Abstract
Background
Ultrasonography (US) is the mainstay of biliary tract imaging, but few recent studies have tested its ability to diagnose acute cholecystitis (AC). Our objective was to determine how well a US diagnosis of AC correlates with the intraoperative diagnosis. We hypothesize that US underestimates this diagnosis, potentially leading to unexpected findings in the operating room (OR).
Methods
This retrospective review included all patients admitted to the acute care surgical service of a tertiary hospital in 2011 with suspected biliary pathology who underwent US and subsequent cholecystectomy. We determined the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of US using the intraoperative diagnosis as the gold standard. Further analysis identified which US findings were most predictive of an intraoperative diagnosis of AC. We used a recursive partitioning method with random forests to identify unique combinations of US findings that, together, are most predictive of AC.
Results
In total, 254 patients underwent US for biliary symptoms; 152 had AC diagnosed, and 143 (94%) of them underwent emergency surgery (median time to OR 23.03 hr). Ultrasonography predicted intraoperative findings with a sensitivity of 73.2%, specificity of 85.5% and PPV of 93.7%. The NPV (52.0%) was quite low. The US indicators most predictive of AC were a thick wall, a positive sonographic Murphy sign and cholelithiasis. Recursive partitioning demonstrated that a positive sonographic Murphy sign is highly predictive of intraoperative AC.
Conclusion
Ultrasonography is highly sensitive and specific for diagnosing AC. The poor NPV confirms our hypothesis that US can underestimate AC.
Abstract
Contexte
L’échographie est la pierre angulaire de l’imagerie des voies biliaires, mais peu d’études récentes ont vérifié sa capacité de diagnostiquer la cholécystite aiguë (CA). Notre objectif était de déterminer dans quelle mesure le diagnostic échographique de la CA est en corrélation avec son diagnostic peropératoire. Selon notre hypothèse, l’échographie sous-estime ce diagnostic, ce qui pourrait entraîner des résultats inattendus au bloc opératoire.
Méthodes
Cette revue rétrospective a inclus tous les patients admis en 2011 au service chirurgical d’urgence d’un hôpital de soins tertiaires pour une pathologie biliaire présumée et qui ont subi une échographie, suivie d’une cholécystectomie. Nous avons déterminé la sensibilité, la spécificité, la valeur prédictive positive (VPP) et la valeur prédictive négative (VPN) de l’échographie, avec le diagnostic peropératoire comme base de référence. Une analyse plus approfondie a permis d’établir quels paramètres échographiques étaient les plus prédictifs d’un diagnostic peropératoire de CA. Nous avons utilisé la méthode de partitionnement récursif avec forêts aléatoires pour recenser les différents paramètres échographiques qui, ensemble, permettent le mieux de prédire la CA.
Résultats
En tout, 254 patients ont subi une échographie pour des symptômes biliaires; 152 ont reçu un diagnostic de CA et 143 ont subi une intervention chirurgicale d’urgence (temps médian avant l’arrivée au bloc opératoire 23,03 h). L’échographie a permis de prédire le diagnostic peropératoire avec une sensibilité de 73,2 %, une spécificité de 85,5 % et une VPP de 93,7 %. La VPN (52,0 %) était plutôt faible. Les paramètres échographiques les plus prédictifs de la CA sont une paroi épaisse, un signe de Murphy échographique positif et la cholélithiase. Le partitionnement récursif a démontré qu’un signe de Murphy échographique positif est une solide prédicteur de la CA peropératoire.
Conclusion
L’échographie est hautement sensible et spécifique pour le diagnostic de la CA. La piètre VPN confirme notre hypothèse selon laquelle l’échographie pourrait sous-estimer la CA.
Cholelithiasis is a common finding, present in 10%–15% of the general population. Among patients with cholelithiasis, 1%–4% will become symptomatic per year.1 Acute cholecystitis (AC) will develop in up to 30% of these patients, with cholelithiasis being the inciting pathology in 90%–95% of cases.1,2 Acute cholecystitis is one of the most common reasons for emergency admission to general surgical services.3,4 A history of recurrent or unrelenting right upper quadrant pain, fever, nausea and vomiting along with physical examination findings of right upper quadrant tenderness, positive Murphy sign and an elevated white blood cell (WBC) count are classic for AC.5,6 However, patients often have a nonspecific presentation, where the history and physical examination are insufficient to establish the diagnosis.5–8 Imaging is therefore an important part of the diagnostic process. Ultrasonography (US) is the mainstay of biliary tract imaging, as it is readily available, is inexpensive to perform and has a high sensitivity and specificity for AC (81% and 83%, respectively).2,8–11 However, there have been few recent studies assessing its ability to diagnose AC.
