Abstract
“Vaccell” is a dendritic cell (DC)-based cancer vaccine which has been established in Japan. The DCs play central roles in deciding the direction of host immune reactions as well as antigen presentation. We have demonstrated that DCs treated with a streptococcal immune adjuvant OK-432, produce interleukin-12, induce Th1-dominant state, and elicit anti-tumor effects, more powerful than those treated with the known DC-maturating factors. We therefore decided to mature DCs by the OK-432 for making an effective DC vaccine, Vaccell. The 255 patients with inoperable pancreatic cancer who received standard chemotherapy combined with DC vaccines, were analyzed retrospectively. Survival time of the patients with positive delayed type hypersensitivity (DTH) skin reaction was significantly prolonged as compared with that of the patients with negative DTH. The findings strongly suggest that there may be “Responders” for the DC vaccine in advanced pancreatic cancer patients. We next conducted a small-scale prospective clinical study. In this trial, we pulsed HLA class II-restricted WT1 peptide (WT1-II) in addition to HLA class I-restricted peptide (WT1-I) into the DCs. Survival of the patients received WT1-I and -II pulsed DC vaccine was significantly extended as compared to that of the patients received DCs pulsed with WT1-I or WT1-II alone. Furthermore, WT1-specific DTH positive patients showed significantly improved the overall survival as well as progression-free survival as compared to the DTH negative patients. The activation of antigen-specific immune responses by DC vaccine in combination with standard chemotherapy may be associated with a good clinical outcome in advanced pancreatic cancer. We are now planning a pivotal study of the Vaccell in appropriate protocols in Japan.
Keywords: Dendritic cell, Cancer vaccine, Pancreatic cancer, Cancer immunotherapy, Anti-cancer immunity
Core tip: Dendritic cell (DC)-based cancer vaccine is expected as a strategy to augment the antigen-specific anti-cancer immune response. Vaccell, a DC vaccine which was optimized to fight against a cancer, has been developed in Japan. Here, we reviewed the development and clinical effects of the “Vaccell”. We conducted large-scale retrospective observations as well as prospective clinical trials, and obtained the findings strongly suggesting that there are “Responders” that the clinical benefits are provided by the DC vaccine. We are now planning a pivotal study of the Vaccell in Japan.
INTRODUCTION
Recently, a clinical benefit of certain immunotherapies for cancer was proved. Especially, anti-cancer effect of the blocking antibodies for checkpoint molecules such as PD-1 and CTLA-4[1-4], is promising, and editors of the Science journal selected the “Cancer Immunotherapy” as a top of breakthrough of the year 2013[5].
On the other hand, although cancer vaccine that is one of the antigen-specific immunotherapy, has been expected for the patients with malignancies resistant to standard treatment, the therapeutic effect has not been evidenced. Even the certain cancer vaccines which showed clinical effects in early phase clinical studies[6-9], has not demonstrated clear clinical benefits in pivotal phase III trials.
The dendritic cell (DC)-based vaccine is expected as a strategy to augment the effects of cancer vaccine. On July 16, 2007, the Swiss Institute of Public Health has approved the world’s first therapeutic vaccine for brain cancer DCVax®-Brain (Northwest Biotherapeutics Inc.), and on April 29, 2010, the United States Food and Drug Administration (FDA) has approved sipuleucel-T (Dendreon Corp.) which is a cancer vaccine for the treatment of hormone refractory prostate cancer. Sipuleucel-T is the only vaccine approved so far by the US FDA to treat cancer. Phase III studies of several DC vaccine for cancer are now ongoing.
Here, we review the development and clinical effects of the DC vaccine “Vaccell” which has been established in Japan.
A Japanese DC vaccine “Vaccell”
Vaccell, a Japanese dendritic cell-based vaccine, is declared with “the vaccine which was optimized to fight against a cancer”. What is “optimized”?
