Abstract
Sixty years ago, Steele, Richardson and Olszewski designated progressive supranuclear palsy (PSP) as a new clinicopathological entity in their seminal paper. Since then, in addition to the classic Richardson’s syndrome (RS), different clinical phenotypic presentations have been linked with this four-repeat tauopathy. The clinical heterogeneity is associated with variability of regional distribution and severity of abnormal tau accumulation and neuronal loss. In PSP subtypes, the presence of certain clinical pointers may be useful for antemortem prediction of the underlying PSP-tau pathology. Midbrain atrophy on conventional MRI correlates with the clinical phenotype of RS but is not predictive of PSP pathology. Cerebrospinal fluid biomarkers and tau ligand positron emission tomography are promising biomarkers of PSP. A multidisciplinary approach to meet the patients’ complex needs is the current core treatment strategy for this devastating disorder.
Keywords: Progressive supranuclear palsy, Richardson’s syndrome, Corticobasal syndrome, Tauopathy, Atypical parkinsonism
INTRODUCTION
In 1964, an unusual syndrome of supranuclear gaze palsy, progressive axial rigidity, pseudobulbar palsy and mild dementia was described [1]. In this seminal paper, extensive subcortical neurofibrillary degeneration predominantly found in the globus pallidus, subthalamic nucleus, substantia nigra and cerebellar dentate nucleus were characterised as the pathological substrates of the new clinicopathologic entity of progressive supranuclear palsy (PSP), also known as Steele-Richardson-Olszewski syndrome [1]. Steele [2] predicted that ‘clinical variants of the syndrome are likely to occur as the disease affects different nuclei at different times and to different degrees’. Since then, increasing recognition of phenotypic heterogeneity has been linked to the regional severity of abnormal tau accumulation and neuronal loss [3], although all PSP regardless of clinical variants share similar neuropathologic features and fulfill the neuropathologic criteria for PSP [4]. In most cases, the evaluation of histological findings cannot lead to deduction of the clinical phenotype due to significant overlap in regional pathologies. Tau-immunoreactive tufted astrocytes are the pathognomonic histological feature, commonly observed in the precentral gyrus, striatum, superior colliculus, thalamus, subthalamic nucleus and red nucleus (Figure 1). Globose neurofibrillary tangles (NFTs) in the brain stem nuclei, flame-shaped NFTs, coiled bodies, neuronal loss and gliosis are other accompanying findings [3]. PSP-tau is comprised predominantly of 4-repeat tau [5]. High frequency of concomitant pathologies such as Alzheimer disease and argyrophilic grains in PSP may partly contribute to the clinical heterogeneity [6].
Figure 1.
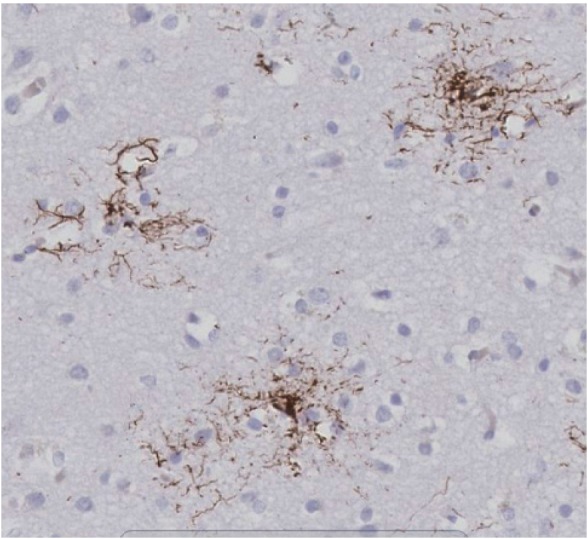
Tau immunohistochemistry using anti-tau (AT8) antibody shows tufted astrocytes in the frontal cortex of a case with pathologically confirmed progressive supranuclear palsy (× 20 magnification).
The aim of this review is to apply the recent advances in PSP in the clinical approach of patients including bedside examination, investigation and management.
EPIDEMIOLOGY, NATURAL HISTORY AND AETIOLOGY
The prevalence of PSP is 5.8–6.5 per 100,000 [7-9]. Patients with the classic PSP-Richardson syndrome (PSP-RS) usually develop their first symptoms in their mid-60s and the condition gradually progresses from symptom onset to death over an average of 7 years [10]. Clinical subtypes of PSP-parkinsonism (PSP-P) and PSP-pure akinesia with gait freezing (PSP-PAGF) have a more benign course with a survival period of a decade or more [11] and both subtypes have an overall tau burden less than those in PSP-RS and the distribution of abnormal tau is relatively restricted to the brain stem [12,13]. The phenotypes of PSP-P and PSP-PAGF are sometimes referred as the ‘brain stem’ variants of PSP, as opposed to the ‘cortical’ variants which present with predominant cortical features including PSP-corticobasal syndrome (PSP-CBS), PSP-behavioural variant of frontotemporal dementia (PSP-bvFTD) and PSP-progressive non-fluent aphasia (PSP-PNFA) [3].
A study of disease progression in 110 pathologically confirmed PSP showed that intervals from disease onset to the development of frequent falls was 3.9 (± 2.5) years, cognitive impairment 4.2 (± 2.9) years, unintelligible speech 6 (± 2.5) years, residential care 6.1 (± 3.0) years, urinary catheter 6.3 (± 3.1) years, wheelchair dependence 6.4 (± 2.7) years and severe dysphagia 6.4 (± 2.4) years [14]. A PSP rating scale with 28 items in six categories provides useful quantitative assessment in clinical practice and research trials [15]. Mean progression rate is + 11.3 points per year with the scores ranging from 0–100. The motor subscale of the Unified Parkinson’s Disease Rating Scale is also a reliably clinimetric scale to assess motor disability in PSP [16].
The cause of PSP is unknown. Advanced age is the only established risk factor [7]. To date, head injury has not been established as a risk factor of PSP [17]. A prevalence study found 24% of PSP cases had early histological evidence of chronic traumatic encephalopathy (CTE) [18], a neurodegenerative consequence of repetitive head injury previously referred as dementia pugilistica in boxers [19]. Whether the CTE-tau pathology began following the onset of PSP as a result of frequent falls is not known [20]. Geographical clusters of patients with PSP-like syndrome on Guam and Guadeloupe have probable links to environmental causes [21,22]. These neurodegenerative tauopathies have clinical features atypical to the classic RS and the Guam-parkinsonian dementia complex is pathologically distinct from PSP [23].