Most published studies analyzing the diagnostic ability of US use the pathological findings as the definitive diagnosis. Very few studies use intraoperative findings as the gold standard. However, it is important to consider the anticipated severity of disease and surgical difficulty, as they may substantially impact operative plans, including the operative time of day, availability of intraoperative fluoroscopy for cholangiograms, surgical assistant skill and even surgeon selection. Arguably, in the current era of immediate cholecystectomy for AC, the patient’s symptomatology and the intraoperative findings are far more relevant to the treating surgeon than the final pathological diagnosis.
Classically, AC was managed with conservative antibiotic therapy and interval cholecystectomy in the following weeks. It was previously thought that the rates of complications and conversions were higher in the acute setting.12,13 Recently, there has been a paradigm shift away from the classical approach toward immediate surgical management. This is reflected in the recent Cochrane review that concluded that early cholecystectomy is safe and has the advantage of a shorter overall hospital stay.14 The early approach is further supported by a new large retrospective cohort study demonstrating decreased risk of major bile duct injuries, death and shorter hospital stays with early cholecystectomy.15 Additionally, a multicentre randomized controlled trial in 2013 confirmed decreased morbidity, decreased length of hospital stay, and decreased costs in the immediate cholecystectomy group (within 24 hr of admission).16 In keeping with this, our institutional practice has shifted to performing urgent cholecystectomy (within 24–48 hr) upon admission of patients with AC unless a patient’s anesthetic risk is deemed prohibitive.
With the institution of acute care surgery in Winnipeg, Man., there was consolidation of emergency surgery care in 3 hospitals. The Acute Care Surgical Service (ACSS) is the largest acute care surgery service in Winnipeg and was established at St. Boniface General Hospital (SBGH) in April, 2008. As a result of regionalization of care, the ACSS saw a 221% increase in patient volume after its inception.17 Included in this expanded case volume was a 149% increase in biliary tract disease and a 162% increase in AC. A surgeon leads the ACSS team for a 7-day period from Monday to Sunday, 8 am to 4 pm. A separate surgeon manages the service overnight on home call. A dedicated resident team, generally with a single senior general surgery resident and a varying number of junior residents, staff the ACSS for 4-week periods at a time. In 2011, the ACSS had a dedicated daytime operating room (OR) during the week from 7:30 am to 4 pm. The OR time was then shared with other surgical services in the evenings and on weekends on a case priority basis.
The objective of our study was to determine how well a US diagnosis of AC correlates with the intraoperative diagnosis. We hypothesized that US underestimates the frequency and severity of AC in the emergency setting, which could lead to unexpected findings in the OR.
Methods
Institutional and University of Manitoba ethics review board approvals were granted before the study began.
Inclusion criteria
All patients who were admitted to the ACSS of a tertiary hospital, SBGH, in 2011 were retrospectively reviewed as a sample of convenience from a larger data set evaluating Winnipeg’s ACSS patient outcomes. Patients were included in the analysis if they were admitted with suspected biliary pathology, underwent diagnostic US and had a subsequent cholecystectomy. Patients were suspected to have cholecystitis based on a history of recurrent or unrelenting right upper quadrant pain; any combination of fever, nausea and vomiting; a physical exam of right upper quadrant tenderness; positive Murphy sign; and an elevated WBC count.5,6 Charts were identified according to diagnostic codes for biliary tract disease and then selected by procedure code for cholecystectomy. Patients with suspected or confirmed acalculous cholecystitis were excluded from the analysis. Patients with AC diagnosed using computed tomography (CT), magnetic resonance imaging (MRI) or alternative imaging modalities were also excluded.
Ultrasound findings
Diagnostic abdominal US was undertaken upon presentation with suspected biliary pathology. All US scans were performed in the radiology department by a certified ultrasonographer and reported by a tertiary care US radiologist. Features noted on abdominal US included cholelithiasis, an immobile calculus or one lodged in the gallbladder neck, pericholecystic fluid, thick gallbladder wall (including the measured thickness in millimetres), gallbladder distension, a positive sonographic Murphy sign, intramural air and perforation.2,6,8 The overall radiological impression or diagnosis was also recorded. Major US criteria used in our institution to diagnose AC are the combination of cholelithiasis (especially an immobile calculus), wall thickening (> 3 mm) and a positive sonographic Murphy sign.18,19 Minor indicators include pericholecystic fluid and gallbladder distension. These criteria are locally agreed upon among the US radiologist staff as there are no universally accepted US diagnostic criteria. Intramural air and perforation were considered indicators of severe or complicated AC.
