Abstract
Objective
Parkinsonism and other movement disorders have previously been reported in the acquired hepatocerebral degeneration associated with portosystemic shunting. However, there is no study to date about their prevalence as has been noted in general practice.
Methods
One hundred and forty-three patients with hepatic cirrhosis from the gastroenterology clinic and internal medicine wards were enrolled. Liver data included the diagnoses, etiologies, assessments of complications, and treatments for cirrhosis. Hepatic encephalopathy was classified with regard to the West Haven criteria for semi-quantitative grading for mental status. Neurological examination results and abnormal involuntary movements were recorded as primary outcomes. Neuro-radiology was used for the detection of severe brain lesions.
Results
Alcoholism was the most common cause of liver cirrhosis. Eighty-three patients (58%) presented with movement disorders. Asterixis was found in one of the cases. The most common movement disorder seen was an intentional tremor at 37.1%, which was followed by bradykinesia, Parkinsonism, and postural tremors at 29.4%, 10.5%, and 6.3%, respectively. The prevalence of movement disorders simultaneously increased with a high Child-Turcotte-Pugh score. The hepatic encephalopathy was grade 1 and 2. With the inclusion of age-range adjustments, we found that alcoholic cirrhosis and hepatic encephalopathy are statistically significant factors [p < 0.05, odds ratio (OR) = 6.41, 95% confidence interval (CI) 1.38–29.71 and p < 0.001, OR = 13.65, 95% CI 4.71–39.54] for the development of movement disorders in non-Wilsonian cirrhotic patients.
Conclusions
Intentional tremor is a common abnormal movement. Alcoholic cirrhosis and hepatic encephalopathy are significant risk factors in the development of movement disorders in non-Wilsonian cirrhotic patients.
Keywords: Movement disorders, Hepatic cirrhosis, Intention tremor, Alcoholic cirrhosis, Hepatic encephalopathy
Hepatic cirrhosis refers to the replacement of normal liver parenchyma by fibrotic tissue and regenerative nodules. When there is liver function decompensation, the development of systemic complications and, to some extent, neurological complications occur. The most common neurological condition related to hepatic cirrhosis is hepatic encephalopathy. Regarding movement disorders, asterixis and negative myoclonus are commonly seen. However, there are reports of different abnormal movements established in cirrhotic patients.
The well-known co-existence of hepatic cirrhosis and abnormal movements in Wilson’s disease is the result of an autosomal recessive genetic disease of copper metabolism that results in the accumulation of copper in body tissues. These include the brain and liver [1]. In non-Wilsonian cirrhotic patients, the chronic hepatocerebral degeneration that is associated with impaired liver function and portosystemic shunting was documented by Victor et al. [2] in 1965. Cognitive abnormalities, Parkinsonism, and predominant rigidity are the clinical characteristics of hepatocerebral degeneration. One recent study has reported that the prevalence of cirrhosis-related Parkinsonism is 4.2% [3]. However, the prevalence, risk factors, and relationships to other phenotypes of movement disorders in non-Wilsonian hepatic cirrhotic patients, along with the Child-Turcotte-Pugh (CTP) scores [4] and the etiologies of hepatic cirrhosis, are unclear. This cross-sectional study aims to identify the prevalence of neurological deficits, phenotypes of movement disorders, their association to the causes of the cirrhosis, CTP scores and other risk factors of hospital-based, non-Wilsonian cirrhosis patients.
MATERIALS & METHODS
Population
The research was conducted after receiving approval by the Ethics Committee of the Faculty of Medicine, Srinakharinwirot University. Cirrhotic patients of the gastroenterology clinic and internal medicine in-patient departments between February 2013 and February 2014 were invited to participate in this study. First, patients visited gastroenterologists and internists as usual, and those doctors introduced the research to them. If patients were interested in being volunteers, they would visit a research assistant to get information. The second step was registration and making the appointment with the neurologist. Two gastroenterologists (CP and WC) were responsible for the diagnoses, etiologies, assessment of complications, and medical treatments for patients with confirmed liver cirrhosis. One neurologist (MK) examined patients who met the inclusion criteria. The patients’ ages were between 20–70 years. The diagnosis of non-Wilsonian hepatic cirrhosis was confirmed by hepatitis virus serology tests, routine hepatic function tests, radiological findings of the upper abdomen, and/or pathology as found in liver biopsies. All volunteers were required to have stable clinical symptoms and be fully conscious for the neurological examination and movement disorders assessment. They were required to sign a consent form for participation. Due to this requirement, some severe forms of hepatic encephalopathy, such as confusion, total disorientation, stupor, and coma, that are found in hepatic encephalopathy grades 3 and 4 were excluded. We also ruled out patients with Wilson’s disease and anyone who continued to experience any emergent conditions, for example, active gastrointestinal bleeding, septicemia, and/or coma. We also excluded any subjects who were unwilling to take part in the study.
