Abstract
Pancreatic fluid collections (PFCs) are a frequent complication of pancreatitis. It is important to classify PFCs to guide management. The revised Atlanta criteria classifies PFCs as acute or chronic, with chronic fluid collections subdivided into pseudocysts and walled-off pancreatic necrosis (WOPN). Establishing adequate nutritional support is an essential step in the management of PFCs. Early attempts at oral feeding can be trialed in patients with mild pancreatitis. Enteral feeding should be implemented in patients with moderate to severe pancreatitis. Jejunal feeding remains the preferred route of enteral nutrition. Symptomatic PFCs require drainage; options include surgical, percutaneous, or endoscopic approaches. With the advent of newer and more advanced endoscopic tools and expertise, and an associated reduction in health care costs, minimally invasive endoscopic drainage has become the preferable approach. An endoscopic ultrasonography-guided approach using a seldinger technique is the preferred endoscopic approach. Both plastic stents and metal stents are efficacious and safe; however, metal stents may offer an advantage, especially in infected pseudocysts and in WOPN. Direct endoscopic necrosectomy is often required in WOPN. Lumen apposing metal stents that allow for direct endoscopic necrosectomy and debridement through the stent lumen are preferred in these patients. Endoscopic retrograde cholangio pancreatography with pancreatic duct (PD) exploration should be performed concurrent to PFC drainage. PD disruption is associated with an increased severity of pancreatitis, an increased risk of recurrent attacks of pancreatitis and long-term complications, and a decreased rate of PFC resolution after drainage. Any pancreatic ductal disruption should be bridged with endoscopic stenting.
Keywords: Pancreatic fluid collection, Pancreatic fluid collection, Pseudocyst, Walled-off pancreatic necrosis, Walled-off pancreatic necrosis, Pancreatitis
Core tip: Pancreatic fluid collections are a frequent complication of pancreatitis. Management includes correctly classifying these collections, initiating early enteral feeding, and draining symptomatic collections. Endoscopic ultrasound with stent placement is the technique of choice. Both metal and plastic stents are efficacious, though metal stents may offer an advantage. When necrosis is present within the collection, direct endoscopic necrosectomy may be required in addition to drainage. Lumen apposing metal stents allow for direct endoscopic necrosectomy through the stent and are preferred in these patients. When a pancreatic duct leak is suspected, endoscopic investigation and stenting is mandated.
INTRODUCTION
Pancreatic fluid collections (PFCs) area frequent complication of pancreatitis. It is estimated that 5%-15% of pancreatitis episodes are complicated by the development of pseudocysts[1]. PFCs can also result from inflammation due to trauma, post surgery, post transplant or pancreatic ductal obstruction. Fifteen percent of pancreatitis episodes are complicated by pancreatic necrosis, and approximately 33% (range 16%-47%) of those with necrosis are complicated by infected necrosis[2]. Management of these collections can pose a challenge. Traditionally, the management has primarily been surgical. However, with new understanding of the pathophysiology paired with new technological advancements, the pendulum has swung towards an emphasis on a minimally invasive approach with a progression to more invasive options as necessary.
CLASSIFICATION OF PANCREATIC FLUID COLLECTIONS
Correctly classifying PFCs is critical for optimizing treatment and management. The first widespread classification system was developed in 1993 by an international consensus meeting in Atlanta, Georgia and became referred to as the Atlanta Criteria[3]. This criteria classified pancreatic fluid collections as acute or chronic collections, with chronic collections being further divided into pancreatic necrosis, pseudocysts, and pancreatic abscesses.
However, with improving pathophysiologic understanding and improving diagnostic tools, it became clear that a more detailed organizational system was required. More specifically, one that distinguished between collections containing fluid alone vs those arising from necrosis and/or containing solid components. As such, a new classification system was developed known as the revised Atlanta criteria[4]. Similar to the original Atlanta Criteria, PFCs are classified as acute (< 4 wk after the pancreatitis episode) or chronic (> 4 wk after the pancreatitis episode). However, in the revised criteria, both acute and chronic collections are further subdivided based on the presence of necrosis within the collection. Acute collections are divided into: acute peripancreatic fluid collections (APFC) and acute necrotic collections (ANC); chronic fluid collections are divided into: pseudocysts or walled-off pancreatic necrosis (WOPN). These new classifications are important because the treatment and management varies depending on the type of collection.
ENTERAL FEEDING
The first step in the management of any PFC is ensuring adequate nutritional support. In mild to moderate acute pancreatitis, oral feeding can be initiated when symptoms are controlled. In severe cases, patients have traditionally been kept nil per os (npo) due to concerns for worsening pancreatic inflammation if normal pancreatic digestion were to be enacted during oral intake[5]. However, prolonged npo in the catabolic stress state of pancreatitis leads to a negative nitrogen balance and nutritional deficiency that became recognized to be associated with a higher mortality rate due to loss of function and structural integrity of vital organs[6]. As a result, total parental nutrition (TPN) became the standard of care in patients with severe acute pancreatitis in an attempt to avoid pancreatic stimulation while still providing nutritional support[5,6].
ENTERAL FEEDING VS TPN
This approach was questioned when studies began showing that complete bowel rest is associated with intestinal mucosal atrophy leading to increased intestinal permeability and bacterial translocation[7]. Furthermore, a metabolically deprived gut absorbs endotoxins and other bacterial products stimulating endogenous cytokines which increases the likelihood of nosocomial infections, sepsis, and organ failure[8]. The use of TPN was further called into question with the emergence of data showing that enteral feeding distal to the ligament of Treitz causes negligible pancreatic stimulation and therefore may be safe in patients with severe pancreatitis[9].
In 2010, the Cochrane Collaboration published their results of a meta-analysis comparing randomized trials of enteral nutrition vs TPN in patients with severe acute pancreatitis[6]. Eternal nutrition was associated with a significant reduction in mortality, multi-system organ failure, and systemic infections with a trend towards shorter length of hospital stay. Based on these findings, enteral nutrition was recommended as the standard of care for nutritional support in acute pancreatitis[6].
In addition to improved morbidity and mortality rates, enteral nutrition is associated with a lower overall cost compared to TPN. In a study of 24 patients with severe acute pancreatitis, enteral nutrition was associated with savings of $5553.06 per patient (P = 0.08). Though not statistically significant, there was a medium to large effect size (d = 0.61) suggesting that the difference between the two groups would likely have been significantin a larger sample size[10].
EARLY VS LATE ENTERAL FEEDING
The timing of initiation of enteral feeding in severe acute pancreatitis has been debated. In a recent meta-analysis, patients receiving early initiation of enteral nutrition (defined as within 48 h of admission) had significantly lower rates of infectious complications (OR = 0.45, 95%CI: 0.15-0.77, P < 0.05), organ failure (OR = 0.27, 95%CI: 0.14-0.50, P < 0.05), length of hospitalization [mean difference -2.18 d, 95%CI: (-3.48)-(-0.87), P < 0.05], and mortality (OR = 0.31, 95%CI: 0.14-0.71, P < 0.05) compared to those with delayed enteral nutrition or TPN[11]. However, the exact time at whichenteral feeding should be initiated is not yet established.
JEJUNAL VS GASTRIC ENTERAL FEEDING
Though enteral nutrition distal to the ligament of Treitz is thought to decrease pancreatic stimulation, placement of a nasojejunal tube requires endoscopy for placement and is more cumbersome than a nasogastric tube which can be placed bedside (Figure 1). Studies have been performed to evaluate the safety of nasogastric feeds compared to nasojejunal feeds. A meta-analysis of these studies showed no difference in mortality and tolerance between the two types of feeding; however, this analysis was limited by the small sample size (157 patients in the 3 studies included for analysis) and the lack of verification of placement of the nasojejunal tube distal to ligament of Treitz in two of the three studies[12]. A recent non-inferiority trial of 78 patients randomized to nasogastric or radiologically-confirmed nasojejunal feeding was recently published, showing non-inferiority of nasogastric feeds. However, there was a higher rate of infectious complications, need for surgical intervention for infected necrosis, and mortality in the nasogastric feeding group[13]. A prospective, randomized controlled trial evaluating nasogastric vs nasojejunal feeding called the Study on Nutrition in Acute Pancreatitis is currently underway, which will provide further evidence on this subject. Until more high-quality data is available, nasojejunal feeding remains the preferred route of enteral nutrition.
Figure 1.
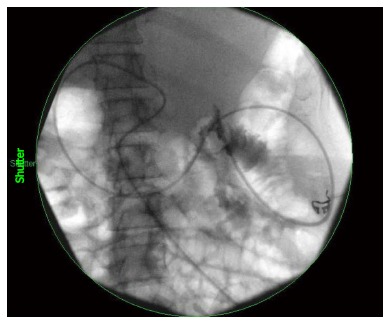
Fluoroscopic image of a percutaneous nasojejunal feeding tube beyond the ligament of Treitz.
