Abstract
In the last decade the impressive expansion of our knowledge of the vast microbial community that resides in the human intestine, the gut microbiota, has provided support to the concept that a disturbed intestinal ecology might promote development and maintenance of symptoms in irritable bowel syndrome (IBS). As a correlate, manipulation of gut microbiota represents a new strategy for the treatment of this multifactorial disease. A number of attempts have been made to modulate the gut bacterial composition, following the idea that expansion of bacterial species considered as beneficial (Lactobacilli and Bifidobacteria) associated with the reduction of those considered harmful (Clostridium, Escherichia coli, Salmonella, Shigella and Pseudomonas) should attenuate IBS symptoms. In this conceptual framework, probiotics appear an attractive option in terms of both efficacy and safety, while prebiotics, synbiotics and antibiotics still need confirmation. Fecal transplant is an old treatment translated from the cure of intestinal infective pathologies that has recently gained a new life as therapeutic option for those patients with a disturbed gut ecosystem, but data on IBS are scanty and randomized, placebo-controlled studies are required.
Keywords: Irritable bowel syndrome, Gut microbiota, Probiotics, Prebiotics, Synbiotics, Antibiotics, Fecal transplantation
Core tip: In the last decade, the gut microbiota has provided support to the concept that a disturbed intestinal ecology could promote development and maintenance of symptoms in irritable bowel syndrome (IBS). As a correlate, manipulation of gut microbiota represents a new strategy for the treatment of this multifactorial disease. Probiotics appear an attractive option in terms of both efficacy and safety, while prebiotics, synbiotics and antibiotics still need formation. Fecal transplant has recently gained a new life as therapeutic option for those patients with an altered gut ecosystem, but data on IBS are scanty and randomized, placebo-controlled studies are required.
INTRODUCTION
Irritable bowel syndrome (IBS) is a disorder characterized by chronic abdominal pain and discomfort associated with alterations of bowel habits in the absence of a demonstrable pathology[1,2]. Other common symptoms are abdominal distension, bloating and flatulence, straining and urgency. IBS is a common gastrointestinal (GI) disorders in the industrialized world with a 10%-15% prevalence in the general population[3]. This high prevalence together with the associated co-morbidities has a significant impact on both patients and society, especially in terms of quality of life and medical costs[4].
IBS is a heterogeneous functional disorder that, depending on the prevailing bowel habit, has been subtyped into IBS with constipation (C-IBS), IBS with diarrhea (D-IBS), mixed, alternate IBS (A-IBS) with both constipation and diarrhea plus unsubtyped IBS with neither constipation nor diarrhea[1,2]. Alterations of bowel habits are likely related to dysregulation of the autonomic system in the gut, whereas symptoms of abdominal pain and discomfort are thought to involve additional changes in the bidirectional communication between the gut and the brain, known as “gut-brain axis”, that cause a modified perception of visceral events in the form of hyperalgesia or allodynia[5,6]. The etiology of IBS is incompletely understood and evidence is growing that IBS might be a post-inflammatory and stress-correlated condition[7,8]. Both host and environmental factors, including diet, play a key role in triggering symptoms. Among the host factors, central alterations (i.e., aberrant stress responses, psychiatric co-morbidity and cognitive dysfunctions) and peripheral alterations (i.e., intestinal dysmotility, visceral hypersensitivity, low-grade immune activation and altered intestinal barrier function) are both involved[9]. Despite considerable research efforts, the treatment of IBS remains a significant challenge mainly due to its poorly defined pathophysiology.
HUMAN MICROBIOTA
Human microbiota is a complex living ecosystem consisting of unicellular microbes, mainly bacterial, but also metagenomic archaeal (i.e., Methanobrevibacter), viral (i.e., bacteriophages) and eukaryotic (i.e., yeast), that occupies almost every mucosal and cutaneous surfaces of our body. It has been estimated that microbes that stably live in human body amount to 100 trillion cells, ten-fold the number of human cells[10] and the majority inhabits the gut where the intestinal microbiota is widely regarded as a virtual organ that actively influences and mediates several physiological functions. These living microorganisms encode for over three millions of genes, the so-called “microbiome”[11], outfitting the human genoma by approximately 100-fold[12]. The intestinal microbiota is composed by 17 families, 50 genera and more than 1000 species of bacteria: its composition varies among individuals, changes during life and depends on environmental factors, mainly lifestyle, diet, drugs, stress and invasive medical procedures. The intestinal microbiota is dominated by four main phyla: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. In the adult life Bacteroidetes and Firmicutes are usually prevalent, whereas Actinobacteria and Proteobacteria are less represented. In the healthy status, the gut microbiota interacts with the human host in a mutualistic relationship, the host intestine provides the bacteria with an environment to grow and the bacterial eco-system contributes to maintain homeostasis within the host by modulating several physiological functions such as gut development[13], nutrient processing and digestion[14,15], immune cell development and immune responses[16-18], resistance to pathogens[19,20], control of host energy and lipid metabolism[14,15] and brain development and function. Changes in bacterial number and composition, the so-called dysbiosis, may induce a dysregulation of this deep relationship and cause the appearance of a spectrum of diseases including metabolic syndrome, diabetes, cancer, inflammatory conditions, neurological pathologies and psychiatric disorders.
Throughout its communication with gastrointestinal epithelial, immune and nerve cells, the gut microbiota generates and releases molecules that can signal to distant organs. It is now recognized that a significant portion of the metabolites circulating in mammalian blood derives from the intestinal microbial community[21-25] and the presence or absence of the gut microbiota influences the metabolic profile in regions distant from the gut such as the brain[26]. Moreover, it releases factors that target specific neuronal systems involved in the gut-brain axis, generating neurotransmitters and neuromodulators as dopamine, noradrenaline, acetylcholine and gamma-aminobutyric acid (GABA)[27-31]. Direct contact of certain probiotics (i.e., Lactobacillus acidophilus) with epithelial cells induce the expression of opioid and cannabinoid receptors in the gut and contribute to the modulation and restoration of the normal perception of visceral pain[32]. Finally, as the result of intestinal microbial colonization, metabolism, and subsequent fermentation, the human microbiota produces a significant proportion of the gases present in the gut, including carbon dioxide (CO2), hydrogen (H2), methane (CH4), and hydrogen sulfide (H2S). Since H2S has been recently recognized as a gaseous neuromodulator/neurotransmitter capable of modulating intestinal inflammation and sensitivity[33-37], it may be hypothesized that the intestinal microbiota plays a significant role in modulating visceral pain also by producing this gaseous mediator. The term microbiota-gut-brain axis is now currently used to indicate the deep correlation among these three functional “organs”.
MICROBIOTA AND IBS
IBS can be considered a multifactorial syndrome in which several pathogenic mechanisms are involved. Gut microbiota interferes with normal intestinal functions at diverse levels, acting as both cause and target of abnormalities of intestinal motility, sensitivity and neuroimmune signaling, including alterations of mucosal barrier and pattern recognition receptors expression, as well as dysfunctions of hypothalamus-pituitary-adrenal (HPA) axis.
Perturbation in microbiota composition
In recent years, perturbations in the intestinal microbiota have being linked to the pathophysiology of IBS (Figure 1), thought that studies investigating the composition of intestinal microbiota in IBS have produced non univocal results[38]. Nevertheless the majority of data support the notion that the composition of luminal and mucosal microbiota differs among specific subgroups of IBS patients and healthy individuals[39-63] (Table 1). Using culture-based techniques and a 16S rRNA gene-based phylogenetic microarray analysis, it has been demonstrated that the diversity of microbial population is reduced, the proportion of specific bacterial groups is altered and the degree of variability in the microbiota composition is different in IBS patients when compared with healthy subjects. Furthermore, a higher degree of temporal instability of the microbiota among IBS patients has been detected. Examples of these modifications are a decreased amount of Lactobacilli and Bifidobacteria along with an increased amount of aerobes relatively to anaerobes in IBS patients. Finally mucosal bacteria have also been found to be more abundant in IBS patients than in healthy controls (Table 1). Consistent with this view, the clinical guidance regarding the modulation of intestinal microbiota in IBS provided by the Rome Team Working Group has recently concluded that there is good evidence supporting the concept that the intestinal microbiota is perturbed in patients with IBS[64].
Figure 1.
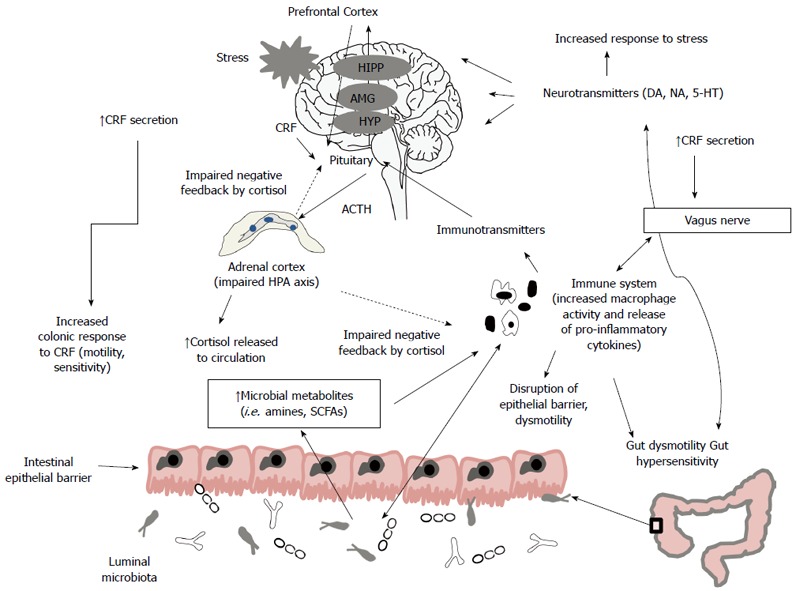
Gut microbiota influences the bidirectional communication between the enteric nervous system and the central nervous system, modulating gut development and several physiological functions, including intestinal motility, sensitivity, secretion and immunity. In irritable bowel syndrome (IBS), the altered composition and/or activity of microbiota may induce a disruption of this communication leading to activation of immune system and production of pro-inflammatory cytokines, production of microbial metabolites as short-chain fatty acids (SCFAs) that are toxic at high concentration, activation of hypothalamic-pituitary-adrenal (HPA) axis with increase of cortisol that feeds back to the pituitary, hypothalamus (HYP), amygdala (AMG), hippocampus (HIPP) and prefrontal cortex to shut off the HPA axis and increase of corticotropin releasing factor (CRF). These effects lead to alterations of intestinal motility and sensation, disruption of epithelial barrier and impaired production of neurotransmitters with an increased response to stressful events. On turn, stress may provoke systemic pro-inflammatory cytokines production that activates the HPA axis that signals to both enteric nervous system and the central nervous system and may alter microbiota composition.
Table 1.
Perturbations in the intestinal microbiotain irritable bowel syndrome patients
| Ref. | Methods | N° of Pts. | Diagnostic criteria | Results in IBS in comparison with healthy subjects |
| Dlugosz et al[38] | qPCR (small bowel) | 35 | Rome II | No differences |
| Balsari et al[39] | ↓Coliforms | |||
| ↓Lactobacilli | ||||
| ↓ Bifidobacteria | ||||
| Si et al[40] | Culture | 25 | Rome II | ↓Bifidobacteria |
| ↑Enterobacteriaceae | ||||
| Malinen et al[41] | qPCR | 27 | Rome II | ↑ Veillonellain C-IBS |
| ↓Lactobacillus in D-IBS | ||||
| Mättö et al[42] | Culture/DGGE | 26 | Rome II | ↑ Aerobes |
| Temporal instability | ||||
| Swidsinski et al[43] | FISH | 20 | A-IBS | ↑ Mucosal bacteria |
| C-IBS | ↑Eubacteriumrectale | |||
| D-IBS | ↑Clostridium coccoides | |||
| Maukonen et al[44] | PCR-DGGE | 16 | A-IBS | Higher instability of the bacterial population |
| C-IBS | ↑Clostridial groups | |||
| D-IBS | ||||
| Kassinen et al[45] | 16S ribosomal RNA gene cloning | 24 | Rome II | Significant differences in several bacterial genera belonging to the genera Coprococcus, Collinsella and Coprobacillus |
| Lyra et al[46] | 16S ribosomal RNA gene cloning | 20 | A-IBS C-IBS D-IBS | ↑Clostridium thermosuccinogenes in D-IBS ↑Ruminococcus torques in D-IBS ↑Ruminococcus bromii-like in C-IBS |
| Krogius-Kurikka et al[47] | 16S ribosomal RNA gene cloning | 10 | Rome II | ↑Proteobacteria and Firmicutes in D-IBS |
| ↓Actinobacteria and Bacteroidetes in D-IBS | ||||
| Kerckhoffs et al[48] | FISH, PCR | 41 | A-IBS | ↓Bifidobacteria |
| C-IBS | ||||
| D-IBS | ||||
| Carroll et al[49] | 16S ribosomal RNA gene cloning | 2 | Rome III | ↑Bacteroidetes ↑Proteobacteria |
| Salonen et al[50] Review | ||||
| Tana et al[51] | Culture | 26 | Rome II | ↑Lactobacillus |
| qPCR | ↑ Veillonella | |||
| Carroll et al[52] | qPCR | 10 | Rome III | ↓Aerobicbacteria |
| D-IBS | ↑Lactobacillus | |||
| Codling et al[53] | 16S rRNA-DGGE | 47 | Rome II | ↓Bacterial richness |
| Ponnusamy et al[54] | rRNA-specific 16S rRNA-DGGE | 54 | Rome II | Same total bacterial quantity Higher diversity of Bacteroidetes and Lactobacilli |
| PCR | Lower diversity of Bifidobacter and Clostridium coccoides | |||
| Kerckhoffs et al[55] | 16S rRNA-DGGE | 37 | Rome II | ↑Pseudomonas aeruginosa |
| qPCR | ||||
| Rajilić-Stojanović et al[56] | 16S rRNA | 62 | Rome II | ↑Ratio of the Firmicutes to Bacteroidetes |
| qPCR | ↑Dorea, Ruminococcus, Clostridium spp | |||
| ↓Bacteroidetes, Bifidobacterium, Faecalibacterium spp | ||||
| Carroll et al[57] | 16S rRNA | 16 | Rome III D-IBS | Lower biodiversity of microbes |
| Carroll et al[58] | 16S rRNA | 23 | D-IBS | ↓Bacterial richness |
| ↑Enterobacteriaceae | ||||
| ↑Proteobacteria | ||||
| ↓Faecalibacterium | ||||
| Parkes et al[59] | FISH | 47 | Rome III | More total bacteria numbers |
| ↑Bacteroides, Clostridia coccoides-Eubacteriumrectale | ||||
| Jeffery et al[60] | 16S rRNA | 37 | Rome II | A sub-group of IBS showed normal-like microbiota |
| A sub-group of IBS showed large microbiota-wide changes with ↑Firmicutes and ↓Bacteroidetes | ||||
| Chassard et al[61] | FISH/16S rDNA | 14 | Rome II | ↑Enterobacteriaceae |
| Functional approaches | Rome III | ↑Suphate-reducing bacteria | ||
| C-IBS | ↓Lactic acid bacteria population (bifidobacteria and to a lesser extent, lactobacilli) | |||
| König et al[62] Review | ||||
| Sundin et al[63] | 16s rRNA | 19 | Rome III | ↑Bacteroidetes in the PI-IBS group (13 patients) ↑Firmicutes (more specifically Clostridium in IBS) |
IBS: Irritable bowel syndrome; IBS-D: Diarrhea predominant IBS; IBS-C: Constipation predominant IBS; A-IBS: Alternate IBS; PI-IBS: Post-infectious-IBS; DGGE: Denaturing gradient gel electrophoresis; PCR: Polymerase chain reaction; FISH: Fluorescence in situ hybridization.