Data
The data collection was done in two divisions. The first included general data, gender, and age. This division addressed preexisting underlying conditions and confirmed hepatic cirrhosis. The second division consisted of a neurological assessment that included the patients’ degree of hepatic encephalopathy, general neurological examinations, and examination for movement disorders.
Cirrhosis history
The age of onset and duration of cirrhosis were recorded. The etiologies of cirrhosis were classified by serology tests for the hepatitis B and C virus, patients’ history of alcohol consumption and drug use, and tests for autoimmunity. The diagnosis of non-alcoholic steatohepatitis cirrhosis was determined by exclusion of other causes, along with evidence of transaminitis, and features of metabolic syndrome. The serum ceruloplasmin and urinary copper excretion were assessed for patients younger than 40 years of age and for patients diagnosed with cryptogenic cirrhosis. If patients had at least two causes of cirrhosis, they would be counted again in the combined-cause group. An ultrasonography, abdominal computed tomography (CT), and tissue biopsies for cases with suspected tumors confirmed liver cirrhosis. All patients underwent an esophago-gastro-duodenoscopy to evaluate severity of varices and portal hypertensive gastropathy. Any history of gas-trointestinal bleeding, bacterial peritonitis, and the prevalence of the hepatocellular carcinoma were recorded. Ascites were graded to be absent, controlled, or refractory. The results of routine laboratory tests, including complete blood count, platelet count, prothrombin time, serum albumin, and serum bilirubin, were recorded. Although the grade of hepatic encephalopathy was initially estimated by gastroenterologists, it was reevaluated by a neurologist. Thus, the CTP scores were documented by both a gastroenterologist and a neurologist.
Neurological assessment
The diagnosis of hepatic encephalopathy was made as a clinical diagnosis, as the serum ammonia test and electroencephalography (EEG) are unavailable in our hospital. There are also no standard mental state tests or questionnaires to evaluate hepatic encephalopathy that have been validated in a Thai version. Thus, the hepatic encephalopathy was classified using the West Haven criteria for semi-quantitative grading of a patient’s mental status. Neurological examinations included evaluations of speech, olfactory function, ocular movements, auditory function, facial expression, motor power, pain and proprioception, deep tendon reflexes, ankle clonus and Babinski’s sign. Movement disorders were primary outcomes. Asterixis, postural tremors, intention tremors, Parkinsonism, chorea, dystonia, and gait performance were evaluated. In diagnosing Parkinsonism, patients had to present with at least two of the following signs; rest tremors, rigidity, bradykinesia and postural instability. For patients with some degree of sluggishness on manual dexterity (finger tapping, opening or closing the hand, and rapid alternate movements) and/or leg agility with or without rigidity, we categorized this phenotype as bradykinesia. The patients’ gait was observed for signs of ataxia and other patterns of gait disorders.
Neuro-radiological assessment
The neuro-radiological study was not provided for all patients due to the limitation of research funds. However, we sent some patients for CTs or magnetic resonance imaging (MRIs) of the brain if their neurological examinations revealed apparent neurological deficits that could result in movement disorders. Patients with histories of cerebrovascular disease were at risk with radiologic studies, and as such, we reviewed old MRI/CTs of the brain from volunteers who had prior studies done at the initiation of our research.
Statistical analysis
We used the standard version of SPSS for Windows, version 11.5.0 (Copyright SPSS Inc., Chicago, IL, USA, 1989–2002) for the statistical analysis. Regarding the correlation between independent variables and outcomes, we used either the chi-square or Fisher-Exact tests with p-value of less than 0.05 for statistical significance. A logistic regression with p-value of less than 0.05 was used to determine the significant risk factors related to neurological deficits and movement disorders. Significant risk factors were identified using the odds ratio (OR) or the 95% confidence interval (CI). The correlation of each independent variable and outcome was separately analyzed piece by piece. Then, we integrated all significant variables and variables with p < 0.25 using the logistic regression analysis with age-range adjustment to clarify real statistically significant factors associated with movement disorders.
