Abstract
Inflammatory bowel diseases (IBDs) are a group of chronic inflammatory conditions mainly of the colon and small intestine. Crohn’s disease (CD) and ulcerative colitis (UC) are the most frequent types of IBD. IBD is a complex disease which arises as a result of the interaction of environmental, genetic and immunological factors. It is increasingly thought that alterations of immunological reactions of the patients to their own enterable bacteria (microfilm) may contribute to inflammation. It is characterized by mucosal and sub mucosal inflammation, perpetuated by infiltration of activated leukocytes. CD may affect the whole gastrointestinal tract while UC only attacks the large intestine. The therapeutic goal is to achieve a steroid-free long lasting remission in both entities. UC has the possibility to be cured by a total colectomy, while CD never can be cured by any operation. A lifelong intake of drugs is mostly necessary and essential. Medical treatment of IBD has to be individualized to each patient and usually starts with anti-inflammatory drugs. The choice what kind of drugs and what route administered (oral, rectal, intravenous) depends on factors including the type, the localization, and severity of the patient’s disease. IBD may require immune-suppression to control symptoms such as prednisolone, thiopurines, calcineurin or sometimes folic acid inhibitors or biologics like TNF-α inhibitors or anti-integrin antibodies. For both types of disease (CD, UC) the same drugs are available but they differ in their preference in efficacy between CD and UC as 5-aminosalicylic acid for UC or budesonide for ileocecal CD. As therapeutic alternative the main mediators of the disease, namely the activated pro-inflammatory cytokine producing leukocytes can be selectively removed via two apheresis systems (Adacolumn and Cellsorba) in steroid-refractory or dependent cases. Extracorporeal photopheresis results in an increase of regulatory B cells, regulatory CD8+ T cells and T-regs Type 1. Both types of apheresis were able to induce clinical remission and mucosal healing accompanied by tapering of steroids.
Keywords: Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Extracorporeal treatment, Step up/top down, Psychological aspects in inflammatory bowel disease, Current medical treatment of inflammatory bowel disease, Future therapeutical strategies
Core tip: This review describes current and future therapeutic strategies in Crohn’s disease and ulcerative colitis and outlines the most important publications in this field. It comprises surgical, medical and extracorporeal treatment options. All described treatment options are carefully reviewed regarding therapeutic effects and side effects. Extracorporeal treatment options are a potent measure to withdraw patients from steroids. Standard treatment as well as innovative therapeutic approaches, like autologous stem cell transplantation are addressed, which revealed promising results in therapy refractory patients.
INTRODUCTION
Inflammatory bowel diseases (IBDs) are a group of inflammatory conditions of the colon and small intestine. Crohn’s disease (CD) and ulcerative colitis (UC) are the leading entities in IBD. While UC primarily affects the colon, CD can be related to the whole gut. The disease was named after gastroenterologist Burrill Bernard Crohn who described a series of patients with inflammation of the terminal ileum of the small intestine, the area most commonly affected by the illness, in 1932, together with two other colleagues at Mount Sinai Hospital in New York[1]. We will never know who described UC for the 1st time although the disease was primarily referred to by name in 1859 by Sir Samuel Wilkes. Among his major discoveries, Wilks recognized UC, differentiating it from bacterial dysentery. His work was confirmed by Sir Arthur Hirst 1931[2]. IBD is a complex disease which arises as a result of the interaction of genetic dispositions, environment and alterations in the function of the immune system[3]. It is also increasingly thought that altered immunological reactions to patient’s own enteral bacteria may contribute to inflammatory gut diseases[4]. IBD affected individuals have been found to have 30%-50% reduced biodiversity of commensalism bacteria such as a decrease in Firmicutes (namely Lachnospiraceae) and Bacteroidetes[5]. Further evidence of the role of gut flora in the cause of IBD-besides animal and in vitro studies - is that IBD affected individuals are more likely to have been prescribed antibiotics in the 2-5 year period before their diagnosis than unaffected individuals[6,7].
The enteral bacteria can be altered by environmental factors, such as diets or oral medications (antibiotics or oral iron preparations)[8].
Genetics
There is strong evidence to suggest a genetic basis for IBD, including familial clustering and racial and ethnic differences in risk for IBD. Ten to 20% of affected individuals will have family history of IBD, with the highest risk among first-degree relatives. A strong association between HLA B27 and ankylosing spondylitis is known since the early 1970s which is also classified as extra intestinal complication in patients with IBD (Table 1)[9-11]. The genetic contribution is poorly understood and seems to arise from the small contribution of dozens of genes. In 2012, 163 IBD susceptibility loci were confirmed which means that 163 different alleles may increase the susceptibility to the disease. These 163 loci explain from 8.2% to a 13.6% of variance in CD and 4.1% to 7.5% in UC. The 163 loci were related to 300 known genes. The most well-known and frequent gene associated with CD is the NOD2/CARD15 gene[12-14].
Table 1.
Complications[11]
|
Crohn’s disease |
Ulcerative colitis |
|||
| Females | Males | Females | Males | |
| Primary sclerosing cholangitis | 0.3% | 0.4% | 3.2% | 0.9% |
| Ankylosing spondylitis | 0.7% | 2.7% | 1.0% | 3.0% |
| Pyoderma gangrenosum | 1.2% | 1.3% | 0.8% | 1.5% |
| Erythema nodosum | 1.9% | 0.6% | 0.8% | 0.7% |
Environmental factors
There is evidence that IBD is primarily a disease of the developed countries. The rise in certain regions (i.e., India, China) parallelizes the industrialization of these countries. It seems likely that environmental factors may also influence the normal intestinal commensal flora and thus trigger an inappropriate mucosal immune response.
A number of environmental risk factors have been explored, including smoking, appendectomy, oral contraceptives, diet, breastfeeding, infections/vaccinations, antibiotics, and childhood hygiene. However, most of these identified risk factors have demonstrated inconsistent findings so that further investigations are warranted. Smoking and infections in childhood may trigger IBD especially CD[15-18].
Immune system
The intestinal immune system defends against pathogens and entry of excessive intestinal microbes; simultaneously, a state of immune tolerance to resident intestinal microbes must be maintained. Perturbation of this balance is associated with intestinal inflammation in various mouse models and is thought to predispose humans to IBD. The immune system continuously monitors resident microbiota and utilizes constitutive antimicrobial mechanisms to maintain immune homeostasis. There is increasing evidence that intestinal microbes influence host immune development, immune responses, and susceptibility to human diseases such as IBD[19].
An imbalanced intestinal immune defense and intestinal immune tolerance is one of the risks for developing IBD. A numerous of inflammatory and anti-inflammatory cytokines as well as immune active cells, like T-and B-cells, T-regs are involved in this intact mechanism. Although UC and CD can usually be differentiated on the basis of clinical, radiographic, endoscopic, and histological findings, these conditions can be difficult to distinguish in about 10% to 15% of IBD patients[20]. Numerous studies have investigated the utility of 2 serologic markers in differentiating between UC and CD: Atypical perinuclear anti-neutrophil cytoplasmic antibody (pANCA) and anti-Saccharomyces cerevisiae antibody (ASCA). Unlike the pANCA or cytoplasmic ANCA found in vasculitis, the IBD-associated pANCA has an “atypical” perinuclear staining pattern. This atypical pANCA is detected in about 40% to 80% of UC patients but only in 5% to 25% of CD patients[21,22]. ASCA, on the other hand, is detected in 40% to 68% of CD patients[21,22] but only in about 6% to 12% of UC patients[20,23]. Table 2, based on a meta-analysis of 60 studies comprising 7860 IBD patients, summarizes the sensitivity and specificity of pANCA/ASCA combinations for UC and CD[22]. The diagnostic and therapeutic value of serological markers (more than pANCA/ASCA) was reviewed by Andrea T Kuna[24] in 2013. Due to the lack of sensitivity, serological markers were not advised for their use in the diagnosis of IBD but rather in differentiating CD from UC. The most important clinical utility of serological markers could be in stratifying patients according to risk for aggressive disease phenotype or postoperative complications. At the current time, there is no usefulness of serological markers in monitoring the treatment of IBD patients[24].
Table 2.
Sensitivity and specificity of atypical perinuclear anti-neutrophil cytoplasmic antibody/anti-Saccharomyces cerevisiae antibody combinations for ulcerative colitis and Crohn’s disease in patients with inflammatory bowel disease[24]
| Marker |
UC |
CD |
||
| Sensitivity | Specificity | Sensitivity | Specificity | |
| pANCA+/ASCA- | 51% | 94% | - | - |
| pANCA-/ASCA+ | - | - | 55% | 93% |
pANCA: Atypical perinuclear anti-neutrophil cytoplasmic antibody; ASCA: Anti-Saccharomyces cerevisiae antibody; CD: Crohn’s disease; UC: Ulcerative colitis.
Calprotectin level in feces, produced from granulocytes, is a useful marker to measure disease activity and can predict disease recurrence. It is more precise than the common used markers like CRP and ESR[25].
Laboratory findings
Beside chemical parameters like increase of unspecific inflammatory markers as CRP (activity marker) and erythrocyte sedimentation rate, iron deficiency and anemia in different severities, there is also found a distinguished pattern of specific cytokines.
In IBD, increased amounts of soluble and membrane-bound TNF are produced by various immune and stromal cell populations, such as macrophages, dendritic cells (DCs), effector T cells, adipocytes and fibroblasts. TNF has been shown to exert various pro-inflammatory functions in the inflamed mucosa in IBD. In particular, TNF induces hypervascularization and angiogenesis, augments pro-inflammatory cytokine production by macrophages and T cells. TNF-specific antibodies may alleviate disease by simultaneously suppressing several pro-inflammatory pathways in patients with IBD. There are many proteins involved in gastrointestinal angiogenesis, including pro-inflammatory cytokines, vascular growth factors, and adhesion molecules[26].
A promising approach in getting more insight in the pathophysiology of IBD may be the development of new biomarkers. The techniques available for biomarkers development are genomics and proteomics. In the future it is expected that all these biomarkers will be implemented in an integrated molecular diagnostic and prognostic approach[27].
The heterogeneous nature of IBD implicates heterogeneous therapeutic strategies. Acute flares as well as the chronic status are treated with a variety of medications. Current therapies include the use of corticosteroids, anti-inflammatories, immune suppressive drugs, antibiotics and biologicals.
CD
The active disease is categorized into mild-, moderate- and severe localized ileocaecal disease, colonic disease, extensive small bowel disease and esophageal and gastroduodenal disease. It is graded by the Crohn’s Disease Activity Index (CDAI) which ranges between 150 and 450 points and more for heavily active disease and acute flare, established in 1978[28]. This calculator is primary a research tool, but is increasingly used to define responses and remissions (< 150 pt)[29]. Besides CDAI, there is a variety of scoring systems which partly are very time consuming and require compliance of the patients (exception: Bradshaw index). Thus their use is rather limited to clinical trials (Tables 3 and 4)[30,31].