A part of the abnormal composition of gut microbiota in IBS, others factors support the notion that intestinal flora plays a key role in determining IBS. First, there is increasing evidence of an activation of the intestinal immune system in IBS leading in a micro-inflammation, with studies demonstrating increased concentrations of mucosal intra-epithelial lymphocytes[65,66], mast cells[66-69] and 5-hydroxytryptamine-secreting enterochromaffin cells[65]. Gut microbiota influences mucosal inflammation in inflammatory bowel disease (IBD) patients, i.e., ulcerative colitis (UC) and Crohn’s disease. Animal-based studies emphasize the critical role of gut microbiota in the balance between immunosuppression and inflammation in the GI tract, involving Toll-Like Receptor (TLRs) signaling pathways[70]. Indeed, helmintic based treatment with Heligmosomoides polygyrus could ameliorate colonic inflammation in murine model of IBDs, shifting the composition of intestinal bacteria[71]. Furthermore, Kuehbacher et al[72] analyzed the gut microbiota of 73 patients with IBD demonstrating that alteration of TM7 bacteria and the genetically determined antibiotic resistance of TM7 species in these patients, could be a relevant part of a more general alteration of bacterial microbiota in IBD patients, i.e., as a promoter of inflammation at early stages[72]. Recent studies demonstrate that reductions in protective bacteria and increases in inflammatory bacteria are associated with pouch inflammation in patients with UC who underwent pouch surgery[73]. Moreover, the presence of Ruminococcus gnavus, Bacteroides vulgatus and Clostridium perfringens and the absence of Blautia and Roseburia in faecal samples of patients with UC before surgery is associated with a higher risk of pouchitis after ileal pouch-anal anastomosis[74]. Given the evidence for the role of intestinal microbiota in the profound inflammatory state in IBD, it might be speculated that luminal antigens should play a similar role in development of subclinical inflammation in IBS. Second, it has been reported that approximately 10% of IBS patients refer that their symptoms began following an episode of infectious diarrhea[75-77], the so-called post infectious IBS (PI-IBS), a condition with a clear infective trigger that may alter the normal intestinal microbiota. Third, there is a strong association between IBS and prior use of antibiotics[78]. Fourth, the intestinal microbiota strongly interacts with exogenous factors, in particular diet, which may directly or indirectly cause IBS symptoms[79]. Fifth, it is well known that alteration of gut microbiota could interfere with behavior and mood in humans[80,81]; on the other hand, psychiatric disorders such as anxiety and depression are highly present as co-morbidities in individuals with IBS[82]. An high Firmicutes: Bacteroides ratio is found in some IBS patients and appears to correlate with depression and anxiety[60], while in another study it has been reported that in IBS patients with clinically significant anxiety, daily treatment with a prebiotic galactooligosaccharide mixture for 4 wk reduced anxiety scores and had a significant positive impact on quality of life[83]. Taken together, these factors support the notion that an imbalance in the intestinal microbiota composition may directly or indirectly interfere with the normal function of the microbiota-gut-brain axis, leading to the development of central and peripheral abnormalities of either intestinal motility and viscero-sensory network.
Microbiota and colo-intestinal motility
Although in the past decades the alterations of intestinal and colonic motility have been considered to play a major role in the development of symptoms in IBS patients[84-86], their influence has been reduced over time as intestinal manometry failed to identify any diagnostic abnormality in IBS patients[87,88]. The abnormal manometric findings found in IBS patients are heterogeneous and range from minimal changes to severe qualitative abnormalities. For example, the incidence of “clustered” contractions is similar in healthy subjects and IBS patients and greatly varies overtime[89,90], acute psychologic stress alters duodeno-jejunal motility in both healthy subjects and IBS patients[91] and more than half of IBS patients has an entirely normal 24-h manometry[92]. Nonetheless gut microbiota modulates gut motor function, which in turn can alter the intestinal microbiota composition. Indeed, not only germ-free animals show profound altered motility patterns that is reversed upon reconstitution with normal flora[93,94], but the influence of the intestinal microbiota on small intestinal myoelectric activity is species-dependent[95,96]. The modulatory effects of microbiota on colo-intestinal motility may be dependent on interaction of bacteria with the gastrointestinal tract through receptors on the epithelial cell such as TLRs and nucleotide oligomerization domain (NOD) receptors and, although bacterial translocation, defined as passage of viable bacteria to mesenteric lymph nodes or other organs, is minimal[97], secreted products of bacteria normally gain access to the submucosa to stimulate the mucosal immune system and to induce changes in intestinal immunity and physiology. In this view, specific bacteria have been reported to induce significant changes in colo-intestinal motility. Bacteroides thetaiotaomicron, a common gut commensal in mice and humans, was found to alter expression of genes involved in smooth-muscle function and neurotransmission[98], soluble factors from the probiotic Escherichia coli Nissle 1917 enhance colonic contractility by direct stimulation of smooth muscle cells[99] and lipopolysaccharide (LPS) from a pathogenic strain of Escherichia coli impairs colonic muscle cell contractility[100]. Finally, exposure of human colonic muscle cells to Lactobacillus rhamnosus GG resulted in a significant dose- and time-dependent impairment of acetylcholine-stimulated contraction[101] and in the restoration of the intrinsic myogenic response in a model of LPS-induced alterations of muscle cells[102].
Microbiota and visceral sensitivity
The connections among gut and brain involve several integrated structures that transport the sensorial information from peripheral (gut) to the central (CNS) stations. Each stimulus from splanchnic visceral afferences (i.e., distensive, chemical, thermal, osmotic) pass throughout the gut intrinsic innervation enteric nervous system (ENS), is received in the spinal dorsal horn and is transmitted to supraspinal sites, the final integration of the painful perception occurring in the cortex[103]. These complex communications connect to the extrinsic innervation [the autonomic nervous system (ANS)] which interacts with the HPA axis affecting the visceral sensory motor functions[104]. Vagal afferences activation plays a modulatory role on the spinal visceral pain pathway[105]. Visceral hypersensitivity may develop at several levels of the brain-gut axis, i.e., ENS, spinal cord and supraspinal sites[106] and plays a key role in the pathogenesis of IBS, the main physiopathological alteration being represented by a reduced pain threshold to rectal distension[6,107,108]. Moreover, an altered rectal compliance[109,110] and/or an increased sensorial colonic response to intestinal lipid perfusion may be present[110-112]. An abnormal central processing of intestinal stimuli could be the cause of visceral hypersensitivity, as indicated by brain imaging studies that have shown an altered vascularization of certain areas of the CNS in response to intestinal distension in IBS patients, such as the anterior cingulated cortex, the amygdale and the dorso-medial frontal cortex[113]. At supraspinal sites, interactions with emotional or stressful stimuli can modulate the visceral sensitivity resulting to increased pain perception[114]. Recent data have shown that gut microbiota may directly modulate several systems involved in visceral hypersensitivity. Indeed, antibiotics-induced intestinal dysbiosis modified colonic pain-related and motor responses by upregulation of TLR4 and TLR7 and downregulation of the antinociceptive cannabinoid 1 and mu-opioid receptor in mice[115]. Moreover, manipulations of the commensal microbiota by stressful events was able to enhance the local expression of visceral sensory-related systems within the colon, as cannabinoid receptor type 2 and tryptophan hydroxylase isoform 1 (TPH1), leading to an excitatory modulation of visceral sensitivity[116]. Functional dysbiosis caused visceral hypersensitivity in patients affected by IBS (including PI-IBS), SIBO and chronic constipation by acting on local or systemic immune activation and altered intestinal fermentation[117], and gut commensals modulate the activations of intestinal sensory endings[118]. Probiotic strains directly modulate visceral perception of nociceptive stimuli. For example, Lactobacillus reuteri inhibited the autonomic response to colorectal distension in rats through effects on enteric nerves[119], modulated vagal afferents[120] and decreased the in vitro and in vivo activation of the transient receptor potential vanilloid 1 (TRPV1) channel which activity may mediate nociceptive signals[121].
Microbiota and autonomic nervous system, hypothalamic-pituitary-adrenal axis, enteric nervous system, mucosal barrier and neuro-immune signalling
The bidirectional communication network among central and peripheral regions includes the CNS, the spinal cord, the ANS, the ENS and the HPA axis. The autonomic system, via the sympathetic and parasympathetic branches, drives both afferent and efferent signals, while the HPA axis modulates the adaptive responses of the organism to stressors of any kind[122,123]. Stressful events, as well as elevated systemic pro-inflammatory cytokines, activate this system that, through secretion of the corticotropin-releasing factor (CRF) from the hypothalamus, stimulates adrenocorticotropic hormone secretion from pituitary gland that, in turn, leads to cortisol (an hormone that has a predominantly anti-inflammatory role on the systemic and GI immune system) release from the adrenal glands. Both neural and hormonal responses induce activation of several effector cells including immune cells, epithelial cells, enteric neurons, smooth muscle cells, interstitial cells of Cajal and enterochromaffin cells. Once activated, these systems exert a profound influence on gut microbiota composition both indirectly by modulating several GI functions (including motility, secretions, maintenance of intestinal permeability and integrity of immune response) and directly via signaling molecules[124]. On the other hand, these systems are under the influence of the gut microbiota composition that interacts not only locally with intestinal cells and ENS, but also directly with CNS through neuroendocrine and metabolic pathways[125-130].
Microbiota and pattern recognition receptors
The balance of innate signaling in the intestine is crucial to homeostasis and microbiota integrity is essential in maintaining the neuro-immune function in the GI tract. The detection of pathogens by the host is obtained through the families of pattern recognition receptors (PRRs) that recognize conserved molecular structures known as pathogen-associated molecular patterns and induce production of innate effector molecules. These signaling receptors can be divided into three families: TLRs, retinoic acid inducible gene I-like receptors, and NOD-like receptors. The TLR family is the best characterized, and 13 receptors have been reported in mice and humans[131]. These PRRs play a key role in detecting pathogens and inducing the innate response. In particular, TLRs respond to specific microbial ligands and to harmful signals produced by the host during infection, initiating a downstream cascade that activates both innate and adaptive immunity. This cascade includes epithelial cell proliferation, secretion of IgA into the gut lumen and production of α-defensins, β-defensins and other bactericidal substances termed antimicrobial peptides (AMPs)[132-134]. Gut microbiota, through PRRs, can modulate the expression of genes involved in inflammatory and pain responses and the production of AMPs. In turn, the expression of PRRs affects the structure of gut microbiota in both health and disease. For example, alterations in the composition of the commensal microbiota as seen in dysbiosis may induce profound change in TLR4 and TLR7 expression, leading in alteration of colonic motility and sensitivity[115], while microbiota protects against ischemia/reperfusion-induced intestinal injury through NOD2 signaling[135]. In turn, TLR signaling maintains segregation between bacteria and the epithelium through production of AMPs[136-138], but deficiency in PRRs such as NOD2 and TLR5 can alter the gut microbiota composition in mice[139].
