Abstract
Immunization therapy targeting α-synuclein has emerged as a promising approach for Parkinson’s disease and perhaps for other synucleinopathies. Several antibodies have shown therapeutic effects in mouse models of synucleinopathies and have alleviated the pathological and behavioral phenotypes of these mice. The mechanisms through which the immunization therapy works were initially puzzling, especially given that α-synuclein is a typical cytosolic protein. Recent studies, however, suggested that extracellular α-synuclein is an important pathogenic entity, and hence, a target for immunotherapy. Here, we review the literature describing immunization therapy for synucleinopathies in mouse models and provide current thoughts on the potential mechanisms underlying the therapeutic effects of α-synuclein immunotherapy.
Keywords: Synucleinopathy, α-synuclein, Aggregate transmission, Microglia activation, Immunotherapy
INTRODUCTION
α-synuclein is a neuronal protein highly expressed in presynaptic terminals and is thought to be involved in the regulation of synaptic functions [1]. More attention has been placed on the pathogenic functions of this protein in a group of neurodegenerative diseases referred to as synucleinopathies. These diseases, which include Parkinson’s disease (PD), dementia with Lewy bodies, multiple system atrophy, and a large proportion of Alzheimer’s disease, are pathologically characterized by abnormal accumulation of α-synuclein aggregates. The link between α-synuclein and disease is particularly strong in PD, in which aggregated α-synuclein accumulates in structures known as Lewy bodies and Lewy neurites [2]. Several missense mutations [3-7] and gene multiplication mutations [8-11] in SNCA, the gene for α-synuclein, have been linked to familial forms of PD. Furthermore, SNCA is the gene that is most strongly and consistently associated with sporadic PD [12,13].
PD is clinically characterized by parkinsonian motor symptoms, including resting tremor, muscle tone rigidity, bradykinesia, and postural instability [14]. However, PD patients also manifest a variety of non-motor symptoms, such as autonomic dysfunctions, sensory abnormalities, psychiatric symptoms, sleep disorders, and dementia [15]. The majority of PD patients develop these symptoms sequentially as the disease progresses. Strikingly, an analysis of Lewy bodies revealed a progressive spreading of α-synuclein aggregates with disease progression, and the pattern in which the aggregates spread through the brain seemed to correlate with the clinical progression of the disease [16]. These findings strongly suggest that the spread of α-synuclein aggregates drives the disease progression, and therefore, stopping the spread of α-synuclein aggregates might halt the disease progression. Recent studies provide strong evidence that cell-to-cell propagation of α-synuclein aggregates is the underlying mechanism for the spreading of Lewy pathology [17].
Studies during the past twenty years testify to the importance of α-synuclein and its aggregation in the initiation and progression of PD, and probably other synucleinopathies, making this protein the most promising therapeutic target for these diseases. However, α-synuclein-targeting drugs have yet to be developed. In this review, we propose that immunotherapy for α-synuclein might be a promising approach for developing anti-synucleinopathy therapy and explain how this approach might work mechanistically.
ACTIVE AND PASSIVE IMMUNIZATION OF THE SYNUCLEINOPATHY MODEL MICE
In recent years, immunotherapy has emerged as a promising approach for targeting and clearing protein aggregate pathology in neurodegenerative diseases [18-22]. In a study performed ten years ago, which assessed the feasibility of PD immunotherapy, a transgenic mouse model for synucleinopathies was actively immunized with recombinant α-synuclein protein. The mice successfully generated antibodies against α-synuclein, and the behavioral deficits, α-synuclein deposition and neurodegeneration in the brains of these mice were significantly ameliorated [23]. Likewise, passive immunization with a monoclonal antibody with the epitope of the C-terminal part of α-synuclein decreased the accumulation of α-synuclein aggregates, as well as reduced the behavioral deficits in an α-synuclein transgenic mouse model [24]. Interestingly, administration of antibodies against α-synuclein oligomers reduced α-synuclein levels in both cell lysates and conditioned media [25].
Initially, the effects of immunization in the synucleinopathy models were puzzling and unexplainable, given the cytosolic nature of the target protein [26]; no rational explanation could be provided for how antibodies access α-synuclein proteins. In the following sections, we will discuss recent progress toward resolving this issue.