ENTERAL FEEDING FORMULATIONS
Enteral nutrition is available in a variety of formulations, including standard, elemental, and semi-elemental with the latter two more commonly used based upon the assumption that they result in less pancreatic stimulation. Standard enteral formulas are, however, significantly cheaper and proven effective[14]. Windsor et al[15] randomized patients with severe acute pancreatitis to TPN vs standard enteral formulas and found that patients receiving standard enteral formulas had improved clinical outcomes compared to those receiving TPN, including decreased rates of systemic inflammatory response syndrome, sepsis, and multi-system organ failure. Makola et al[14] also examined the efficacy of enteral formulas in acute pancreatitis and demonstrated that it is associated with an improvement in the severity of pancreatitis, a higher albumin, and a trend towards a normal BMI.
INDICATIONS FOR DRAINAGE OF PFCS
In the initial Atlanta criteria, PFCs were recommended for drainage based on the presence of symptoms and/or complications such as abdominal pain, gastrointestinal obstruction, vascular compression, biliary obstruction, or infection, as well as on the size of the collection. However, in the revised criteria, size alone does not necessitate treatment; only symptomatic PFCs are recommended for drainage. Historically, drainage has been managed via surgical techniques. But with the advent of newer and more advanced endoscopic tools and expertise, and an associated reduction in health care costs, minimally invasive endoscopic drainage has become the preferable approach[16].
PANCREATIC PSEUDOCYSTS
As described in the revised Atlanta criteria, a pseudocyst is an encapsulated fluid collection, without the presence of solid debris, that develops as a consequence of pancreatitis a minimum of 4 wk after the initial injury[3].
SURGICAL DRAINAGE
Surgical cystogastrostomy involves an open or laparoscopic procedure in which an anastomosis is created between the lumen of the cyst cavity and the stomach or small bowel using suturing or stapling devices[11]. Depending on the location, a cystojejunostomy can also be a surgical alternative.
Historically, surgical drainage was an efficacious therapy, with published pseudocyst recurrence rates between 2.5%-5% post-drainage, but complication rates approaching 30% in some studies[16]. As endoscopic therapies emerged, initial studies comparing surgical cystogastrostomy to endoscopic cystogastrostomy showed grossly equivalent success rates, defined as pseudocyst resolution, and comparable complication rates[17,18]. However, as endoscopic techniques improved, endoscopic therapy became the preferred initial treatment approach. A randomized comparative trial by Varadarajulu et al[19] looking at surgical vs endoscopic cystogastrostomy found that while the two techniques yielded similar technical success and complication rates, endoscopic therapy was associated with a shorter hospital stay, a lower overall cost, and better mental health and physical health component scores among patients.
PERCUTANEOUS DRAINAGE
Percutaneous drainage involves placement of an external drainage catheter into the pseudocyst using real-time imaging guidance, usually with computed tomography (CT) or ultrasound (US) with fluoroscopy. Initial studies comparing surgical drainage to percutaneous drainage found both procedures to be efficacious[20,21]. However, more recent comparative studies have generally favored percutaneous drainage[22], with some studies even demonstrating a mortality benefit[23]. Percutaneous drainage has also recently been compared to endoscopic drainage. A recent study directly comparing percutaneous vs endoscopic management retrospectively reviewed 81 patients. This study found equal technical success rates and adverse events rates between the techniques, but a decreased re-intervention rate, a shorter hospital stay, and a decreased number of follow-up abdominal imaging studies among patients drained endoscopically[24].
CONVENTIONAL TRANSMURAL DRAINAGE
Conventional transmural drainage was the endoscopic procedure of choice to drain PFCs in the early era of endoscopic PFC management. This procedure consists of endoscopically visualizing the PFC bulge in the gastric wall, creating a fistulous tract between the pseudocyst cavity and the gastric lumen using a seldinger technique, advancing a guidewire into the pseudocyst cavity, dilating the tract, and finally deploying one or more plastic stents to secure apposition and allow for continuous drainage[25].
This concept was first introduced into the medical literature in 1975 in a case report by Rogers et al[26]. It was expanded upon by Kozarek et al[27] in 1985 in a case series of 4 patients who underwent endoscopic cystogastrostomy needle decompression and by Cremer et al[28] in 1986 in which they described 13 patients who underwent cystogastrostomy with trans-nasal drain placement. The first large series evaluating this technique was published in 1989 and consisted of a 7-year follow-up study of 33 patients who underwent endoscopic cystogastrostomy or cystoduodenostomy with a success rate of 82%, recurrence rate of 12%, and complication rate of 2%[29]. In 1995, Binmoeller et al[30] published a similar study of 53 patients with a success rate of 87%, recurrent rate of 21%, and complication rate of 11%. A series of subsequent studies from the early 2000s demonstrated similar results, reporting success rates between 70%-100% and complication rates ranging from 2%-40%, mainly bleeding, perforation, and infection due to stent occlusion or migration[29-39].
One of the limitations of this technique was the need for the PFC to be bulging into the gastric wall. It is estimated that no bulge is present in 42%-48% of PFCs, limiting the efficacy and safety of this technique in almost half of all cases[40]. However, with the incorporation of echoendoscopy, this limitation was overcome.
EUS-GUIDED TRANSMURAL DRAINAGE
The use of EUS in pseudocyst drainage provides endoscopists with the ability to identify and avoid vascular structures between the cyst and the gastric lumen, to measure the distance between the lumen and the cystic lesion and ensure that adequate apposition can be obtained, to localize non-bulging pseudocysts that are otherwise unidentifiable using endoscopy alone, and to confirm the lack of solid or necrotic components within the pseudocyst cavity (Figure 2). This technique first emerged in the medical literature in 1992 by Grimm et al[41] and 1996 by Wiersema[42], both of whom described a single case of successful endoscopic pseudocyst drainage using an echoendoscope. Several larger case series looking at 27 patients[30] and 35 patients[43] documented success rates of 78% and 89% with complication rates of 7% and 4% respectively, significantly lower than with conventional transmural drainage (CTD). Since then, a multitude of studies have validated these initial findings, with early studies quoting success rates ranging from 80%-100% and complication rates averaging around 10%, mainly bleeding and perforation[25,30,35,40,43-47].
Figure 2.
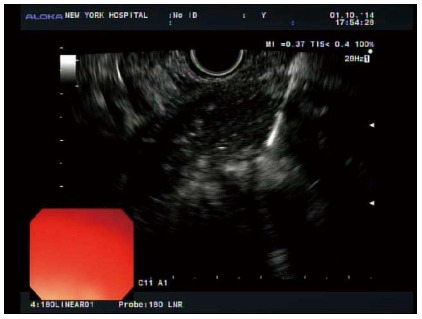
Endosonographic visualization of accessing a pancreatic fluid collection with a fine needle aspiration needle.
More recent studies have further subdivided pancreatic pseudocysts into simple vs infected pseudocysts. Sadik et al[48] noted a 94% success rate and 5% complication rate in simple pseudocysts vs 80% success rate and 30% complication rate in infected pseudocysts. Similarly, Varadarajulu et al[49] found a 93.5% success rate and 5% complication rate vs a 63% success rate and 16% complication rate in sterile vs infected pseudocysts. This suggests that while EUS-guided drainage is still efficacious, infected pseudocysts are more difficult to drain and associated with a higher complication rate.
Several studies have directly compared EUS-guided PFC drainage to CTD. A study by Kahaleh et al[25] showed equal efficacy and safety between the two techniques when conventional drainage was used for bulging lesions and EUS-guided drainage was used for all other lesions. Subsequently, two prospective, randomized studies by Varadarajulu et al[50] and Park et al[51] found significantly higher technical success rates with EUS-guided drainage, and a trend towards a better safety profile although statistical significance was not reached.
FULLY COVERED SELF-EXPANDING METAL STENTS
Fully-covered self-expanding metal stents (FCSEMS) offer a variety of advantages over traditional plastic stents. Firstly, they allow for a larger drainage lumen, which decreases the risk of stent occlusion and theoretically the need for repeat procedures. And secondly, they allow for shorter procedure times since they require a single access of the cyst for deployment, rather than the multiple access points required for the deployment of multiple plastic stents.