Frontotemporal dementia with parkinsonism due to autosomal dominant mutations in the MAPT gene (FTDP-17T) is clinically and pathologically heterogeneous [24]. Specifically, FTDP-17T due to exon 10 coding or splicing shares the most similarity to PSP, both clinically and pathologically [25]. Although PSP is considered a sporadic condition, FTDP-17T probably provides the best clues as to the etiology of PSP [26].
The strong association between the H1c haplotype and PSP was confirmed by a genome-wide study (GWAS) of PSP which identified the presence of independent association signals at the MAPT locus representing both the H1/H2 haplotypes and the rs242557 MAPT SNP, related to the H1c sub-haplotype [27]. Non-MAPT risk factors associated with PSP, EIF2AK3, MOBP and STX-6, were also found in the PSP GWAS [27]. The protein functions of these candidate genes provide insights to the biochemical basis of the pathophysiological mechanisms.
The findings of seeding and spreading of transmissible tau neuropathology in transgenic mouse brains including PSP-tau support the notion of a cell-to-cell propagation mechanism of different ‘strains’ of fibrillary tau leading to distinct patterns of neuronal and glial pathology which is disease- and neural network-specific [28].
PSP-RS
The current operational criteria are only limited to the clinical diagnosis of PSP-RS and no accepted guidelines for the clinical diagnosis of other phenotypic presentations of PSP are currently available (Table 1). The National Institute of Neurological Disorders and Stroke (NINDS) criteria for ‘probable’ PSP describes a gradual progressive disorder with an age of onset over 40 years, falls within the first year, vertical supranuclear gaze palsy or slowing of vertical saccades [29].
Table 1.
Clinical features of PSP-RS, PSP-P, PSP-PAGF, PSP-CBS, PSP-PNFA, PSP-bvFTD, PSP-C, Parkinson’s disease, and MSA-P
| PSP-RS | PSP-P | PSP-PAGF | PSP-CBS | PSP-PNFA | PSP-bvFTD | PSP-C | Parkinson’s disease | MSA-P | |
|---|---|---|---|---|---|---|---|---|---|
| Rigidity | Axial > limb | Limb > axial | Axial | Limb > axial | + | + | Axial > limb | Limb > axial | Limb > axial |
| Early postural instability and/or falls | +++ | - | + | -/+ | - | - | +++ | - | - |
| Early eye movement abnormalities | +++ | ++ | +/- | ++ | + | + | +++ | - | -/+ |
| Early cognitive decline | ++ | - | - | +++ | +++ | +++ | ++ | - | - |
| Early frontal behaviour | ++ | - | - | ++ | ++ | +++ | ++ | -/+ | -/+ |
| Non-fluent aphasia and/or apraxia of speech | + | - | - | ++ | +++ | ++ | - | - | - |
| Limb dystonia | + | + | -/+ | +++ | + | + | (limb and truncal ataxia) | + | + |
| Pyramidal and Babinski’s signs | + | + | + | ++ | + | + | - | - | ++ |
| Levodopa response | - | ++ | - | - | - | - | - | +++ | ++ |
| Dysautonomia | - | - | - | - | - | - | - | + | +++ |
MSA-P: multiple system atrophy-parkinsonism, PSP: progressive supranuclear palsy, PSP-C: PSP with predominant cerebellar ataxia, PSP-CBS: PSP-corticobasal syndrome, PSP-bvFTD: PSP-behavioural variant of frontotemporal dementia, PSP-P: PSP-parkinsonism, PSP-PAGF: PSP-pure akinesia with gait freezing, PSP-PNFA: PSP-progressive non-fluent aphasia, PSP-RS: PSP-Richardson’s syndrome, -: absent, -/+: rare, +: occasional or mild, ++: usual or moderate, +++: frequent or severe.
Patients in their late 50’s or 60’s usually present with insidious onset of non-specific symptoms such as blurred vision, dry eyes, photophobia, dizziness, unsteadiness, falls and fatigue. Family members may comment on apathy, depression, irritability and softening of speech. Predominant behavioural and cognitive features are the presenting features without motor symptoms in a fifth of patients indistinguishable to frontotemporal dementia. Correct diagnosis is commonly delayed to 3–4 years after symptom onset [7].
As the disease progresses, the characteristic features of postural instability with unprovoked falls, mostly backwards, become disabling which render the patients wheelchair-bound to prevent injuries resulting from falls. Gait is slightly wide-based and may initially be misdiagnosed as cerebellar ataxia. Gait ignition failure and freezing of gait are common. Frontalis overactivity, reduced eye blink, focal dystonia of the procerus muscle (procerus sign), axial rigidity, upright extended posture and sometimes, retrocollis, give a characteristic appearance which can be immediately recognisable to an experienced neurologist as the patient enters the consultant room. Motor recklessness caused by frontal impairment further contributes to falls and injuries. Repetitive finger tapping is small in amplitude (hypokinesia) with good speed and without decrement, which differs distinctively from criteria-defined bradykinesia with fatigue and decrements in Parkinson’s disease [30]. Orthostatic hypotension is not a feature of PSP but urinary symptoms including urgency, retention and incontinence, constipation and erectile dysfunction are common as the disease progresses. Sleep abnormalities including rapid eye movement (REM) sleep behavior disorder, are more commonly observed in synucleinopathies such as multiple system atrophy (MSA) and Parkinson’s disease, but can also occur in up to 35% of patients with PSP [31].
Inability to read is a frequent and disabling symptom due to saccadic eye movement disorder. Square-wave jerks, in which the eyes oscillate horizontally across the midline during visual fixation is an early eye sign. It is also observed in MSA, cerebellar disorders and occasionally in Parkinson’s disease [32]. Careful ocular examination also reveals impairment of convergence and defective pupillary responses with accommodation [33]. Slowing of vertical saccades with or without ‘round the houses’ sign (curved trajectory of vertical saccades) [34] are followed by supranuclear gaze palsy with restriction of the range of vertical gaze [35]. Pretarsal blepharospasm and apraxia of eyelid opening are other common features [36]. Visual grasping (eye deviation and intermittent head turns towards object the patients have walked past) is sometimes mistaken as cervical dystonia [37-40].
Disinhibition in PSP can be demonstrated by bilateral impaired antisaccade task (inability to look in the direction opposite to the visual stimulus places on a horizontal visual plane which is usually the examiner’s waving hands on either side of the patient) and impaired Stroop test performance [40].