EXTRACELLULAR α-SYNUCLEIN
Secretion of α-synuclein from neuronal cells
α-synuclein is a typical cytosolic protein and is mostly present in the cytosolic fractions of brain homogenates and neuronal cell homogenates. However, a small portion of cellular α-synuclein is present in the lumen of vesicles [27], the identity of which is yet to be elucidated. These vesicular α-synuclein proteins were secreted from neuronal cells through unconventional exocytosis [28], which collectively refers to endoplasmic reticulum/Golgi-independent exocytosis. The precise mechanism of the exocytosis, however, is unknown. Recently, exosome-associated exocytosis [29] and exophagy (autophagosome-mediated exocytosis) [30] have been suggested as the mechanisms underlying α-synuclein secretion. However, the results of some studies contradict these proposals [31], and the amount of secreted α-synuclein that is associated with extracellular vesicles explains only a very small fraction of the total amount of α-synuclein secreted.
Although the mechanisms of exocytosis are unknown, we do know several conditions under which α-synuclein secretion is enhanced. These conditions, which include proteasome inhibition [28], lysosomal inhibition [32], autophagy inhibition [33], mitochondrial inhibition, oxidative modifications [34,35], and heat shock [29] which commonly affect cellular proteostasis (protein folding homeostasis). A large portion of secreted α-synuclein is oligomeric, whereas the cytosolic α-synuclein is mostly monomers [36]. From these results, we speculate that exocytosis of α-synuclein, and perhaps many other proteins that go through the same pathway, is part of the cellular response to the misfolding of the protein. More work needs to be done to resolve this problem.
Pathogenic actions of extracellular α-synuclein
After secretion from neuronal cells, α-synuclein can act on neighboring cells. Extracellular α-synuclein can be internalized into neuronal cells [37-39]. These proteins undergo endosomal trafficking [37-39] and are delivered to lysosomes where they are degraded [40]. If the internalized α-synuclein can survive the lysosomal degradation, which could result from lysosomal dysfunction, it can induce aggregation of endogenous α-synuclein proteins. Under certain conditions, this aggregate transmission coincides with neuronal cell death, both in cell cultures and in vivo [38,41,42]. However, neurodegeneration does not always occur with aggregate transmission [39,43].
Extracellular α-synuclein also acts on glial cells. α-synuclein released from neurons is transferred to astrocytes, where it induces pro-inflammatory responses [44]. Extracellular α-synuclein can also activate microglia and subsequently trigger inflammatory responses. In microglia, oligomeric forms of neuronreleased α-synuclein interact with toll-like receptor 2 (TLR2) and activate the TLR2 signaling pathway [36]. The interaction between TLR2 and α-synuclein seems to be highly conformation-selective; only certain types of oligomers interact with and activate TLR2, whereas monomers, fibrils, and some oligomer types do not. There seem to be other receptors for α-synuclein in microglia. One of these receptors might be β1-integrin, the activation of which is responsible for the morphological changes and migration of microglia [45].
Although extracellular α-synuclein accounts for only a minor portion of the total brain α-synuclein, its actions on neighboring neurons and glia suggest that it might be an excellent therapeutic target for PD and other synucleinopathies.
MECHANISMS UNDERLYING ANTI-α-SYNUCLEIN IMMUNOTHERAPY
The mechanisms underlying the therapeutic effects of immunization against α-synuclein remain elusive. It has become increasingly clear that extracellular α-synuclein itself and the cellular events that lead to its generation and clearance are promising therapeutic targets for PD. Here, we discuss two mechanisms through which immunotherapy might work.
Clearance of extracellular α-synuclein
Extracellular α-synuclein aggregates can be internalized to neurons and glia. Among the cell types that can internalize α-synuclein aggregates, microglia exhibit the most rapid clearance of these proteins [46]. More recently, it was shown that antibody assisted in the clearance of extracellular α-synuclein, resulting in reduced neuronal and glial accumulation of α-synuclein [47]. This antibody treatment also ameliorated neurodegeneration and behavioral deficits in a mouse model of synucleinopathy. That study showed that microglia became better scavengers for extracellular α-synuclein aggregates in the presence of specific antibodies against α-synuclein. Antibody-α-synuclein immune complexes entered microglia through the Fcγ receptors, which led to efficient delivery of these immune complexes to lysosomes, hence resulting in fast degradation. By clearing extracellular α-synuclein, antibody treatment significantly reduced the extent of cell-to-cell transmission of the protein in mouse models, suggesting that this approach is effective in slowing the disease progression. Furthermore, clinical advantages might be expected if extracellular α-synuclein is selectively targeted by immunotherapy with the intraneuronal α-synuclein being left intact.
