Abstract
In the early 1900s, numerous seminal publications reported that high rates of cancer occurred in certain occupations. During this period, work with infectious agents produced only meager results which seemed irrelevant to humans. Then in the 1980s ground breaking evidence began to emerge that a variety of viruses also cause cancer in humans. There is now sufficient evidence of carcinogenicity in humans for human T-cell lymphotrophic virus, human immunodeficiency virus, hepatitis B virus, hepatitis C virus, human papillomavirus, Epstein-Barr virus, and human herpes virus 8 according to the International Agency for Research on Cancer (IARC). Many other causes of cancer have also been identified by the IARC, which include: Sunlight, tobacco, pharmaceuticals, hormones, alcohol, parasites, fungi, bacteria, salted fish, wood dust, and herbs. The World Cancer Research Fund and the American Institute for Cancer Research have determined additional causes of cancer, which include beta carotene, red meat, processed meats, low fibre diets, not breast feeding, obesity, increased adult height and sedentary lifestyles. In brief, a historical review of the discoveries of the causes of human cancer is presented with extended discussions of the difficulties encountered in identifying viral causes of cancer.
Keywords: Infections, Causes, Cancer, Carcinogens, Historical, Etiology, International agency for research on cancer
Core tip: The International Agency for Research on Cancer has worked for around 45 years evaluating the scientific literature, concerning the potential of around 1000 different agents to cause cancer. Those agents which were determined to definitively cause cancer in humans are reviewed from a historical perspective. It is reviewed how there were many complexities in identifying infectious agents as causes of cancer. The author incidentally discovered while writing this review that natural factors are an additional and relatively underappreciated cause of cancer.
INTRODUCTION
The question of “what causes cancer” has intrigued people for generations. In 1950, the World Health Organization sponsored an international symposium, and the attendees were intrigued by the dramatic variations in the types of cancer found in different areas of the world[1]. It was learned that people who migrated to other countries, developed types of cancer common to their adopted countries, rather than their homelands. This implied that most cancers were caused by exposures in the environment, rather than inherited genetic factors. The symposium led to the creation of the International Agency for Research on Cancer (IARC) in 1965 which was instructed to conduct multidisciplinary investigations of the causes of human cancers[1,2]. The assessments of the IARC were initially based only on epidemiological evidence[3], and then later the criteria were extended to include experimental evidence[4].
There has been a widespread notion that synthetic agents are the cause of most cancers, so this review begins with a review of the discoveries of occupational and pharmaceutical agents which cause cancer, wherein it becomes evident how this opinion arose. The next section recounts how there has also been a strong suspicion that infectious agents cause cancer, and includes a description of the exhaustive search for viruses which cause cancer. This is followed by a section which discusses natural factors and non-viral infectious agents which have been demonstrated to cause cancer.
Numerous resources were frequently consulted, and influenced the selection of topics for discussion in this review. The historical treatise by Shimkin[5], the historic milestones outlined by Sirica[6], and the monograph by Ludwig Gross[7] were consulted many times. Most importantly, the monographs by the IARC were used to identify which agents have been determined to cause cancer in humans, and were frequently used to identify the earliest and most influential studies. “Food, Nutrition, Physical Activity and Prevention of Cancer: A Global Perspective” is an expert report published by the World Cancer Research Fund and the American Institute for Research on Cancer[8] which provided supplementary analyses for a variety of natural agents.
OCCUPATIONAL, PHARMACEUTICAL, AND TOBACCO
Early studies
Early epidemiological studies: The earliest carcinogens to be identified were generally associated with specific occupations. Bernardino Ramazzini[9] observed in 1713 that nuns suffered from high rates of breast cancer which he attributed to their celibate life. Percivol Pott[10] documented in 1775 that chimney sweeps frequently developed cancer of the scrotum which he deduced to be caused by their heavy exposure to soot. A century afterwards, reports emerged that a variety of other occupations were associated with increased rates of cancer. Richard von Volkmann[11] diagnosed three cases of scrotal cancer in 1875 among coal tar distillers in Germany, which was quickly followed by similar reports by other physicians[12]. Joseph Bell[13] described two cases of scrotal cancer among shale oil workers in Scotland in 1876, and commented that the cancer was quite common among shale oil workers. Harting and Hesse documented in 1879 that miners in the Black Forest regions of Schneeberg in Germany and Joachimsthal in Czechoslovakia suffered from a high mortality due to lung cancer[14,15]. Ludwig Rhen[16,17] reported in 1895 that long term dye workers in Germany frequently perished of bladder cancer. Wilhelm Conrad Röntgen[18] discovered X-rays in 1895, which were heralded as a phenomenal discovery, because they permitted the painless visualization of bones. The early radiologists routinely tested the performance of their equipment by exposing their hands. Then a few days after a prolonged exposure, an extremely painful skin condition termed radiodermatitis developed[19,20]. A decade after Röntgen’s discovery of X-rays, case reports began emerging from many diverse areas of the world, that radiologists were succumbing to skin cancers[21,22].
A few non-occupational agents were also identified during this period. John Hill[23] reported in 1761 that immoderate use of tobacco snuff was associated with the occurrence of nasal cancers[24]. Sir Johnathan Hutchinson[25] observed in 1881 that patients who used a tonic which contained arsenic for extended durations frequently developed keratosis lesions which sometimes progressed to skin cancer.
Early experimental studies: In the late 1800s, there were three fundamental theories of the cause of cancer[26-28]. Virchow proposed that cancer was a product of chronic irritation[28,29]; Lobstein and Recamier, and later Cohnheim hypothesized that cancer was the result of displaced embryonal tissue[28,29]; others surmised that cancer was caused by an infectious (or parasitic) agent[27,28,30]. Numerous researchers attempted to induce cancer in experimental animals, based on one of these theories. However, experiments to produce tumors with irritating chemicals produced only benign growths[31]. Work to prove Cohnheim’s theory by transplanting embryonal or fetal tissue into adult hosts similarly failed to induce malignant growths[32]. A broad range of microbes were identified in cancerous growths. However attempts to extract the microbes and produce cancers, could not induce cancers reproducibly[28]. Experimental induction of cancer was considered to be important, because this was expected facilitate the development of preventative measures and effective treatments[32].
In 1908, Ellermann and Bang[33,34] reported that a cell-free filtrate caused a leukemia in chickens, and Peyton Rous[35,36] reported that a cell-free filtrate produced a sarcoma in chickens shortly afterwards. However, work with chickens seemed to be irrelevant to humans, so efforts to produce experimental cancer based on the other theories continued unabated.
Jean Clunet designed an experiment which simulated the procedures of early radiologists, who developed radiation burns after prolonged exposures to X-rays[37,38]. He administered X-rays to four rats, at dosages sufficient to induce epidermal ulcerations, then allowed the lesions to heal for a few days, and repeated the exposure[37,38]. Cancer developed in one of two surviving rats at the site of ulceration. However, the experiment was not widely accepted as a success for three reasons: Only one rat developed cancer, the tumor resembled spontaneous tumors of rats, and other experimenters had difficulty reproducing the experiment[39].
Katsusaburo Yamagiwa was a young associate professor, whom the Japanese government considered to have good potential. They sent him to Virchow’s Institute in Germany, where he studied pathology from 1892-1894[26]. von Volkmann’s study of skin cancers among coal tar workers had become very well known by the time of Yamagiwa’s studies, with numerous other investigators reporting additional cases[40,41]. Yamagiwa was intrigued by these reports, so he devised to induce skin cancer in rabbits, by exposing them to conditions which resembled occupational exposure to coal tar. The ears of rabbit’s were not known to be susceptible to spontaneous cancers, so he decided to apply tar to their ears. He reasoned that, since previous attempts to induce experimental tumors produced only benign lesions which regressed, then he should continue to apply tar when the benign lesions emerged. He surmised that the application of tar to benign lesions could promote further changes, which would progress to malignancy[40,41].
When Yamagiwa returned to Japan, he applied tar to the ears of 137 rabbits, and repeated the application every two or three days. Seven rabbits eventually developed cancerous lesions. The average cancer developed after five months of tarring; some cancers only emerged after a year of tar application[40,41]. Yamagiwa recorded the occurrence of metastasis in two of the rabbits, which confirmed the malignant nature of the tumors, and the experiment became widely regarded as the first successful experimental induction of cancer.
Numerous experimenters attempted to replicate Yamagiwa’s experiment. Many endeavors to reproduce the experiment failed, which led investigators to decipher why Yamagiwa’s experiment was efficacious. It became evident that many previous experimenters failed because they did not continue their treatments for a sufficient duration. Woglom colorfully reflected in 1926, that Yamagiwa and Ichikawa were possessed of “infinite patience”, because they continued to apply tar for many months without evidence that cancers would develop[42]. Murray Shear[43] similarly conjectured that other investigators had terminated their experiments early, because they thought they were hopelessly “kicking a dead horse”. Another reason for failure was because an insusceptible species had been chosen, since it was not appreciated that most carcinogens are species specific[42]. A further reason for Yamagiwa’s success was that he chose to use many animals, because only seven of 137 rabbits developed cancer, and only two developed metastases.
1920-1950
Kennaway’s experimental work: During 1920-1950, additional evidence emerged that synthetic agents were the cause of human cancer. Ernest Kennaway[44] studied many modifications of Yamagiwa’s experiment. He was intrigued by observations that some fractions of coal tar induced cancer, while other fractions were ineffective. He suspected that this was due to an active component, which was present in only minute quantities, similar the vitamins in foods, or hormones in tissues which were first discovered during this period. An intense search was undertaken to identify “the cancer producing compound in coal-tar” under his direction[45,46]. Kennaway and Hieger[47] identified dibenz(a,h)anthracene in 1930 as the first pure chemical compound to induce cancer in experimental animals. This was followed by the isolation of benzo(a)pyrene as the major “cancer producing compound of coal-tar” in 1933[48]. Many other chemicals were identified that induced cancer in experimental animals during this period which are too numerous to describe, but were reviewed in 1947[49].
Occupational studies: A few additional reports of occupational carcinogens emerged during this period. During 1915-1929, young women in the United States were recruited to paint watch dials with a new florescent paint. The paint contained minute quantities of the newly discovered element named radium which illuminated the dials at night. The women ingested the isotope incidentally by pointing their paint brushes with their mouths. Shortly after they began the work, it was reported that many of them developed decreased levels of polymorphonuclear leukocytes and lymphocytes. A few years later, it was reported that numerous women were diagnosed with necrosis of the jaw bones which were frequently fatal[50]. After further follow-up, in 1929, it was recounted that the survivors commonly succumbed to osteosarcomas of the jaw bones[51,52]. X-rays, coal tar dyes and radium appeared to be astonishing discoveries when they were first introduced, but then horrendous diseases developed in those exposed to the new discoveries. The notion that synthetic agents were the cause of cancer was emerging.
During this period, the Report of the Chief Inspector of Factories and Workshops in England, described an increased incidence of nasal cancer among workers in a large nickel refining company in South Wales[53]. Machle and Gregorius reported that men in the United States, whose employment involved industrial exposure to the fumes of chromate, developed lung cancer at a 25 fold higher rate compared to workers in other industries[54].
1950-1980
Numerous notable occupational studies: A strong groundswell of interest in cancer began to emerge in the 1950s and the disease received more systematic study. Some carcinogens were reported which caused very high rates of cancer. Robert Case worked to identify which of the 100s of chemicals used in the dyestuffs industry in England and Wales caused the high rates of bladder cancer among dye workers. Case analyzed the various combinations of chemicals had been used by persons who developed bladder cancer, and after a meticulous analysis, he deduced that 30%-50% of the workers who had long term exposure to β-naphthylamine developed bladder cancer[55]. He also estimated that about 10% of workers exposed to benzidine developed bladder cancer[55].
Another study reported that seventeen percent of a subset of workers involved in the production of 4-aminobiphenyl, (a chemical that was used as an antioxidant in the rubber industry), developed bladder cancer[56]. Six of eighteen (30%) of workers exposed to bis(chloromethyl)ether, (an important intermediate in the synthesis of organic compounds), were reported to have developed lung cancer after only six years of exposure[57,58]. A similar compound, chloromethyl methyl ether, induced lung cancer in 14 of 91 (15%) of exposed workers[59,60]. Thirty three cases of the extremely rare mesothelioma were reported among the residents of the asbestos mining area of North Western Cape Province in South Africa[61]. Karin is a small village in the Anatolian region of Turkey, which has high deposits of erionite, an asbestos-like mineral, which occurs naturally near the surface of the earth in the region. Erionite is easily cut into large blocks which have been traditionally used for construction of homes and multiple other purposes in the region[62]. Eighty-two of 179 (45.8%) deaths in the village of Karain have been reported to be due to mesothelioma[63,64].
There were discoveries of additional occupational exposures which cause cancer which were also significant, though less striking. Three cases of a very rare cancer, angiosarcoma of the liver, occurred among workers employed in the manufacture of vinyl chloride[65]. A case series of workers occupationally exposed to benzene, reported a high rate of hemocytoblastic leukemia[66]. The cause of the high rates of lung cancer among miners had been a mystery. Arsenic and cobalt were each been suspected, but during this period, it was deduced that radon emitted from uranium was the principal cause[67]. A follow-up of the British veterans of the 1914-1918 war was published. The report estimated that the veterans who were exposed to mustard gas had a twofold increase in death due to cancers of the lung and pleura over the expected number[68]. Follow-up of the survivors of the Hiroshima and Nagasaki atomic bomb explosions of 1945 was published, and it was reported that they had increased rates of leukemia, as well as other types of cancer[69].
The cigarette smoking and lung cancer controversy: Sophisticated methods for statistical analysis were developed to detect additional causes of cancer during this period. By the mid-1940s, it was evident that the rate of lung cancer was increasing at epidemic proportions, but the cause was unknown. Austin Bradford Hill sought to study medicine, but was unable when he contracted tuberculosis, so he completed a BSc in economics by correspondence while convalescing. Following this, Professor Major Greenwood mentored him in statistical methods[70,71]. Hill developed an interest in formulating mathematical methods to discern the health effects of exposure to chemicals introduced since 1900. He became involved in a committee, which designed a comprehensive epidemiological study to attempt to decipher the cause of the increasing rates of lung cancer[72]. Hill hired Richard Doll, a young physician who preferred to work with mathematics over patients[72]. A case-control study was designed which would investigate the effects of a wide variety of exposures that were new to the 1950s, which included automobile exhaust, road tars, atmospheric pollution, and cigarette smoking[72]. Doll and Hill[72,73] were surprised when their analysis showed that only cigarette smoking was correlated with the incidence of lung cancer. Doll himself was a cigarette smoker, as the harmful effects of smoking were generally unsuspected[74]. They decided to follow up with a prospective trial, in order to test for possible undetected flaws of the case-control study. After 29 mo of follow-up, thirty five lung cancer deaths occurred among 24, 389 men. The highest proportion of lung cancer cases occurred among the heaviest smokers in both the case-control and prospective studies which was interpreted as confirming that smoking was the cause[75]. Numerous other studies of smoking and lung cancer were published both before and after Doll and Hill’s studies, which reported similar results. The studies were reviewed by the Royal College of Physicians of London in Great Britain in 1962[76], the United States Surgeon General in 1964[77] and more recently by Doll[78].
The studies of smoking and lung cancer were initially received with incredulity by the medical community. Firstly, because smoking was considered a benign activity, with some physicians recommending cigarette smoking because there were suggestions that smoking had various health benefits[74,79,80]. Secondly, the usefulness of mathematics to discern the cause of a disease, was a new discipline and not universally accepted[81-83]. The conventional approach to demonstrate disease causation was experimentation. The controversy concerning whether cigarette smoking causes lung cancer prompted extensive discussions of the criteria to determine whether exposure to an agent causes a disease[81,84], and was the impetus for Hill to develop a seminal list of principles to discern whether epidemiological associations are causal[85]. The smoking and lung cancer controversy contributed strongly to the establishment of modern cancer epidemiology[1].
Pharmaceutical studies: Beginning in the late 1960s, pharmaceuticals began to be frequently identified as carcinogens. Users of high doses of analgesic mixtures containing phenacetin were reported to develop high rates of carcinoma of the renal pelvis[86]. Organ transplant recipients, who used the immunosuppressant drug azathioprine, were reported to develop high rates of lymphomas[87]. Studies of women who took diethylstilboestrol during pregnancy revealed that their female children had high rates of the extremely rare adenocarcinoma of the vagina in adulthood[88]. Postmenopausal women, who used estrogen replacement therapy, were reported to have a high risk of developing endometrial cancer[89]. Four of 5 patients who received high cumulative doses (200 g or more) of chlornaphazine, a chemical related to β-naphthylamine for treatment of Hodgkin’s lymphoma, developed invasive bladder carcinomas[90,91].
Research concerning chemical warfare agents had shown that the sulphur and nitrogen mustards (β-chloroethyl sulphides and amines) exerted strong cytotoxic activity on rapidly proliferating tissue, especially lymphoid tissue, bone marrow and epithelium of the gastrointestinal tract[92]. Subsequently, numerous analogues were developed for use as therapeutic agents for the treatment of cancer. Melphalan[93], busulfan[94], and cyclophosphamide[95] were each shown to be associated with increased rates of acute nonlymphocytic leukemia (ANLL).
Medical radioisotopes were also found to be associated with high rates of cancer. Patients suffering polycythaemia vera were treated with radioactive phosphorus (32PO4) during this period. They developed high rates of leukemia[96]. Patients who were administered a contrast medium which contained radioactive thorium, for imaging purposes, developed high rates of the rare angiosarcoma of the liver[97,98]. The use of thorium was curiously preceded, by numerous cautions predicting its probable carcinogenic effects due to its radioactivity[99,100].
1980-present
Sophisticated occupational studies: The most obvious agents were already identified in previous periods, so many studies published after 1980 were frequently re-examinations of previously tested agents. Cogliano et al[101] described how the IARC relaxed the criteria which are required to classify agents as carcinogenic during this period. They recounted how agents which lacked epidemiologic evidence of carcinogenicity were permitted to be classified as group 1 carcinogens based only on mechanistic evidence. The author observed that epidemiological studies generally became larger, employed more sophisticated methods of analysis and frequently only reported modest increases.
Ortho-toluidine is an aromatic amine which belongs to the same class of chemicals as β-naphthylamine. It is used in the synthesis of dyes, herbicides, synthetic rubber and other chemicals[102]. Ward et al[103] reported that six of 73 workers exposed to ortho-toluidine for over ten years in a synthetic rubber manufacturing plant developed bladder cancer which yielded a standardized incidence ratio of 27.2.
A study of workers in Vermont granite manufacturing plants, reported that exposure to silica dust was associated with increased rates of lung cancer. A standardized mortality ratio (SMR) of 1.81 was reported for workers exposed to silica dust for over 30 years[104]. Exposure to diesel exhaust was also reported to be associated with a higher risk of lung cancer. A cohort of 54973 United States railway workers who were exposed to diesel exhaust for 24.8 years were estimated to have a relative risk of lung cancer of 1.40 based on 4351 lung cancer deaths[105].
Sulfuric acid has been used to remove oxides from the surfaces of steel in preparation for painting and other coating processes. Exposures to mists of sulfuric acid have been found to cause laryngeal cancer. A study of 879 steelworkers who were exposed to mists of sulfuric acid for a mean of 9.5 years reported a standardized incidence ratio of 2.30 for laryngeal cancer based on nine cases[106].
Exposure to formaldehyde was determined to cause nasopharyngeal carcinoma (NPC) during this period. Eight of 25619 workers succumbed to nasopharyngeal cancer after exposure to formaldehyde for a median of 35 years. A SMR of 2.10 was estimated from this study[107].
1,3-butadiene is a chemical used in the production of synthetic rubbers and polymers, which has been ascertained to cause non-Hodgkin’s lymphoma. Four of three hundred and sixty-four men involved in 1,3-butadiene production died of non-Hodgkin’s lymphomas for a SMR of 5.77[108,109].
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), is a large complex molecule which occurs as a by-product of some industrial chemical reactions, which has received much media attention as a probable carcinogen. Fingerhut et al[110] conducted a study of mortality among twelve plants in the United States, which produced chemicals contaminated with TCDD. They reported a slight increase in all cancers combined, though the increase was limited to workers with the heaviest exposures to TCDD[110].
Trichloroethylene is a solvent which has been used in the dry cleaning industry and for degreasing metal machine parts that has been determined to cause renal cell cancer. A case-control study from France reported an odds ratio of 1.96 for the development renal cell carcinoma among workers with high cumulative exposure, after adjusting for various factors[111].
Ethylene oxide is used mainly as a disinfectant and sterilizing agent in medical facilities for the manufacture of sterile disposable items, which has been determined to cause lymphatic and haematopoietic cancers. The IARC examined the epidemiological evidence concerning exposure to ethylene oxide, and concluded that mortality from lymphatic and haematopoietic cancers were “only marginally elevated”[112].
Beryllium is a metal with a high strength and excellent electrical conductivity which is commonly used in electronics, which has been concluded to cause lung cancer. A study of 689 patients with beryllium lung disease estimated a SMR of 2.0 for the mortality due to lung cancer based on 28 deaths[113]. The IARC has concluded that beryllium is a group 1 carcinogen[114], though various investigators have challenged the conclusion[115].
4,4’-Methylenebis(2-chlorobenzenamine) (MOCA) is a chemical used in the synthesis of some polyurethane products which has been designated as a bladder carcinogen. Screening of 540 workers exposed to MOCA detected two noninvasive papillary tumors of the bladder[116]. The IARC designated MOCA as carcinogenic to humans based on mechanistic rationale[117].
Some of the early nuclear industry personnel in Russia were exposed to high levels of plutonium. However, the reports were originally published in Russian in “classified” reports or journals, which were unavailable to western scientists. The 1990s witnessed a relaxation of this secrecy, and it was reported that workers exposed to high levels of plutonium developed high rates of lung, liver and bone cancers[118,119]. The Chernobyl accident in 1986 resulted in release of iodine-131 into the atmosphere, which was followed by increased rates of childhood thyroid cancers in the nearby areas of Belarus, Russian Federation and the Ukraine[120].