Frontal lobe dysfunction is the most consistent deficit in PSP [41]. Behavioural change, apathy, executive dysfunction, emotional lability with uncontrollable laughter or crying, aggressive outbursts, compulsive behaviour and inappropriate sexual behavior are sometimes encountered. The frontal assessment battery is useful to capture frontal impairments with lexical fluency and Luria motor sequencing being the two most useful parameters [42]. A score of less than 12 (out of 18) has a sensitivity of 77% and specificity of 87% in differentiating frontal lobe dysfunction from amnestic dementia [43]. Letter fluency is more impaired than semantic fluency in PSP-RS in contrast to Alzheimer’s disease [44]. Inability to recall more than seven initial letter words supports the diagnosis of PSP-RS [45]. Positive applause sign (when the patient claps hands more than 3 times exceeding the examiner’s demonstration and instruction) is more commonly observed in PSP than in corticobasal degeneration (CBD), FTD and Parkinson’s disease and is a sign of motor perseveration [46].
Despite executive and inhibition deficits being the most prominent cognitive features, a third of PSP patients also have memory impairment including poor episodic memory and visuospatial functions [41]. The Addenbrooke’s cognitive examinationrevised (ACE-R) [45] and the dementia rating scales [47] are more sensitive tools than the mini-mental state examination to capture these deficits.
In the late stages, swallowing difficulties, severe dysphonia and dysarthria, emotional lability, inspiratory sighs, stereotyped moaning or groaning occur. Pseudobulbar palsy with slow spastic tongue movements, reduced gag reflex, brisk jaw and facial reflexes, are common findings. Severe nuchal rigidity frequently precludes the performance of the doll’s eyes maneuver to establish the presence of a vertical supranuclear gaze palsy and is disproportionate to the moderately increased tone in the limbs. One fifth of patients have unilateral or bilateral positive Babinski sign [10]. Unilateral limb dystonia or arm levitations are observed in a minority of patients and do not equate to the presence of alien limb phenomenon, CBS or the underlying diagnosis of corticobasal degeneration [48,49].
Akinetic mutism, complete ophthalmoplegia, significant rigidity with or without contractures, immobility are inevitably observed in the terminal disease. Pneumonia, respiratory failure, pulmonary embolism and urinary tract infection are common causes of death.
PSP SUBTYPES
Clinicopathological studies have led to the recognition of other clinical phenotypes associated with PSP-tau pathology. The clinical pictures of these PSP subtypes are most distinct in the first 2 years of presentation. As the disease progresses, overlap of clinical features, emergence of new phenotype and temporal evolution to the classic RS in later stages are frequently observed. RS is still considered as the classic and most frequent clinical presentation of cases with PSP-tau pathology, comprising of 50% or more of PSP cases with post-mortem confirmation [11]. The second most common subtype is PSP-P and may be observed in up to a third of all PSP cases [10]. PSP-PAGF, cortical PSP variants (PSP-CBS, PSP-bvFTD, PSP-PNFA) and the recently described PSP-C are relatively rare and each phenotypic subtype makes up of less than 5% of all PSP cases. A multicentre clinicopathological series reported clinical heterogeneity beyond a pure RS in the majority (87%) of cases within the first two years of presentation, and almost 40% of cases could not be classified into a particular phenotype [50]. This study implies that heterogeneous presentations may be more common in PSP than has previously been indicated by other series in the literature and highlights the need for effective diagnostic biomarkers [51].
Globular glial tauopathies (GGTs) are a group of 4-repeat tauopathies characterised neuropathologically by widespread, globular glial inclusions (GGIs) with the latter being predominantly negative for Gallyas silver staining in contrast to the Gallyas-positive tau lesions in PSP [52]. The clinical presentations of GGTs are heterogeneous with clinical diagnoses ranging from frontotemporal dementia, Pick’s disease, PSP, CBS, motor neuron disease (MND) and primary lateral sclerosis [52]. A subgroup of GGT was previously referred as atypical PSP with corticospinal tract degeneration (PSP-CST) [53]. It is now increasingly recognized that this condition, previously referred as PSP-CST [53], sporadic multiple system tauopathy with dementia [54] and sporadic 4R tauopathy with frontotemporal lobar degeneration, parkinsonism and motor neuron disease (FTLD-P-MND) [55], all conform to the unified neuropathological diagnosis of GGT with GGIs being a consistent and defining histological feature. GGT is considered a pathological entity that is distinct from PSP.
PSP-P
This subgroup is frequently misdiagnosed clinically as Parkinson’s disease. Patients with PSP-P presents with asymmetric limb bradykinesia and rigidity and do not have supranuclear vertical gaze palsy in the early stage [10]. Patients may have a jerky postural tremor or a rest tremor. Half of the patients have moderate levodopa response but the benefit rarely sustains for more than a few years. As the disease advances, the clinical picture usually becomes more like RS. Early clinical pointers that favour PSP-P over Parkinson’s disease are rapid progression, prominent axial symptoms and an attenuated response to levodopa [10]. The pattern of hypokinesia without decrement on repetitive finger tapping is another clue to the diagnosis of PSP [30]. Falls and cognitive decline occur later in PSP-P than in PSP-RS and are considered favourable prognostic features which may explain a longer survival of an average of 9 years in PSP-P [14].
A proportion of patients have overlapped features of both Parkinsonism (asymmetric bradykinesia, levodopa response, rigidity and tremor) and RS (early postural instability, subtle eye movement abnormalities and frontal subcortical deficits) in the early stages, but are categorised as PSP-P because of the predominant clinical picture of asymmetrical Parkinsonism. In a small number of patients, a pure Parkinson’s disease-like syndrome predominates until death and eye movement abnormalities never appear [10,56]. A sustained levodopa response, levodopa-induced choreiform dyskinesia and long disease duration characterise this far end of the clinical spectrum of PSP-P. Until clinical diagnostic criteria for PSP subtypes is available, the antemortem diagnosis of PSP-P relies on careful clinical examination, accurate clinical documentation, diagnostic revision and in some cases, a correct prediction of PSP pathology may not be possible without postmortem.