Physical blocking of extracellular α-synuclein
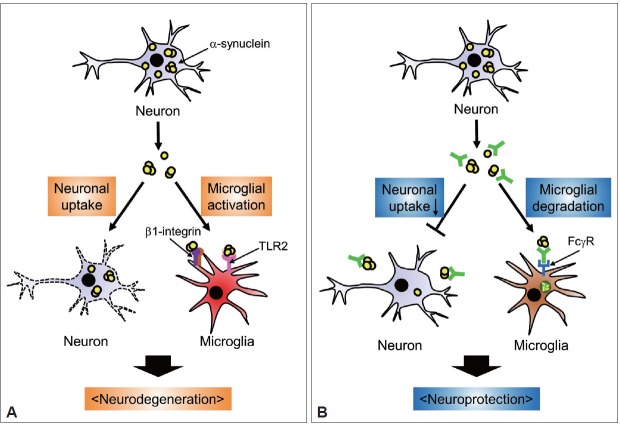
In addition to the accelerated clearance of extracellular α-synuclein, antibodies could directly block cell-to-cell transmission of the protein. Tran et al. [48] recently showed that several antibodies against α-synuclein physically blocked intercellular transmission of α-synuclein aggregates in cell cultures and animal models. Therefore, from the results thus far, we propose a working model in which antibodies capture extracellular α-synuclein aggregates and physically interfere with the transfer of the protein to neighboring cells, thus enhancing the efficiency of its uptake into microglia for clearance (Figure 1).
Figure 1.
Proposed mechanisms of anti-α-synuclein immunotherapy targeting extracellular α-synuclein. A: Pathogenic roles of extracellular α-synuclein. Cellular α-synuclein is released from neuronal cells into the extracellular space. Extracellular α-synuclein aggregates can be taken up by neighboring neurons, where aggregation of the protein is transmitted. In addition, neuron-released α-synuclein can induce microglial activation and migration by stimulating TLR2 and β1-integrin, respectively. These direct (neuron-to-neuron) and indirect (microglia-mediated) functions of extracellular α-synuclein may account for the neurodegeneration observed in synucleinopathies. B: Mechanisms of anti-α-synuclein immunotherapy. Administration of antibodies targeting α-synuclein may have a neuroprotective effect by 1) blocking the direct transfer of extracellular α-synuclein into neurons and 2) facilitating Fcγ receptor-mediated internalization of extracellular α-synuclein into microglia for subsequent lysosomal degradation. Accelerated clearance would prevent the pathogenic actions of extracellular α-synuclein on neurons and microglia. TLR2: toll-like receptor 2.
PERSPECTIVES
Studies so far have been encouraging in terms of targeting α-synuclein for PD immunotherapy. However, we are facing a number of potential issues or problems in developing immunotherapy for PD. First, immunotherapy should not interfere with the physiological function of α-synuclein. Generation of conformation-specific antibodies for the “pathogenic” forms of α-synuclein would allow us to overcome this possible problem. Second, delivery of antibodies to the brain parenchyma might be a problem. Engineering the antibodies so they can penetrate the blood-brain barrier might be a necessary step. Finally, the efficacy of immunotherapy needs to be validated in non-human primate models. For passive immunization, the antibodies need to be “humanized”, and thus, the toxicity and efficacy of the humanized antibodies have to be tested in non-human primates.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C0093).
