Abstract
Pancreatic cancer (PC) would become the second leading cause of cancer death in the near future, despite representing only 3% of new cancer diagnosis. Survival improvement will come from a better knowledge of risk factors, earlier diagnosis, better integration of locoregional and systemic therapies, as well as the development of more efficacious drugs rising from a deeper understanding of disease biology. For patients with unresectable, non-metastatic disease, combined strategies encompassing primary chemotherapy and radiation seems to be promising. In fit patients, new polychemotherapy regimens can lead to better outcomes in terms of slight but significant survival improvement associated with a positive impact on quality of life. The upfront use of these regimes can also increase the rate of radical resections in borderline resectable and locally advanced PC. Second line treatments showed to positively affect both overall survival and quality of life in fit patients affected by metastatic disease. At present, oxaliplatin-based regimens are the most extensively studied. Nonetheless, other promising drugs are currently under evaluation. Presently, in addition to surgery and conventional radiation therapy, new locoregional treatment techniques are emerging as alternative options in the multimodal approach to patients or diseases not suitable for radical surgery. As of today, in contrast with other types of cancer, targeted therapies failed to show relevant activity either alone or in combination with chemotherapy and, thus, current clinical practice does not include them. Up to now, despite the fact of extremely promising results in different tumors, also immunotherapy is not in the actual therapeutic armamentarium for PC. In the present paper, we provide a comprehensive review of the current state of the art of clinical practice and research in PC aiming to offer a guide for clinicians on the most relevant topics in the management of this disease.
Keywords: Pancreatic cancer, Chemotherapy, Radio-frequency, Stereotactic radiotherapy, Irreversible electroporation
Core tip: This review focuses on the current clinical practice in the treatment of pancreatic cancer (PC), and outlines research topics. PC is still a highly lethal disease, for a usual presentation stage not manageable with curative surgery. Up to now, new targeted therapies have not shown any positive impact on its dismal prognosis. Only slight improvements ensued from the availability of more active polychemotherapy regimens. From the point of view of a multimodal approach, in addition to surgery, new locoregional techniques are nowadays available, suitable for combination with systemic treatments, to increase disease control and survival.
INTRODUCTION
In contrast to the general trend of increase in cancer survival, advances have been slow for pancreatic cancer (PC). Therefore PC is actually the fourth cause of cancer death, and it is expected it will be the second cause of cancer death by 2030 in Western countries. The American Cancer Society estimated that there will be 48960 new cases of PC in the United States in 2015, with 40560 deaths[1,2]. Despite surgery, locoregional therapy, chemotherapy and molecular therapies, the overall median survival is less than 1 year from diagnosis, highlighting the need for better therapeutic options. In fact, PC is frequently undiagnosed until the sudden appearance of prominent clinical symptoms and signs for advanced disease. Only in 10%-20% of cases the disease is resectable or borderline resectable, therefore suitable to surgery associated with neoadjuvant or adjuvant treatment, with curative purposes. In the last years different ablative techniques such as irreversible electroporation (IRE), radio-frequency ablation (RFA) and stereotactic body radiation therapy (SBRT) caught the attention of the scientific community. Such techniques may be an alternative to surgery in patients with a locally advanced disease, poor response to systemic therapy, and with a locoregional rather than metastatic growth pattern.
This review aims to explore the major questions still open regarding the management of the disease. We identified studies and systematic reviews by searching PubMed, ClinicalTrials.gov, and the Cochrane database from database inception to April 2015.
ARE THERE PROVEN RISK FACTORS IN PC? CAN WE PREVENT IT?
Current knowledge and unmet needs
Facing such a dismal prognosis cancer, a frequent question from patients is “Why? Why to me“. PC has a multifactor etiology, whose better knowledge could be helpful to identify groups of people worthy of surveillance trials. A study on 117 meta-analytical and pooled reports estimated risk factors and the fraction of PCs attributable to them[3]. There is a moderately sized association between a family history of PC in first degree relatives, with multivariate-adjusted odds ratios (OR) of 1.8[4], justifying 5%-10% of cases. There is a significant association between PC risk and AB0 phenotypes (OR = 1.4 in non 0 blood type), and up to 19.5% of all cases of PC in populations with European ancestry could be attributable to a non-0 blood group[5]. Moreover, a multistage genome-wide association study[6] identified multiple susceptibility alleles to be further evaluated. A study of Maisonneuve and Lowenfels[7] however suggests that nearly two thirds of PC are due to potentially avoidable causes. The strongest associations are with tobacco smoking, that is the greatest behavioral risk factor for PC, and Helicobacter pylori infection, with estimated population attributable fractions of 11%-32% and 4%-25% respectively. Besides carcinogens, smoking also generates agents perpetuating inflammatory response, and heightens the risk of chronic pancreatitis. A higher risk of PC indeed is associated with chronic pancreatitis. In this perspective, Helicobacter pylori infection could have a role in pancreas carcinogenesis, through the induction of autoimmune pancreatitis[8]. Heavy alcohol intake, defined as a daily consumption of over 30 g, has a strong association with PC, with an attributable increased risk of 20%-30%. All or many of these risk factors could concur through complex interactions involving different pathways[9]. Diabetes, obesity and reduced adiponectin level are all related to insulin resistance, and probably share common pathways, which can be responsible for attributable fraction up to 16%-19%, with the opposite postulated protective effect of higher physical activity[10].
The strongest evidence for a protective effect is for atopic allergy, especially hay fever or allergy to animals, that could reduce PC risk up to 20%-30%[11]. A number of other postulated risk factors or protective factors like meat and fruit intake, or vitamin D circulating levels have a lower level of evidence and deserve further studies.
Cystic lesions occasionally detected with non-invasive abdominal imaging, prescribed for unrelated indications, deserve a separate discussion. Prevalence range of incidental pancreatic cysts in the adult population is from 2.2%-5.9% (depending on imaging technique)[12]. Their correct management is crucial for preventing and early treating of the disease. Especially intraductal papillary mucinous neoplasms can have a progression model similar to that of colonic polyps, with the risk of transforming into invasive cancer, more likely in cases with involvement of main pancreatic duct or with multiple lesions. But only a few of them actually progress to malignancy. Their optimal management is still controversial, based more on experts’ opinions than on evidence from randomised studies. This uncertainty about the prediction of future behavior is due to a lack of accurate diagnostic tools and prognostic factors. It exposes patients to a risk of overtreatment with unnecessary high-risk surgery, undertreatment or expensive long term imaging follow-up[13].
IS INTEGRATED APPROACH (SURGERY, RADIOTHERAPY AND CHEMOTHERAPY) THE GOLD STANDARD IN LOCALLY ADVANCED PC? WHAT IS TODAY THE ROLE OF SURGERY?
Current knowledge
Nearly 30%-40% patients at diagnosis have a borderline-resectable (BRPC) or locally advanced PC (LAPC)[14]. But despite the absence of distant metastasis, the overall survival (OS) of these patients is absolutely poor[15,16], and only radical surgery can give a chance for a cure[17]. Selected patients can have an improved outcome with a multimodal approach, combining chemotherapy with radiation therapy or surgery. The selection of a population of patients suitable to multimodal approach, however, needs an accurate identification of LAPC and BRPC. LAPC refers to cases with an extended involvement of adjacent structures[18]; whereas BRPC comprises a subset of patients eligible to an upfront resection, but with a high risk of residual microscopic disease (R1, according to the International Union Against Cancer Classification) caused by an involvement of nearby structures, such as superior mesenteric artery or celiac artery, not allowing a removal of the tumour without an arterial resection, thus greatly increasing the risk of R1 or R2 surgery. R0 resection only can cure PC. Unfortunately, there is a wide heterogeneity in the literature regarding the definition of resectability criteria. Moreover, BRPC patients are an ill-represented population in the majority of chemotherapy clinical trials.
In this context, upfront resection has been rated as a 2B recommendation in the National Comprehensive Cancer Network Guideline[18-21].
Although lacking high level evidence, there is a general consensus for neo-adjuvant chemotherapy, estimated able to convert to R0 resection 33% of LAPC/BRPC patients[22,23].
This therapeutic strategy has been historically based on fluoropyrimidines, 5-fluorouracil (5-FU) or capecitabine, combined with radiation and recently on gemcitabine induction followed by concomitant chemo-radiation with either gemcitabine or fluoropyrimidines[24,25]., At present, there are no data about the better neo-adjuvant chemotherapy regimen. But based on the observed results in the metastatic settings, FOLFIRINOX or gemcitabine plus nab - paclitaxel with or without subsequent chemoradiation might represent promising options. However, especially FOLFIRINOX suits only to fit patients, for high rate of G3-G4 toxicities[26-29]. The results of ongoing Alliance A021101 pilot trial (NCT01821612) could help clarify the role of a multimodal strategy of neoadjuvant FOLFIRINOX, followed by chemoradiation [50.4 Gy external beam radiation therapy (EBRT) with concomitant capecitabine], definitive surgery and postoperative adjuvant gemcitabine in BRPC patients.