A study by Penn et al[52] looked at 20 patients with symptomatic pancreatic pseudocysts which were drained under EUS guidance with placement of biliary FCSEMS (Wallflex; Boston Scientific, Natick, MA). They found a 100% technical success rate and a 70% rate of complete pseudocyst resolution without recurrence. Three patients experienced complications (15%) requiring surgery in 2 of the 3, and stent migration was noted in 3 patients, all of whom still achieved pseudocyst resolution. Similarly, a case series looking at 18 patients with symptomatic pseudocysts drained with FCSEMS (Wallflex; Boston Scientific) under EUS-guidance showed a 78% rate of complete pseudocyst resolution (14 patients); however, 16% of patients required surgery for ongoing sepsis or ineffective drainage[53]. A case series looking at 20 patients with infected pseudocysts drained with biliary FCSEMS and/or esophageal CSEMS reported a 100% technical success rate and a complete clinical success rate of 85%[54]. In this series, 1 patient had stent migration and 1 patient had a superinfection treated with medical therapy[55].
FCSEMS with antimigratory fins (Viabil, Conmed, city, state) have also been proven efficacious (Figure 3). Talreja et al[56] reported a 78% clinical success rate with complete resolution after pseudocyst drainage in 18 patients. In their series, 1 patient had stent migration, though still achieved pseudocyst resolution. Berzosa et al[57] evaluated the same stent for pseudocyst drainage in 5 patients and found an 83% resolution rate without recurrence at 18 wk.
Figure 3.
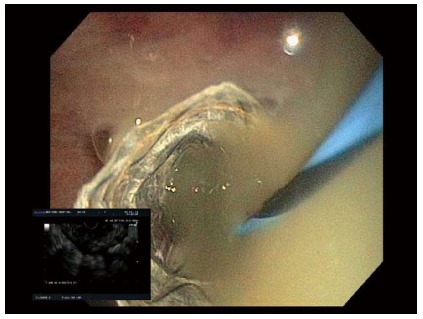
Endoscopic visualization of a biliary fully covered self-expanding metal stent to drain an infected pseudocyst.
PLASTIC STENTS VS METAL STENTS
Despite the advantages that FCSEMS hold over traditional plastic stents, direct comparison has not consistently shown them to be superior. A recent meta-analysis that included 698 patients found no difference in treatment success, adverse events, or recurrence rates between pseudocysts drained with multiple plastic stents vs metal stents[18,58]. However, a more recent study by Sharaiha et al[59] of 230 patients found that pseudocysts drained with plastic stents were 2.5 times more likely to report adverse events than when FCSEMS were used. Similarly, complete pseudocyst resolution was 89% with plastic stents compared to 98% with FCSEMS. Given the cost differential between metal and plastic stents, further randomized controlled trials are needed prior to final recommendations on the best approach.
NOVEL LUMEN-APPOSING METAL STENT
In 2013, a new FCSEMS received FDA approval for use in drainage of PFCs (Axios; Boston Scientific, Boston, MA). The design of the stent includes two 21 mm or 24 mm flanges on either side of a 10 mm or 15 mm diameter lumen to help decrease the risk of stent migration. The first clinical data using this stent came from a study by Itoi et al[60] in 2012 looking at 15 patients with symptomatic pseudocysts. Success rate in the trial was 100%, with zero percent recurrence at 11 mo follow-up and the only complication being stent migration in 1 patient without clinical sequelae (Figure 4).
Figure 4.
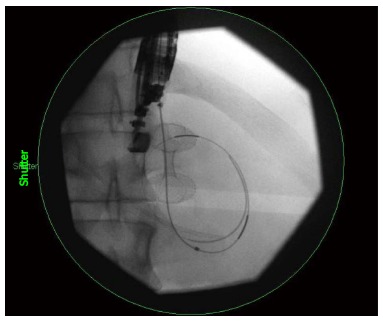
Fluoroscopic visualization of a lumen apposing metal stent being deployed into a pancreatic pseudocyst.
Several additional studies validated this initial reported success. A prospective study by Shah et al[61] looking at 33 patients found a technical success rate of 91% with a cyst resolution rate of 93%. Gornals et al[62] looked at 9 patients who underwent pseudocyst drainage with placement of a lumen-apposing metal stent (LAMS) and reported a technical success 89%, a pseudocyst resolution rate of 100%, and 1 significant complication (pneumothorax). Walter et al[63] published their data of 15 patients with a clinical success rate of 93%, resolution rate of 100%, and 1 significant complication (perforation). And most recently, Rinninella et al[64] documented a 98% cyst resolution rate with adverse events in 2 out of 41 patients.
In summary, pancreatic pseudocysts can be efficaciously managed endoscopically. Although conventional endoscopic drainage can be safely used for bulging pseudocysts, the majority of pseudocysts are drained under EUS-guidance to allow for safer access and a decrease in complications. Metal stents, including the newly emerged lumen-apposing metal stent, carry an advantage over plastic stents in pseudocyst drainage, but given the increased cost and lack of definitive evidence as to their superiority, further trials are needed (Table 1).
Table 1.
Pancreatic fluid collection
| Cases | Procedure used | Device used | Clinical success rates | Technical success rates | Complications | |
| Pancreatic pseudocysts | ||||||
| Hookey et al[35], 2006 | 116 | Conventional Transmural drainage | Stents | 88% | 88% | 11% complication rate |
| Antillon et al[40], 2006 | 33 | EUS-Guided Transmural drainage | Double-pigtail Stent | 94% | 82% | 2 major complications and 3 minor complications |
| Azar et al[44], 2006 | 23 | EUS-Guided Transmural drainage | Double-pigtail Stent | 91% | 91% | |
| Lopes et al[46], 2007 | 51 | EUS-guided Transmural drainage | Straight/Double-pigtail Stent | 94% | 94% | 17.7% stent migration, stent obstruction |
| Barthet et al[45], 2008 | 50 | EUS-guided Transmural drainage | Double-pigtail Stent/Straight Polyethylene | 90% | 98% | 18% morbidity, 5 superinfections |
| Talreja et al[56], 2008 | 18 | EUS-guided drainage | Covered self-expanding metal stent | 95% | 78% | Superinfection (5), bleeding (2), and inner migration (1). |
| Berzosa et al[57], 2012 | 7 | Single-step endoscopic ultrasonography-guided drainage | Single-self expandable metal stent | 100% | 83% | |
| Fabbri et al[95], 2012 | 22 | EUS-guided drainage | Covered self-expanding metal stent | 77% | 77% | |
| Penn et al[52], 2012 | 20 | EUS-guided drainage | Fully covered self-expandable metallic stents | 70% | 70% | Pseudocyst infection (2), post transmural drainage fever and post-ERCP pancreatitis(1) |
| Itoi et al[60], 2012 | 15 | EUS-guided drainage | Novel lumen-apposing, self-expandable metal stent (Axios) | 100% | 100% | |
| Weilert et al[53], 2012 | 18 | EUS-guided drainage | Fully covered self-expanding metal stent | 78% | 78% | |
| Varadarajulu et al[19], 2013 | 20 | Endoscopic Cystogastrostomy | Plastic stents | 95% | 90% | |
| Sarkaria et al[97], 2014 | 17 | EUS-guided drainage | Fully covered esophageal self-expandable metallic stents | 88% | 88% | Perforation during tract dilation (1) |
| Shah et al[61], 2015 | 33 | EUS-guided drainage | Lumen-apposing, covered, self-expanding metal stent; Axios | 91% | 93% | Abdominal pain (n = 3), spontaneous stent migration, back pain (n = 1), access-site infection, and stent dislodgement (n = 1) |
| Walter et al[63], 2015 | 61 | EUS-guided drainage | Axios | 93% | 98% | stent migration (n = 3), stent dislodgement during necrosectomy (n = 3), stent removal during surgery (n = 2), or refusal by the patient (n = 2) |
| Mukai et al[99], 2015 | 2 | EUS-guided drainage/ Direct endoscopic necrosectomy | novel flared-type biflangedmetal stent | 100% | 100% | There was 1 psuedocyst recurrence in cystogramy |
| Rinninella et al[64], 2015 | 18 | EUS-guided drainage | Lumen-apposing, self-expanding metal stent (Axios) | 100% | ||
| Sharaiha et al[59], 2015 | 230 | EUS-guided transmural drainage | 118 DP plastic stents/112 FCSEMS | 75%-90% | < 90% | |
| Walled-off Necrosis | ||||||
| Seewald et al[89], 2005 | 13 | Direct endoscopic necrosectomy | Double-pigtail stent | 91% | 91% | Minor bleeding after balloon dilation, Necrosectomy (4) |
| Charnley et al[92], 2006 | 13 | Direct endoscopic necrosectomy | Double-pigtail stents | 92.3% | 92.3% | |
| Voermans et al[90], 2007 | 25 | Direct endoscopic necrosectomy | Double-pigtail stents | 93% | 93% | Surgery(2), Hemorrhage (1), perforation of cyst wall (1) |
| Papachristou et al[88], 2007 | 53 | Direct endoscopic necrosectomy | Double-pigtail stents | 81% | 81% | Twelve patients (23%) required open operative intervention a median of 47 d (range, 5–540) after initial endoscopic drainage/debridement, due to persistence of WOPN (n = 3), recurrence of a fluid collection (n = 2), cutaneous fistula formation (n = 2), or technical failure, persistence of pancreatic pain, colonic obstruction, perforation, and flank abscess (n = 1 each) |
| Escourrou et al[91], 2008 | 13 | Direct endoscopic necrosectomy | Double-pigtail stents | 100% | 100% | bleeding (n = 3), transient aggravation of sepsis (n = 3) |
| Seifert et al[93], 2009 | 93 | Transmural endoscopic necresectomy | Multiple Stents | 80% | 80% | Bleeding (13), Perforations of the necrosis (5), fistula formation (2), air embolism (2), complications at other organs (2) |
| Gardner et al[102], 2009 | 45 | 25 used direct endoscopic neserectomy and 20 used conventional standard endoscopic drainage | Multiple Stents | 45% | 88% for DEN and 45% for Standard endoscopy drainage | |
| Gardner et al[94], 2011 | 104 | Direct endoscopic necrosectomy | Multiple Stents | 91% | 91% | 14%; included 5 retrogastric perforations/pneumoperitoneum |
| Attam et al[98], 2014 | 10 | endoscopic transluminal necrosectomy and transmural drain | Novel large-bore, fully covered metal through-the-scope esophageal stent | 90% | 100% | |
| Smoczyński et al[100], 2014 | 112 | Endoscopic drainage | Multiple Stents | 84% | 93% | Stoma bleeding (19), GI Perforation (4), collection perforation (2), sepsis (1), stent migration (3) |
| Sarkaria et al[97], 2014 | 17 | EUS-guided drainage | Fully covered esophageal self-expandable metallic stents | 83% | 83% | |
| Mukai et al[99], 2015 | 19 | EUS-guided drainage and DEN for PFCs using the novel flared-type BFMS | novel flared-type biflangedmetal stent | 100% | 100% | |
| Rinninella et al[64], 2015 | 52 | EUS guidance FCSEMS/LACSEMS | Axios Stent | 90.4% | 100% | 3 patients required surgery due to infection/1 patient had a perforated wall |
| Walter et al[63], 2015 | 46 | EUS guided drainage | Axios Stent | 81% | 81% | 9% |
EUS: Endoscopic ultrasonography; GI: Gastrointestinal; DEN: Direct endoscopic necrosectomy; PFCs: Pancreatic fluid collections.