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
REFERENCES
- 1.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 4.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 5.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 6.Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord. 2013;28:811–813. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 7.Lesage S, Anheim M, Letournel F, Bousset L, Honoré A, Rozas N, et al. G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol. 2013;73:459–471. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 8.Ibáñez P, Bonnet AM, Débarges B, Lohmann E, Tison F, Pollak P, et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 9.Ross OA, Braithwaite AT, Skipper LM, Kachergus J, Hulihan MM, Middleton FA, et al. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol. 2008;63:743–750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 11.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 12.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 13.Simón-Sánchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–154. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer I. Neuropathology and neurochemistry of nonmotor symptoms in Parkinson’s disease. Parkinsons Dis. 2011;2011:708404. doi: 10.4061/2011/708404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Bae EJ, Lee SJ. Extracellular α--synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10:92–98. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- 18.Atwal JK, Chen Y, Chiu C, Mortensen DL, Meilandt WJ, Liu Y, et al. A therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivo. Sci Transl Med. 2011;3:84ra43. doi: 10.1126/scitranslmed.3002254. [DOI] [PubMed] [Google Scholar]
- 19.Delrieu J, Ousset PJ, Caillaud C, Vellas B. ‘Clinical trials in Alzheimer’s disease’: immunotherapy approaches. J Neurochem. 2012;120 Suppl 1:186–193. doi: 10.1111/j.1471-4159.2011.07458.x. [DOI] [PubMed] [Google Scholar]
- 20.Gros-Louis F, Soucy G, Larivière R, Julien JP. Intracerebroventricular infusion of monoclonal antibody or its derived Fab fragment against misfolded forms of SOD1 mutant delays mortality in a mouse model of ALS. J Neurochem. 2010;113:1188–1199. doi: 10.1111/j.1471-4159.2010.06683.x. [DOI] [PubMed] [Google Scholar]
- 21.Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A, et al. Immunotherapy for Alzheimer’s disease: from anti-β-amyloid to tau-based immunization strategies. Immunotherapy. 2012;4:213–238. doi: 10.2217/imt.11.170. [DOI] [PubMed] [Google Scholar]
- 22.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Näsström T, Gonçalves S, Sahlin C, Nordström E, Screpanti Sundquist V, Lannfelt L, et al. Antibodies against alpha-synuclein reduce oligomerization in living cells. PLoS One. 2011;6:e27230. doi: 10.1371/journal.pone.0027230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 27.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113:1263–1274. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- 29.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, et al. Cell-produced alphasynuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ejlerskov P, Rasmussen I, Nielsen TT, Bergström AL, Tohyama Y, Jensen PH, et al. Tubulin polymerization-promoting protein (TPPP/p25α) promotes unconventional secretion of α-synuclein through exophagy by impairing autophagosome-lysosome fusion. J Biol Chem. 2013;288:17313–17335. doi: 10.1074/jbc.M112.401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grathwohl SA, Steiner JA, Britschgi M, Brundin P. Mind the gut: secretion of α-synuclein by enteric neurons. J Neurochem. 2013;125:487–490. doi: 10.1111/jnc.12191. [DOI] [PubMed] [Google Scholar]
- 32.Bae EJ, Yang NY, Song M, Lee CS, Lee JS, Jung BC, et al. Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. Nat Commun. 2014;5:4755. doi: 10.1038/ncomms5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HJ, Cho ED, Lee KW, Kim JH, Cho SG, Lee SJ. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp Mol Med. 2013;45: doi: 10.1038/emm.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae EJ, Ho DH, Park E, Jung JW, Cho K, Hong JH, et al. Lipid peroxidation product 4-hydroxy-2-nonenal promotes seeding-capable oligomer formation and cell-to-cell transfer of α-synuclein. Antioxid Redox Signal. 2013;18:770–783. doi: 10.1089/ars.2011.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HJ, Baek SM, Ho DH, Suk JE, Cho ED, Lee SJ. Dopamine promotes formation and secretion of non-fibrillar alpha-synuclein oligomers. Exp Mol Med. 2011;43:216–222. doi: 10.3858/emm.2011.43.4.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, Glabe C, et al. Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen C, Angot E, Bergström AL, Steiner JA, Pieri L, Paul G, et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(Pt 4):1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim C, Cho ED, Kim HK, You S, Lee HJ, Hwang D, et al. β1-integrin-dependent migration of microglia in response to neuron-released α-synuclein. Exp Mol Med. 2014;46:e91. doi: 10.1038/emm.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HJ, Suk JE, Bae EJ, Lee SJ. Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008;372:423–428. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 47.Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, et al. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32:13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran HT, Chung CH, Iba M, Zhang B, Trojanowski JQ, Luk KC, et al. Α-synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 2014;7:2054–2065. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]