Intraoperative findings
All patients had an attempted laparoscopic cholecystectomy. Intraoperative observations, methods and diagnoses were taken from the dictated operative report. For the purposes of this study, we defined a “difficult” cholecystectomy as one where a retrograde or fundus-down approach was used, a partial or subtotal cholecystectomy was performed, a drain was placed, a fifth laparoscopic port was inserted, or conversion to open cholecystectomy was required.
Statistical analysis
We determined the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of US diagnosis using the intraoperative diagnosis as the gold standard. Further analysis identified which individual US criteria were most predictive of an intraoperative diagnosis of AC, of gangrenous AC and a difficult operation. We compared the pathological diagnosis from the official pathology report with the US diagnosis for the correlation. Logistic regression was used to analyze the effect of age, sex, body mass index (BMI), diabetes, time to OR and degree of wall thickness on US reliability using interaction terms, which, if significant, would indicate that the reliability of US for predicting AC was dependent on these factors. We used a recursive partitioning method with random forests to identify unique combinations of US findings that, together, are most predictive of AC. Recursive partitioning is a nonparametric modelling approach that allows us to identify complex nonlinear associations between sets of potential risk factors and the dependent variable.20 This enables flexible exploratory modelling without any a priori distributional assumptions, which may be important if risk factors combine their effects in unexpected ways. We used the party package of R version 3.0.2 to perform the recursive partitioning and SAS version 9.3 (SAS Institute) for all other analyses.
Results
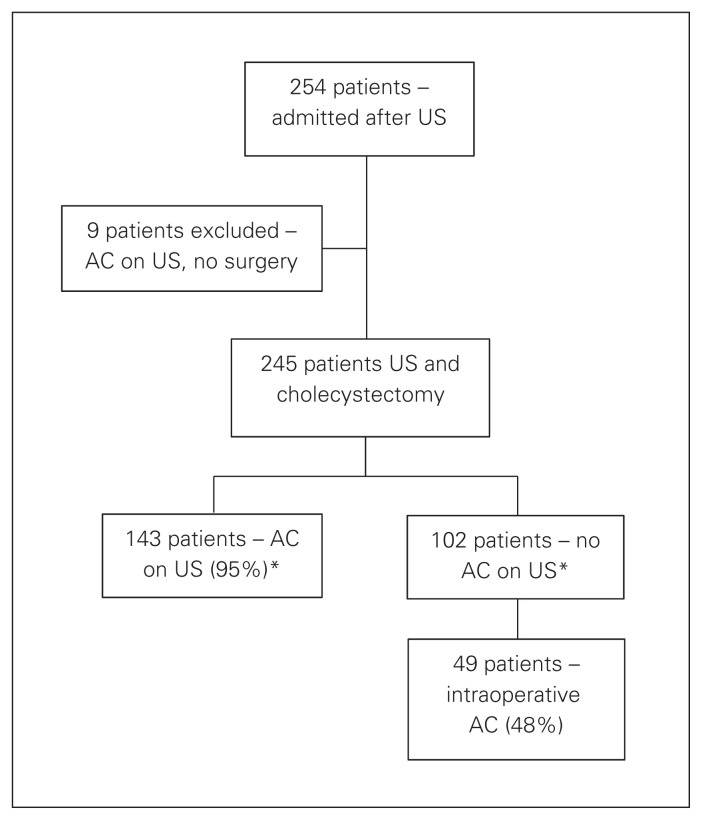
In total, 254 patients were admitted to the ACSS and underwent urgent abdominal US to investigate suspected biliary symptoms; 152 patients received definitive diagnoses of AC based on US, and 143 (94%) of these patients underwent emergency surgery (median time to OR 23.03 hr). The 9 patients with AC diagnosed US who did not undergo a cholecystectomy were excluded from the study cohort, leaving 245 patients who met the inclusion criteria (Fig. 1).
Fig. 1.
Flow of patients through the study. AC = acute cholecystitis; US = ultrasonography. *Median time to the operating room was 23.03 hours.