MANAGEMENT OF WOPN
WOPN is a PFC that contains solid necrotic debris surrounded by a clearly defined capsule with or without concurrent fluid[4]. Although a small percentage of WOPN will resolve spontaneously, the majority of collections will require drainage.
SURGICAL DRAINAGE
Open surgical debridement has historically been the therapy for WOPN[65,66]. Surgical management consists of 4 principal approaches, all involving accessing the pancreatic bed but differing in the surgical approach. The standard approaches include access via the lesser sac, the gastrocolic-omentum, or trans-mesenterially through the transverse mesocolon[67]. Once the necrosectomy has been performed, the options are: (1) necrosectomy alongside open packing[68]; (2) planed, staged re-laparotomies with repeat lavage[69]; (3) closed continuous lavage of the lesser sac and retro-peritoneum[65]; and (4) closed packing[70].
Open necrosectomy is associated with high morbidity (34% to 95%) and mortality (6% to 25%) rates[71-76], and a plethora of adverse events including organ failure, perforation, wound infections, hemorrhage, chronic pancreatico-cutaneous and entero-cutaneous fistulae, and abdominal wall hernias[65,67,70,72,73]. With the development of laparoscopic surgery, minimally invasive procedures supplanted open debridement as the surgical option of choice. Laparoscopic debridement can be performed using 2 approaches: trans-peritoneal (anterior) or retroperitoneal (posterior)[66]. The trans-peritoneal approach involves an anterior access through the stomach or the bowel to drain the collection. The retroperitoneal approach uses a mini-lumbotomy, usually left-sided, through which a laparoscope is introduced to remove the necrotic debris under direct visualization. Currently, the trans-peritoneal approach is rarely used due to increased technical difficulty and the risk of contamination of the peritoneal cavity[77]. Additionally, a retroperitoneal approach can be performed with minimal or no gas insufflation and avoids the complications associated with severing the peritoneum[78,79].
PERCUTANOUS DRAINAGE
Percutaneous drainage for WOPN involves placement of a catheter into the collection under US guidance with fluoroscopy or CT guidance. Ideally, a retroperitoneal approach is taken. After placement and aspiration of as much fluid as possible, 12 French drains are left in place and irrigated with 10-20 mL of sterile saline 3 times daily. The catheters can be upsized to a maximum of 28 French as the patient’s follow-up requires[80].
Traditionally, the success rate of percutaneous drainage alone (defined as survival without the need for additional surgical necrosectomy) ranged from 35%-84%, with mortality rates ranging from 5.6%-34% and morbidity ranges of 11%-42%, most commonly due to pancreatico-cutaneous fistulas and pancreatico-enteric fistulas which occur in an as many as 20% of cases[81-85]. Consequently, percutaneous drainage is more often used as an adjunct therapy, often serving as the first step of a step-up approach to endoscopic or surgical drainage[65,76,81]. The Dutch PANTER trial illustrated this concept by comparing open necrosectomy with a less-invasive step-up approach in 88 patients[86]. In the step-up approach, patients first underwent percutaneous drainage of the collection followed by minimally invasive retroperitoneal necrosectomy if clinical improvement was not achieved. Results showed that the minimally invasive approach was associated with an overall decreased mortality rate, fewer major and long-term complications, and reduced overall healthcare costs. Of note, percutaneous drainage alone without subsequent necrosectomy was achieved only in 30% of patients.
ENDOSCOPIC NECROSECTOMY
The endoscopic technique for drainage of WOPN is called direct endoscopic necrosectomy (DEN). As in pseudocyst drainage, EUS is used to identify and access the collection, a wire is coiled within the cavity lumen, and the fistulous tract is created. However, unlike pseudocyst drainage, the tract is then dilated enough to allow for passage of an endoscope into the collection. Mechanical cleaning with removal of necrotic debris is then performed.
Nasocystic drainage is typically performed to facilitate liquefaction of the debris and improve drainage[31].
Hydrogen peroxide (H2O2) can be used to facilitate removal of necrotic debris[15]. H2O2 is infused into the cavity during endoscopy in a 1:5 or 1:10 dilution with normal saline, allowing for enhanced necrotic tissue dislodgement and debris extraction during endoscopy. The use of H2O2 has been shown to decrease procedure time, reduce complication rates, and decrease the total number of necrosectomy sessions until resolution. Some adverse events have been reported including bleeding, perforation, and self-limited pneumoperitoneum. However, these complications are rare, especially after the incorporation of carbon dioxide for peri-procedural insufflation.
The first experiences with endoscopic necrosectomy were done through the deployment of plastic stents and placement of a nasocystic drain without direct mechanical debridement. This was first described by Baron et al[87] in 1996, in which 11 patients underwent WOPN drainage with an overall success rate of 81% and a complication rate of 36% (bleeding and infection). Papachristou et al[88] reported similar findings in 2007 in a study of 53 patients, with an overall success rate of 81% and a complication rate of 21%.
Seewald et al[89] introduced the concept of dilation of the fistulous tract to allow for advancement of an endoscope into the necrotic cavity and mechanical removal of debris. They described a 91% WOPN resolution rate in 13 patients, with 2 patients having recurrence on 4 mo follow-up necessitating surgical resection. Voermans et al[90] documented a 93% success rate in 25 patients, with only 2 patients requiring surgical intervention for bleeding and perforation. Smaller studies by Escourrou et al[91] and Charnley et al[92] found similar results.
The first multicenter study evaluating endoscopic necrosectomy was performed by Seifert et al[93]. In this study of 93 patients, an 80% clinical success rate was achieved with a 23% complication rate and 7.5% mortality rate. A second multicenter study was published by Gardner et al[94] in 2011 looking at 104 patients with WOPN. Successful resolution was achieved in 91% of patients, with a complication rate of 14% including 3 patients requiring surgical intervention either for bleeding or failed resolution, 5 patients dying of other causes prior to WOPN resolution, and 1 peri-procedural death due to hypotension.
FULLY-COVERED SELF-EXPANDING METAL STENTS
Biliary FCSEMS provide a larger stent lumen for drainage of WOPN, but are limited in that they do not permit passage of an endoscope (Figure 5). Fabbri et al[95] published results of 2 patients with WOPN drained with biliary FCSEMS (Wallflex, Boston Scientific). In 1 patient, the WOPN completely resolved; in the second patient, the stent migrated leading to widespread sepsis and need for surgical intervention. Berzosa et al[57] also looked at 2 patients with WOPN drained with biliary FCSEMS (Viable, ConMed). The WOPN resolved in both patients with no recurrence after 18 wk follow-up.
