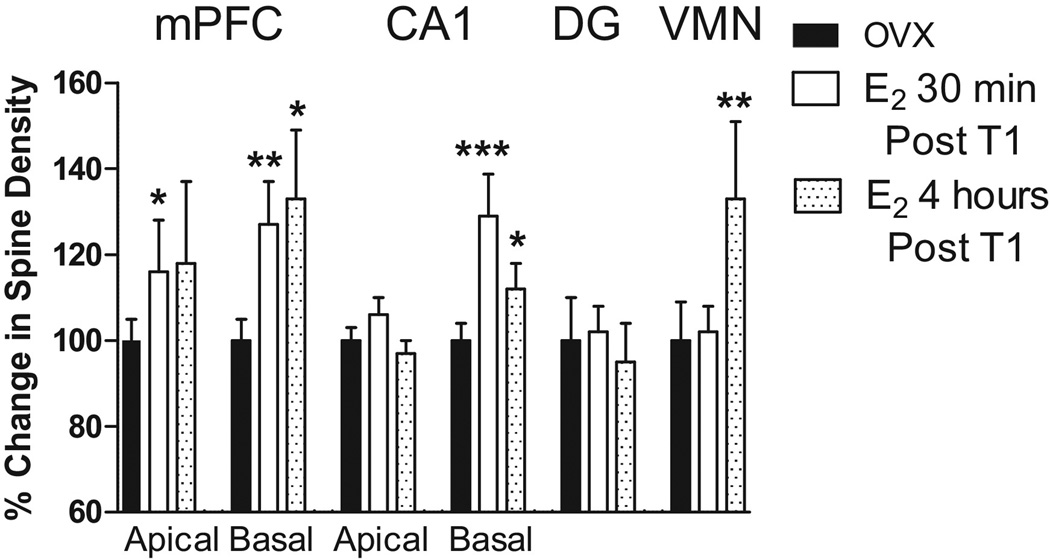
We have shown that 30 min following S.C. estradiol administration to OVX females, spine density is increased by 15–30% in apical and basal medial prefrontal cortex (PFC) dendrites and in basal, but not, apical CA1 hippocampal dendrites (Figure 3). No changes were found in dendritic spines on granule cells of the hippocampal dentate gyrus or in ventromedial nucleus cells of the hypothalamus (VMN), two ER containing areas. Dendritic spine density remained elevated 4 h following estradiol administration in the PFC and CA1, and the VMN showed a 20% increase in spine density at this time post estradiol. However, spine changes were not evident in the DG at either 30 min or 4 h post estradiol. Christensen et al [3] reported increased spine density in the arcuate nucleus 4 h post estradiol, but earlier time points were not examined, and Phan et al [24] found increased CA1 spines 40 min post estradiol. Thus, increased spine density following estradiol may appear more rapidly in estrogen responsive areas important for memory, the PFC and hippocampus, than areas involved in reproduction, such as the VMN and arcuate nucleus, but further temporal evaluations of hypothalamic sites is necessary. This dose of estradiol, 20 ug/kg, enhances memory when given immediately following and up to 45 min, but not 60 min, after a learning trial (T1) for recognition memory [25]. Thus, the increased spine densities present in the PFC and hippocampus 30 min. following estradiol treatment may contribute to enhanced memory consolidation. Memory retention is also enhanced at 4 h following 20 ug/kg of estradiol [14, 25; Figure 2B]. Therefore, the increased spine densities present 4h following estradiol treatment may contribute to enhanced memory retention. However, the exact relationship of spines to memory consolidation and retention requires further investigation and evaluation.
Figure 3. Rapid effects of estradiol on spine density.
Ovx rats received a T1 recognition trial and were immediately injected with vehicle or 20 ug/kg of estradiol and sacrificed 30 min or 4 h later. Secondary basal dendrites and tertiary apical dendrites were analyzed from pyramidal cells in the CA1 region of the dorsal hippocampus and layer II/III of the PFC. Primary dendrites from VMN and dentate granule cells were also counted. Spines were counted under oil at 100× and represent all visible spines. Entries show effects of estradiol treatments as % change + S.E.M. in spine density from vehicle treated ovx rats. CA1 and PFC results from [25]; DG 30 min results from Luine [13]; DG 4 h and VMN results from Frankfurt and Luine [64]. * p < 0.05; ** p < 0.01; *** p < 0.001 by Student’s t-test.