RESULTS
One hundred and forty three (143) volunteers participated in this study. The confirmations of hepatic cirrhosis were predominantly acquired with the use of abdominal ultrasonography (115 patients, 80.4%), CT scans of the abdomen (12 patients, 8.4%), and CT scans of the abdomen with hepatic tissue biopsies (16 patients, 11.2%). There were 99 male (69.2%) and 44 female (30.8%) patients. The age range is 37–70 years, with a mean age of 56.42 years. The duration of their cirrhosis, from the first diagnosis to study date, was between 0.03 and 251 months (mean = 33.63 months). The data classified by gender are presented in Table 1.
Table 1.
Data on non-Wilsonian cirrhotic patients classified by gender (one patient can have more than 1 causes of cirrhosis, complications of the portal hypertension and other medical illnesses)
| Factors | Male (n = 99) | Female (n = 44) | Total (M + F) | p value | |
|---|---|---|---|---|---|
| Hepatitis B | 46 (46.5%) | 12 (27.3%) | 58 | < 0.05* | |
| Hepatitis C | 16 (16.2%) | 11 (25.0%) | 27 | 0.15 | |
| Alcoholism | 59 (59.6%) | 11 (25.0%) | 70 | < 0.001* | |
| NASH | 6 (6.1%) | 12 (27.3%) | 18 | < 0.05* | |
| Cryptogenic | 1 (1.0%) | 1 (2.3%) | 2 | 0.52 | |
| Combined cause | 28 (28.3%) | 3 (6.8%) | 31 | < 0.05* | |
| History of alcohol consumption | 71 (71.7%) | 13 (29.5%) | 84 | < 0.001* | |
| Portal hypertension | 75 (75.8%) | 33 (75%) | 108 | 0.54 | |
| Gastroesophageal varices | 48 (48.5%) | 23 (52.3%) | 71 | 0.40 | |
| Portal hypertensive gastropathy | 34 (34.3%) | 18 (40.9%) | 52 | 0.28 | |
| Hepatocellular carcinoma | 19 (19.2%) | 2 (4.5%) | 21 | < 0.05* | |
| Hepatic encephalopathy | 37 (37.4%) | 18 (40.9%) | 55 | 0.41 | |
| Diabetes mellitus | 34 (34.3%) | 17 (38.6%) | 51 | 0.37 | |
| Hypertension | 41 (41.4%) | 16 (36.4%) | 57 | 0.35 | |
| Previous history of stroke | 3 (3.0%) | 0 (0%) | 3 | 0.32 | |
| Renal insufficiency | 3 (3.0%) | 2 (4.5%) | 5 | 0.48 | |
| Coronary artery disease | 1 (1.0%) | 1 (2.3%) | 2 | 0.52 | |
| Hyperlipidemia | 33 (33.3%) | 14 (31.8%) | 47 | 0.50 |
% within sex.
statistically significant. M: male, F: female, NASH: non-alcoholic steatohepatitis.
Patients with a history of alcohol use stated consumption levels in different quantities. This group included both patients who were presently drinkers and those who no longer drank. For the current drinkers, most of them were trying to stop drinking, so they occasionally drank, and the quantities of alcohol intake were uncertain. No patients with alcohol withdrawal symptoms participated in the study.
Most of the volunteers are in the group with the CTP A score (69.9%). Fifty-five patients (38.5%) met the criteria for hepatic encephalopathy. Forty-six patients (32.2%) were at grade 1, and 9 patients (6.3%) were grade 2. No patients met the criteria for grades 3 or 4. The percentages of cirrhotic patients with and without neurological deficits are presented in Table 2.
Table 2.