Figure 5.
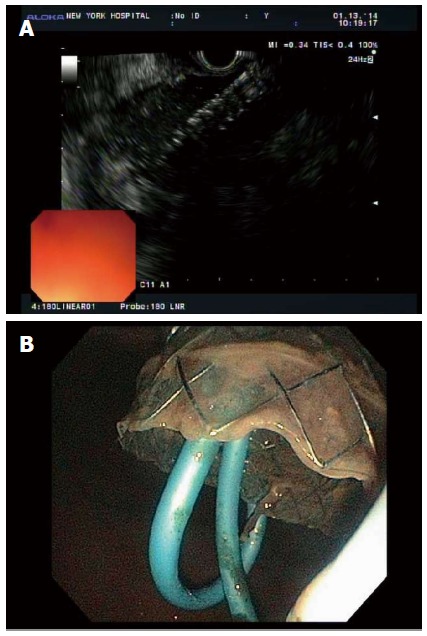
Endosonographic visualization (A) and endoscopic visualization (B) of a biliary fully covered self-expanding metal stent being deployed into walled-off pancreatic necrosis.
Esophageal FCSEMS have a larger lumen diameter and allow for passage of the endoscope through the lumen of the stent after deployment (Figure 6A). The first reported case of WOPN drainage using an esophageal FCSEMS was published by Antillon et al[96]. Sarkaria et al[97] published results of 17 patients who underwent WOPN drainage with placement of an esophageal stent, 88% of whom demonstrated complete resolution with an average of 5 endoscopic sessions and 2 of whom ultimately required surgical intervention. No major complications were reported. Attam et al[98] found similar results in 10 patients using a through-the-scope esophageal FCSEMS in which resolution was achieved in 90% of patients after an average of 3 endoscopic sessions. Two patients required stent revision due to persistent infection in long-term follow-up, and 1 patient died of gastrointestinal bleeding from a pseudoaneurysm. Esophageal FCSEMS are a promising concept in the endoscopic management of WOPN. However, the development of lumen apposing metal stent may supplant the utilization of esophageal FCSEMS.
Figure 6.
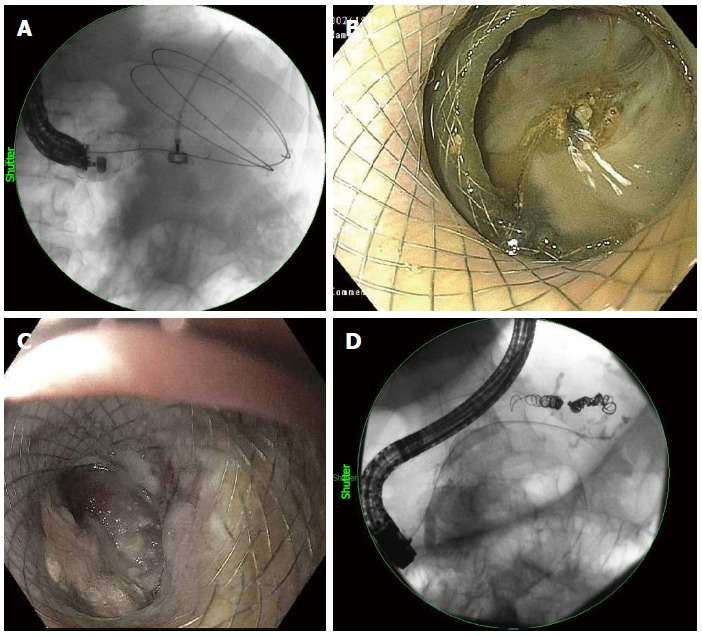
Fluoroscopic visualization of an esophageal fully-covered self-expanding metal stents deployed into walled-off necrosis (A), a pancreatic duct leak (D), endoscopic visualization of a lumen-apposing metal stent deployed into walled-off necrosis (B, C).
LAMS
The previously mentioned LAMS (Axios, Xlumena) also allows for passage of an endoscope through the lumen of the stent into the cavity for mechanical necrosectomy. Only a small number of studies have been published specifically evaluating the use of LAMS for drainage of WOPN. Shah et al[61] achieved WOPN resolution in 10 of 11 patients using a LAMS for drainage. Walter et al[63] looked at 46 patients with WOPN and found a clinical success rate of 81%, with an overall major complication rate of 9% due to infection from stent occlusion, all managed endoscopically with only 3 patients ultimately requiring surgical intervention for persistent infection. Most recently, Rinninella et al[64] documented a 90% clinical success rate in 52 patients, with a 5.4% complication rate. Additional multi-center studies are needed, but LAMS represent a promising advance in the endoscopic management of WOPN (Figure 6B and C).
Cumulatively, these studies illustrate that while endoscopic necrosectomy is efficacious, it is a complicated procedure requiring a high-level of skill in endoscopy with complications occurring even in the most experienced of hands and requiring the presence of a strong multi-disciplinary team to be successful. The incorporation of metal stents that allow for a large drainage lumen and the advancement of an endoscope through the stent lumen for DEN is a major advance, which may ultimately improve efficacy and decrease complications associated with these procedures (Table 1)[99-102].
ENDOSCOPY VS PERCUTAENOUS OR SURGICAL DRAINAGE
A recent randomized multicenter trial from 2012 directly compared endoscopic necrosectomy and surgical necrosectomy (video-assisted retroperitoneal debridement with open laparoscopic necrosectomy for rescue) in 22 patients[103]. Their results showed that endoscopic therapy was associated with a lower post-procedure inflammatory response (as demonstrated by interleukin levels), a lower complication rate, fewer pancreatic fistulae developments, and less pancreatic enzyme use on 6 mo follow-up. Amore recent study from 2014 directly compared a step-up approach starting with percutaneous drainage and escalating to more invasive therapy as needed to DEN in 24 patients[104]. Their results demonstrated a resolution rate of 92% vs 25% in the necrosectomy vs percutaneous drainage group, with 9 of 12 patients requiring surgery after percutaneous drainage alone. Additionally, less antibiotic use, pancreatic insufficiency, and hospitalization was seen in the endoscopic necrosectomy group.
ERCP FOR PANCREATIC DUCT EXPLORATION
An important component in the management of PFCs is ensuring the integrity of the pancreatic duct (PD) via ERCP. Disruptions in the PD are associated with an increased severity of pancreatitis, an increased risk of recurrent attacks of pancreatitis and long-term complications, and a decreased rate of PFC resolution after drainage[105-110] (Figure 6D).
PD DISRUTPION AND SEVERITY OF PANCREATITIS
A PD disruption has been shown to be associated with a more severe course of pancreatitis. A retrospective review of 105 patients with acute pancreatitis found that nearly half of patients with severe pancreatitis had concurrent PD disruption, while a normal PD was noted in 100% of patients with mild pancreatitis[105]. Similarly, in a retrospective review of 144 patients with severe pancreatitis, Lau et al[109] found that patients with a PD leak were 3.4 times more likely to have pancreatic necrosis. Thus, assessing for a PD disruption in patients with pancreatitis is an important prognosticating step.
PD DISRUPTION AND RECURRENT PANCREATITIS/LONG-TERM COMPLICATIONS
In addition to predicting the severity of pancreatitis, a PD disruption can also predict the likelihood of long-term complications and recurrent episodes of pancreatitis. Howard et al[106] looked at 14 patients with WOPN who developed recurrent pancreatitis after initially-successful debridement, and found that all 14 patients had a pancreatic duct abnormality on either ERCP or MRCP. No other predictive factor of recurrence was identified. Nealon et al[107] demonstrated that in 174 patients with severe pancreatitis, long-term complications such as sepsis and recurrent pancreatitis occurred in 36%-38% vs 0% and 62%-89% vs 7% of patients with an abnormal PD compared to those with a normal PD.
PD DISRUPTION AND PFC RESOLUTION
Assessing for PD disruptions can also predict treatment success. In the same study as above-mentioned, Nealon et al[107] demonstrated that altered PD anatomy is directly correlated with a decreased rate of pseudocyst resolution. In 563 patients with pseudocysts, they found that spontaneous resolution occurred only in 0%-5% of patients with a ductal disruption compared to 87% of patients with a normal pancreatic duct. Similarly, Trevino et al[108] demonstrated improved PFC resolution of both pseudocysts and WOPN in patients who underwent PFC drainage with transpapillary PD stenting compared with PFC drainage alone (97.5% vs 80%). Of note, undergoing ERCP was not associated with any increase in mortality, the need for necrosectomy, or hospital length of stay.