MODULATION OF THE INTESTINAL MICROBIOTA IN IBS
Probiotics
The term “probiotic” as originally defined by FAO/WHO refers to “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”[140]. However, in order to be beneficial, probiotic bacteria must be able to survive along the gastrointestinal tract, to resist to gastric acid, bile and pancreatic juice action and to demonstrate functional efficacy[141]. Several clinical trials that have investigated the therapeutic benefits of probiotics on either overall or specific IBS symptoms, but these studies are highly heterogeneous. Thus, although a number of meta-analysis or systemic reviews indicate that probiotics may be helpful in the treatment of IBS symptoms, their conclusions vary because of inadequate sample size, poor study design and use of various probiotic strains in the reviewed studies. This review has been focused on a number of meta-analysis and extensive reviews, published in the last 5 years, that have screened randomized and controlled trials (RCTs) conducted on IBS patients by using different probiotic strains.
Moayyedi et al[142] have examined 18 RCTs including 1650 patients with IBS and, although there was a significant heterogeneity among studies, a preference toward probiotic treatment was detected. Indeed, probiotic administration was significantly better than placebo at improving overall symptoms. No major difference was apparent between various types of probiotics used, with Lactobacillus (three trials, 140 patients)[143-145], Bifidobacterium (two trials, 422 patients)[146,147], Streptococcus (one trial, 54 patients)[148] and combinations of probiotics (four trials, 319 patients)[149-152], all showing a trend towards benefit, with no side effects reported. Moreover probiotics showed a statistically significant effect in improving individual symptoms such as pain, flatulence and bloating, but not urgency.
Lactic acid bacteria (LAB)[153], the most commonly used bacteria in probiotic preparations that include both typical and atypical species, covering Lactobacilli, Bifidobacteria, Enterococci and Streptococci have also been widely used in clinical trials. Analysis of 42 RCTs by Clarke et al[153] indicates that, despite a significant studies heterogeneity, 34 studies reported beneficial effects on at least one pre-specified endpoints or symptoms. Indeed, 20 of the 34 trials involving LAB reported improvement in abdominal pain/discomfort, 12 of the 24 trials reported improvement on abdominal bloating/distension, and benefits over placebo were reported in 13 of the 24 trials assessed using an index of defecatory function. Both Bifidobacteria[147,154-157] and Lactobacilli[144,145,154,158-165] were found effective in ameliorating IBS symptoms, while the beneficial effects of the multispecies LAB preparations, including the multistrain preparation VSL#3, were less evident[149,150,152,166-171].
A more strictly selected list of 16 RCTs was evaluated by Brenner et al[172]. These Authors found that 11 trials were inadequately blinded, of too short duration, of too small sample size, and/or lacked intention to treat analysis; they concluded that only two of the studies - those using Bifidobacterium infantis 35624[147-154] - showed significant improvements in abdominal pain/discomfort, bloating/distension and/or bowel movements, compared to the placebo. However, none of the studies provided quantifiable data about both tolerability and adverse events.
A systematic review by Hungin et al[173] has selected 19 studies and included 1807 patients. The majority of these studies tended to include all IBS subtypes, with only two studies focusing on C-IBS[156,157], and three studies focusing on D-IBS[149,174,175]. Although reported trials were extremely different for probiotic strains (above all Lactobacilli and Bifidobacteria, but also Streptococcus salivaris, Saccharomyces boulardii and probiotic mixtures such as VSL#3), study design and definition of treatment response with a responder rates of 18%-80% in IBS group and 5%-50% in healthy controls, this review[149,156,157,173-188] detected several positive effects of probiotics on IBS symptoms and health-related quality of life. Probiotics had a favorable safety profile with no difference in adverse events among the 23 specific probiotic treatments and placebo[173].
Another systematic review with meta-analysis has been recently published by Didari et al[189]. They retrieved 11748 publication on probiotics, but only 15 were used for the meta-analysis, 9 of which were reviewed in deep. Again, the majority of the study was excluded for poor clinical design, lack of inclusion criteria, time limitation, and lack of a control group and use of probiotics in combination with herbal medications or prebiotics. The 15 trials used for the meta-analysis included 882 patients with D-IBS, C-IBS and A-IBS according to Rome II, Rome III, International Classification of Health Problems in Primary Care and World Organization of Family Doctors criteria[145,152,155,166,169,170,174-177,179,190-193]. Although the studies differ in term of bacterial strain used, probiotic dosage, duration of either treatment or follow-up and endpoints/outcome, probiotics were more effective than placebo in reducing abdominal pain after 8 and 10 wk of treatment; the effect was higher at week 8, suggesting a reduced effectiveness with long-term use. Furthermore, probiotics administration improved general IBS symptoms and the severity of symptoms was decreased, although not significantly in comparison with placebo. Few adverse events were reported in both probiotics and placebo groups. The same results have been reached by the extensive review of other 9 studies that, according to Rome II or Rome III criteria, included 324 patients with C-IBS, D-IBS and A-IBS[156,161,167,182,194-198].
As several trials have demonstrated the superiority of probiotics over placebo in controlling IBS symptoms (above all, Lactobacilli and Bifidobacteria, but also other species including bacterial mixtures such as VSL#3), there is now a general agreement on their efficacy as a therapy for this elusive syndrome (extensively discussed in Reference 112 and Table 2). However, given the controversies in IBS pathophysiology, patient heterogeneity, or lack of clear and reproducible evidence for gut microbiota abnormalities in patients with IBS, additional RCTs with appropriate end points and design are needed for determining to which extent (and in which IBS subpopulations) probiotics are a useful therapeutic strategy in the management of IBS symptoms.
Table 2.
Efficacy of probiotics in irritable bowel syndrome[112]
| Authors' statement | Parameter scored | Conclusion | Grade of evidence for effect | Ref. |
| 1 | Global symptom assessment | Specific probiotics help relieve overall symptom burden in some patients with IBS | High | [147,149,150,152,156-157,169, 174,176-178] |
| 2 | Global symptom assessment | Specific probiotics help relieve overall symptom burden in some patients with C-IBS | Low | [147,156-157] |
| 3 | Global symptom assessment | Specific probiotics help relieve overall symptom burden in some patients with D-IBS | Moderate | [147,149,174-175] |
| 4 | Abdominal Pain | Specific probiotics help reduce abdominal pain in some patients with IBS | High | [145,147,149-150,152,155-157, 168-169,174-119] |
| 5 | Bloating/distension | Specific probiotics help reduce bloating/distension in some patients with IBS | Moderate | [147,149-150,155-156,168-170, 174-177,179-183] |
| 6 | Flatus | Probiotics tested to date do not help reduce flatus in patients with IBS | Low | [147,149-150,156,168,174-175, 178-179,184] |
| 7 | Constipation | Specific probiotics may help reduce constipation in some patients with IBS | Low | [155-156,183,185] |
| 8 | Bowel habit | Specific probiotics help improve frequency and/or consistency of bowel movements in some patients with IBS | Moderate | [145,147,149-150,152,155-157, 168-170,174-180,182,185-186,188-189] |
| 9 | Diarrhoea | Probiotics tested to date do not reduce diarrhea in patients with IBS | Very low | [174,179,181-183,185,187] |
| 12 | Health-related quality of life | With specific probiotics, improvement of symptoms has been shown to lead to improvement in some aspects of health-related quality of life | Moderate | [147,150,152,155,169-170,174,176-177,179-180,183-184,186] |
IBS: Irritable bowel syndrome; D-IBS: Diarrhea predominant IBS; C-IBS: Constipation predominant IBS.
Putative mechanisms of action of probiotics
As the pathogenesis of IBS is multifactorial, probiotics have been shown effective in modulating several mechanisms that might have a mechanistic role in IBS pathogenesis, including effects on composition of intestinal microbiota, gastrointestinal dysmotility, visceral hypersensitivity, altered gut epithelium and immune function, luminal metabolism, dysfunction of gut-brain axis, psychological distress.
Composition of intestinal microbiota in IBS
Only few trials on IBS patients have examined the composition of intestinal bacteria before and after the supplementation therapy, therefore the effect (if any) of probiotics administration on resident microbiota is not fully understood. However, it has been suggested that probiotics might reshape the intestinal eco-system generating an ecological milieu that is unfavorable for the growth of harmful species by increasing the number of Lactobacilli and Bifidobacteria[199] that will stabilize the intestinal microbiota[174,179]. As bacteria compete for nutrients and produce substances that directly affect the growth of other bacteria, probiotics can provide a two-fold protection against a broad range of pathogens, including certain forms of Clostridium, Escherichia coli, Salmonella, Shigella and Pseudomonas: aside from competing for the nutrients, probiotics also produce metabolites (i.e., lactic acid, short chain fatty acids and hydrogen peroxide) and soluble factors (i.e., bacteriocins as sakacin, lactocin, amylovorin, acidophilin, bifidin, bifidocin) that are inhibitory for some pathogenic bacteria[200,201]. Moreover, probiotics reduce the adherence of pathogenic bacteria by promoting the production of mucins[202-204].
Gastrointestinal dysmotility
From almost 4 decades, the assumption that IBS is characterized by impaired intestinal motor functions[205-207] and by gas retention[208] has driven the treatment of IBS to small bowel and colonic dysmotilities. In this conceptual framework probiotics are thought to directly affect the intestinal motility. Indeed, Bifidobacterium Lactis HN019 and Bifidobacterium lactis DN-173 010 decreased intestinal transit time in adult constipated patients[209] and a recent meta-analysis of randomized controlled trials have revealed that Bifidobacterium lactis, but not for Lactobacillus casei Shirota, reduced whole gut transit time and increased stool frequency in constipated patients[210]. Moreover, fermented dairy product containing Bifidobacterium lactis DN-173 010 reduced distension in association with acceleration of gastrointestinal transit and improvement of symptoms in IBS with constipation[156], daily Bifidobacterium lactis supplementation decreases WGTT and the frequency of functional GI symptoms in a dose-dependent manner in subjects suffering from irregular bowel movements and flatulence[185,211] and a combination of probiotics (Bacillus subtilis and Streptococcus faecium) was effective for relief of symptoms in patients with non-diarrheal-type IBS[212]. Probiotics are usefull also on D-IBS, as a probiotic mixture containg Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus rhamnosus, Bifidobacterium breve, Bifidobacterium lactis, Bifidobacterium longum, and Streptococcus thermophilus has shown to be effective in controlling symptoms[174], but the effect on intestinal motility and transit time in this subtype of patients is less proven[149].
Visceral hypersensitivity
Mechanistic data provided mainly by animal studies highlight that probiotics exert a direct anti-nociceptive action through the modulation of bacterial metabolites production (i.e., neurotransmitters, neuroactive substances including GABA and serotonin) on sensitive nerve endings in the gut mucosa[26-31,130], or by targeting specific central neurosensitive pathways. For instance, various strains of probiotics have been shown effective in reducing visceral nociceptive reflex responses in several experimental models of IBS[213,214] by directly modulating a number of central anti-nociceptive and pro-nociceptive pathways[32,214-216]. However, only few studies have investigated the effects of probiotics on visceral sensitivity in humans. Indeed, in healthy subjects a non-fermented milk product contained Bifidobacterium animalis subsp Lactis, Streptococcus thermophiles, Lactobacillus bulgaricus and Lactococcus lactis subsp Lactis was able to affect activity of brain regions that control central processing of emotion and sensation[81]; on the other hand, in IBS patients the multispecies probiotic Winclove 801 containing six bacterial species (Bifidobacterium lactis W52, Lactobacillus casei W56, Lactobacillus salivarius W57, Lactococcus lactis W58, Lactobacillus acidophilus NCFM and Lactobacillus rhamnosus W71) failed to ameliorate visceral hypersensitivity in comparison with placebo[217]. However, this limited information is insufficient to translate the animals findings to “hypersensitive” disease states involving disturbances in the gut/brain axis such as IBS.
Epithelial barrier/intestinal inflammation/immune activation
Despite IBS being considered as a “functional”, non-organic disease, it is widely accepted that persistence of IBS-like symptoms occurs in a small percentage of patients after a documented episode of intestinal bacterial or viral infection. The fact that IBS could be a state of “low grade inflammation” is gaining acceptance based on the fact that animal and epidemiological studies indicate that IBS is characterized by an increased intestinal permeability, a biomarker of impaired epithelial barrier function. Further on, an increased activity of innate immune (mainly represented by accumulation of mast cells and antigen-presenting cells such as dendritic cells and macrophages) and an activated adaptive immune response in the intestinal mucosa and in blood, including an increased levels of systemic or mucosal cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-8, IL-12, associated to a decrease of anti-inflammatory cytokines (i.e., IL-10) has been described in IBS[218-220]. Probiotics appear effective in reducing the inflammatory components of IBS. Thus, probiotics administered either as a single strain or in combination, maintain the integrity of intestinal epithelial barrier in inflammatory models[221-225] and in humans[161,175], modulate both innate and adaptive immunity[226,227], restore the imbalanced ratio between pro-inflammatory and anti-inflammatory cytokines (i.e., IL-10/IL-12)[154] and decrease the levels of pro-inflammatory cytokine as TNF-α and IFN-γ[228-230]. Human studies have demonstrated that a probiotic combination containing Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1 and Bifidobacterium longum MM2 175 induced a less inflammatory cytokine profile in older adults[231], Saccharomyces boulardii supplementation induced a significant decrease in blood and tissue levels of proinflammatory cytokines IL-8 and TNF-α and an increase in anti-inflammatory IL-10 levels, as well as an increase in the tissue IL-10/IL-12 ratio ameliorating the quality of life of D-IBS[232] and the symptomatic response induced by Bifidobacterium infantis 35624 in IBS was associated with normalization of the ratio of an anti-inflammatory to a proinflammatory cytokine[154].