3.3. Intra-hippocampal synthesis of estrogens
Estradiol is synthesized in discrete regions of the brain by the enzyme aromatase from androgen precursors such as testosterone and/or by direct de novo synthesis from cholesterol. Levels of estradiol in the hippocampus, hypothalamus and PFC are higher than in the circulation. For example, Kato et al [26] reported that hippocampal 17β-estradiol levels are 4 nM in female rats during proestrus while circulating concentrations are only 0.1 nM and that hippocampal levels change over the estrus cycle. Furthermore, estradiol remained measureable in the hippocampus following ovariectomy. The in situ production of estradiol indicates that it may act as a neurosteroid as well as a gonadal steroid, and some evidence shows that it may also be a neuromodulator [27, 28]. Brain-synthesized estradiol was first measured in birds and is now recognized to mediate neural functions in regions of a number of avian species [28, 29]. In addition, in vitro studies report that hippocampal estradiol synthesis is important for the maintenance of hippocampal spines, synapses and synaptic proteins in cultures from rats [30]. These demonstrations suggest a completely new role for hippocampally derived estrogens in neural function.
An important question for the present review is whether neurally derived estrogens contribute to learning and memory. Support for this idea comes from earlier studies showing that stimulation of hippocampal slices with NMDA significantly increases estradiol synthesis [31] and that forebrain estradiol levels in songbirds are rapidly regulated by voltage-gated calcium channels [32]. A recent experiment provides evidence that hippocampally derived estradiol may contribute to spatial memory. Direct infusion into the hippocampus of an aromatase inhibiter impaired spatial memory in a food-finding task in male zebra finches [33]. Thus, locally derived estradiol may contribute to learning and memory, but extensive experimentation will be required to determine the relative contribution of ovarian and hippocampally derived estrogens in memory regulation. Ovarian steroids may prime the hippocampus or prefrontal cortex to respond to locally synthesized estradiol so that learning events are potentiated. On the other hand, ovarian derived estrogens may simply be necessary for the general maintenance of the neurons and not contribute to cognitive processes.
4. Function(s) of rapid, estrogen induced changes in memory
A looming question concerning the rapid effects of estradiol on memory and spinogenesis is whether these changes are functional, and if so, for what purpose(s). The next sections provide mainly speculations which are designed to spark research interest.
4.1. Endocrine disrupters
We have found that endocrine disrupters affect cognition and spine density and may be one way that hormones or hormone-like agents may rapidly affect neural function. Bisphenol A (BPA) is a known endocrine disruptor documented to have estrogenic, anti- estrogenic, androgenic, and anti-androgenic effects [34, 35] on various hormone- induced physiological and behavioral phenomena. Given immediately following a learning trial for recognition memory, BPA impaired NOR and NOP in male rats [36] and impaired NOR in female rats during proestrus [25]. Given 30 min before the learning trial, BPA blocked enhanced NOR and NOP in OVX females that received estradiol immediately after the sample trial [25]. Impaired memory following BPA is associated with 10 and 23% decreases in spine density in male CA1 and PFC, respectively, 30–40 min following BPA injection [36]. In females, BPA blocked estrogen dependent increases in basal CA1 spine density, but did not alter PFC spine density, at 4 h after estradiol. Thus, BPA may cause rapid, deleterious effects on memory by acting through estrogen dependent mechanisms which normally activate memory. It is important to note that similar memory impairments could arise in humans after ingesting food or water from BPA containing bottles or cans.
4.2. Phytoestrogens
Phytoestrogens are naturally occurring estrogens which are present in plants such as soy and clover and may cause rapid, enhancing effects on memory. We have found that chronic ingestion of phytoestrogens, present in rat chow, enhances recognition memory in OVX rats and increases CA1 and PFC spine density [37], but we have not tested acute effects of these estrogens. It can, however, be speculated that ingestion of these natural estrogens might have been beneficial to our ancestor hunter-gathers or to grazing animals today because ingestion of phytoestrogen containing plants could rapidly enhance remembrance of the location of these protein rich food sources, an idea which requires testing.