For LAPC affected patients as well, a combined approach in LAPC could allow radical resection also in cases not eligible to upfront surgery. In several studies[30,31], and a meta-analysis by Gillen et al[23], gemcitabine-based combination regimens allowed a higher resection rate than single agent chemotherapy (33% vs 27%). In this meta-analysis OS was almost doubled in patients who finally underwent surgical resection of their tumour (20.5 mo vs 10.2 mo). Moreover, three meta-analyses have suggested a survival advantage in patients treated with gemcitabine-based chemo-radiation (CRT)[32-34].
Nevertheless, the role of chemoradiation in LAPC is still unclear, for conflicting results of clinical trials. Indeed, two studies reported improved OS with CRT (Gastrointestinal Tumor Study Group 9283[35] and ECOG 4201[36]) and Huguet et al[37] reviewed two perspective trials finding a survival advantage in patients treated with chemotherapy and chemoradiation vs patients treated with chemotherapy alone. Other interesting results were recently reported by Sherman et al[38] using docetaxel and capecitabine followed by gemcitabine and capecitabine combined with radiation therapy and surgery[38]. In this phase 2 trial, 20 out of 45 treated patients (44%) had R0 resection.
On the opposite, Chauffert et al[39] reported no advantage in OS and more toxicity with the addition of CRT to chemotherapy, and preliminary results of the international phase 3 GERCOR LAP-07 study demonstrated improved local control with the addition of chemoradiation to chemotherapy, but no difference in OS[40].
Waiting for definitive evidence about the usefulness of CRT, at present, the most widespread approach in fit patients is to start with induction chemotherapy followed by chemoradiation in absence of disease progression at the time of first radiological evaluation. This approach has two advantages: It avoids unnecessary radiotherapy in the nearly 30% of patients who undergo widespread disease progression during initial treatment, and it permits to test patient’s tolerance to chemotherapy alone, before adding the relevant toxicities of a radiation concomitant to chemotherapy. Radiotherapy[41].
Standard dose radiation therapy is usually 50.4 Gy in 1.8-Gy fractions, although some trials reported the use of a 30-36 Gy in 3-Gy fractions schedule[42]. Better outcomes could come from the use of newer radiotherapy techniques like intensity-modulated radiation therapy (IMRT) and SBRT, suited to deliver higher biological dose[42]. Indeed, in a phase 2 multi-institutional trial, SBRT was feasible without unexpected toxicities and obtained a 1-year local progression-free survival (PFS) of 78%[43].
Unmet needs
An agreement about an unambiguous, rigorous definition of the BRPC could help to reach a homogeneous approach to borderline resectable disease, thus allowing comparison among different trials results. Despite multimodal treatments, not all BRPC and LAPC will become resectable up to R0, missing their chance for a cure. Deeper exploration of combination regimens is necessary to improve this outcome, especially through the identification of prognostic factors and biomarkers to predict the response or the resistance to the different treatments. At present, little evidence is available. As an example, SMAD4-deleted tumours are associated with widespread disease, whereas SMAD4-proficient tumours are associated with a more locally aggressive disease[44]. Nevertheless, the impact of SMAD4 on treatment outcome is far to be defined. In any case, a multidisciplinary management in high-expertise centers can increase the chance of cure for all patients with PC, and even more for those with BRPC and LAPC.
WHAT IS THE BEST FIRST LINE CHEMOTHERAPY IN INOPERABLE PC?
Current knowledge
Despite recent advances in our understanding of the molecular biology of PC, there has been limited progress in therapeutic options for metastatic disease, and traditional chemotherapy outcomes, even though improved, are still disappointing. The overall median survival from diagnosis is still less than 1 year, underscoring the need for the development of newer therapeutic options. The goals of chemotherapy are: The improvement of survival, the control of symptoms and the need to ensure a good quality of life for the patient. In the past, several studies have demonstrated the superiority of chemotherapy compared to best supportive care alone (BSC) and fluorouracil (5-FU), in different doses, schedules, and combination regimens, has been considered the cornerstone in the palliative treatment of metastatic PC[45]. Since 1997, gemcitabine monotherapy has represented the standard of care for patients with metastatic PC, when Burris et al[46] demonstrated that it was superior to 5-FU in terms of clinical benefit/efficacy, outcome measures and safety profile in patients with a baseline Karnofsky performance status (PS) ≥ 50. Gemcitabine subsequently represented a backbone in chemotherapy, in clinical trials investigating more intensive combination regimens. Due to its good tolerability and demonstrated efficacy, from 1997 to 2010 several studies had combined it with many other active cytotoxic agents, including fluorouracil[39], capecitabine[47], cisplatin[48], epirubicin[49], docetaxel[50-52], oxaliplatin[31], irinotecan[53,54], and pemetrexed[55]; but up to now, no conclusive results about an effective impact on survival. In contrast to other tumour types, with the exception of the negligible benefit showed by erlotinib[56], tested targeted therapies as cetuximab[57], bevacizumab[58], axitinib[59], tipirarnib[60], oftrametinib[61], trastuzumab[62], have largely failed to show any significant benefit when added to standard chemotherapy in metastatic PC.
In 2011 a combination regimen of leucovorin, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) obtained a meaningful survival benefit over single agent gemcitabine. FOLFIRINOX, providing a significant survival improvement of 4.3 mo in comparison to gemcitabine alone[63]. The median OS, PFS, and objective response rate (ORR) were significantly higher with FOLFIRINOX compared with gemcitabine alone (median OS, 11.1 mo vs 6.8 mo; PFS, 6.4 mo vs 3.3 mo; ORR, 32% vs 9%). FOLFIRINOX, however, showed an unfavourable toxicity profile compared to gemcitabine alone, including grade 3/4 neutropenia (46% vs 21%), febrile neutropenia (5.4% vs 1.2%), thrombocytopenia (9.1% vs 3.6%), sensory neuropathy (9% vs 0%), vomiting (15% vs 8%), fatigue (23% vs 18%), and diarrhea (13% vs 2%). Only well-selected patients with metastatic PC can therefore bear such a treatment without heavy side effects.
In 2013, nab-paclitaxel in combination with gemcitabine showed an improved median survival of almost two months (1.8), compared to gemcitabine alone[64]. It also increased OS at 1 and 2 years, with a tolerable toxicity profile. Grade 3/4 adverse events occurred, as expected, more often with the combination therapy and included neutropenia (38% vs 27%), febrile neutropenia (3% vs 1%), fatigue (17% vs 7%), diarrhea (6% vs 1%), and neuropathy (17% vs 1%). In September 2013, nab-paclitaxel in combination with gemcitabine was approved by the FDA for first-line treatment of metastatic PC of the pancreas.
Unmet needs and proposals
Clinical trials results suggest that combination chemotherapy with regimens FOLFIRINOX or gemcitabine plus nab-paclitaxel are an acceptable option for patients with good PS, good pain management, and adequate nutritional intake. It is still not clear which is the best: FOLFIRINOX or gemcitabine and nab-paclitaxel? The median OS obtained in the two different trials was 11.1 mo with FOLFIRINOX and 8.5 mo with gemcitabine plus nab-paclitaxel. A direct comparison of the results of the two trials, conducted on different populations, is impossible.
In our opinion, both FOLFIRINOX and gemcitabine plus nab-paclitaxel are reasonable choices for first-line therapy in patients with Eastern Cooperative Oncology Group (ECOG) PS 0 or 1. For a better tolerability, the combination of gemcitabine and nab-paclitaxel could be an option also for patients with a slight worse PS, who cannot tolerate a FOLFIRINOX regimen, or in patients who have received FOLFIRINOX as neoadjuvant treatment. However, in common clinical practice only a small number of patients with metastatic PC presents with good PS. For the other patients gemcitabine monotherapy is still the only therapeutic option (Figure 1).
Figure 1.
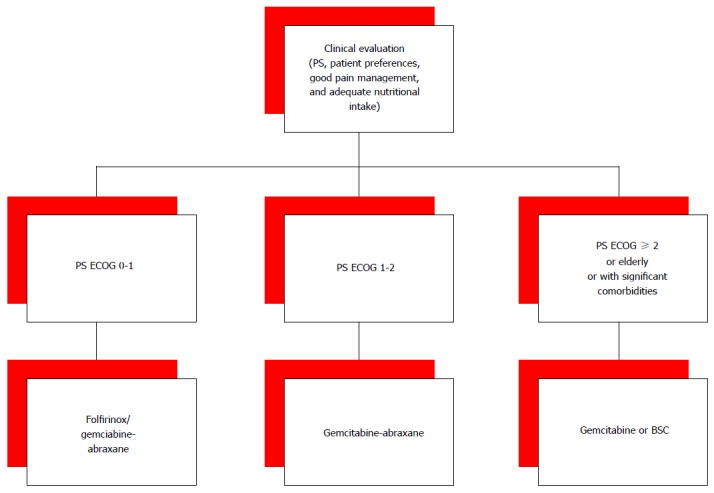
Proposal for the choice of the first line. PS ECOG: The eastern cooperative oncology group score of performance status; BSC: Best supportive care.
Additional therapeutic advances are expected from studies evaluating strategies for depletion stromal, inhibition pathways of cancer (i.e., Hedgehog, RAS-RAF-MAPK and PI3K-AKT), new chemotherapeutic drugs (i.e., MM-398 irinotecan encapsulated into liposomal-based nano particles)[65,66], or the new era of immunotherapy. The identification of biomarkers continues to be clinically challenging but essential in order to tailor therapy to specific patients’ subgroups in which the maximal antitumour effect from novel agents can be obtained.