Neurological deficits found in non-Wilsonian hepatic cirrhosis patients
| Neurological deficits | Present (%) | Normal (%) |
|---|---|---|
| Mild dysarthria | 2.8 | 97.2 |
| Hyposmia | 24.5 | 75.5 |
| Facial palsy | 4.9 | 95.1 |
| Impaired hearing function | 9.1 | 90.9 |
| Decreased motor strength (grade 4) | 7.7 | 92.3 |
| Decreased pain sensation | 10.5 | 89.5 |
| Impaired proprioception | 5.6 | 94.4 |
| Abnormal reflexes | 14 | 86 |
Neuro-imaging
Twenty-six patients had MRIs or CT scans of the brain. Only one patient showed congenital dysgenesis of the left temporoparietal lobe. Despite the disability of his right arm, other limbs functioned normally. Nineteen patients showed a mild to moderate cerebellar atrophy. Twelve patients had multi-focal lacunar infarctions. The distributions of lacunes were present in subcortical white matter and periventricular areas.
Movement disorders
There were 83 (58%) patients with abnormal movements. Asterixis or negative myoclonus was found in one patient. The most common form of abnormal movement was an intention tremor at 37.1%, which was followed by bradykinesia, Parkinsonism, and postural tremors at 29.4%, 10.5%, and 6.3%, respectively. There were two patients presenting with cervical dystonia and three patients who had presented a task-specific tremor, simple tics, left hemifacial spasm. Most of the patients had never noticed their movement disorders because they could maintain daily activities so well. Two persons with mild cervical dystonia had experienced this symptom for ten years without clinical progression. One patient with hemifacial spasm had noticed it for five years but declined treatment. Male subjects significantly experienced movement disorders more than female subjects (p < 0.05). The prevalence of all phenotypes of movement disorders was significant in alcoholic cirrhotic patients, which was confirmed by logistic regression analysis (p < 0.001, OR = 4.46, 95% CI 2.17–9.16).
The prevalence of movement disorders in each CTP score group is illustrated in Figure 1. Portal hypertension was a significant risk factor for the development of movement disorders (p < 0.05). The prevalence of hepatocellular carcinoma showed no significance.
Figure 1.
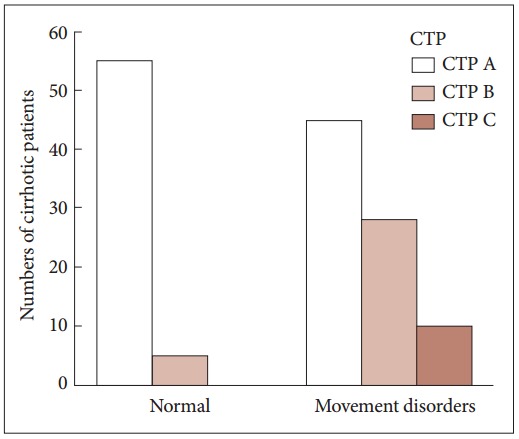
The bar chart illustrates the increase of the prevalence of movement disorders with high CTP scores. CTP: Child-Turcotte-Pugh.
The hepatic encephalopathy showed a significant susceptibility in the development of movement disorders (p < 0.001). Pre-existing medical illnesses, a history of alcohol consumption and various age ranges have been analyzed as confounders. With regard to age range, patients were grouped as being of 37–49, 50–59, and 60–70 years of age. The results of the chi-square and Fisher’s exact as well as the logistic regression analysis confirm the significance of the history of alcohol consumption after adjustments were made for the differing age ranges (p < 0.05).
Neurological deficits that influenced movement disorders were decrease in motor power, impairment of pain sensation and impaired proprioception. We categorized them into two confounding factors of motor and sensory deficit and motor or sensory deficit. The analysis showed no significance in both factors on the prevalence of movement disorders. Results of the univariate analysis are shown in Table 3.
Table 3.