Table 3.
Crohn’s disease activity index scoring system
| Clinical or laboratory variable | Multiplication factor |
| Number of liquid or soft stools each day for seven days | × 2 |
| Abdominal pain (graded from 0-3 on severity) each day for seven days | × 5 |
| General wellbeing, subjectively assessed from 0 (well) to 4 (terrible) each day for seven days | × 7 |
| Presence of complications1 | × 20 |
| Taking Lomotil or opiates for diarrhea | × 30 |
| Presence of an abdominal mass (0 as none, 2 as questionable, 5 as definite) | × 10 |
| Hematocrit of < 47% in men and < 42% in women | × 6 |
| Percentage deviation from standard weight | × 1 |
One point each is added for each set of complications.
Table 4.
Harvey-Bradshaw index
| General well-being | Very well | Below average | Poor | Very poor | Terrible |
| 0 | 1 | 2 | 3 | 4 | |
| Abdominal pain | None | Mild | Moderate | Severe | |
| 0 | 1 | 2 | 3 | ||
| # liquid stools/d | # | # | # | # | # |
| Abdominal mass | None | Dubious | Definite | Tender | |
| 0 | 1 | 2 | 3 |
A score of less than 5 is generally considered to represent clinical remission.
CD is a chronic disease (as well as UC) and the therapeutic goal is to achieve sustained, steroid-free remission. Medical therapy is considered to be the treatment modality of choice for most patients. According to the severity of CD current therapy strategies include nutritional approaches, anti-inflammatory drugs, immunosuppression, chemotherapy and biologicals. The therapeutic decision is influenced by the extent of severity, the presence of septic complications and extra-intestinal manifestations. The surgical management is reserved for individuals who fail medical treatment or develop potentially life-threatening complications. The surgical management has changed substantially during the last 10 years. Surgical treatment of CD is solely symptomatic. In addition, medical therapy always precedes surgery and almost always continues afterwards. The indications for surgical treatment are failure of medical treatment and progressive complications (e.g., fistula, abscess, obstruction)[32]. Hulten described 1988 disease recurrence of about 50% within 10 years post operation[33]. Bernell described a significantly higher relative risk for recurrence after first resection in CD in women and when the small bowel or the continuous ileocolonic was affected[34,35].
When operating on advanced CD, usually associated with abscess or fistula, a significantly higher complication rate (49%) was reported than after surgery for otherwise uncomplicated CD (12% complication rate)[36].
Postoperative medical treatment can hardly prevent recurrence of CD, therefore an aggressive medical intervention is recommended when objective signs of active disease are found in endoscopy or X ray[37]. There is still a need for preventive strategies[37]. A recent investigation postulated that TNF-α blockers are most effective in treatment and prevention of postoperative disease recurrence[38-41]. Currently there is no consent among the experts concerning the optimal time between TNF-α blocker treatment and surgery[42].
Recommended by the ECCO guidelines 2010[42] surgery should be considered as a primary treatment option in selected cases as localized ileocaecal disease with obstruction but without inflammation[19,43-45]. Patients with a maximum of 40 cm affected bowel, obstructions and clinical symptoms who failed to respond to steroids (CDAI > 220) will also require surgery during the course of disease[46-49]. In general the methods depend on the extent, the localization and severity of morphological complications (ECCO 2010)[42].
Medical treatment of active CD should always be balanced between the potential of the chosen drugs and their side effects. It has to take the state of the disease (e.g., relapse, steroid-refractoriness, quality of life) the severity and extra-intestinal manifestations (Table 2) into account.
A nutritional approach inclusive probiotics was shown to be less effective in these patients even with a mild pattern of disease[50,51]. Neither Omega-3 fatty acid diet, nor probiotics nor nutritional supplementation were convincing in modulating the disease positively[52-54], although a recent study showed a disease controlling effect from whey and soy proteins especially when the patients were treated with TNF-α antibodies and azathioprine[55]. A randomized controlled trial conducted by Takagi has shown the effectiveness of half elemental diet in maintenance therapy in selected patients[56].
However a benefit in reducing pain can be attributed to n-3 unsaturated fatty acids[57]. Thus nutritional therapy as supplementation to medical treatment may be helpful in induction and maintenance of remission or controlling symptoms especially in children[50,56].
It is important to be aware that a considerable portion of patients suffer only from a mild type of CD and need no medical therapy as pointed out in a systematic review of clinical trials by Su et al[58]. In general the initial therapy should consist of steroids as immune-modulating agent and mesalazine as antiphlogistic medication. Mesalazine is an amino-derivative of salicylic acid [5-amino-salicylic-acid (5-ASA)]. Beside antiphlogistic activity, which is due to a suppressive effect on pro-inflammatory cytokines (IL1, TNF-α) by inhibition of interleukin-1 stimulated Re1A phosphorylation[59] and by inhibiting macrophage chemotaxis, 5-ASA is also thought to be an antioxidant and traps free radicals which are found to be present in CD[60]. The therapeutic effect of 5-ASA in mild to moderate CD is discussed controversially in the literature. Tromm found no difference in the efficacy to induce remission in moderate CD of 5-ASA compared to budesonide. Remission rate for budesonide was 69% vs 62% for 5-ASA[61]. These data have to compare with a previous meta-analysis which showed 5-ASA no more effective than placebo[62]. The minimal efficient dose is 4 g/d. High dose (6 g/d) for active CD is currently under investigation[42]. One medication of choice to induce remission in mild to moderate CD is budesonide, a synthetic glucocorticoid with limited systemic bioavailability due to extensive first-pass hepatic metabolism. It is effective for induction of remission and causes almost no side effects due to its low bioavailability. It seems to be superior to 5-ASA in moderate disease[63]. Both are also applied as topical treatment in mild types of disease. The systemic administration of corticosteroids/prednisolone is of course much more effective in induction of clinical remission[64,65], but commonly causes more side effects than budesonide[63,64,66]. Two recent studies support this observation even in high dose 5-ASA therapy[67]. The risk to develop Cushing syndrome due to systemic steroid therapy is known at a daily dose of 7.5 mg prednisolone. Therefore, disease control under dose reduction or discontinuation of steroids should be achieved, especially as steroids commonly fail to maintain clinical remission in the majority of patients with active disease[68]. Thus the early onset of the monoclonal antibody anti TNF-α may help to achieve clinical remission even in steroid free or steroid naive conditions[69]. TNF-α is a cell signaling protein which is involved in systemic inflammation.
It is produced mainly by activated macrophages[70]. Antibodies to tumor necrosis factor (anti TNF-α) are highly efficient immune-suppressive drugs. TNF-α inhibitors offer a targeted strategy that contrasts with the nonspecific immune-suppressive agents traditionally used to treat most inflammatory diseases. Anti TNF-α suppresses immune responses in CD by binding to membrane-bound and soluble TNF (mTNF)[71]. Several trials prove the efficacy of Anti TNF-α in achieving clinical remission[72-74]. A recent study, conducted by a Danish group, confirmed the results from previous investigations. Among 492 patients with CD and 267 patients with UC, 74%/13%/14% and 65%/12%/24% were responders, partial responders and non-responders to anti-TNF therapy, respectively[75]. Atreya R developed a method to predict the response rate of this therapy, in brief: Topical antibody administration in 25 patients with CD led to detection of intestinal membrane-bound TNF (mTNF) immune cells during confocal laser endo-microscopy. Patients with high numbers of mTNF cells showed significantly higher short-term response rates (92%) at week 12 upon subsequent anti-TNF therapy as compared to patients with low amounts of mTNF cells (15%). These data indicate that molecular imaging with fluorescent antibodies has the potential to predict therapeutic responses to biological treatment and can be used for personalized medicine in CD and other autoimmune or inflammatory disorders[76]. Due to their mode of action TNF-α-blockers may cause expected and paradoxical side effects, like “de novo psoriasis”, described by Joyau et al[77]. In general patients treated with TNF-α-blockers are at increased risk to develop life threatening opportunistic infections[78-80]. There are also reports of developing rare white blood cell cancer (hepatosplenic T-cell lymphoma) in combination with thiopurines[81].
The disease modulating relevance of antibiotics is restricted to their use in septic complications. The efficacy of metronidazole and ciprofloxacin alone is similar to mesalazine (5-ASA), but inferior to steroids[82,83]. A meta-analysis of 6 trials showed no convincing positive influence on the disease[82-84].
Thiopurines are purine antimetabolites which are widely used in the treatment of autoimmune disorders (e.g., CD, rheumatoid arthritis), and organ transplant recipients[85]. The leading substances are azothioprine (AZA) and mercaptopurine (6-MP). Azathioprine acts as a prodrug for mercaptopurine, inhibiting an enzyme required for the synthesis of DNA. Thus, it most strongly affects proliferating cells, such as the T- and B-cells of the immune system[86,87]. The main adverse effects of thiopurines are bone marrow suppression, hepatotoxicity and pancreatitis and may result in a withdrawal of this drug[86,88] AZA was shown to be efficient in inducing and maintaining remission after tapering of steroids. It is used in the management of moderately to severely or chronically active CD and in corticosteroid-dependent patients[89,90]. Azathioprine treatment is associated with an increased risk of lymphoma, but it is unclear if this is due to the drug or to a predisposition related to CD[91,92]. A recent study provides evidence that In CD, treatment with azathioprine shortly after diagnosis was no more likely to result in corticosteroid-free remission than standard care or placebo[93,94]. No such investigations were performed for 6-MP but as 6-MP is a metabolite of AZA it is considered equivalent. Thioguanine, a 3rd related drug may be an alternative in patients, who are refractory or intolerant to AZA and 6-MP (occurs in up to 15% of long term exposed patients)[92,95]. Patients who relapse under thiopurine therapy should have their dose optimized. Also a switch to TNF-α blockers or MTX should be considered. A long term combination of AZA/6-MP and TNF-α blockers should be avoided in young male patients due to the risk of hepatosplenic T-cell lymphoma[43].
Methotrexate (MTX) is a folic acid inhibitor and thus interferes with cell-growth[96]. It is used predominantly for immunosuppression in autoimmune diseases and as chemotherapy. In CD it has - to date - remained in treatment algorithms as a salvage therapy for patients who have failed to respond or became intolerant to azathioprine[97]. However, its use is not so common in the clinical routine for CD but MTX seems to be sufficient in maintenance therapy. Recently a user’s guide was published which favors the safe and efficient use of MTX in several clinical conditions of CD and UC. The authors Swaminath et al[97] provided an ease to use algorithm. MTX is largely used as a second line therapy after AZA failure.
Cyclosporin A (CSA), tacrolimus and mycophenolatmofetil currently play only a minor role CD as evidence for induction and maintenance of remission is lacking[42].
Current treatment algorithm for CD is displayed in the Guidelines for the Management of CD by Ye et al[98] and IBD Study Group of the Korean Association for the Study of the Intestinal Diseases.