PSP-PAGF
PAGF is characterised by pronounced gait ignition failure and start hesitation which remains as the isolated clinical picture for several years [13]. Hypophonia, facial hypomimia and fast micrographia may be subtle accompanying symptoms. As the condition progresses, freezing of gait, stuttering or stammering speech gradually develop. Axial rigidity and absence of limb rigidity are distinctive features. Late features include slowing of vertical saccades or vertical supranuclear gaze palsy, blepharospasm, postural instability and falls are useful pointers for PSP-PAGF. Pronounced frontal subcortical impairment, bradyphrenia, asymmetric bradykinesia, rigidity, tremor, levodopa response are not observed in this phenotype. The median disease duration of PSP-PAGF is 11 years making this the most benign PSP subtype [11].
Other underlying causes of a clinical presentation of PAGF are subcortical white matter ischaemia (Binswanger leukoaraiosis), normal pressure hydrocephalus [57], Parkinson’s disease and dementia with Lewy bodies.
PSP-CBS
The underlying pathologies of CBS are heterogeneous but 70% of cases have a tauopathy including CBD and PSP [58]. CBS, however, is a rare presentation of PSP and PSP-CBS comprises of only 4% of PSP cases [59].
Patients with CBS present with progressive functional difficulties with the use of one limb caused by a combination of limb apraxia, parietal sensory impairment, dystonia, myoclonus, levodopa-unresponsive rigidity and bradykinesia and occasionally alien limb phenomenon [60]. Delayed initiation of horizontal saccades is characteristic for CBS and can be observed also in PSP-CBS and is more pronounced when gaze is directed toward the side of the apraxic limb [59]. PNFA, apraxia of speech (AOS), orobuccal apraxia are common associated features [61,62]. Pyramidal and Babinski’s signs are observed in half of PSP-CBS cases [59]. Overlap of clinical features with RS is common especially in mid and late stages. Postural instability in the first year of disease onset, supranculear downgaze palsy in patients with CBS are helpful clues to the underling PSP pathology. The median disease duration of PSP-CBS is 7.3 years, the same as PSP-RS.
PSP-PNFA
PNFA is a language disorder characterised by nonfluent speech with hesitancy, agrammatism and phonemic errors can be the predominant and sometimes isolated clinical presentation of PSP [61]. It is frequently accompanied by AOS which is a motor speech disorder featuring slow, segmented and groping speech with errors in timing and abnormal prosody [61]. Progressive agraphia is sometimes a presenting feature preceding the speech impairment [63]. PNFA and AOS may also be observed in other phenotypes such as RS, CBS and bvFTD, but when both PNFA and AOS co-exist as the predominant clinical features, it is highly suggestive of FTLD-tau as the underlying pathology [61]. PNFA in isolation without AOS is associated with FTLD-tau (including PSP, CBD and Pick’s) in over 50%, FTLD-TAR DNA-binding protein-43 (FTLD-TDP) [64] in 20%, and some cases with FTLD-TDP pathology may carry progranulin mutation or C9orf72 expansion [65]. Other pathologies such as Alzheimer’s disease and dementia with Lewy bodies have also been reported as the cause of PNFA [66].
PSP-bvFTD
The most common cause of bvFTD is FTLD-TDP but other causes are recognized including Pick’s disease, CBD, PSP, Alzheimer’s disease or familial Alzheimer’s disease [64]. Only less than 4% of bvFTD cases have PSP pathology [67]. Insidious behavioural, personality changes, emotional blunting, lack of empathy, aggressive outbursts, distractibility, hyperphagia, neglected hygiene, socially inappropriate, disinhibited and compulsive behaviours and loss of insight are characteristic features. Typical features of RS may emerge in later stages and if present are useful clinical pointers to the underling PSP pathology [68].
PSP-C
Cerebellar ataxia as the predominant early presenting feature is increasingly recognized as a very rare subtype of PSP (PSP-C) which is associated with severe neuronal loss with gliosis and higher densities of coiled bodies in the cerebellar dentate nucleus compared to PSP-RS [69,70]. Reported cases with pathologically confirmed PSP-C are mostly Japanese [69,71], akin to the prevalent cerebellar subtype of MSA in that region. The clinical findings of progressive truncal and limb ataxia in PSP-C are distinct from unsteadiness due to postural instability observed in classic RS [69]. Cardinal features of PSP may be observed early in the disease and are eventually present in all PSP-C cases. A Japanese series of 4 PSP-C cases identified that older age of onset (PSP-C: 68.8 ± 4.4 years vs. MSA-C: 58.3 ± 7.4 years), early falls within the first 2 years, vertical supranuclear gaze palsy without dysautonomia were predictive of PSP-C in individuals with late onset cerebellar ataxia [69].
INVESTIGATIONS
Differentiate diagnoses especially potentially treatable causes should be considered when assessing patients with postural instability and supranuclear gaze palsy. Conventional MRI of the brain is useful to exclude extensive cerebrovascular disease, leukodystrophy, normal pressure hydrocephalus, structural midbrain lesions and, rarely, manganese intoxication (with T1 hyperinstensity in globus pallidus) which can all masquerade as PSP. Laboratory tests for syphilis and HIV serology, autoimmune profile, paraneoplastic disease autoantibodies (e.g., Ma antibodies [72]), antibodies for stiff-person syndrome (including DPPX-IgG [73]) and Niemann Pick Type C may be considered. Cerebrospinal fluid (CSF) study of PCR for Tropheryma Whipplei is occasionally sent. In patients with rapid disease progression from symptom onset to akinetic mutism in less than a year, CSF14-3-3 protein, cortical high signal on diffusion-weighted MRI images and diffuse slowing of EEG without or without periodic triphasic waves are suggestive of Creutzfeldt-Jakob disease [74]. CSF neurodegenerative biomarkers for Alzheimer’s disease including an elevated total tau protein and reduced β-amyloid 1-42 protein support the diagnosis of Alzheimer’s disease [75]. A CSF study suggested that C and N terminal fragments of tau are promising biomarkers of PSP pathology [76]. Early disease onset, positive family history [77] or concurrent clinical syndrome such as MND or FTD may point to mutations in MAPT [78], progranulin [79] or C9orf72 expansion [80].
In specialist centres, formal neuropsychometry testing is routinely arranged to assess the performance of different cognitive domains. Swallowing assessment and, in patients with more advanced disease, video fluoroscopy are valuable investigations to identify and manage patients who are at risk of aspiration.