Luminal metabolism
The gut microbiota produces several metabolites including short-chain fatty acids (SCFAs), neurotransmitters, metabolites of bile acids, and cytokines that target enteric cells via specific receptors and signal to the brain via afferent vagal or endocrine pathways. SCFAs like acetate, propionate and butyrate derive from the fermentation of undigested and unabsorbed carbohydrates, i.e., resistant starches and dietary fibers and are used as a fuel by the colonic microbiota. While propionate is largely taken up by the liver and acetate enters the systemic circulation to be metabolized by the peripheral tissues, butyrate works as major energy source for colonocytes. Butyrate modulates epithelial proliferation, apoptosis and cell differentiation in the large intestine[233], inhibits nuclear factor kappa B activation[234] and stimulates intestinal mucus production[235], thereby supporting the mucosal barrier function. Furthermore, butyrate plays a major role in inflammation-related repairs[236], offers protection against colonic carcinogenesis in rats[237] while in humans improves visceral perception[238], suggesting a possible beneficial effect in “hypersensitive” disorders such as IBS.
In contrast acetic and propionic acid-producing bacteria (i.e., Veionella and Lactobacilli spp) have been reported in IBS patients[51] leading to enhanced production of SCFAs that are toxic at high concentrations and stimulate 5-HT release from the intestinal nerve endings[239]. As 5-HT initiates high-amplitude colonic contractions, accelerates intestinal transit and increases colonic motility, i.e., all possible features of IBS, it might be speculated that these fermentation products play a role in IBS symptoms. However, fecal concentrations of SCFAs in IBS patients differ only slightly in comparison to healthy subjects[51,240] and IBS symptoms show only a slight correlation with SCFAs fecal concentrations. Moreover, studies that have examined the effect of probiotics supplementation on fecal SCFAs in humans have provided conflicting results. Thus, the probiotic Lactobacillus paracasei DG modulated fecal butyrate concentration in healthy subjects[241], while bifidobacteria-fermented milk increased fecal butyrate, propionate and short-chain fatty acid concentrations but ameliorated symptoms in patients affects by UC[242]. Finally, the Bifidobacterium lactis Bb12 increased the fecal level of acetate and lactate in preterm infants[243].
However studies demonstrating a strong correlation between the assumption of probiotics and the level of fecal SCFAs in IBS patients are lacking.
Dysfunction of the brain-gut axis and psychological distress
There are evidence that the gut microbiota modulates the CNS via the ENS, the ANS, the HPA axis and vice versa[124,213]: a deep correlation that influences both brain development and responses, intestinal motility, sensitivity, secretions and immunity. An intestinal dysbiosis may lead to alteration of brain-gut axis and probiotics may restore the normal interaction among all components of this pathway. Several beneficial actions of probiotics on brain-gut axis including the maintenance of intestinal barrier that “protects” the ENS, the effects of certain probiotics and their products on intestinal sensory and motor nerves of ENS, ANS and on several receptors (i.e., opioid and cannabinoid receptors) and the modulation of cytokines profile leading to an anti-inflammatory action have been mentioned earlier in this review. Importantly, the HPA axis is a neuroendocrine system essential for the normal stress response to challenges in vertebrates which integrity is important in the pathogenesis of IBS[244,245]. There is evidence that certain probiotics directly influence the exaggerated HPA axis response observed in several experimental models of IBS. Indeed, Bifidobacterium animalis subsp lactis BB-12® and Propionibacterium jensenii 702 induced activation of neonatal stress pathways and an imbalance in gut microflora but also improved the immune environment of stressed animals and protected against stress-induced disturbances in adult gut microflora[246], while probiotic preparation containing live Lactobacillus rhamnosus strain R0011 and Lactobacillus helveticus strain R0052 improved gut dysfunction induced by maternal separation, at least in part by normalisation of HPA axis activity[247]. Moreover, probiotics alleviate anxiety- and depression-related behavior that are a typical feature of IBS, as Lactobacillus rhamnosus (JB-1) reduced the stress-induced elevation in corticosterone in stressed animals via modulation of GABA receptors implicated in anxiety behavior[216], Bifidobacterium longum 1714 had a positive impact on cognition in stressed mice[248] and the probiotic mixture VSL#3 induced an increase in brain-derived neurotrophic factor (BDNF) expression and reduced age-related alterations in the hippocampus in a murine model of deterioration in cognitive functions[249].
Probiotics and gene expression: a new mechanism of action?
Finally, probiotics administration might result in a central (i.e., CNS) and peripheral (i.e., intestinal) reprogramming of genes. In the maternal separation (MS) rat model of IBS, Bifidobacterium breve 6330 influenced hippocampal BDNF gene expression[250], while the probiotic mixture VSL#3 downregulated the colonic expression of several genes (i.e., TPH1, CCL2, CCR2, NOS3, NTRK1, BDKRB1, IL10, TNFRSF1B and TRPV4) encoding for proteins involved in nociception[214]; maternal probiotic intervention also increased the gene expression of ileal mucin-2 (MUC2)[230], indicating that the mechanism of action of probiotics is deeper and more complex than previously thought.
PREBIOTICS
The Food and Agriculture Organization of the United Nations (FAO) defines prebiotic as “a nonviable food component that confers a health benefit on the host associated with modulation of the microbiota”[251]. They represent an alternative strategy for reprogramming the gut microbiota by providing regular doses of a specific substrate engineered to be readily metabolized by specific desirable bacteria, thereby encouraging their growth in contrast to the development of harmful microbial species. Also known as “functional” foods[252], they escape absorption in the small bowel and enter the colon where they provide for nutrients for specific bacteria, mainly bifidobacteria and lactobacilli. Several prebiotics belong to the group of non-digestible carbohydrates: monosaccharides (i.e., fructose), disaccharides (i.e., lactose), oligosaccharides [(i.e., fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS)], and polyols (e.g., sorbitol), the so-called Fermentable Oligo- Di- and Mono-saccharides And Polyols or FODMAPs, the prototype of which is inulin, a non-digestible carbohydrate naturally present in a large variety of plants that, when enzymatically hydrolyzed, produces oligofructose[252]. All of them occur in many fruits, cereals, vegetables and in human milk, which contains more than 1000 oligosaccharides[253]. Furthermore, long-chain polyunsaturated fatty acids have been tested as active prebiotics[254].
Experimental data[255,256] and human studies have shown a beneficial effect of prebiotic supplementation in different pathological conditions, including infections[254], allergies[257,258], pregnancy-related disorders[259-260], metabolic disorders[261,262], hepatic and gastrointestinal diseases including cirrhosis[263], IBD[264] and chronic constipation[265]. However, few studies have evaluated the efficacy of prebiotics in IBS and existing results are conflicting. Two trials, using oligofructose in one case[266] and fructo-oligosaccharides in the other[267], failed to confirm any beneficial effects of prebiotics, while two other studies have demonstrated symptoms improvement, with FOS ameliorating symptoms[268] and GOS lowering flatulence and bloating while also improving the anxiety score[83]. The clinical response may be dependent on the prebiotic type and dose, taking into account that low doses may be ineffective and high doses may stimulate colonic gas production. Indeed, some reports indicate that prebiotics may exacerbate IBS symptoms. Thus, lactulose and bran, that are successfully used for constipation, tend to produce substantial amounts of gas and abdominal pain and may aggravate symptoms of IBS[269-271]. Although fructose and sorbitol mixtures produce a modest increase in stool weight, they often increase flatulence and bloating in both healthy volunteers and IBS patients[272,273]. Ong et al[274] have recently shown that a diet rich in FODMAPs increases abdominal pain, bloating and flatulence in IBS patients, focusing the attention on the necessity of restricting fermentable carbohydrates in these patients[275-277]. Finally, fructose, sorbitol and a range of poorly absorbed polyhydric alcohols enter the gut where they retain a substantial amount of water causing unwanted diarrhea. However, low FODMAP diet recommended for reduction of IBS symptoms will have adverse effects on colonic luminal microenvironment and microbiota in healthy populations, reducing the absolute and relative bacterial abundance and diversity[278].
SYNBIOTICS
An important safety feature of probiotics is that they have a short lifespan within the gut and need repeated doses to keep a constant level. Indeed, an alternative strategy to maintain constant levels of beneficial bacteria within the gut is the contemporary administration of probiotic strains and prebiotics, the so-called synbiotic therapy. Few open label trials[64,279] and PCT studies have evaluated the efficacy of synbiotics in both functional pain[280] and IBS, all demonstrating a superiority of the probiotic/prebiotic combinations vs placebo[281-283]. Although the concept of combining prebiotic with probiotic is theoretically attractive, the limited experience and the poor quality of published studies do not allow any recommendation on their use in IBS.
ANTIBIOTICS
The alteration in the gut microbiota composition is increasingly considered to be a relevant pathogenetic factor in IBS, leading to the use of antibiotics as a treatment for this multifactorial syndrome. In addition, a SIBO may be relevant to at least a subset of IBS patients, thus justifying an antimicrobial approach. However, it should be considered that lactose hydrogen breath test, routinely used as a surrogate marker for SIBO, has a low sensitivity and specificity[284-286]. The first antibiotic investigated in a clinical trial was neomycin, which is not adsorbed in the gut. Pimentel et al[285] have demonstrated that neomycin caused a 50% improvement in global IBS symptoms compared to placebo-treated patients, but also induced a rapid clinical resistance. Other broad-spectrum antibiotics reducing bacterial overgrowth have been challenged in the treatment of IBS, including tetracycline, amoxicillin clavulanate, metronidazole and fluoroquinolones such as norfloxacin[287,288]; however, these drugs are absorbable causing local and systemic side effects and their use in IBS patients is not recommended. The semisynthetic, antibacterial, rifamycin-derivative rifaximin has virtually no systemic absorption, is effective in improving IBS symptoms with low bacterial resistance profile and has a favorable side-effects profile.Since the first trial published almost a decade ago[289], several trials have shown that rifaximin is an effective treatment for IBS. The strongest evidence comes from two large-scale, multicenter studies: TARGET 1 and TARGET 2[290], which included a total of 1260 patient affected by IBS without constipation. In these phase 3, double-blind, placebo-controlled trials, significantly more patients in the rifaximin group than in the placebo group had adequate relief of global IBS symptoms during the first 4 wk of treatment (40.7% vs 31.7% respectively in the two studies combined). In addiction, more patients in the rifaximin group than in the placebo group had adequate relief of bloating, abdominal pain and stool consistency (40.2% vs 30.2% respectively in the two studies combined). The incidence of adverse events was similar. In general, available metanalysis tend to show a beneficial profile of rifaximin vs placebo on global IBS symptoms and bloating[291]. Furthermore, a pooled analysis by Schoenfeld et al[292] has confirmed the safety and tolerability profile of rifaximin in comparison with placebo, with similar incidence of drug-related adverse events. In conclusion, rifaximin is the only antibiotic which can play a role in the treatment of IBS, but limitations should be considered: (1) it is effective in less than 50% of patients; (2) large phase III study has included only patients without constipation, indeed studies on C-IBS are needed; (3) its long-term effects have not been investigated; and (4) the effect of rifaximin on gut microbiota composition is essentially unknown.
FECAL TRANSPLANTATION IN IBS: A NEW LIFE FOR AN OLD TREATMENT?
Fecal microbiota transplant (FMT) is an interesting strategy proposed for a large spectrum of diseases[293], including recurrent Clostridium Difficile infection (CDI) resistant to conventional antibiotic therapies (that represents the main gastroenterological indication for FMT), chronic constipation, IBD, recurrent metabolic syndrome, multiple sclerosis, autism and chronic fatigue syndrome. A systematic review of 325 cases of FMT for CDI suggested a lower success rate for upper gut administration (76%), as compared with colonoscopy (89%) and enema (95%) administration[294]. Moreover, a large case series of CDI patients has showed rapid response and a cure rate of nearly 90%, without significant adverse event rate[295]. Colonic infusion of donor human intestinal flora can reverse UC in a small series of selected patients, determining endoscopic and histological remission[296]. This report was followed by a number of small studies of FMT in children and adults with UC, CD, and pouchitis with mixed results[297-302]. It has been recently demonstrated that sensitivity to colonic distension of IBS patients can be transferred to rats by the fecal microbiota transplant[303].
From a pure technical point of view, FMT is an easy technique that requires a healthy donor (usually a patient’s family member or an anonymous donor), with no risk factors for transmissible diseases or any issues that may alter the cellular composition, particularly prior antibiotic use. The FMT Working Group have recently published guidelines for FMT donor selection criteria and screening tests[304]. The steps for an adequate treatment of the fecal material include dilution in saline solutions, homogenization and filtration, while the route of administration can be naso-duodenal, transcolonoscopic or enema based. Naso-duodenal administration is the method patients favour the lest. Colonoscopy allows direct assessment of the colonic mucosa for the assessment of disease severity and exclusion of coexistent pathology. Enema administration is effective, cheap and safe and carries less procedural or institutional admission costs.