UC
UC is graded into 4 disease activities (mild, moderate, sever and remission) and divided into 3 different distribution patterns (proctitis, left-sided, pancolitis).
The severity of the disease is classified by a clinical activity index (CAI)-Rachmilewitz index or Mayo score including stool frequency, rectal bleeding, the endoscopic activity of the colon, and a physician rating of disease activity. Each of these items is given a number from 0 to 3, with 3 being the highest rating for disease activity[99]. A drop to less than 2 points is defined as a clinical relevant remission[99].
The therapeutic goal in UC is to induce steroid-free clinical long-term remission or to increase intervals of acute flare[100]. A cancer surveillance in UC patients is strongly recommended as these individuals have an elevated risk to develop a colon cancer within 10 years. Advanced endoscopic and imaging techniques are warranted to optimize the diagnosis as cancer in UC is a clear indication for surgery[100,101].
Therapeutic management of active UC
The choice of the appropriate medication depends on severity, the previous course (relapsed or persistent active disease) and the localization of the disease. The topical use of 5-ASA (mesalazine) is still the treatment of choice for proctitis or left sided mild to moderate disease or topical steroids although topical steroids (budesonide) were found to be less effective than topical mesalazine[102]. The systemic use of aminosalicyl-derivatives is additionally recommended in more extensive or severe cases[100]. Mesalazine is comparable with newer substances (balsalazide) in its efficacy to induce remission and is better tolerated[103-105]. As previously described two studies have shown that patients treated with oral mesalazine 4 g/d and 1 g/d mesalazine enema experienced a shorter time to resolution of rectal bleeding than those treated with oral therapy alone (P = 0.0025)[106,107]. A smaller study of patients with frequently relapsing disease found that dose escalation of oral mesalazine combined with the addition of topical 5-ASA significantly reduced the number of disease recurrences and courses of steroids (P < 0.0001)[108]. Patients on high dose sulfasalazine (prodrug of mesalazine) require folic supplementation (1 mg/d) to maintain normal cell division. This is of specific importance for patients who receive additionally MTX[96]. Steroids have been a mainstay of UC therapy for many years, based on a thoroughly established efficacy profile for the induction of remission[109,110]. For severe UC, and in patients refractory to 5-ASA, the need for systemic steroids is a general knowledge[100,111-113]. The combination of oral steroids and 5-ASA in escalating doses in case of treatment failure with 5-ASA alone is strongly recommended[109]. Steroids should be tapered as soon as possible as they usually fail to maintain remission and have a problematic safety profile[42,100,114,115]. A therapeutic challenge in UC (similar to CD) is the steroid-dependent or steroid-refractory patient. In this setting additional immune-modulating therapy with thiopurines is recommended[100,111,116-118]. In contrast to CD calcineurin-inhibitors as CSA or tacrolimus play a disease modulating role in UC. Due to the binding-mechanism to calcineurin, transcriptions for pro-inflammatory cytokines IL-2 and TNF-α are inhibited. Side effects as renal impairment or hypomagnesaemia are common in about 50% of the patients, but the main concerns are opportunistic infections. Arts found an incidence of 3.5% (3/86) to die of such infections[119].
Thiopurines [azathioprine (AZA)/mercaptopurine (6-MP)] have limited utility in the acute setting of UC, but are recommended in the maintenance therapy of UC and have a steroid sparing effect[100,111]. CSA in combination with thiopurines are effective to induce remission and avoid colectomy in patients at risk[120]. The treatment with TNF-α blockers (infliximab, adalimumab) is discussed controversial in the literature. Two large well conducted placebo-controlled trials described the induction and maintenance of steroid- free remission in about 26%[121] and 13%[122] at 12 mo of enrolled patients. These data could not be confirmed by a real life observation[111,123]. Only a short term clinical response was observed. A recently presented study suggested a benefit from a combination therapy of TNF-α blockers and thiopurines in early stage of active UC. A steroid free response was seen in 40% of patients at week 16[124,125]. The major drawbacks of TNF-α blockers are already discussed in CD section. Although luminal bacteria are thought to play a major role in pathogenesis of IBD the use of antibiotics in UC is also restricted to the therapy of complications due to infections[114].
Surgical interventions
The cumulative risk for colectomy in relation to time of diagnosis has been reported as 13.1% in 5 years[126]. A recent population based UC study observed the global risk for colectomy by 8.7% over 10 years[100]. About 27% of patients with severe UC require colectomy[114,126]. Early surgery, within 3 mo of diagnosis is increasingly performed in patient > 65 years but should not be favored as older patients (> 50 years) have a reduced risk to need colectomy during their course of disease[127-129]. They are more likely to respond to pharmacologicals than younger people[130]. Emergency colectomy or ileostomy due to toxic megacolon, bleeding or perforation is associated with a complication risk or death of 5%[131].
Elective surgery is indicated in chronic therapy refractory cases or when signs of dysplasia are found. Here the common surgery therapy is total proctocolectomy with ileal-pouch anal anastomosis (IPAA)[100,111]. This surgical intervention may have a high curative potential in UC but high rates (up to 20%) of postoperative complications are still emerging problems[100,132-134].
High dose steroids should be weaned before surgery because they are a risk factor for complications[111]. Neither thiopurines nor calcineurin inhibitors seem to increase the risk of postoperative complications while biologicals may be associated with a higher complication rate[111].
Maintenance therapy
Generally maintenance therapy is recommended for all patients. The goal is to keep a steroid free clinical and endoscopic remission. The medication of choice again is 5-ASA (masalazine) topically and/or orally depending on the site and severity of inflammation as long term treatment since this may reduce the risk of colon cancer[100,111]. In steroid dependent patients thiopurines (AZA) are steroid sparing medications. MTX is generally not recommended for UC as the beneficial effect is not proven, although in selected patients (intolerant to the other immunosuppressants) MTX was seen to be effective[100,111,114,135,136]. A large retrospective cohort study of 91 patients reported that one-third of patients were successfully weaned off steroids with MTX therapy. MTX may be considered in the long-term management of patients with UC on steroids[137]. TNF-α blockers are recommended in patients who were treated with this medication initially and achieved remission[121].
A detailed figure of current treatment algorithm for UC is published by Meier and Sturm[100] in World J Gastroenterology 2011.
NON-PHARMOCOLOGICAL OPTIONS IN IBD
Granulocyte monocyte apheresis
A special challenge in this setting is the steroid refractory and steroid dependent type of IBD as well as azathioprine-intolerant or - resistant patients. These patients are at high risk to undergo surgery. Thus a Japanese group developed a non-pharmacological therapeutic alternative to conventional therapy. It is an apheresis system, which removes activated monocytes/macrophages (one of the main disease mediators), from the patient’s blood circulation (GMA = granulocyte monocyte apheresis). Currently two systems are available, the Adacolumn® system which is approved by the Japanese Ministry of Health and Welfare and is CE marked in Japan and Europe by the TUV since 1999[138,139], and the Cellsorba® System. It is also approved by the Japanese Ministry of Health and Welfare since 1989[140]. Both systems are defined to selectively remove activated WBC, especially granulocytes, monocytes and macrophages (source of TNF-α) from the patients’ blood circulation without compromising patients’ peripheral blood counts[141]. With the Cellsorba® System additionally activated platelets are removed. The action is based on columns (cellulose-acetate beads in Adasystem and polyester fibers, respectively in Cellsorba) in both systems. The severity of the disease (CD, UC) and mucosal damage correlate with the excess of mucosal granulocyte infiltration[142-144]. Both systems have a great immune-modulating effect. The exact mode of action is not yet sufficiently understood, but certainly, a modulation of the immune system takes place[145]. As a result, less pro-inflammatory cytokines are released. Furthermore, the production of interleukin-1-receptor-antagonist with its anti-inflammatory property is increased and the apoptosis of granulocytes boosted. The decreased LECAM-1-expression on leucocytes impedes the leukotaxis to the inflamed tissue and CD10-negative immature granulocytes appear in the peripheral blood[141,146,147]. Another effect to be mentioned is the removal of the peripheral DCs[148] and the leachate of regulatory T-cells (T-regs)[30,149,150]. Pro-inflammatory cytokines decrease while anti-inflammatory cytokines increase[151]. The induction of clinical and morphological remission of IBD can be explained by this mechanism of action.
Most clinical trials are conducted using the Adacolumn® system for GMA in both, UC and CD whereby UC is the leading entity. The best responders seem to be steroid naive patients followed by steroid dependent patients or steroid refractory patients with a short history of disease[152,153]. A recent study tried to define predictive factors to identify patients with UC who are likely to respond to GMA[154]. In this trial 43 patients with active UC were enrolled. Best responders to GMA were those treated immediately (< 49.5 d) after relapse. Also an initial low WBC count (< 10 G/L) was predictive for a good response. They conclude that in these selected patients GMA is efficient as mono-therapy inducing remission (defined by CAI). Sacco investigated 118 steroid dependent or refractory patients (UC n = 83, CD n = 35). GMA was efficient in inducing and maintaining remission during a follow up of 12 mo, irrespective the entity or the steroid status (UC: CAI = 6; CD: CDAI < 150)[155].
Also a combination therapy (thiopurines and GMA) promises a rapid induced high remission rate in patients with active early diagnosed CD[156]. In this study 22 steroid and biological naïve patients were treated with thiopurines and GMA. The rate of mucosal healing was 50% after 1 year.
A meta-analysis by Yoshino et al[157] 2014 revealed that intensive granulocyte and monocyte adsorption apheresis is a safe and effective treatment with higher rates of clinical remission and response for UC compared with corticosteroids.
GMA treatment is known to have an excellent safety profile. Almost no side effects occur[153,158]. Up to now no treatment had to be discontinued because of adverse events[158]. There is a strong recommendation to introduce GMA at an early state of disease before patients develop extensive mucosal damage and become dependent or refractory to drugs (similar to a TNF-α therapy)[159].
There is still a controversy regarding the optimal treatment schedule. Five sessions in 5 wk vs 10 sessions in 8 wk was shown to be similar efficient[160]. Therefore Vecchi recommended in his review the perpetuation of the traditional treatment schedule of 5 sessions in 5 wk (1/wk) as it is seems not to be inferior to the more intensive version, more convenient to the patients and more cost effective[158].
Still more randomized sham controlled double blind trials with a long term surveillance to evaluate the long term outcome, treatment schedules and cost effectiveness (equipment, pharmacological therapy, avoidance of surgical intervention) are required, although several reports attest comparable costs to conventional therapy as the good safety profile should comprise higher costs[161].
According to current data the American Society for Apheresis (ASFA) assigned 2013 GMA in UC to category II/III with a recommendation level of Grade 1B/2B (strong to weak recommendation, moderate quality evidence) and the CD to category III, recommendation 1B (strong, but randomized controlled trials with important limitations)[162].
ASFA defines its categories as below
Cat II: Disorders for which apheresis is accepted as second-line therapy; either as a stand alone treatment or in conjunction with other modes of treatment[162].