Univariate analysis of factors associated with the prevalence of movement disorders in non-Wilsonian cirrhotic patients
| Variables | p value | Odds ratio | 95% confidence interval |
|---|---|---|---|
| Gender (male) | 0.04* | 2.10* | 1.02-4.31* |
| Hepatitis B | 0.35 | 0.72 | 0.37-1.43 |
| Hepatitis C | 0.11 | 0.50 | 0.21-1.182 |
| Alcoholism | < 0.001* | 4.46* | 2.17-9.16* |
| NASH | 0.08 | 0.41 | 0.14-1.13 |
| Combined cause | 0.41 | 1.41 | 0.62-3.22 |
| Portal hypertension | 0.03* | 2.26* | 1.04-4.92* |
| Hepatocellular carcinoma | 0.18 | 1.98 | 0.72-5.46 |
| Hepatic encephalopathy | < 0.001* | 10.38* | 4.21-25.55* |
| History of alcohol consumption | 0.001* | 3.44* | 1.71-6.92* |
| Diabetes mellitus | 0.39 | 1.35 | 0.67-2.72 |
| Hypertension | 0.70 | 0.87 | 0.44-1.72 |
| Previous history of stroke | 0.99 | - | - |
| Renal insufficiency | 0.92 | 1.08 | 0.17-6.71 |
| Coronary artery disease | 0.99 | - | - |
| Hyperlipidemia | 0.92 | 0.96 | 0.47-1.95 |
| Motor and sensory deficits | 0.80 | 0.85 | 0.24-2.95 |
| Motor or sensory deficits | 0.49 | 1.40 | 0.52-3.76 |
statistically significant. NASH: non-alcoholic steatohepatitis.
Variables and confounders with p values < 0.25 are included in the logistic regression analysis. After adjustments for age ranges (10 year groups), alcoholic cirrhosis and hepatic encephalopathy continue to be statistically significant factors for the development of movement disorders in non-Wilsonian cirrhotic patients (alcoholic cirrhosis p < 0.05, OR = 6.41, 95% CI 1.38–29.71, hepatic encephalopathy p < 0.001, OR = 13.65, 95% CI 4.71–39.54). The details of the multivariate analysis are shown in Table 4.
Table 4.
Multivariate analysis of factors associated with the prevalence of movement disorders in non-Wilsonian cirrhotic patients with age-range adjustment
| Variables | p value | Odds ratio | 95% confidence interval |
|---|---|---|---|
| Gender (male) | 0.11 | 2.39 | 0.81-7.07 |
| Hepatitis C | 0.60 | 0.72 | 0.21-2.42 |
| Alcoholism | 0.01* | 6.41* | 1.38-29.71* |
| NASH | 0.15 | 0.36 | 0.09-1.44 |
| Portal hypertension | 0.61 | 1.29 | 0.46-3.60 |
| Hepatocellular carcinoma | 0.80 | 1.17 | 0.31-4.33 |
| Hepatic encephalopathy | < 0.001* | 13.65* | 4.71-39.54* |
| History of alcohol consumption | 0.32 | 0.46 | 0.10-2.14 |
| Age range (10 years) | 0.11 | 1.72 | 0.88-3.36 |
statistically significant. NASH: non-alcoholic steatohepatitis.
DISCUSSION
Previous case reports have shown various phenotypes of abnormal movements in non-Wilsonian cirrhotic patients. These studies have reported on choreoathetosis [4], orofacial dyskinesias [5], extraocular muscle dystonia [6] and series of Parkinsonism [7-10]. According to the literature, patients who developed movement disorders had cases of severely decompensated cirrhosis. The factors associated with movement disorders were proposed to be a result of neuronal loss or morphological changes of astrocytes in the cerebral cortex and basal ganglia. These were proven by histopathology obtained from autopsies [4,5]. Because of Parkinsonism, some reports hypothesized that manganese intoxication could cause these movement disorders [11,12].
Asterixis and negative myoclonus are well known abnormal movements that are seen in daily clinical practice. Physicians consider asterixis as an indicator of hepatic encephalopathy, while Parkinsonism is widely accepted to be a result of acquired hepatocerebral degeneration. Research on this subject, although rare, lists one report showing the estimated percentage of Parkinsonism related to cirrhosis at 21.6% [7]. No study has reported the overall prevalence of various movement disorders that develop in cirrhotic patients.
Our study included more than 100 non-Wilsonian cirrhotic patients; most of them were seen at our hospital as outpatients. Patients more than 70 years of age were considered unsuitable for the research, because of the high possibility of dementia due to the normal aging process. Although only young cirrhotic and cryptogenic cirrhotic patients were thoroughly investigated for the exclusion of Wilson’s disease, strong evidence of the etiologies of hepatic failure and an advanced age confirmed that there were no patients with Wilson’s disease included in the study. As expected, alcoholic cirrhosis was the primary diagnosis for the majority of the patients in our study. This result is similar to a previous survey that was done in our hospital [13] (prevalence of cirrhosis registered for our population of patients) and also reflected the consequences of alcohol addiction, especially in males, that is prevalent here in Thailand [14,15]. All subjects were not compatible with Chronic Acquired Hepatocerebral Degeneration, as they did not present with severe cognitive or behavioral abnormalities. This study represents the general population of hepatic cirrhosis patients as seen in daily practice.