Additional pharmaceutical studies: Additional pharmaceuticals were identified as carcinogens. Patients who had been treated with MOPP (nitrogen mustard, vincristine, procarbazine, and prednisone) for Hodgkin’s disease, were reported to have an increase in ANLL[121]. A randomized trial of polycythemia vera patients reported a thirteen fold increase in ANLL among those receiving the nitrogen mustard drug chlorambucil, which was strongly related to the dosage[122]. A dose-response relationship was reported between the use of the nitrosourea drug semustine and the occurrence of ANLL[123,124]. Patients who were treated with cyclosporine immunosuppressive therapy, developed increased frequencies of lymphoma, Kaposi’s sarcoma and skin cancer[125]. Patients who used tamoxifen were reported to have an increased risk of endometrial cancer[126], but to have a reduced risk of recurrence of breast cancer[127].
Etoposide is a semi-synthetic derivative of an extract of the roots and rhizomes of species of the genus Podophyllin[128]. Ratain et al[129] reported that 4 of 21 lung cancer patients who survived longer than one year, of a chemotherapy regimen which included etoposide, developed ANLL. Thiotepa and treosulfan are alkylating agents used in chemotherapy which have each been shown to increase the risk of various types of leukemia[130-132].
The use of combined (estrogen/progestin) oral contraceptives was reported to be associated with decreases in ovarian and endometrial cancers[133,134]. Epidemiological studies concerning the association between oral contraceptive use and breast cancer produced inconsistent results which were difficult to interpret. Some studies reported higher rates, others reported lower rates, and still others reported no effect. A reanalysis of 53297 women with breast cancer and 100239 controls from 54 epidemiological studies reported a relative risk of 1.24 (95%CI: 1.15-1.33) among current users, and a relative risk of 1.07 (95%CI: 1.02-1.3) among previous users who discontinued usage for 5-9 years[135].
Emerging studies of possible new synthetic carcinogens
Having considered the past and present advancements in our understanding of the causes of cancer, it is interesting to consider a few promising areas which are presently under investigation. The effect of prenatal exposures on the rates of cancers in both childhood and also in adulthood is generating interest. The concept of prenatal exposure has been well demonstrated in principle. Prenatal exposure to diethylstilboestrol has been demonstrated, to cause the rare adenocarcinoma of the vagina in the female offspring of mothers who used the drug during pregnancy[88]. A considerable amount of literature indicates that many chemicals can have biological effects at extremely low levels, at levels far below those currently recognized by government regulatory agencies such as the United States Environmental Protection Agency (EPA)[136-138]. Evidence is emerging that in utero exposure to xenoestrogens causes reproductive cancers. Bisphenol A (BPA) is a xenoestrogen which has been widely used in the manufacture of polycarbonate plastics, which are used as food storage containers, and epoxy resins which are used to line food and beverage cans[139,140]. BPA has been shown to leach into food products at very low levels[141,142], and to accumulate in the amniotic fluid of pregnant women[143]. When BPA was administered to pregnant nonhuman primates at levels similar to human exposure levels, the histology of the mammary glands of the newborn females was altered[144]. Rats which received in utero exposure of BPA, at 1/20000 the level currently estimated by the EPA as the lowest observable adverse effects level, developed mammary gland ductal hyperplasia and carcinoma in situ[145]. Male rat fetuses which were exposed to low levels of BPA displayed an increased propensity to develop prostatic intraepithelial neoplasias[146]. It will be interesting to follow the research concerning in utero exposure to BPA, as well as other xenoestrogens as possible contributors to the high rates of breast and prostate cancers in developed countries[147-149].
A very recent review has highlighted the fact that most research has analyzed the effects of single agents, while human exposures involve complex combinations of agents, and that combinations of agents may have synergistic effects. They encourage researchers to investigate the effects of low doses of combinations of chemicals[150].
VIRAL
Early experimental studies
Belief in a contagious cause of cancer began in classical times, when it was hypothesized that a single infectious organism caused every kind of cancer. A tremendous amount of work was performed searching for a contagious cause, but nothing was found which could be confirmed[151-153]. In the late 1800s Pasteur and Koch demonstrated the contagious origin of many diseases (see Figure 1)[9,11,12,16,17,35,36,40,41,51,67,73,137,154-174] which prompted many more searches for a microbial cause of cancer[151-153]. In 1900, the first human virus was identified, with the isolation of the yellow fever virus[159,160]. Shortly afterwards, Borrel[175,176] hypothesized that cancer had a viral etiology. Following this, Vilhelm Ellermann and Oluf Bang[33,34,177] reported that a cell-free filtrate induced leukemia in chickens, and Peyton Rous[35,36] reported that another cell-free filtrate caused a sarcoma in chickens. However, these early reports kindled little interest, because many orthodox pathologists did not accept the notion that leukemia was a form of cancer. Furthermore, the chicken seemed to be too different from humans for the work to be relevant[153,178].
Figure 1.
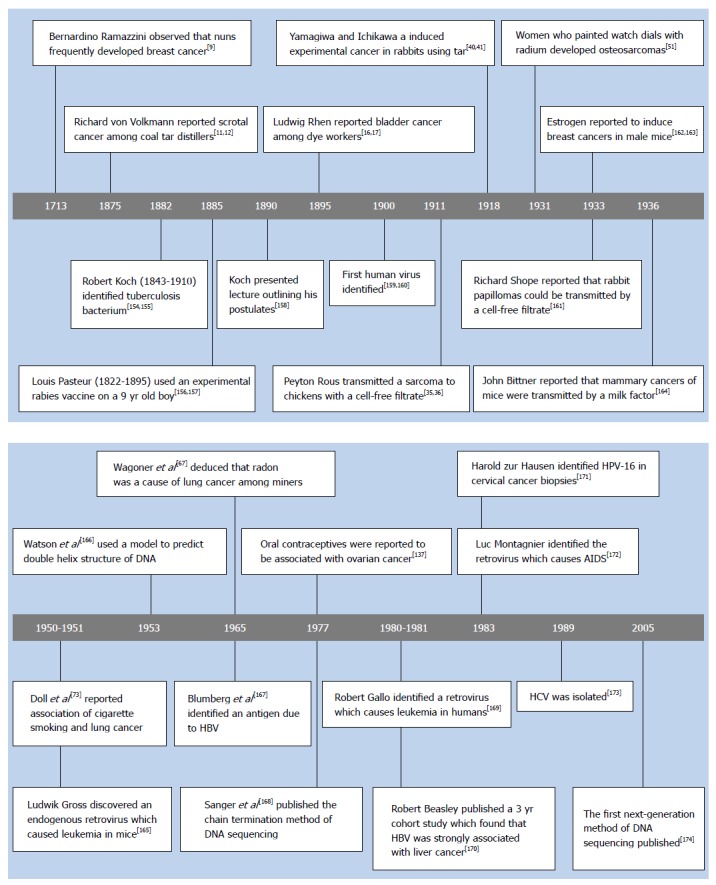
Some milestone publications concerning the causes of cancer, microbiology, and technology.
A well studied observer reflected in 1903[27] that the number of reports which claimed to have identified a microbial cause of cancer peaked in 1887, and then steadily decreased each year thereafter. Interest faded when none of the results could be confirmed[28,179]. The search for an infectious cause of cancer became so intense, and extended for so long, that it eventually “led to wide acceptance of the dogma that cancer did not, and could not have an infectious agent as its cause”[153,180]. An infectious cause came to be considered as ruled out by most well respected investigators when the first studies of chicken cancers were published[181-183]. Furthermore, numerous reports had emerged that a variety of industrial exposures, such as chimney soot, coal tar, dye chemicals, and X-rays caused cancer in humans when these studies emerged, which further eroded interest in the virus studies.
1920-1950
Additional experimental studies: Rous worked with the chicken virus for a decade, and then became discouraged so he abandoned the work[180]. Following this, William E Gye published an independent investigation of the virus, which incited fierce controversies[184-186]. He argued that the work was an embarrassment to both sides. Those in favour of the parasitic theory found little support in the work, because it was not evident how the agent could provide a unifying concept for the tremendous variety of tumors which occur in so many different species[184,186]. The virus only induced one type of cancer in one species. Those opposed to the parasitic theory argued that the Rous sarcoma virus (RSV) was purifiable by chemical methods which no living organism could survive[186]. Other opponents argued that the sarcoma was a disease peculiar to chickens, not really a cancer, and therefore not relevant[184,185].
Methods to successfully transplant cancerous tissue were developed in the late 1800s. Carol O Jensen and Leo Leob independently produced a considerable amount of research on the nature of transplanted cancers by 1903[187]. They each searched for evidence to prove/disprove the infectious cause of cancer theory. Jensen reported that cancers which were transplanted as whole cells frequently survived in a new host, but when cancer cells were crushed in a mortar, the inoculation was never able to produce cancers in the recipients[188,189]. This seemed to be definitive evidence that the transplanted cancers were not the product of transmission of an infection.
The first crude electron microscope (EM) was only developed in 1933[190], and the RSV was not visualized until 1947[191]. The EM only became consistently reliable for the examination of biological specimens in the 1950s[192]. Hesitation to accept the discoveries of viral induced cancers in chickens is understandable.
A few additional viruses were identified which induced cancer during this period. Shope demonstrated that rabbit papillomas could be transmitted with a cell-free filtrate in 1933[161]. Bittner reported that mammary carcinomas of mice could be induced by an infectious agent transmitted in the milk in 1936[164]. Lucke reported in 1938 that a virus caused renal adenocarcinoma in leopard frogs[193]. The reports of Shope and Bittner were the first evidence that viruses could induce tumors in mammals, but interest in synthetic industrial agents as causes of cancer had become overwhelmingly convincing.
1950-1980
A golden era for animal experimental studies: Endogenous viruses were discovered in the 1950s. Mice had been systematically bred for genetic inheritances of high and low frequencies of leukemia in the 1920s and 1930s[194]. In 1951 Gross[165] reported that a cell-free extract from the high frequency Ak strain, induced leukemia in the low frequency C3H strain. In related works, normal chicken embryos were reported to contain an antigen, which was indistinguishable from an antigen of a virus which caused leukemia in chickens in 1967[195]. There was an intriguing report, that either ionizing radiation or chemical carcinogens could induce the replication and spontaneous release of an avian leukemia virus from normal uninfected cells in 1971[196], and another report indicated that the murine leukemia virus could be similarly induced[197,198]. These curious reports became comprehensible when it was found in 1972, that retroviruses frequently integrate into the host cell DNA[199]. Selective breeding for high rates of cancer using the principles of genetics had unintentionally selected for latent endogenous retroviruses which were transmitted vertically[200].
In 1964 Jarrett et al[201,202] discovered a retrovirus which caused lymphomas in common domestic cats, which has been estimated to account for as many as 70% of the lymphomas occurring in domestic cats[203]. During this period, virus particles were detected in cultured human Burkitt’s lymphoma cells[204]. An antigen was discovered in a hemophiliac patient, which would later be found to be produced by the hepatitis B virus (HBV)[167]. Breast milk of women who were at high risk of developing breast cancer, was reported to contain particles which were morphologically similar to the MMTV, and to have reverse transcriptase activity[205].
The virus cancer program: The discovery of the mouse leukemia virus[165], along the chicken leukemia virus[33,34], and the feline lymphoma virus[201,202], made it seem reasonable that similar agents could induce leukemia in other species[206]. There were many other discoveries leading up to, and during this period, and additional reasons which fuelled interest in a possible role of viruses in human cancers[7,207]. Some were predicting that viruses would be found to have a universal role in all cancers, including those induced by radiation and chemicals. Others advocated a more bridled enthusiasm. They accepted that viruses were involved in some cancers, but they did not accept the hypothesis that viruses were involved in all cancers[207].
In 1968 the Congress of the United States considered the evidence that viruses induce cancers in animals to be overwhelming. They expected the viruses responsible for human cancers to be found soon, so they began investing ten million dollars a year in the virus cancer program at the National Cancer Institute[208,209]. Much work focussed on searching for human oncogenic retroviruses, analogous to the endogenous retroviruses which were found in animals. A decade was spent searching for the suspected viruses, but very few were found, and those which were found could not be grown in culture. Adding further to the disillusionment, some agents were eventually shown to be contaminants[200-211], while other agents could not withstand the increasingly sophisticated methods of analysis which were being developed[212,213]. The notion became widespread again that human cancers were not caused by viruses. The mood became analogous to the scepticism of the early 1900s[214]. Suspected cancer causing viruses had previously been frequently termed “tumor viruses”. Naysayers became so confident that a virus would never be found which caused human cancer, that they mockingly called them “rumor viruses”[212,215].
1980-present
Major advances in the establishment of the roles of viruses in human cancer occurred during this period for numerous reasons. Laboratory methods were improved which enabled the analysis of much lower levels of viruses. The epidemiological methods which had been developed to evaluate the association of tobacco smoking with lung cancer were revised for analysis of infectious agents with other types of cancer. However each virus presented unique difficulties, as described below.
Human T-cell leukemia virus: Robert Gallo’s only sibling was diagnosed with leukemia when she was only five years of age, and Robert was eleven. Influenced by his sister’s physician, Gallo enrolled as a medical student in 1960, and studied the biochemistry of blood cells[216]. In 1970, it was discovered that RNA viruses replicate using the reverse transcriptase enzyme[217,218], and Gallo decided to work developing enzyme assays to detect viral reverse transcriptase, as a more sensitive method of detecting possible cancer causing viruses[219] (The EM had become the gold standard, but it was tedious and time consuming).
Gallo et al[219] were determined to search for a retroviral cause of human leukemia when other researchers were abandoning the search. They were provoked by the discoveries of a retrovirus which caused leukemia in nonhuman primates[220], and another retrovirus which caused leukemia in cattle[221]. The discovery of the bovine leukemia retrovirus intrigued them because it induced leukemia with only very low levels of replication. They reasoned that human leukemia could have similarly low levels of replication, and therefore be difficult to isolate[210].
They eventually found a retrovirus in a T-cell line established from a patient with a cutaneous T-cell lymphoma, which they termed the human T-cell leukemia retrovirus (HTLV-1) in 1980[169,210]. Hinuma et al[222] reported in 1982 that 140 of 142 patients with adult T-cell leukemia (ATL) were positive for the virus from certain areas of Japan. Southern blot analysis showed that all cases contained the virus, and that it was integrated into the host genome in monoclonal form, indicating that infection and integration occurred before clonal expansion of the tumor cells[223]. The discovery of HTLV-1 has been reviewed[210,224,225].
The virus causes only a particular type of leukemia, which is common in some areas of Japan, but it is rare in the United States. Parkin et al[226] estimated that the global fraction of leukemia due to HTLV-1 is only about 1%, but the principle that viruses could cause human cancer, was finally proven. Convincing evidence that a retrovirus causes common forms of leukemia has still not emerged, though evidence concerning other infectious agents continues to be intriguing[227].
The mechanism by which HTLV-1 causes leukemia is only partially understood. The virus integrates into the host genome as a component of its regular replication cycle. It is found as a single integrated provirus in around 80% of ATL patients, with the remaining ATL patients displaying either two integration sites or multiple clones[228]. The virus codes for a protein termed Tax, which has multiple effects which are predicted to induce transformation[229]. However, it is perplexing that about 60% of patients with ATL have lost expression of the tax gene[230], so investigators have been searching for another mechanism to account for how HTLV-1 causes ATL. It has been reported that the HTLV-1 basic leucine zipper factor (HBZ) gene is transcribed in all cases of ATL[231]. HBZ RNA promotes T cell proliferation[231], and transgenic mice which express the HBZ gene in CD4+ cells develop lymphomas[232]. For recent reviews of HTLV-1 replication and ATL see[233,234].
Human immunodeficiency virus: In the summer of 1981, the first reports of a mysterious new syndrome emerged. Young male homosexuals in New York City and California were reported to have phenomenally high rates of Pneumocystis carinii pneumonia[235], and unprecedented rates of Kaposi’s sarcoma (KS)[236].
The etiology of the acquired immunodeficiency syndrome (AIDS) perplexed the medical community and many bizarre theories were suggested. Max Essex suggested to Gallo that some feline leukemia retrovirus variants caused immunosuppressive syndromes[224,237]. Gallo reasoned that HTLV sometimes caused immunosuppression, and that the geographic distribution of HTLV across Africa resembled the distribution of AIDS in Africa[238]. He wondered if AIDS could be caused by a retrovirus closely related to HTLV. He decided to abandon the search for retroviral causes of human leukemia/lymphoma, in order to investigate whether the cause of AIDS was a retrovirus, and possibly intercept an impending worldwide epidemic[215]. Luc Montagnier et al[239] at the Pasteur institute in France had also been searching for oncogenic human retroviruses, when the AIDS epidemic emerged. Inspired by Gallo’s theory, they decided to redirect their resources to search for a retroviral cause of AIDS as well. Using a reverse transcriptase assay Luc Montagnier and Francois Barre-Sinoussi reported identification of a retrovirus in the lymph node biopsy of a patient in the early stages of AIDS in May of 1983[172].
Montagnier et al[240] reported detection of antibodies to the retrovirus in the serum of 60% of patients with the pre-AIDS lymphadenopathy syndrome, but in only 20% of patients with AIDS, so the significance of their discovery was initially uncertain. Following this, Sarngadharan et al[241] reported that they had developed an improved method to detect antibodies to the virus, and that they found around 90% of cases with AIDS were seropositive in May of 1984. This was followed a few months later by two independent reports of detection of antibodies to the virus in around 90% of patients with AIDS[242,243], which confirmed the virus as the cause of AIDS. Shaw et al[224] also developed a cell line capable of yielding sufficient quantity of the virus to be studied and they produced a commercial blood test for the virus in May of 1984[244]. Montagnier et al[245] reported development of a comparable method of production of the virus in July of 1984, and a reliable method to detect antibodies in Oct of 1984[246]. Of course the virus was later termed the human immunodeficiency virus (HIV).
Luc Montagnier and Francoise Barre-Sinoussi received the prestigious Nobel Prize for their identification of the virus in 2008 (http://nobelprize.org). A succinct review of the chronology of events concerning the discovery of HIV has been published[247]. Gallo and Montagnier have both written detailed accounts of their personal reflections of this period[248,249]. Robin Weiss has also written a concise informative review of the discovery of HIV[250].
Around 50% of patients diagnosed with AIDS, were also diagnosed with KS at the time of their AIDS diagnosis in the early 1980s[251,252], which would seem to be unequivocal proof that HIV causes KS. However, KS cells were found to be curiously not infected with HIV[253]. Large trials also demonstrated that non-Hodgkin’s lymphoma occurred in around 4% of patients with AIDS at the time of their diagnosis, and is the second most common cancer among AIDS patients[254]. However, B cells of AIDS related lymphomas have also been reported to be devoid of the virus[255,256].
The mechanism of how HIV causes cancers is not straightforward. The virus infects T cells and macrophages which release an HIV encoded protein known as Tat, which is taken up by other cell types in the microenvironment. Tat has numerous effects which are also predicted to promote angiogenesis and carcinogenesis[257-259]. It appears that immunosuppression caused by HIV is a potent cofactor in KS and lymphomas, rather than a cause of these cancers, since they also occur at increased rates in transplant patients [Also see the discussion of human herpes virus 8 (HHV-8) below for additional discussion of KS].
The IARC concluded in 2012 that HIV causes not only Kaposi sarcoma and non-Hodgkin’s lymphoma, but also Hodgkin’s lymphoma, and cancers of the cervix, anus, and conjunctiva[260].
HBV: Baruch Blumberg was formally educated as a physician in the United States, and relates that his education left him “woefully ignorant about viruses”[261,262]. In the early 1960s he was searching for genetic polymorphisms which could account for differences among individuals in their susceptibility to diverse diseases. Harvey Alter was assisting him in this, when they found a precipitin in a serum which they were hoping was caused by a genetic polymorphism[167,263]. Following this, Blumberg became intensely interested in studying every detail about the precipitin. After many months of study, they noticed that a couple of patients who were negative for the antigen became positive, and that the patients coincidentally developed hepatitis[264-266]. Blumberg collaborated with some experienced electron microscopists and reported visualization of small particles in the serum of the patients which were later identified as the surface antigen of HBV[267].
It was not evident that HBV caused hepatocellular carcinoma (HCC) when it was discovered. The geographical correlation between the distributions of the rates of HBV infection and HCC became recognized a decade after the discovery of the antigen[268]. However, aflatoxin had been previously demonstrated to induce liver cancers in rodents[269] and it was proven to cause liver cancers in primates as well[270], so aflatoxin seemed more likely to be the cause of HCC in humans. Furthermore, serum antigens of the virus were not reproducibly detectable at the time of diagnosis of HCC using the methods that were available in the 1970s[271,272]. Moreover, viral antigens were only present at much reduced levels in the tumor tissue compared to the surrounding normal liver tisue[273-275]. In spite of this evidence, Robert Beasley was determined to definitively test whether HBV caused HCC. He decided to study a healthy population without HCC, and to test them for serum HBV antigens using newly developed and highly sensitive radioimmunoassay techniques. He devised to follow subjects prospectively to determine which individuals developed HCC. It was difficult to get funding for a large study when the evidence seemed to indicate that aflatoxin was the cause of liver cancer. He recruited 22707 healthy male government employees in Taiwan, and tested them for a variety of indicators of HBV infection[170]. After three years of follow-up, forty one deaths due to HCC occurred. Only 15% of the employees were seropositive for hepatitis B surface antigen (HBsAg), but forty of the 41 men who developed HCC were seropositive for the antigen. The relative risk of HCC among men who were seropositive for HBsAg was calculated to be 223 (95%CI: 28-1479)[170]. This was evidence which could not be dismissed[276]. Vaccination of Taiwanese children has resulted in a pronounced decrease in the incidence of childhood HCC[277,278], which is confirmation that HBV causes HCC.
While epidemiological studies have provided convincing evidence that HBV causes HCC, investigations of the mechanisms of how it causes cancer have been less straightforward. A variety of mechanisms have been proposed, which can be divided into three general categories; viral proteins, inflammation and genetic instability. The virus produces oncogenic proteins; HBsAg and HBx have received the most intensive investigations. Mice which are transgenic for HBsAg or HBx have been reported to develop liver cancers[279,280]. The second general mechanism involves the immune response to the virus which results in a state of chronic inflammation, which produces cirrhosis that deteriorates into HCC[281,282]. The third general mechanism involves the integration of the virus into the host genome, which generates genetic instability. The normal replication cycle of HBV does not include integration into the host cell genome. However, the virus has been found integrated into the host cell genome in around 80% of HCC cases associated with HBV[283]. The integrated virus is fragmented and rearranged, so that it cannot produce infectious particles[284-286]. However, the integrated virus is attributed to induce chromosomal instability of the host cell[287]. Each of these mechanisms likely causes a few cases of HCC, with most cases involving two or all three of the above mechanisms. Cases of HCC which develop without cirrhosis are caused by viral integration and viral proteins. Cases which develop without viral integration are caused by inflammation/cirrhosis and viral proteins.