NEUROIMAGING
Atrophy of the midbrain and superior cerebellar peduncle and dilatation of the third ventricle are the characteristic findings of PSP on conventional MRI [81]. Midbrain atrophy is increasingly recognized as a radiological marker for a clinical RS phenotype but it is not predictive of PSP pathology [82]. The hummingbird sign on the midsagittal plane with rostral midbrain atrophy and concavity is observed in 67% of pathologically confirmed PSP cases [83]. Midbrain to pons ratio of less than 0.52 using AP diameter measurements was useful to differentiate PSP-RS from MSA, however, no PSP subtypes were included in the study [84].
Diffusion tensor imaging studies showed white matter tract degeneration especially in the superior cerebellar peduncles and superior longitudinal fasciculus has been associated with RS [85]. Functional MRI studies consistently demonstrated disruption of network connectivity between the cerebellum, midbrain, thalamus and premotor cortex [86].
Focal midbrain hypometabolism on fluorodeoxyglucose (FDG)-positron emission tomography (PET) has been identified in patients with typical PSP, referred to as the pimple sign which corresponds to midbrain atrophy on MRI and can be considered as a radiological biomarker for the clinical RS phenotype [87]. Another study showed that this focal midbrain region together with the caudate, thalamus and supplementary motor area are affected in pathologically confirmed PSP-RS cases [88].
Dopamine transporter single photon emission computed tomography (SPECT) imaging shows reduced tracer uptake in the striatum which is a useful finding to differentiate PSP from other mimics such as cerebrovascular disease and normal pressure hydrocephalus [89]. PET imaging with tau ligand is a promising radiologic tool for diagnostic and longitudinal follow-up [90].
TREATMENT
Most specialist centres give trials of levodopa and amantadine but unfortunately their symptomatic benefits are limited [91]. Zolpidem, a GABA agonist, may improve motor function, dysarthria and ocular abnormalities according to anecdotal evidence from case reports [92]. Selective serotonin re-uptake inhibitors (SSRIs) are effective at treating depression, obsessive-compulsive behaviour and emotional lability but may worsen apathy [93]. Memantine may provide symptomatic benefit in patients with PNFA [94].
Botulinum toxin injection to the pretarsal muscles is effective to alleviate eye closure symptoms in patients with blepharospasm and apraxia of eyelid opening. Regular administration of artificial tears is beneficial for eye irritation and dry eyes. Gastrostomy feeding introduced in an appropriate disease stage is helpful to maintain nutrition, hydration and prevent significant weight loss and aspiration pneumonia, however, suitable counselling of the patients and relatives is essential to ensure an informed decision is made.
A multidisciplinary team approach with input of swallowing and language therapist, dietician, physiotherapist, psychologist, palliative care team, occupational therapist and social worker (allocation of local health care service) is extremely important to ensure the needs of the patients are met. The charity-led support group such as PSP association in the UK is a valuable resource for families, caregivers and patients (http://www.pspassociation.org.uk/).
Disease-modifying therapeutic trials in the past years have improved our insights in the natural course of this devastating disorder [95-98]. Although these trial medications have not proven to be effective, recent knowledge in tau seeding, propagations and strains in PSP and other tauopathies will lead to potential new therapeutic targets to halt the disease progression [99].
Acknowledgments
HL is funded by the CBD Solution Research Grant.
Footnotes
Conflicts of Interest
The author has no financial conflicts of interest.
REFERENCES
- 1.Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 2.Steele JC. Progressive supranuclear palsy. Brain. 1972;95:693–704. [PubMed] [Google Scholar]
- 3.Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23:394–400. doi: 10.1097/WCO.0b013e32833be924. [DOI] [PubMed] [Google Scholar]
- 4.Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55:97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Buée-Scherrer V, Buée L, Leveugle B, Perl DP, Vermersch P, Hof PR, et al. Pathological tau proteins in postencephalitic parkinsonism: comparison with Alzheimer’s disease and other neurodegenerative disorders. Ann Neurol. 1997;42:356–359. doi: 10.1002/ana.410420312. [DOI] [PubMed] [Google Scholar]
- 6.Dugger BN, Adler CH, Shill HA, Caviness J, Jacobson S, Driver-Dunckley E, et al. Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord. 2014;20:525–529. doi: 10.1016/j.parkreldis.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC. Prevalence and natural history of progressive supranuclear palsy. Neurology. 1988;38:1031–1034. doi: 10.1212/wnl.38.7.1031. [DOI] [PubMed] [Google Scholar]
- 8.Kawashima M, Miyake M, Kusumi M, Adachi Y, Nakashima K. Prevalence of progressive supranuclear palsy in Yonago, Japan. Mov Disord. 2004;19:1239–1240. doi: 10.1002/mds.20149. [DOI] [PubMed] [Google Scholar]
- 9.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354:1771–1775. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- 10.Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain. 2005;128(Pt 6):1247–1258. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- 11.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8:270–279. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 12.Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, et al. Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain. 2007;130(Pt 6):1566–1576. doi: 10.1093/brain/awm104. [DOI] [PubMed] [Google Scholar]
- 13.Williams DR, Holton JL, Strand K, Revesz T, Lees AJ. Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy. Mov Disord. 2007;22:2235–2241. doi: 10.1002/mds.21698. [DOI] [PubMed] [Google Scholar]
- 14.O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131(Pt 5):1362–1372. doi: 10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- 15.Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. 2007;130(Pt 6):1552–1565. doi: 10.1093/brain/awm032. [DOI] [PubMed] [Google Scholar]