To date, only anecdotal data have been reported about the efficacy of FMT for IBS treatment, results being far conclusive. The first case series on FMT in IBS has been published in 1989 by Borody et al[305] demonstrating approximately a 50% of relief in symptoms of IBS after FMT; however, the study also included IBD and CDI patients and did not show any distinction within the results between these different diseases. Since that publication, the lead Author has treated with FMT more than 300 D-IBS patients whose symptoms had failed to respond to conventional therapies[306]. Other preliminary studies indicate a beneficial effect in patients with chronic constipation (i.e., relief in defecation and reduction of bloating)[307], but these data need confirmation in rigorous clinical trials. Pinn et al[308] have reported promising results using FMT in IBS patients with refractory disease: 70% of the patients achieving resolution or improvement of the symptoms. Taken together, these data indicate that FMT is a promising treatment option for serious and recurrent CDI[309,310]. However, many questions should be answered before it may be recommended as routine standard treatment of IBS[311] and randomized, double-blinded, placebo-controlled trials are required.
CONCLUSION
Evidence regarding the manipulation of gut microbiota composition as an effective cure for IBS is increasing and, to date, probiotic supplementation and antimicrobial therapy with not absorbable antibiotics are promising treatment. However, all meta-analysis point out to the weakness of the majority of the studies and recommend additional RCT trials to confirm the positive findings reported by small studies. Specifically, additional information on type of probiotic, doses, side effects and time of administration are required, as well as data on patient subtypes. Prebiotics are often burdened by unwanted side effects and synbiotics lack of sufficient numbers of clinical trials on their efficacy and safeness. FMT might be a reasonable option for treating IBS, as it is an inexpensive and easy treatment, but standardized controlled trials are necessary to ascertain which patients are eligible, the most effective regiment as well as the most acceptable method of administration of the donor’s microbiota. For these therapeutic options, a careful selection of patients, a close monitoring of clinical data and side effects and a long-term follow-up are necessary, as well as more information on modification of host microbiota composition.
Footnotes
Conflict-of-interest statement: The Authors declare no conflict of interest
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 1, 2015
First decision: October 14, 2015
Article in press: December 30, 2015
P- Reviewer: Bellini M, Engin AB S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Simrén M, Svedlund J, Posserud I, Björnsson ES, Abrahamsson H. Health-related quality of life in patients attending a gastroenterology outpatient clinic: functional disorders versus organic diseases. Clin Gastroenterol Hepatol. 2006;4:187–195. doi: 10.1016/s1542-3565(05)00981-x. [DOI] [PubMed] [Google Scholar]
- 5.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 6.Distrutti E, Salvioli B, Azpiroz F, Malagelada JR. Rectal function and bowel habit in irritable bowel syndrome. Am J Gastroenterol. 2004;99:131–137. doi: 10.1046/j.1572-0241.2003.04012.x. [DOI] [PubMed] [Google Scholar]
- 7.De Giorgio R, Barbara G. Is irritable bowel syndrome an inflammatory disorder? Curr Gastroenterol Rep. 2008;10:385–390. doi: 10.1007/s11894-008-0073-0. [DOI] [PubMed] [Google Scholar]
- 8.Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41–48. doi: 10.1016/j.bbi.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 10.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 11.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 13.Murgas Torrazza R, Neu J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol. 2011;31 Suppl 1:S29–S34. doi: 10.1038/jp.2010.172. [DOI] [PubMed] [Google Scholar]
- 14.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 15.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 16.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P, Rizzello F, Campieri M, Brigidi P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol. 2008;125:286–292. doi: 10.1016/j.ijfoodmicro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 21.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antunes LC, Han J, Ferreira RB, Lolić P, Borchers CH, Finlay BB. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother. 2011;55:1494–1503. doi: 10.1128/AAC.01664-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 25.Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, Fischbach MA, Sonnenburg JL. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013;7:1933–1943. doi: 10.1038/ismej.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, Benno Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci. 2013;7:9. doi: 10.3389/fnsys.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 28.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33:574–581. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- 29.Forsythe P, Kunze WA. Voices from within: gut microbes and the CNS. Cell Mol Life Sci. 2013;70:55–69. doi: 10.1007/s00018-012-1028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 31.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 32.Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 33.Medani M, Collins D, Docherty NG, Baird AW, O’Connell PR, Winter DC. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm Bowel Dis. 2011;17:1620–1625. doi: 10.1002/ibd.21528. [DOI] [PubMed] [Google Scholar]
- 34.Schemann M, Grundy D. Role of hydrogen sulfide in visceral nociception. Gut. 2009;58:744–747. doi: 10.1136/gut.2008.167858. [DOI] [PubMed] [Google Scholar]
- 35.Distrutti E. Hydrogen sulphide and pain. Inflamm Allergy Drug Targets. 2011;10:123–132. doi: 10.2174/187152811794776240. [DOI] [PubMed] [Google Scholar]
- 36.Distrutti E, Cipriani S, Renga B, Mencarelli A, Migliorati M, Cianetti S, Fiorucci S. Hydrogen sulphide induces micro opioid receptor-dependent analgesia in a rodent model of visceral pain. Mol Pain. 2010;6:36. doi: 10.1186/1744-8069-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- 38.Dlugosz A, Winckler B, Lundin E, Zakikhany K, Sandström G, Ye W, Engstrand L, Lindberg G. No difference in small bowel microbiota between patients with irritable bowel syndrome and healthy controls. Sci Rep. 2015;5:8508. doi: 10.1038/srep08508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–194. [PubMed] [Google Scholar]
- 40.Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802–1805. doi: 10.3748/wjg.v10.i12.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 42.Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213–222. doi: 10.1016/j.femsim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maukonen J, Satokari R, Mättö J, Söderlund H, Mattila-Sandholm T, Saarela M. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol. 2006;55:625–633. doi: 10.1099/jmm.0.46134-0. [DOI] [PubMed] [Google Scholar]
- 45.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Lyra A, Rinttilä T, Nikkilä J, Krogius-Kurikka L, Kajander K, Malinen E, Mättö J, Mäkelä L, Palva A. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J Gastroenterol. 2009;15:5936–5945. doi: 10.3748/wjg.15.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerckhoffs AP, Samsom M, van der Rest ME, de Vogel J, Knol J, Ben-Amor K, Akkermans LM. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol. 2009;15:2887–2892. doi: 10.3748/wjg.15.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carroll IM, Ringel-Kulka T, Siddle JP, Klaenhammer TR, Ringel Y. Characterization of the fecal microbiota using high-throughput sequencing reveals a stable microbial community during storage. PLoS One. 2012;7:e46953. doi: 10.1371/journal.pone.0046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salonen A, de Vos WM, Palva A. Gastrointestinal microbiota in irritable bowel syndrome: present state and perspectives. Microbiology. 2010;156:3205–3215. doi: 10.1099/mic.0.043257-0. [DOI] [PubMed] [Google Scholar]
- 51.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519, e114-e115. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 52.Carroll IM, Chang YH, Park J, Sartor RB, Ringel Y. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog. 2010;2:19. doi: 10.1186/1757-4749-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Codling C, O’Mahony L, Shanahan F, Quigley EM, Marchesi JR. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci. 2010;55:392–397. doi: 10.1007/s10620-009-0934-x. [DOI] [PubMed] [Google Scholar]
- 54.Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol. 2011;60:817–827. doi: 10.1099/jmm.0.028126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerckhoffs AP, Ben-Amor K, Samsom M, van der Rest ME, de Vogel J, Knol J, Akkermans LM. Molecular analysis of faecal and duodenal samples reveals significantly higher prevalence and numbers of Pseudomonas aeruginosa in irritable bowel syndrome. J Med Microbiol. 2011;60:236–245. doi: 10.1099/jmm.0.022848-0. [DOI] [PubMed] [Google Scholar]
- 56.Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 57.Carroll IM, Ringel-Kulka T, Keku TO, Chang YH, Packey CD, Sartor RB, Ringel Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799–G807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–530, e248. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parkes GC, Rayment NB, Hudspith BN, Petrovska L, Lomer MC, Brostoff J, Whelan K, Sanderson JD. Distinct microbial populations exist in the mucosa-associated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:31–39. doi: 10.1111/j.1365-2982.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- 60.Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 61.Chassard C, Dapoigny M, Scott KP, Crouzet L, Del’homme C, Marquet P, Martin JC, Pickering G, Ardid D, Eschalier A, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828–838. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- 62.König J, Brummer RJ. Alteration of the intestinal microbiota as a cause of and a potential therapeutic option in irritable bowel syndrome. Benef Microbes. 2014;5:247–261. doi: 10.3920/BM2013.0033. [DOI] [PubMed] [Google Scholar]
- 63.Sundin J, Rangel I, Fuentes S, Heikamp-de Jong I, Hultgren-Hörnquist E, de Vos WM, Brummer RJ. Altered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment Pharmacol Ther. 2015;41:342–351. doi: 10.1111/apt.13055. [DOI] [PubMed] [Google Scholar]
- 64.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 67.Guilarte M, Santos J, de Torres I, Alonso C, Vicario M, Ramos L, Martínez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O’Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 69.Weston AP, Biddle WL, Bhatia PS, Miner PB. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38:1590–1595. doi: 10.1007/BF01303164. [DOI] [PubMed] [Google Scholar]
- 70.He Q, Wang L, Wang F, Li Q. Role of gut microbiota in a zebrafish model with chemically induced enterocolitis involving toll-like receptor signaling pathways. Zebrafish. 2014;11:255–264. doi: 10.1089/zeb.2013.0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walk ST, Blum AM, Ewing SA, Weinstock JV, Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuehbacher T, Rehman A, Lepage P, Hellmig S, Fölsch UR, Schreiber S, Ott SJ. Intestinal TM7 bacterial phylogenies in active inflammatory bowel disease. J Med Microbiol. 2008;57:1569–1576. doi: 10.1099/jmm.0.47719-0. [DOI] [PubMed] [Google Scholar]
- 73.Reshef L, Kovacs A, Ofer A, Yahav L, Maharshak N, Keren N, Konikoff FM, Tulchinsky H, Gophna U, Dotan I. Pouch Inflammation Is Associated With a Decrease in Specific Bacterial Taxa. Gastroenterology. 2015;149:718–727. doi: 10.1053/j.gastro.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 74.Machiels K, Sabino J, Vandermosten L, Joossens M, Arijs I, de Bruyn M, Eeckhaut V, Van Assche G, Ferrante M, Verhaegen J, et al. Specific members of the predominant gut microbiota predict pouchitis following colectomy and IPAA in UC. Gut. 2015:Epub ahead of print. doi: 10.1136/gutjnl-2015-309398. [DOI] [PubMed] [Google Scholar]