Cat III: Optimum role of apheresis therapy is not established. Decision making should be individualized.
Centrifugal lymphocytapheresis
In the 80ties Bicks et al[163] described successful treatment of active CD by centrifugal lymphocytapheresis (CLA) and lymphoplasmapheresis[164]. Nowadays these treatment options are replaced by selective adsorption systems (Adacolumn and Cellsorba).
Extracorporealphotopheresis
Two uncontrolled case series have been published suggesting that extracorporealphotopheresis (ECP) can promote remission for a proportion of patients in the category of steroid and/or immunosuppressant intolerant or refractory CD[165,166].
The domain of ECP is T-cell mediated diseases, like T-cell lymphoma or acute or chronic graft vs host disease (GvHD). The mode of action is, like for all extracorporeal treatments, elusive. The mechanism of this treatment is likely due to the induction of anticlonotypic immunity directed against pathogenic clones of T lymphocytes. Treatment induces apoptotic death of pathogenic T-cells, and it is postulated that activation of antigen-presenting cells has important effects in this process[167]. More recently, it has been suggested that ECP may induce Ag-specific immune-modulation via regulatory T-cells, which could explain its efficacy in immune-mediated diseases and lack of toxicities. The frequency of T-regs was significantly increased in the blood of ECP-treated patients[168]. In vitro these cells exerted suppressive activity and showed features of T-regs. The best-characterized subtypes of T-regs are those expressing CD4 and CD25[149,150]. It is also suggested that ECP induces IL-10 producing regulatory B cells, regulatory CD8+ T cells and IL-10 producing T-regs Type 1[167,169,170]. IL-10, also known as cytokine-synthesis inhibitory factor, is a potent anti-inflammatory cytokine[171]. Abreu et al[165] observed in 28 patients with moderate-to-severely active CD (mean baseline CDAI 324) who were refractory to or intolerant of immunosuppressants and/or anti-TNF agents that ECP was well tolerated and induced clinical response (50%) and remission (25%) in patients with CD[165]. Reinisch confirmed these results in 31 CD patients, ECP permitted reduction or discontinuation in steroid dependent or - refractory patients[166].
As sham controlled trials are still missing ASFA recommends assigned ECP in CD to category III with a recommendation level 2[162].
Currently ECP plays a minor role in the treatment of CD (weak recommendation due to low quality of evidence).
FUTURE ASPECTS IN THE MANAGEMENT OF IBD
CD and UC are complex disorders which need complex therapeutic strategies and a continuous development of treatment managements, new drugs and alternative measures. Despite the high prevalence of mental health co-morbidities in IBD, psychological illness remains largely under treated, with studies showing that 60% of IBD patients experiencing mental health problems do not receive adequate help. Therapeutic approaches must always be chosen in agreement with the patients to increase the patients’ compliance[172].
Due to the complexity of the disease a collaboration of medical specialists is necessary to cover all complications and to improve treatment success[173].
A new scoring system for CD, the Lémann score, is developed to allow a better identification of patients with severe epithelial damage and those with rapid progression of damage. This system monitors the cumulative damage. It measures cumulative structural bowel progression at a specific time point, based on medical and disease history, by endoscopy and other imaging methods[173,174]. This instrument can also be used to assess the effect of various medical therapies on the progression of bowel damage.
The big hope is set on advances in biologicals with different mechanism of action (i.e., anti TNF vaccination, TNF gene silencing and TNF neutralizing nanobodies) so that in case of treatment failure other biologicals or even combinations are available[175]. As mentioned earlier (in the CD section), laboratory assays to identify TNF-α blocker responders and non- responders would be very helpful in treatment optimization[176]. Further drugs targeting other pro-inflammatory cytokines are in evaluation[175]. Another therapeutic approach is given by vedolizumab, a biological which targets cell adhesion molecules (CAMs), a monoclonal antibody that inhibits mucosal leukocyte infiltration[177]. In contrast to natalizumab (α4-integrin-inhibitor, Tysabri®) vedolizumab (α4β7 integrin-inhibitor, Entyvio®) modulates the adaptive immune system without systemic side effects[178].
Enteric bacteria, viruses or fungi may induce IBD, thus fecal microbiota transplantation or fecal bacterio-therapy (from a healthy individual) is also a therapeutic option in IBD[179]. Autologous hematopoietic stem cell transplantation (aHSCT) has been used as a treatment for severe, active and therapy-refractory autoimmune diseases for more than 13 years[180,181]. In 2012 the EBMT published guidelines for the selection of patients with autoimmune diseases[182]. Among disorders like multiple sclerosis, systemic lupus erythematosus, myasthenia gravis also severe active CD refractory to conventional therapy has been proposed as a potential indication for aHSCT[180]. Meanwhile several studies were conducted, which revealed promising results. The majority of so treated patients showed clinical and endoscopic remission within 6 mo, and remained free of medical therapy for at least one year. In relapsing patients a disease control with low dose steroids and immune-suppressive therapy was achieved[183,184].
Another point in therapeutic care in IBD is a harmonization between different countries and centers. Two retrospective cross sectional studies from 2009 describe country and care-setting specific therapeutic variations. The impact on outcome is not yet clear[185,186].
Step up and top down strategy
The current clinical practice and recommended treatment for CD is the “step-up” approach which refers to a sequential treatment strategy that often begins with a less potent and less toxic treatment strategy, such as topical steroids or aminosalicylates with escalation to the highly effective but potentially more toxic treatment strategies[42,187]. The top down strategy refers to the use highly effective but potentially more toxic treatment strategies early in the course of a chronic illness to prevent disease progression and achieve remission[188].
The introduction of biologic therapy, and particularly the use of anti TNF-α therapy, has provided a powerful tool in the treatment and management of IBD. The prevention of structural damage by achievement of “mucosal healing”, however, is associated with the more “aggressive” treatment and an earlier use of immune-suppressants and biologicals[189]. A recent study from D’Haens[190] has provided evidence suggesting that reversing the treatment paradigm from a “step-up” to a “top-down” approach may positively alter the natural course of this illness by mucosal healing[188,190]. Several further studies show, that the onset of biologicals in the early course of disease results in better mucosal healing, earlier tapering of steroids, less complications and less need for surgery. Also the incidence of relapse is reduced[191]. On the other hand one must be aware, that the early use of biologicals, especially TNF-α blockers, may result in an increased risk of complications, particularly server life threatening infections. LIN described that about 30% of patients might be over-treated by the top down strategy[188]. Thus it is certainly a crucial point to identify high-risk patients who clearly would benefit from the early use of a more aggressive treatment so that the expected benefit outweighs increased risk for probably severe side effects.
In UC conventional treatment strategies (step-up) are still favored as aminosalycylate derivatives (5-ASA) seem to prevent cancer, the most important complication in UC. Therefore, at present, there is little rationale for a top-down approach to managing UC. Although, Sandborn[192] did not exclude that a top down approach for a selected subgroup of patients with UC (patients at high risk to develop complicated or therapy refractory disease) may profit from the administration of biologicals or other immune-suppressants at early stage of the disease.
CONCLUSION
Up to now there is no cure for IBD. This review describes shortly current and advanced therapeutic options for patients with IBD. They all have limitations due to side effects, refractoriness or unresponsiveness of the patients due to known and unknown causes. There are still a number of individuals in whom the current strategies are insufficient in controlling symptoms. Further studies are in progress to develop new therapeutic options or to improve those already in use in order to achieve durable remission in the majority of patients. The future strategy aims at early hard therapy of patients at risk for severe course of IBD- even with combined immunosuppression, if necessary. After achieving complete remission- endoscopic and biologic (comprising normal stool calprotectin) - tapering of immunosuppression might be possible. A lot of new pharmacological and immune modifying therapies are currently studied in phase II and III studies in IBD and are the hope for therapy refractory patients. Even for patients with short bowel syndrome a new therapeutic approach with a GLP-2 analog - teduglutide - is on the market and fighting for coverage by insurance companies[193]. Patients with malnutrition and weight loss of more than 5% within 3 mo should be treated with appropriate medication but also with oral nutritional therapy to avoid further complications like opportunistic infections, long hospitalization and higher mortality. Serum levels of Vit B12, folic acid, Vit D and zinc should be monitored carefully[194]. Figure 1 comprises current therapeutic options in IBD including alternative strategies, like extracorporeal techniques and autologous stem cell transplantation.
Figure 1.
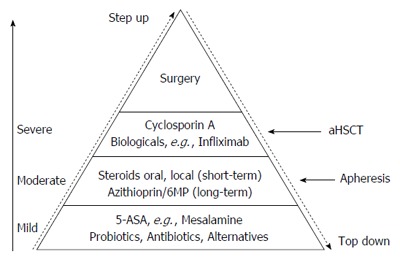
Therapeutic approaches in inflammatory bowel disease. 5-ASA: 5-amino-salicylate-acid; aHSCT: Autologous stem cell transplantation.