Human papillomavirus: Harold zur Hausen was intrigued by the fact that epidemiological evidence indicated that the risk of cervical cancer was correlated with sexual promiscuity, which suggested an infectious cause[288,289]. Herpes simplex virus type 2 seemed like a plausible agent, so he analyzed cervical cancer biopsies for the virus. However, the samples were found to be devoid of the virus, so he decided to investigate papillomaviruses[289]. The early methods of analysis for human papillomavirus (HPV) were crude and did not produce convincing evidence of a causal role. In the mid-1970s recombinant DNA technology was developed, and zur Hausen’s group used the technology to sequence the virus. They discovered that the HPV exists as a variety of different types[290,291]. They identified HPV-16 and HPV-18 in cervical cancer biopsies[171,292]. Following this, the early epidemiological studies still did not consistently identify HPV DNA in cervical cancers, because the methods of analysis of HPV DNA varied substantially in sensitivity and specificity[293,294]. As the sensitivity of the techniques increased, HPV DNA became detectable in 97%-98% of cervical cancer biopsies[295,296].
HPV-16 and HPV-18 produce a number of proteins (E5, E6, and E7) which have various oncogenic activities[297,298]. E6 induces chromosomal instability by binding to the tumor suppressor protein p53, interfering with its normal function and inducing its degradation[299]. E7 also induces chromosomal instability by interfering with the normal functioning of the retinoblastoma family of proteins and inducing their degradation[300,301]. HPV replicates as an episome, in a cycle that does not involve integration into the host’s cell genome[302]. However, the virus has been frequently found integrated into the host cell’s genome. It is not known precisely how the virus becomes integrated, but it likely results when DNA breaks occur, which are likely promoted by episomal E6 and E7[303,304]. The integrated virus is found in a truncated form, though E6 and E7 usually remain intact, and continue to be transcribed. Numerous reports have found that E6 and E7 are transcribed at higher levels when it is integrated into the host genome[305], and the proteins produced by the integrated virus may have increased stability[306]. Studies which have analyzed the frequencies of integration in premalignant and invasive cancers have consistently reported higher rates of integration among invasive cancers[307,308]. It has also been consistently reported that the infectious episomal form of the virus is detected less frequently in invasive cancers than in premalignant lesions[308].
Recent prospective cohort studies have reported strong associations among women in whom HPV was detected on multiple occasions, which is consistent with cervical cancer developing from persistent infections. Analysis of ten years of follow-up of a cohort in Copenhagen, reported that 13.6% (167/1229) of women who were positive for high risk HPV at enrolment developed high grade lesions (severe dysplasia or carcinoma in situ), while 20.0% (83/414) of women who tested positive at enrolment and tested positive again two years later developed high grade lesions[309].
Confirmation of the causative role of HPV in cervical cancer is expected to emerge, with the successful clinical trials of vaccines for HPV. Vaccine efficacy for a bivalent vaccine (HPV 16, 18) was tested in over 10000 HPV naïve women who were followed for 4 years. The efficacy was 64.9% (95%CI: 52.7-74.2) for protection against CIN2 irrespective of HPV DNA in the lesion, and 93.2% (95%CI: 78.9-98.7) for prevention of CIN3 irrespective of HPV DNA in the lesion. Seven cases of adenocarcinoma in situ occurred in the unvaccinated controls, whereas zero cases occurred in the vaccinated women[310]. Results of the efficacy of HPV vaccination against cervical cancer are expected to emerge in 5-10 years[311].
Initial studies with HPV focussed on identification of the cause of cervical cancer. Further studies have shown that mucosotrophic HPV types also cause cancers of the vulva, vagina, penis, oropharynx, oral cavity, and tonsil[312,313]. Zur Hausen was awarded the Nobel Prize for his work with HPV in 2008 (http://nobelprize.org). Robin Weiss has written a concise reflection of zur Hausen’s work[250].
Hepatitis C virus: After the discoveries of HAV and HBV, it was found that most cases of transfusion associated hepatitis were caused by neither HAV nor HBV[314]. A non-A non-B (NANB) infectious agent was suspected of causing transfusion associated hepatitis. The NANB infectious agent was also suspected of causing HCC, based on case reports of transfusion recipients who developed chronic hepatitis, which progressed to cirrhosis, and then HCC[315,316]. However, the identification of hepatitis C virus (HCV) was excruciatingly difficult. The NANB hepatitis agent could be transmitted to chimpanzees; however efforts to isolate the virus using traditional methods, based on antigens and antibodies were without success for over fifteen years. Numerous tests were published, but none could be confirmed by independent laboratories[317,318]. The results were so perplexing and inconsistent, that it was speculated that the agent might only be present at very low levels[318], and/or the immune response could be very weak and therefore difficult to identify[318,319].
Finally, a massive systematic search was initiated by Michael Houghton et al[320] at the Chiron Corporation together with Daniel Bradley at the Centers for Disease Control. Their approach was to clone the agent before isolating it, using a “blind” immunoassay. The identification began in the early 1980s, and utilized the recombinant DNA techniques which had been recently developed. Michael Houghton et al[320] searched intensely for the elusive NANB hepatitis agent(s), using many different approaches to screen hundreds of millions of cDNA clones. After seven years, they identified the elusive agent, as a single stranded RNA molecule of about 10000 nucleotides, and termed it HCV[173]. Choo et al[321] have written a detailed review of the discovery of HCV.
After the identification of the clone, the development of an ELISA test for the detection of circulating antibodies was promptly published[322], and many reports of high rates of detection of antibodies to HCV in the serum of patients with HCC soon followed[323,324].
HCV is considered to cause HCC, though conclusive proof has been elusive. HCV induced HCC evolves through a progression of chronic hepatitis, to cirrhosis, to HCC which generally requires 20-30 years, or longer to develop, so prospective trials have been few. A meta-analysis of HCV positive cirrhotics reported that 17%-30% of patients developed HCC over five years of observation[325]. An eleven year prospective study followed 925 patients with antibodies to HCV. They reported a cumulative risk of HCC of 1.1% among those with undetectable HCV RNA levels and 14.7% among those with the high serum levels of HCV RNA[326].
Confirmation that HCV causes HCC has also been elusive. Treatment of HCV infection with α-interferon (or α-interferon plus ribavirin) results in decreased rates of HCC[327]. However, the demonstration that α-interferon reduces the incidence of HCC is complicated by the evidence that α-interferon has anti-carcinogenic effects independent of its antiviral effects[328-330]. A meta-analysis by Kimer et al[331] reported that the rate of HCC was reduced in both sustained virological responders [risk ratio (RR) = 0.15, 95%CI: 0.05-0.45] and also in non-responders (RR = 0.57, 95%CI: 0.37-0.85). The improved prognosis among non-responders is consistent with α-interferon having an anti-carcinogenic effect in addition to its anti-viral effect. Interferon-free treatment regimens are very recently becoming widely prescribed[332], so the long term effect of viral clearance by these treatments should provide a definitive confirmation of the causative role of HCV in HCC.
HCV has an interesting and complex life cycle. The virus replicates using an error prone polymerase which lacks proofreading activity. Consequently, HCV circulates in an individual as a heterogeneous population of sequences or quasispecies, which is very complex for the immune system to clear[333]. A systematic review estimated that 75% of individuals fail to eradicate the virus, because their immune response is only partially effective[334]. The principal mechanism by which HCV causes HCC is considered to be the chronic immune inflammatory response[335]. Around 40% of patients with chronic HCV develop cirrhosis after 30 years[336]. While HCC develops mostly among cirrhotics, it also develops at low rates among patients devoid of cirrhosis[337], which is interpreted as evidence that the virus may possess some directly carcinogenic effects. The virus replicates in the cytoplasm and does not integrate into the host genome as a component of its replication cycle or incidentally, unlike some of the viruses discussed above.
Epstein-barr virus
Burkitt’s lymphoma: Dr Burkitt was an English missionary physician posted in Uganda, when he became impressed by the unusually high incidence of a very aggressive jaw and abdominal tumor among the local children. He published an account of it in 1958, then returned home to England in 1961, and presented public lectures on his recent studies[338]. A young Tony Epstein was performing electron microscopy studies of tumor viruses during this period, when he happened to decide to sit in on a lecture of Dr Burkitt’s. Afterwards, Epstein requested Dr Burkitt to ship biopsy samples to him from Uganda[339], and a few years later Epstein et al[204] reported that they detected virus particles in cell cultures of the biopsy samples.
Henle et al[340] reported in 1969, that patients with BL had higher antibody titres to the Epstein-Barr virus (EBV) viral capsid antigen (VCA) than controls. This was followed by a seven year prospective study of 42000 Ugandan children, 14 of whom developed BL in 1978. This study found that high antibody titres to EBV VCA preceded the development of BL[341]. The virus was found to be present in monoclonal episomes in BL cells, which is consistent with the infection of a single normal cell occurring before the transformation and expansion of the tumor[342].
The above discussion has been limited to the endemic BL, which occurs principally in certain areas of Africa, and is strongly associated with malaria[343]. Malaria is discussed further in the natural factors section below. A sporadic form of the tumor occurs outside of Africa, in areas where malaria is rare, at much lower rates. The sporadic form of BL usually lacks detectable levels of EBV in the tumors. For detailed reviews of BL see the following references[344-346].
NPC: The incidence of NPC varies widely throughout the world; however EBV is consistently present in the undifferentiated form of NPC in various geographic regions[347,348]. High serum EBV antibodies have been found to precede the development of NPC[349], and to be informative in predicting the onset of NPC[350]. The virus is found in monoclonal episomes in every tumor cell, indicating that infection of the cells occurred prior to clonal expansion[342,351]. Administration of EBV-specific cytotoxic T-lymphocytes has induced remissions of cases of NPC which are refractory to conventional radiotherapy and chemotherapy[352,353].
Deciphering the mechanism of how EBV causes NPC has been complex. The virus has a dual tropism for B cells and epithelial cells. The life cycle is furthermore complicated by possessing a variety of different latency cycles as well as a lytic cycle[354]. The virus is present in NPC cells in a latent cycle. The virus produces a latent membrane protein 1 which has strong carcinogenic effects, but is only present at extremely low levels in most NPC biopsies[355]. NPC biopsies are characterized by high levels of untranslated EBV RNA which are termed EBER 1 and EBER 2[355]. NPC biopsies also have high levels of rightward transcripts derived from the BamHI A region (BARTs) of the EBV genome[356]. The BamHI A transcripts are multiple spliced which yield numerous microRNAs[357]. It is unclear precisely how EBERs and BamHI A transcripts might induce malignant changes, but in recent years evidence has emerged that untranslated RNA, especially microRNAs, can have strong effects on cellular metabolism[358]. The mechanisms whereby EBV could induce malignancy, have been recently reviewed[355,359].
EBV has also been demonstrated to cause non-Hodgkin lymphomas among immunosuppressed patients, and some cases of Hodgkin lymphoma[360]. While EBV was discovered before HBV, HCV, HPV-16, HPV-18, and HIV, work to prevent cancers caused by EBV has dawdled behind achievements to prevent cancers caused by other viruses. A commercial vaccine for EBV is only in the early stages of development[361].
HHV-8: The initial identification of HHV-8 consisted of only a few epidemiological observations at the Centers for Disease Control, which were followed up by two or three scientists with very limited laboratory experience, who worked for only around three months[362]. Using polymerase chain reaction-based technology, the causative agent was identified as a new herpes virus, with homology to both EBV and herpesvirus saimiri[363]. However, definitive evidence that it causes KS has been challenging.
HHV-8 DNA is present in 91%-96% of KS biopsies[364]. Prospective studies which followed patients with AIDS (or transplant recipients) reported that 20%-50% of persons with detectable levels of HHV-8 in their peripheral blood develop KS[365-368]. Two studies reported that low levels of HHV-8 specific T-cell responses are associated with a high risk of developing KS among immunosuppressed patients[369,370].
Studies of antiherpes drugs usage for cytomegalovirus retinitis and other cytomegalovirus induced disease among HIV patients provide additional evidence that HHV-8 causes KS. Three large studies conducted before the widespread use of HAART, reported decreases in the number of cases of KS among HIV patients who were treated with the antiherpes drugs ganciclovir or foscarnet for cytomegalovirus infections (Reviewed by Casper et al[371]).
Not everyone has agreed that HHV-8 has been established as the cause of KS, or that KS is a form of cancer. It has been argued that KS is polyclonal reactive process, rather than a true malignancy. Furthermore, studies concerning the clonality of the virus indicate that some are monoclonal, but most are oligoclonal[372]. Many objections to whether KS is a true cancer, and whether HHV-8 is the cause are settled if it is considered that most KS lesions are preneoplastic with a propensity to progress to cancer[373]. HHV-8 is also attributed to cause a rare cancer termed primary effusion lymphoma[374]. For recent reviews see the following[374,375].
Emerging studies of possible new viral carcinogens
Tremendous progress has been made in understanding the causes of liver and cervical cancers since the 1980s. However, our understanding of breast, prostate, and colon cancers has made relatively little progress. It is striking that the rates of breast cancer are so high, and that few if any, agents have been identified which strongly increase or decrease the risk. The author hypothesizes that an initiating event occurs in all females during fetal development, which renders human breast tissue susceptible to the promoting effects of estrogen and a diet high in protein and calories.
It was decided during the early drafts of writing this review, that discussions of genetics would be beyond the scope of this review. However, it is ambiguous whether some elements of the human genome should be considered as infectious or genetic. The International Human Genome Sequencing Consortium has reported that only around 1.5% of the human genome consists of protein coding sequences. Around 50% of the human genome consists of repeat sequences which have been traditionally dismissed as uninteresting “junk”. Most of the repeat regions are classified as transposable elements, which consist of; short interspersed elements, long interspersed elements (LINEs), long terminal repeat (LTR) retrotransposons, and DNA transposons[376]. These elements appear to be ancient with uncertain origins, though they have considerable homology with viruses[377]. The LTR retrotransposons resemble ancient retroviruses, and are commonly termed human endogenous retroviruses (HERV). Most HERV have multiple mutations and deletions which render them unable to replicate for general reviews see[212,378,379]. However, a few HERV copies appear to be complete, and sometimes produce viral proteins, though complete viral particles are only rarely produced[380]. Many women with breast cancer have increased expression of HERV in both their serum and cancers[381,382]. Melanoma biopsies have also been repeatedly reported to have high levels of expression of HERV[212,383].
LINEs are much more active than HERV. LINEs make copies of themselves which are inserted into random regions of the genome with deleterious effects[384]. Recent studies have reported a high frequency of novel insertions of LINEs in lung and colon cancer samples[385,386]. Transposable elements could cause cancer by mutagenesis and altering regulation of host genes[387,388].
LINEs and HERVs are silenced by epigenetic mechanisms, though they are frequently active during early development[389,390]. Do altered activation patterns of LINEs and HERVs during early development increase the risk of breast and other cancers?
NATURAL FACTORS AND NON-VIRAL INFECTIOUS AGENTS
When high rates of cancer were reported in a variety of occupations in the early 1900s, it seemed self-evident the cause of cancer was synthetic agents. Then research since the 1980s firmly established viruses as another important cause of cancer. In addition, there are a variety of other agents which cause cancer which the author has categorized as “natural factors and non-viral infectious agents”.
Hormones
Interest in the notion that natural factors cause cancer is generally dated to have commenced in the 1930s. Estrone was identified in the urine of pregnant women in 1929[391], which was followed by an extensive research effort which resulted in the elucidation of the structures of cholesterol, bile acids, and the sex hormones[392]. In the early 1930s, Lacassagne administered weekly injections of estrone to three castrated male mice, and reported that every mouse injected with estrone developed mammary adenocarcinoma[162,163]. The experiment drew widespread interest among scientists, because estrone is a natural hormone which is produced endogenously in females. The experiment particularly was striking because male mice are not susceptible to spontaneous mammary tumors, and the cancers developed in each of the treated mice. The renowned Alexander Haddow considered Lacassagne’s study to be the first report of a “natural” carcinogen[49]; though a variety of natural agents were recognized to cause cancer in animals by some well-studied cancer researchers when Lacassagne’s publication appeared[393].
Bernardino Ramazzini was an Italian physician and professor who observed that each occupation was characterized by a distinct pattern of diseases. Ramazzini observed that nuns have high rates of breast cancer in 1713[9], but the reasons for this have been complex to decipher. Lane-Claypon[394] performed a case-control study which concluded that women who had multiple childbirths were less likely to develop breast cancer[395]. MacMahon et al[396] performed an international collaborative study and concluded that a younger age at the first childbirth was associated with a decreased risk. Breast feeding was also suspected of decreasing the risk, but it was difficult to definitively decipher. An international collaborative group recently estimated that the risk of breast cancer decreases by 4.3% for every 12 mo of breast feeding[397]. There is strong evidence that all of the above factors affect the risk of breast cancer, however the interaction of these factors has not been deciphered, and there is only a consensus for breast feeding reducing the risk[398].
Ultraviolet radiation
Paul Unna was a general physician who took a strong interest in skin diseases[399]. He built a clinic in order to diagnose and treat patients with skin diseases, and published a textbook of dermatology. In the publication he described the histological changes which accompanied the clinical conditions which were encountered by dermatologists. He described a “diffuse cyanotic redness” which occurred on the face and hands of sailors after long term exposure to the “weather” which is implied to be due to ultraviolet light. He described both the clinical and histological changes as the lesions progressed to become cancerous. Following this, he compares the course of the sailor’s skin disease with xeroderma pigmentosum in adults who had been exposed to the sun and developed skin cancer[400,401].
Parasites
Schistosoma haematobium (S. haematobium) is a parasitic fluke, which is endemic in Africa and Southwestern Asia. It is spread through human skin contact with infected water[402]. Ferguson reported a case series of S. haematobium and bladder cancer in Egypt in 1911[403]. He documented that 40% of males over 5 years of age were infected with the parasite, and described 40 patients with bladder cancer with ova of the fluke present mostly in the portal vein, bladder or tumor. Opisthorchis viverrini (O. viverrini) is another parasitic fluke, which is contracted by eating raw fish. The infection rates are endemic in Thailand and other east Asian countries. O. viverrini was suspected of being a carcinogen by Stewart[404] in 1931; however the first case series did not appear until the report by Bhamarapravati and Viranuvatti[405] in 1966, who reported that the infection was associated with the bile duct cancer known as cholangiocarcinoma in Thailand[406].
Dr. Burkitt began working in a teaching hospital in Kampala Uganda in the mid-1950s, when he encountered a high number of children with very rapidly growing lymphomas. The lymphomas were frequently characterized by massive malignant growths that occurred in the abdomen and jaw areas. With funds of only £ 25 ($75), he prepared leaflets and photographs, which were sent to health care workers across Africa. The leaflets described the clinical features of the lymphoma, in order to survey the geographical distribution of the cancer. This was followed up with a 10000 mile safari, for which he described his research resources as: A photograph album illustrating the tumor; a second hand Ford stationwagon; the companionship of Dr. Clifford Nelson, “a Canadian doctor”; and Dr. Ted Williams “a mission doctor with a life-time of African experience and an expert car mechanic”[338]. The lymphoma rates were found to be high in a belt stretching from 10° north to 10° south of the equator, and rare at altitudes of over 5000 feet above sea level. This corresponded to the distribution of the areas which were holoendemic for malaria which is attributed to cause the lymphoma[343].
Fungus
In 1960, turkey poults in England were dying at high rates of a mysterious acute hepatic necrosis, which resulted in dramatic economic loses. A Brazilian groundnut component of the diet was identified as the cause of the illnesses[407]. Lancaster et al[269] reported that rats were peculiarly resistant to the acute necrosis, but after extended feeding they developed liver tumors without cirrhosis. Adamson et al[270] reported that the toxin induced liver tumors in primates as well. The carcinogenic factor was identified as aflatoxin B1 (AFB1), a toxin which is synthesized by the fungus Aspergillus flavus[408]. Following this, it was been difficult to ascertain whether aflatoxins are carcinogenic to humans. The rates of liver cancer are high in hot humid tropical regions of the world where aflatoxin contaminates stored foodstuffs. However, the prevalence of aflatoxin contamination is highly heterogeneous in areas of high contamination. It was necessary to determine the exposure of specific individuals, which was not performed in early studies. Furthermore, areas with high levels of aflatoxin contamination frequently have high rates of HBV infection, which also required consideration[409,410].
A study from Shanghai, China collected blood and urine samples from 18000 middle aged men and followed them prospectively for four years[411,412]. A nested case-control analysis of the cohort estimated a relative risk of 3.4 for men with urinary metabolites of aflatoxin only, a relative risk of 7.3 among men with seropositivity for HBsAg only, and a relative risk of 59 for men who had both urinary metabolites of aflatoxin and were seropositive for HBsAg. They concluded that aflatoxin is carcinogenic, and that there is a strong positive interaction between aflatoxin and HBV. Wu et al[413] have written a succinct review of the evidence that aflatoxin causes liver cancer.
Bacteria
Robin Warren was a pathologist at the Royal Perth Hospital who possessed an attention for detail. He was examining gastric biopsy specimens in 1979, when he thought he saw small spiral shaped bacteria growing on the surface[414,415]. Dogma stated that the stomach is sterile, so there was much opposition to the notion of viable bacteria growing in the stomach[414]. The editors at the Lancet wanted to publish the findings, but were delayed for months because no reviewer could be found who thought the work was credible[414,415]. The bacteria were reported to be present in 80% of patients with gastric ulcers and in 100% of patients with duodenal ulcers[416]. It was later found that helicobacter pylori (H. pylori) survives in the stomach by residing in the gastric mucosa which provides a partially effective physical barrier from the acidic pH and by synthesizing small amounts of ammonia which neutralizes hydrochloric acid[417,418]. H. pylori curiously have a trophy for healthy mucosal epithelium. Among patients with duodenal ulcers, the bacteria is interestingly present at considerably low frequencies in the ulcer crater and acutely inflamed edges, compared to the healthy gastric antrum where it is present at very high frequencies[419]. As gastric tissue progresses through atrophic gastritis to intestinal metaplasia and carcinoma, the bacterium is similarly detected at decreasing frequencies in metaplasias and carcinomas compared to the healthy gastric tissue[420-422].