WHAT IS THE ROLE OF THE LINES OF CHEMOTHERAPY SUBSEQUENT TO THE FIRST?
Current knowledge
Outside the context of clinical trials[67], median OS in patients with metastatic PC is 2.8-5.7 mo. However, despite the aggressiveness of this disease, in recent years the better results obtained with first-line chemotherapy have allowed a wider use of second-line treatments. In a retrospective study, the French and British oncologists analysed data of 400 patients treated for metastatic PC between 2009 and 2012. They collected patients' information about sex, age, PS, comorbidities, cancer-directed treatment, supportive care, adverse events and complications. The most common used first-line chemotherapy regimens were gemcitabine alone (46%), FOLFIRINOX (20.1%), gemcitabine/capecitabine (10.8%), and gemcitabine/oxaliplatin (9.5%). Approximately 40% of patients received second-line systemic therapy, whereas less than 20% received third-line systemic therapy[68]. About 45% of patients in phase II-III trial PRODIGE 4-ACCORD 11 received second-line therapy. FOLFIRINOX, despite significantly higher chemotherapy-related adverse events, allowed a better Quality of Life (QoL) than gemcitabine[69]. Since the QoL of patients with metastatic PC is more influenced by disease symptoms than by chemotherapy-related toxicity, the second-line chemotherapy could be a good option for selected patients. In a phase II study, oxaliplatin-based regimen showed some activity in metastatic PC patients after failure of first-line chemotherapy with gemcitabine[70]. The CONKO-01 randomised phase III multicenter study compared OFF (oxaliplatin, folinic acid and 5-FU 24 h) to BSC in patients with PC progressing while on gemcitabine therapy. Stratification included duration of first-line therapy, PS, and tumour stage. Trial terminated prematurely, after the accrual of 46 patients instead of 165 planned, probably for patients and physicians unwillingness to a randomisation in a BSC arm. Median second-line survival was 4.82 mo with OFF treatment, compared to 2.30 mo with BSC. Median OS for the sequence GEM-OFF and for GEM-BSC was 9.09 and 7.9 mo, respectively. The OFF regimen was well tolerated with 13% of grade II/III gastrointestinal toxicities. This randomised trial has supported the hypothesis of the benefit of second-line chemotherapy in comparison to BSC alone, for patients with PC[71]. A further phase III trial, CONKO-003, evaluated the effect on survival of oxaliplatin added to 5-FU, on second-line therapy. This trial randomised 168 patients with disease progression during first-line gemcitabine therapy, to folinic acid and 5-FU or oxaliplatin and 5-FU (OFF). In the OFF arm, the median OS and TTP were significantly extended in comparison to the 5-FU arm. The toxicities were similar between the two groups except for neurotoxicity, in 38.2% of OFF group patients[72]. However, oxaliplatin-based regimens for second-line chemotherapy have not given only positive outcomes. The PANCREOX trial randomised 108 patients after first-line gemcitabine, to mFOLFOX6 vs infusional 5-FU and folinic acid (5-FU/LV). The study showed no difference in median PFS (3.1 mo vs 2.9 mo, P = 0.99). Moreover mFOLFOX6 arm had a shorter OS and a higher patients number in mFOLFOX6 group withdrew for adverse events, thus the conclusion could be that this regimen is too toxic for this patients[73]. Irinotecan, alone or in combination with other drugs, could be another promising option for second-line therapy, in patients with metastatic PC after failure of gemcitabine. Also FOLFIRI has been proved, by some phase II trials, to be a safe and potentially active regimen in this setting[74,75]. But a more interesting aspect is the availability of a new irinotecan formulation, encapsulated into liposome-based nanoparticles, potentially increasing drug stability and sustaining drug release in the tumour area. The NAPOLI-1 trial, a multicentre, open- label, three-arm, randomised phase III trial, randomised 417 patients affected with metastatic PC, after prior gemcitabine-based therapy, to nano-liposomal irinotecan (MM-398) alone, or combined with 5-FU/LV, in comparison to 5-FU/LV. The combination of MM-398 + 5-FU/LV significantly improved OS, PFS, TTF, and ORR in comparison to 5-FU/LV. Median OS was 6.1 and 4.2 mo respectively. And median PFS 3.1 and 1.5 mo. MM-398 alone did not demonstrate any statistical improvement in efficacy. Many phase II trials have investigated other therapeutic options as taxanes[76,77], capecitabine[78], S1[79], FOLFIRI and FOLFOX[80], FOLFIRINOX[81,82], nab-paclitaxel[83] for the treatment of chemorefractory patients, but more confirmation studies are needed.
Unmet needs and proposals
Given the evidence of some benefit from second-line therapy, questions still remain about which optimal drugs and regimens and for which patients. Moreover, available second-lines therapies further questions concern the optimal treatment sequences. For patients who received FOLFIRINOX in the first-line setting, the second-line option is often a gemcitabine-based therapy. The association nab-paclitaxel with gemcitabine proved to be effective in the front-line setting, but lack efficacy data in second-line setting. While, for the patients who received nab-paclitaxel and gemcitabine in the first-line setting, an oxaliplatin-based treatment may be considered in the second-line (Figure 2). Choosing second-lines options very aggressive behaviour of PC and the relatively rapid QoL deterioration have not to be forgotten. The choice of second-line treatment should always be done with close attention to PS, patient’s age, the presence of comorbidities, and patient preferences.
Figure 2.
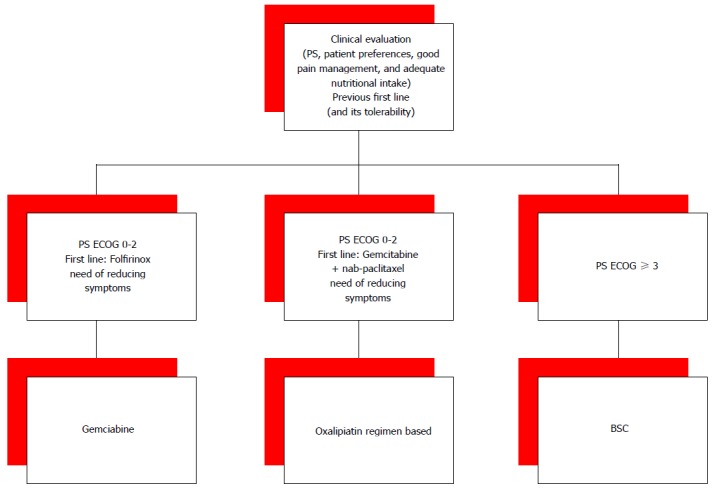
Proposal for the choice of the second line. PS ECOG: The eastern cooperative oncology group score of performance status; BSC: Best supportive care.
ARE LOCOREGIONAL TREATMENTS ACHIEVABLE ALTERNATIVES TO SURGERY? ARE THEY USEFUL IN LAPC OR METASTATIC PC?
Current knowledge
Roughly 40% of PC diagnosis are of LAPC, because non-metastatic but unresectable disease, not suited to surgery with radical intent. So far, in this setting, sole palliative chemotherapy can only give slight survival improvement. But there are further options of several innovative local ablative therapies, including RFA, IRE, SBRT, high-intensity focused ultrasound (HIFU), microwave ablation (MWA), photodynamic therapy (PDT), and cryoablation (Table 1). Ablative therapies based on thermal tumour damage include RFA, HIFU, cryoablation and MWA while IRE, PDT and SBRT are non-thermal ablative methods. Actually, despite their proven safety, feasibility and reproducibility, novel ablative methods in LAPC or metastatic PC have still to demonstrate a benefit on survival in large prospective randomised studies[84].
Table 1.
Ongoing clinical trials about locoregional treatments in locally advanced pancreatic cancer
| Combination | Inclusion criteria | Start | Clinical trial identifier1 | Expected end of accrual |
| FOLFIRINOX + SBRT | T ≤ 7 cm, non-metastatic | November 14 | NCT02292745 | November 20 |
| RFA | Unresectable, also metastatic | June 14 | NCT02166190 | June 16 |
| Cryoablation | Borderline resectable/locally advanced | November 14 | NCT02336672 | November 16 |
| Radioembolization | unresectable/failure of celiac alcholization | Not available | NCT01786850 | Not available |
| Irreversible electroporation (PAN.FIRE) | T < 5 cm, non-metastatic | September 13 | NCT01939665 | June 16 |
RFA: Radiofrequency ablation; SBRT: Stereotactic body radiation therapy;
Data Available from: URL: http// www.clinicaltrials.gov (last access 2015 May 24).