- 75.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535–544. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- 76.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 77.Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, Zaitoun A, Palva A, Spiller RC, de Vos WM. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–1745. doi: 10.1136/gutjnl-2013-305994. [DOI] [PubMed] [Google Scholar]
- 78.Villarreal AA, Aberger FJ, Benrud R, Gundrum JD. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ. 2012;111:17–20. [PubMed] [Google Scholar]
- 79.Rajilić-Stojanović M, Jonkers DM, Salonen A, Hanevik K, Raes J, Jalanka J, de Vos WM, Manichanh C, Golic N, Enck P, et al. Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena? Am J Gastroenterol. 2015;110:278–287. doi: 10.1038/ajg.2014.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401, 1401.e1-e4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651–660. doi: 10.1007/s00406-014-0502-z. [DOI] [PubMed] [Google Scholar]
- 83.Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508–518. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- 84.Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987;92:1885–1893. doi: 10.1016/0016-5085(87)90620-2. [DOI] [PubMed] [Google Scholar]
- 85.Suttor VP, Prott GM, Hansen RD, Kellow JE, Malcolm A. Evidence for pelvic floor dyssynergia in patients with irritable bowel syndrome. Dis Colon Rectum. 2010;53:156–160. doi: 10.1007/DCR.0b013e3181c188e8. [DOI] [PubMed] [Google Scholar]
- 86.Serra J, Villoria A, Azpiroz F, Lobo B, Santos J, Accarino A, Malagelada JR. Impaired intestinal gas propulsion in manometrically proven dysmotility and in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:401–406, e91-e92. doi: 10.1111/j.1365-2982.2009.01447.x. [DOI] [PubMed] [Google Scholar]
- 87.Cogliandro RF, Antonucci A, De Giorgio R, Barbara G, Cremon C, Cogliandro L, Frisoni C, Pezzilli R, Morselli-Labate AM, Corinaldesi R, et al. Patient-reported outcomes and gut dysmotility in functional gastrointestinal disorders. Neurogastroenterol Motil. 2011;23:1084–1091. doi: 10.1111/j.1365-2982.2011.01783.x. [DOI] [PubMed] [Google Scholar]
- 88.Lindfors P, Törnblom H, Sadik R, Björnsson ES, Abrahamsson H, Simrén M. Effects on gastrointestinal transit and antroduodenojejunal manometry after gut-directed hypnotherapy in irritable bowel syndrome (IBS) Scand J Gastroenterol. 2012;47:1480–1487. doi: 10.3109/00365521.2012.733955. [DOI] [PubMed] [Google Scholar]
- 89.Staumont G, Delvaux M, Fioramonti J, Berry P, Bueno L, Frexinos J. Differences between jejunal myoelectric activity after a meal and during phase 2 of migrating motor complexes in healthy humans. Dig Dis Sci. 1992;37:1554–1561. doi: 10.1007/BF01296502. [DOI] [PubMed] [Google Scholar]
- 90.Quigley EM, Donovan JP, Lane MJ, Gallagher TF. Antroduodenal manometry. Usefulness and limitations as an outpatient study. Dig Dis Sci. 1992;37:20–28. doi: 10.1007/BF01308337. [DOI] [PubMed] [Google Scholar]
- 91.Kellow JE, Langeluddecke PM, Eckersley GM, Jones MP, Tennant CC. Effects of acute psychologic stress on small-intestinal motility in health and the irritable bowel syndrome. Scand J Gastroenterol. 1992;27:53–58. doi: 10.3109/00365529209011167. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt T, Hackelsberger N, Widmer R, Meisel C, Pfeiffer A, Kaess H. Ambulatory 24-hour jejunal motility in diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 1996;31:581–589. doi: 10.3109/00365529609009131. [DOI] [PubMed] [Google Scholar]
- 93.Caenepeel P, Janssens J, Vantrappen G, Eyssen H, Coremans G. Interdigestive myoelectric complex in germ-free rats. Dig Dis Sci. 1989;34:1180–1184. doi: 10.1007/BF01537265. [DOI] [PubMed] [Google Scholar]
- 94.Collins S, Verdu E, Denou E, Bercik P. The role of pathogenic microbes and commensal bacteria in irritable bowel syndrome. Dig Dis. 2009;27 Suppl 1:85–89. doi: 10.1159/000268126. [DOI] [PubMed] [Google Scholar]
- 95.Husebye E, Hellström PM, Midtvedt T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig Dis Sci. 1994;39:946–956. doi: 10.1007/BF02087542. [DOI] [PubMed] [Google Scholar]
- 96.Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G368–G380. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- 97.Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol. 1999;473:11–30. doi: 10.1007/978-1-4615-4143-1_2. [DOI] [PubMed] [Google Scholar]
- 98.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 99.Bär F, Von Koschitzky H, Roblick U, Bruch HP, Schulze L, Sonnenborn U, Böttner M, Wedel T. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol Motil. 2009;21:559–566, e16-e17. doi: 10.1111/j.1365-2982.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- 100.Guarino MP, Sessa R, Altomare A, Cocca S, Di Pietro M, Carotti S, Schiavoni G, Alloni R, Emerenziani S, Morini S, et al. Human colonic myogenic dysfunction induced by mucosal lipopolysaccharide translocation and oxidative stress. Dig Liver Dis. 2013;45:1011–1016. doi: 10.1016/j.dld.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Guarino MP, Altomare A, Stasi E, Marignani M, Severi C, Alloni R, Dicuonzo G, Morelli L, Coppola R, Cicala M. Effect of acute mucosal exposure to Lactobacillus rhamnosus GG on human colonic smooth muscle cells. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 2:S185–S190. doi: 10.1097/MCG.0b013e31817e1cac. [DOI] [PubMed] [Google Scholar]
- 102.Ammoscato F, Scirocco A, Altomare A, Matarrese P, Petitta C, Ascione B, Caronna R, Guarino M, Marignani M, Cicala M, et al. Lactobacillus rhamnosus protects human colonic muscle from pathogen lipopolysaccharide-induced damage. Neurogastroenterol Motil. 2013;25:984–e777. doi: 10.1111/nmo.12232. [DOI] [PubMed] [Google Scholar]
- 103.Kellow JE, Azpiroz F, Delvaux M, Gebhart GF, Mertz HR, Quigley EM, Smout AJ. Applied principles of neurogastroenterology: physiology/motility sensation. Gastroenterology. 2006;130:1412–1420. doi: 10.1053/j.gastro.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 104.Mayer EA. Emerging disease model for functional gastrointestinal disorders. Am J Med. 1999;107:12S–19S. doi: 10.1016/s0002-9343(99)00277-6. [DOI] [PubMed] [Google Scholar]
- 105.Grundy D, Al-Chaer ED, Aziz Q, Collins SM, Ke M, Taché Y, Wood JD. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130:1391–1411. doi: 10.1053/j.gastro.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 106.Feng B, La JH, Schwartz ES, Gebhart GF. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1085–G1098. doi: 10.1152/ajpgi.00542.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ritchie JA, Ardran GM, Truelove SC. Observations on experimentally induced colonic pain. Gut. 1972;13:841. [PubMed] [Google Scholar]
- 108.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 109.Prior A, Maxton DG, Whorwell PJ. Anorectal manometry in irritable bowel syndrome: differences between diarrhoea and constipation predominant subjects. Gut. 1990;31:458–462. doi: 10.1136/gut.31.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zar S, Benson MJ, Kumar D. Rectal afferent hypersensitivity and compliance in irritable bowel syndrome: differences between diarrhoea-predominant and constipation-predominant subgroups. Eur J Gastroenterol Hepatol. 2006;18:151–158. doi: 10.1097/00042737-200602000-00007. [DOI] [PubMed] [Google Scholar]
- 111.Distrutti E, Hauer SK, Fiorucci S, Pensi MO, Morelli A. Intraduodenal lipids increase perception of rectal distension in IBS patients. Gastroenterology. 2000;118:A138 (abs). [Google Scholar]
- 112.Caldarella MP, Milano A, Laterza F, Sacco F, Balatsinou C, Lapenna D, Pierdomenico SD, Cuccurullo F, Neri M. Visceral sensitivity and symptoms in patients with constipation- or diarrhea-predominant irritable bowel syndrome (IBS): effect of a low-fat intraduodenal infusion. Am J Gastroenterol. 2005;100:383–389. doi: 10.1111/j.1572-0241.2005.40100.x. [DOI] [PubMed] [Google Scholar]
- 113.Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 114.Hertig VL, Cain KC, Jarrett ME, Burr RL, Heitkemper MM. Daily stress and gastrointestinal symptoms in women with irritable bowel syndrome. Nurs Res. 2007;56:399–406. doi: 10.1097/01.NNR.0000299855.60053.88. [DOI] [PubMed] [Google Scholar]
- 115.Aguilera M, Cerdà-Cuéllar M, Martínez V. Antibiotic-induced dysbiosis alters host-bacterial interactions and leads to colonic sensory and motor changes in mice. Gut Microbes. 2015;6:10–23. doi: 10.4161/19490976.2014.990790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aguilera M, Vergara P, Martínez V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neurogastroenterol Motil. 2013;25:e515–e529. doi: 10.1111/nmo.12154. [DOI] [PubMed] [Google Scholar]
- 117.Theodorou V, Ait Belgnaoui A, Agostini S, Eutamene H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes. 2014;5:430–436. doi: 10.4161/gmic.29796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mao YK, Kasper DL, Wang B, Forsythe P, Bienenstock J, Kunze WA. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat Commun. 2013;4:1465. doi: 10.1038/ncomms2478. [DOI] [PubMed] [Google Scholar]
- 119.Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Wang Y, Tougas G, Bienenstock J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55:191–196. doi: 10.1136/gut.2005.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J, Kunze W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2013;304:G211–G220. doi: 10.1152/ajpgi.00128.2012. [DOI] [PubMed] [Google Scholar]
- 121.Perez-Burgos A, Wang L, McVey Neufeld KA, Mao YK, Ahmadzai M, Janssen LJ, Stanisz AM, Bienenstock J, Kunze WA. The TRPV1 channel in rodents is a major target for antinociceptive effect of the probiotic Lactobacillus reuteri DSM 17938. J Physiol. 2015;593:3943–3957. doi: 10.1113/JP270229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 123.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 124.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 126.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–264, e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 128.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 129.Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61:1284–1290. doi: 10.1136/gutjnl-2011-300474. [DOI] [PubMed] [Google Scholar]
- 130.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jenkins DG, Quigley BM. The y-intercept of the critical power function as a measure of anaerobic work capacity. Ergonomics. 1991;34:13–22. doi: 10.1080/00140139108967284. [DOI] [PubMed] [Google Scholar]
- 133.Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5:1465–1483. doi: 10.1002/emmm.201201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McClure R, Massari P. TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Front Immunol. 2014;5:386. doi: 10.3389/fimmu.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perez-Chanona E, Mühlbauer M, Jobin C. The microbiota protects against ischemia/reperfusion-induced intestinal injury through nucleotide-binding oligomerization domain-containing protein 2 (NOD2) signaling. Am J Pathol. 2014;184:2965–2975. doi: 10.1016/j.ajpath.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Davies JM, Abreu MT. Host-microbe interactions in the small bowel. Curr Opin Gastroenterol. 2015;31:118–123. doi: 10.1097/MOG.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Murata K, Tomosada Y, Villena J, Chiba E, Shimazu T, Aso H, Iwabuchi N, Xiao JZ, Saito T, Kitazawa H. Bifidobacterium breve MCC-117 Induces Tolerance in Porcine Intestinal Epithelial Cells: Study of the Mechanisms Involved in the Immunoregulatory Effect. Biosci Microbiota Food Health. 2014;33:1–10. doi: 10.12938/bmfh.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014;142:24–31. doi: 10.1111/imm.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mu C, Yang Y, Zhu W. Crosstalk Between The Immune Receptors and Gut Microbiota. Curr Protein Pept Sci. 2015;16:622–631. doi: 10.2174/1389203716666150630134356. [DOI] [PubMed] [Google Scholar]
- 140.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 141.Food and Agriculture Organization/World Health Organization. Evaluation of health and nutritional properties of probiotics in food, including powder milk with the live lactic acid bacteria. Report of a Joint FAO/WHO Expert Consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Available from: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf.