At present, clinical manifestations are the most useful way to make therapeutic decisions.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests’ for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 7, 2015
First decision: August 4, 2015
Article in press: January 11, 2016
P- Reviewer: Tomizawa M S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
References
- 1.Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: a pathologic and clinical entity. 1932. Mt Sinai J Med. 2000;67:263–268. [PubMed] [Google Scholar]
- 2.Crohn BB. An historic note on ulcerative colitis. Gastroenterology. 1962;42:366–367. [PubMed] [Google Scholar]
- 3.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- 5.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29:79–84. doi: 10.1097/MOG.0b013e32835a4b3e. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein CN. Antibiotic use and the risk of Crohn’s disease. Gastroenterol Hepatol (N Y) 2013;9:393–395. [PMC free article] [PubMed] [Google Scholar]
- 8.Kotanko P, Carter M, Levin NW. Intestinal bacterial microflora--a potential source of chronic inflammation in patients with chronic kidney disease. Nephrol Dial Transplant. 2006;21:2057–2060. doi: 10.1093/ndt/gfl281. [DOI] [PubMed] [Google Scholar]
- 9.Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1:904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 10.Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704–706. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- 11.Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore) 1976;55:401–412. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Gaya DR, Russell RK, Nimmo ER, Satsangi J. New genes in inflammatory bowel disease: lessons for complex diseases? Lancet. 2006;367:1271–1284. doi: 10.1016/S0140-6736(06)68345-1. [DOI] [PubMed] [Google Scholar]
- 13.Jess T, Riis L, Jespersgaard C, Hougs L, Andersen PS, Orholm MK, Binder V, Munkholm P. Disease concordance, zygosity, and NOD2/CARD15 status: follow-up of a population-based cohort of Danish twins with inflammatory bowel disease. Am J Gastroenterol. 2005;100:2486–2492. doi: 10.1111/j.1572-0241.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 14.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394–400. doi: 10.1016/j.autrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Mikhailov TA, Furner SE. Breastfeeding and genetic factors in the etiology of inflammatory bowel disease in children. World J Gastroenterol. 2009;15:270–279. doi: 10.3748/wjg.15.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2010;6:339–346. [PMC free article] [PubMed] [Google Scholar]
- 18.Ng SC, Woodrow S, Patel N, Subhani J, Harbord M. Role of genetic and environmental factors in British twins with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:725–736. doi: 10.1002/ibd.21747. [DOI] [PubMed] [Google Scholar]
- 19.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savige J, Dimech W, Fritzler M, Goeken J, Hagen EC, Jennette JC, McEvoy R, Pusey C, Pollock W, Trevisin M, et al. Addendum to the International Consensus Statement on testing and reporting of antineutrophil cytoplasmic antibodies. Quality control guidelines, comments, and recommendations for testing in other autoimmune diseases. Am J Clin Pathol. 2003;120:312–318. doi: 10.1309/WAEP-ADW0-K4LP-UHFN. [DOI] [PubMed] [Google Scholar]
- 21.Bossuyt X. Serologic markers in inflammatory bowel disease. Clin Chem. 2006;52:171–181. doi: 10.1373/clinchem.2005.058560. [DOI] [PubMed] [Google Scholar]
- 22.Reese GE, Constantinides VA, Simillis C, Darzi AW, Orchard TR, Fazio VW, Tekkis PP. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–2422. doi: 10.1111/j.1572-0241.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 23.Seibold F, Stich O, Hufnagl R, Kamil S, Scheurlen M. Anti-Saccharomyces cerevisiae antibodies in inflammatory bowel disease: a family study. Scand J Gastroenterol. 2001;36:196–201. doi: 10.1080/003655201750065960. [DOI] [PubMed] [Google Scholar]
- 24.Kuna AT. Serological markers of inflammatory bowel disease. Biochem Med (Zagreb) 2013;23:28–42. doi: 10.11613/BM.2013.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang JY, Ouyang Q, Li GD, Xiao NP. Clinical value of fecal calprotectin in determining disease activity of ulcerative colitis. World J Gastroenterol. 2008;14:53–57. doi: 10.3748/wjg.14.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eder P, Korybalska K, Linke K, Witowski J. Angiogenesis-related proteins--their role in the pathogenesis and treatment of inflammatory bowel disease. Curr Protein Pept Sci. 2015;16:249–258. doi: 10.2174/1389203716666150224150756. [DOI] [PubMed] [Google Scholar]
- 27.Cioffi M, Rosa AD, Serao R, Picone I, Vietri MT. Laboratory markers in ulcerative colitis: Current insights and future advances. World J Gastrointest Pathophysiol. 2015;6:13–22. doi: 10.4291/wjgp.v6.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 29.Irvine EJ, Feagan B, Rochon J, Archambault A, Fedorak RN, Groll A, Kinnear D, Saibil F, McDonald JW. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology. 1994;106:287–296. doi: 10.1016/0016-5085(94)90585-1. [DOI] [PubMed] [Google Scholar]
- 30.C Leitner G, Worel N, Vogelsang H. Selective Granulocyte and Monocyte Apheresis as a Non-Pharmacological Option for Patients with Inflammatory Bowel Disease. Transfus Med Hemother. 2012;39:246–252. doi: 10.1159/000341801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sostegni R, Daperno M, Scaglione N, Lavagna A, Rocca R, Pera A. Review article: Crohn’s disease: monitoring disease activity. Aliment Pharmacol Ther. 2003;17 Suppl 2:11–17. doi: 10.1046/j.1365-2036.17.s2.17.x. [DOI] [PubMed] [Google Scholar]
- 32.Malafosse M. [Crohn’s disease: current surgical treatment] Bull Acad Natl Med. 2007;191:1143–1156; discussion 1157-1158. [PubMed] [Google Scholar]
- 33.Hultén L. Surgical treatment of Crohn’s disease of the small bowel or ileocecum. World J Surg. 1988;12:180–185. doi: 10.1007/BF01658051. [DOI] [PubMed] [Google Scholar]
- 34.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn’s disease. Br J Surg. 2000;87:1697–1701. doi: 10.1046/j.1365-2168.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- 35.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38–45. doi: 10.1097/00000658-200001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strong SA. Surgical treatment of inflammatory bowel disease. Curr Opin Gastroenterol. 2002;18:441–446. doi: 10.1097/00001574-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Rutgeerts P. Review article: recurrence of Crohn’s disease after surgery - the need for treatment of new lesions. Aliment Pharmacol Ther. 2006;24 Suppl 3:29–32. doi: 10.1111/j.1365-2036.2006.03056.x. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn’s disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015;148:64–76.e2; quiz e14. doi: 10.1053/j.gastro.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorrentino D. State-of-the-art medical prevention of postoperative recurrence of Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2013;10:413–422. doi: 10.1038/nrgastro.2013.69. [DOI] [PubMed] [Google Scholar]
- 40.Sorrentino D, Fogel S, Van den Bogaerde J. Surgery for Crohn’s disease and anti-TNF agents: the changing scenario. Expert Rev Gastroenterol Hepatol. 2013;7:689–700. doi: 10.1586/17474124.2013.842895. [DOI] [PubMed] [Google Scholar]
- 41.Vuitton L, Koch S, Peyrin-Biroulet L. Preventing postoperative recurrence in Crohn’s disease: what does the future hold? Drugs. 2013;73:1749–1759. doi: 10.1007/s40265-013-0128-x. [DOI] [PubMed] [Google Scholar]
- 42.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Solem CA, Loftus EV, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 44.Tromm A, Tromm CD, Hüppe D, Schwegler U, Krieg M, May B. Evaluation of different laboratory tests and activity indices reflecting the inflammatory activity of Crohn’s disease. Scand J Gastroenterol. 1992;27:774–778. doi: 10.3109/00365529209011182. [DOI] [PubMed] [Google Scholar]
- 45.Vermeire S, Van Assche G, Rutgeerts P. The role of C-reactive protein as an inflammatory marker in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:580–586. doi: 10.1038/ncpgasthep0359. [DOI] [PubMed] [Google Scholar]
- 46.Graadal O, Nygaard K. [Crohn disease. Long-term effects of surgical treatment] Tidsskr Nor Laegeforen. 1994;114:1603–1605. [PubMed] [Google Scholar]
- 47.Kim NK, Senagore AJ, Luchtefeld MA, MacKeigan JM, Mazier WP, Belknap K, Chen SH. Long-term outcome after ileocecal resection for Crohn’s disease. Am Surg. 1997;63:627–633. [PubMed] [Google Scholar]
- 48.Nordgren SR, Fasth SB, Oresland TO, Hultén LA. Long-term follow-up in Crohn’s disease. Mortality, morbidity, and functional status. Scand J Gastroenterol. 1994;29:1122–1128. doi: 10.3109/00365529409094898. [DOI] [PubMed] [Google Scholar]
- 49.Weston LA, Roberts PL, Schoetz DJ, Coller JA, Murray JJ, Rusin LC. Ileocolic resection for acute presentation of Crohn’s disease of the ileum. Dis Colon Rectum. 1996;39:841–846. doi: 10.1007/BF02053980. [DOI] [PubMed] [Google Scholar]
- 50.Donnellan CF, Yann LH, Lal S. Nutritional management of Crohn’s disease. Therap Adv Gastroenterol. 2013;6:231–242. doi: 10.1177/1756283X13477715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheil B, Shanahan F, O’Mahony L. Probiotic effects on inflammatory bowel disease. J Nutr. 2007;137:819S–824S. doi: 10.1093/jn/137.3.819S. [DOI] [PubMed] [Google Scholar]
- 52.Feagan BG, Sandborn WJ, Mittmann U, Bar-Meir S, D’Haens G, Bradette M, Cohen A, Dallaire C, Ponich TP, McDonald JW, et al. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA. 2008;299:1690–1697. doi: 10.1001/jama.299.14.1690. [DOI] [PubMed] [Google Scholar]
- 53.Rolfe VE, Fortun PJ, Hawkey CJ, Bath-Hextall F. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826. doi: 10.1002/14651858.CD004826.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Verma S, Kirkwood B, Brown S, Giaffer MH. Oral nutritional supplementation is effective in the maintenance of remission in Crohn’s disease. Dig Liver Dis. 2000;32:769–774. doi: 10.1016/s1590-8658(00)80353-9. [DOI] [PubMed] [Google Scholar]
- 55.Machado JF, Oya V, Coy CS, Morcillo AM, Severino SD, Wu C, Sgarbieri VC, Vilela MM. Whey and soy protein supplements changes body composition in patients with Crohn’s disease undergoing azathioprine and anti-TNF-alpha therapy. Nutr Hosp. 2015;31:1603–1610. doi: 10.3305/nh.2015.31.4.8362. [DOI] [PubMed] [Google Scholar]
- 56.Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, Takahashi H, Takahashi S, Kinouchi Y, Hiwatashi N, et al. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment Pharmacol Ther. 2006;24:1333–1340. doi: 10.1111/j.1365-2036.2006.03120.x. [DOI] [PubMed] [Google Scholar]