The DCs play central roles in deciding the direction of host immune reactions as well as antigen presentation. The DCs are “headquarters” of antigen-specific host immune responses[10,11]. The antigen presentation have to be done under the helper T-cell 1 (Th1) condition that is interferon (IFN)- and interleukin (IL)-12-rich condition for inducing antigen-specific cytotoxic T lymphocytes (CTLs) and eliciting anti-cancer effects (Figure 1A), however it is difficult to predict what kinds of immune responses, e.g., Th1, Th2, Th17 and regulatory T cells (Treg), will be induced by the dendritic cells in patients’ bodies. This is the serious problem of the conventional cancer vaccines which are the methods only using cancer antigens such as peptides, proteins and whole cells without DCs. The strategy to perform “optimization” of DCs in Th1-inducing type by processing DCs ex vivo should be reasonable.
Figure 1.
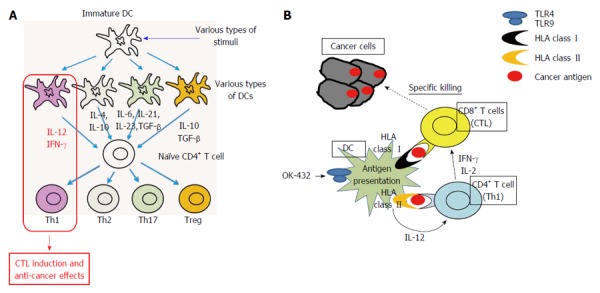
The dendritic cells play central roles in deciding the direction of host immune reactions as well as antigen presentation. A: Role of various dendritic cells in CD4+ T-cell differentiation; B: Antigen presentation and CTL induction under Th1 condition. CTL: Cytotoxic T lymphocytes; DC: Dendritic cell.
We have reported that DCs stimulated with a streptococcal immune adjuvant OK-432 which has been developed in Japan 1970’[12], produce IL-12, induce Th1-dominant state, and elicit anti-tumor effect, that these effects are more powerful than those of the known DC-maturating factors such as tumor necrosis factor (TNF)- and LPS, and that these reactions are caused via Toll-like receptor signaling (Figure 1B)[13-17].
A Th1-inducing type of DC vaccine, Vaccell is made as follows. Peripheral blood monocytes were cultured in medium containing granulocyte macrophage-colony stimulating factor (GM-CSF) and IL-4 to generate immature DCs. Five days later, the DCs were stimulated with the OK-432 and prostaglandin E2 for 24 h. The DCs were then pulsed with peptide antigens according to the HLA pattern. Based on previous reports, Several antigens such as WT1 and MUC1, were selected to be pulsed into DCs[18] (Figure 2A).
Figure 2.
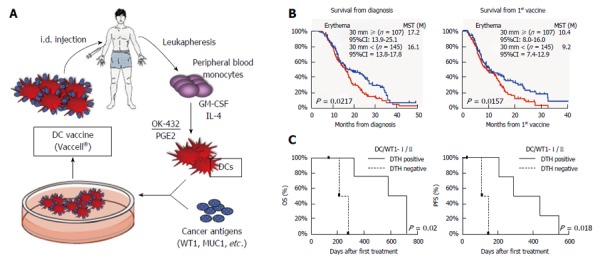
A Th1-inducing type of dendritic cell vaccine, Vaccell is made as follows. A: Preparation of the DC vaccine “Vaccell”; B and C: Vaccell for pancreatic cancer patients; B: Two hundred fifty five patients who received standard chemotherapy combined with DC vaccines were analyzed. DTH skin reaction after vaccination was an independent prognostic factor for better survival; B: The WT1-specific DTH positive patients showed significantly improved OS and PFS compared with the DTH negative patients[20,21]. DC: Dendritic cell; DTH: Delayed type hypersensitivity; PFS: Progressive-free survival; OS: Overall survival.