Stereotactic body radiotherapy: In the last few years, SBRT gained increasing interest for its better and longer lasting outcomes, as well less toxicity than conventional EBRT. The SBRT can selectively deliver a higher dose of radiation to a target lesion, in single or multiple sessions. When using SBRT it is of paramount importance the precise delineation of the therapeutic target and the correct evaluation of possible target motion, in particular for pancreas, in a site affected from breathing movements. For this reason, the treatment planning uses four-dimensional diagnostic imaging. SBRT may be delivered using non-isocentric technique, IMRT, or volumetric-modulated arc therapy[85]. Despite the above mentioned characteristics which seem to improve some of the major limits of EBRT, the role of SBRT in LAPC and BRPC is not clearly defined yet, though some interesting preliminary evidence of its activity has been recently reported. As an example, in a single centre institution experience, the authors reported a median OS of 18.4 mo and median PFS of 9.8 mo in 88 patients affected by LAPC and BRPC treated with SBRT (2-30 Gy in five fractions on the planning target volume) with an acceptable toxicity profile (3.4% of > G3 gastrointestinal toxicity)[86]. Furthermore, SBRT led to improved pain control in five out of six studies in which this outcome has been evaluated[87]. Moreover, as previously mentioned, SBRT can be delivered in association with chemotherapy with interesting preliminary evidence of activity (e.g., gemcitabine). Some trials are currently ongoing trying to better clarify the role of SBRT in PC and its activity in association with more recent combination regimens (e.g., SBRT with FOLFIRINOX, NCT 02292745).
RFA is the commonest thermal ablative technique used to treat tumours. It causes both direct thermal destructive effect and stimulation of antitumour immunity, through the expression of heat shock protein. RFA appears to be an attractive treatment for LAPC. According to the experience of Spiliotis et al[88] it should not be offered as an option for resectable PC, but it has shown to improve survival in 25 consecutive patients with inoperable LAPC who underwent palliative therapy with or without RFA. Median OS was 13 mo in patients receiving palliative therapy alone, compared to 33 mo in those who received RFA too (P = 0.0048). Moreover, RFA could be an option for patients with liver only metastasis in locally controlled PC. In a retrospective review by Park et al[89], RFA of liver metastasis was performed on 34 patients with PC, after the pancreatectomy or at the same time of the pancreatectomy. Median OS after liver metastasis treatment was 14 mo. In multivariate analysis, a single < 2 cm diameter liver metastasis and good or moderate differentiation were independent predictors for longer patient survival (P = 0.27, P = 0.16)[90].
Pancreatic Cryo Ablation (PCA) is a technique that uses single (or multiple) argon based probe in order to freeze the tumour. In most cases two cycles of freezing are used. It is currently used in several centers in the Far East for unresectable and often metastatic pancreatic ductal adenocarcinoma. Reported complications include acute pancreatitis, bleeding, leakage of bile, and delayed gastric emptying. No randomised trials have evaluated the efficacy of cryoablation, but Niu et al[91] retrospectively assessed the effect of comprehensive cryosurgery (ablation of intrapancreatic and extrapancreatic tumours) plus immunotherapy in 106 metastatic PC patients (cryoimmunotherapy: 31 patients, cryotherapy: 36 patients, immunotherapy: 17 patients and chemotherapy: 22 patients). Median OS was higher in the cryoimmunotherapy (13 mo) and cryotherapy groups (7 mo) than in the chemotherapy group (3.5 mo; both P < 0.001) and was higher in the cryoimmunotherapy group than in the cryotherapy (P < 0.05) and immunotherapy groups (5 mo; P < 0.001). In both the cryoimmunotherapy and cryotherapy groups, median OS was higher after multiple cryoablations than after a single cryoablation (P = 0.0048 and 0.041, respectively). A single institution retrospective review suggested effectiveness of PCA in palliation of cancer pain, on 62 patients, in combination with celiac plexus block. Some slight adverse effects (e.g., increased serum amylase, abdominal distension and nausea, abdominal bleeding) had disappeared by 3 wk, spontaneously or after symptomatic treatment. A significant difference was found between pretreatment and post-treatment pain frequency (P = 0.0019), regardless of the presence of advanced (P = 0.0096) or metastatic (P = 0.0072) cancer, and pain control was reported to last for more than 8 wk, without severe side effects[92].
Radio Embolization (RE) is a form of brachytherapy, which involves the direct intra-arterial delivery of radioactive isotopes close to or into a tumour. RE with intravascular yttrium-90 microspheres has been shown to be a safe and efficacious treatment of unresectable primary and metastatic hepatic tumours. RE is well tolerated with minimal toxicity. Patients may experience a short lasting post embolization syndrome, characterized by fatigue, nausea, abdominal pain, and/or a transient rise in liver function tests. RE for the treatment of liver metastasis from PC is investigational[93].
MWA is an emerging modality, performed either under percutaneous ultrasound guidance or through a laparotomy. Although operative temperatures may be higher with MW than with RFA, heat sink effects are less prominent, with less procedure related pain. Multiple probes can be used at the same time, reducing operative time. In a retrospective series[94], 10 patients with unresectable LAPC were treated with MW and palliative bypass surgery. In 5 of them MW was administered percutaneously, while in the other 5 it was delivered during laparotomy. One late major complication occurred, without any visceral injury being detected. No patient underwent further surgery. All patients had an improvement in QOL. In conclusion, MW ablation is a feasible approach in the palliative treatment of PC, but further studies are necessary.
Trans Artherial Chemo Embolization it is an interventional radiology procedure, of intra-arterial catheter-based chemotherapy. The selective administration of small drug-coated particles allow high doses directly to the tumour bed while sparing the surrounding liver tissue. For reported very limited experience, regarding liver metastasis from PC, its use is purely investigational[95].
PDT is a minimally invasive and safe method of treating cancer using an intravenous adinistered photosensitizer, activated by a specific wavelength of light, to kill tumour cells. The activated photosensitizer, produces singlet oxygen from molecular oxygen, which in turn causes tumour necrosis. There is also indirect cell death caused by induced hypoxia through tumour vasculature damage, without significant damage to connective tissues. The VERTPAC-01 trial investigated the safety and efficacy of PDT in 15 patients with LAPC using Verteroporphin. In 11 of 13 assessable patients, tumour size was stable at 1 mo, and in 6 of them stability was maintained at 3 mo. The technique proved to be feasible and safe and the authors concluded that it warrants further studies and may have a role in the multimodal treatment of PC[96].
HIFU The intention of a HIFU treatment is to deliver ultrasound energy to a well-defined targeted volume at depth, and to induce complete coagulation necrosis of the tumour. It can be administered with continuous or pulsed modality. HIFU doesn’t need the placement of a needle and it is characterized by a low rate of adverse events[87]. In a recent trial of HIFU, administered in addition chemotherapy or chemoradiotherapy to 30 patients with stage III/IV PC, the rate of symptom relief effect was 66.7% and the disease control-rate was 86% (mainly stable disease). The procedure was well tolerated, with moderate adverse events occurred in 10% of cases, mainly pseudocyst formation and mild pancreatitis[97,98].
IRE is a nonthermal ablative technique that uses ultrashort pulsed but very strong electrical fields. Formation of nanopores and micropores in the lipid bilayer of cell membranes induces cancer cells apoptosis[99,100]. No results of randomised trial are currently available. In the largest prospective series in LAPC, 54 patients have undergone an open approach IRE for unresectable cancer. The outcomes were compared to those obtained in 85 matched stage III patients, treated with chemotherapy and radiation therapy alone. The IRE procedure was given in addition to standard treatment: Chemotherapy or chemoradiation therapy in forty-nine (90%) patients and chemotherapy or chemoradiation after IRE in forty patients (73%). The 90 d mortality was 2%. IRE was associated with an increase in local progression-free survival (14 mo vs 6 mo; P = 0.01), distant progression-free survival (15 vs mo 9 mo; P = 0.02), and OS (20 mo vs 13 mo; P = 0.03)[101]. In a percutaneous approach IRE study, in 14 patients IRE was performed. All patients had received chemotherapy or radiation previously. Two patients underwent surgical resection with margin-negative and both had long disease-free survival (11 and 14 mo). There were no procedure-related deaths[100]. IRE appears to be feasible and safe, but it doesn’t improve OS compared with standard treatments, because of rapid progression of distant metastasis. IRE could be used as an additional treatment when surgical resection is possible but with hight risk of margin-positive (R1).
Regional intra-arterial chemotherapy: Intra-arterial chemotherapy aims both to increase drug concentrations in tumours tissues and to maintain low systemic drug levels. A recent meta-analysis and systematic review of randomised controlled trials included 155 patients receiving Regional Intra-Arterial Chemotherapy (RIAC) and 143 patients receiving systemic chemotherapy[102]. The RIAC efficacy seems to be evidenced by response rates of 58.06% with RIAC vs 29.37% with systemic treatment. Also, clinical benefit seems to be in favor of the RIAC (78.06% vs 29.37% respectively). The median survival time with RIAC (5-21 mo) was longer than for systemic chemotherapy (2.7-14 mo). Side effects were fewer in patients treated with RIAC (49.03%) than in those treated with systemic chemotherapy (71.33%), but the only statistically significant difference was for hematological side effects (60.87% vs 85.71% respectively). Despite these results, RIAC is not commonly used in clinical practice because it is invasive and requests hospitalization, with consequent risks of complications. A possible application of this technique, to further explore, could be in the neoadjuvant setting, in order to increase the resection rates and then probably OS with local advanced PC[103].