- 142.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 143.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–1147. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 144.Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 145.Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, Kim YH, Kim JJ, Rhee JC, Rhee PL. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53:2714–2718. doi: 10.1007/s10620-007-0196-4. [DOI] [PubMed] [Google Scholar]
- 146.Long ZR, Yu CH, Yang Y, Wang HN, Chi XX. [Clinical observation on acupuncture combined with microorganism pharmaceutical preparations for treatment of irritable bowel syndrome of constipation type] Zhongguo Zhenjiu. 2006;26:403–405. [PubMed] [Google Scholar]
- 147.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 148.Gade J, Thorn P. Paraghurt for patients with irritable bowel syndrome. A controlled clinical investigation from general practice. Scand J Prim Health Care. 1989;7:23–26. doi: 10.3109/02813438909103666. [DOI] [PubMed] [Google Scholar]
- 149.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, Zinsmeister AR. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 150.Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–394. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 151.Lorenzo-Zúñiga V, Llop E, Suárez C, Alvarez B, Abreu L, Espadaler J, Serra J. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J Gastroenterol. 2014;20:8709–8716. doi: 10.3748/wjg.v20.i26.8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147–152. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 153.Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome--focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35:403–413. doi: 10.1111/j.1365-2036.2011.04965.x. [DOI] [PubMed] [Google Scholar]
- 154.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 155.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 156.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 157.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475–486. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 158.Andriulli A, Neri M, Loguercio C, Terreni N, Merla A, Cardarella MP, Federico A, Chilovi F, Milandri GL, De Bona M, et al. Clinical trial on the efficacy of a new symbiotic formulation, Flortec, in patients with irritable bowel syndrome: a multicenter, randomized study. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 2:S218–S223. doi: 10.1097/MCG.0b013e31817fadd6. [DOI] [PubMed] [Google Scholar]
- 159.Lönnermark E, Friman V, Lappas G, Sandberg T, Berggren A, Adlerberth I. Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J Clin Gastroenterol. 2010;44:106–112. doi: 10.1097/MCG.0b013e3181b2683f. [DOI] [PubMed] [Google Scholar]
- 160.Gawrońska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25:177–184. doi: 10.1111/j.1365-2036.2006.03175.x. [DOI] [PubMed] [Google Scholar]
- 161.Francavilla R, Miniello V, Magistà AM, De Canio A, Bucci N, Gagliardi F, Lionetti E, Castellaneta S, Polimeno L, Peccarisi L, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010;126:e1445–e1452. doi: 10.1542/peds.2010-0467. [DOI] [PubMed] [Google Scholar]
- 162.Bauserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147:197–201. doi: 10.1016/j.jpeds.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 163.Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33:1302–1310. doi: 10.1111/j.1365-2036.2011.04665.x. [DOI] [PubMed] [Google Scholar]
- 164.O’Sullivan MA, O’Morain CA. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Dig Liver Dis. 2000;32:294–301. doi: 10.1016/s1590-8658(00)80021-3. [DOI] [PubMed] [Google Scholar]
- 165.Stevenson C, Blaauw R, Fredericks E, Visser J, Roux S. Randomized clinical trial: effect of Lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome. Nutrition. 2014;30:1151–1157. doi: 10.1016/j.nut.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 166.Enck P, Zimmermann K, Menke G, Müller-Lissner S, Martens U, Klosterhalfen S. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome--a randomized controlled trial with primary care physicians. Neurogastroenterol Motil. 2008;20:1103–1109. doi: 10.1111/j.1365-2982.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 167.Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Sibal A, Romano C, Canani RB, Lionetti P, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 168.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 169.Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, Corfe BM. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- 170.Simrén M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–227. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 171.Lyra A, Krogius-Kurikka L, Nikkilä J, Malinen E, Kajander K, Kurikka K, Korpela R, Palva A. Effect of a multispecies probiotic supplement on quantity of irritable bowel syndrome-related intestinal microbial phylotypes. BMC Gastroenterol. 2010;10:110. doi: 10.1186/1471-230X-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033–1049; quiz 1050. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 173.Hungin AP, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Rubin G, Winchester C, de Wit N. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Aliment Pharmacol Ther. 2013;38:864–886. doi: 10.1111/apt.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–227. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 175.Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 176.Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.-coli preparation (DSM17252) compared to placebo. Z Gastroenterol. 2009;47:209–214. doi: 10.1055/s-2008-1027702. [DOI] [PubMed] [Google Scholar]
- 177.Søndergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46:663–672. doi: 10.3109/00365521.2011.565066. [DOI] [PubMed] [Google Scholar]
- 178.Sen S, Mullan MM, Parker TJ, Woolner JT, Tarry SA, Hunter JO. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47:2615–2620. doi: 10.1023/a:1020597001460. [DOI] [PubMed] [Google Scholar]
- 179.Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 180.Guyonnet D, Schlumberger A, Mhamdi L, Jakob S, Chassany O. Fermented milk containing Bifidobacterium lactis DN-173 010 improves gastrointestinal well-being and digestive symptoms in women reporting minor digestive symptoms: a randomised, double-blind, parallel, controlled study. Br J Nutr. 2009;102:1654–1662. doi: 10.1017/S0007114509990882. [DOI] [PubMed] [Google Scholar]
- 181.Ojetti V, Gigante G, Gabrielli M, Ainora ME, Mannocci A, Lauritano EC, Gasbarrini G, Gasbarrini A. The effect of oral supplementation with Lactobacillus reuteri or tilactase in lactose intolerant patients: randomized trial. Eur Rev Med Pharmacol Sci. 2010;14:163–170. [PubMed] [Google Scholar]
- 182.Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010;10:16. doi: 10.1186/1471-230X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.S Kim L, Hilli L, Orlowski J, Kupperman JL, Baral M, F Waters R. Efficacy of probiotics and nutrients in functional gastrointestinal disorders: a preliminary clinical trial. Dig Dis Sci. 2006;51:2134–2144. doi: 10.1007/s10620-006-9297-8. [DOI] [PubMed] [Google Scholar]
- 184.Hong KS, Kang HW, Im JP, Ji GE, Kim SG, Jung HC, Song IS, Kim JS. Effect of probiotics on symptoms in korean adults with irritable bowel syndrome. Gut Liver. 2009;3:101–107. doi: 10.5009/gnl.2009.3.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Waller PA, Gopal PK, Leyer GJ, Ouwehand AC, Reifer C, Stewart ME, Miller LE. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol. 2011;46:1057–1064. doi: 10.3109/00365521.2011.584895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kalman DS, Schwartz HI, Alvarez P, Feldman S, Pezzullo JC, Krieger DR. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol. 2009;9:85. doi: 10.1186/1471-230X-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pitkala KH, Strandberg TE, Finne Soveri UH, Ouwehand AC, Poussa T, Salminen S. Fermented cereal with specific bifidobacteria normalizes bowel movements in elderly nursing home residents. A randomized, controlled trial. J Nutr Health Aging. 2007;11:305–311. [PubMed] [Google Scholar]
- 188.Larsen CN, Nielsen S, Kaestel P, Brockmann E, Bennedsen M, Christensen HR, Eskesen DC, Jacobsen BL, Michaelsen KF. Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults. Eur J Clin Nutr. 2006;60:1284–1293. doi: 10.1038/sj.ejcn.1602450. [DOI] [PubMed] [Google Scholar]
- 189.Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072–3084. doi: 10.3748/wjg.v21.i10.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kruis W, Chrubasik S, Boehm S, Stange C, Schulze J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int J Colorectal Dis. 2012;27:467–474. doi: 10.1007/s00384-011-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012–4018. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. doi: 10.1186/1471-230X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot JM, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol. 2012;18:2067–2075. doi: 10.3748/wjg.v18.i17.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hun L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med. 2009;121:119–124. doi: 10.3810/pgm.2009.03.1984. [DOI] [PubMed] [Google Scholar]
- 195.Dolin BJ. Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Find Exp Clin Pharmacol. 2009;31:655–659. doi: 10.1358/mf.2009.31.10.1441078. [DOI] [PubMed] [Google Scholar]
- 196.Hong YS, Hong KS, Park MH, Ahn YT, Lee JH, Huh CS, Lee J, Kim IK, Hwang GS, Kim JS. Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:415–425. doi: 10.1097/MCG.0b013e318207f76c. [DOI] [PubMed] [Google Scholar]
- 197.Choi CH, Jo SY, Park HJ, Chang SK, Byeon JS, Myung SJ. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679–683. doi: 10.1097/MCG.0b013e318204593e. [DOI] [PubMed] [Google Scholar]
- 198.Michail S, Kenche H. Gut microbiota is not modified by Randomized, Double-blind, Placebo-controlled Trial of VSL#3 in Diarrhea-predominant Irritable Bowel Syndrome. Probiotics Antimicrob Proteins. 2011;3:1–7. doi: 10.1007/s12602-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol. 2001;152:735–741. doi: 10.1016/s0923-2508(01)01254-2. [DOI] [PubMed] [Google Scholar]
- 200.Gibson GR, Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994;77:412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- 201.Liévin V, Peiffer I, Hudault S, Rochat F, Brassart D, Neeser JR, Servin AL. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut. 2000;47:646–652. doi: 10.1136/gut.47.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mattar AF, Teitelbaum DH, Drongowski RA, Yongyi F, Harmon CM, Coran AG. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int. 2002;18:586–590. doi: 10.1007/s00383-002-0855-7. [DOI] [PubMed] [Google Scholar]
- 203.Kim Y, Kim SH, Whang KY, Kim YJ, Oh S. Inhibition of Escherichia coli O157: H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J Microbiol Biotechnol. 2008;18:1278–1285. [PubMed] [Google Scholar]
- 204.Da Silva S, Robbe-Masselot C, Ait-Belgnaoui A, Mancuso A, Mercade-Loubière M, Salvador-Cartier C, Gillet M, Ferrier L, Loubière P, Dague E, et al. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. Am J Physiol Gastrointest Liver Physiol. 2014;307:G420–G429. doi: 10.1152/ajpgi.00290.2013. [DOI] [PubMed] [Google Scholar]
- 205.Snape WJ, Carlson GM, Cohen S. Colonic myoelectric activity in the irritable bowel syndrome. Gastroenterology. 1976;70:326–330. [PubMed] [Google Scholar]
- 206.Whitehead WE, Engel BT, Schuster MM. Irritable bowel syndrome: physiological and psychological differences between diarrhea-predominant and constipation-predominant patients. Dig Dis Sci. 1980;25:404–413. doi: 10.1007/BF01395503. [DOI] [PubMed] [Google Scholar]
- 207.Ansari R, Sohrabi S, Ghanaie O, Amjadi H, Merat S, Vahedi H, Khatibian M. Comparison of colonic transit time between patients with constipation-predominant irritable bowel syndrome and functional constipation. Indian J Gastroenterol. 2010;29:66–68. doi: 10.1007/s12664-010-0015-2. [DOI] [PubMed] [Google Scholar]
- 208.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–19. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Miller LE, Ouwehand AC. Probiotic supplementation decreases intestinal transit time: meta-analysis of randomized controlled trials. World J Gastroenterol. 2013;19:4718–4725. doi: 10.3748/wjg.v19.i29.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100:1075–1084. doi: 10.3945/ajcn.114.089151. [DOI] [PubMed] [Google Scholar]
- 211.Eskesen D, Jespersen L, Michelsen B, Whorwell PJ, Müller-Lissner S, Morberg CM. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12®, on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638–1646. doi: 10.1017/S0007114515003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Choi CH, Kwon JG, Kim SK, Myung SJ, Park KS, Sohn CI, Rhee PL, Lee KJ, Lee OY, Jung HK, et al. Efficacy of combination therapy with probiotics and mosapride in patients with IBS without diarrhea: a randomized, double-blind, placebo-controlled, multicenter, phase II trial. Neurogastroenterol Motil. 2015;27:705–716. doi: 10.1111/nmo.12544. [DOI] [PubMed] [Google Scholar]
- 213.Johnson AC, Greenwood-Van Meerveld B, McRorie J. Effects of Bifidobacterium infantis 35624 on post-inflammatory visceral hypersensitivity in the rat. Dig Dis Sci. 2011;56:3179–3186. doi: 10.1007/s10620-011-1730-y. [DOI] [PubMed] [Google Scholar]
- 214.Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One. 2013;8:e63893. doi: 10.1371/journal.pone.0063893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609, 609.e1-e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 216.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ludidi S, Jonkers DM, Koning CJ, Kruimel JW, Mulder L, van der Vaart IB, Conchillo JM, Masclee AA. Randomized clinical trial on the effect of a multispecies probiotic on visceroperception in hypersensitive IBS patients. Neurogastroenterol Motil. 2014;26:705–714. doi: 10.1111/nmo.12320. [DOI] [PubMed] [Google Scholar]
- 218.Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 219.Bashashati M, Rezaei N, Bashashati H, Shafieyoun A, Daryani NE, Sharkey KA, Storr M. Cytokine gene polymorphisms are associated with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2012;24:1102–e566. doi: 10.1111/j.1365-2982.2012.01990.x. [DOI] [PubMed] [Google Scholar]
- 220.Hughes PA, Zola H, Penttila IA, Blackshaw LA, Andrews JM, Krumbiegel D. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066–1074. doi: 10.1038/ajg.2013.120. [DOI] [PubMed] [Google Scholar]
- 221.Martín R, Chain F, Miquel S, Natividad JM, Sokol H, Verdu EF, Langella P, Bermúdez-Humarán LG. Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. Hum Vaccin Immunother. 2014;10:1611–1621. doi: 10.4161/hv.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 223.Shi CZ, Chen HQ, Liang Y, Xia Y, Yang YZ, Yang J, Zhang JD, Wang SH, Liu J, Qin HL. Combined probiotic bacteria promotes intestinal epithelial barrier function in interleukin-10-gene-deficient mice. World J Gastroenterol. 2014;20:4636–4647. doi: 10.3748/wjg.v20.i16.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nébot-Vivinus M, Harkat C, Bzioueche H, Cartier C, Plichon-Dainese R, Moussa L, Eutamene H, Pishvaie D, Holowacz S, Seyrig C, et al. Multispecies probiotic protects gut barrier function in experimental models. World J Gastroenterol. 2014;20:6832–6843. doi: 10.3748/wjg.v20.i22.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Laval L, Martin R, Natividad JN, Chain F, Miquel S, Desclée de Maredsous C, Capronnier S, Sokol H, Verdu EF, van Hylckama Vlieg JE, et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes. 2015;6:1–9. doi: 10.4161/19490976.2014.990784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci USA. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rodes L, Khan A, Paul A, Coussa-Charley M, Marinescu D, Tomaro-Duchesneau C, Shao W, Kahouli I, Prakash S. Effect of probiotics Lactobacillus and Bifidobacterium on gut-derived lipopolysaccharides and inflammatory cytokines: an in vitro study using a human colonic microbiota model. J Microbiol Biotechnol. 2013;23:518–526. doi: 10.4014/jmb.1205.05018. [DOI] [PubMed] [Google Scholar]