The DCs were cryopreserved and kept until the day of administration. The phenotype CD14−/low/HLA-DR+/HLA-ABC+/CD80+/CD83+/CD86+/CD40+/CCR7+ was taken to define mature DCs. The DCs were prepared by well-trained technical staff in each institutional cell processing center under the Standard Operating Procedure (SOP). Regarding release criteria, testing for sterility, mycoplasma (PCR method), and endotoxin was done using the supernatant or cell suspension just before the tube filling.
Vaccell has become to be served for lots of patients with various malignancies, and then not only large-scale retrospective observations but also prospective clinical trials have been done or are ongoing.
Clinical effects of the Vaccell mainly in pancreatic cancer
At the point of writing, we have published 13 original articles related to the clinical application of Vaccel. Five articles described for pancreatic cancer[19-23], 2 for biliary tract cancer[24,25], 1 for lung cancer[26], 1 for ovarian cancer[27], 1 for pediatric patient with relapsed leukemia[28], 1 for gastric cancer[29], 1 for malignant glioma[30], and 1 for several types of advanced cancers treated with radiation therapy in combination with DC vaccine[31]. Here, we introduce the Vaccell’s effects in pancreatic cancer patients in which the analysis has been progressed to most among these cases.
First, the clinical and immunological evaluation of DC-based immunotherapy in combination with standard chemotherapy mainly gemcitabine and S-1, in 49 patients with inoperable, advanced pancreatic carcinoma has been done retrospectively[19]. Prolongation of survival in this cohort was highly likely (median OS: 360 d from the 1st vaccination). There were the patients whose numbers of Tregs were decreased in peripheral blood, and the overall survival (OS) from 1st vaccination tended to be prolonged in these patients.
We have conducted the next retrospective observation by expanding sample size as a multicenter analysis[20]. The 255 patients with inoperable pancreatic cancer who received standard chemotherapy combined with peptide-pulsed DC vaccines, were analyzed. Relapse cases were excluded. The median OS from diagnosis was 16.5 mo and that from the 1st vaccination was 9.9 mo. Interestingly, The median survival time (MST) of the patients with positive delayed type hypersensitivity (DTH) skin reaction was significantly prolonged compared to that of the patients with negative DTH (P = 0.0157 by log-rank test) (Figure 2B). These findings strongly suggest that there may be “Responders” for the DC vaccine in advanced pancreatic patients. This is the first report of a multicenter clinical study suggesting the feasibility and possible clinical benefit of an add-on DC vaccine in patients with advanced pancreatic combined with standard chemotherapy.
Based on the results of the above retrospective analysis, we conducted 2 small-scale prospective clinical studies for advanced pancreatic cancer patients. One protocol is the combination therapy with gemcitabine and DC vaccine pulsed with only HLA class I-restricted WT1 peptide[23]. In 10 patients, the disease control associated with a low neutrophil⁄ lymphocyte (N/L) ratio was observed in all 3 patients with DTH positivity. In another protocol of gemcitabine in combination with DC vaccine for pancreatic cancer, we loaded the WT1-specific HLA class II-restricted epitope (WT1-II) as well as the HLA class I-restricted epitope (WT1-I) into the DCs[21,22]. Ten stage IV patients with pancreatic ductal adenocarcinoma who showed HLA-positive for A*02:01, A*02:06, A*24:02, DRB1*04:05, DRB1*08:03, DRB1*15:01, DRB1*15:02, DPB1*05:01, or DPB1*09:01 were enrolled. The survival of 7 patients received WT1-I and -II pulsed DC vaccine was significantly extended compared to that of the 3 patients received DC vaccine pulsed with WT1-I or WT1-II alone [P = 0.036 in OS, P = 0.010 in Progressive-free survival (PFS)]. WT1-specific DTH positive patients showed significantly improved OS and PFS as compared to the DTH negative patients (P = 0.021 in OS, P = 0.018 in PFS) (Figure 2C). In particular, all 3 patients with strong DTH reactions (erythema > 5 mm) had a median OS of 717 d. In addition, it was also observed in this protocol that the decreased N/L ratio may be prognostic markers of longer survival (P = 0.018).