The prevalence of all phenotypes of movement disorders (above 50%) shows that nearly half of all cirrhotic patients have experienced neurological problems without awareness. The risk of movement disorders increases with higher CTP scores, and hepatic encephalopathy is a major factor. Alcoholic cirrhosis is a significant risk factor for the development of movement disorders. Alcohol toxicity and its effects on the brain are widely known [16,17]. However, when evaluating the history of alcohol consumption after reanalysis of patients with alcoholic cirrhosis and hepatic encephalopathy, it has been postulated that the drawbacks of alcohol’s effects on the brain increases when the liver becomes fibrotic.
Only one cirrhotic patient developed asterixis, whereas 38.5% of cases met the West-Haven criteria for low-grade hepatic encephalopathy (grade 1 and 2). This reveals the poor sensitivity of a negative myoclonus for the early detection of encephalopathy. Intentional tremors are the most common observations [2,18]. When considering the significant association between the prevalence of all movement disorders and a presence of alcoholic cirrhosis, the research team proposes that alcohol causes intense neuronal damage of the cerebellum with heavy intake. Neuronal cells of the cerebellar hemispheres are, in probability, more sensitive to chronic alcohol intoxication than other areas. Consequently, fine coordination is affected more than posture and balance [19]. We provide a subgroup analysis of each movement disorder phenotype and the risk factors in the next report.
Although there were 21 patients with hepatocellular carcinoma, overall movement disorders have no relation to this cancer. The paraneoplastic syndromes of the hepatocellular carcinoma are hypercholesterolemia, hypercalcemia, and erythrocytosis [20]. No report of an abnormal movement as the presenting symptom of the paraneoplastic syndrome in hepatocellular carcinoma was recorded.
There were some limitations in this study. The first was a personal bias, as only one neurologist evaluated the patients’ neurological signs. This may result in an over-estimation of the signs evaluated. However, we recorded video images of the positive outcome cases and then had them evaluated and confirmed by another specialist. Second, the etiology of patients’ hepatic cirrhosis was not blinded to the investigators. Third, there was no healthy population set as a control group for the comparison of the neurological signs. Finally, a lack of laboratorybased evidence, such as serum ammonia, EEG, or neuroradiology studies that should be available for all patients, might result in a bias in the diagnosis of hepatic encephalopathy. However, we hope that findings from this research will be beneficial for physicians working in community-based hospitals. This will be of significant benefit in Southeast Asia or in other areas where advanced investigations are unavailable.
In conclusion, the most common movement disorder in non-Wilsonian cirrhotic patients found in our study was an intention tremor. Significant risk factors contributing to the prevalence of movement disorders are alcoholic cirrhosis and hepatic encephalopathy. Portal hypertension has no significant effect on movement disorders.
Acknowledgments
This work was supported by the Faculty of Medicine, Srinakharinwirot University (grant number 433/2556).