Patients with gastric cancer have been reported to have a higher rate of seropositivity for H. pylori than normal persons. A meta-analysis of 19 studies (5 cohort and 14 case-control) reported a summary odds ratio of 1.92 (95%CI: 1.32-2.78) for the occurrence of gastric cancer among those who were seropositive for H. pylori[423]. The association was reported to be considerably stronger when the samples for serology were collected more than ten years before the diagnosis of cancer[424].
Wood dust, alcohol
Woodworkers in the furniture industry of the Oxford region of England were reported to have high rates of nasal adenocarcinoma[425]. The increased rates were observed mostly among persons exposed to hardwood rather than softwood dust[426]. Men who worked in trades which consumed high amounts of alcohol, such as brewers and inn keepers, were reported to have high rates of cancer of the oesophagus[427].
Food preparation technique
It was reported that the incidence of NPC was high in areas of Southern China, where the consumption of salted fish was high[428]. It was later found that the process of preparing salted fish in the high incidence areas is susceptible to bacterial contamination[429], and that nitrosamines are produced during the salting process[430,431]. Rats which were fed Cantonese style salted fish developed cancers of the nasal and paranasal regions[432,433]. Some studies have reported that the consumption of Cantonese-style salted fish during weaning is associated with a higher risk of development of NPC than consumption during adulthood[434].
Herb
A group of middle aged women were involved in a weight loss regimen in Belgium that involved consumption of a mixture of Chinese herbs which included the Aristolochia species. They developed high rates of renal fibrosis that frequently progressed to urothelial carcinomas of the renal pelvis and ureter after a very short latency[435]. In a series of ten patients who received renal transplants for nephropathy due to Aristolochia consumption, four were diagnosed with a multifocal high grade carcinoma in situ of the renal pelvis and ureter[435]. The exposure had lasted for an average of 20 mo, and the cancers were diagnosed after only 2-6 years after ceasing the regimen[435]. In another series, 17 of 39 patients with end stage nephropathy were diagnosed with upper urinary tract urothelial carcinomas[436]. Upper urinary tract urothelial carcinomas had been previously reported to occur at high rates in phenacetin analgesic abusers[86], and were characteristic of those living in the Balkans where aristolochic species contaminate wheat fields[437,438] and in Taiwan where consumption of aristolochic herbs is common[439,440]. Urothelial carcinomas of the upper urinary tract were rare in other regions of the world[441], so the imported herb was promptly identified as the cause.
WCRF/AICR analyses of diet, obesity, exercise, and supplements
The World Cancer Research Fund and the American Institute for Research on Cancer (WCRF/AICR) undertook comprehensive meta-analyses of the potential of foods, nutrition, and physical activity to prevent cancer (www.wcrf.org). Experimental work in the 1940s and 1950s demonstrated that caloric restriction dramatically reduced the incidence of most spontaneous and induced tumors of mice[442,443]. However it is difficult to accurately estimate caloric intake of humans on an individual level, and the epidemiologic studies yielded unclear results. Body weight is much more reliably measured, and is considered a reflection of total energy balance; the sum of energy intake and energy expenditure. Cancers of the oesophagus, kidney and endometrium have been consistently reported to be associated with overweight and obesity[444,445]. There is also convincing evidence that a sedentary lifestyle increases the risk of colon, and probably breast and endometrial cancers[446,447]. Consumption of red and processed meats has been estimated to cause around a 20% increase in risk of colorectal cancer with daily consumption of moderate amounts[448,449]. Increased adult height is also associated with an increased risk of colorectal cancer[450]. Consumption of a high fibre diet has been estimated to cause around a 10% decrease in risk of colorectal cancer[451].
There has been much interest in the potential of nutritional supplements to prevent cancer[452]. Epidemiological studies have shown that populations with higher serum levels of β carotene have reduced rates of lung cancer. However, investigators were confounded when a large randomized double-blind study reported that smokers who took β carotene supplements had higher rates of lung cancers compared to those who took a placebo[453]. Numerous studies confirmed that consumption of β carotene supplements increased the risk of lung cancer in smokers, though there is no evidence that β carotene supplements increase the rate among non-smokers[454].
The WCRF/AICR used the designation of “convincing” to indicate their highest level of certainty that an agent increases or decreases the risk of cancer[455]. The discussion above was limited to factors which received the designation of “convincing”. Consensus concerning the effect of diet and nutritional supplement usage is an evolving area. As new studies are published which include additional patients and are better designed, the WCRF/AIRC expects their consensus to further evolve. They concluded that there was “convincing” evidence that diets high in fruits and vegetables decreased the rates of a variety of cancers, based predominantly on evidence of case-control studies in a 1997 publication. However a number of cohort studies have been published since 1997 which have weakened the evidence, such that the evidence is no longer considered to be “convincing”, so it has been revised in subsequent publications[456,457]. There is good evidence that consumption of dairy products reduces the risk of colorectal cancer, however there is also fairly consistent evidence that consumption of dairy products increases the risk of prostate cancer[458]. This is considered to be a complex problem, so dairy products have not been designated. Recent analysis has concluded that consumption of coffee reduces the risk of liver cancer among men, though the evidence is designated as “probable” rather than “convincing”[459].
Natural verses synthetic
It is evident that numerous natural agents cause cancer in humans. Many natural components of foods have been reported to be carcinogenic to rodents[460,461]. A variety of toxins are well recognized to be produced by plants[462-464]. Bruce Ames et al[462] surveyed the natural chemicals which have been tested to cause cancer in rodents, and concluded that about half of those tested induced tumors[465]. Concerning the synthetic chemicals which have been tested, about half of them similarly induced tumors[462,465] (Brambilla et al[466] independently concluded that around 50% of pharmaceuticals cause cancer). Ames et al[462] concluded that natural chemicals are just as carcinogenic as synthetic chemicals.
It is frequently not obvious which substances are synthetic. Soot, coal tar, asbestos, erionite, arsenic, uranium, radon, and radium could each be considered natural because they occur naturally, though usually at very low levels. The author posits that the level of exposure is more important than whether a substance is natural or synthetic. The dose-response relationship is strongly supported by studies with both experimental animals and humans[467-469]. According to the dose-response relationship, when the exposure levels are low, the rates of cancers are low, and when the exposures are high, the rates of cancer are high. Whether the dose-response curve is linear is controversial, and the shape of the curve may change at low dosages, but the principle of increasing rates of cancer for increasing levels of exposure is well established for most exposures[138].
Recognition of soot as a carcinogen only emerged when young boys were exposed to high levels with the advent of chimney sweeping as an occupation. Radium occurs naturally in the mineral pitchblende. Its carcinogenic activity only became obvious when it was refined from pitchblende, ingested by watch dial painters, and then induced osteosarcomas of the jaw bones among dial painters. It could be argued that occupational levels of exposure are unnatural, though it is relevant to mention that a great number of occupational exposures are not associated with cancer[470].
Emerging studies of possible new natural carcinogens
The variety of natural agents which have been documented to cause cancer is considerable. While viruses have received an intense amount of research for their possible roles in cancer, bacteria have received considerably less. The colon contains 1011-1012 bacteria per gram of fecal material[471]. The recent development of high-throughput technologies is permitting detailed analysis of the microbial composition of the feces as well as the mucosa of the colon[472,473]. Interesting associations of certain bacterial species with colorectal cancers have been reported[474-476]. The microbiota of the colon can be modified with antibiotics, prebiotics and probiotics, so it will be interesting to observe how this research develops.
CONCLUSION
Some occupational carcinogens were readily identified, because they were akin to natural experiments, in which a few people in the community were exposed to uncommon substances with very rare types of cancer occurring in some occupations. Scrotal cancer was practically unheard of, except among chimney sweeps, and then later among men with occupational exposure to coal tar or shale oil[12]. Mesothelioma was encountered so seldom, that before the report of 33 cases among asbestos miners in South Africa, some pathologists had argued that tumors of the mesothelium did not occur[61,477,478]. It had been argued that tumors ascribed to mesothelial origin were likely misdiagnosed tumors of other cell types, which had metastasized to the mesothelium. Occupational exposures which caused very rare cancers were promptly recognized.
Other occupational exposures caused excessively high rates of common cancers. Around 50% of the early miners of Schneeberg and Joachimsthal were estimated to have developed lung cancer[479]. A small group of men who worked distilling β-naphthylamine in England were reported to have 90%-100% incidence of bladder cancer[480-482]. Barling reflected in 1926, that with the exception of Hall-Edwards, all of the other early radiologists whom he knew, had succumbed to skin cancers[483]. Occupational exposures which caused extremely high rates of common cancers were also readily recognized.
The notion that synthetic agents are the cause of cancer evolved from the early identification of occupational (or synthetic) agents. Infectious agents, as well as other naturally occurring agents, were usually more widespread geographically. The exposure to naturally occurring agents generally extended temporally for generations, so their effects were not readily evident.
Proof of most infectious causes of cancer required both technical advancements to identify the agents and large epidemiological studies to determine whether the agent is associated with increased rates of cancer. A few of the earliest infectious agents were identified with simple methods, but others could not be elucidated until more advanced methods were developed. The ova of S. haematobium and O. viverrini were identified with simple laboratory techniques and low powered light microscopes[403,484]. EBV was discovered using the EM[339], which only became consistently reliable in the 1950s[192]. HTLV and HIV were discovered using enzyme assays for reverse transcriptase, which were developed following the discovery that RNA viruses replicate using this enzyme[217,218]. HPV-16, HPV-18 and HCV were identified using recombinant DNA technology shortly after it became available in the mid-1970s. Modern techniques, such as next generation DNA sequencing introduced in 2005[174] have the potential to lead to the identification of additional infectious causes of cancer.
Each of the early theories concerning the cause of cancer which were described in the introduction, have been demonstrated to have some validity. Cancers are now considered to be caused by both irritation and infectious agents. Cohnheim’s theory of embryonic rests also appears to have some validity, as there is evidence that tumor cells originate from stem cells[485]. The range of identified human carcinogens now includes occupational exposures, pharmaceuticals, X-rays, natural factors, non-viral infectious agents, and viruses.
Important contributions were sometimes made by persons who were not formally well qualified and occasionally by researchers not specifically searching for causes of cancer. Those who made important contributions were usually characterized by meticulous attention to detail and those who investigated viruses often persisted in their study in the presence of adversity.
It is interesting to reflect, that contrary to the dogma of the early 1900s, that infectious agents did not and could not cause cancer, it is now evident that they do. Discoveries of the roles of infectious agents as causes of cancer have contributed significantly to our ability to prevent cancer since 1980. Cervical cancer is the second most common cancer among women in the developing world[486]. Essentially all cervical cancers are now attributed to HPV, as cervical cancer seldom occurs without exposure to HPV[487]. Few chemical carcinogens share this distinction of being essential, for the development of specific cancer. Cervical cancer accounts for 5.2% of the world cancer burden cancer[488]. Liver cancer is the third most common cancer among men in the developing world[486]. Liver cancers caused by HBV and HCV are estimated to account for 4.9% of the total world burden of cancer[488]. H. pylorus has been estimated to cause around 2/3 of the cases of gastric cancer[486], and is estimated to cause 5.5% of the world cancer burden[488]. The global burden of cancers due to infectious agents has been estimated to be 17.8%[488].
Infectious agents were expected to be present in the diseased tissue according to Koch’s postulates, but liver cancer caused by HBV is often devoid of the virus, except for the integrated virus which is truncated and unable to produce infectious particles. HPV follows a similar pattern in cervical cancers, while H pylora is usually completely devoid in gastric cancer tissues. Infectious agents did not cause cancer in a manner predicted by microbiologists of the early 1900s.
The IARC designated 109 agents as group 1 carcinogens in their update of November 7, 2012. Group 1 is defined as having “sufficient evidence of carcinogenicity in humans”. About half of these agents have been discussed in this review. Selection of carcinogens for inclusion/exclusion was designed to include the widest possible variety of agents without producing an excessively long list. If a mixture was found to be carcinogenic and later a component of the mixture was also reported to be carcinogenic, then usually only the mixture or the pure agent was reviewed. If one route of exposure was determined to be carcinogenic (e.g., dermal exposure to coal tar), then usually other routes of exposure were not discussed (e.g., inhalation of fumes of coal tar). About half of the agents listed as group 1 carcinogens were excluded based on these principles. The complete list of the 109 group 1 carcinogens is available at the IRAC website (www.iarc.fr) or (http://monographs.iarc.fr/ENG/Classification/index.php last accessed January 27, 2013).
Additional information concerning the above discussed carcinogens can be found in the IARC monograph series. Cumulative indexes are provided at the end of recent volumes of the monographs, and also at their website (http://www.iarc.fr). The National Toxicology Program has independently compiled a similar list of carcinogens (http://ntp.niehs.nih.gov/ntp/roc/twelfth/roc12.pdf). Foods, supplements, physical activity, and obesity have been comprehensively reviewed in the publication titled Food, Nutrition, Physical Activity and Prevention of Cancer: A Global Perspective which is available at the World Cancer Research Fund website (http://www.wcrf.org).
Footnotes
Conflict-of-interest statement: I declare that I have no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 22, 2015
First decision: September 22, 2015
Article in press: November 25, 2015
P- Reviewer: Eleftheriadis N, Micale N S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.Higginson J, Muir CS, Munoz M. Introduction to epidemiology. In: Human Cancer: Epidemiology and Environmental Causes., editor. Cambridge, England: Cambridge University Press; 1992. pp. xvii–xxv, esp xx, xxii, xxiii. [Google Scholar]
- 2.Shimkin MB. International Agency for Research on Cancer: Higginson. In: Contrary to Nature: being an Illustrated commentary on Some Persons and Events of Historical Importance in the Development of Knowledge concerning Cancer., editor. NIH Publication No. 76-720. Washington, (DC): US Department of Health, Education and Welfare; 1977. pp. 485–486. [Google Scholar]
- 3.IARC (International Agency for Research on Cancer) Background and purpose of the IARC programme on the evaluation of the carcinogenic risk of chemicals to man. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man., editor. Volume 1. Lyon: International Agency for Research on Cancer; 1972. pp. 8–14, esp 11. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 4.IARC (International Agency for Research on Cancer) Background and purpose of the IARC programme on the evaluation of the carcinogenic risk of chemicals to man. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man., editor. Volume 16, Some Aromatic Amines and related nitro compounds – hair dyes, colouring agents and miscellaneous chemicals. Lyon: International Agency for Research on Cancer; 1978. pp. 9–20. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 5.Shimkin MB. Contrary to Nature: being an Illustrated commentary on Some Persons and Events of Historical Importance in the Development of Knowledge concerning Cancer. NIH Publication No. 76-720. Washington, (DC): US Department of Health, Education and Welfare; 1977. [Google Scholar]
- 6.Sirica AE. Introduction: chronology of significant events in the study of neoplasia. In: Sirica AE, editor , The Pathobiology of Neoplasia, editors. New York: Plenum Press; 1989. pp. 1–24. [Google Scholar]
- 7.Gross L. Oncogenic viruses, second edition. Oxford: Permagon Press; 1970. [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective, Washington, DC: AIRC, 2007. Available from: http://www.dietandcancerreport.org/expert_report/report_contents/index.php.
- 9.Ramazzini B. Chapter XX. Wet nurses. and reprinted in 1964. New York. In: Diseases of Workers, translated from the Latin text De Morbis Artificum of 1713 by Wilmer Cave Wright, with an introduction by George Rosen, M.D., Ph.D. Translation first published in; 1940. pp. Hafner Publishing Company, 1713: 167–201, esp 191. [Google Scholar]
- 10.Pott P. Cancer scroti. In: Chirurgical observations relative to the cataract, polypus of the nose, the cancer of the scrotum, the different kinds of ruptures, and mortification of the toes and feet. London: Hawes, Clarke, and Collins, 1775: 63-68 [Google Scholar]
- 11.von Volkmann R. Uber theer-und russkrebs. Beliner klinische Wochenschrift. 1874;11:218. [Google Scholar]
- 12.Waldron HA. A brief history of scrotal cancer. Br J Ind Med. 1983;40:390–401. doi: 10.1136/oem.40.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell J. Paraffin epithelioma of the scrotum. Edinb Med J. 1876;22:135–137. [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg M, Selikoff IJ. Lung cancer in the Schneeberg mines: a reappraisal of the data reported by Harting and Hesse in 1879. Ann Occup Hyg. 1993;37:5–14. doi: 10.1093/annhyg/37.1.5. [DOI] [PubMed] [Google Scholar]
- 15.Shimkin MB. Lung cancer among miners: Harting and Hesse. In: Contrary to Nature: being an Illustrated commentary on Some Persons and Events of Historical Importance in the Development of Knowledge concerning Cancer., editor. NIH Publication No. 76-720. Washington, (DC): US Department of Health, Education and Welfare; 1977. pp. 161–162. [Google Scholar]
- 16.Rhen L. Blasengeschwulste bei fuchsin-arbeitern. Archiv Fur Klinische Chirurgie. 1895;50:588–600 [in German]. [Google Scholar]
- 17.Rhen L. Bladder tumors in fuchsin workers (from German). Archiv Fur Klinische Chirurgie 50: 588-600, 1895. In: Shimkin MB, editor. Some Classics of Experimental Oncology. 50 Selections, 1775-1965, NIH Publication No. 80-2150. Washington, (DC): U. S. Department of Health and Human Services; 1980. pp. 44–51. [Google Scholar]
- 18.Röntgen WC. On a new kind of rays. Science. 1896;3:227–231. doi: 10.1126/science.3.59.227. [DOI] [PubMed] [Google Scholar]
- 19.Brown P. The cause and the effect. In: American martyrs to science through the Roentgen rays., editor. Springfield, (Il): Charles C Thomas; 1936. pp. 3–31. [Google Scholar]
- 20.Pitkin JT. Danger of the X-ray operation. Transactions. American Roentgen Ray Society, 1903; 4: 232-259 [Google Scholar]
- 21.Haagensen CD. An exhibit of important books, papers, and memorabilia illustrating the evolution of the knowledge of cancer. Am J Cancer. 1933;18:42–126, esp 107. [Google Scholar]
- 22.Porter CA, White CJ. I. Multiple Carcinomata following Chronic X-ray Dermatitis. Ann Surg. 1907;46:649–671. doi: 10.1097/00000658-190711000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill J. Cautions against the immoderate use of snuff. London: R Baldwin, 1761 [Google Scholar]
- 24.Redmond DE. Tobacco and cancer: the first clinical report, 1761. N Engl J Med. 1970;282:18–23. doi: 10.1056/NEJM197001012820105. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson J. On some examples of arsenic-keratosis of the skin and arsenic cancer. Transactions of the Pathological Society of London. 1888;39:352–363. [Google Scholar]
- 26.Henschen F. Yamagiwa’s tar cancer and its historical significance. From Percival Pott to Katsusaburo Yamagiwa. Gan. 1968;59:447–451. [PubMed] [Google Scholar]