Unmet needs and proposals
Locoregional therapies alternative to surgery and radiation, for unresectable PC, are attractive and a number of studies demonstrated that they are feasible and reproducible. All of them should be considered as having a complementary role in the multimodal management care model. In metastatic setting, few data are available, most of them concerning RFA, these could be a safe and feasible strategy for extending survival in selected patients. Albeit several studies have anyway shown improved outcomes (changes in stage, diagnosis, or treatment plan), long-term survival data are lacking. Large prospective randomised studies are mandatory to assess the efficacy of these techniques and define their role/position in future treatment algorithms for the management of LAPC. Their main interest is in the context of a multidisciplinary-team patient evaluation, that is the best option to help patients cope with this challenging cancer[104-106].
IS MOLECULAR BIOLOGY THE NEW ROUTE IN DIAGNOSIS AND THERAPY?
Current knowledge
PC has a mean of 50 to 60 somatic mutations in protein-coding genes and at least 4 to 6 of them are driver mutation-driven in proto-oncogenes or tumour suppressor genes[106]. In addition, these somatic mutations are distributed in several key molecular pathways, probably ten or more[107], thus facilitating the acquisition of both intrinsic and secondary resistance to chemotherapy and targeted agents.
The commonest genome aberrations of PC are[108]: (1) the chromosomal rearrangements, widespread among the cancer genome and very common; (2) the KRAS oncogene mutated in nearly 90% of PC; (3) the tumour-suppressor genes TP53, SMAD4 e CDKN2A inactivated in more than 50% of cases.
Some key features of PC have been recently elucidated by the results of whole genome analyses of 100 cases of PC[109]. In particular, according to structural variations profiles and implicated molecular mechanisms underlying, PC can be classified into 4 subtypes defined as: (1) “stable”, 20% of cases, with low (< 50) structural variation events and frequent aneuploidy, suggesting defects in cell cycle/mitosis mechanisms; (2) “locally rearranged”, 30% of all samples, exhibiting a significant focal event in 1 or 2 chromosomes. In nearly one-third of cases it was present a gain of known oncogenes, mainly KRAS, SOX9 and GATA6, but also therapeutic target genes as ERBB2, MET, CDK6, PIK3CA, but with a low individual prevalence; (3) “scattered”, 36% of samples, exhibiting a moderate range of non-random chromosomal damage and less than 200 structural variation events; (4) “unstable”, 14% of cases, with a large (> 200) number of structural variation events suggesting defects in DNA maintenance including both mutations in BRCA pathway and mutations in other pathways involved in genomic instability, with a possible association with sensitivity to platinum agents and PARP inhibitors.
Moreover, the techniques of circulating cell-free tumour DNA (cfct-DNA) or circulating tumour cells, even if there are still very limited data in PC, seem a very interesting and promising way to study dynamically the global amount of cancer mutation. The cfct-DNA can be detected in respectively > 75% and 48% of patients with advanced or localized PC[110].
Unfortunately, up to now no single targeted agent, in preliminary clinical and preclinical data, has demonstrated to have a relevant impact on the natural history of metastatic PC. Strategies employed in these trials have involved mainly the inhibition of EGFR-MEK pathway and farnesyl-transferase. Targeted agents have been studied in PC mainly in combination with standard chemotherapy, in most cases gemcitabine. The association of chemotherapy with targeted agents blocking a single pathway in a molecularly unselected PC population has not led to relevant increase of treatment outcomes as, for example, in the case of erlotinib, tipifarnib, anti MEK-drugs like selumetinib and trametinib, trastuzumab and bevacizumab. Strategies involving a multiple blockade seem more promising due to the complexity of PC genome: Available clinical data and ongoing trials in this setting are described in Tables 2 and 3[111-115]. Data about multiple pathway inhibition strategies are available only in preclinical models[116].
Table 2.
Available clinical results about multitarget inhibition in pancreatic adenocarcinoma
| Combination | Molecular targets | Frequence of mutation1 | Setting/combination | Results |
| Everolimus + Erlotinib | mTOR + EGFR | +, + | Phase II, 16 patients, chemo-refractory | No responses |
| (Javle 2010) | ||||
| Bevacizumab + Erlotinib | VEGF + EGFR | +, + | Phase III, 301 patients, plus GEM + ERLO | No increase in OS respect GEM+ ERLO |
| (Van Cutsem 2009) | ||||
| Cixutumumab + Erlotinib | IGF-1R + EGFR | +, + | Phase Ib/II, 126 patients, plus GEM | No increase in PFS and OS respect GEM + ERLO |
| (Philip 2014) | ||||
| Sunitinb (Bergmann 2015) | VEGFR + PDGFR | +, + | Phase II, 106 patients, 1st line, plus GEM | No increase in TTP and OS respect GEM |
GEM: Gemcitabine; ERLO: Erlotinib; Nab-P: Nab-paclitaxel; OS: Overall survival; PFS: Progression-free survival; mTOR: Mammalian target of rapamycin; EGFR: Epidermal growth factor receptor; IGF-1R: Insulin-like growth factor 1 receptor; VEGFR: Vascular endothelial growth factor receptor; PDGFR: Platelet-derived growth factor receptor; TTP: Time to progression.
Table 3.
Ongoing clinical trials about multitarget inhibition in pancreatic adenocarcinoma
| Combination | Target | Frequence of mutation1 | Setting | Clinical trial identifier2 | Expected end of accrual |
| Dovitinib | FGRFR + PDGFR + VEGFR | +, +, + | Phase II, + GEM and CAPE | NCT01497392 | Sep-16 |
| Trastuzumab + Erlotinib | EGFR2 + EGFR | +, + | Phase II, + GEM | NCT01204372 | Apr-15 |
| MEK162 + Ganitumab | MEK1 + IGF-1R | +, + | Phase II, multi-disease, chemorefractory | NCT01562899 | Apr-15 |
GEM: Gemcitabine; MEK 1: Mitogen-activated extracellular signal regulated kinase 1; CAPE: Capecitabine.
To obtain this parameter, a mean between the frequency of somatic mutations in the target was calculated from the paper by Biankin et al[108] and Waddel et al[109], Figure 1. Three parameter were possible: +++ ≥ 75%, ++ > 50%, + ≤ 50%;
Data Available from: URL: http// www.clinicaltrials.gov (last access 2015 May 24).
A very peculiar feature of PC is its ability to promote the growth of a complex peritumoural stroma, with desmoplasia and altered vascularization. This surrounding environment greatly hinders antitumour drugs to reach active concentration into the tumour[117]. As far as inhibition of stroma is concerned, some recent preclinical data showed a possible benefit from hyaluronidase, an enzyme able to dissolve extracellular matrix[118], which is being tested in association with chemotherapy. Moreover, in a preclinical model, the concurrent administration of gemcitabine plus saridegib, a multiple Hedgehog signalling pathway inhibitor, increased vascular density and intratumoural concentration of gemcitabine. Clinical data about these approaches are resumed in Table 4[119-122]. Results from phase II and phase III trials exploring other treatment targets, as Hedgehog pathway, angiogenesis and immune regulation are expected in the next years[111]. Both the genomic instability and the complex tumour-stroma interactions promote the development of a relevant spatial and temporal molecular heterogeneity[123].
Table 4.
Available and ongoing clinical results about drugs targeting mainly stroma in pancreatic adenocarcinoma
| Combination | Target (s) | Setting | Clinical trial identifier1 | Expected end of accrual |
| Demcizumab | Cancer stem cells by DLL4 inhibition | Phase Ib, plus GEM +/- Nab-P | NCT01189929 | Concluded. presented at ASCO 2014: Increase in ORR, cardiovascular toxicity |
| (Gracian 2014) | ||||
| Ruxolitinib (Hurwitz 2014) | Inflammation by JAK/STAT inhibition | Phase II, 2nd line, plus CAPE | NCT01423604 | Concluded. presented at ASCO 2014: Benefit in patients with elevated CRP |
| PEGPH20 | HA by Pegylated-hyaluronidase | Phase II, 1st line, plus GEM | NCT01453153 | Concluded. presented at ASCO 2013: ORR 33%, especially in patients with high HA expression |
| (Hingorani 2013)S | ||||
| ‘’ | ‘’ | Phase II, plus GEM + Nab-P | NCT01839487 | July 16 |
CAPE: Capecitabine; CRP: C-reactive protein; GEM: Gemcitabine; HA: Hyaluronic acid; ORR: Objective response rate; DLL4: Delta like ligand 4; ASCO: American society of clinical oncology; JAK/STAT: Janus kinase/signal transducer and activator of transcription; Nab-P: Nab-paclitaxel.
Data Available from: URL: http// www.clinicaltrials.gov (last access 2015 May 24).
PC stem cells (PCSCs) are believed to promote tumour growth and progression through a number of mechanisms, including differentiation into bulk tumour cells, metastasis, alteration of adjacent stromal cells, and evasion of conventional therapies. Possible strategies to target PCSCs involve inhibiting specific proteins and pathways, such as c-Met, Alk-4, Notch pathway and gamma-secretase. These approaches are in a preclinical stage of development[124] (Table 3).
Regarding epigenetic modifications, key tumour suppressors genes, with a well-established role in PC, may be altered through hypermethylation. And permissive histone modifications may be the cause of oncogenes upregulation. Moreover, factors involved in tumour invasiveness can be aberrantly expressed through deregulated microRNA. In this perspective, a potential therapeutical target in order to modify epigenetics is the enzyme enhancer of zeste homolog 2 (codified by the gene EZH2) which, when overexpressed, contributes to PC growth. Only preclinical data are available[125].