CONCLUSION
Pancreatitis can frequently result in the development of fluid collections, ranging from simple pseudocysts to WOPN. The initial step in management of these collections is ensuring adequate nutritional support is provided. Enteral nutrition is preferred over parenteral nutrition, with post-pancreatic jejunal feeding being the optimal enteral route in patients with moderate or severe disease. Symptomatic PFCs require drainage. Endoscopic drainage can be successfully accomplished with improved safety and efficacy as compared to surgical or radiologic approaches. Furthermore, patients with WOPN can safely undergo endoscopic necrosectomy, obviating the need for surgical exploration. Lastly, in all patients with suspected PD disruption, ERCP with PD exploration should be performed and if MRCP is available, it should be used accordingly to rule out pancreatic duct disruption in low probability patients.
In summary, all forms of PFC can be safely and effectively managed by a variety of endoscopic procedures.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 20, 2015
First decision: November 5, 2015
Article in press: December 30, 2015
P- Reviewer: Ercolani G, Iso Y, Kleeff J, Sharma SS S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Poornachandra KS, Bhasin DK, Nagi B, Sinha SK, Rana SS, Shafiq N, Greer K, Gupta R, Kang M, Malhotra S, et al. Clinical, biochemical, and radiologic parameters at admission predicting formation of a pseudocyst in acute pancreatitis. J Clin Gastroenterol. 2011;45:159–163. doi: 10.1097/MCG.0b013e3181dd9d14. [DOI] [PubMed] [Google Scholar]
- 2.Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 3.Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 4.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 5.Goodgame JT, Fischer JE. Parenteral nutrition in the treatment of acute pancreatitis: effect on complications and mortality. Ann Surg. 1977;186:651–658. doi: 10.1097/00000658-197711000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;(1):CD002837. doi: 10.1002/14651858.CD002837.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415; 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 8.Oláh A, Romics L. Enteral nutrition in acute pancreatitis: a review of the current evidence. World J Gastroenterol. 2014;20:16123–16131. doi: 10.3748/wjg.v20.i43.16123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corcoy RSJ, Domingo P. Nutrition in patients with severe acute pancreatitis. Nutrition. 1988;4:269–275. [Google Scholar]
- 10.Mutch KL, Heidal KB, Gross KH, Bertrand B. Cost-analysis of nutrition support in patients with severe acute pancreatitis. Int J Health Care Qual Assur. 2011;24:540–547. doi: 10.1108/09526861111160571. [DOI] [PubMed] [Google Scholar]
- 11.Li JY, Yu T, Chen GC, Yuan YH, Zhong W, Zhao LN, Chen QK. Enteral nutrition within 48 hours of admission improves clinical outcomes of acute pancreatitis by reducing complications: a meta-analysis. PLoS One. 2013;8:e64926. doi: 10.1371/journal.pone.0064926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang YS, Fu HQ, Xiao YM, Liu JC. Nasogastric or nasojejunal feeding in predicted severe acute pancreatitis: a meta-analysis. Crit Care. 2013;17:R118. doi: 10.1186/cc12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh N, Sharma B, Sharma M, Sachdev V, Bhardwaj P, Mani K, Joshi YK, Saraya A. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis: a noninferiority randomized controlled trial. Pancreas. 2012;41:153–159. doi: 10.1097/MPA.0b013e318221c4a8. [DOI] [PubMed] [Google Scholar]
- 14.Makola D, Krenitsky J, Parrish C, Dunston E, Shaffer HA, Yeaton P, Kahaleh M. Efficacy of enteral nutrition for the treatment of pancreatitis using standard enteral formula. Am J Gastroenterol. 2006;101:2347–2355. doi: 10.1111/j.1572-0241.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- 15.Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, Welsh F, Guillou PJ, Reynolds JV. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–435. doi: 10.1136/gut.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parks RW, Tzovaras G, Diamond T, Rowlands BJ. Management of pancreatic pseudocysts. Ann R Coll Surg Engl. 2000;82:383–387. [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MD, Walsh RM, Henderson JM, Brown N, Ponsky J, Dumot J, Zuccaro G, Vargo J. Surgical versus nonsurgical management of pancreatic pseudocysts. J Clin Gastroenterol. 2009;43:586–590. doi: 10.1097/MCG.0b013e31817440be. [DOI] [PubMed] [Google Scholar]
- 18.Melman L, Azar R, Beddow K, Brunt LM, Halpin VJ, Eagon JC, Frisella MM, Edmundowicz S, Jonnalagadda S, Matthews BD. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc. 2009;23:267–271. doi: 10.1007/s00464-008-0196-2. [DOI] [PubMed] [Google Scholar]
- 19.Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583–590.e1. doi: 10.1053/j.gastro.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Heider R, Meyer AA, Galanko JA, Behrns KE. Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg. 1999;229:781–787; discussion 787-789. doi: 10.1097/00000658-199906000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spivak H, Galloway JR, Amerson JR, Fink AS, Branum GD, Redvanly RD, Richardson WS, Mauren SJ, Waring JP, Hunter JG. Management of pancreatic pseudocysts. J Am Coll Surg. 1998;186:507–511. doi: 10.1016/s1072-7515(98)00088-x. [DOI] [PubMed] [Google Scholar]
- 22.Lang EK, Paolini RM, Pottmeyer A. The efficacy of palliative and definitive percutaneous versus surgical drainage of pancreatic abscesses and pseudocysts: a prospective study of 85 patients. South Med J. 1991;84:55–64. doi: 10.1097/00007611-199101000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Adams DB, Anderson MC. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg. 1992;215:571–576; discussion 576-578. doi: 10.1097/00000658-199206000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akshintala VS, Saxena P, Zaheer A, Rana U, Hutfless SM, Lennon AM, Canto MI, Kalloo AN, Khashab MA, Singh VK. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc. 2014;79:921–928; quiz 983.e2, 983.e5. doi: 10.1016/j.gie.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–359. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 26.Rogers BH, Cicurel NJ, Seed RW. Transgastric needle aspiration of pancreatic pseudocyst through an endoscope. Gastrointest Endosc. 1975;21:133–134. doi: 10.1016/s0016-5107(75)73821-x. [DOI] [PubMed] [Google Scholar]
- 27.Kozarek RA, Brayko CM, Harlan J, Sanowski RA, Cintora I, Kovac A. Endoscopic drainage of pancreatic pseudocysts. Gastrointest Endosc. 1985;31:322–327. doi: 10.1016/s0016-5107(85)72215-8. [DOI] [PubMed] [Google Scholar]
- 28.Cremer M, Deviere J. Endoscopic management of pancreatic cysts and pseudocysts. Gastrointest Endosc. 1986;32:367–368. doi: 10.1016/s0016-5107(86)71892-0. [DOI] [PubMed] [Google Scholar]
- 29.Cremer M, Deviere J, Engelholm L. Endoscopic management of cysts and pseudocysts in chronic pancreatitis: long-term follow-up after 7 years of experience. Gastrointest Endosc. 1989;35:1–9. doi: 10.1016/s0016-5107(89)72677-8. [DOI] [PubMed] [Google Scholar]
- 30.Binmoeller KF, Seifert H, Walter A, Soehendra N. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:219–224. doi: 10.1016/s0016-5107(95)70095-1. [DOI] [PubMed] [Google Scholar]
- 31.Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7–17. doi: 10.1067/mge.2002.125106. [DOI] [PubMed] [Google Scholar]
- 32.Beckingham IJ, Krige JE, Bornman PC, Terblanche J. Long term outcome of endoscopic drainage of pancreatic pseudocysts. Am J Gastroenterol. 1999;94:71–74. doi: 10.1111/j.1572-0241.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 33.Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, Bruno M. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977–983. doi: 10.1055/s-2005-870336. [DOI] [PubMed] [Google Scholar]
- 34.De Palma GD, Galloro G, Puzziello A, Masone S, Persico G. Endoscopic drainage of pancreatic pseudocysts: a long-term follow-up study of 49 patients. Hepatogastroenterology. 2002;49:1113–1115. [PubMed] [Google Scholar]
- 35.Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635–643. doi: 10.1016/j.gie.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Sharma SS, Bhargawa N, Govil A. Endoscopic management of pancreatic pseudocyst: a long-term follow-up. Endoscopy. 2002;34:203–207. doi: 10.1055/s-2002-20292. [DOI] [PubMed] [Google Scholar]