Table 1 lists the demographic and clinical characteristics of our study cohort. Of note, the majority of patients were women (65.5%), and the mean BMI was 30.8. Importantly, of the 245 patients who underwent cholecystectomy, the overall intraoperative complication rate was low (3.3%). There were no bile duct injuries, 8 (3.3%) patients had intraoperative bleeding, and 9 (3.7%) patients experienced bile leaks necessitating a postoperative endoscopic retrograde cholangiopancreatography (ERCP). These bile leaks were all attributed to cystic duct stump leaks on ERCP. Our conversion to open rate was 1.1%, and the rate of subtotal cholecystectomy was 7.8% (Table 1). Ultrasonography predicted intraoperative findings with a sensitivity of 73.2%, specificity of 85.5% and PPV 93.7%. The NPV (52.0%) was quite low. The 102 patients without signs of AC on US underwent cholecystectomies for various pathology, including biliary colic, choledocholithiasis, cholangitis and gallstone pancreatitis. Those in whom biliary colic was diagnosed on admission to the ACSS had an operative rate of 96.7%. Patients with biliary obstruction (choledocholithiasis, cholangitis, gallstone pancreatitis) had an operative rate of 60.3%. Of the 102 patients with other biliary pathology who underwent a cholecystectomy, 49 had intraoperative findings suggestive of AC (false negative rate of 48.0%; Fig. 1). There were no conversions to open cholecystectomy in this group. The ability of US to predict intraoperative findings is summarized in Table 2.
Table 1.
Patient demographic and clinical characteristics
| Characteristic | Mean (range) or % |
|---|---|
| Age, yr | 47.9 (18–92) |
| Male sex | 34.5 |
| BMI | 30.8 (16.0–59.2) |
| Diabetes | 10.6 |
| Procedure duration, min | 96.9 (26–229) |
| Overall complication rate | 3.3 |
| Conversion rate | 1.1 |
| Subtotal cholecystectomy | 7.8 |
| Bile duct injuries | 0 |
| Bile leaks (stump leaks) | 3.7 |
| Intraoperative cholangiogram | 3.3 |
BMI = body mass index.
Table 2.
Agreement between US and intraoperative diagnosis of AC
| Factor | Intraoperative AC | No intraoperative AC | Total | PPV/NPV (%) |
|---|---|---|---|---|
| US AC | 134 | 9 | 143 | PPV 93.7 |
| No US AC | 49 | 53 | 102 | NPV 52.0 |
| Total | 183 | 62 | 245 | — |
| Sensitivity/specificity, % | Sensitivity 73.2 | Specificity 85.5 | — | |
AC = acute cholecystitis; NPV = negative predictive value; PPV = positive predictive value; US = ultrasonography.
The intraoperative and pathological diagnoses of AC correlated 65.7% of the time. However, when acute and chronic cholecystitis were combined as a diagnosis of “cholecystitis” and compared with a combined pathological diagnosis of “cholecystitis,” the correlation was 95.7%. The intraoperative diagnosis underestimated the pathological diagnosis of “cholecystitis” 4.3% of the time.
The individual US indicators most predictive of AC were cholelithiasis (sensitivity 90.0%, specificity 4.6%, PPV 75.3%, NPV 12.5%), a thickened gallbladder wall (sensitivity 71.4, specificity 72.3, PPV 89.3%, NPV 43.9%) and a positive sonographic Murphy sign (sensitivity 59.5%, specificity 86.2%, PPV 93.3%, NPV 39.7%). These findings are summarized in Table 3. While the PPVs of our minor US criteria (immobile calculus in the gallbladder neck, gallbladder distension and pericholecystic fluid) were very high (PPV 90.5%, 90.0% and 94.3%, respectively), the sensitivities were very low (36.2%, 34.3% and 15.7%, respectively), suggesting these are of limited diagnostic utility for AC.
Table 3.