- 27.Morris H. The Bradshaw Lecture ON CANCER AND ITS ORIGIN: Delivered at the Royal College of Surgeons on December 9th. Br Med J. 1903;2:1505–1511. doi: 10.1136/bmj.2.2241.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberling C. Three hypotheses. In: The Riddle of Cancer, (Translated from French by William H., editors. Woglom) London: Yale University Press; 1944. pp. 17–37, esp 33-37. [Google Scholar]
- 29.Triolo VA. Nineteenth century foundations of cancer research advances in tumor pathology, nomenclature, and theories of oncogenesis. Cancer Res. 1965;25:75–106. [PubMed] [Google Scholar]
- 30.Plimmer HG. The parasitic theory of cancer. Br Med J. 1903;2:1511–1515. doi: 10.1136/bmj.2.2241.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woglom WH. Chapter II. Attempts to produce tumors. In: Studies in cancer and allied subjects., editor. Vol I. The study of Experimental Cancer. A review. New York: Columbia University Press; 1913. pp. 20–38, esp 20. [Google Scholar]
- 32.Krumbhaar EB. Experimental cancer: a historical retrospect. Ann Med Hist. 1925;7:132–140. [PMC free article] [PubMed] [Google Scholar]
- 33.Ellermann V, Bang O. Experimentelle leukamie bei huhnern. Zentralblatt für Bacteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene (1, Abt.; Originale), 1908; 46: 595-609 [Google Scholar]
- 34.Ellermann V, Bang O. Experimental leukemia in chickens II (from German) Zeitschrift fur hygiene und infektionskrakheiten 63: 231-273, 1909. In: Shimkin MB, editor. Some Classics of Experimental Oncology. 50 Selections, 1775-1965, NIH Publication No. 80-2150. Washington, (DC): U. S. Department of Health and Human Services; 1980. pp. 328–360. [Google Scholar]
- 35.Rous P. Transmission of a malignant new growth by means of a cell-free filtrate. JAMA. 1911;56:198. [PubMed] [Google Scholar]
- 36.Rous P. A Sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marie P, Clunet J, Raulot-Lapointe G. Contribution a l’etudte du development des tumeurs malignes sur les ulcers de Roentgen, Tumeur maligne developpee sur une radiodermite experimentale chez le rat blanc. Bulletin de L’Association Francaise pour l’etude du Cancer. 1910;3:404–426 [in French]. [Google Scholar]
- 38.Mustacchi P, Shimkin MB. Radiation cancer and Jean Clunet. Cancer. 1956;9:1073–1074. doi: 10.1002/1097-0142(195611/12)9:6<1073::aid-cncr2820090602>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Leitch A. A British Medical Association Lecture on the experimental inquiry into the causes of cancer. Br Med J. 1923;2:1–7. doi: 10.1136/bmj.2.3262.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamagiwa K, Ichikawa K. Experimental study of the pathogenesis of carcinoma. J Cancer Res. 1918;3:1–29, esp 1-3, 20. doi: 10.3322/canjclin.27.3.174. [DOI] [PubMed] [Google Scholar]
- 41.Yamagiwa K, Ichikawa K. Experimental study of the pathogenesis of carcinoma. CA Cancer J Clin. 1918;27:174–181. doi: 10.3322/canjclin.27.3.174. [DOI] [PubMed] [Google Scholar]
- 42.Woglom WH. Experimental tar cancer. Arch Pathol Lab Med. 1926;2:533–576, 733-752, esp 536. [Google Scholar]
- 43.Shear MJ. Yamagiwa’s tar cancer and its historical significance--from Yamagiwa to Kennaway. Gan. 1969;60:121–127. [PubMed] [Google Scholar]
- 44.Kennaway EL. On the cancer-producing factor in tar. Br Med J. 1924;1:564–567. doi: 10.1136/bmj.1.3300.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook JW. Ernest Laurence Kennaway, 1881-1958. Biogr Mem Fellows R Soc. 1958;4:139–154. [Google Scholar]
- 46.Kennaway E. The identification of a carcinogenic compound in coal-tar. Br Med J. 1955;2:749–752. doi: 10.1136/bmj.2.4942.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kennaway EL, Hieger I. Carcinogenic substances and their fluorescence spectra. Br Med J. 1930;1:1044–1046. doi: 10.1136/bmj.1.3622.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook JW, Hewitt CL, Hieger I. The isolation of a cancer producing hydrocarbon from coal tar. J Chem Soc. 1933;Part 1:395–405. [Google Scholar]
- 49.Haddow A, Kon GA. Chemistry of carcinogenic compounds. Br Med Bull. 1947;4:314–326. doi: 10.1093/oxfordjournals.bmb.a072819. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman FL. Radium (mesothorium) necrosis. JAMA. 1925;85:961–965. [Google Scholar]
- 51.Martland HS. The occurrence of malignancy in radioactive persons. Am J Cancer. 1931;15:2435–2516. [Google Scholar]
- 52.Martland HS, Humphries RE. Osteogenic sarcomas in dial painters using luminous paint. Arch Pathol. 1929;7:406–417. [Google Scholar]
- 53.Bridge JC. Annual Report of the Chief Inspector of Factories for the Year 1932. London: HMSO; 1933. pp. 103–104. [Google Scholar]
- 54.Machle W, Gregorius F. Cancer of the respiratory system in the United States chromate producing industry. Public Health Rep. 1948;63:1114–1127. [PubMed] [Google Scholar]
- 55.Case RA, Hosker ME, McDonald DB, Pearson JT. Tumours of the urinary bladder in workmen engaged in the manufacture and use of certain dyestuff intermediates in the British chemical industry. I. The role of aniline, benzidine, alpha-naphthylamine, and beta-naphthylamine. Br J Ind Med. 1954;11:75–104. doi: 10.1136/oem.11.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melick WF, Escue HM, Naryka JJ, Mezera RA, Wheeler EP. The first reported cases of human bladder tumors due to a new carcinogen-xenylamine. J Urol. 1955;74:760–766. doi: 10.1016/S0022-5347(17)67344-0. [DOI] [PubMed] [Google Scholar]
- 57.Thiess AM, Hey W, Zeller H. [Toxicology of dichlorodimethylether--suspected cancerogenic effect in man] Zentralbl Arbeitsmed. 1973;23:97–102. [PubMed] [Google Scholar]
- 58.IARC (International Agency for Research on Cancer) Bis(chloromethyl)ether. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man., editor. Volume 4, Some Aromatic Amines, Hydrazine and Related Substances, N-nitroso Compounds and Miscellaneous Alkylating Agents. Lyon: International Agency for Research on Cancer; 1974. pp. 231–238, esp 236. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 59.Figueroa WG, Raszkowski R, Weiss W. Lung cancer in chloromethyl methyl ether workers. N Engl J Med. 1973;288:1096–1097. doi: 10.1056/NEJM197305242882104. [DOI] [PubMed] [Google Scholar]
- 60.Weiss W. Epidemic curve of respiratory cancer due to chloromethyl ethers. J Natl Cancer Inst. 1982;69:1265–1270. [PubMed] [Google Scholar]
- 61.Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carbone M, Emri S, Dogan AU, Steele I, Tuncer M, Pass HI, Baris YI. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7:147–154. doi: 10.1038/nrc2068. [DOI] [PubMed] [Google Scholar]
- 63.Baris YI, Sahin AA, Ozesmi M, Kerse I, Ozen E, Kolacan B, Altinörs M, Göktepeli A. An outbreak of pleural mesothelioma and chronic fibrosing pleurisy in the village of Karain/Urgüp in Anatolia. Thorax. 1978;33:181–192. doi: 10.1136/thx.33.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baris YI, Grandjean P. Prospective study of mesothelioma mortality in Turkish villages with exposure to fibrous zeolite. J Natl Cancer Inst. 2006;98:414–417. doi: 10.1093/jnci/djj106. [DOI] [PubMed] [Google Scholar]
- 65.Creech JL, Johnson MN. Angiosarcoma of liver in the manufacture of polyvinyl chloride. J Occup Med. 1974;16:150–151. [PubMed] [Google Scholar]
- 66.Vigliani EC, Saita G. Benzene and leukemia. N Engl J Med. 1964;271:872–876. doi: 10.1056/NEJM196410222711703. [DOI] [PubMed] [Google Scholar]
- 67.Wagoner JK, Archer VE, Lundin FE, Holaday DA, Lloyd JW. Radiation as the cause of lung cancer among uranium miners. N Engl J Med. 1965;273:181–188. doi: 10.1056/NEJM196507222730402. [DOI] [PubMed] [Google Scholar]
- 68.Case RA, Lea AJ. Mustard gas poisoning, chronic bronchitis, and lung cancer; an investigation into the possibility that poisoning by mustard gas in the 1914-18 war might be a factor in the production of neoplasia. Br J Prev Soc Med. 1955;9:62–72. doi: 10.1136/jech.9.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Folley JH, Borges W, Yamawaki T. Incidence of leukemia in survivors of the atomic bomb in Hiroshima and Nagasaki, Japan. Am J Med. 1952;13:311–321. doi: 10.1016/0002-9343(52)90285-4. [DOI] [PubMed] [Google Scholar]
- 70.Doll R. Sir Austin Bradford Hill and the progress of medical science. BMJ. 1992;305:1521–1526. doi: 10.1136/bmj.305.6868.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilkinson L. Sir Austin Bradford Hill: medical statistics and the quantitative approach to prevention of disease. Addiction. 1997;92:657–666. [PubMed] [Google Scholar]
- 72.Doll R. Conversation with Sir Richard Doll. Br J Addict. 1991;86:365–377. doi: 10.1111/j.1360-0443.1991.tb03410.x. [DOI] [PubMed] [Google Scholar]
- 73.Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950;2:739–748. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doll R. Tobacco: a medical history. J Urban Health. 1999;76:289–313. doi: 10.1007/BF02345669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doll R, Hill AB. The mortality of doctors in relation to their smoking habits; a preliminary report. Br Med J. 1954;1:1451–1455. doi: 10.1136/bmj.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Royal College of Physicians of London. Smoking and Health: Summary and Report of the Royal College of Physicians of London on Smoking in Relation to Cancer of the Lung and Other Diseases. London: Pitman; 1962. [PubMed] [Google Scholar]
- 77.US Surgeon General. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Public Health Service Publication No. 1103. Washington, (DC): U. S. Government Printing Office; 1964. [Google Scholar]
- 78.Doll R. Uncovering the effects of smoking: historical perspective. Stat Methods Med Res. 1998;7:87–117. doi: 10.1177/096228029800700202. [DOI] [PubMed] [Google Scholar]
- 79.Burnham JC. American physicians and tobacco use: two Surgeons General, 1929 and 1964. Bull Hist Med. 1989;63:1–31. [PubMed] [Google Scholar]
- 80.Charlton A. Medicinal uses of tobacco in history. J R Soc Med. 2004;97:292–296. doi: 10.1258/jrsm.97.6.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parascandola M. Two approaches to etiology: the debate over smoking and lung cancer in the 1950s. Endeavour. 2004;28:81–86. doi: 10.1016/j.endeavour.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Talley C, Kushner HI, Sterk CE. Lung cancer, chronic disease epidemiology, and medicine, 1948-1964. J Hist Med Allied Sci. 2004;59:329–374. doi: 10.1093/jhmas/jrh088. [DOI] [PubMed] [Google Scholar]
- 83.Vandenbroucke JP. Clinical investigation in the 20th century: the ascendancy of numerical reasoning. Lancet. 1998;352 Suppl 2:SII12–SII16. doi: 10.1016/s0140-6736(98)90293-8. [DOI] [PubMed] [Google Scholar]
- 84.Susser M. What is a cause and how do we know one? A grammar for pragmatic epidemiology. Am J Epidemiol. 1991;133:635–648. doi: 10.1093/oxfordjournals.aje.a115939. [DOI] [PubMed] [Google Scholar]
- 85.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 86.Bengtsson U, Angervall L, Ekman H, Lehmann L. Transitional cell tumors of the renal pelvis in analgesic abusers. Scand J Urol Nephrol. 1968;2:145–150. doi: 10.3109/00365596809135358. [DOI] [PubMed] [Google Scholar]
- 87.Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc. 1969;1:106–112. [PMC free article] [PubMed] [Google Scholar]
- 88.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 89.Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–1167. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- 90.Thiede T, Christensen BC. [Bladder tumors induced by chlornaphazine treatment] Ugeskr Laeger. 1975;137:661–666. [PubMed] [Google Scholar]
- 91.IARC (International Agency for Research on Cancer) Chlornaphazine. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100A: A Review of Human Carcinogens: Pharmaceuticals., editor. Lyon: International Agency for Research on Cancer; 2012. pp. 333–335. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 92.Gilman A, Philips FS. The Biological Actions and Therapeutic Applications of the B-Chloroethyl Amines and Sulfides. Science. 1946;103:409–436. doi: 10.1126/science.103.2675.409. [DOI] [PubMed] [Google Scholar]
- 93.Kyle RA, Pierre RV, Bayrd ED. Multiple myeloma and acute myelomonocytic leukemia. N Engl J Med. 1970;283:1121–1125. doi: 10.1056/NEJM197011192832101. [DOI] [PubMed] [Google Scholar]
- 94.Stott H, Fox W, Girling DJ, Stephens RJ, Galton DA. Acute leukaemia after busulphan. Br Med J. 1977;2:1513–1517. doi: 10.1136/bmj.2.6101.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reimer RR, Hoover R, Fraumeni JF, Young RC. Acute leukemia after alkylating-agent therapy of ovarian cancer. N Engl J Med. 1977;297:177–181. doi: 10.1056/NEJM197707282970402. [DOI] [PubMed] [Google Scholar]
- 96.Osgood EE. Contrasting incidence of acute monocytic and granulocytic leukemias in p32-treated patients with polycythemia vera and chronic lymphocytic leukemia. J Lab Clin Med. 1964;64:560–573. [PubMed] [Google Scholar]
- 97.Dahlgren S. Thorotrast tumours. A review of the literature and report of two cases. Acta Pathol Microbiol Scand. 1961;53:147–161. [PubMed] [Google Scholar]
- 98.Da Horta JS, Da Motta LC, Abbatt JD, Roriz ML. Malignancy and other late effects following administration of thorotrast. Lancet. 1965;2:201–205. doi: 10.1016/s0140-6736(65)90692-6. [DOI] [PubMed] [Google Scholar]
- 99.Council on Pharmacy and Chemistry. Thorotrast. JAMA. 1932;99:2183–2185. [Google Scholar]
- 100.Martland HS, Conlon P, Knef JP. Some unrecognized dangers in the use and handling of radioactive substances, with special reference to storage of insoluble products of radium and mesothorium in the reticulo-endothelial system. JAMA. 1925;85:1769–1776. [Google Scholar]
- 101.Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827–1839. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.IARC (International Agency for Research on Cancer) Ortho-toluidine. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100F: A Review of Human Carcinogens: Chemical Agents and Related Occupations., editor. Lyon: International Agency for Research on Cancer; 2012. pp. 93–100. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 103.Ward E, Carpenter A, Markowitz S, Roberts D, Halperin W. Excess number of bladder cancers in workers exposed to ortho-toluidine and aniline. J Natl Cancer Inst. 1991;83:501–506. doi: 10.1093/jnci/83.7.501. [DOI] [PubMed] [Google Scholar]
- 104.Costello J, Graham WG. Vermont granite workers’ mortality study. Am J Ind Med. 1988;13:483–497. doi: 10.1002/ajim.4700130408. [DOI] [PubMed] [Google Scholar]
- 105.Garshick E, Laden F, Hart JE, Rosner B, Smith TJ, Dockery DW, Speizer FE. Lung cancer in railroad workers exposed to diesel exhaust. Environ Health Perspect. 2004;112:1539–1543. doi: 10.1289/ehp.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steenland K, Schnorr T, Beaumont J, Halperin W, Bloom T. Incidence of laryngeal cancer and exposure to acid mists. Br J Ind Med. 1988;45:766–776. doi: 10.1136/oem.45.11.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hauptmann M, Lubin JH, Stewart PA, Hayes RB, Blair A. Mortality from solid cancers among workers in formaldehyde industries. Am J Epidemiol. 2004;159:1117–1130. doi: 10.1093/aje/kwh174. [DOI] [PubMed] [Google Scholar]
- 108.Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Cogliano V. Carcinogenicity of 1,3-butadiene, ethylene oxide, vinyl chloride, vinyl fluoride, and vinyl bromide. Lancet Oncol. 2007;8:679–680. doi: 10.1016/s1470-2045(07)70235-8. [DOI] [PubMed] [Google Scholar]
- 109.Ward EM, Fajen JM, Ruder AM, Rinsky RA, Halperin WE, Fessler-Flesch CA. Mortality study of workers in 1,3-butadiene production units identified from a chemical workers cohort. Environ Health Perspect. 1995;103:598–603. doi: 10.1289/ehp.95103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fingerhut MA, Halperin WE, Marlow DA, Piacitelli LA, Honchar PA, Sweeney MH, Greife AL, Dill PA, Steenland K, Suruda AJ. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. N Engl J Med. 1991;324:212–218. doi: 10.1056/NEJM199101243240402. [DOI] [PubMed] [Google Scholar]
- 111.Guha N, Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Baan R, Mattock H, Straif K. Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol. 2012;13:1192–1193. doi: 10.1016/s1470-2045(12)70485-0. [DOI] [PubMed] [Google Scholar]
- 112.IARC (International Agency for Research on Cancer) Ethylene oxide. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 60: Some Industrial Chemicals., editor. Lyon: International Agency for Research on Cancer; 1994. pp. 73–159, esp 137. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 113.Steenland K, Ward E. Lung cancer incidence among patients with beryllium disease: a cohort mortality study. J Natl Cancer Inst. 1991;83:1380–1385. doi: 10.1093/jnci/83.19.1380. [DOI] [PubMed] [Google Scholar]
- 114.IARC (International Agency for Research on Cancer) Beryllium and beryllium compounds. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100C: A Review of Human Carcinogens: Arsenic, Metals , Fibres and Dusts., editors. Lyon: International Agency for Research on Cancer; 2012. pp. 95–120. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 115.Boffetta P, Fryzek JP, Mandel JS. Occupational exposure to beryllium and cancer risk: a review of the epidemiologic evidence. Crit Rev Toxicol. 2012;42:107–118. doi: 10.3109/10408444.2011.631898. [DOI] [PubMed] [Google Scholar]
- 116.Ward E, Halperin W, Thun M, Grossman HB, Fink B, Koss L, Osorio AM, Schulte P. Bladder tumors in two young males occupationally exposed to MBOCA. Am J Ind Med. 1988;14:267–272. doi: 10.1002/ajim.4700140304. [DOI] [PubMed] [Google Scholar]
- 117.IARC (International Agency for Research on Cancer) 4,4’-Methylenebis(2-chlorobenzenamine) In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100F: A Review of Human Carcinogens: Chemical Agents and Related Occupations., editor. Lyon: International Agency for Research on Cancer; 2012. pp. 73–82. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 118.IARC (International Agency for Research on Cancer) IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. Volume 78, Ionizing Radiation, Part 2: Some Internally Deposited Nucleotides. Lyon: International Agency for Research on Cancer; 2001. p. esp 194, 195, 469. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 119.Koshurnikova NA, Bolotnikova MG, Ilyin LA, Keirim-Markus IB, Menshikh ZS, Okatenko PV, Romanov SA, Tsvetkov VI, Shilnikova NS. Lung cancer risk due to exposure to incorporated plutonium. Radiat Res. 1998;149:366–371. [PubMed] [Google Scholar]
- 120.Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21. doi: 10.1038/359021a0. [DOI] [PubMed] [Google Scholar]
- 121.Pedersen-Bjergaard J, Larsen SO. Incidence of acute nonlymphocytic leukemia, preleukemia, and acute myeloproliferative syndrome up to 10 years after treatment of Hodgkin’s disease. N Engl J Med. 1982;307:965–971. doi: 10.1056/NEJM198210143071601. [DOI] [PubMed] [Google Scholar]
- 122.Berk PD, Goldberg JD, Silverstein MN, Weinfeld A, Donovan PB, Ellis JT, Landaw SA, Laszlo J, Najean Y, Pisciotta AV, et al. Increased incidence of acute leukemia in polycythemia vera associated with chlorambucil therapy. N Engl J Med. 1981;304:441–447. doi: 10.1056/NEJM198102193040801. [DOI] [PubMed] [Google Scholar]
- 123.Boice JD, Greene MH, Killen JY, Ellenberg SS, Keehn RJ, McFadden E, Chen TT, Fraumeni JF. Leukemia and preleukemia after adjuvant treatment of gastrointestinal cancer with semustine (methyl-CCNU) N Engl J Med. 1983;309:1079–1084. doi: 10.1056/NEJM198311033091802. [DOI] [PubMed] [Google Scholar]
- 124.Boice JD, Greene MH, Killen JY, Ellenberg SS, Fraumeni JF, Keehn RJ, McFadden E, Chen TT, Stablein D. Leukemia after adjuvant chemotherapy with semustine (methyl-CCNU)--evidence of a dose-response effect. N Engl J Med. 1986;314:119–120. doi: 10.1056/NEJM198601093140214. [DOI] [PubMed] [Google Scholar]
- 125.Penn I, Brunson ME. Cancers after cyclosporine therapy. Transplant Proc. 1988;20:885–892. [PubMed] [Google Scholar]
- 126.Hardell L. Pelvic irradiation and tamoxifen as risk factors for carcinoma of corpus uteri. Lancet. 1988;2:1432. doi: 10.1016/s0140-6736(88)90629-0. [DOI] [PubMed] [Google Scholar]
- 127.Baum M, Brinkley DM, Dossett JA, McPherson K, Patterson JS, Rubens RD, Smiddy FG, Stoll BA, Wilson A, Lea JC, et al. Controlled trial of tamoxifen as adjuvant agent in management of early breast cancer. Interim analysis at four years by Nolvadex Adjuvant Trial Organisation. Lancet. 1983;1:257–261. [PubMed] [Google Scholar]
- 128.IARC (International Agency for Research on Cancer) Etoposide in combination with cisplatin and bleomycin. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100A: A Review of Human Carcinogens: Pharmaceuticals., editor. Lyon: International Agency for Research on Cancer; 2012. pp. 91–105. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 129.Ratain MJ, Kaminer LS, Bitran JD, Larson RA, Le Beau MM, Skosey C, Purl S, Hoffman PC, Wade J, Vardiman JW. Acute nonlymphocytic leukemia following etoposide and cisplatin combination chemotherapy for advanced non-small-cell carcinoma of the lung. Blood. 1987;70:1412–1417. [PubMed] [Google Scholar]
- 130.Kaldor JM, Day NE, Pettersson F, Clarke EA, Pedersen D, Mehnert W, Bell J, Høst H, Prior P, Karjalainen S. Leukemia following chemotherapy for ovarian cancer. N Engl J Med. 1990;322:1–6. doi: 10.1056/NEJM199001043220101. [DOI] [PubMed] [Google Scholar]
- 131.IARC (International Agency for Research on Cancer) Thiotepa. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100A: A Review of Human Carcinogens: Pharmaceuticals., editor. Lyon: International Agency for Research on Cancer; 2012. pp. 163–169. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 132.IARC (International Agency for Research on Cancer) Treosulfan. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100A: A Review of Human Carcinogens: Pharmaceuticals., editor. Lyon: International Agency for Research on Cancer; 2012. pp. 171–174. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 133.Newhouse ML, Pearson RM, Fullerton JM, Boesen EA, Shannon HS. A case control study of carcinoma of the ovary. Br J Prev Soc Med. 1977;31:148–153. doi: 10.1136/jech.31.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551–554. doi: 10.1056/NEJM198003063021004. [DOI] [PubMed] [Google Scholar]
- 135.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 136.Kaiser J. Endocrine disrupters. Panel cautiously confirms low-dose effects. Science. 2000;290:695–697. doi: 10.1126/science.290.5492.695. [DOI] [PubMed] [Google Scholar]