4.3. Stress resilience in female rats
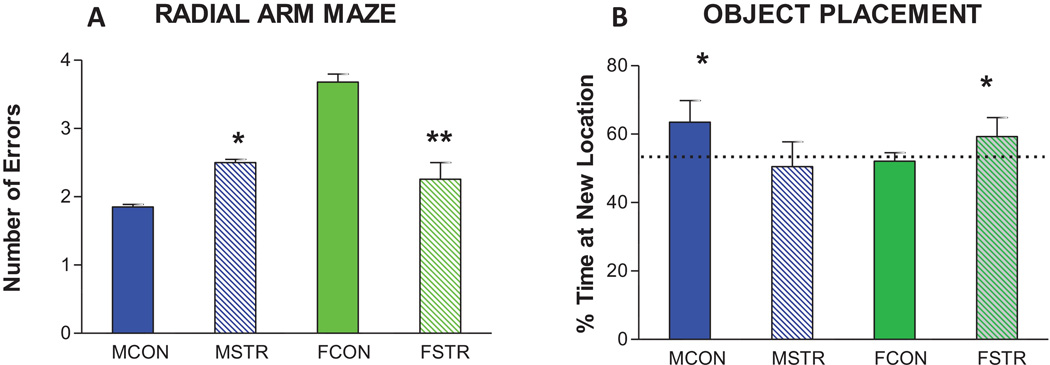
There is mounting evidence that females are more resilient to stress than males, especially with respect to cognitive function where chronic stress impairs male, but either has no effect on, or enhances, female cognition. Moreover, estradiol may contribute to this sex difference. In male rodents, 1–3 weeks of stress elicited by daily restraint or different daily stressors (unpredictable chronic stress, UCS) generally results in impaired learning and memory. Impairments in male learning have been shown using spatial learning and memory tasks like the Morris water maze and eight-arm radial task (Figure 4A). On the radial arm maze, stressed male rats make more mistakes/trial and take longer to reach a learning criterion than unstressed rats [38]. In the water maze, a similar pattern of impaired learning is shown after chronic stressors [39].
Figure 4. Sex differences in chronic stress effects on spatial memory tasks.
A. Adult male and female rats received 21 days of daily (6h) restraint stress and were tested on the radial arm maze. Stress significantly increased errors in males, but significantly decreased errors in females. Data pooled from Luine et al. [38] for males and from Bowman et al. [49] for females. * P < 0.05; ** P < 0.01.
B. Adult male and female rats received 21 days of daily (6h) restraint stress and were tested on the NOP task. % time at new location is plotted. Stressed males could not significant discriminate locations but stressed females could. * P < 0.05. Data from Beck and Luine [42].
In tests assessing only memory, chronic stress also generally impairs male performance [see Conrad, 40 for review]. Stressed male rats and mice are unable to significantly discriminate between known and new objects in the NOR test [41, 42] or on a test of temporal order recognition memory (TORM) [43 and Figure 5A]. Spatial memory in males is also impaired by chronic stress in NOP (Figure 4B) [41, 44, 45] and the Y-maze [46, 47, 48]. Thus, chronic stress impairs learning and memory in male rodents in a variety of tasks.
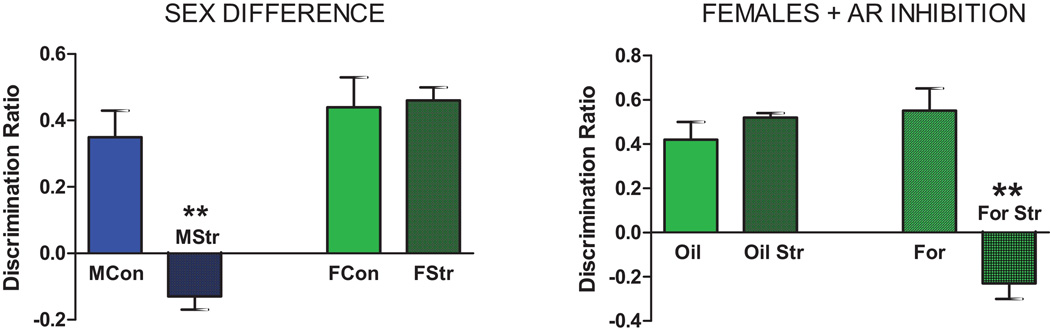
Figure 5. Stress effects on Temporal Order Recognition Memory (TORM) task.