Sonographic indicators predictive of intraoperative diagnosis of AC
| Sonographic indicator | No. | Sensitivity,% | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|---|
| Diagnosis of AC | |||||
| Cholelithiasis | 245 | 90.0 | 4.6 | 75.3 | 12.5 |
| Thick wall | 172 | 71.4 | 72.3 | 89.3 | 43.9 |
| Murphy sign | 137 | 59.5 | 86.2 | 93.3 | 39.7 |
| Stone in neck | 84 | 36.2 | 87.7 | 90.5 | 29.8 |
| Distended | 80 | 34.3 | 87.7 | 90.0 | 29.2 |
| Pericholecystic fluid | 35 | 15.7 | 96.9 | 94.3 | 26.3 |
| Diagnosis of difficult operation* | |||||
| Cholelithiasis | 245 | 93.6 | 9.3 | 22.9 | 83.3 |
| Thick wall | 172 | 71.0 | 41.4 | 25.9 | 83.2 |
| Murphy sign | 137 | 50.0 | 51.2 | 22.8 | 78.0 |
| Stone in neck | 84 | 48.4 | 74.9 | 35.7 | 83.4 |
| Distended | 80 | 33.9 | 72.6 | 26.3 | 79.2 |
| Pericholecystic fluid | 35 | 14.5 | 87.9 | 25.7 | 78.1 |
| Diagnosis of gangrenous AC | |||||
| Cholelithiasis | 245 | 85.2 | 8.1 | 9.2 | 83.3 |
| Thick wall | 172 | 77.8 | 40.7 | 12.5 | 94.4 |
| Murphy sign | 137 | 70.4 | 53.6 | 14.2 | 94.3 |
| Stone in neck | 84 | 37.0 | 70.2 | 11.9 | 91.1 |
| Distended | 80 | 44.4 | 72.6 | 15.0 | 92.3 |
| Pericholecystic fluid | 35 | 18.5 | 87.9 | 14.3 | 90.8 |
AC = acute cholecystitis; NPV = negative predictive value; PPV = positive predictive value.
Difficult operation: fundus-down dissection, drain, partial cholecystectomy, conversion, or fifth port insertion.
Table 3 also lists the individual US signs that are most predictive of a difficult operation. The most predictive signs were cholelithiasis (sensitivity 93.6%, specificity 9.3%, PPV 22.9%, NPV 83.3%), thickened gallbladder wall (sensitivity 71.0%, specificity 41.4%, PPV 25.9%, NPV 83.2%) and a stone in the gallbladder neck (sensitivity 48.4%, specificity 74.9%, PPV 35.7%, NPV 83.4%).
The individual US findings found to be most predictive of an intraoperative diagnosis of gangrenous cholecystitis are listed in Table 3. These values, however, should be interpreted with caution as they are derived from a sample of only 3 patients with a US diagnosis of gangrenous cholecystitis.
The logistic regression model revealed that the selected patient demographic and clinical characteristics had no statistically significant effect on the accuracy of US diagnosis (BMI p = 0.24, age p = 0.42, sex p = 0.67, diabetes p = 0.94, time to OR p = 0.29, degree of wall thickness p = 0.81; Table 4).
Table 4.
Logistic regression — moderator effect on US diagnosis of AC
| Moderator | p value |
|---|---|
| BMI | 0.24 |
| Age | 0.42 |
| Sex | 0.67 |
| Diabetes | 0.94 |
| Time from US to OR | 0.29 |
| Degree of wall thickness | 0.81 |
AC = acute cholecystitis; BMI = body mass index; OR = operating room; US = ultrasonography.
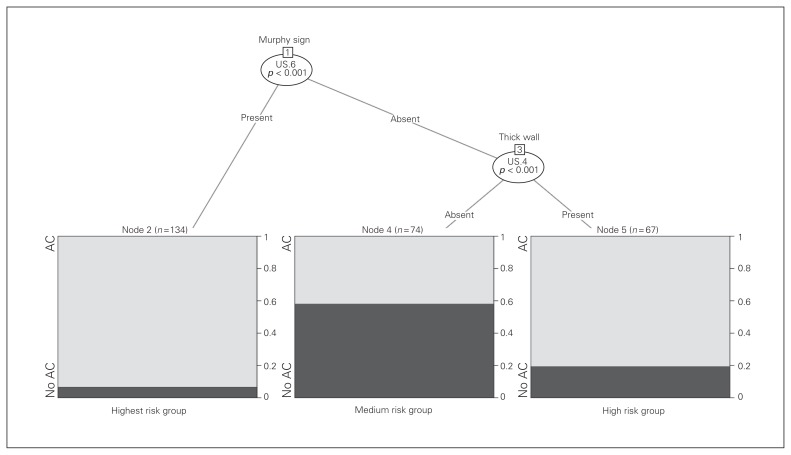
Recursive partitioning (Fig. 2) demonstrated that a positive sonographic Murphy sign was independently predictive of a high risk of intraoperative AC and, when absent, the risk of AC depends on the presence of a thickened gallbladder wall.