- 16.Cubo E, Stebbins GT, Golbe LI, Nieves A, Leurgans S, Goetz CG, et al. Application of the Unified Parkinson’s Disease Rating Scale in progressive supranuclear palsy: factor analysis of the motor scale. Mov Disord. 2000;15:276–279. doi: 10.1002/1531-8257(200003)15:2<276::aid-mds1010>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Golbe LI, Rubin RS, Cody RP, Belsh JM, Duvoisin RC, Grosmann C, et al. Follow-up study of risk factors in progressive supranuclear palsy. Neurology. 1996;47:148–154. doi: 10.1212/wnl.47.1.148. [DOI] [PubMed] [Google Scholar]
- 18.Ling H, Holton JL, Shaw K, Davey K, Lashley T, Revesz T. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol. 2015;130:891–893. doi: 10.1007/s00401-015-1496-y. [DOI] [PubMed] [Google Scholar]
- 19.Ling H, Hardy J, Zetterberg H. Neurological consequences of traumatic brain injuries in sports. Mol Cell Neurosci. 2015;66(Pt B):114–122. doi: 10.1016/j.mcn.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Ling H, Kara E, Revesz T, Lees AJ, Plant GT, Martino D, et al. Concomitant progressive supranuclear palsy and chronic traumatic encephalopathy in a boxer. Acta Neuropathol Commun. 2014;2:24. doi: 10.1186/2051-5960-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caparros-Lefebvre D, Sergeant N, Lees A, Camuzat A, Daniel S, Lannuzel A, et al. Guadeloupean parkinsonism: a cluster of progressive supranuclear palsy-like tauopathy. Brain. 2002;125(Pt 4):801–811. doi: 10.1093/brain/awf086. [DOI] [PubMed] [Google Scholar]
- 22.Hirano A, Kurland LT, Krooth RS, Lessell S. Parkinsonism-dementia complex, an endemic disease on the island of Guam. I. Clinical features. Brain. 1961;84:642–661. doi: 10.1093/brain/84.4.642. [DOI] [PubMed] [Google Scholar]
- 23.Geddes JF, Hughes AJ, Lees AJ, Daniel SE. Pathological overlap in cases of parkinsonism associated with neurofibrillary tangles. A study of recent cases of postencephalitic parkinsonism and comparison with progressive supranuclear palsy and Guamanian parkinsonism-dementia complex. Brain. 1993;116(Pt 1):281–302. doi: 10.1093/brain/116.1.281. [DOI] [PubMed] [Google Scholar]
- 24.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 25.Morris HR, Osaki Y, Holton J, Lees AJ, Wood NW, Revesz T, et al. Tau exon 10 +16 mutation FTDP-17 presenting clinically as sporadic young onset PSP. Neurology. 2003;61:102–104. doi: 10.1212/01.wnl.0000072325.27824.a5. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Gong CX. Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener. 2008;3:8. doi: 10.1186/1750-1326-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, Klei L, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Ling H, Massey LA, Lees AJ, Brown P, Day BL. Hypokinesia without decrement distinguishes progressive supranuclear palsy from Parkinson’s disease. Brain. 2012;135(Pt 4):1141–1153. doi: 10.1093/brain/aws038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sixel-Döring F, Schweitzer M, Mollenhauer B, Trenkwalder C. Polysomnographic findings, video-based sleep analysis and sleep perception in progressive supranuclear palsy. Sleep Med. 2009;10:407–415. doi: 10.1016/j.sleep.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Rascol O, Sabatini U, Simonetta-Moreau M, Montastruc JL, Rascol A, Clanet M. Square wave jerks in parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 1991;54:599–602. doi: 10.1136/jnnp.54.7.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leigh RJ, Riley DE. Eye movements in parkinsonism: it’s saccadic speed that counts. Neurology. 2000;54:1018–1019. doi: 10.1212/wnl.54.5.1018. [DOI] [PubMed] [Google Scholar]
- 34.Quinn N. The “round the houses” sign in progressive supranuclear palsy. Ann Neurol. 1996;40:951. doi: 10.1002/ana.410400630. [DOI] [PubMed] [Google Scholar]
- 35.Rivaud-Péchoux S, Vidailhet M, Gallouedec G, Litvan I, Gaymard B, Pierrot-Deseilligny C. Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology. 2000;54:1029–1032. doi: 10.1212/wnl.54.5.1029. [DOI] [PubMed] [Google Scholar]
- 36.Golbe LI, Davis PH, Lepore FE. Eyelid movement abnormalities in progressive supranuclear palsy. Mov Disord. 1989;4:297–302. doi: 10.1002/mds.870040402. [DOI] [PubMed] [Google Scholar]
- 37.Bisdorff AR, Bronstein AM, Wolsley C, Lees AJ. Torticollis due to disinhibition of the vestibulo-collic reflex in a patient with Steele-Richardson-Olszewski syndrome. Mov Disord. 1997;12:328–336. doi: 10.1002/mds.870120311. [DOI] [PubMed] [Google Scholar]
- 38.Ghika J, Tennis M, Growdon J, Hoffman E, Johnson K. Environment-driven responses in progressive supranuclear palsy. J Neurol Sci. 1995;130:104–111. doi: 10.1016/0022-510x(95)00015-t. [DOI] [PubMed] [Google Scholar]
- 39.Murdin L, Bronstein AM. Head deviation in progressive supranuclear palsy: enhanced vestibulo-collic reflex or loss of resetting head movements? J Neurol. 2009;256:1143–1145. doi: 10.1007/s00415-009-5090-x. [DOI] [PubMed] [Google Scholar]
- 40.Rafal RD, Posner MI, Friedman JH, Inhoff AW, Bernstein E. Orienting of visual attention in progressive supranuclear palsy. Brain. 1988;111(Pt 2):267–280. doi: 10.1093/brain/111.2.267. [DOI] [PubMed] [Google Scholar]
- 41.Brown RG, Lacomblez L, Landwehrmeyer BG, Bak T, Uttner I, Dubois B, et al. Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain. 2010;133(Pt 8):2382–2393. doi: 10.1093/brain/awq158. [DOI] [PubMed] [Google Scholar]
- 42.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 43.Slachevsky A, Villalpando JM, Sarazin M, Hahn-Barma V, Pillon B, Dubois B. Frontal assessment battery and differential diagnosis of frontotemporal dementia and Alzheimer disease. Arch Neurol. 2004;61:1104–1107. doi: 10.1001/archneur.61.7.1104. [DOI] [PubMed] [Google Scholar]