A. Sex Differences in response - Male and female rats received 1 week of daily (6h) restraint stress and were tested on the TORM task one day following the last stress. Entries are the Discrimination Ratio. Males were impaired by stress, but females were unaffected. ** P < 0.01
B. Effects of aromatase inhibition on TORM task in females - Control and stressed female rats received S.C. oil or the aromatase inhibitor formestane, 2 mg/kg, 1 h before daily stress and were tested on the TORM task one day following the last stress. Oil treated control, formastane treated control and stressed subjects all significantly discriminated while formestane + stressed subjects did not. These results suggest that estradiol provides resilience to stress in females. ** P< 0.005. Data redrawn from Wei et al [43].
When female rodents are given the same stress regimens and tested on the same tasks, a different pattern emerges. On the radial arm maze, stressed females perform better than unstressed females (Figure 4A). Stressed females make fewer mistakes/trial and reach learning criterions more rapidly than unstressed females [49]. Similarly, chronic stress enhances female rodent performance in the water maze [39]. In recognition memory tasks, stressed females show better performance than unstressed females in NOP (Figure 4B) [41, 44] and memory is either not affected or enhanced on the Y-maze (Figure 1C) [50, 51], NOR [42, 44, 50, 52] and TORM [43 and Figure 5A] following chronic stress. In addition, no impairments were found when females were given longer periods of stress: radial arm maze after 28 days of stress [49] and NOR and NOP after 35 days of stress [53]. Thus, female rodents appear to show resilience to chronic stress, at least in terms of learning and memory. However, it should be noted that fewer experiments have been conducted in female rodents than in males and that most experiments in females utilized restraint stress. Thus, stress effects on female cognition need further investigation and confirmation.
Several lines of evidence support the idea that estradiol, of both ovarian and neural origin, may be responsible for the cognitive resilience to chronic stress exhibited by females. Because females have high circulating estradiol levels as compared to males and only males were cognitively impaired by stress, we hypothesized that ovariectomized (OVX) females would not show stress resilience. This hypothesis was only partially confirmed because OVX + stressed rats no longer showed enhanced radial-arm maze performance following 21 days of daily restraint stress, but their performance was not impaired by stress as in males [54]. In addition, McLaughlin et al [55] showed that OVX + chronically stressed rats still showed some improvements on the Y maze as compared to non-stressed subjects. Yet, when estradiol was given to OVX-stressed females, radial arm maze performance was enhanced [54]. At the time that these experiments were completed, it was hypothesized that resilience to stress in females must be due to both activating effects of estradiol at adulthood and enduring, organizing effects of estradiol during development in females. However, it is now known, as discussed above, that significant concentrations of estradiol are synthesized locally in the hippocampus following OVX, and thus, neuronally derived estradiol may contribute to stress resilience.
Further information on female rats’ cognitive resilience to stress was provided by Zhen and colleagues who explored sex differences to stress in the TORM task [43]. As indicated earlier and shown in Figure 5A, chronic stress impairs males but not females in TORM. Estrogen involvement in this sex difference was implicated because stress impaired TORM in females when estrogen receptors were inhibited or knocked out in the prefrontal cortex, and impairments of performance in stressed males were prevented by estradiol administration. To further test estrogen’s involvement, females were injected S.C. with the aromatase inhibitor formestane which blocks both peripheral and neural synthesis of estradiol [43]. Under this condition, stress impaired TORM in females. When compared to experiments with RAM and Y maze in females where only peripheral estrogen was removed by OVX (described above), these results provide some evidence that neurally derived estradiol provides resilience against the detrimental effects of chronic stress on memory.