Fig. 2.
Recursive partitioning demonstrated that a positive sonographic Murphy sign was independently predictive of a high risk of intraoperative acute cholecystitis (AC) and, when absent, the risk of AC depends on the presence of a thickened gallbladder wall. US = ultrasonography.
Discussion
Since the 1970s, US has been shown to be a fast, accurate, accessible and cost-effective modality for imaging of the biliary tract.21 No other imaging modality is more sensitive or specific for the detection of gallstones (sensitivity 97%, specificity 95%).10 However, the utility of US in diagnosing AC remains questionable, as the literature contains variable results.4 Because of this, AC remains very much a clinical diagnosis, with US providing a diagnostic adjunct.22 Additionally, many studies use a pathological diagnosis as the gold standard, which may not correlate with clinical findings. The literature is additionally limited by many single-institution, retrospective, small data sets.10
Our study included one of the largest data sets (n = 245) in the current literature as well as a high operative rate (94%) for AC with a short time interval from US to OR (median 23.03 hr). This short interval should optimize the correlation between the US findings and intraoperative findings by reducing the time for progression or resolution of disease. Early cholecystectomy is our institutional practice, which is supported by the most up-to-date literature, suggesting more favourable outcomes, including reduced major bile duct injuries and death, as well as shorter length of overall hospital stay.14,15 This literature includes a Cochrane review,14 a large retrospective cohort study of more than 14 000 patients,15 and a large multicentre randomized controlled trial.16 Interestingly, many of the studies on US for the diagnosis of AC are from the 1980s and 1990s, a time when the standard practice was a delayed cholecystectomy.9 The disadvantage of the shift toward early cholecystectomy is the increased frequency of difficult cholecystectomies encountered in the emergency setting. This increases the need for an accurate diagnosis and prediction of the severity of AC in order to allow the surgeon to be adequately prepared with appropriate equipment and assistance, to consider the operative time of day and even consultation or referral to another surgeon. Despite difficult emergency cholecystectomies, our 1-year cohort had a very low conversion rate of 1.1% and a low rate of subtotal cholecystectomy of 7.8%. We felt these results are a reflection of the skill set of our ACSS surgeons, 4 of whom have fellowship training in minimally invasive surgery. Subtotal or partial cholecystectomy is used in our institution as a technique to reduce the morbid complications associated with difficult gallbladders, particularly common bile duct injuries, and reflects the recent trend and growing acceptance of this approach.23 This practice is further supported by the recent systematic review and meta-analysis of more than 1200 subtotal cholecystectomies confirming that this technique, for difficult gallbladders, achieves morbidity rates comparable to total cholecystectomy in uncomplicated cases and is, therefore, an important tool in the approach to the difficult gallbladder.23 Our study, however, like many in the literature, is still limited as a single-institution, retrospective analysis.
A recent systematic review and meta-analysis of imaging in AC suggested that the diagnostic accuracy of US in AC was lower than that reported in previous studies (sensitivity 81%, specificity 83%).9 This is contrasted to an older meta-analysis stating a sensitivity of 88% (95% CI 0.74–1.00) and a specificity of 80% (95% CI 0.62–0.98), adjusted for verification bias.10 Our study is in keeping with previously published data with a specificity of 85.5%, though our sensitivity was lower at 73.2%. The low NPV of 52.0% nonetheless confirms our hypothesis that US can underdiagnose AC, suggesting that consistent major and minor US criteria should be set for a sonographic diagnosis to improve its accuracy. Instead of using the pathological diagnosis as the reference standard, we used the intraoperative diagnosis. Dynamic in vivo imaging was therefore compared with direct intraoperative observation of the in vivo gallbladder where the gallbladder is intact, perfused, unaltered by fixatives and electrocautery, and unaffected by pathological sampling error. Also, the intraoperative findings are perhaps more clinically and surgically relevant given that an accurate prediction of the intraoperative findings could help a surgeon to appropriately prepare for operative challenges, potential complications, additional equipment, availability of intraoperative fluoroscopy for cholangiograms and adequate assistance. A limitation to our data, however, is that they are subject to verification bias, as they were retrospectively collected and the decision to proceed with the gold standard (cholecystectomy) was reliant on the results of the diagnostic test (US). Verification bias would result in an overestimation of the sensitivity of the US and underestimation of the specificity.10 It is possible that a cohort of patients who presented with biliary symptoms underwent US that found no AC and were subsequently discharged without operative intervention. These patients may have then proceeded to elective cholecystectomy with intraoperative findings of cholecystitis. This group would not have been captured by our study cohort and therefore reflects an additional bias of our study. However, if included, this group would have further increased the false-negative rate of US, thus our results may appear better than reality. Ultrasonography is known to be a user-dependent imaging modality, which is a disadvantage of this technique. We did not control for the user-dependency of US in our analysis, which does limit our results. This was primarily owing to the retrospective nature of the study and would be easier to account for in a prospective trial.