Unmet needs and proposals
Although presenting a molecular landscape shared with other neoplasms (e.g., colorectal and breast cancer) PC has a worse prognosis. As described above, the very complex genomic landscape with the simultaneous activation of multiple relevant pathways and the complexity of cancer microenvironment could be key factors in determining the disappointing results of targeted agents in PC. From a clinical point of view, due to the increasing availability of targeted agents, a deeper understanding of PC’s biology is desirable and remains the mainstay of clinical research in PC.
The recent availability of next-generation sequencing techniques and the creation of joined multicenter working groups has greatly increased the knowledge of the mutational landscape of PC and raised the possibility to perform a personalized medicine even in such as “distressing” setting[126].
Starting from the current knowledges, possible research strategies to improve the results of targeted agents could be the simultaneous inhibition of multiple pathways, the combination of stroma targeting agents with other possibly effective drugs (e.g., chemotherapy), targeting PCSCs, targeting epigenetic alterations.
Moreover, the heterogeneity of the disease has to be taken in account. A better knowledge of pathways and targets and of distinct genetic features can help in defining prognostic and predictive factors to select or stratify patients accrued in clinical trials. As an example, a significant proportion of subtype 2/locally arranged PC harbor mutations in “druggable” genes (as ERBB2 and MET) and many subtype 4/unstable PCs have defects in DNA repair mechanisms suggesting the hypothesis this group could have a particular sensitivity to platinum agents and PARP inhibitors, to prospectively test in further trials.
CONCLUSION
Despite new biomolecular knowledge and the efforts to define new therapeutic approaches in PC in all the setting of care, there are still many unresolved issues. In fact, starting from the definition of resectable disease to the evaluation of the best locoregional treatment in LAPC, everything today is constantly evolving in clinical practice and there is still no uniformity of view from center to center. Moreover in the era of cancer treatment based on specific molecular alterations and of immunotherapy rather than chemotherapy, PC seems to go against the grain. Disappointing results of targeted therapy studies have not allowed us to add new weapons to systemic treatments, and immunotherapy is still object of clinical trials. Furthermore, the high biological aggressiveness of PC and the incomplete knowledge of the biology of this disease have hampered the development of new more efficacious strategies of target selection and drug development. Hence, PC is still an undefeated enemy, with high and early mortality, high genetic complexity and lack of prognostic and predictive factors that can drive the clinical decision. Efforts to define and validate prognostic and predictive factors as well as the genetic and molecular basis that can help the oncologist in everyday clinical practice must be carried over. A multidisciplinary team is crucial in order to rapidly and effectively translate clinical and preclinical findings into valuable and applicable data for the clinical setting.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest regarding this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 2, 2015
First decision: July 31, 2015
Article in press: November 25, 2015
P- Reviewer: Fukuda S, Kamisawa T S- Editor: Qiu S L- Editor: A E- Editor: Jiao XK
References
- 1.AIRTUM Working Group. Italian cancer figures, report 2014: Prevalence and cure of cancer in Italy. Epidemiol Prev. 2014;38:1–122. [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44:186–198. doi: 10.1093/ije/dyu240. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs EJ, Chanock SJ, Fuchs CS, Lacroix A, McWilliams RR, Steplowski E, Stolzenberg-Solomon RZ, Arslan AA, Bueno-de-Mesquita HB, Gross M, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Int J Cancer. 2010;127:1421–1428. doi: 10.1002/ijc.25148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolpin BM, Kraft P, Gross M, Helzlsouer K, Bueno-de-Mesquita HB, Steplowski E, Stolzenberg-Solomon RZ, Arslan AA, Jacobs EJ, Lacroix A, et al. Pancreatic cancer risk and ABO blood group alleles: results from the pancreatic cancer cohort consortium. Cancer Res. 2010;70:1015–1023. doi: 10.1158/0008-5472.CAN-09-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolpin BM, Rizzato C, Kraft P, Kooperberg C, Petersen GM, Wang Z, Arslan AA, Beane-Freeman L, Bracci PM, Buring J, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet. 2014;46:994–1000. doi: 10.1038/ng.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowenfels AB, Maisonneuve P. Can we prevent pancreatic disease? Clin Gastroenterol Hepatol. 2014;12:1645–1646. doi: 10.1016/j.cgh.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Bulajic M, Panic N, Löhr JM. Helicobacter pylori and pancreatic diseases. World J Gastrointest Pathophysiol. 2014;5:380–383. doi: 10.4291/wjgp.v5.i4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol. 2009;9:411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Tsugane S. Insulin resistance and cancer: epidemiological evidence. Endocr Relat Cancer. 2012;19:F1–F8. doi: 10.1530/ERC-12-0142. [DOI] [PubMed] [Google Scholar]
- 11.Olson SH, Hsu M, Satagopan JM, Maisonneuve P, Silverman DT, Lucenteforte E, Anderson KE, Borgida A, Bracci PM, Bueno-de-Mesquita HB, et al. Allergies and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Case-Control Consortium. Am J Epidemiol. 2013;178:691–700. doi: 10.1093/aje/kwt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ip IK, Mortele KJ, Prevedello LM, Khorasani R. Focal cystic pancreatic lesions: assessing variation in radiologists’ management recommendations. Radiology. 2011;259:136–141. doi: 10.1148/radiol.10100970. [DOI] [PubMed] [Google Scholar]
- 13.Del Chiaro M, Verbeke C. Cystic tumors of the pancreas: Opportunities and risks. World J Gastrointest Pathophysiol. 2015;6:29–32. doi: 10.4291/wjgp.v6.i2.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 15.Tempero MA, Arnoletti JP, Behrman SW, Ben-Josef E, Benson AB, Casper ES, Cohen SJ, Czito B, Ellenhorn JD, Hawkins WG, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10:703–713. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, Donehower RC, Pawlik TM, Ziegler MA, Cai H, et al. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol. 2013;31:886–894. doi: 10.1200/JCO.2012.44.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puleo F, Maréchal R, Demetter P, Bali MA, Calomme A, Closset J, Bachet JB, Deviere J, Van Laethem JL. New challenges in perioperative management of pancreatic cancer. World J Gastroenterol. 2015;21:2281–2293. doi: 10.3748/wjg.v21.i8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. [accessed 2015 May] Available from: http//www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
- 19.Mehta VK, Poen JC, Ford JM, Oberhelman HA, Vierra MA, Bastidas AJ, Fisher GA. Protracted venous infusion 5-fluorouracil with concomitant radiotherapy compared with bolus 5-fluorouracil for unresectable pancreatic cancer. Am J Clin Oncol. 2001;24:155–159. doi: 10.1097/00000421-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Small W, Berlin J, Freedman GM, Lawrence T, Talamonti MS, Mulcahy MF, Chakravarthy AB, Konski AA, Zalupski MM, Philip PA, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008;26:942–947. doi: 10.1200/JCO.2007.13.9014. [DOI] [PubMed] [Google Scholar]
- 21.Stokes JB, Nolan NJ, Stelow EB, Walters DM, Weiss GR, de Lange EE, Rich TA, Adams RB, Bauer TW. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol. 2011;18:619–627. doi: 10.1245/s10434-010-1456-7. [DOI] [PubMed] [Google Scholar]
- 22.Patel M, Hoffe S, Malafa M, Hodul P, Klapman J, Centeno B, Kim J, Helm J, Valone T, Springett G. Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol. 2011;104:155–161. doi: 10.1002/jso.21954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mondo EL, Noel MS, Katz AW, Schoeniger LO, Hezel AF. Unresectable locally advanced pancreatic cancer: treatment with neoadjuvant leucovorin, fluorouracil, irinotecan, and oxaliplatin and assessment of surgical resectability. J Clin Oncol. 2013;31:e37–e39. doi: 10.1200/JCO.2012.44.0339. [DOI] [PubMed] [Google Scholar]
- 25.Arvold ND, Ryan DP, Niemierko A, Blaszkowsky LS, Kwak EL, Wo JY, Allen JN, Clark JW, Wadlow RC, Zhu AX, et al. Long-term outcomes of neoadjuvant chemotherapy before chemoradiation for locally advanced pancreatic cancer. Cancer. 2012;118:3026–3035. doi: 10.1002/cncr.26633. [DOI] [PubMed] [Google Scholar]
- 26.Faris JE, Blaszkowsky LS, McDermott S, Guimaraes AR, Szymonifka J, Huynh MA, Ferrone CR, Wargo JA, Allen JN, Dias LE, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543–548. doi: 10.1634/theoncologist.2012-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN, Blaszkowsky LS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christians KK, Tsai S, Mahmoud A, Ritch P, Thomas JP, Wiebe L, Kelly T, Erickson B, Wang H, Evans DB, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist. 2014;19:266–274. doi: 10.1634/theoncologist.2013-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marthey L, Sa-Cunha A, Blanc JF, Gauthier M, Cueff A, Francois E, Trouilloud I, Malka D, Bachet JB, Coriat R, et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol. 2015;22:295–301. doi: 10.1245/s10434-014-3898-9. [DOI] [PubMed] [Google Scholar]