- 44.Bak TH, Rogers TT, Crawford LM, Hearn VC, Mathuranath PS, Hodges JR. Cognitive bedside assessment in atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2005;76:420–422. doi: 10.1136/jnnp.2003.029595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rittman T, Ghosh BC, McColgan P, Breen DP, Evans J, Williams-Gray CH, et al. The Addenbrooke’s Cognitive Examination for the differential diagnosis and longitudinal assessment of patients with parkinsonian disorders. J Neurol Neurosurg Psychiatry. 2013;84:544–551. doi: 10.1136/jnnp-2012-303618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubois B, Slachevsky A, Pillon B, Beato R, Villalponda JM, Litvan I. “Applause sign” helps to discriminate PSP from FTD and PD. Neurology. 2005;64:2132–2133. doi: 10.1212/01.WNL.0000165977.38272.15. [DOI] [PubMed] [Google Scholar]
- 47.Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP. Performance on the dementia rating scale in Parkinson’s disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:1215–1220. doi: 10.1136/jnnp.74.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barclay CL, Bergeron C, Lang AE. Arm levitation in progressive supranuclear palsy. Neurology. 1999;52:879–882. doi: 10.1212/wnl.52.4.879. [DOI] [PubMed] [Google Scholar]
- 49.Oide T, Ohara S, Yazawa M, Inoue K, Itoh N, Tokuda T, et al. Progressive supranuclear palsy with asymmetric tau pathology presenting with unilateral limb dystonia. Acta Neuropathol. 2002;104:209–214. doi: 10.1007/s00401-002-0531-y. [DOI] [PubMed] [Google Scholar]
- 50.Respondek G, Stamelou M, Kurz C, Ferguson LW, Rajput A, Chiu WZ, et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord. 2014;29:1758–1766. doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- 51.Lang AE. Clinical heterogeneity in progressive supranuclear palsy: challenges to diagnosis, pathogenesis and future therapies. Mov Disord. 2014;29:1707–1709. doi: 10.1002/mds.26105. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed Z, Bigio EH, Budka H, Dickson DW, Ferrer I, Ghetti B, et al. Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol. 2013;126:537–544. doi: 10.1007/s00401-013-1171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Josephs KA, Katsuse O, Beccano-Kelly DA, Lin WL, Uitti RJ, Fujino Y, et al. Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol. 2006;65:396–405. doi: 10.1097/01.jnen.0000218446.38158.61. [DOI] [PubMed] [Google Scholar]
- 54.Bigio EH, Lipton AM, Yen SH, Hutton ML, Baker M, Nacharaju P, et al. Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J Neuropathol Exp Neurol. 2001;60:328–341. doi: 10.1093/jnen/60.4.328. [DOI] [PubMed] [Google Scholar]
- 55.Fu YJ, Nishihira Y, Kuroda S, Toyoshima Y, Ishihara T, Shinozaki M, et al. Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, Parkinsonism, and motor neuron disease: a distinct clinicopathological and biochemical disease entity. Acta Neuropathol. 2010;120:21–32. doi: 10.1007/s00401-010-0649-2. [DOI] [PubMed] [Google Scholar]
- 56.Birdi S, Rajput AH, Fenton M, Donat JR, Rozdilsky B, Robinson C, et al. Progressive supranuclear palsy diagnosis and confounding features: report on 16 autopsied cases. Mov Disord. 2002;17:1255–1264. doi: 10.1002/mds.10211. [DOI] [PubMed] [Google Scholar]
- 57.Magdalinou NK, Ling H, Smith JD, Schott JM, Watkins LD, Lees AJ. Normal pressure hydrocephalus or progressive supranuclear palsy? A clinicopathological case series. J Neurol. 2013;260:1009–1013. doi: 10.1007/s00415-012-6745-6. [DOI] [PubMed] [Google Scholar]
- 58.Ling H, O’Sullivan SS, Holton JL, Revesz T, Massey LA, Williams DR, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010;133(Pt 7):2045–2057. doi: 10.1093/brain/awq123. [DOI] [PubMed] [Google Scholar]
- 59.Ling H, de Silva R, Massey LA, Courtney R, Hondhamuni G, Bajaj N, et al. Characteristics of progressive supranuclear palsy presenting with corticobasal syndrome: a cortical variant. Neuropathol Appl Neurobiol. 2014;40:149–163. doi: 10.1111/nan.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathew R, Bak TH, Hodges JR. Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry. 2012;83:405–410. doi: 10.1136/jnnp-2011-300875. [DOI] [PubMed] [Google Scholar]
- 61.Josephs KA, Duffy JR. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol. 2008;21:688–692. doi: 10.1097/WCO.0b013e3283168ddd. [DOI] [PubMed] [Google Scholar]
- 62.Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukui T, Lee E. Progressive agraphia can be a harbinger of degenerative dementia. Brain Lang. 2008;104:201–210. doi: 10.1016/j.bandl.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain. 2011;134(Pt 9):2565–2581. doi: 10.1093/brain/awr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris JM, Jones M. Pathology in primary progressive aphasia syndromes. Curr Neurol Neurosci Rep. 2014;14:466. doi: 10.1007/s11910-014-0466-4. [DOI] [PubMed] [Google Scholar]
- 66.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6:88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128(Pt 9):1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 68.Hassan A, Parisi JE, Josephs KA. Autopsy-proven progressive supranuclear palsy presenting as behavioral variant frontotemporal dementia. Neurocase. 2012;18:478–488. doi: 10.1080/13554794.2011.627345. [DOI] [PubMed] [Google Scholar]
- 69.Kanazawa M, Tada M, Onodera O, Takahashi H, Nishizawa M, Shimohata T. Early clinical features of patients with progressive supranuclear palsy with predominant cerebellar ataxia. Parkinsonism Relat Disord. 2013;19:1149–1151. doi: 10.1016/j.parkreldis.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 70.Kanazawa M, Shimohata T, Toyoshima Y, Tada M, Kakita A, Morita T, et al. Cerebellar involvement in progressive supranuclear palsy: a clinicopathological study. Mov Disord. 2009;24:1312–1318. doi: 10.1002/mds.22583. [DOI] [PubMed] [Google Scholar]
- 71.Iwasaki Y, Mori K, Ito M, Tatsumi S, Mimuro M, Yoshida M. An autopsied case of progressive supranuclear palsy presenting with cerebellar ataxia and severe cerebellar involvement. Neuropathology. 2013;33:561–567. doi: 10.1111/neup.12012. [DOI] [PubMed] [Google Scholar]
- 72.Adams C, McKeon A, Silber MH, Kumar R. Narcolepsy, REM sleep behavior disorder, and supranuclear gaze palsy associated with Ma1 and Ma2 antibodies and tonsillar carcinoma. Arch Neurol. 2011;68:521–524. doi: 10.1001/archneurol.2011.56. [DOI] [PubMed] [Google Scholar]