The activation of WT1-specific immune responses by DC vaccine pulsed with WT1-I as well as with WT1-II in combination with standard chemotherapy may be associated with disease stability in advanced pancreatic cancer.
DTH skin reaction was associated with good clinical outcome or clinical response in pancreatic cancer patients received DC vaccine in a large-scale retrospective observation as well as in 2 small-scale prospective studies. Moreover, N/L ratio was a marker for clinical benefit both in 2 prospective studies. In retrospective analysis of 255 cases, N/L ratio was not statistically significant, but tended to be a good prognostic marker. It is a notable thing that the much similar result was provided in a large-scale retrospective observation and in the 2 independent prospective trials. Additionally, a number granulocytic myeloid-derived suppressor cells (MDSCs) was a marker for poor clinical response[23]. Rodriguez et al[32] reported that MDSCs are a subpopulation of activated granulocytes. We have also observed that the infiltration of CD66b-positive neutrophils in a tumor site may be a negative prognostic marker (authors’ personal observation, manuscript in preparing). Granulocytic MDSCs may be a core population of neutrophils which may suppress anti-tumor immunity.
Previously, there have been some reports describing the advantage of the use of HLA-class II-restricted antigen epitope(s)[33], while it is also suggested the possibility that HLA-class II-restricted epitope may induce suppressive immune responses such as Th2, Th17 and Tregs. I believe that it is reasonable and useful to make a cancer vaccine using the DCs optimized ex vivo for Th1-inducing type.
CONCLUSION
To provide more effective cancer immunotherapy for patients: (1) development of the strategy and technology for cancellation of immunosuppression such as checkpoint inhibitors; and (2) establishment of biomarkers to discriminate between responder and non-responder of the certain immunotherapy in addition to development of more powerful cancer vaccine. Cancer vaccine may play a significant role for enhancing the anti-cancer effect of checkpoint inhibitory antibodies[34,35]. The combination therapy using cancer vaccine and checkpoint inhibitors is a promising strategy. Moreover, searching of the biomarkers is a significant theme not only for the prediction of the therapeutic effects of immunotherapies but also for the development of the novel strategy of cancellation of the immunosuppression.
Furthermore, as described above, the results of retrospective observation of past cases are extremely useful to prepare an appropriate prospective protocol.
I believe that there are some populations (relatively large populations) of cancer patients who can obtain clinical benefit by DC vaccine. DC vaccine has a potential to elicit anti-tumor effect, and we therefore have to make DC vaccine a standard treatment for cancer patients.
We will be able to make the DC vaccine much more effective by expanding DC number, by identifying more effective antigens, by developing predictive biomarkers, and by combining with the therapies for cancelling an immunosuppressive condition as well as for tumor mass reduction.
We are now planning phase II/III studies of Vaccell in appropriate protocols.
ACKNOWLEDGMENTS
We thank all of the members of the DC Vaccine Study Group at the J-SICT (the Japan Society of Immunotherapy and Cell Therapy) for carrying out clinical studies, and for giving me critical comments for the DC vaccines.