- 30.Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, Alberts S, O’Dwyer P, Haller D, Catalano P, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu CP, Shi J, Chen YX, Xie WF, Lin Y. Gemcitabine in the chemoradiotherapy for locally advanced pancreatic cancer: a meta-analysis. Radiother Oncol. 2011;99:108–113. doi: 10.1016/j.radonc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Li CP, Chao Y, Chi KH, Chan WK, Teng HC, Lee RC, Chang FY, Lee SD, Yen SH. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys. 2003;57:98–104. doi: 10.1016/s0360-3016(03)00435-8. [DOI] [PubMed] [Google Scholar]
- 34.Crane CH, Abbruzzese JL, Evans DB, Wolff RA, Ballo MT, Delclos M, Milas L, Mason K, Charnsangavej C, Pisters PW, et al. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys. 2002;52:1293–1302. doi: 10.1016/s0360-3016(01)02740-7. [DOI] [PubMed] [Google Scholar]
- 35.Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 36.Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 38.Sherman WH, Chu K, Chabot J, Allendorf J, Schrope BA, Hecht E, Jin B, Leung D, Remotti H, Addeo G, et al. Neoadjuvant gemcitabine, docetaxel, and capecitabine followed by gemcitabine and capecitabine/radiation therapy and surgery in locally advanced, unresectable pancreatic adenocarcinoma. Cancer. 2015;121:673–680. doi: 10.1002/cncr.29112. [DOI] [PubMed] [Google Scholar]
- 39.Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L, Azzedine A, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 40.Huguet F, Hammel P, Vernerey D, Goldstein D, Van Laethem JL, Glimelius B, Spry N, Paget-Bailly S, Bonnetain F, Louvet C. Impact of chemoradiotherapy (CRT) on local control and time without treatment in patients with locally advanced pancreatic cancer (LAPC) included in the international phase III LAP 07 study. J Clin Oncol. 2014;32:abst 4001. [Google Scholar]
- 41.Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol. 2013;24:2484–2492. doi: 10.1093/annonc/mdt239. [DOI] [PubMed] [Google Scholar]
- 42.Franke AJ, Rosati LM, Pawlik TM, Kumar R, Herman JM. The role of radiation therapy in pancreatic ductal adenocarcinoma in the neoadjuvant and adjuvant settings. Semin Oncol. 2015;42:144–162. doi: 10.1053/j.seminoncol.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, Iacobuzio-Donahue CA, Griffith ME, Pawlik TM, Pai JS, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 45.Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2006;19:CD002093. doi: 10.1002/14651858.CD002093.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 47.Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 48.Colucci G, Labianca R, Di Costanzo F, Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolò M, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645–1651. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 49.Reni M, Cereda S, Bonetto E, Viganò MG, Passoni P, Zerbi A, Balzano G, Nicoletti R, Staudacher C, Di Carlo V. Dose-intense PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) in advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2007;59:361–367. doi: 10.1007/s00280-006-0277-7. [DOI] [PubMed] [Google Scholar]
- 50.Reni M, Cereda S, Rognone A, Belli C, Ghidini M, Longoni S, Fugazza C, Rezzonico S, Passoni P, Slim N, et al. A randomized phase II trial of two different 4-drug combinations in advanced pancreatic adenocarcinoma: cisplatin, capecitabine, gemcitabine plus either epirubicin or docetaxel (PEXG or PDXG regimen) Cancer Chemother Pharmacol. 2012;69:115–123. doi: 10.1007/s00280-011-1680-2. [DOI] [PubMed] [Google Scholar]
- 51.Fine RL, Fogelman DR, Schreibman SM, Desai M, Sherman W, Strauss J, Guba S, Andrade R, Chabot J. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: a retrospective analysis. Cancer Chemother Pharmacol. 2008;61:167–175. doi: 10.1007/s00280-007-0473-0. [DOI] [PubMed] [Google Scholar]
- 52.De Jesus-Acosta A, Oliver GR, Blackford A, Kinsman K, Flores EI, Wilfong LS, Zheng L, Donehower RC, Cosgrove D, Laheru D, et al. A multicenter analysis of GTX chemotherapy in patients with locally advanced and metastatic pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2012;69:415–424. doi: 10.1007/s00280-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, Potamianou A, Ziras N, Boukovinas J, Varthalitis J, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95:587–592. doi: 10.1038/sj.bjc.6603301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rocha Lima CM, Green MR, Rotche R, Miller WH, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 55.Oettle H, Richards D, Ramanathan RK, van Laethem JL, Peeters M, Fuchs M, Zimmermann A, John W, Von Hoff D, Arning M, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639–1645. doi: 10.1093/annonc/mdi309. [DOI] [PubMed] [Google Scholar]
- 56.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 57.Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 60.Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 61.Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, Oh DY, Liu Y, Redhu S, Steplewski K, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50:2072–2081. doi: 10.1016/j.ejca.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 62.Harder J, Ihorst G, Heinemann V, Hofheinz R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S, et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106:1033–1038. doi: 10.1038/bjc.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 64.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaanan A, Trouilloud I, Markoutsaki T, Gauthier M, Dupont-Gossart AC, Lecomte T, Aparicio T, Artru P, Thirot-Bidault A, Joubert F, et al. FOLFOX as second-line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC Cancer. 2014;14:441. doi: 10.1186/1471-2407-14-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66:3271–3277. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 67.Carrato A, Falcone A, Ducreux M, Valle JW, Parnaby A, Djazouli K, Alnwick-Allu K, Hutchings A, Palaska C, Parthenaki I. A Systematic Review of the Burden of Pancreatic Cancer in Europe: Real-World Impact on Survival, Quality of Life and Costs. J Gastrointest Cancer. 2015;46:201–211. doi: 10.1007/s12029-015-9724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smyth EN, Bapat B, Ball DE, André T, Kaye JA. Metastatic Pancreatic Adenocarcinoma Treatment Patterns, Health Care Resource Use, and Outcomes in France and the United Kingdom Between 2009 and 2012: A Retrospective Study. Clin Ther. 2015;37:1301–1316. doi: 10.1016/j.clinthera.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Boige V, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23–29. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 70.Novarino A, Satolli MA, Chiappino I, Giacobino A, Bellone G, Rahimi F, Milanesi E, Bertetto O, Ciuffreda L. Oxaliplatin, 5-fluorouracil, and leucovorin as second-line treatment for advanced pancreatic cancer. Am J Clin Oncol. 2009;32:44–48. doi: 10.1097/COC.0b013e31817be5a9. [DOI] [PubMed] [Google Scholar]
- 71.Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dörken B, Riess H, Oettle H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676–1681. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 72.Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Görner M, Mölle M, Greten TF, Lakner V, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423–2429. doi: 10.1200/JCO.2013.53.6995. [DOI] [PubMed] [Google Scholar]
- 73.Gill S, Ko YJ, Cripps MC, BeauDOIn A, Dhesy-Thind SK, Zulfiqar M. PANCREOX: a randomised phase 3 study of 5-FU/LV with or without oxaliplatin for second-line advanced pancreatic cancer (APC) in patients (pts) who have received gemcitabine (GEM)-based chemotherapy (CT) J Clin Oncol. 2014;32:abstr 4022. doi: 10.1200/JCO.2016.68.5776. [DOI] [PubMed] [Google Scholar]
- 74.Zaniboni A, Aitini E, Barni S, Ferrari D, Cascinu S, Catalano V, Valmadre G, Ferrara D, Veltri E, Codignola C, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol. 2012;69:1641–1645. doi: 10.1007/s00280-012-1875-1. [DOI] [PubMed] [Google Scholar]
- 75.Neuzillet C, Hentic O, Rousseau B, Rebours V, Bengrine-Lefèvre L, Bonnetain F, Lévy P, Raymond E, Ruszniewski P, Louvet C, et al. FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts. World J Gastroenterol. 2012;18:4533–4541. doi: 10.3748/wjg.v18.i33.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cereda S, Reni M. Weekly docetaxel as salvage therapy in patients with gemcitabine-refractory metastatic pancreatic cancer. J Chemother. 2008;20:509–512. doi: 10.1179/joc.2008.20.4.509. [DOI] [PubMed] [Google Scholar]
- 77.Oettle H, Arnold D, Esser M, Huhn D, Riess H. Paclitaxel as weekly second-line therapy in patients with advanced pancreatic carcinoma. Anticancer Drugs. 2000;11:635–638. doi: 10.1097/00001813-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Xiong HQ, Varadhachary GR, Blais JC, Hess KR, Abbruzzese JL, Wolff RA. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008;113:2046–2052. doi: 10.1002/cncr.23810. [DOI] [PubMed] [Google Scholar]