Whether acute increases in ovarian or neural estradiol contribute to rapid enhancements in memory or sex differences in acute stress responses is currently unclear. However, acute stress (1 h. of restraint) immediately increases estradiol in the hypothalamus of OVX or proestrous rats [56]. Hypothalamic aromatase was also increased in proestrous rats, and plasma estradiol was increased in all females, but brain areas involved in cognition were not sampled. However, Lu et al [57] found that acute foot shock decreased plasma estradiol in PE and estrus rats while increasing it in males. When the Trier Social Stress Tests was administered to humans, both male and female subjects showed an immediate post-test increase in plasma estradiol and testosterone [58]. Thus, increases in estradiol may influence cognition following acute stress, another observation requiring experimental validation.
Thus, it could be speculated that stress dependent increases in estrogens might be advantageous in the classic scenario of an antelope escaping across the savannah from a lioness. Stress dependent, increased estradiol might promote the memory of the location of the attack or some details of its overall circumstance. For humans, similar stress effects might enhance memories for the location of traffic jams or dangerous situations in cities. Enhanced release of corticosterone during acute stress is associated with memory enhancements [59], but a possible role for peripheral and/or central estrogen has not been investigated and should provide fertile ground for investigations.
The possibility of estrogens enhancing memory during stressful situation raises the question of whether these effects occur exclusively in females. Estradiol is not associated with cognitive resilience following chronic stress in males, but it is synthesized in vitro by hippocampal cultures from male rats which suggests that estradiol might influence cognition [31]. Effects of estradiol in males have not been extensively investigated. In the NOP task, we found that two day treatments with testosterone, but not estradiol, enhanced performance [13], and Gibbs [60] found that both estradiol and testosterone influenced cognitive performance on the T-maze with each hormone influencing distinct cognitive domains. Leranth and colleagues [reviewed in 61] reported that androgens, but not estrogens, increase spine and spine synapse density in male hippocampi. In addition, inhibition of aromatase in hippocampal cultures results in synapse loss in female but not male cultures [30]. However, these experiments were conducted after chronic treatments, and possible rapid effects of hormones have received little investigation in males. We recently began investigating possible rapid effects of gonadal hormones in castrate male rats and found that both estradiol and testosterone increased memory in the NOP task when given immediately after a training trial [62]. Thirty min after treatment, spine densities on pyramidal cells in CA1 were increased 40% by testosterone and 28% by estradiol, and these remained elevated at 2h. Moreover, Graham and Milad [63] recently showed that inhibition of estradiol synthesis with S.C. fadrazole prior to or immediately after extinction training significantly impaired fear recall during testing in male rats. Thus, some limited data suggests that gonadal hormones may act acutely in male rats to enhance memory, but further studies are necessary to verify these effects and understand their possible relationship to cognition in males.
4.5. Role of neural cross-talk
A final, general role for estrogen’s rapid effects on cognition may be to activate signaling mechanisms which in turn facilitate genomic responses. Thus, this nuclear crosstalk could serve as a “priming mechanism” or a form of coincidence detection for long term changes associated with classic receptor mechanisms. Some support for this concept is the observation that following long term OVX, larger doses or longer treatment times are often necessary to activate estrogen dependent responses [2], but this idea requires extensive validation.
5. Conclusions
New data concerning membrane mediated effects of estradiol have altered our understanding of the mechanisms and scope by which estradiol influences neural functions. It is now recognized that estrogens can rapidly enhance cognition through mechanisms that do not involve classical ERs and genomic mechanisms. This review has examined the possible role of rapid changes in signaling pathways and increases in hippocampal dendritic spines in rapidly altering memory. While rodent models robustly show these effects, it is unknown whether they are relevant physiologically in either rats or humans. It is hypothesized that membrane mediated effects of estradiol might underlie effects of endocrine disruptors and naturally occurring phytoestrogens. In addition, estrogens may contribute to sex differences in cognitive responses to stress. Thus, a multitude of new avenues of research have recently been opened for understanding estradiol’s powerful effects on neural function.