- 73.Tobin WO, Lennon VA, Komorowski L, Probst C, Clardy SL, Aksamit AJ, et al. DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology. 2014;83:1797–1803. doi: 10.1212/WNL.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petrovic IN, Martin-Bastida A, Massey L, Ling H, O’Sullivan SS, Williams DR, et al. MM2 subtype of sporadic Creutz-feldt-Jakob disease may underlie the clinical presentation of progressive supranuclear palsy. J Neurol. 2013;260:1031–1036. doi: 10.1007/s00415-012-6752-7. [DOI] [PubMed] [Google Scholar]
- 75.Borroni B, Premi E, Agosti C, Alberici A, Cerini C, Archetti S, et al. CSF Alzheimer’s disease-like pattern in corticobasal syndrome: evidence for a distinct disorder. J Neurol Neurosurg Psychiatry. 2011;82:834–838. doi: 10.1136/jnnp.2010.221853. [DOI] [PubMed] [Google Scholar]
- 76.Wagshal D, Sankaranarayanan S, Guss V, Hall T, Berisha F, Lobach I, et al. Divergent CSF τ alterations in two common tauopathies: Alzheimer’s disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2015;86:244–250. doi: 10.1136/jnnp-2014-308004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujioka S, Van Gerpen JA, Uitti RJ, Dickson DW, Wszolek ZK. Familial progressive supranuclear palsy: a literature review. Neurodegener Dis. 2014;13:180–182. doi: 10.1159/000354975. [DOI] [PubMed] [Google Scholar]
- 78.Rohrer JD, Paviour D, Vandrovcova J, Hodges J, de Silva R, Rossor MN. Novel L284R MAPT mutation in a family with an autosomal dominant progressive supranuclear palsy syndrome. Neurodegener Dis. 2011;8:149–152. doi: 10.1159/000319454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tremolizzo L, Bertola F, Casati G, Piperno A, Ferrarese C, Appollonio I. Progressive supranuclear palsy-like phenotype caused by progranulin p.Thr272fs mutation. Mov Disord. 2011;26:1964–1966. doi: 10.1002/mds.23749. [DOI] [PubMed] [Google Scholar]
- 80.Le Ber I, Camuzat A, Guillot-Noel L, Hannequin D, Lacomblez L, Golfier V, et al. C9ORF72 repeat expansions in the frontotemporal dementias spectrum of diseases: a flow-chart for genetic testing. J Alzheimers Dis. 2013;34:485–499. doi: 10.3233/JAD-121456. [DOI] [PubMed] [Google Scholar]
- 81.Ling H, Lees AJ. How can neuroimaging help in the diagnosis of movement disorders? Neuroimaging Clin N Am. 2010;20:111–123. doi: 10.1016/j.nic.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Whitwell JL, Jack CR, Jr, Parisi JE, Gunter JL, Weigand SD, Boeve BF, et al. Midbrain atrophy is not a biomarker of progressive supranuclear palsy pathology. Eur J Neurol. 2013;20:1417–1422. doi: 10.1111/ene.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Massey LA, Micallef C, Paviour DC, O’Sullivan SS, Ling H, Williams DR, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord. 2012;27:1754–1762. doi: 10.1002/mds.24968. [DOI] [PubMed] [Google Scholar]
- 84.Massey LA, Jäger HR, Paviour DC, O’Sullivan SS, Ling H, Williams DR, et al. The midbrain to pons ratio: a simple and specific MRI sign of progressive supranuclear palsy. Neurology. 2013;80:1856–1861. doi: 10.1212/WNL.0b013e318292a2d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitwell JL, Avula R, Master A, Vemuri P, Senjem ML, Jones DT, et al. Disrupted thalamocortical connectivity in PSP: a resting-state fMRI, DTI, and VBM study. Parkinsonism Relat Disord. 2011;17:599–605. doi: 10.1016/j.parkreldis.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gardner RC, Boxer AL, Trujillo A, Mirsky JB, Guo CC, Gennatas ED, et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol. 2013;73:603–616. doi: 10.1002/ana.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Botha H, Whitwell JL, Madhaven A, Senjem ML, Lowe V, Josephs KA. The pimple sign of progressive supranuclear palsy syndrome. Parkinsonism Relat Disord. 2014;20:180–185. doi: 10.1016/j.parkreldis.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 88.Zalewski N, Botha H, Whitwell JL, Lowe V, Dickson DW, Josephs KA. FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J Neurol. 2014;261:710–716. doi: 10.1007/s00415-014-7256-4. [DOI] [PubMed] [Google Scholar]
- 89.Kägi G, Bhatia KP, Tolosa E. The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatry. 2010;81:5–12. doi: 10.1136/jnnp.2008.157370. [DOI] [PubMed] [Google Scholar]
- 90.Kepe V, Bordelon Y, Boxer A, Huang SC, Liu J, Thiede FC, et al. PET imaging of neuropathology in tauopathies: progressive supranuclear palsy. J Alzheimers Dis. 2013;36:145–153. doi: 10.3233/JAD-130032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stamelou M, de Silva R, Arias-Carrión O, Boura E, Höllerhage M, Oertel WH, et al. Rational therapeutic approaches to progressive supranuclear palsy. Brain. 2010;133(Pt 6):1578–1590. doi: 10.1093/brain/awq115. [DOI] [PubMed] [Google Scholar]
- 92.Cotter C, Armytage T, Crimmins D. The use of zolpidem in the treatment of progressive supranuclear palsy. J Clin Neurosci. 2010;17:385–386. doi: 10.1016/j.jocn.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 93.Hughes LE, Rittman T, Regenthal R, Robbins TW, Rowe JB. Improving response inhibition systems in frontotemporal dementia with citalopram. Brain. 2015;138(Pt 7):1961–1975. doi: 10.1093/brain/awv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boxer AL, Lipton AM, Womack K, Merrilees J, Neuhaus J, Pavlic D, et al. An open-label study of memantine treatment in 3 subtypes of frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2009;23:211–217. doi: 10.1097/WAD.0b013e318197852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN; NNIPPS Study Group. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain. 2009;132(Pt 1):156–171. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014;13:676–685. doi: 10.1016/S1474-4422(14)70088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tolosa E, Litvan I, Höglinger GU, Burn D, Lees A, Andrés MV, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014;29:470–478. doi: 10.1002/mds.25824. [DOI] [PubMed] [Google Scholar]
- 98.Golbe LI. The tau of PSP: a long road to treatment. Mov Disord. 2014;29:431–434. doi: 10.1002/mds.25855. [DOI] [PubMed] [Google Scholar]
- 99.Lewis J, Dickson DW. Propagation of tau pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2015 Nov 17; doi: 10.1007/s00401-015-1507-z. [Epub]. http://dx.doi.org/10.1007/s00401-015-1507-z. [DOI] [PubMed]