- 57.Tokuyama S, Nakamoto K. Unsaturated fatty acids and pain. Biol Pharm Bull. 2011;34:1174–1178. doi: 10.1248/bpb.34.1174. [DOI] [PubMed] [Google Scholar]
- 58.Su C, Lichtenstein GR, Krok K, Brensinger CM, Lewis JD. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn’s disease. Gastroenterology. 2004;126:1257–1269. doi: 10.1053/j.gastro.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 59.Egan LJ, Mays DC, Huntoon CJ, Bell MP, Pike MG, Sandborn WJ, Lipsky JJ, McKean DJ. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999;274:26448–26453. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- 60.Langmead L, Rampton DS. Review article: herbal treatment in gastrointestinal and liver disease--benefits and dangers. Aliment Pharmacol Ther. 2001;15:1239–1252. doi: 10.1046/j.1365-2036.2001.01053.x. [DOI] [PubMed] [Google Scholar]
- 61.Tromm A, Bunganič I, Tomsová E, Tulassay Z, Lukáš M, Kykal J, Bátovský M, Fixa B, Gabalec L, Safadi R, et al. Budesonide 9 mg is at least as effective as mesalamine 4.5 g in patients with mildly to moderately active Crohn’s disease. Gastroenterology. 2011;140:425–434.e1; quiz e13-14. doi: 10.1053/j.gastro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Hanauer SB. Review article: aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 Suppl 4:60–65. doi: 10.1111/j.1365-2036.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- 63.Seow CH, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;(3):CD000296. doi: 10.1002/14651858.CD000296.pub3. [DOI] [PubMed] [Google Scholar]
- 64.Otley A, Steinhart AH. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2005;(4):CD000296. doi: 10.1002/14651858.CD000296.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Silverman J, Otley A. Budesonide in the treatment of inflammatory bowel disease. Expert Rev Clin Immunol. 2011;7:419–428. doi: 10.1586/eci.11.34. [DOI] [PubMed] [Google Scholar]
- 66.Campieri M, Ferguson A, Doe W, Persson T, Nilsson LG. Oral budesonide is as effective as oral prednisolone in active Crohn’s disease. The Global Budesonide Study Group. Gut. 1997;41:209–214. doi: 10.1136/gut.41.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;(2):CD006792. doi: 10.1002/14651858.CD006792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho GT, Chiam P, Drummond H, Loane J, Arnott ID, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24:319–330. doi: 10.1111/j.1365-2036.2006.02974.x. [DOI] [PubMed] [Google Scholar]
- 69.D’Haens G. Anti-TNF treatment in Crohn’s disease: toward tailored therapy? Am J Gastroenterol. 2010;105:1140–1141. doi: 10.1038/ajg.2010.15. [DOI] [PubMed] [Google Scholar]
- 70.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 71.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, Gjertson D, Braun J, Reed E, Singh RR. TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol. 2008;126:121–136. doi: 10.1016/j.clim.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;(1):CD006893. doi: 10.1002/14651858.CD006893. [DOI] [PubMed] [Google Scholar]
- 73.Peyrin-Biroulet L. [Anti-TNF therapy and Crohn’s disease] Gastroenterol Clin Biol. 2008;32:478–481. doi: 10.1016/j.gcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Peyrin-Biroulet L, Oussalah A, Boucekkine T, Bigard MA. TNF antagonists in the treatment of inflammatory bowel disease: results of a survey of gastroenterologists in the French region of Lorraine. Gastroenterol Clin Biol. 2009;33:23–30. doi: 10.1016/j.gcb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Bank S, Andersen PS, Burisch J, Pedersen N, Roug S, Galsgaard J, Turino SY, Brodersen JB, Rashid S, Avlund S, et al. Effectiveness of anti-tumour necrosis factor-α therapy in Danish patients with inflammatory bowel diseases. Dan Med J. 2015;62:pii: A4994. [PubMed] [Google Scholar]
- 76.Atreya R, Neumann H, Neufert C, Waldner MJ, Billmeier U, Zopf Y, Willma M, App C, Münster T, Kessler H, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med. 2014;20:313–318. doi: 10.1038/nm.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joyau C, Veyrac G, Dixneuf V, Jolliet P. Anti-tumour necrosis factor alpha therapy and increased risk of de novo psoriasis: is it really a paradoxical side effect? Clin Exp Rheumatol. 2012;30:700–706. [PubMed] [Google Scholar]
- 78.Deepak P, Stobaugh DJ, Ehrenpreis ED. Infectious complications of TNF-α inhibitor monotherapy versus combination therapy with immunomodulators in inflammatory bowel disease: analysis of the Food and Drug Administration Adverse Event Reporting System. J Gastrointestin Liver Dis. 2013;22:269–276. [PubMed] [Google Scholar]
- 79.Salmon-Ceron D, Tubach F, Lortholary O, Chosidow O, Bretagne S, Nicolas N, Cuillerier E, Fautrel B, Michelet C, Morel J, et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis. 2011;70:616–623. doi: 10.1136/ard.2010.137422. [DOI] [PubMed] [Google Scholar]
- 80.Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol. 2006;2:602–610. doi: 10.1038/ncprheum0336. [DOI] [PubMed] [Google Scholar]
- 81.Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265–267. doi: 10.1097/MPG.0b013e31802f6424. [DOI] [PubMed] [Google Scholar]
- 82.Colombel JF, Lémann M, Cassagnou M, Bouhnik Y, Duclos B, Dupas JL, Notteghem B, Mary JY. A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn’s disease. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID) Am J Gastroenterol. 1999;94:674–678. doi: 10.1111/j.1572-0241.1999.935_q.x. [DOI] [PubMed] [Google Scholar]
- 83.Ursing B, Alm T, Bárány F, Bergelin I, Ganrot-Norlin K, Hoevels J, Huitfeldt B, Järnerot G, Krause U, Krook A, et al. A comparative study of metronidazole and sulfasalazine for active Crohn’s disease: the cooperative Crohn’s disease study in Sweden. II. Result. Gastroenterology. 1982;83:550–562. [PubMed] [Google Scholar]
- 84.Borgaonkar MR, MacIntosh DG, Fardy JM. A meta-analysis of antimycobacterial therapy for Crohn’s disease. Am J Gastroenterol. 2000;95:725–729. doi: 10.1111/j.1572-0241.2000.01842.x. [DOI] [PubMed] [Google Scholar]
- 85.Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008;64:753–767. doi: 10.1007/s00228-008-0478-6. [DOI] [PubMed] [Google Scholar]
- 86.Konidari A, Matary WE. Use of thiopurines in inflammatory bowel disease: Safety issues. World J Gastrointest Pharmacol Ther. 2014;5:63–76. doi: 10.4292/wjgpt.v5.i2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maltzman JS, Koretzky GA. Azathioprine: old drug, new actions. J Clin Invest. 2003;111:1122–1124. doi: 10.1172/JCI18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mazor Y, Koifman E, Elkin H, Chowers Y, Krivoy N, Karban A, Efrati E. Risk factors for serious adverse effects of thiopurines in patients with Crohn’s disease. Curr Drug Saf. 2013;8:181–185. doi: 10.2174/15748863113089990033. [DOI] [PubMed] [Google Scholar]
- 89.Biancone L, Tosti C, Fina D, Fantini M, De Nigris F, Geremia A, Pallone F. Review article: maintenance treatment of Crohn’s disease. Aliment Pharmacol Ther. 2003;17 Suppl 2:31–37. doi: 10.1046/j.1365-2036.17.s2.20.x. [DOI] [PubMed] [Google Scholar]
- 90.Sandborn WJ. Azathioprine: state of the art in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1998;225:92–99. doi: 10.1080/003655298750027290. [DOI] [PubMed] [Google Scholar]
- 91.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neurath M. Thiopurines in IBD: What Is Their Mechanism of Action? Gastroenterol Hepatol (N Y) 2010;6:435–436. [PMC free article] [PubMed] [Google Scholar]
- 93.Cosnes J, Bourrier A, Laharie D, Nahon S, Bouhnik Y, Carbonnel F, Allez M, Dupas JL, Reimund JM, Savoye G, et al. Early administration of azathioprine vs conventional management of Crohn’s Disease: a randomized controlled trial. Gastroenterology. 2013;145:758–765.e2; quiz e14-15. doi: 10.1053/j.gastro.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 94.Cosnes J, Seksik P. Early azathioprine in Crohn’s disease. Inflamm Bowel Dis. 2013;19:674–675. doi: 10.1097/MIB.0b013e318281d697. [DOI] [PubMed] [Google Scholar]
- 95.Derijks LJ, Gilissen LP, de Boer NK, Mulder CJ. 6-Thioguanine-related hepatotoxicity in patients with inflammatory bowel disease: dose or level dependent? J Hepatol. 2006;44:821–822. doi: 10.1016/j.jhep.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 96.Rossi S. Australian medicines handbook 2013. Australian: Australian Medicines Handbook Pty Ltd; 2013. [Google Scholar]
- 97.Swaminath A, Taunk R, Lawlor G. Use of methotrexate in inflammatory bowel disease in 2014: A User’s Guide. World J Gastrointest Pharmacol Ther. 2014;5:113–121. doi: 10.4292/wjgpt.v5.i3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ye BD, Yang SK, Shin SJ, Lee KM, Jang BI, Cheon JH, Choi CH, Kim YH, Lee H. Guidelines for the management of Crohn’s disease. Korean J Gastroenterol. 2012;59:141–179. doi: 10.4166/kjg.2012.59.2.141. [DOI] [PubMed] [Google Scholar]
- 99.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851–1858. doi: 10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- 100.Meier J, Sturm A. Current treatment of ulcerative colitis. World J Gastroenterol. 2011;17:3204–3212. doi: 10.3748/wjg.v17.i27.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsumoto T, Kudo T, Jo Y, Esaki M, Yao T, Iida M. Magnifying colonoscopy with narrow band imaging system for the diagnosis of dysplasia in ulcerative colitis: a pilot study. Gastrointest Endosc. 2007;66:957–965. doi: 10.1016/j.gie.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 102.Cottone M, Renna S, Modesto I, Orlando A. Is 5-ASA still the treatment of choice for ulcerative colitis? Curr Drug Targets. 2011;12:1396–1405. doi: 10.2174/138945011796818126. [DOI] [PubMed] [Google Scholar]
- 103.Ford AC, Khan KJ, Achkar JP, Moayyedi P. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in Ulcerative Colitis: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:167–176; author reply 177. doi: 10.1038/ajg.2011.410. [DOI] [PubMed] [Google Scholar]
- 104.Kamm MA, Sandborn WJ, Gassull M, Schreiber S, Jackowski L, Butler T, Lyne A, Stephenson D, Palmen M, Joseph RE. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132:66–75; quiz 432-433. doi: 10.1053/j.gastro.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 105.Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006;(2):CD000543. doi: 10.1002/14651858.CD000543.pub2. [DOI] [PubMed] [Google Scholar]