- 137.Calabrese EJ, Blain RB. The hormesis database: the occurrence of hormetic dose responses in the toxicological literature. Regul Toxicol Pharmacol. 2011;61:73–81. doi: 10.1016/j.yrtph.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 138.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nerín C, Fernández C, Domeño C, Salafranca J. Determination of potential migrants in polycarbonate containers used for microwave ovens by high-performance liquid chromatography with ultraviolet and fluorescence detection. J Agric Food Chem. 2003;51:5647–5653. doi: 10.1021/jf034330p. [DOI] [PubMed] [Google Scholar]
- 140.Chang CM, Chou CC, Lee MR. Determining leaching of bisphenol A from plastic containers by solid-phase microextraction and gas chromatography-mass spectrometry. Anal Chim Acta. 2005;539:41–47. [Google Scholar]
- 141.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Biles JE, McNeal TP, Begley TH, Hollifield HC. Determination of bisphenol-A in reusable polycarbonate food-contact plastics and migration to food-simulating liquids. J Agr Food Chem. 1997;45:3541–3544. [Google Scholar]
- 143.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 144.Tharp AP, Maffini MV, Hunt PA, VandeVoort CA, Sonnenschein C, Soto AM. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci USA. 2012;109:8190–8195. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007;23:383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat Rev Endocrinol. 2010;6:363–370. doi: 10.1038/nrendo.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Goodson WH, Lowe L, Carpenter DO, Gilbertson M, Manaf Ali A, Lopez de Cerain Salsamendi A, Lasfar A, Carnero A, Azqueta A, Amedei A, et al. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcinogenesis. 2015;36 Suppl 1:S254–S296. doi: 10.1093/carcin/bgv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shimkin MB. Bacteriology of cancer. In: Contrary to Nature: being an Illustrated commentary on Some Persons and Events of Historical Importance in the Development of Knowledge concerning Cancer., editor. NIH Publication No. 76-720. Washington, (DC): US Department of Health, Education and Welfare; 1977. pp. 175–176. [Google Scholar]
- 152.Shimkin MB. Contagiousness of cancer: Zacutus and Tulp. In: Contrary to Nature: being an Illustrated commentary on Some Persons and Events of Historical Importance in the Development of Knowledge concerning Cancer., editor. NIH Publication No. 76-720. Washington, (DC): US Department of Health, Education and Welfare; 1977. pp. 69–70. [Google Scholar]
- 153.Epstein MA. Historical background. Philos Trans R Soc Lond B Biol Sci. 2001;356:413–420. doi: 10.1098/rstb.2000.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koch R. Die Aetiologie der Tuberculose. Berliner Klinische Wochenschrift. 1882;19:221–230 [In German] Translated and edited in reference #155. [Google Scholar]
- 155.Brock TD. The etiology of tuberculosis. In: Milestones in Microbiology., editor. Englewood Cliffs, (NJ): Prentice-Hall, Inc; 1961. pp. 109–115. [Google Scholar]
- 156.Pasteur L. Methode pour prevenir la rage après morsure. Comptes rendus de l’Academie des Sciences. 1885;101:765–774 [in French]. [Google Scholar]
- 157.Smith KA. Louis pasteur, the father of immunology? Front Immunol. 2012;3:68. doi: 10.3389/fimmu.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grafe A. Microscopes and cell culture fail. In: A history of Experimental Virology, Berlin: Springer-Verlag; 1991. pp. 30–46. [Google Scholar]
- 159.Reed W, Carroll J, Agramonte A, Lazear JW. The Etiology of Yellow Fever-A Preliminary Note. Public Health Pap Rep. 1900;26:37–53. [PMC free article] [PubMed] [Google Scholar]
- 160.Levy JA, Fraenkel-Conrat H, Owens RA. General concepts of virology. In: Virology, Englewood Cliffs, (NJ): Prentice Hall; 1994. pp. 1–25, esp 3-5. [Google Scholar]
- 161.Shope RE, Hurst EW. Infectious papillomatosis of rabbits: with a note on the histopathology. J Exp Med. 1933;58:607–624. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lacassagne A. Appearance of breast cancer in male mice injected with folliculin (from French) Comptes Rendus Hebdomadaires Des Seances de Academie Des Sciences. 1932;195:630–632. [Google Scholar]
- 163.Lacassagne A. Appearance of breast cancer in male mice injected with folliculin (from French). Comptes Rendus Hebdomadaires Des Seances de Academie Des Sciences. 195: 630-632, 1932. In: Shimkin MB, editor. Some Classics of Experimental Oncology. 50 Selections, 1775-1965, NIH Publication No. 80-2150. Washington, (DC): U. S. Department of Health and Human Services; 1980. pp. 281–288. [Google Scholar]
- 164.Bittner JJ. Some possible effects of nursing on the mammary gland tumor incidence in mice. Science. 1936;84:162. doi: 10.1126/science.84.2172.162. [DOI] [PubMed] [Google Scholar]
- 165.Gross L. “Spontaneous” leukemia developing in C3H mice following inoculation in infancy, with AK-leukemic extracts, or AK-embrvos. Proc Soc Exp Biol Med. 1951;76:27–32. [PubMed] [Google Scholar]
- 166.Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 167.Blumberg BS, Alter HJ, Visnich S. A “new” antigen in leukemia sera. JAMA. 1965;191:541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- 168.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 171.Dürst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 173.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 174.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Borrel A. Epithelioses infectieuses et epitheliomas. Annales de Institut Pasteur (Paris) 1903;17:81–122. [Google Scholar]
- 176.Shimkin MB. Virus hyspothesis: Borrel and Oberling. In: Contrary to Nature: being an Illustrated commentary on Some Persons and Events of Historical Importance in the Development of Knowledge concerning Cancer., editor. NIH Publication No. 76-720. Washington, (DC): US Department of Health, Education and Welfare; 1977. pp. 213–214. [Google Scholar]
- 177.Schmeisser HC. Spontaneous and experimental leukemia of the fowl. J Exp Med. 1915;22:820–838. doi: 10.1084/jem.22.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shope RE. Evolutionary episodes in the concept of viral oncogenesis. Perspect Biol Med. 1966;9:258–274. doi: 10.1353/pbm.1966.0022. [DOI] [PubMed] [Google Scholar]
- 179.Ewing J. Cancer problems. Harvey Lect 1907- 1908;3:34–88, esp 34-47. [Google Scholar]
- 180.Andrewes CH. Francis Peyton Rous 1879-1970. Biogr Mem Fellows R Soc. 1971;17:643–662. doi: 10.1098/rsbm.1971.0025. [DOI] [PubMed] [Google Scholar]
- 181.White CP. Appendix. In: Lectures on the pathology of cancer., editor. Manchester: University Press; 1908. pp. 69–72. [Google Scholar]
- 182.British Medical Journal Publishing Group. The international cancer conference. BMJ. 1910;ii:1266–1268. [Google Scholar]
- 183.Bashford EF. Cancer research. Br Med J. 1911;1:1008–1009. [PMC free article] [PubMed] [Google Scholar]
- 184.Gye WE. Origin of cancer. Lancet. 1925;ii(5316):109–117. [Google Scholar]
- 185.Andrewes CH. Viruses in relation to the aetiology of tumors. Lancet. 1934;ii:63–69. [Google Scholar]
- 186.Andrewes CH. Viruses in relation to the aetiology of tumors. Lancet. 1934;ii:117–124. [Google Scholar]
- 187.Triolo VA. Nineteenth century foundations of cancer research. origins of experimental research. Cancer Res. 1964;24:4–27. [PubMed] [Google Scholar]
- 188.Jensen CO. Experimentelle untersuchungen uber krebs bei mausen. Zentralblatt für Bacteriologie, Parasitenkunde und Infektionskrankheiten. 1903;34:28–34, 122-143. [Google Scholar]
- 189.Jensen CO. Experimental studies on cancer in mice (from German). Zentralblatt für Bacteriologie, Parasitenkunde und Infektionskrankheiten 34: 28-34 and 122-143, 1903. In: Shimkin MB, editor. Some Classics of Experimental Oncology. 50 Selections, 1775-1965, NIH Publication No. 80-2150. Washington, (DC): U. S. Department of Health and Human Services; 1980. pp. 78–105, esp 87,88. [Google Scholar]
- 190.Ruska E. Nobel lecture. The development of the electron microscope and of electron microscopy. Biosci Rep. 1987;7:607–629. doi: 10.1007/BF01127674. [DOI] [PubMed] [Google Scholar]
- 191.Claude A, Porter KR, Pickels EG. Electron microscope studies of chicken tumor cells. Cancer Res. 1947;7:421–430. [Google Scholar]
- 192.de Harven E. Electron microscopy: remarks on 40 years of ultrastructural explorations. CA Cancer J Clin. 1977;27:281–288. doi: 10.3322/canjclin.27.5.281. [DOI] [PubMed] [Google Scholar]
- 193.Lucké B. Carcinoma in the leopard frog: its probable causation by a virus. J Exp Med. 1938;68:457–468. doi: 10.1084/jem.68.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gross L. Chapter 8. The development of inbred strains of mice, and its impact on experimental cancer research. In: Oncogenic Viruses, second edition., editors. Oxford: Permagon Press; 1970. pp. 229–237. [Google Scholar]
- 195.Dougherty RM, Di Stefano HS, Roth FK. Virus particles and viral antigens in chicken tissues free of infectious avian leukosis virus. Proc Natl Acad Sci USA. 1967;58:808–817. doi: 10.1073/pnas.58.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Weiss RA, Friis RR, Katz E, Vogt PK. Induction of avian tumor viruses in normal cells by physical and chemical carcinogens. Virology. 1971;46:920–938. doi: 10.1016/0042-6822(71)90091-2. [DOI] [PubMed] [Google Scholar]
- 197.Lowy DR, Rowe WP, Teich N, Hartley JW. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971;174:155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- 198.Coffin J. Endogenous viruses. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA Tumor Viruses. Molecular Biology of Tumor Viruses, second edition. New York: Cold Harbor Laboratory; 1982. pp. 1109–1203. [Google Scholar]
- 199.Varmus HE, Weiss RA, Friis RR, Levinson W, Bishop JM. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells) Proc Natl Acad Sci USA. 1972;69:20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gardner MB. Historical Background. In: Stephenson JR, editor. Molecular Biology of RNA tumor viruses. New York, NY: Academic Press, Inc; 1980. pp. 1–46, esp 10. [Google Scholar]
- 201.Jarrett WF, Martin WB, Crighton GW, Dalton RG, Stewart MF. Transmission experiments with leukemia (lymphosarcoma) Nature. 1964;202:566–567. doi: 10.1038/202566a0. [DOI] [PubMed] [Google Scholar]
- 202.Jarrett WF, Crawford EM, Martin WB, Davie F. A virus-like particle associated with leukemia (lymphosarcoma) Nature. 1964;202:567–569. doi: 10.1038/202567a0. [DOI] [PubMed] [Google Scholar]
- 203.Hardy WD. Oncogenic viruses of cats: the feline leukaemia and sarcoma viruses. In: Holzworth J, editor. Diseases of the Cat: Medicine and Surgery. Philadelphia: W. B. Saunders Company; 1987. pp. 246–248. [Google Scholar]
- 204.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from burkitt’s lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 205.Moore DH, Charney J, Kramarsky B, Lasfargues EY, Sarkar NH, Brennan MJ, Burrows JH, Sirsat SM, Paymaster JC, Vaidya AB. Search for a human breast cancer virus. Nature. 1971;229:611–614. doi: 10.1038/229611a0. [DOI] [PubMed] [Google Scholar]
- 206.Karpas A. Human retroviruses in leukaemia and AIDS: reflections on their discovery, biology and epidemiology. Biol Rev Camb Philos Soc. 2004;79:911–933. doi: 10.1017/s1464793104006505. [DOI] [PubMed] [Google Scholar]
- 207.Dmochowski L. Viruses and tumors; an old problem in the light of recent advances. Bacteriol Rev. 1959;23:18–40. doi: 10.1128/br.23.1.18-40.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Weinberg RA. Chapter 7. Very special cancer viruses. Big government and the search for the human cancer agent. In: Racing to the Beginning of the Road., editor. New York: Harmony Book; 1996. pp. 66–84, esp 73, 81, 82. [Google Scholar]
- 209.Gardner MB. The Virus Cancer Program of the 1970s: a personal and retrospective view. Lab Anim Sci. 1994;44:101–113. [PubMed] [Google Scholar]
- 210.Gallo RC. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene. 2005;24:5926–5930. doi: 10.1038/sj.onc.1208980. [DOI] [PubMed] [Google Scholar]
- 211.Gardner MB, Rasheed S, Shimzu S, Rongey RW, Henderson BE, McAllister RM, Klement V, Charman HP, Gilden RV, Herbling RL, et al. Search for RNA tumor virus in humans. In: Hiatt HH, Watson JD, Winsten JA, editors. Origins of Human Cancer, Book B, Mechanisms of Carcinogenesis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1977. pp. 1235–1251. [Google Scholar]
- 212.Voisset C, Weiss RA, Griffiths DJ. Human RNA “rumor” viruses: the search for novel human retroviruses in chronic disease. Microbiol Mol Biol Rev. 2008;72:157–96, table of contents. doi: 10.1128/MMBR.00033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Weiss RA. Chapter 11. The search for human RNA tumor viruses. In: Weiss R, Teich N, Varmus H, Coffin J, editors , et al., editors. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1982. pp. 1205–1281. [Google Scholar]
- 214.Klein G. Perspectives in studies of human tumor viruses. Front Biosci. 2002;7:d268–d274. doi: 10.2741/A726. [DOI] [PubMed] [Google Scholar]
- 215.Gallo RC. Chapter 5 Success, defeat, success. In: Virus hunting: AIDS, Cancer and the Human Retrovirus: a Story of Scientific Discovery, et al., editors. New York, (NY): Basic Books, Harper Collins; 1991. pp. 82–98, esp 85. [Google Scholar]
- 216.Gallo RC. Chapter 1. Becoming a physician, becoming a scientist. In: Virus hunting: AIDS, Cancer and the Human Retrovirus: a Story of Scientific Discovery, et al., editors. New York, (NY): Basic Books, Harper Collins; 1991. pp. 13–25, esp 16-21. [Google Scholar]
- 217.Temin HM, Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970;226:1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- 218.Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970;226:1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- 219.Gallo RC. Chapter 4. The story of retroviruses and cancer: from poultry to people. In: Virus hunting: AIDS, Cancer and the Human Retrovirus: a Story of Scientific Discovery, et al., editors. New York, NY: Basic Books, Harper Collins; 1991. pp. 59–81, esp 69-71. [Google Scholar]
- 220.Kawakami TG, Huff SD, Buckley PM, Dungworth DL, Synder SP, Gilden RV. C-type virus associated with gibbon lymphosarcoma. Nat New Biol. 1972;235:170–171. doi: 10.1038/newbio235170a0. [DOI] [PubMed] [Google Scholar]
- 221.Kettmann R, Mammerickx M, Dekegel D, Ghysdael J, Portetelle D, Burny A. Biochemical approach to bovine leukemia. Acta Haematol. 1975;54:201–209. doi: 10.1159/000208076. [DOI] [PubMed] [Google Scholar]
- 222.Hinuma Y, Komoda H, Chosa T, Kondo T, Kohakura M, Takenaka T, Kikuchi M, Ichimaru M, Yunoki K, Sato I, et al. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982;29:631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- 223.Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA. 1984;81:2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shaw GM, Broder S, Essex M, Gallo RC. Human T-cell leukemia virus: its discovery and role in leukemogenesis and immunosuppression. Adv Intern Med. 1984;30:1–27. [PubMed] [Google Scholar]
- 225.Yoshida M. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene. 2005;24:5931–5937. doi: 10.1038/sj.onc.1208981. [DOI] [PubMed] [Google Scholar]
- 226.Parkin DM, Pisani P, Munoz N, Ferlay J. The global health burden of infection associated cancers. Cancer Surv. 1999;33:5–33. [Google Scholar]
- 227.Eden T. Aetiology of childhood leukaemia. Cancer Treat Rev. 2010;36:286–297. doi: 10.1016/j.ctrv.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 228.Cook LB, Melamed A, Niederer H, Valganon M, Laydon D, Foroni L, Taylor GP, Matsuoka M, Bangham CR. The role of HTLV-1 clonality, proviral structure, and genomic integration site in adult T-cell leukemia/lymphoma. Blood. 2014;123:3925–3931. doi: 10.1182/blood-2014-02-553602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Romanelli MG, Diani E, Bergamo E, Casoli C, Ciminale V, Bex F, Bertazzoni U. Highlights on distinctive structural and functional properties of HTLV Tax proteins. Front Microbiol. 2013;4:271. doi: 10.3389/fmicb.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Takeda S, Maeda M, Morikawa S, Taniguchi Y, Yasunaga J, Nosaka K, Tanaka Y, Matsuoka M. Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int J Cancer. 2004;109:559–567. doi: 10.1002/ijc.20007. [DOI] [PubMed] [Google Scholar]
- 231.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci USA. 2006;103:720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Satou Y, Yasunaga J, Zhao T, Yoshida M, Miyazato P, Takai K, Shimizu K, Ohshima K, Green PL, Ohkura N, et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011;7:e1001274. doi: 10.1371/journal.ppat.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Niederer HA, Bangham CR. Integration site and clonal expansion in human chronic retroviral infection and gene therapy. Viruses. 2014;6:4140–4164. doi: 10.3390/v6114140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yasunaga J, Matsuoka M. Molecular mechanisms of HTLV-1 infection and pathogenesis. Int J Hematol. 2011;94:435–442. doi: 10.1007/s12185-011-0937-1. [DOI] [PubMed] [Google Scholar]
- 235.Gottlieb MS, Schanker HM, Fan PT, Saxon A, Weisman JD, Pozalski I. Pneumocystis pneumonia--Los Angeles. MMWR Morb Mortal Wkly Rep. 1981;30:250–252. [PubMed] [Google Scholar]
- 236.Centers for Disease Control (CDC) Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30:305–308. [PubMed] [Google Scholar]
- 237.Gallo RC. Historical essay. The early years of HIV/AIDS. Science. 2002;298:1728–1730. doi: 10.1126/science.1078050. [DOI] [PubMed] [Google Scholar]
- 238.Gallo RC. A reflection on HIV/AIDS research after 25 years. Retrovirology. 2006;3:72. doi: 10.1186/1742-4690-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Montagnier L. Historical essay. A history of HIV discovery. Science. 2002;298:1727–1728. doi: 10.1126/science.1079027. [DOI] [PubMed] [Google Scholar]
- 240.Montagnier L, Chermann JC, Barre-Sinoussi F, Chamaret S, Gruest J, Nugeyre MT, Rey F, Dauguet C, Axler-Blin C, Vezinet-Brun F, et al. A new human T-lymphocytic retrovirus: characterization and possible role in lymphadenopathy and acquired immune deficiency syndromes. In: Human T Cell Leukemia/Lymphoma disorders, Gallo RC, Essex ME, Gross L, et al., editors. Cold Spring Harbor, (NY): Cold Spring Harbor Laboratory Press; 1984. pp. 363–379. [Google Scholar]
- 241.Sarngadharan MG, Popovic M, Bruch L, Schüpbach J, Gallo RC. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984;224:506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- 242.Cheingsong-Popov R, Weiss RA, Dalgleish A, Tedder RS, Shanson DC, Jeffries DJ, Ferns RB, Briggs EM, Weller IV, Mitton S. Prevalence of antibody to human T-lymphotropic virus type III in AIDS and AIDS-risk patients in Britain. Lancet. 1984;2:477–480. doi: 10.1016/s0140-6736(84)92562-5. [DOI] [PubMed] [Google Scholar]
- 243.Levy JA, Hoffman AD, Kramer SM, Landis JA, Shimabukuro JM, Oshiro LS. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 244.Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 245.Montagnier L, Gruest J, Chamaret S, Dauguet C, Axler C, Guétard D, Nugeyre MT, Barré-Sinoussi F, Chermann JC, Brunet JB. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984;225:63–66. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- 246.Brun-Vézinet F, Rouzioux C, Montagnier L, Chamaret S, Gruest J, Barré-Sinoussi F, Geroldi D, Chermann JC, McCormick J, Mitchell S. Prevalence of antibodies to lymphadenopathy-associated retrovirus in African patients with AIDS. Science. 1984;226:453–456. doi: 10.1126/science.6238406. [DOI] [PubMed] [Google Scholar]
- 247.Gallo RC, Montagnier L. The chronology of AIDS research. Nature. 1987;326:435–436. doi: 10.1038/326435a0. [DOI] [PubMed] [Google Scholar]
- 248.Gallo RC. Virus hunting: AIDS, Cancer and the Human Retrovirus: a Story of Scientific Discovery. New York, (NY): Basic Books, Harper Collins; 1991. [Google Scholar]
- 249.Montagnier L. Virus: the Co-discoverer of HIV tracks its Rampage and Charts the Future; translated from French by Stephen Sartarelli, English translation copyright 2000. (Originally published in French as “Des Virus et Des Hommes” in 1994. ) New York, (NY): W. W. Norton & Company, Inc; 2000. [Google Scholar]
- 250.Weiss RA. On viruses, discovery, and recognition. Cell. 2008;135:983–986. doi: 10.1016/j.cell.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Des Jarlais DC, Stoneburner R, Thomas P, Friedman SR. Declines in proportion of Kaposi’s sarcoma among cases of AIDS in multiple risk groups in New York City. Lancet. 1987;2:1024–1025. doi: 10.1016/s0140-6736(87)92586-4. [DOI] [PubMed] [Google Scholar]
- 252.Rutherford GW, Schwarcz SK, Lemp GF, Barnhart JL, Rauch KJ, Warner WL, Piland TH, Werdegar D. The epidemiology of AIDS-related Kaposi’s sarcoma in San Francisco. J Infect Dis. 1989;159:569–572. doi: 10.1093/infdis/159.3.569. [DOI] [PubMed] [Google Scholar]
- 253.Delli Bovi P, Donti E, Knowles DM, Friedman-Kien A, Luciw PA, Dina D, Dalla-Favera R, Basilico C. Presence of chromosomal abnormalities and lack of AIDS retrovirus DNA sequences in AIDS-associated Kaposi’s sarcoma. Cancer Res. 1986;46:6333–6338. [PubMed] [Google Scholar]
- 254.Dal Maso L, Franceschi S. Epidemiology of non-Hodgkin lymphomas and other haemolymphopoietic neoplasms in people with AIDS. Lancet Oncol. 2003;4:110–119. doi: 10.1016/s1470-2045(03)00983-5. [DOI] [PubMed] [Google Scholar]
- 255.Shiramizu B, Herndier BG, McGrath MS. Identification of a common clonal human immunodeficiency virus integration site in human immunodeficiency virus-associated lymphomas. Cancer Res. 1994;54:2069–2072. [PubMed] [Google Scholar]
- 256.Huysentruyt LC, McGrath MS. Mechanisms of AIDS-related lymphoma pathogenesis. Future Virology. 2012;7:229. [Google Scholar]
- 257.Barillari G, Ensoli B. Angiogenic effects of extracellular human immunodeficiency virus type 1 Tat protein and its role in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Clin Microbiol Rev. 2002;15:310–326. doi: 10.1128/CMR.15.2.310-326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.De Falco G, Bellan C, Lazzi S, Claudio P, La Sala D, Cinti C, Tosi P, Giordano A, Leoncini L. Interaction between HIV-1 Tat and pRb2/p130: a possible mechanism in the pathogenesis of AIDS-related neoplasms. Oncogene. 2003;22:6214–6219. doi: 10.1038/sj.onc.1206637. [DOI] [PubMed] [Google Scholar]
- 259.Johri MK, Mishra R, Chhatbar C, Unni SK, Singh SK. Tits and bits of HIV Tat protein. Expert Opin Biol Ther. 2011;11:269–283. doi: 10.1517/14712598.2011.546339. [DOI] [PubMed] [Google Scholar]