Footnotes
Conflict-of-interest statement: Yonemitsu Y is a previous member of the Board of Directors on Science and Medicine at tella Inc; all remaining authors have declared no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 7, 2015
First decision: July 31, 2015
Article in press: November 17, 2015
P- Reviewer: De Berardinis P S- Editor: Qiu S L- Editor: A E- Editor: Wang CH
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 6.Vansteenkiste J, Zielinski M, Linder A, Dahabreh J, Gonzalez EE, Malinowski W, Lopez-Brea M, Vanakesa T, Jassem J, Kalofonos H, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol. 2013;31:2396–2403. doi: 10.1200/JCO.2012.43.7103. [DOI] [PubMed] [Google Scholar]
- 7.Kruit WH, Suciu S, Dreno B, Mortier L, Robert C, Chiarion-Sileni V, Maio M, Testori A, Dorval T, Grob JJ, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol. 2013;31:2413–2420. doi: 10.1200/JCO.2012.43.7111. [DOI] [PubMed] [Google Scholar]
- 8.Butts C, Maksymiuk A, Goss G, Soulières D, Marshall E, Cormier Y, Ellis PM, Price A, Sawhney R, Beier F, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol. 2011;137:1337–1342. doi: 10.1007/s00432-011-1003-3. [DOI] [PubMed] [Google Scholar]
- 9.Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu TE, Bosquée L, Trigo JM, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto H, Shoin S, Koshimura S, Shimizu R. Studies on the anticancer and streptolysin S-forming abilities of hemolytic streptococci. Jpn J Microbiol. 1967;11:323–326. doi: 10.1111/j.1348-0421.1967.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto M, Oshikawa T, Tano T, Ohe G, Furuichi S, Nishikawa H, Ahmed SU, Akashi S, Miyake K, Takeuchi O, et al. Involvement of Toll-like receptor 4 signaling in interferon-gamma production and antitumor effect by streptococcal agent OK-432. J Natl Cancer Inst. 2003;95:316–326. doi: 10.1093/jnci/95.4.316. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto M, Furuichi S, Nishioka Y, Oshikawa T, Tano T, Ahmed SU, Takeda K, Akira S, Ryoma Y, Moriya Y, et al. Expression of toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of OK-432, a streptococcal preparation. Cancer Res. 2004;64:5461–5470. doi: 10.1158/0008-5472.CAN-03-4005. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed SU, Okamoto M, Oshikawa T, Tano T, Sasai A, Kan S, Hiroshima T, Ohue H, Moriya Y, Ryoma Y, et al. Anti-tumor effect of an intratumoral administration of dendritic cells in combination with TS-1, an oral fluoropyrimidine anti-cancer drug, and OK-432, a streptococcal immunopotentiator: involvement of toll-like receptor 4. J Immunother. 2004;27:432–441. doi: 10.1097/00002371-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto M, Oshikawa T, Tano T, Ahmed SU, Kan S, Sasai A, Akashi S, Miyake K, Moriya Y, Ryoma Y, et al. Mechanism of anticancer host response induced by OK-432, a streptococcal preparation, mediated by phagocytosis and Toll-like receptor 4 signaling. J Immunother. 2006;29:78–86. doi: 10.1097/01.cji.0000192106.32206.30. [DOI] [PubMed] [Google Scholar]
- 17.Oshikawa T, Okamoto M, Tano T, Sasai A, Kan S, Moriya Y, Ryoma Y, Saito M, Akira S, Sato M. Antitumor effect of OK-432-derived DNA: one of the active constituents of OK-432, a streptococcal immunotherapeutic agent. J Immunother. 2006;29:143–150. doi: 10.1097/01.cji.0000189028.18288.6f. [DOI] [PubMed] [Google Scholar]
- 18.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41:195–205. doi: 10.1097/MPA.0b013e31822398c6. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Shimodaira S, Nagai K, Ogasawara M, Takahashi H, Abe H, Tanii M, Okamoto M, Tsujitani S, Yusa S, et al. Prognostic factors related to add-on dendritic cell vaccines on patients with inoperable pancreatic cancer receiving chemotherapy: a multicenter analysis. Cancer Immunol Immunother. 2014;63:797–806. doi: 10.1007/s00262-014-1554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20:4228–4239. doi: 10.1158/1078-0432.CCR-14-0314. [DOI] [PubMed] [Google Scholar]