- 79.Sudo K, Nakamura K, Yamaguchi T. S-1 in the treatment of pancreatic cancer. World J Gastroenterol. 2014;20:15110–15118. doi: 10.3748/wjg.v20.i41.15110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoo C, Hwang JY, Kim JE, Kim TW, Lee JS, Park DH, Lee SS, Seo DW, Lee SK, Kim MH, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101:1658–1663. doi: 10.1038/sj.bjc.6605374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee MG, Lee SH, Lee SJ, Lee YS, Hwang JH, Ryu JK, Kim YT, Kim DU, Woo SM. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with advanced pancreatic cancer who have progressed on gemcitabine-based therapy. Chemotherapy. 2013;59:273–279. doi: 10.1159/000356158. [DOI] [PubMed] [Google Scholar]
- 82.Assaf E, Verlinde-Carvalho M, Delbaldo C, Grenier J, Sellam Z, Pouessel D, Bouaita L, Baumgaertner I, Sobhani I, Tayar C, et al. 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology. 2011;80:301–306. doi: 10.1159/000329803. [DOI] [PubMed] [Google Scholar]
- 83.Hosein PJ, de Lima Lopes G, Pastorini VH, Gomez C, Macintyre J, Zayas G, Reis I, Montero AJ, Merchan JR, Rocha Lima CM. A phase II trial of nab-Paclitaxel as second-line therapy in patients with advanced pancreatic cancer. Am J Clin Oncol. 2013;36:151–156. doi: 10.1097/COC.0b013e3182436e8c. [DOI] [PubMed] [Google Scholar]
- 84.Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:2267–2278. doi: 10.3748/wjg.v20.i9.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trakul N, Koong AC, Chang DT. Stereotactic body radiotherapy in the treatment of pancreatic cancer. Semin Radiat Oncol. 2014;24:140–147. doi: 10.1016/j.semradonc.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Moningi S, Dholakia AS, Raman SP, Blackford A, Cameron JL, Le DT, De Jesus-Acosta AM, Hacker-Prietz A, Rosati LM, Assadi RK, et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann Surg Oncol. 2015;22:2352–2358. doi: 10.1245/s10434-014-4274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rombouts SJ, Vogel JA, van Santvoort HC, van Lienden KP, van Hillegersberg R, Busch OR, Besselink MG, Molenaar IQ. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surg. 2015;102:182–193. doi: 10.1002/bjs.9716. [DOI] [PubMed] [Google Scholar]
- 88.Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55–60. doi: 10.1007/s00423-006-0098-5. [DOI] [PubMed] [Google Scholar]
- 89.Park JB, Kim YH, Kim J, Chang HM, Kim TW, Kim SC, Kim PN, Han DJ. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol. 2012;23:635–641. doi: 10.1016/j.jvir.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 90.Fegrachi S, Besselink MG, van Santvoort HC, van Hillegersberg R, Molenaar IQ. Radiofrequency ablation for unresectable locally advanced pancreatic cancer: a systematic review. HPB (Oxford) 2014;16:119–123. doi: 10.1111/hpb.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Niu L, Chen J, He L, Liao M, Yuan Y, Zeng J, Li J, Zuo J, Xu K. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;42:1143–1149. doi: 10.1097/MPA.0b013e3182965dde. [DOI] [PubMed] [Google Scholar]
- 92.Niu L, Wang Y, Yao F, Wei C, Chen Y, Zhang L, Chen J, Li J, Zuo J, Xu K. Alleviating visceral cancer pain in patients with pancreatic cancer using cryoablation and celiac plexus block. Cryobiology. 2013;66:105–111. doi: 10.1016/j.cryobiol.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 93.Dominello M, Bowers J, Zaki M, Konski A. Radiotherapy and radioembolization for liver metastases. Ann Palliat Med. 2014;3:104–113. doi: 10.3978/j.issn.2224-5820.2014.04.05. [DOI] [PubMed] [Google Scholar]
- 94.Carrafiello G, Ierardi AM, Fontana F, Petrillo M, Floridi C, Lucchina N, Cuffari S, Dionigi G, Rotondo A, Fugazzola C. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24:1513–1520. doi: 10.1016/j.jvir.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Farshid P, Darvishi A, Naguib N, Bazrafshan B, Paul J, Mbalisike E, Vogl TJ. Repetitive chemoembolization of hypovascular liver metastases from the most common primary sites. Future Oncol. 2013;9:419–426. doi: 10.2217/fon.12.191. [DOI] [PubMed] [Google Scholar]
- 96.Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M, Kent E, Bown SG, Hasan T, Pogue BW, et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer. 2014;110:1698–1704. doi: 10.1038/bjc.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sofuni A, Moriyasu F, Sano T, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Tanaka R, et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J Gastroenterol. 2014;20:9570–9577. doi: 10.3748/wjg.v20.i28.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu F. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:16480–16488. doi: 10.3748/wjg.v20.i44.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al Efishat M, Wolfgang CL, Weiss MJ. Stage III pancreatic cancer and the role of irreversible electroporation. BMJ. 2015;350:h521. doi: 10.1136/bmj.h521. [DOI] [PubMed] [Google Scholar]
- 100.Narayanan G, Hosein PJ, Arora G, Barbery KJ, Froud T, Livingstone AS, Franceschi D, Rocha Lima CM, Yrizarry J. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23:1613–1621. doi: 10.1016/j.jvir.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Martin RC, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20 Suppl 3:S443–S449. doi: 10.1245/s10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 102.Liu F, Tang Y, Sun J, Yuan Z, Li S, Sheng J, Ren H, Hao J. Regional intra-arterial vs. systemic chemotherapy for advanced pancreatic cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2012;7:e40847. doi: 10.1371/journal.pone.0040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davis JL, Pandalai PK, Ripley RT, Langan RC, Avital I. Expanding surgical treatment of pancreatic cancer: the role of regional chemotherapy. Pancreas. 2012;41:678–684. doi: 10.1097/MPA.0b013e318249955a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pawlik TM, Laheru D, Hruban RH, Coleman J, Wolfgang CL, Campbell K, Ali S, Fishman EK, Schulick RD, Herman JM. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–2088. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gardner TB, Barth RJ, Zaki BI, Boulay BR, McGowan MM, Sutton JE, Ripple GH, Colacchio TA, Smith KD, Byock IR, et al. Effect of initiating a multidisciplinary care clinic on access and time to treatment in patients with pancreatic adenocarcinoma. J Oncol Pract. 2010;6:288–292. doi: 10.1200/JOP.2010.000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Teague A, Lim KH, Wang-Gillam A. Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Ther Adv Med Oncol. 2015;7:68–84. doi: 10.1177/1758834014564775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Javle MM, Shroff RT, Xiong H, Varadhachary GA, Fogelman D, Reddy SA, Davis D, Zhang Y, Wolff RA, Abbruzzese JL. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer. 2010;10:368. doi: 10.1186/1471-2407-10-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 114.Philip PA, Goldman B, Ramanathan RK, Lenz HJ, Lowy AM, Whitehead RP, Wakatsuki T, Iqbal S, Gaur R, Benedetti JK, et al. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727) Cancer. 2014;120:2980–2985. doi: 10.1002/cncr.28744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bergmann L, Maute L, Heil G, Rüssel J, Weidmann E, Köberle D, Fuxius S, Weigang-Köhler K, Aulitzky WE, Wörmann B, et al. A prospective randomised phase-II trial with gemcitabine versus gemcitabine plus sunitinib in advanced pancreatic cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Eur J Cancer. 2015;51:27–36. doi: 10.1016/j.ejca.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 116.Alagesan B, Contino G, Guimaraes AR, Corcoran RB, Deshpande V, Wojtkiewicz GR, Hezel AF, Wong KK, Loda M, Weissleder R, et al. Combined MEK and PI3K inhibition in a mouse model of pancreatic cancer. Clin Cancer Res. 2015;21:396–404. doi: 10.1158/1078-0432.CCR-14-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rucki AA, Zheng L. Pancreatic cancer stroma: understanding biology leads to new therapeutic strategies. World J Gastroenterol. 2014;20:2237–2246. doi: 10.3748/wjg.v20.i9.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gracian AC, Jameson MB, Grande E, Cooray P, Parnis F, Grimison P, Jeffery M, Stagg RJ, Dupont J, Tebbutt NC. A phase 1b study of the anticancer stem cell agent demcizumab (DEM) and gemcitabine (GEM) with or without paclitaxel protein bound particles (nab-paclitaxel) in patients with pancreatic cancer. J ClinOncol. 2014;32:abstr 279. [Google Scholar]
- 121.Hurwitz H, Uppal N, Wagner SA, Bendell JC, Beck JC, Wade S, Nemunaitis JJ, Stella PJ, Pipas JM, Wainberg ZA, et al. A randomized double-blind phase 2 study of ruxolitinib (RUX) or placebo (PBO) with capecitabine (CAPE) as second-line therapy in patients (pts) with metastatic pancreatic cancer (mPC) J ClinOncol. 2014;32:abstr 4000. doi: 10.1200/JCO.2015.61.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin S, Gladkov O, Holcombe RF, Jiang P, et al. A phase Ib study of gemcitabine plus PEGPH20 (pegylated recombinant human hyaluronidase) in patients with stage IV previously untreated pancreatic cancer. J Clin Oncol. 2013;31:abstr 4010. [Google Scholar]
- 123.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abel EV, Simeone DM. Biology and clinical applications of pancreatic cancer stem cells. Gastroenterology. 2013;144:1241–1248. doi: 10.1053/j.gastro.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 125.McCleary-Wheeler AL, Lomberk GA, Weiss FU, Schneider G, Fabbri M, Poshusta TL, Dusetti NJ, Baumgart S, Iovanna JL, Ellenrieder V, et al. Insights into the epigenetic mechanisms controlling pancreatic carcinogenesis. Cancer Lett. 2013;328:212–221. doi: 10.1016/j.canlet.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]