- 37.Smits ME, Rauws EA, Tytgat GN, Huibregtse K. The efficacy of endoscopic treatment of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:202–207. doi: 10.1016/s0016-5107(95)70092-7. [DOI] [PubMed] [Google Scholar]
- 38.Weckman L, Kylänpää ML, Puolakkainen P, Halttunen J. Endoscopic treatment of pancreatic pseudocysts. Surg Endosc. 2006;20:603–607. doi: 10.1007/s00464-005-0201-y. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez Cortes E, Maalak A, Le Moine O, Baize M, Delhaye M, Matos C, Devière J. Endoscopic cystenterostomy of nonbulging pancreatic fluid collections. Gastrointest Endosc. 2002;56:380–386. doi: 10.1016/s0016-5107(02)70042-4. [DOI] [PubMed] [Google Scholar]
- 40.Antillon MR, Shah RJ, Stiegmann G, Chen YK. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797–803. doi: 10.1016/j.gie.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Grimm H, Binmoeller KF, Soehendra N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc. 1992;38:170–171. doi: 10.1016/s0016-5107(92)70384-8. [DOI] [PubMed] [Google Scholar]
- 42.Wiersema MJ. Endosonography-guided cystoduodenostomy with a therapeutic ultrasound endoscope. Gastrointest Endosc. 1996;44:614–617. doi: 10.1016/s0016-5107(96)70022-6. [DOI] [PubMed] [Google Scholar]
- 43.Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473–477. doi: 10.1055/s-2001-14967. [DOI] [PubMed] [Google Scholar]
- 44.Azar RR, Oh YS, Janec EM, Early DS, Jonnalagadda SS, Edmundowicz SA. Wire-guided pancreatic pseudocyst drainage by using a modified needle knife and therapeutic echoendoscope. Gastrointest Endosc. 2006;63:688–692. doi: 10.1016/j.gie.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 45.Barthet M, Lamblin G, Gasmi M, Vitton V, Desjeux A, Grimaud JC. Clinical usefulness of a treatment algorithm for pancreatic pseudocysts. Gastrointest Endosc. 2008;67:245–252. doi: 10.1016/j.gie.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524–529. doi: 10.1080/00365520601065093. [DOI] [PubMed] [Google Scholar]
- 47.Varadarajulu S, Wilcox CM, Tamhane A, Eloubeidi MA, Blakely J, Canon CL. Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007;66:1107–1119. doi: 10.1016/j.gie.2007.03.1027. [DOI] [PubMed] [Google Scholar]
- 48.Sadik R, Kalaitzakis E, Thune A, Hansen J, Jönson C. EUS-guided drainage is more successful in pancreatic pseudocysts compared with abscesses. World J Gastroenterol. 2011;17:499–505. doi: 10.3748/wjg.v17.i4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080–2088. doi: 10.1007/s11605-011-1621-8. [DOI] [PubMed] [Google Scholar]
- 50.Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2008;68:1102–1111. doi: 10.1016/j.gie.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 51.Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842–848. doi: 10.1055/s-0029-1215133. [DOI] [PubMed] [Google Scholar]
- 52.Penn DE, Draganov PV, Wagh MS, Forsmark CE, Gupte AR, Chauhan SS. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 2012;76:679–684. doi: 10.1016/j.gie.2012.04.457. [DOI] [PubMed] [Google Scholar]
- 53.Weilert F, Binmoeller KF, Shah JN, Bhat YM, Kane S. Endoscopic ultrasound-guided drainage of pancreatic fluid collections with indeterminate adherence using temporary covered metal stents. Endoscopy. 2012;44:780–783. doi: 10.1055/s-0032-1309839. [DOI] [PubMed] [Google Scholar]
- 54.Fabbri C, Luigiano C, Maimone A, Polifemo AM, Tarantino I, Cennamo V. Endoscopic ultrasound-guided drainage of pancreatic fluid collections. World J Gastrointest Endosc. 2012;4:479–488. doi: 10.4253/wjge.v4.i11.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarantino I, Barresi L, Fazio V, Di Pisa M, Traina M. EUS-guided self-expandable stent placement in 1 step: a new method to treat pancreatic abscess. Gastrointest Endosc. 2009;69:1401–1403. doi: 10.1016/j.gie.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, Kahaleh M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video) Gastrointest Endosc. 2008;68:1199–1203. doi: 10.1016/j.gie.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 57.Berzosa M, Maheshwari S, Patel KK, Shaib YH. Single-step endoscopic ultrasonography-guided drainage of peripancreatic fluid collections with a single self-expandable metal stent and standard linear echoendoscope. Endoscopy. 2012;44:543–547. doi: 10.1055/s-0031-1291710. [DOI] [PubMed] [Google Scholar]
- 58.Navaneethan U. 734: Endoscopic Transmural Drainage of Pancreatic Pseudocysts: Multiple Plastic Stents Vs Metal Stents - A Systematic Review and Meta-Analysis. Gastrointest Endosc. 2014;79:AB 167–AB 8. [Google Scholar]
- 59.Sharaiha RZ, DeFilippis EM, Kedia P, Gaidhane M, Boumitri C, Lim HW, Han E, Singh H, Ghumman SS, Kowalski T, et al. Metal versus plastic for pancreatic pseudocyst drainage: clinical outcomes and success. Gastrointest Endosc. 2015;82:822–827. doi: 10.1016/j.gie.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 60.Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastrointest Endosc. 2012;75:870–876. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 61.Shah RJ, Shah JN, Waxman I, Kowalski TE, Sanchez-Yague A, Nieto J, Brauer BC, Gaidhane M, Kahaleh M. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015;13:747–752. doi: 10.1016/j.cgh.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 62.Gornals JB, De la Serna-Higuera C, Sánchez-Yague A, Loras C, Sánchez-Cantos AM, Pérez-Miranda M. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013;27:1428–1434. doi: 10.1007/s00464-012-2591-y. [DOI] [PubMed] [Google Scholar]
- 63.Walter D, Will U, Sanchez-Yague A, Brenke D, Hampe J, Wollny H, López-Jamar JM, Jechart G, Vilmann P, Gornals JB, et al. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a prospective cohort study. Endoscopy. 2015;47:63–67. doi: 10.1055/s-0034-1378113. [DOI] [PubMed] [Google Scholar]
- 64.Rinninella E, Kunda R, Dollhopf M, Sanchez-Yague A, Will U, Tarantino I, Gornals Soler J, Ullrich S, Meining A, Esteban JM, et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: a large retrospective study (with video) Gastrointest Endosc. 2015;82:1039–1046. doi: 10.1016/j.gie.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Aranda-Narváez JM, González-Sánchez AJ, Montiel-Casado MC, Titos-García A, Santoyo-Santoyo J. Acute necrotizing pancreatitis: Surgical indications and technical procedures. World J Clin Cases. 2014;2:840–845. doi: 10.12998/wjcc.v2.i12.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freeman ML, Werner J, van Santvoort HC, Baron TH, Besselink MG, Windsor JA, Horvath KD, vanSonnenberg E, Bollen TL, Vege SS. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41:1176–1194. doi: 10.1097/MPA.0b013e318269c660. [DOI] [PubMed] [Google Scholar]
- 67.Karakayali FY. Surgical and interventional management of complications caused by acute pancreatitis. World J Gastroenterol. 2014;20:13412–13423. doi: 10.3748/wjg.v20.i37.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bradley EL. Management of infected pancreatic necrosis by open drainage. Ann Surg. 1987;206:542–550. doi: 10.1097/00000658-198710000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarr MG, Nagorney DM, Mucha P, Farnell MB, Johnson CD. Acute necrotizing pancreatitis: management by planned, staged pancreatic necrosectomy/debridement and delayed primary wound closure over drains. Br J Surg. 1991;78:576–581. doi: 10.1002/bjs.1800780518. [DOI] [PubMed] [Google Scholar]
- 70.Fernández-del Castillo C, Rattner DW, Makary MA, Mostafavi A, McGrath D, Warshaw AL. Débridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228:676–684. doi: 10.1097/00000658-199811000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wittau M, Scheele J, Gölz I, Henne-Bruns D, Isenmann R. Changing role of surgery in necrotizing pancreatitis: a single-center experience. Hepatogastroenterology. 2010;57:1300–1304. [PubMed] [Google Scholar]