- 22.Takakura K, Koido S, Kan S, Yoshida K, Mori M, Hirano Y, Ito Z, Kobayashi H, Takami S, Matsumoto Y, et al. Prognostic markers for patient outcome following vaccination with multiple MHC Class I/II-restricted WT1 peptide-pulsed dendritic cells plus chemotherapy for pancreatic cancer. Anticancer Res. 2015;35:555–562. [PubMed] [Google Scholar]
- 23.Mayanagi S, Kitago M, Sakurai T, Matsuda T, Fujita T, Higuchi H, Taguchi J, Takeuchi H, Itano O, Aiura K, et al. Phase I pilot study of Wilms tumor gene 1 peptide-pulsed dendritic cell vaccination combined with gemcitabine in pancreatic cancer. Cancer Sci. 2015;106:397–406. doi: 10.1111/cas.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koido S, Kan S, Yoshida K, Yoshizaki S, Takakura K, Namiki Y, Tsukinaga S, Odahara S, Kajihara M, Okamoto M, et al. Immunogenic modulation of cholangiocarcinoma cells by chemoimmunotherapy. Anticancer Res. 2014;34:6353–6361. [PubMed] [Google Scholar]
- 25.Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, Yanagida E, Shibamoto Y, Ogasawara M, Tsujitani S, et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg. 2013;17:1609–1617. doi: 10.1007/s11605-013-2286-2. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi H, Okamoto M, Shimodaira S, Tsujitani S, Nagaya M, Ishidao T, Kishimoto J, Yonemitsu Y. Impact of dendritic cell vaccines pulsed with Wilms’ tumour-1 peptide antigen on the survival of patients with advanced non-small cell lung cancers. Eur J Cancer. 2013;49:852–859. doi: 10.1016/j.ejca.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Chiba A, Izawa H, Yanagida E, Okamoto M, Shimodaira S, Yonemitsu Y, Shibamoto Y, Suzuki N, Nagaya M. The feasibility and clinical effects of dendritic cell-based immunotherapy targeting synthesized peptides for recurrent ovarian cancer. J Ovarian Res. 2014;7:48. doi: 10.1186/1757-2215-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito S, Yanagisawa R, Yoshikawa K, Higuchi Y, Koya T, Yoshizawa K, Tanaka M, Sakashita K, Kobayashi T, Kurata T, et al. Safety and tolerability of allogeneic dendritic cell vaccination with induction of Wilms tumor 1-specific T cells in a pediatric donor and pediatric patient with relapsed leukemia: a case report and review of the literature. Cytotherapy. 2015;17:330–335. doi: 10.1016/j.jcyt.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi M, Sakabe T, Chiba A, Nakajima A, Okamoto M, Shimodaira S, Yonemitsu Y, Shibamoto Y, Suzuki N, Nagaya M. Therapeutic effect of intratumoral injections of dendritic cells for locally recurrent gastric cancer: a case report. World J Surg Oncol. 2014;12:390. doi: 10.1186/1477-7819-12-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai K, Shimodaira S, Maejima S, Udagawa N, Sano K, Higuchi Y, Koya T, Ochiai T, Koide M, Uehara S, et al. Dendritic cell-based immunotherapy targeting Wilms’ tumor 1 in patients with recurrent malignant glioma. J Neurosurg. 2015;123:989–997. doi: 10.3171/2015.1.JNS141554. [DOI] [PubMed] [Google Scholar]
- 31.Shibamoto Y, Okamoto M, Kobayashi M, Ayakawa S, Iwata H, Sugie C, Mitsuishi Y, Takahashi H. Immune-maximizing (IMAX) therapy for cancer: Combination of dendritic cell vaccine and intensity-modulated radiation. Mol Clin Oncol. 2013;1:649–654. doi: 10.3892/mco.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosa DS, Ribeiro SP, Cunha-Neto E. CD4+ T cell epitope discovery and rational vaccine design. Arch Immunol Ther Exp (Warsz) 2010;58:121–130. doi: 10.1007/s00005-010-0067-0. [DOI] [PubMed] [Google Scholar]
- 34.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutz ER, Kinkead H, Jaffee EM, Zheng L. Priming the pancreatic cancer tumor microenvironment for checkpoint-inhibitor immunotherapy. Oncoimmunology. 2014;3:e962401. doi: 10.4161/21624011.2014.962401. [DOI] [PMC free article] [PubMed] [Google Scholar]