Acknowledgements
Hunter College supported the writing of this review. The empirical studies described from the lab were supported by R25-GM-60665 (MBRS/RISE) and S06-GM-60654 (SCORE) grants from NIH. I thank all previous undergraduate and graduate students, especially Tomoko Inagaki and Luis Jacome, for their assistance with the experiments, and Dr. Maya Frankfurt for helpful discussions and critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luine VN. Estradiol and cognition function: Past, present and future. Hormones and Behavior. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 3.Christensen AP, Dewing P, Micevych P. Membrane-Initiated Estradiol Signaling Induces Spinogenesis Required for Female Sexual Receptivity. J. Neurosci. 2011;30:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Front Neuroendocrinol. 2012;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol. Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- 5.Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrinol. 2012;33:342–363. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ervin KS, Mulvale E, Gallagher N, Roussel V, Choleris E. Activation of the G protein-coupled estrogen receptor, but not estrogen receptor α or β, rapidly enhances social learning. Psychoneuroendocrinol. 2015;58:51–66. doi: 10.1016/j.psyneuen.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Luine V, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and spine density changes. Frontiers in Neuroendocrinology. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frick KM. Molecular mechanisms underlying the memory enhancing effects of estradiol. Horm. Behav. 2015 May 7; doi: 10.1016/j.yhbeh.2015.05.001. pii: S0018-506X(15)00072-0. doi: 10.1016/j.yhbeh.2015.05.001. [Epub ahead of print]. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luine V, Richards ST, Wu VY, Beck K. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm. Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 10.Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol. Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- 11.Jacome LF, Gautreaux C, Inagaki T, Mohan G, Arellanos A, MacLusky N, Alves S, Lubbers L, Luine VN. ERβ Agonists Enhance Recognition Memory and Alter Monoamines in Ovariectomized Rats. Neurobiology of Learning and Memory. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuscher JJ, Fortress AM, Kim J, Frick KM. Regulation of object recognition and object placement by ovarian sex steroid hormones. Behav Brain Res. 2015;285:140–157. doi: 10.1016/j.bbr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luine V. Recognition memory tasks in neuroendocrine research. Behavioural Brain Research. 2015;285:158–164. doi: 10.1016/j.bbr.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inagaki, Gautreaux C, Luine V. Acute Estrogen Treatment Facilitates Recognition Memory Consolidation and Alters Monoamine levels in Memory-related Brain Areas. Horm. Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGaugh JL. Dissociating learning and performance: drug and hormone enhancement of memory storage. Brain Res. Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- 16.Arevalo M, Azcoitia I, Gonzalez-Burgod I, Garcia-Segura LM. Signalling mechanisms regulating synaptic plasticity and memory by estradiol. Horm Behav. 2015 Apr 25; doi: 10.1016/j.yhbeh.2015.04.016. 2015 pii: S0018-506X(15)00067-7. doi: 10.1016/j.yhbeh.2015.04.016. [Epub ahead of print] Review. [DOI] [PubMed] [Google Scholar]
- 17.Almey A, Cannel E, Bertram K, Filardo E, Milern TA, Brake WG. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology. 2014;155:4422–4432. doi: 10.1210/en.2014-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweat JD. Mechanisms of Memory, Second Edition The NMDA Receptor 191–208. San Diego: Academic Press; 2010. [Google Scholar]
- 19.Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neurosci. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frick KM. Building a better hormone therapy?: How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav. Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramar EA, Babayan AH, Gall CM, Lynch G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neurosci. 2013;239:3–16. doi: 10.1016/j.neuroscience.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Front Neuroendocrinol. 2014;35:530–549. doi: 10.1016/j.yfrne.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharm. 2012;37:2299–2309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inagaki T, Frankfurt M, Luine V. Estrogen-Induced Memory Enhancements are Blocked by Acute Bisphenol A in Adult Female Rats: Role of Dendritic Spines. Endocrinology. 2012;153:3357–3367. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013 Oct 18;7:149. doi: 10.3389/fncir.2013.00149. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr. Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog. Brain Res. 2010;181:209–232. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- 30.Vierk R, Brandt N, Rune GN. Hippocampal estradiol synthesis and its significance for hippocampal synaptic stability in male and female animals. Neuroscience. 