- 229.Urbanska AM, Paul A, Bhathena J, Prakash S. Suppression of tumorigenesis: modulation of inflammatory cytokines by oral administration of microencapsulated probiotic yogurt formulation. Int J Inflam. 2010;2010:894972. doi: 10.4061/2010/894972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Barouei J, Moussavi M, Hodgson DM. Perinatal maternal probiotic intervention impacts immune responses and ileal mucin gene expression in a rat model of irritable bowel syndrome. Benef Microbes. 2015;6:83–95. doi: 10.3920/BM2013.0011. [DOI] [PubMed] [Google Scholar]
- 231.Spaiser SJ, Culpepper T, Nieves C, Ukhanova M, Mai V, Percival SS, Christman MC, Langkamp-Henken B. Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2 Ingestion Induces a Less Inflammatory Cytokine Profile and a Potentially Beneficial Shift in Gut Microbiota in Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. J Am Coll Nutr. 2015;34:459–469. doi: 10.1080/07315724.2014.983249. [DOI] [PubMed] [Google Scholar]
- 232.Abbas Z, Yakoob J, Jafri W, Ahmad Z, Azam Z, Usman MW, Shamim S, Islam M. Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: a randomized trial. Eur J Gastroenterol Hepatol. 2014;26:630–639. doi: 10.1097/MEG.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 233.Boren J, Lee WN, Bassilian S, Centelles JJ, Lim S, Ahmed S, Boros LG, Cascante M. The stable isotope-based dynamic metabolic profile of butyrate-induced HT29 cell differentiation. J Biol Chem. 2003;278:28395–28402. doi: 10.1074/jbc.M302932200. [DOI] [PubMed] [Google Scholar]
- 234.Ogawa H, Rafiee P, Fisher PJ, Johnson NA, Otterson MF, Binion DG. Butyrate modulates gene and protein expression in human intestinal endothelial cells. Biochem Biophys Res Commun. 2003;309:512–519. doi: 10.1016/j.bbrc.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 235.Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1168–G1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 236.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McIntyre A, Gibson PR, Young GP. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993;34:386–391. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, Venema K, Brummer RJ. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009;21:952–e76. doi: 10.1111/j.1365-2982.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 239.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 240.Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280–286. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 241.Ferrario C, Taverniti V, Milani C, Fiore W, Laureati M, De Noni I, Stuknyte M, Chouaia B, Riso P, Guglielmetti S. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. 2014;144:1787–1796. doi: 10.3945/jn.114.197723. [DOI] [PubMed] [Google Scholar]
- 242.Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004;20:1133–1141. doi: 10.1111/j.1365-2036.2004.02268.x. [DOI] [PubMed] [Google Scholar]
- 243.Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr Res. 2008;64:418–422. doi: 10.1203/PDR.0b013e318181b7fa. [DOI] [PubMed] [Google Scholar]
- 244.Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 245.Kennedy PJ, Cryan JF, Quigley EM, Dinan TG, Clarke G. A sustained hypothalamic-pituitary-adrenal axis response to acute psychosocial stress in irritable bowel syndrome. Psychol Med. 2014;44:3123–3134. doi: 10.1017/S003329171400052X. [DOI] [PubMed] [Google Scholar]
- 246.Barouei J, Moussavi M, Hodgson DM. Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS One. 2012;7:e46051. doi: 10.1371/journal.pone.0046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 249.Distrutti E, O’Reilly JA, McDonald C, Cipriani S, Renga B, Lynch MA, Fiorucci S. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS One. 2014;9:e106503. doi: 10.1371/journal.pone.0106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.O’Sullivan E, Barrett E, Grenham S, Fitzgerald P, Stanton C, Ross RP, Quigley EM, Cryan JF, Dinan TG. BDNF expression in the hippocampus of maternally separated rats: does Bifidobacterium breve 6330 alter BDNF levels? Benef Microbes. 2011;2:199–207. doi: 10.3920/BM2011.0015. [DOI] [PubMed] [Google Scholar]
- 251.Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, Brunser O, Tuohy K. FAO Technical meeting on prebiotics. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 2:S156–S159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- 252.Roberfroid MB. Functional foods: concepts and application to inulin and oligofructose. Br J Nutr. 2002;87 Suppl 2:S139–S143. doi: 10.1079/BJNBJN/2002529. [DOI] [PubMed] [Google Scholar]
- 253.Boehm G, Stahl B, Jelinek J, Knol J, Miniello V, Moro GE. Prebiotic carbohydrates in human milk and formulas. Acta Paediatr Suppl. 2005;94:18–21. doi: 10.1111/j.1651-2227.2005.tb02149.x. [DOI] [PubMed] [Google Scholar]
- 254.Chatchatee P, Lee WS, Carrilho E, Kosuwon P, Simakachorn N, Yavuz Y, Schouten B, Graaff PL, Szajewska H. Effects of growing-up milk supplemented with prebiotics and LCPUFAs on infections in young children. J Pediatr Gastroenterol Nutr. 2014;58:428–437. doi: 10.1097/MPG.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Neyrinck AM, Van Hée VF, Bindels LB, De Backer F, Cani PD, Delzenne NM. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br J Nutr. 2013;109:802–809. doi: 10.1017/S0007114512002206. [DOI] [PubMed] [Google Scholar]
- 256.Yasuda A, Inoue K, Sanbongi C, Yanagisawa R, Ichinose T, Tanaka M, Yoshikawa T, Takano H. Dietary supplementation with fructooligosaccharides attenuates allergic peritonitis in mice. Biochem Biophys Res Commun. 2012;422:546–550. doi: 10.1016/j.bbrc.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 257.Arslanoglu S, Moro GE, Boehm G, Wienz F, Stahl B, Bertino E. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26:49–59. [PubMed] [Google Scholar]
- 258.Hayen SM, Kostadinova AI, Garssen J, Otten HG, Willemsen LE. Novel immunotherapy approaches to food allergy. Curr Opin Allergy Clin Immunol. 2014;14:549–556. doi: 10.1097/ACI.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 259.Dasopoulou M, Briana DD, Boutsikou T, Karakasidou E, Roma E, Costalos C, Malamitsi-Puchner A. Motilin and gastrin secretion and lipid profile in preterm neonates following prebiotics supplementation: a double-blind randomized controlled study. JPEN J Parenter Enteral Nutr. 2015;39:359–368. doi: 10.1177/0148607113510182. [DOI] [PubMed] [Google Scholar]
- 260.Armanian AM, Sadeghnia A, Hoseinzadeh M, Mirlohi M, Feizi A, Salehimehr N, Saee N, Nazari J. The Effect of Neutral Oligosaccharides on Reducing the Incidence of Necrotizing Enterocolitis in Preterm infants: A Randomized Clinical Trial. Int J Prev Med. 2014;5:1387–1395. [PMC free article] [PubMed] [Google Scholar]
- 261.Magrone T, Jirillo E. Childhood obesity: immune response and nutritional approaches. Front Immunol. 2015;6:76. doi: 10.3389/fimmu.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mirmiran P, Bahadoran Z, Azizi F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J Diabetes. 2014;5:267–281. doi: 10.4239/wjd.v5.i3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–447. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 264.Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol. 2014;7:473–487. doi: 10.2147/CEG.S27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Collado Yurrita L, San Mauro Martín I, Ciudad-Cabañas MJ, Calle-Purón ME, Hernández Cabria M. Effectiveness of inulin intake on indicators of chronic constipation; a meta-analysis of controlled randomized clinical trials. Nutr Hosp. 2014;30:244–252. doi: 10.3305/nh.2014.30.2.7565. [DOI] [PubMed] [Google Scholar]
- 266.Hunter JO, Tuffnell Q, Lee AJ. Controlled trial of oligofructose in the management of irritable bowel syndrome. J Nutr. 1999;129:1451S–1453S. doi: 10.1093/jn/129.7.1451S. [DOI] [PubMed] [Google Scholar]
- 267.Olesen M, Gudmand-Hoyer E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am J Clin Nutr. 2000;72:1570–1575. doi: 10.1093/ajcn/72.6.1570. [DOI] [PubMed] [Google Scholar]
- 268.Paineau D, Payen F, Panserieu S, Coulombier G, Sobaszek A, Lartigau I, Brabet M, Galmiche JP, Tripodi D, Sacher-Huvelin S, et al. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br J Nutr. 2008;99:311–318. doi: 10.1017/S000711450779894X. [DOI] [PubMed] [Google Scholar]
- 269.Hasler WL. Lactulose breath testing, bacterial overgrowth, and IBS: just a lot of hot air? Gastroenterology. 2003;125:1898–1900; discussion 1900. doi: 10.1053/j.gastro.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 270.Snook J, Shepherd HA. Bran supplementation in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8:511–514. doi: 10.1111/j.1365-2036.1994.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 271.Badiali D, Corazziari E, Habib FI, Tomei E, Bausano G, Magrini P, Anzini F, Torsoli A. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig Dis Sci. 1995;40:349–356. doi: 10.1007/BF02065421. [DOI] [PubMed] [Google Scholar]
- 272.Nelis GF, Vermeeren MA, Jansen W. Role of fructose-sorbitol malabsorption in the irritable bowel syndrome. Gastroenterology. 1990;99:1016–1020. doi: 10.1016/0016-5085(90)90621-7. [DOI] [PubMed] [Google Scholar]
- 273.Symons P, Jones MP, Kellow JE. Symptom provocation in irritable bowel syndrome. Effects of differing doses of fructose-sorbitol. Scand J Gastroenterol. 1992;27:940–944. doi: 10.3109/00365529209000167. [DOI] [PubMed] [Google Scholar]
- 274.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 275.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 276.Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510–1518. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 277.Tuck CJ, Muir JG, Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: role in irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2014;8:819–834. doi: 10.1586/17474124.2014.917956. [DOI] [PubMed] [Google Scholar]
- 278.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 279.Saneian H, Pourmoghaddas Z, Roohafza H, Gholamrezaei A. Synbiotic containing Bacillus coagulans and fructo-oligosaccharides for functional abdominal pain in children. Gastroenterol Hepatol Bed Bench. 2015;8:56–65. [PMC free article] [PubMed] [Google Scholar]
- 280.Tsuchiya J, Barreto R, Okura R, Kawakita S, Fesce E, Marotta F. Single-blind follow-up study on the effectiveness of a symbiotic preparation in irritable bowel syndrome. Chin J Dig Dis. 2004;5:169–174. doi: 10.1111/j.1443-9573.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 281.Rogha M, Esfahani MZ, Zargarzadeh AH. The efficacy of a synbiotic containing Bacillus Coagulans in treatment of irritable bowel syndrome: a randomized placebo-controlled trial. Gastroenterol Hepatol Bed Bench. 2014;7:156–163. [PMC free article] [PubMed] [Google Scholar]
- 282.Bittner AC, Croffut RM, Stranahan MC. Prescript-Assist probiotic-prebiotic treatment for irritable bowel syndrome: a methodologically oriented, 2-week, randomized, placebo-controlled, double-blind clinical study. Clin Ther. 2005;27:755–761. doi: 10.1016/j.clinthera.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 283.Quigley EM. Therapies aimed at the gut microbiota and inflammation: antibiotics, prebiotics, probiotics, synbiotics, anti-inflammatory therapies. Gastroenterol Clin North Am. 2011;40:207–222. doi: 10.1016/j.gtc.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 284.Riordan SM, McIver CJ, Walker BM, Duncombe VM, Bolin TD, Thomas MC. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795–1803. [PubMed] [Google Scholar]
- 285.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 286.Bratten JR, Spanier J, Jones MP. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol. 2008;103:958–963. doi: 10.1111/j.1572-0241.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 287.Schmulson M, Bielsa MV, Carmona-Sánchez R, Hernández A, López-Colombo A, López Vidal Y, Peláez-Luna M, Remes-Troche JM, Tamayo JL, Valdovinos MA. Microbiota, gastrointestinal infections, low-grade inflammation, and antibiotic therapy in irritable bowel syndrome: an evidence-based review. Rev Gastroenterol Mex. 2014;79:96–134. doi: 10.1016/j.rgmx.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 288.Rezaie A, Nikfar S, Abdollahi M. The place of antibiotics in management of irritable bowel syndrome: a systematic review and meta-analysis. Arch Med Sci. 2010;6:49–55. doi: 10.5114/aoms.2010.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 290.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 291.Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35; quiz 36. doi: 10.1038/ajg.2011.355. [DOI] [PubMed] [Google Scholar]
- 292.Schoenfeld P, Pimentel M, Chang L, Lembo A, Chey WD, Yu J, Paterson C, Bortey E, Forbes WP. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther. 2014;39:1161–1168. doi: 10.1111/apt.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Konturek PC, Haziri D, Brzozowski T, Hess T, Heyman S, Kwiecien S, Konturek SJ, Koziel J. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J Physiol Pharmacol. 2015;66:483–491. [PubMed] [Google Scholar]
- 294.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 295.Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693–702. doi: 10.1097/MCG.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 296.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 297.Zhang FM, Wang HG, Wang M, Cui BT, Fan ZN, Ji GZ. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn’s disease. World J Gastroenterol. 2013;19:7213–7216. doi: 10.3748/wjg.v19.i41.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Suskind DL, Brittnacher MJ, Wahbeh G, Shaffer ML, Hayden HS, Qin X, Singh N, Damman CJ, Hager KR, Nielson H, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis. 2015;21:556–563. doi: 10.1097/MIB.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Suskind DL, Singh N, Nielson H, Wahbeh G. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2015;60:27–29. doi: 10.1097/MPG.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 300.Vermeire S, Joossens M, Verbeke K, Wang J, Machiels K, Sabino J, Ferrante M, Van Assche G, Rutgeerts P, Raes J. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J Crohns Colitis. 2015:Epub ahead of print. doi: 10.1093/ecco-jcc/jjv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H, Cloney D, Kugathasan S. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 302.Landy J, Walker AW, Li JV, Al-Hassi HO, Ronde E, English NR, Mann ER, Bernardo D, McLaughlin SD, Parkhill J, et al. Variable alterations of the microbiota, without metabolic or immunological change, following faecal microbiota transplantation in patients with chronic pouchitis. Sci Rep. 2015;5:12955. doi: 10.1038/srep12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Crouzet L, Gaultier E, Del’Homme C, Cartier C, Delmas E, Dapoigny M, Fioramonti J, Bernalier-Donadille A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil. 2013;25:e272–e282. doi: 10.1111/nmo.12103. [DOI] [PubMed] [Google Scholar]
- 304.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 306.Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep. 2013;15:337. doi: 10.1007/s11894-013-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Andrews PJ, Borody TJ. “Putting back the bugs”: bacterial treatment relieves chronic constipation and symptoms of irritable bowel syndrome. Med J Aust. 1993;159:633–634. doi: 10.5694/j.1326-5377.1993.tb138063.x. [DOI] [PubMed] [Google Scholar]
- 308.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am J Gastroenterol. 2014;109:1831–1832. doi: 10.1038/ajg.2014.295. [DOI] [PubMed] [Google Scholar]
- 309.Borody TJ, Brandt LJ, Paramsothy S. Therapeutic faecal microbiota transplantation: current status and future developments. Curr Opin Gastroenterol. 2014;30:97–105. doi: 10.1097/MOG.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lo Vecchio A, Cohen MB. Fecal microbiota transplantation for Clostridium difficile infection: benefits and barriers. Curr Opin Gastroenterol. 2014;30:47–53. doi: 10.1097/MOG.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 311.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil. 2015;27:19–29. doi: 10.1111/nmo.12479. [DOI] [PubMed] [Google Scholar]