A 2004 study attempted to reduce verification bias by performing a prospective study of US compared with both the intraoperative and pathological diagnosis of AC.4 Furthermore, this was one of the few studies comparing US diagnosis to intraoperative findings. They proceeded with cholecystectomy if the clinical picture suggested AC and the US confirmed gallstones, regardless of signs of inflammation on US.4 Their results demonstrated a much lower sensitivity and specificity of 60% and 77%, respectively, than previously published data, but their results are closer to ours.4 However, they had a very high rate of noninflamed gallbladders at cholecystectomy (15 of 55, 27.3%).4 This same group also suggested the importance of a positive sonographic Murphy sign to the diagnostic accuracy of US by showing the sensitivity improved from 54% to 60% and the specificity improved from 67% to 77% when the radiologist was aware of the presence or absence of this sign.4 This is in alignment with our findings, where a thickened gallbladder wall and positive sonographic Murphy sign were most predictive of AC (sensitivity 71.4%, specificity 72.3%, and sensitivity 59.5%, specificity 86.1%, respectively). Our recursive partitioning model concurrently demonstrates that a positive sonographic Murphy sign independently was highly predictive of AC. Recursive partitioning is a powerful technique for evaluating unique combinations of potential risk factors that may interact in unexpected ways. However, a limitation to recursive partitioning models is that they can be sensitive to mild perturbations in the data. This may result in arriving at a slightly different conclusion with another sample from the same population. Interestingly, our analysis was the first to demonstrate that potential moderators of diagnostic accuracy (BMI, age, sex, diabetes, time to OR, and degree of gallbladder wall thickness) had no statistically significant effect on the ability of US to diagnose AC.
Conclusion
Ultrasonography is highly sensitive and specific for diagnosing AC; however, the low NPV confirms our hypothesis that US can underestimate the diagnosis of AC. The most predictive individual US signs for AC are a positive sonographic Murphy sign, thickened gallbladder wall and cholelithiasis, which is consistent with the literature.18,19 Independently, a positive sonographic Murphy sign is highly predictive of AC. These signs should be considered as major criteria for sonographic diagnosis of AC.
Acknowledgements
The authors thank Brenden Dufault of the George and Fay Yee Centre for Healthcare Innovation, College of Medicine, University of Manitoba, for his contribution to the statistical analysis of this study.
Footnotes
Competing interests: None declared.
Funding: Funding for this project was sourced from the University of Manitoba Department of Surgery GFT Research Grant.
Contributors: All authors designed the study. S. Stogryn and J. Metcalfe acquired and analyzed the data, which A. Vergis and K. Hardy also analyzed. All authors wrote and reviewed the article and approved the final version for publication.