- 106.Marteau P, Probert CS, Lindgren S, Gassul M, Tan TG, Dignass A, Befrits R, Midhagen G, Rademaker J, Foldager M. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut. 2005;54:960–965. doi: 10.1136/gut.2004.060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Probert CS, Dignass AU, Lindgren S, Oudkerk Pool M, Marteau P. Combined oral and rectal mesalazine for the treatment of mild-to-moderately active ulcerative colitis: rapid symptom resolution and improvements in quality of life. J Crohns Colitis. 2014;8:200–207. doi: 10.1016/j.crohns.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 108.Frieri G, Pimpo M, Galletti B, Palumbo G, Corrao G, Latella G, Chiaramonte M, Caprilli R. Long-term oral plus topical mesalazine in frequently relapsing ulcerative colitis. Dig Liver Dis. 2005;37:92–96. doi: 10.1016/j.dld.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 109.Probert C. Steroids and 5-aminosalicylic acids in moderate ulcerative colitis: addressing the dilemma. Therap Adv Gastroenterol. 2013;6:33–38. doi: 10.1177/1756283X12461395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. doi: 10.1016/j.crohns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 112.Regueiro M, Loftus EV, Steinhart AH, Cohen RD. Medical management of left-sided ulcerative colitis and ulcerative proctitis: critical evaluation of therapeutic trials. Inflamm Bowel Dis. 2006;12:979–994. doi: 10.1097/01.mib.0000231495.92013.5e. [DOI] [PubMed] [Google Scholar]
- 113.Regueiro M, Loftus EV, Steinhart AH, Cohen RD. Clinical guidelines for the medical management of left-sided ulcerative colitis and ulcerative proctitis: summary statement. Inflamm Bowel Dis. 2006;12:972–978. doi: 10.1097/01.mib.0000231496.92013.85. [DOI] [PubMed] [Google Scholar]
- 114.Garud S, Peppercorn MA. Ulcerative colitis: current treatment strategies and future prospects. Therap Adv Gastroenterol. 2009;2:99–108. doi: 10.1177/1756283X09102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23; quiz 524. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 116.Ghosh S, Chaudhary R, Carpani M, Playford RJ. Is thiopurine therapy in ulcerative colitis as effective as in Crohn’s disease? Gut. 2006;55:6–8. doi: 10.1136/gut.2005.074401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gisbert JP, Linares PM, McNicholl AG, Maté J, Gomollón F. Meta-analysis: the efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment Pharmacol Ther. 2009;30:126–137. doi: 10.1111/j.1365-2036.2009.04023.x. [DOI] [PubMed] [Google Scholar]
- 118.Gisbert JP, Luna M, Maté J, González-Guijarro L, Cara C, Pajares JM. Choice of azathioprine or 6-mercaptopurine dose based on thiopurine methyltransferase (TPMT) activity to avoid myelosuppression. A prospective study. Hepatogastroenterology. 2006;53:399–404. [PubMed] [Google Scholar]
- 119.Arts J, D’Haens G, Zeegers M, Van Assche G, Hiele M, D’Hoore A, Penninckx F, Vermeire S, Rutgeerts P. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10:73–78. doi: 10.1097/00054725-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 120.Cheifetz AS, Stern J, Garud S, Goldstein E, Malter L, Moss AC, Present DH. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol. 2011;45:107–112. doi: 10.1097/MCG.0b013e3181e883dd. [DOI] [PubMed] [Google Scholar]
- 121.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 122.Sandborn WJ, Van Asche GA, Reinisch W, Colombel JF, D’Haens GR, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar R. Induction and maintenance of clinical remission by adalimumab in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2011;140 Suppl 1:S123–S124. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 123.Gies N, Kroeker KI, Wong K, Fedorak RN. Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort. Aliment Pharmacol Ther. 2010;32:522–528. doi: 10.1111/j.1365-2036.2010.04380.x. [DOI] [PubMed] [Google Scholar]
- 124.Panaccione R, Loftus EV, Binion D, McHugh K, Alam S, Chen N, Guerette B, Mulani P, Chao J. Efficacy and safety of adalimumab in Canadian patients with moderate to severe Crohn’s disease: results of the Adalimumab in Canadian SubjeCts with ModErate to Severe Crohn’s DiseaSe (ACCESS) trial. Can J Gastroenterol. 2011;25:419–425. doi: 10.1155/2011/724813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sokol H, Seksik P, Carrat F, Nion-Larmurier I, Vienne A, Beaugerie L, Cosnes J. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut. 2010;59:1363–1368. doi: 10.1136/gut.2010.212712. [DOI] [PubMed] [Google Scholar]
- 126.Marchioni Beery R, Kane S. Current approaches to the management of new-onset ulcerative colitis. Clin Exp Gastroenterol. 2014;7:111–132. doi: 10.2147/CEG.S35942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chapman JR, Larson DW, Wolff BG, Dozois EJ, Cima RR, Pemberton JH, Crownhart BS, Larson DR. Ileal pouch-anal anastomosis: does age at the time of surgery affect outcome? Arch Surg. 2005;140:534–539; discussion 539-540. doi: 10.1001/archsurg.140.6.534. [DOI] [PubMed] [Google Scholar]
- 128.Pinto RA, Canedo J, Murad-Regadas S, Regadas SF, Weiss EG, Wexner SD. Ileal pouch-anal anastomosis in elderly patients: is there a difference in morbidity compared with younger patients? Colorectal Dis. 2011;13:177–183. doi: 10.1111/j.1463-1318.2009.02097.x. [DOI] [PubMed] [Google Scholar]
- 129.Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, Bernklev T, Henriksen M, Sauar J, Vatn MH, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study) Scand J Gastroenterol. 2009;44:431–440. doi: 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 130.Sanjay Bandyopadhyay K. Ulcerative Colitis: Current management strategy. Medicine Update. 2010;20:497–509. [Google Scholar]
- 131.Bernstein CN, Ng SC, Lakatos PL, Moum B, Loftus EV. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19:2001–2010. doi: 10.1097/MIB.0b013e318281f3bb. [DOI] [PubMed] [Google Scholar]
- 132.Hueting WE, Buskens E, van der Tweel I, Gooszen HG, van Laarhoven CJ. Results and complications after ileal pouch anal anastomosis: a meta-analysis of 43 observational studies comprising 9,317 patients. Dig Surg. 2005;22:69–79. doi: 10.1159/000085356. [DOI] [PubMed] [Google Scholar]
- 133.Loftus EV, Delgado DJ, Friedman HS, Sandborn WJ. Colectomy and the incidence of postsurgical complications among ulcerative colitis patients with private health insurance in the United States. Am J Gastroenterol. 2008;103:1737–1745. doi: 10.1111/j.1572-0241.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 134.Waljee A, Waljee J, Morris AM, Higgins PD. Threefold increased risk of infertility: a meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis. Gut. 2006;55:1575–1580. doi: 10.1136/gut.2005.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oren R, Arber N, Odes S, Moshkowitz M, Keter D, Pomeranz I, Ron Y, Reisfeld I, Broide E, Lavy A, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology. 1996;110:1416–1421. doi: 10.1053/gast.1996.v110.pm8613046. [DOI] [PubMed] [Google Scholar]
- 136.Paoluzi OA, Pica R, Marcheggiano A, Crispino P, Iacopini F, Iannoni C, Rivera M, Paoluzi P. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751–1759. doi: 10.1046/j.1365-2036.2002.01340.x. [DOI] [PubMed] [Google Scholar]
- 137.Khan N, Abbas AM, Moehlen M, Balart L. Methotrexate in ulcerative colitis: a nationwide retrospective cohort from the Veterans Affairs Health Care System. Inflamm Bowel Dis. 2013;19:1379–1383. doi: 10.1097/MIB.0b013e31828133e8. [DOI] [PubMed] [Google Scholar]
- 138.Sands BE, Sandborn WJ, Feagan B, Löfberg R, Hibi T, Wang T, Gustofson LM, Wong CJ, Vandervoort MK, Hanauer S. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400–409. doi: 10.1053/j.gastro.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 139.Saniabadi AR, Hanai H, Suzuki Y, Ohmori T, Sawada K, Yoshimura N, Saito Y, Takeda Y, Umemura K, Kondo K, et al. Adacolumn for selective leukocytapheresis as a non-pharmacological treatment for patients with disorders of the immune system: an adjunct or an alternative to drug therapy? J Clin Apher. 2005;20:171–184. doi: 10.1002/jca.20046. [DOI] [PubMed] [Google Scholar]
- 140.Shibata H, Kuriyama T, Yamawaki N. Cellsorba. Ther Apher Dial. 2003;7:44–47. doi: 10.1046/j.1526-0968.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 141.Kashiwagi N, Sugimura K, Koiwai H, Yamamoto H, Yoshikawa T, Saniabadi AR, Adachi M, Shimoyama T. Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci. 2002;47:1334–1341. doi: 10.1023/a:1015330816364. [DOI] [PubMed] [Google Scholar]
- 142.Grisham MB, Yamada T. Neutrophils, nitrogen oxides, and inflammatory bowel disease. Ann N Y Acad Sci. 1992;664:103–115. doi: 10.1111/j.1749-6632.1992.tb39753.x. [DOI] [PubMed] [Google Scholar]
- 143.Grisham MB, Granger N. Mechanisms of neutrophil-mediated tissue injury. In: MacDermott RP, Stenson WF, editors. Inflammatory Bowel Disease. New York, Elsevier; 1992. pp. 225–239. [Google Scholar]
- 144.Sandborn WJ. Preliminary data on the use of apheresis in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12 Suppl 1:S15–S21. doi: 10.1097/01.mib.0000195387.26892.22. [DOI] [PubMed] [Google Scholar]
- 145.Hanai H, Takeda Y, Eberhardson M, Gruber R, Saniabadi AR, Winqvist O, Lofberg R. The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: a concise review. Clin Exp Immunol. 2011;163:50–58. doi: 10.1111/j.1365-2249.2010.04279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kashiwagi N, Nakano M, Saniabadi AR, Adachi M, Yoshikawa T. Anti-inflammatory effect of granulocyte and monocyte adsorption apheresis in a rabbit model of immune arthritis. Inflammation. 2002;26:199–205. doi: 10.1023/a:1016523914161. [DOI] [PubMed] [Google Scholar]
- 147.Kuijpers TW, Tool AT, van der Schoot CE, Ginsel LA, Onderwater JJ, Roos D, Verhoeven AJ. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78:1105–1111. [PubMed] [Google Scholar]
- 148.Waitz G, Petermann S, Liebe S, Emmrich J, Ramlow W. Reduction of dendritic cells by granulocyte and monocyte adsorption apheresis in patients with ulcerative colitis. Dig Dis Sci. 2008;53:2507–2515. doi: 10.1007/s10620-007-0168-8. [DOI] [PubMed] [Google Scholar]