- 260.IARC(International Agency for Research on Cancer) Human immunodeficiency virus-1. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100B: A Review of Human Carcinogens: Biological Agents, et al., editors. Lyon: International Agency for Research on Cancer; 2012. pp. 215–253. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http: //www.iarc.fr. [Google Scholar]
- 261.Blumberg BS. The Hunt for a Killer Virus. Princeton: Princeton University Press; 2002. [Google Scholar]
- 262.Blumberg BS. Identifying the hepatitis B virus. In: The Hunt for a Killer Virus, et al., editors. Princeton: Princeton University Press; 2002. pp. 106–118, esp 106. [Google Scholar]
- 263.Blumberg BS. The Discovery of Australia Antigen. In: The Hunt for a Killer Virus, et al., editors. Princeton: Princeton University Press; 2002. pp. 72–83, esp 77-79. [Google Scholar]
- 264.Alter HJ. The road not taken or how I learned to love the liver: a personal perspective on hepatitis history. Hepatology. 2014;59:4–12. doi: 10.1002/hep.26787. [DOI] [PubMed] [Google Scholar]
- 265.Blumberg BS. Australia antigen and the biology of hepatitis B. Science. 1977;197:17–25. doi: 10.1126/science.325649. [DOI] [PubMed] [Google Scholar]
- 266.Blumberg BS. What is Australia Antigen? In: The Hunt for a Killer Virus, et al., editors. Princeton: Princeton University Press; 2002. pp. 84–105. [Google Scholar]
- 267.Bayer ME, Blumberg BS, Werner B. Particles associated with Australia antigen in the sera of patients with leukaemia, Down’s Syndrome and hepatitis. Nature. 1968;218:1057–1059. doi: 10.1038/2181057a0. [DOI] [PubMed] [Google Scholar]
- 268.Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- 269.Lancaster MC, Jenkins FP, Philip JM. Toxicity associated with certain samples of groundnuts. Nature. 1961;192:1095–1096. [Google Scholar]
- 270.Adamson RH, Correa P, Dalgard DW. Occurrence of a primary liver carcinoma in a Rhesus monkey fed aflatoxin B 1. J Natl Cancer Inst. 1973;50:549–553. doi: 10.1093/jnci/50.2.549. [DOI] [PubMed] [Google Scholar]
- 271.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 272.Simons MJ, Yap EH, Yu M, Shanmugaratnam K. Australia antigen in Singapore Chinese patients with hepatocellular carcinoma and comparison groups: influence of technique sensitivity on differential frequencies. Int J Cancer. 1972;10:320–325. doi: 10.1002/ijc.2910100212. [DOI] [PubMed] [Google Scholar]
- 273.Hsu HC, Wu TT, Sheu JC, Wu CY, Chiou TJ, Lee CS, Chen DS. Biologic significance of the detection of HBsAg and HBcAg in liver and tumor from 204 HBsAg-positive patients with primary hepatocellular carcinoma. Hepatology. 1989;9:747–750. doi: 10.1002/hep.1840090515. [DOI] [PubMed] [Google Scholar]
- 274.Kew MC, Ray MB, Desmet VJ, Desmyter J. Hepatitis-B surface antigen in tumour tissue and non-tumorous liver in black patients with hepatocellular carcinoma. Br J Cancer. 1980;41:399–406. doi: 10.1038/bjc.1980.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Peters RL, Afroudakis AP, Tatter D. The changing incidence of association of hepatitis B with hepatocellular carcinoma in California. Am J Clin Pathol. 1977;68:1–7. doi: 10.1093/ajcp/68.1.1. [DOI] [PubMed] [Google Scholar]
- 276.Beasley RP. Rocks along the road to the control of HBV and HCC. Ann Epidemiol. 2009;19:231–234. doi: 10.1016/j.annepidem.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 277.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 278.Chien YC, Jan CF, Kuo HS, Chen CJ. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev. 2006;28:126–135. doi: 10.1093/epirev/mxj010. [DOI] [PubMed] [Google Scholar]
- 279.Chisari FV, Klopchin K, Moriyama T, Pasquinelli C, Dunsford HA, Sell S, Pinkert CA, Brinster RL, Palmiter RD. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989;59:1145–1156. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- 280.Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 281.Blackadar CB. Re: Hepatocellular carcinoma and other liver diseases among Greenlanders chronically infected with hepatitis B virus: a population-based study. J Natl Cancer Inst. 2012;104:1515–1516; author reply 1516. doi: 10.1093/jnci/djs361. [DOI] [PubMed] [Google Scholar]
- 282.Blackadar CB. Systematic review of hepatocellular carcinoma mortality rates among hepatitis B virus-infected renal transplant recipients, with supplemental analyses of liver failure and all-cause mortality. Int J Infect Dis. 2013;17:e24–e36. doi: 10.1016/j.ijid.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 283.Buendia MA. Hepatitis B viruses and hepatocellular carcinoma. Adv Cancer Res. 1992;59:167–226. doi: 10.1016/s0065-230x(08)60306-1. [DOI] [PubMed] [Google Scholar]
- 284.Matsubara K, Tokino T. Integration of hepatitis B virus DNA and its implications for hepatocarcinogenesis. Mol Biol Med. 1990;7:243–260. [PubMed] [Google Scholar]
- 285.Bréchot C, Gozuacik D, Murakami Y, Paterlini-Bréchot P. Molecular bases for the development of hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) Semin Cancer Biol. 2000;10:211–231. doi: 10.1006/scbi.2000.0321. [DOI] [PubMed] [Google Scholar]
- 286.IARC (International Agency for Research on Cancer) Section 4.2.1. Integration of HBV DNA. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, et al., editors. Volume 59, Hepatitis Viruses. Lyon: International Agency for Research on Cancer; 1994. pp. 117–120. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http: //www.iarc.fr. [Google Scholar]
- 287.Tokino T, Fukushige S, Nakamura T, Nagaya T, Murotsu T, Shiga K, Aoki N, Matsubara K. Chromosomal translocation and inverted duplication associated with integrated hepatitis B virus in hepatocellular carcinomas. J Virol. 1987;61:3848–3854. doi: 10.1128/jvi.61.12.3848-3854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rotkin ID. A comparison review of key epidemiological studies in cervical cancer related to current searches for transmissible agents. Cancer Res. 1973;33:1353–1367. [PubMed] [Google Scholar]
- 289.zur Hausen H. Viruses in human tumors--reminiscences and perspectives. Adv Cancer Res. 1996;68:1–22. [PubMed] [Google Scholar]
- 290.Koutsky LA, Galloway DA, Holmes KK. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988;10:122–163. doi: 10.1093/oxfordjournals.epirev.a036020. [DOI] [PubMed] [Google Scholar]
- 291.zur Hausen H. Roots and perspectives of contemporary papillomavirus research. J Cancer Res Clin Oncol. 1996;122:3–13. doi: 10.1007/BF01203067. [DOI] [PubMed] [Google Scholar]
- 292.Boshart M, Gissmann L, Ikenberg H, Kleinheinz A, Scheurlen W, zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984;3:1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Schiffman MH, Castle P. Epidemiologic studies of a necessary causal risk factor: human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst. 2003;95:E2. doi: 10.1093/jnci/95.6.e2. [DOI] [PubMed] [Google Scholar]
- 294.zur Hausen H. Papillomavirus in anogenital cancer: the dilemma of epidemiologic approaches. J Natl Cancer Inst. 1989;81:1680–1682. doi: 10.1093/jnci/81.22.1680. [DOI] [PubMed] [Google Scholar]
- 295.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 296.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 297.DiMaio D, Petti LM. The E5 proteins. Virology. 2013;445:99–114. doi: 10.1016/j.virol.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tommasino M. The human papillomavirus family and its role in carcinogenesis. Semin Cancer Biol. 2014;26:13–21. doi: 10.1016/j.semcancer.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 299.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 300.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- 301.McLaughlin-Drubin ME, Münger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384:335–344. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Pett M, Coleman N. Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis? J Pathol. 2007;212:356–367. doi: 10.1002/path.2192. [DOI] [PubMed] [Google Scholar]
- 303.Pett MR, Alazawi WO, Roberts I, Dowen S, Smith DI, Stanley MA, Coleman N. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 2004;64:1359–1368. doi: 10.1158/0008-5472.can-03-3214. [DOI] [PubMed] [Google Scholar]
- 304.Melsheimer P, Vinokurova S, Wentzensen N, Bastert G, von Knebel Doeberitz M. DNA aneuploidy and integration of human papillomavirus type 16 e6/e7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteri. Clin Cancer Res. 2004;10:3059–3063. doi: 10.1158/1078-0432.ccr-03-0565. [DOI] [PubMed] [Google Scholar]
- 305.Ho CM, Lee BH, Chang SF, Chien TY, Huang SH, Yan CC, Cheng WF. Integration of human papillomavirus correlates with high levels of viral oncogene transcripts in cervical carcinogenesis. Virus Res. 2011;161:124–130. doi: 10.1016/j.virusres.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 306.Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci USA. 1995;92:1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cullen AP, Reid R, Campion M, Lörincz AT. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991;65:606–612. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Arias-Pulido H, Peyton CL, Joste NE, Vargas H, Wheeler CM. Human papillomavirus type 16 integration in cervical carcinoma in situ and in invasive cervical cancer. J Clin Microbiol. 2006;44:1755–1762. doi: 10.1128/JCM.44.5.1755-1762.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kjaer S, Høgdall E, Frederiksen K, Munk C, van den Brule A, Svare E, Meijer C, Lorincz A, Iftner T. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630–10636. doi: 10.1158/0008-5472.CAN-06-1057. [DOI] [PubMed] [Google Scholar]
- 310.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmerón J, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 311.Rana MM, Huhtala H, Apter D, Eriksson T, Luostarinen T, Natunen K, Paavonen J, Pukkala E, Lehtinen M. Understanding long-term protection of human papillomavirus vaccination against cervical carcinoma: Cancer registry-based follow-up. Int J Cancer. 2013;132:2833–2838. doi: 10.1002/ijc.27971. [DOI] [PubMed] [Google Scholar]
- 312.Arbyn M, de Sanjosé S, Saraiya M, Sideri M, Palefsky J, Lacey C, Gillison M, Bruni L, Ronco G, Wentzensen N, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer. 2012;131:1969–1982. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.IARC (International Agency for Research on Cancer) Human papillomaviruses. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100B: A Review of Human Carcinogens: Biological Agents, et al., editors. Lyon: International Agency for Research on Cancer; 2012. pp. 255–313. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 314.Prince AM, Brotman B, Grady GF, Kuhns WJ, Hazzi C, Levine RW, Millian SJ. Long-incubation post-transfusion hepatitis without serological evidence of exposure to hepatitis-B virus. Lancet. 1974;2:241–246. doi: 10.1016/s0140-6736(74)91412-3. [DOI] [PubMed] [Google Scholar]
- 315.Resnick RH, Stone K, Antonioli D. Primary hepatocellular carcinoma following non-A, non-B posttransfusion hepatitis. Dig Dis Sci. 1983;28:908–911. doi: 10.1007/BF01317042. [DOI] [PubMed] [Google Scholar]
- 316.Gilliam JH, Geisinger KR, Richter JE. Primary hepatocellular carcinoma after chronic non-A, non-B post-transfusion hepatitis. Ann Intern Med. 1984;101:794–795. doi: 10.7326/0003-4819-101-6-794. [DOI] [PubMed] [Google Scholar]
- 317.Purcell RH. Hepatitis C virus: historical perspective and current concepts. FEMS Microbiol Rev. 1994;14:181–191. doi: 10.1111/j.1574-6976.1994.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 318.Shih JW, Mur JI, Alter HJ. Non-A, non-B hepatitis: advances and unfulfilled expectations of the first decade. Prog Liver Dis. 1986;8:433–452. [PubMed] [Google Scholar]
- 319.Bradley DW, Maynard JE. Etiology and natural history of post-transfusion and enterically-transmitted non-A, non-B hepatitis. Semin Liver Dis. 1986;6:56–66. doi: 10.1055/s-2008-1040794. [DOI] [PubMed] [Google Scholar]
- 320.Houghton M. The long and winding road leading to the identification of the hepatitis C virus. J Hepatol. 2009;51:939–948. doi: 10.1016/j.jhep.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 321.Choo QL, Weiner AJ, Overby LR, Kuo G, Houghton M, Bradley DW. Hepatitis C virus: the major causative agent of viral non-A, non-B hepatitis. Br Med Bull. 1990;46:423–441. doi: 10.1093/oxfordjournals.bmb.a072408. [DOI] [PubMed] [Google Scholar]
- 322.Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 323.Bruix J, Barrera JM, Calvet X, Ercilla G, Costa J, Sanchez-Tapias JM, Ventura M, Vall M, Bruguera M, Bru C. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1004–1006. doi: 10.1016/s0140-6736(89)91015-5. [DOI] [PubMed] [Google Scholar]
- 324.Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006–1008. doi: 10.1016/s0140-6736(89)91016-7. [DOI] [PubMed] [Google Scholar]
- 325.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 326.Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH, Liu CJ, Chen PJ, You SL, Wang LY, Chen WJ, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587–4593. doi: 10.1200/JCO.2010.29.1500. [DOI] [PubMed] [Google Scholar]
- 327.Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, Lee WM, Di Bisceglie AM, Bonkovsky HL, Dienstag JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Breitenstein S, Dimitroulis D, Petrowsky H, Puhan MA, Müllhaupt B, Clavien PA. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96:975–981. doi: 10.1002/bjs.6731. [DOI] [PubMed] [Google Scholar]
- 329.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 330.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 331.Kimer N, Dahl EK, Gluud LL, Krag A. Antiviral therapy for prevention of hepatocellular carcinoma in chronic hepatitis C: systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2012;2:e001313. doi: 10.1136/bmjopen-2012-001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol. 2015;62:S87–S99. doi: 10.1016/j.jhep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 333.Farci P. New insights into the HCV quasispecies and compartmentalization. Semin Liver Dis. 2011;31:356–374. doi: 10.1055/s-0031-1297925. [DOI] [PubMed] [Google Scholar]
- 334.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 335.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 336.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 337.McGivern DR, Lemon SM. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30:1969–1983. doi: 10.1038/onc.2010.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Burkitt DP. The discovery of Burkitt’s lymphoma. Cancer. 1983;51:1777–1786. doi: 10.1002/1097-0142(19830515)51:10<1777::aid-cncr2820511003>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 339.Epstein MA. Historical background; Burkitt’s lymphoma and Epstein-Barr virus. IARC Sci Publ. 1985;(60):17–27. [PubMed] [Google Scholar]
- 340.Henle G, Henle W, Clifford P, Diehl V, Kafuko GW, Kirya BG, Klein G, Morrow RH, Munube GM, Pike P, et al. Antibodies to Epstein-Barr virus in Burkitt’s lymphoma and control groups. J Natl Cancer Inst. 1969;43:1147–1157. [PubMed] [Google Scholar]
- 341.de-Thé G, Geser A, Day NE, Tukei PM, Williams EH, Beri DP, Smith PG, Dean AG, Bronkamm GW, Feorino P, et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt’s lymphoma from Ugandan prospective study. Nature. 1978;274:756–761. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]
- 342.Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- 343.Burkitt D. Determining the climatic limitations of a children’s cancer common in Africa. Br Med J. 1962;2:1019–1023. doi: 10.1136/bmj.2.5311.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Niedobitek G, Meru N, Delecluse HJ. Epstein-Barr virus infection and human malignancies. Int J Exp Pathol. 2001;82:149–170. doi: 10.1046/j.1365-2613.2001.iep0082-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Thorley-Lawson DA. EBV the prototypical human tumor virus--just how bad is it? J Allergy Clin Immunol. 2005;116:251–61; quiz 262. doi: 10.1016/j.jaci.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 346.Thorley-Lawson DA, Allday MJ. The curious case of the tumour virus: 50 years of Burkitt’s lymphoma. Nat Rev Microbiol. 2008;6:913–924. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- 347.Klein G. The relationship of the virus to nasopharyngeal carcinoma. In: Epstein MA, Achong BG, et al., editors. The Epstein-Barr Virus. Berlin: Springer-Verlag; 1979. pp. 339–350. [Google Scholar]
- 348.Niedobitek G, Agathanggelou A, Nicholls JM. Epstein-Barr virus infection and the pathogenesis of nasopharyngeal carcinoma: viral gene expression, tumour cell phenotype, and the role of the lymphoid stroma. Semin Cancer Biol. 1996;7:165–174. doi: 10.1006/scbi.1996.0023. [DOI] [PubMed] [Google Scholar]
- 349.Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen CJ, Yang CS. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345:1877–1882. doi: 10.1056/NEJMoa011610. [DOI] [PubMed] [Google Scholar]
- 350.Ji MF, Wang DK, Yu YL, Guo YQ, Liang JS, Cheng WM, Zong YS, Chan KH, Ng SP, Wei WI, et al. Sustained elevation of Epstein-Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br J Cancer. 2007;96:623–630. doi: 10.1038/sj.bjc.6603609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431–441. doi: 10.1016/s1044579x0200086x. [DOI] [PubMed] [Google Scholar]
- 352.Louis CU, Straathof K, Bollard CM, Ennamuri S, Gerken C, Lopez TT, Huls MH, Sheehan A, Wu MF, Liu H, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33:983–990. doi: 10.1097/CJI.0b013e3181f3cbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, Gresik MV, Gee AP, Russell HV, Brenner MK, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus--specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 354.Shah KM, Young LS. Epstein-Barr virus and carcinogenesis: beyond Burkitt’s lymphoma. Clin Microbiol Infect. 2009;15:982–988. doi: 10.1111/j.1469-0691.2009.03033.x. [DOI] [PubMed] [Google Scholar]
- 355.Gourzones C, Busson P, Raab-Traub N. Epstein-Barr virus and the pathogenesis of nasopharyngeal carcinomas. Adv Exp Med Biol. 2013;778:42–60. [Google Scholar]
- 356.Gilligan KJ, Rajadurai P, Lin JC, Busson P, Abdel-Hamid M, Prasad U, Tursz T, Raab-Traub N. Expression of the Epstein-Barr virus BamHI A fragment in nasopharyngeal carcinoma: evidence for a viral protein expressed in vivo. J Virol. 1991;65:6252–6259. doi: 10.1128/jvi.65.11.6252-6259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Lo AK, Dawson CW, Jin DY, Lo KW. The pathological roles of BART miRNAs in nasopharyngeal carcinoma. J Pathol. 2012;227:392–403. doi: 10.1002/path.4025. [DOI] [PubMed] [Google Scholar]
- 358.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 359.Raab-Traub N. Novel mechanisms of EBV-induced oncogenesis. Curr Opin Virol. 2012;2:453–458. doi: 10.1016/j.coviro.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.IARC (International Agency for Research on Cancer) Epstein-Barr Virus. In: Volume 100B, A Review of Human Carcinogens: Biological Agents, IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, et al., editors. Lyon: International Agency for Research on Cancer; 2012. pp. 49–92. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 361.Cohen JI. Epstein-barr virus vaccines. Clin Transl Immunology. 2015;4:e32. doi: 10.1038/cti.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Moore PS, Chang Y. Kaposi’s sarcoma (KS), KS-associated herpesvirus, and the criteria for causality in the age of molecular biology. Am J Epidemiol. 1998;147:217–221. doi: 10.1093/oxfordjournals.aje.a009440. [DOI] [PubMed] [Google Scholar]
- 363.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 364.IARC (International Agency for Research on Cancer) Detection of KSHV/HHV8 in tumor tissue. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, et al., editors. Volume 70, Epstein-Barr Virus Kaposi’s Sarcoma Herpesviruses/Human Herpesvirus 8. Lyon: International Agency for Research on Cancer; 1997. pp. 402–410. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 365.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 366.Moore PS, Kingsley LA, Holmberg SD, Spira T, Gupta P, Hoover DR, Parry JP, Conley LJ, Jaffe HW, Chang Y. Kaposi’s sarcoma-associated herpesvirus infection prior to onset of Kaposi’s sarcoma. AIDS. 1996;10:175–180. doi: 10.1097/00002030-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 367.O’Brien TR, Kedes D, Ganem D, Macrae DR, Rosenberg PS, Molden J, Goedert JJ. Evidence for concurrent epidemics of human herpesvirus 8 and human immunodeficiency virus type 1 in US homosexual men: rates, risk factors, and relationship to Kaposi’s sarcoma. J Infect Dis. 1999;180:1010–1017. doi: 10.1086/315039. [DOI] [PubMed] [Google Scholar]
- 368.Whitby D, Howard MR, Tenant-Flowers M, Brink NS, Copas A, Boshoff C, Hatzioannou T, Suggett FE, Aldam DM, Denton AS. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 369.Guihot A, Dupin N, Marcelin AG, Gorin I, Bedin AS, Bossi P, Galicier L, Oksenhendler E, Autran B, Carcelain G. Low T cell responses to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194:1078–1088. doi: 10.1086/507648. [DOI] [PubMed] [Google Scholar]
- 370.Lambert M, Gannagé M, Karras A, Abel M, Legendre C, Kerob D, Agbalika F, Girard PM, Lebbe C, Caillat-Zucman S. Differences in the frequency and function of HHV8-specific CD8 T cells between asymptomatic HHV8 infection and Kaposi sarcoma. Blood. 2006;108:3871–3880. doi: 10.1182/blood-2006-03-014225. [DOI] [PubMed] [Google Scholar]
- 371.Casper C, Wald A. The use of antiviral drugs in the prevention and treatment of Kaposi sarcoma, multicentric Castleman disease and primary effusion lymphoma. Curr Top Microbiol Immunol. 2007;312:289–307. doi: 10.1007/978-3-540-34344-8_11. [DOI] [PubMed] [Google Scholar]
- 372.Duprez R, Lacoste V, Brière J, Couppié P, Frances C, Sainte-Marie D, Kassa-Kelembho E, Lando MJ, Essame Oyono JL, Nkegoum B, et al. Evidence for a multiclonal origin of multicentric advanced lesions of Kaposi sarcoma. J Natl Cancer Inst. 2007;99:1086–1094. doi: 10.1093/jnci/djm045. [DOI] [PubMed] [Google Scholar]
- 373.Ensoli B, Stürzl M. Kaposi’s sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 1998;9:63–83. doi: 10.1016/s1359-6101(97)00037-3. [DOI] [PubMed] [Google Scholar]
- 374.IARC (International Agency for Research on Cancer) Kaposi Sarcoma Kerpesvirus. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100B: A Review of Human Carcinogens: Biological Agents, et al., editors. Lyon: International Agency for Research on Cancer; 2012. pp. 169–214. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 375.Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Lander ES. Initial impact of the sequencing of the human genome. Nature. 2011;470:187–197. doi: 10.1038/nature09792. [DOI] [PubMed] [Google Scholar]