2014;274:24–32. doi: 10.1016/j.neuroscience.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Kawato S. Adult male rat rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P450 17-alpha and P450 aromatase localized in neurons. Proc. Nat’l. Acad. Sci. USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J. Neurosci. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinol. 2013;154:4707–4714. doi: 10.1210/en.2013-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negish T, Kawasaki K, Takatori A, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Effects of perinatal exposure to bisphenol A on the behavior of offspring in F344 rats. Environ. Toxicol. Pharmacol. 2003;14:99–108. doi: 10.1016/S1382-6689(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 35.Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 36.Eilam-Stock T, Frankfurt M, Serrano P, Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behavioral Neuroscience. 2012;126:175–185. doi: 10.1037/a0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Research. 2006;1126:183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 39.Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 40.Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- 42.Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol. Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- 43.Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol. Psychiatry. 2014;19:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- 44.Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex dependent changes in anxiety, memory, and monoamines following one week of stress. Physiology & Behavior. 2009;97:21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Gomez JL, Lewis M, Luine V. Alcohol Access Alleviates Stress Induced Spatial Memory Impairments in Male Rats. Alcohol. 2012;46:499–504. doi: 10.1016/j.alcohol.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 47.Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez JL, Lewis MJ, Sebastian V, Serrano P, Luine V. Alcohol Administration Blocks Stress-Induced Impairments in Memory and Anxiety and Alters Hippocampal Neurotransmitter Receptor Expression in Male Rats. Hormones & Behavior. 2013;63:659–661. doi: 10.1016/j.yhbeh.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- 50.Conrad CD, Grote KD, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 51.Gomez JL, Luine V. Female Rats Exposed to Stress and Alcohol Show Impaired Memory and Increased Depressive-like Behaviors. Physiology and Behavior. 2014;123:47–54. doi: 10.1016/j.physbeh.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bisagno V, Grillo CA, Piroli GG, Giraldo P, McEwen B, Luine VN. Chronic stress alters amphetamine effects on behavior and synaptophysin levels in female rats. Pharmac. Biochem. Behav. 2004;78:541–550. doi: 10.1016/j.pbb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 53.Bowman RE, Kelly R. Chronically stressed female rats show increased anxiety but no behavioral alterations in object recognition or placement memory: a preliminary examination. Stress. 2012;15:524–532. doi: 10.3109/10253890.2011.645926. [DOI] [PubMed] [Google Scholar]
- 54.Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–441. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 55.McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Hu P, Qi XR, Meng FT, Kalsbeek A, Zhou JN. Acute restraint stress increases intrahypothalamic oestradiol concentrations in conjunction with increased hypothalamic oestrogen receptor β and aromatase mRNA expression in female rats. J. Neuroendocrinol. 2011;23:435–443. doi: 10.1111/j.1365-2826.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- 57.Lu J, Wu XY, Zhu QB, Li J, Shi LG, Wu JL, Zhang QJ, Huang ML, Bao AM. Sex differences in the stress response in SD rats. Behav. Brain Res. 2015;284C:231–237. doi: 10.1016/j.bbr.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Lennartsson AK, Kushnir MM, Bergquist J, Billig H, Jonsdottir IH. Sex steroid levels temporarily increase in response to aute psychosocial stress in healthy men and women. Int. J. Psychophysiol. 2012;84:246–253. doi: 10.1016/j.ijpsycho.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neurosci Biobehav Rev. 2012;36:1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm. Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacLusky NJ, Hauszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neurosci. 2006;138:957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 62.Luine VN, Jacome LF, Lema F, Barateli K, Buitrago D, Frankfurt M. Rapid effects of gonadal hormones on spatial memory and hippocampal spines in male rats. Soc. Neurosci Abs. 2015 doi: 10.1210/en.2015-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn. Mem. 2014;21:347–350. doi: 10.1101/lm.034926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frankfurt M, Luine V. The evolving role of dendritic spines and memory: interaction(s) with estradiol. 2014 doi: 10.1016/j.yhbeh.2015.05.004. [epub ahead of print] review. [DOI] [PMC free article] [PubMed] [Google Scholar]