- 149.Cuadrado E. Granulocyte/monocyte apheresis as immunotherapic tool: cellular adsorption and immune modulation. Autoimmun Rev. 2009;8:292–296. doi: 10.1016/j.autrev.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 150.Cuadrado E, Alonso M, de Juan MD, Echaniz P, Arenas JI. Regulatory T cells in patients with inflammatory bowel diseases treated with adacolumn granulocytapheresis. World J Gastroenterol. 2008;14:1521–1527. doi: 10.3748/wjg.14.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Toya Y, Chiba T, Mizutani T, Sato K, Kasugai S, Matsuda N, Orikasa S, Shibata S, Abiko Y, Akasaka R, et al. The effect of granulocyte and monocyte adsorptive apheresis on serum cytokine levels in patients with ulcerative colitis. Cytokine. 2013;62:146–150. doi: 10.1016/j.cyto.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 152.Hanai H. Positions of selective leukocytapheresis in the medical therapy of ulcerative colitis. World J Gastroenterol. 2006;12:7568–7577. doi: 10.3748/wjg.v12.i47.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yamamoto T, Umegae S, Matsumoto K. Safety and clinical efficacy of granulocyte and monocyte adsorptive apheresis therapy for ulcerative colitis. World J Gastroenterol. 2006;12:520–525. doi: 10.3748/wjg.v12.i4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yokoyama Y, Kawai M, Fukunaga K, Kamikozuru K, Nagase K, Nogami K, Kono T, Ohda Y, Iimuro M, Hida N, et al. Looking for predictive factors of clinical response to adsorptive granulocyte and monocyte apheresis in patients with ulcerative colitis: markers of response to GMA. BMC Gastroenterol. 2013;13:27. doi: 10.1186/1471-230X-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sacco R, Tanaka T, Yamamoto T, Bresci G, Saniabadi AR. Adacolumn leucocytapheresis for ulcerative colitis: clinical and endoscopic features of responders and unresponders. Expert Rev Gastroenterol Hepatol. 2015;9:327–333. doi: 10.1586/17474124.2014.953060. [DOI] [PubMed] [Google Scholar]
- 156.Fukuchi T, Nakase H, Ubukata S, Matsuura M, Yoshino T, Toyonaga T, Shimazu K, Koga H, Yamashita H, Ito D, et al. Therapeutic effect of intensive granulocyte and monocyte adsorption apheresis combined with thiopurines for steroid- and biologics-naïve Japanese patients with early-diagnosed Crohn’s disease. BMC Gastroenterol. 2013;13:124. doi: 10.1186/1471-230X-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yoshino T, Nakase H, Minami N, Yamada S, Matsuura M, Yazumi S, Chiba T. Efficacy and safety of granulocyte and monocyte adsorption apheresis for ulcerative colitis: a meta-analysis. Dig Liver Dis. 2014;46:219–226. doi: 10.1016/j.dld.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 158.Vecchi M, Vernia P, Riegler G, D’Incà R, Annese V, Bagnoli S. Therapeutic landscape for ulcerative colitis: where is the Adacolumn(®) system and where should it be? Clin Exp Gastroenterol. 2013;6:1–7. doi: 10.2147/CEG.S33275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saniabadi AR, Tanaka T, Ohmori T, Sawada K, Yamamoto T, Hanai H. Treating inflammatory bowel disease by adsorptive leucocytapheresis: a desire to treat without drugs. World J Gastroenterol. 2014;20:9699–9715. doi: 10.3748/wjg.v20.i29.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dignass AU, Eriksson A, Kilander A, Pukitis A, Rhodes JM, Vavricka S. Clinical trial: five or ten cycles of granulocyte-monocyte apheresis show equivalent efficacy and safety in ulcerative colitis. Aliment Pharmacol Ther. 2010;31:1286–1295. doi: 10.1111/j.1365-2036.2010.04295.x. [DOI] [PubMed] [Google Scholar]
- 161.Tominaga K, Nakano M, Hoshino M, Kanke K, Hiraishi H. Efficacy, safety and cost analyses in ulcerative colitis patients undergoing granulocyte and monocyte adsorption or receiving prednisolone. BMC Gastroenterol. 2013;13:41. doi: 10.1186/1471-230X-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Szczepiorkowski ZM, Winters JL, Bandarenko N, Kim HC, Linenberger ML, Marques MB, Sarode R, Schwartz J, Weinstein R, Shaz BH. Guidelines on the use of therapeutic apheresis in clinical practice--evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher. 2010;25:83–177. doi: 10.1002/jca.20240. [DOI] [PubMed] [Google Scholar]
- 163.Bicks RO, Groshart KD. The current status of T-lymphocyte apheresis (TLA) treatment of Crohn’s disease. J Clin Gastroenterol. 1989;11:136–138. [PubMed] [Google Scholar]
- 164.Matsumoto T, Fukunaga K, Kamikozuru K, Tozawa K, Yokoyama Y, Kusaka T, Onishi K, Miwa H, Nakamura S. Cytapheresis as a Non-Pharmacological Therapy for Inflammatory Bowel Disease. Transfus Med Hemother. 2008;35:18–23. doi: 10.1159/000111763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abreu MT, von Tirpitz C, Hardi R, Kaatz M, Van Assche G, Rutgeerts P, Bisaccia E, Goerdt S, Hanauer S, Knobler R, et al. Extracorporeal photopheresis for the treatment of refractory Crohn’s disease: results of an open-label pilot study. Inflamm Bowel Dis. 2009;15:829–836. doi: 10.1002/ibd.20833. [DOI] [PubMed] [Google Scholar]
- 166.Reinisch W, Knobler R, Rutgeerts PJ, Ochsenkühn T, Anderson F, von Tirpitz C, Kaatz M, Janneke van der Woude C, Parenti D, Mannon PJ. Extracorporeal photopheresis (ECP) in patients with steroid-dependent Crohn’s disease: an open-label, multicenter, prospective trial. Inflamm Bowel Dis. 2013;19:293–300. doi: 10.1002/ibd.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Oliven A, Shechter Y. Extracorporeal photopheresis: a review. Blood Rev. 2001;15:103–108. doi: 10.1054/blre.2001.0155. [DOI] [PubMed] [Google Scholar]
- 168.Di Biaso I, Di Maio L, Bugarin C, Gaipa G, Dander E, Balduzzi A, Parma M, D’Amico G, Perseghin P, Biondi A, et al. Regulatory T cells and extracorporeal photochemotherapy: correlation with clinical response and decreased frequency of proinflammatory T cells. Transplantation. 2009;87:1422–1425. doi: 10.1097/TP.0b013e3181a27a5d. [DOI] [PubMed] [Google Scholar]
- 169.Maeda A, Schwarz A, Bullinger A, Morita A, Peritt D, Schwarz T. Experimental extracorporeal photopheresis inhibits the sensitization and effector phases of contact hypersensitivity via two mechanisms: generation of IL-10 and induction of regulatory T cells. J Immunol. 2008;181:5956–5962. doi: 10.4049/jimmunol.181.9.5956. [DOI] [PubMed] [Google Scholar]
- 170.Worel N, Leitner G. Clinical Results of Extracorporeal Photopheresis. Transfus Med Hemother. 2012;39:254–262. doi: 10.1159/000341811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Simon R, Mikocka-Walus AA. Psychological Aspects of Inflammatory Bowel Disease: A biopsychosocial approac. London; New York: Routledge; 2014. [Google Scholar]
- 173.Ghosh S, Pariente B, Mould DR, Schreiber S, Petersson J, Hommes D. New tools and approaches for improved management of inflammatory bowel diseases. J Crohns Colitis. 2014;8:1246–1253. doi: 10.1016/j.crohns.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 174.Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, Chowers Y, D’Haens G, Feagan BG, Hibi T, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.D’Haens GR, Sartor RB, Silverberg MS, Petersson J, Rutgeerts P. Future directions in inflammatory bowel disease management. J Crohns Colitis. 2014;8:726–734. doi: 10.1016/j.crohns.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 176.Bendtzen K, Ainsworth M, Steenholdt C, Thomsen OØ, Brynskov J. Individual medicine in inflammatory bowel disease: monitoring bioavailability, pharmacokinetics and immunogenicity of anti-tumour necrosis factor-alpha antibodies. Scand J Gastroenterol. 2009;44:774–781. doi: 10.1080/00365520802699278. [DOI] [PubMed] [Google Scholar]
- 177.McLean LP, Shea-Donohue T, Cross RK. Vedolizumab for the treatment of ulcerative colitis and Crohn’s disease. Immunotherapy. 2012;4:883–898. doi: 10.2217/imt.12.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Krupka N, Baumgart DC. Designing biologic selectivity for inflammatory bowel disease--role of vedolizumab. Drug Des Devel Ther. 2015;9:147–154. doi: 10.2147/DDDT.S50348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503–516. doi: 10.1111/j.1365-2036.2012.05220.x. [DOI] [PubMed] [Google Scholar]
- 180.Hügle T, Daikeler T. Stem cell transplantation for autoimmune diseases. Haematologica. 2010;95:185–188. doi: 10.3324/haematol.2009.017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sales-Campos H, Basso PJ, Alves VB, Fonseca MT, Bonfá G, Nardini V, Cardoso CR. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz J Med Biol Res. 2015;48:96–107. doi: 10.1590/1414-431X20143774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, Denton C, Hawkey C, Labopin M, Mancardi G, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47:770–790. doi: 10.1038/bmt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Burt RK, Craig RM, Milanetti F, Quigley K, Gozdziak P, Bucha J, Testori A, Halverson A, Verda L, de Villiers WJ, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood. 2010;116:6123–6132. doi: 10.1182/blood-2010-06-292391. [DOI] [PubMed] [Google Scholar]
- 184.Hasselblatt P, Drognitz K, Potthoff K, Bertz H, Kruis W, Schmidt C, Stallmach A, Schmitt-Graeff A, Finke J, Kreisel W. Remission of refractory Crohn’s disease by high-dose cyclophosphamide and autologous peripheral blood stem cell transplantation. Aliment Pharmacol Ther. 2012;36:725–735. doi: 10.1111/apt.12032. [DOI] [PubMed] [Google Scholar]
- 185.Cassinotti A, Keshav S, Ardizzone S, Mortensen N, Sampietro G, Fociani P, Duca P, George B, Lazzaroni M, Manes G, et al. IBD care in Europe: A comparative audit of the inpatient management of Crohn’s disease and ulcerative colitis using the national UK IBD audit tool. J Crohns Colitis. 2009;3:291–301. doi: 10.1016/j.crohns.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 186.Colletti RB, Baldassano RN, Milov DE, Margolis PA, Bousvaros A, Crandall WV, Crissinger KD, D’Amico MA, Day AS, Denson LA, et al. Variation in care in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2009;49:297–303. doi: 10.1097/MPG.0b013e3181919695. [DOI] [PubMed] [Google Scholar]
- 187.Panaccione R, Ghosh S. Optimal use of biologics in the management of Crohn’s disease. Therap Adv Gastroenterol. 2010;3:179–189. doi: 10.1177/1756283X09357579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lin MV, Blonski W, Lichtenstein GR. What is the optimal therapy for Crohn‘s disease: step-up or top-down? Expert Rev Gastroenterol Hepatol. 2010;4:167–180. doi: 10.1586/egh.10.4. [DOI] [PubMed] [Google Scholar]
- 189.Rogler G. Top-down or step-up treatment in Crohn’s disease? Dig Dis. 2013;31:83–90. doi: 10.1159/000347190. [DOI] [PubMed] [Google Scholar]
- 190.D’Haens GR. Top-down therapy for IBD: rationale and requisite evidence. Nat Rev Gastroenterol Hepatol. 2010;7:86–92. doi: 10.1038/nrgastro.2009.222. [DOI] [PubMed] [Google Scholar]
- 191.Chen QQ, Yan L, Wan J. Select a suitable treatment strategy for Crohn’s disease: step-up or top-down. EXCLI J. 2014;13:111–122. [PMC free article] [PubMed] [Google Scholar]
- 192.Sandborn WJ. Step-up versus top-down therapy in the treatment of ulcerative colitis. Gastroenterol Hepatol (N Y) 2007;3:16–17. [PMC free article] [PubMed] [Google Scholar]
- 193.Jeppesen PB, Pertkiewicz M, Messing B, Iyer K, Seidner DL, O’keefe SJ, Forbes A, Heinze H, Joelsson B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473–1481.e3. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 194.Fuchssteiner H, Nigl K, Mayer A, Kristensen B, Platzer R, Brunner B, Weiß I, Haas T, Benedikt M, Gröchenig HP, et al. [Nutrition and IBD-Consensus of the Austrian Working Group of IBD (Inflammatory Bowel Diseases) of the ÖGGH] Z Gastroenterol. 2014;52:376–386. doi: 10.1055/s-0034-1366252. [DOI] [PubMed] [Google Scholar]