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
REFERENCES
- 1.Wilson SAK. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver. Brain. 1912;34:295–509. doi: 10.1093/brain/awp193. [DOI] [PubMed] [Google Scholar]
- 2.Victor M, Adams RD, Cole M. The acquired (non-Wilsonian) type of chronic hepatocerebral degeneration. Medicine (Baltimore) 1965;44:345–396. doi: 10.1097/00005792-196509000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Pasqualetti P, Di Lauro G, Festuccia V, Giandomenico G, Casale R. Prognostic value of Pugh’s modification of ChildTurcotte classification in patients with cirrhosis of the liver. Panminerva Med. 1992;34:65–68. [PubMed] [Google Scholar]
- 4.Toghill PJ, Johnston AW, Smith JF. Choreoathetosis in porto-systemic encephalopathy. J Neurol Neurosurg Psychiatry. 1967;30:358–363. doi: 10.1136/jnnp.30.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thobois S, Giraud P, Debat P, Gouttard M, Maurizi A, Perret-Liaudet A, et al. Orofacial dyskinesias in a patient with primary biliary cirrhosis: a clinicopathological case report and review. Mov Disord. 2002;17:415–419. doi: 10.1002/mds.10037. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara J, Gupta D, Foster E, Garman K, Stacy M. Extraocular muscle dystonia due to acquired (non-Wilsonian) hepatocerebral degeneration. Mov Disord. 2008;23:875–878. doi: 10.1002/mds.21841. [DOI] [PubMed] [Google Scholar]
- 7.Burkhard PR, Delavelle J, Du Pasquier R, Spahr L. Chronic parkinsonism associated with cirrhosis: a distinct subset of acquired hepatocerebral degeneration. Arch Neurol. 2003;60:521–528. doi: 10.1001/archneur.60.4.521. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Haque M, Yoshida EM. Transient improvement of acquired hepatocerebral degeneration with parkinsonian symptoms after failed liver transplant: case report and literature review. Exp Clin Transplant. 2011;9:363–369. [PubMed] [Google Scholar]
- 9.Butterworth RF. Parkinsonism in cirrhosis: pathogenesis and current therapeutic options. Metab Brain Dis. 2013;28:261–267. doi: 10.1007/s11011-012-9341-7. [DOI] [PubMed] [Google Scholar]
- 10.Tryc AB, Goldbecker A, Berding G, Rümke S, Afshar K, Shahrezaei GH, et al. Cirrhosis-related Parkinsonism: prevalence, mechanisms and response to treatments. J Hepatol. 2013;58:698–705. doi: 10.1016/j.jhep.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Pomier-Layrargues G, Spahr L, Butterworth RF. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet. 1995;345:735. doi: 10.1016/s0140-6736(95)90909-5. [DOI] [PubMed] [Google Scholar]
- 12.Park HK, Kim SM, Choi CG, Lee MC, Chung SJ. Effect of trientine on manganese intoxication in a patient with acquired hepatocerebral degeneration. Mov Disord. 2008;23:768–770. doi: 10.1002/mds.21957. [DOI] [PubMed] [Google Scholar]
- 13.Rattanamongkolgul S, Wongjitrat C, Puapankitcharoen P. Prevalence of cirrhosis registered in Nakhon Nayok, Thailand. J Med Assoc Thai. 2010;93 Suppl 2:S87–S91. [PubMed] [Google Scholar]
- 14.Assanangkornchai S, Saunders JB, Conigrave KM. Patterns of drinking in Thai men. Alcohol Alcohol. 2000;35:263–269. doi: 10.1093/alcalc/35.3.263. [DOI] [PubMed] [Google Scholar]
- 15.Assanangkornchai S, Sam-Angsri N, Rerngpongpan S, Lertnakorn A. Patterns of alcohol consumption in the Thai population: results of the National Household Survey of 2007. Alcohol Alcohol. 2010;45:278–285. doi: 10.1093/alcalc/agq018. [DOI] [PubMed] [Google Scholar]
- 16.Neiman J, Lang AE, Fornazzari L, Carlen PL. Movement disorders in alcoholism: a review. Neurology. 1990;40:741–746. doi: 10.1212/wnl.40.5.741. [DOI] [PubMed] [Google Scholar]
- 17.Campanella S, Petit G, Maurage P, Kornreich C, Verbanck P, Noël X. Chronic alcoholism: insights from neurophysiology. Neurophysiol Clin. 2009;39:191–207. doi: 10.1016/j.neucli.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Graham DI, Adams JH, Caird FI, Lawson JW. Acquired hepatocerebral degeneration: report of an atypical case. J Neurol Neurosurg Psychiatry. 1970;33:656–662. doi: 10.1136/jnnp.33.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan EV, Rose J, Pfefferbaum A. Physiological and focal cerebellar substrates of abnormal postural sway and tremor in alcoholic women. Biol Psychiatry. 2010;67:44–51. doi: 10.1016/j.biopsych.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang PE, Ong WC, Lui HF, Tan CK. Epidemiology and prognosis of paraneoplastic syndromes in hepatocellular carcinoma. ISRN Oncol. 2013;2013:684026. doi: 10.1155/2013/684026. [DOI] [PMC free article] [PubMed] [Google Scholar]