References
- 1.Halldestam I, Enell EL, Kullman E, et al. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg. 2004;91:734–8. doi: 10.1002/bjs.4547. [DOI] [PubMed] [Google Scholar]
- 2.Bennett GL, Balthazar EJ. Ultrasound and CT evaluation of emergent gallbladder pathology. Radiol Clin North Am. 2003;41:1203–16. doi: 10.1016/s0033-8389(03)00097-6. [DOI] [PubMed] [Google Scholar]
- 3.Stoker J, van Randen A, Laméris W, et al. Imaging patients with acute abdominal pain. Radiology. 2009;253:31–46. doi: 10.1148/radiol.2531090302. [DOI] [PubMed] [Google Scholar]
- 4.Bingener J, Schwesinger WH, Chopra S, et al. Does the correlation of acute cholecystitis on ultrasound and at surgery reflect a mirror image? Am J Surg. 2004;188:703–7. doi: 10.1016/j.amjsurg.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 5.Trowbridge RL, Rutkowski NK, Shojania KG. Does this patient have acute cholecystitis? JAMA. 2003;289:80–6. doi: 10.1001/jama.289.1.80. [DOI] [PubMed] [Google Scholar]
- 6.Hirota M, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:78–82. doi: 10.1007/s00534-006-1159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laméris W, van Randen A, van Es HW, et al. Imaging strategies for detection of urgent conditions in patients with acute abdominal pain: diagnostic accuracy study. BMJ. 2009;338:b2431. doi: 10.1136/bmj.b2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menu Y, Vuillerme M. Non-traumatic abdominal emergencies: imaging and intervention in acute biliary conditions. Eur Radiol. 2002;12:2397–406. doi: 10.1007/s00330-002-1613-x. [DOI] [PubMed] [Google Scholar]
- 9.Kiewiet JJS, Leeuwenburgh MMN, Bipat S, et al. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264:708–20. doi: 10.1148/radiol.12111561. [DOI] [PubMed] [Google Scholar]
- 10.Shea JA, Berlin JA, Escarce JJ, et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med. 1994;154:2573–81. [PubMed] [Google Scholar]
- 11.De Vargas Macciucca M, Lanciotti S, De Cicco ML, et al. Ultrasonographic and spiral CT evaluation of simple and complicated acute cholecystitis: diagnostic protocol assessment based on personal experience and review of the literature. Radiol Med (Torino) 2006;111:167–80. doi: 10.1007/s11547-006-0018-3. [DOI] [PubMed] [Google Scholar]
- 12.Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004;188:205–11. doi: 10.1016/j.amjsurg.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Kum CK, Eypasch E, Lefering R, et al. Laparoscopic cholecystectomy for acute cholecystitis: Is it really safe? World J Surg. 1996;20:43–8. doi: 10.1007/s002689900008. [DOI] [PubMed] [Google Scholar]
- 14.Gurusamy KS, Samraj K. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis [Review] Cochrane Libr. 2009;1:1–63. doi: 10.1002/14651858.CD005440.pub2. [DOI] [PubMed] [Google Scholar]
- 15.de Mestral C, Rotstein OD, Laupacis A, et al. Comparative operative outcomes of early and delayed cholecystectomy for acute cholecystitis: a population-based propensity score analysis. Ann Surg. 2014;259:10–5. doi: 10.1097/SLA.0b013e3182a5cf36. [DOI] [PubMed] [Google Scholar]
- 16.Gutt CN, Encke J, Köninger J, et al. Acute cholecystitis: early versus delayed cholecystectomy, a multicenter randomized trial (ACDC Study, NCT00447304) Ann Surg. 2013;258:385–93. doi: 10.1097/SLA.0b013e3182a1599b. [DOI] [PubMed] [Google Scholar]
- 17.Faryniuk AM, Hochman DJ. Effect of an acute care surgical service on the timeliness of care. Can J Surg. 2013;56:187–91. doi: 10.1503/cjs.022911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draghi F, Ferrozzi G, Calliada F, et al. Power Doppler ultrasound of gallbladder wall vascularization in inflammation: clinical implications. Eur Radiol. 2000;10:1587–90. doi: 10.1007/s003300000371. [DOI] [PubMed] [Google Scholar]
- 19.Ralls PW, Colletti PM, Lapin SA, et al. Real-time sonography in suspected acute cholecystitis. Prospective evaluation of primary and secondary signs. Radiology. 1985;155:767–71. doi: 10.1148/radiology.155.3.3890007. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Singer B. Recursive partitioning and applications. 2nd ed. Springer; 2010. [Google Scholar]
- 21.Prian GW, Norton LW, Eule J, Jr, et al. Clinical indications and accuracy of gray scale ultrasonography in the patient with suspected biliary tract disease. Am J Surg. 1977;134:705–11. doi: 10.1016/0002-9610(77)90307-5. [DOI] [PubMed] [Google Scholar]
- 22.Irkorucu O, Reyhan E, Erdem H, et al. Accuracy of surgeon-performed gallbladder ultrasound in identification of acute cholecystitis. J Invest Surg. 2013;26:85–8. doi: 10.3109/08941939.2012.697977. [DOI] [PubMed] [Google Scholar]
- 23.Elshaer M, Gravante G, Thomas K, et al. Subtotal cholecystectomy for “difficult gallbladders” systematic review and meta-analysis. JAMA Surg. 2015;150:159–68. doi: 10.1001/jamasurg.2014.1219. [DOI] [PubMed] [Google Scholar]