- 377.Xiong Y, Eickbush TH. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990;9:3353–3362. doi: 10.1002/j.1460-2075.1990.tb07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Urnovitz HB, Murphy WH. Human endogenous retroviruses: nature, occurrence, and clinical implications in human disease. Clin Microbiol Rev. 1996;9:72–99. doi: 10.1128/cmr.9.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Stoye JP. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat Rev Microbiol. 2012;10:395–406. doi: 10.1038/nrmicro2783. [DOI] [PubMed] [Google Scholar]
- 380.Hohn O, Hanke K, Bannert N. HERV-K(HML-2), the Best Preserved Family of HERVs: Endogenization, Expression, and Implications in Health and Disease. Front Oncol. 2013;3:246. doi: 10.3389/fonc.2013.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Contreras-Galindo R, Kaplan MH, Leissner P, Verjat T, Ferlenghi I, Bagnoli F, Giusti F, Dosik MH, Hayes DF, Gitlin SD, et al. Human endogenous retrovirus K (HML-2) elements in the plasma of people with lymphoma and breast cancer. J Virol. 2008;82:9329–9336. doi: 10.1128/JVI.00646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wang-Johanning F, Li M, Esteva FJ, Hess KR, Yin B, Rycaj K, Plummer JB, Garza JG, Ambs S, Johanning GL. Human endogenous retrovirus type K antibodies and mRNA as serum biomarkers of early-stage breast cancer. Int J Cancer. 2014;134:587–595. doi: 10.1002/ijc.28389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Schmitt K, Reichrath J, Roesch A, Meese E, Mayer J. Transcriptional profiling of human endogenous retrovirus group HERV-K(HML-2) loci in melanoma. Genome Biol Evol. 2013;5:307–328. doi: 10.1093/gbe/evt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Beck CR, Garcia-Perez JL, Badge RM, Moran JV. LINE-1 elements in structural variation and disease. Annu Rev Genomics Hum Genet. 2011;12:187–215. doi: 10.1146/annurev-genom-082509-141802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Tubio JM, Li Y, Ju YS, Martincorena I, Cooke SL, Tojo M, Gundem G, Pipinikas CP, Zamora J, Raine K, et al. Mobile DNA in cancer. Extensive transduction of nonrepetitive DNA mediated by L1 retrotransposition in cancer genomes. Science. 2014;345:1251343. doi: 10.1126/science.1251343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Lee E, Iskow R, Yang L, Gokcumen O, Haseley P, Luquette LJ, Lohr JG, Harris CC, Ding L, Wilson RK, et al. Landscape of somatic retrotransposition in human cancers. Science. 2012;337:967–971. doi: 10.1126/science.1222077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Rebollo R, Romanish MT, Mager DL. Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annu Rev Genet. 2012;46:21–42. doi: 10.1146/annurev-genet-110711-155621. [DOI] [PubMed] [Google Scholar]
- 388.Kaer K, Speek M. Retroelements in human disease. Gene. 2013;518:231–241. doi: 10.1016/j.gene.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 389.Levin HL, Moran JV. Dynamic interactions between transposable elements and their hosts. Nat Rev Genet. 2011;12:615–627. doi: 10.1038/nrg3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Rowe HM, Trono D. Dynamic control of endogenous retroviruses during development. Virology. 2011;411:273–287. doi: 10.1016/j.virol.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 391.Doisy EA, Veler CD, Thayer SA. Folliculin from the urine of pregnant women. Am J Physiol. 1929;90:329–330. [Google Scholar]
- 392.Fieser LF, Fieser M. Steroids. New York: Reinhold Publishing Corporation; 1959. [Google Scholar]
- 393.Ewing J. The General Pathological Conception of Cancer. Can Med Assoc J. 1935;33:125–135. [PMC free article] [PubMed] [Google Scholar]
- 394.Lane-Claypon JE. A further report on cancer of the breast with special reference to its associated antecedent conditions. London: HMSO; 1926. [Google Scholar]
- 395.Press DJ, Pharoah P. Risk factors for breast cancer: a reanalysis of two case-control studies from 1926 and 1931. Epidemiology. 2010;21:566–572. doi: 10.1097/EDE.0b013e3181e08eb3. [DOI] [PubMed] [Google Scholar]
- 396.MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43:209–221. [PMC free article] [PubMed] [Google Scholar]
- 397.Beral V, Bull D, Doll R, Peto R, Reeves G, Skeeg D, Colditz G, Hulka B, La Vecchia C, Magnusson C, et al. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 398.World Cancer Research Fund/American Institute for Cancer Research. Chapter 6: Growth, development, body composition, 6.3 Lactation. In: Food , Nutrition , Physical Activity, and the Prevention of Cancer: a Global Perspective, et al., editors. Washington, DC: AIRC; 2007. pp. 211–228. Available from: http://www.dietandcancerreport.org/expert_report/report_contents/index.php. [Google Scholar]
- 399.Hollander AW. Development of dermatopathology and Paul Gerson Unna. J Am Acad Dermatol. 1986;15:727–734. doi: 10.1016/s0190-9622(86)80116-5. [DOI] [PubMed] [Google Scholar]
- 400.Unna PG. Die Histopathologie der Hautkrankheiten. Berlin: Hirschwald A; 1894. p. [in German]. [Google Scholar]
- 401.Unna PG. Carcinoma of the sailor’s skin. In: The Histopathology of the Diseases of the Skin by Dr P, et al., editors. G. Unna- translated from the German with assistance of the author, by Norman Walker. New York: Macmillan & Co; 1896. pp. 719–724. [Google Scholar]
- 402.IARC (International Agency for Research on Cancer) Infection with schistosomes (Schistosoma haematobium, S. mansoni and S. japonicum) In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, et al., editors. Volume 61, Schistosomes, liver flukes and helicobacter pylori. Lyon: International Agency for Research on Cancer; 1994. pp. 45–120, esp 45-59. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 403.Ferguson AR. Associated bilharziosis and primary malignant disease of the urinary bladder, with observations on a series of forty cases. J Pathol Bacteriol. 1911;16:76–98. [Google Scholar]
- 404.Stewart MJ. Precancerous lesions of the alimentary tract. Lancet. 1931;218:669–675. [Google Scholar]
- 405.Bhamarapravati N, Virranuvatti V. Liver diseases in Thailand. An analysis of liver biopsies. Am J Gastroenterol. 1966;45:267–275. [PubMed] [Google Scholar]
- 406.IARC (International Agency for Research on Cancer) Infection with liver flukes (Opisthorchis viverrini, O. felineus and Clonorchis sinensis) In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, et al., editors. Volume 61, Schistosomes, liver flukes and helicobacter pylori. Lyon: International Agency for Research on Cancer; 1994. pp. 121–175, esp 136, 139. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 407.Allcroft R. Chapter IX. Aflatoxicosis in farm animals. In: Goldblatt LA, editor , Aflatoxin , scientific background, control , et al., editors. New York: Academic Press; 1969. pp. 237–264. [Google Scholar]
- 408.Sargeant K, Sheridan A, O’Kelly J, Carnaghan RBA. Toxicity associated with ground nuts. Nature. 1961;192:1096–1097. [Google Scholar]
- 409.Groopman JD, Wang JS, Scholl P. Molecular biomarkers for aflatoxins: from adducts to gene mutations to human liver cancer. Can J Physiol Pharmacol. 1996;74:203–209. [PubMed] [Google Scholar]
- 410.Jackson PE, Groopman JD. Aflatoxin and liver cancer. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:545–555. doi: 10.1053/bega.1999.0047. [DOI] [PubMed] [Google Scholar]
- 411.Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 412.Qian GS, Ross RK, Yu MC, Yuan JM, Gao YT, Henderson BE, Wogan GN, Groopman JD. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3:3–10. [PubMed] [Google Scholar]
- 413.Wu F, Groopman JD, Pestka JJ. Public health impacts of foodborne mycotoxins. Annu Rev Food Sci Technol. 2014;5:351–372. doi: 10.1146/annurev-food-030713-092431. [DOI] [PubMed] [Google Scholar]
- 414.Warren JR. Helicobacter: the ease and difficulty of a new discovery (Nobel lecture) ChemMedChem. 2006;1:672–685. doi: 10.1002/cmdc.200600121. [DOI] [PubMed] [Google Scholar]
- 415.Moss SF. The rediscovery of H. pylori bacteria in the gastric mucosa by Robin Warren, and implications of this finding for human biology and disease. Dig Dis Sci. 2013;58:3072–3078. doi: 10.1007/s10620-013-2863-y. [DOI] [PubMed] [Google Scholar]
- 416.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 417.Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–323. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 418.Thomsen LL, Gavin JB, Tasman-Jones C. Relation of Helicobacter pylori to the human gastric mucosa in chronic gastritis of the antrum. Gut. 1990;31:1230–1236. doi: 10.1136/gut.31.11.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Tytgat GN, Rauws EA. Campylobacter pylori and its role in peptic ulcer disease. Gastroenterol Clin North Am. 1990;19:183–196. [PubMed] [Google Scholar]
- 420.Kang HY, Kim N, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Jung HC, Song IS. Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci. 2006;51:2310–2315. doi: 10.1007/s10620-006-9276-0. [DOI] [PubMed] [Google Scholar]
- 421.Hazell SL, Hennessy WB, Borody TJ, Carrick J, Ralston M, Brady L, Lee A. Campylobacter pyloridis gastritis II: Distribution of bacteria and associated inflammation in the gastroduodenal environment. Am J Gastroenterol. 1987;82:297–301. [PubMed] [Google Scholar]
- 422.Robey-Cafferty SS, Ro JY, Cleary KR. The prevalence of Campylobacter pylori in gastric biopsies from cancer patients. Mod Pathol. 1989;2:473–476. [PubMed] [Google Scholar]
- 423.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 424.Webb PM, Law M, Varghese C, Forman D, Yuan JM, Yu M, Ross R, Limburg PJ, Mark SD, Taylor PR, Dawsey SM, Qiao YL, Aromaa A, Knet P, Kosunen TU, Heinonen OP, Virtamo J, Tulinius H, Ogmundsdottir H, Watanabe Y, Ozasa K, Kurata JH, Hansen S, Melby KK, Aase S, Jellum E, Vollset SE, Siman JH, Forsgren A, Berglund G, Floren CH, Lin JT, Chen C J, Forman D, Law M, Wald NJ, Parsonnet J, Friedman GD, Blaser MJ, Nomura A, Stemmermann GN, Helicobacter Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Acheson ED, Cowdell RH, Hadfield E, Macbeth RG. Nasal cancer in woodworkers in the furniture industry. Br Med J. 1968;2:587–596. doi: 10.1136/bmj.2.5605.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.IARC (International Agency for Research on Cancer) Wood dust. In: IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man Volume 100C: A Review of Human Carcinogens: Arsenic, Metals , Fibres and Dusts, et al., editors. Lyon: International Agency for Research on Cancer; 2012. pp. 407–465, esp 443, 449. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http://www.iarc.fr. [Google Scholar]
- 427.Young M, Russell WT. An Investigation into the Statistics of Cancer in Different Trades and Professions. London: His Majesty’s Stationery Office; 1926. [Google Scholar]
- 428.Ho JH. Nasopharyngeal carcinoma (NPC) Adv Cancer Res. 1972;15:57–92. doi: 10.1016/s0065-230x(08)60372-3. [DOI] [PubMed] [Google Scholar]
- 429.Fong YY, Chan WC. Methods for limiting the content of dimethylnitrosamine in Chinese marine salt fish. Food Cosmet Toxicol. 1976;14:95–98. doi: 10.1016/s0015-6264(76)80250-7. [DOI] [PubMed] [Google Scholar]
- 430.Fong YY, Chan WC. Bacterial production of di-methyl nitrosamine in salted fish. Nature. 1973;243:421–422. doi: 10.1038/243421a0. [DOI] [PubMed] [Google Scholar]
- 431.Fong YY, Chan WC. Dimethylnitrosamine in Chinese marine salt fish. Food Cosmet Toxicol. 1973;11:841–845. doi: 10.1016/0015-6264(73)90142-9. [DOI] [PubMed] [Google Scholar]
- 432.Huang DP, Ho JH, Saw D, Teoh TB. Carcinoma of the nasal and paranasal regions in rats fed Cantonese salted marine fish. IARC Sci Publ. 1978;(20):315–328. [PubMed] [Google Scholar]
- 433.Yu MC, Nichols PW, Zou XN, Estes J, Henderson BE. Induction of malignant nasal cavity tumours in Wistar rats fed Chinese salted fish. Br J Cancer. 1989;60:198–201. doi: 10.1038/bjc.1989.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Geser A, Charnay N, Day NE, de-The G, Ho HC. Environmental factors in the etiology of nasopharyngeal carcinoma: report on a case-control study in Hong Kong. IARC Sci Publ. 1978:(20): 213–229. [PubMed] [Google Scholar]
- 435.Cosyns JP, Jadoul M, Squifflet JP, Wese FX, van Ypersele de Strihou C. Urothelial lesions in Chinese-herb nephropathy. Am J Kidney Dis. 1999;33:1011–1017. doi: 10.1016/S0272-6386(99)70136-8. [DOI] [PubMed] [Google Scholar]
- 436.Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, Depierreux MF, De Pauw L, Abramowicz D, Vereerstraeten P, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000;342:1686–1692. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 437.Markovic N, Ignjatovic I, Cukuranovic R, Petrovic B, Kocic B, Stefanovic V. Decreasing incidence of urothelial cancer in a Balkan endemic nephropathy region in Serbia. A surgery based study from 1969 to 1998. Pathol Biol (Paris) 2005;53:26–29. doi: 10.1016/j.patbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 438.Colin P, Koenig P, Ouzzane A, Berthon N, Villers A, Biserte J, Rouprêt M. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009;104:1436–1440. doi: 10.1111/j.1464-410X.2009.08838.x. [DOI] [PubMed] [Google Scholar]
- 439.Hsieh SC, Lin IH, Tseng WL, Lee CH, Wang JD. Prescription profile of potentially aristolochic acid containing Chinese herbal products: an analysis of National Health Insurance data in Taiwan between 1997 and 2003. Chin Med. 2008;3:13. doi: 10.1186/1749-8546-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Yang MH, Chen KK, Yen CC, Wang WS, Chang YH, Huang WJ, Fan FS, Chiou TJ, Liu JH, Chen PM. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology. 2002;59:681–687. doi: 10.1016/s0090-4295(02)01529-7. [DOI] [PubMed] [Google Scholar]
- 441.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. 2011;107:1059–1064. doi: 10.1111/j.1464-410X.2010.09675.x. [DOI] [PubMed] [Google Scholar]
- 442.Albanes D. Caloric intake, body weight, and cancer: a review. Nutr Cancer. 1987;9:199–217. doi: 10.1080/01635588709513929. [DOI] [PubMed] [Google Scholar]
- 443.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 444.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 445.World Cancer Research Fund/American Institute for Cancer Research. Chapter 6: Growth, development, body composition, 6.1 Body fatness. In: Food , Nutrition , Physical Activity, and the Prevention of Cancer: a Global Perspective, et al., editors. Washington, DC: AIRC; 2007. pp. 211–228. Available from: http://www.dietandcancerreport.org/expert_report/report_contents/index.php. [Google Scholar]
- 446.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 447.World Cancer Research Fund/American Institute for Cancer Research. Chapter 5: physical activity. In: Food , Nutrition , Physical Activity, and the Prevention of Cancer: a Global Perspective, et al., editors. Washington, DC: AIRC; 2007. pp. 198–209. Available from: http://www.dietandcancerreport.org/expert_report/report_contents/index.php. [Google Scholar]
- 448.Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6:e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.World Cancer Research Fund/American Institute for Cancer Research. Chapter 4: Foods and drinks, section 4.3 Meat, poultry, fish and eggs. In: Food , Nutrition , Physical Activity, and the Prevention of Cancer: a Global Perspective, et al., editors. Washington, DC: AIRC; 2007. pp. 116–128. Available from: http://www.dietandcancerreport.org/expert_report/report_contents/index.php. [Google Scholar]
- 450.World Cancer Research Fund/American Institute for Cancer Research. Continuous update project report: Food, Nutrition, Physical Activity, and the Prevention of colorectal cancer. Washington, DC: AIRC; 2011. Available from: http://www.wcrf.org/sites/default/files/Colorectal-Cancer-2011-Report.pdf. [Google Scholar]
- 451.Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 453.Heinonen OP, Huttunen JK, Albanes D, Haapakoski J, Palmgren J, Pietinen P, Pikkarainen J, Rautalahti M, Virtamo J, Edwards BK, et al. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 454.World Cancer Research Fund/American Institute for Cancer Research. Chapter 4: Foods and drinks, section 4.10 Dietary constituents and supplements. In: Food , Nutrition , Physical Activity, and the Prevention of Cancer: a Global Perspective, et al., editors. Washington, DC: AIRC; 2007. pp. 179–189. Available from: http://www.dietandcancerreport.org/expert_report/report_contents/index.php. [Google Scholar]
- 455.World Cancer Research Fund/American Institute for Cancer Research. Chapter 3: Judging the evidence, section 3.5 Coming to judgement. In: Food , Nutrition , Physical Activity, and the Prevention of Cancer: a Global Perspective, et al., editors. Washington, DC: AIRC; 2007. pp. 58–61, esp 60. Available from: http://www.dietandcancerreport.org/expert_report/report_contents/index.php. [Google Scholar]
- 456.World Cancer Research Fund/American Institute for Cancer Research. Chapter 4: Foods and drinks, section 4.2 Vegetables, fruits, pulses (legumes), nuts, seeds, herbs, spices. In: Food , Nutrition , Physical Activity, and the Prevention of Cancer: a Global Perspective, et al., editors. Washington, DC: AIRC; 2007. pp. 75–115, esp 113-114. Available from: http://www.dietandcancerreport.org/expert_report/report_contents/index.php. [Google Scholar]
- 457.Norat T, Aune D, Chan D, Romaguera D. Fruits and vegetables: updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention. Cancer Treat Res. 2014;159:35–50. doi: 10.1007/978-3-642-38007-5_3. [DOI] [PubMed] [Google Scholar]
- 458.Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, Kampman E, Norat T. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23:37–45. doi: 10.1093/annonc/mdr269. [DOI] [PubMed] [Google Scholar]
- 459.World Cancer Research Fund/American Institute for Cancer Research. Continuous update project report: Diet, Nutrition, physical activity and liver cancer. 2015. Washington, DC: AIRC; 2015. Available from: http://www.wcrf.org/sites/default/files/Liver-Cancer-2015-Report.pdf. [Google Scholar]
- 460.Hirono I. Natural carcinogenic products of plant origin. Crit Rev Toxicol. 1981;8:235–277. doi: 10.3109/10408448109109659. [DOI] [PubMed] [Google Scholar]
- 461.Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis. 2000;21:387–395. doi: 10.1093/carcin/21.3.387. [DOI] [PubMed] [Google Scholar]
- 462.Ames BN, Profet M, Gold LS. Dietary pesticides (99.99% all natural) Proc Natl Acad Sci USA. 1990;87:7777–7781. doi: 10.1073/pnas.87.19.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Ames BN, Profet M, Gold LS. Nature’s chemicals and synthetic chemicals: comparative toxicology. Proc Natl Acad Sci USA. 1990;87:7782–7786. doi: 10.1073/pnas.87.19.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Cheeke PR. Natural toxicants in feeds, forages, and poisonous plants: second edition. Danville, (Il): Interstate Publishers; 1998. [Google Scholar]
- 465.Gold LS, Slone TH, Stern BR, Manley NB, Ames BN. Rodent carcinogens: setting priorities. Science. 1992;258:261–265. doi: 10.1126/science.1411524. [DOI] [PubMed] [Google Scholar]
- 466.Brambilla G, Mattioli F, Robbiano L, Martelli A. Update of carcinogenicity studies in animals and humans of 535 marketed pharmaceuticals. Mutat Res. 2012;750:1–51. doi: 10.1016/j.mrrev.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 467.Zeise L, Wilson R, Crouch EA. Dose-response relationships for carcinogens: a review. Environ Health Perspect. 1987;73:259–306. doi: 10.1289/ehp.8773259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.IARC (International Agency for Research on Cancer) IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. Volume 75, Ionizing Radiation, Part 1: X- and Gamma (γ)-Radiation, and Neutrons. Lyon: International Agency for Research on Cancer; 2000. Available from: http://www.iarc.fr/en/publications/list/monographs/index.php or http: //www.iarc.fr. [Google Scholar]
- 469.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 470.Hunter D. Diseases of Occupations, sixth edition. London: Hodder & Stoughton; 1978. [Google Scholar]
- 471.Marchesi JR. Human distal gut microbiome. Environ Microbiol. 2011;13:3088–3102. doi: 10.1111/j.1462-2920.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 472.Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57:1605–1615. doi: 10.1136/gut.2007.133603. [DOI] [PubMed] [Google Scholar]
- 473.Proctor LM. The Human Microbiome Project in 2011 and beyond. Cell Host Microbe. 2011;10:287–291. doi: 10.1016/j.chom.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 474.Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10:575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- 475.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Mark EJ, Yokoi T. Absence of evidence for a significant background incidence of diffuse malignant mesothelioma apart from asbestos exposure. Ann N Y Acad Sci. 1991;643:196–204. doi: 10.1111/j.1749-6632.1991.tb24463.x. [DOI] [PubMed] [Google Scholar]
- 478.McCaughey WT. The diagnosis of mesothelioma by panels. Am J Ind Med. 1991;19:121–124. doi: 10.1002/ajim.4700190113. [DOI] [PubMed] [Google Scholar]
- 479.Lorenz E. Radioactivity and lung cancer: critical review of lung cancer in miners of Schneeberg and Joachimsthal. J Natl Cancer Inst. 1944;5:1–15, esp 1. [Google Scholar]
- 480.Case RA. Tumours of the urinary tract as an occupational disease in several industries. Ann R Coll Surg Engl. 1966;39:213–235. [PMC free article] [PubMed] [Google Scholar]
- 481.Doll R. Strategy for detection of cancer hazards to man. Nature. 1977;265:589–596. doi: 10.1038/265589a0. [DOI] [PubMed] [Google Scholar]
- 482.Williams MHC. Occupational tumors of the bladder. In: Raven RW, editor , Cancer , Volume 3, et al., editors. London: Butterworth & Co; 1958. pp. 337–380, esp Tables I, II, 347. [Google Scholar]
- 483.Barling G. Obituary. J. F. Hall-Edwards. Br J Radiol (B. I. R. Sect.) 1926;31:455–459, esp 459. [Google Scholar]
- 484.Stoll NR. Investigations on the control of hookworm disease XV: An effective method of counting hookworm eggs in feces. Am J Hyg. 1923;3:59–70. [Google Scholar]
- 485.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 486.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 487.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]