- 72.Rodriguez JR, Razo AO, Targarona J, Thayer SP, Rattner DW, Warshaw AL, Fernández-del Castillo C. Debridement and closed packing for sterile or infected necrotizing pancreatitis: insights into indications and outcomes in 167 patients. Ann Surg. 2008;247:294–299. doi: 10.1097/SLA.0b013e31815b6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raraty MG, Halloran CM, Dodd S, Ghaneh P, Connor S, Evans J, Sutton R, Neoptolemos JP. Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg. 2010;251:787–793. doi: 10.1097/SLA.0b013e3181d96c53. [DOI] [PubMed] [Google Scholar]
- 74.Parikh PY, Pitt HA, Kilbane M, Howard TJ, Nakeeb A, Schmidt CM, Lillemoe KD, Zyromski NJ. Pancreatic necrosectomy: North American mortality is much lower than expected. J Am Coll Surg. 2009;209:712–719. doi: 10.1016/j.jamcollsurg.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 75.Howard TJ, Patel JB, Zyromski N, Sandrasegaran K, Yu J, Nakeeb A, Pitt HA, Lillemoe KD. Declining morbidity and mortality rates in the surgical management of pancreatic necrosis. J Gastrointest Surg. 2007;11:43–49. doi: 10.1007/s11605-007-0112-4. [DOI] [PubMed] [Google Scholar]
- 76.van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 77.Wroński M, Cebulski W, Słodkowski M, Krasnodębski IW. Minimally invasive treatment of infected pancreatic necrosis. Prz Gastroenterol. 2014;9:317–324. doi: 10.5114/pg.2014.47893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gambiez LP, Denimal FA, Porte HL, Saudemont A, Chambon JP, Quandalle PA. Retroperitoneal approach and endoscopic management of peripancreatic necrosis collections. Arch Surg. 1998;133:66–72. doi: 10.1001/archsurg.133.1.66. [DOI] [PubMed] [Google Scholar]
- 79.van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg. 2007;31:1635–1642. doi: 10.1007/s00268-007-9083-6. [DOI] [PubMed] [Google Scholar]
- 80.Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998;170:969–975. doi: 10.2214/ajr.170.4.9530046. [DOI] [PubMed] [Google Scholar]
- 81.van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011;98:18–27. doi: 10.1002/bjs.7304. [DOI] [PubMed] [Google Scholar]
- 82.Lee JK, Kwak KK, Park JK, Yoon WJ, Lee SH, Ryu JK, Kim YT, Yoon YB. The efficacy of nonsurgical treatment of infected pancreatic necrosis. Pancreas. 2007;34:399–404. doi: 10.1097/MPA.0b013e318043c0b1. [DOI] [PubMed] [Google Scholar]
- 83.Chang YC, Tsai HM, Lin XZ, Chang CH, Chuang JP. No debridement is necessary for symptomatic or infected acute necrotizing pancreatitis: delayed, mini-retroperitoneal drainage for acute necrotizing pancreatitis without debridement and irrigation. Dig Dis Sci. 2006;51:1388–1395. doi: 10.1007/s10620-006-9112-6. [DOI] [PubMed] [Google Scholar]
- 84.Bruennler T, Langgartner J, Lang S, Wrede CE, Klebl F, Zierhut S, Siebig S, Mandraka F, Rockmann F, Salzberger B, et al. Outcome of patients with acute, necrotizing pancreatitis requiring drainage-does drainage size matter? World J Gastroenterol. 2008;14:725–730. doi: 10.3748/wjg.14.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mortelé KJ, Girshman J, Szejnfeld D, Ashley SW, Erturk SM, Banks PA, Silverman SG. CT-guided percutaneous catheter drainage of acute necrotizing pancreatitis: clinical experience and observations in patients with sterile and infected necrosis. AJR Am J Roentgenol. 2009;192:110–116. doi: 10.2214/AJR.08.1116. [DOI] [PubMed] [Google Scholar]
- 86.Besselink MG, van Santvoort HC, Nieuwenhuijs VB, Boermeester MA, Bollen TL, Buskens E, Dejong CH, van Eijck CH, van Goor H, Hofker SS, et al. Minimally invasive ‘step-up approach’ versus maximal necrosectomy in patients with acute necrotising pancreatitis (PANTER trial): design and rationale of a randomised controlled multicenter trial [ISRCTN13975868] BMC Surg. 2006;6:6. doi: 10.1186/1471-2482-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–764. doi: 10.1053/gast.1996.v111.pm8780582. [DOI] [PubMed] [Google Scholar]
- 88.Papachristou GI, Takahashi N, Chahal P, Sarr MG, Baron TH. Peroral endoscopic drainage/debridement of walled-off pancreatic necrosis. Ann Surg. 2007;245:943–951. doi: 10.1097/01.sla.0000254366.19366.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seewald S, Groth S, Omar S, Imazu H, Seitz U, de Weerth A, Soetikno R, Zhong Y, Sriram PV, Ponnudurai R, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm (videos) Gastrointest Endosc. 2005;62:92–100. doi: 10.1016/s0016-5107(05)00541-9. [DOI] [PubMed] [Google Scholar]
- 90.Voermans RP, Veldkamp MC, Rauws EA, Bruno MJ, Fockens P. Endoscopic transmural debridement of symptomatic organized pancreatic necrosis (with videos) Gastrointest Endosc. 2007;66:909–916. doi: 10.1016/j.gie.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 91.Escourrou J, Shehab H, Buscail L, Bournet B, Andrau P, Moreau J, Fourtanier G. Peroral transgastric/transduodenal necrosectomy: success in the treatment of infected pancreatic necrosis. Ann Surg. 2008;248:1074–1080. doi: 10.1097/SLA.0b013e31818b728b. [DOI] [PubMed] [Google Scholar]
- 92.Charnley RM, Lochan R, Gray H, O’Sullivan CB, Scott J, Oppong KE. Endoscopic necrosectomy as primary therapy in the management of infected pancreatic necrosis. Endoscopy. 2006;38:925–928. doi: 10.1055/s-2006-944731. [DOI] [PubMed] [Google Scholar]
- 93.Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, Kreitmair C, Meining A, Wehrmann T, Rösch T. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study) Gut. 2009;58:1260–1266. doi: 10.1136/gut.2008.163733. [DOI] [PubMed] [Google Scholar]
- 94.Gardner TB, Coelho-Prabhu N, Gordon SR, Gelrud A, Maple JT, Papachristou GI, Freeman ML, Topazian MD, Attam R, Mackenzie TA, et al. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73:718–726. doi: 10.1016/j.gie.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 95.Fabbri C, Luigiano C, Cennamo V, Polifemo AM, Barresi L, Jovine E, Traina M, D’Imperio N, Tarantino I. Endoscopic ultrasound-guided transmural drainage of infected pancreatic fluid collections with placement of covered self-expanding metal stents: a case series. Endoscopy. 2012;44:429–433. doi: 10.1055/s-0031-1291624. [DOI] [PubMed] [Google Scholar]
- 96.Antillon MR, Bechtold ML, Bartalos CR, Marshall JB. Transgastric endoscopic necrosectomy with temporary metallic esophageal stent placement for the treatment of infected pancreatic necrosis (with video) Gastrointest Endosc. 2009;69:178–180. doi: 10.1016/j.gie.2008.03.1066. [DOI] [PubMed] [Google Scholar]
- 97.Sarkaria S, Sethi A, Rondon C, Lieberman M, Srinivasan I, Weaver K, Turner BG, Sundararajan S, Berlin D, Gaidhane M, et al. Pancreatic necrosectomy using covered esophageal stents: a novel approach. J Clin Gastroenterol. 2014;48:145–152. doi: 10.1097/MCG.0b013e3182972219. [DOI] [PubMed] [Google Scholar]
- 98.Attam R, Trikudanathan G, Arain M, Nemoto Y, Glessing B, Mallery S, Freeman ML. Endoscopic transluminal drainage and necrosectomy by using a novel, through-the-scope, fully covered, large-bore esophageal metal stent: preliminary experience in 10 patients. Gastrointest Endosc. 2014;80:312–318. doi: 10.1016/j.gie.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 99.Mukai S, Itoi T, Sofuni A, Tsuchiya T, Gotoda T, Moriyasu F. Clinical evaluation of endoscopic ultrasonography-guided drainage using a novel flared-type biflanged metal stent for pancreatic fluid collection. Endosc Ultrasound. 2015;4:120–125. doi: 10.4103/2303-9027.156738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smoczyński M, Marek I, Dubowik M, Rompa G, Kobiela J, Studniarek M, Pieńkowska J, Adrych K. Endoscopic drainage/debridement of walled-off pancreatic necrosis--single center experience of 112 cases. Pancreatology. 2014;14:137–142. doi: 10.1016/j.pan.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 101.Bang JY, Varadarajulu S. Endoscopic ultrasound-guided management of pancreatic pseudocysts and walled-off necrosis. Clin Endosc. 2014;47:429–431. doi: 10.5946/ce.2014.47.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gardner TB, Chahal P, Papachristou GI, Vege SS, Petersen BT, Gostout CJ, Topazian MD, Takahashi N, Sarr MG, Baron TH. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009;69:1085–1094. doi: 10.1016/j.gie.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 103.Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053–1061. doi: 10.1001/jama.2012.276. [DOI] [PubMed] [Google Scholar]
- 104.Kumar N, Conwell DL, Thompson CC. Direct endoscopic necrosectomy versus step-up approach for walled-off pancreatic necrosis: comparison of clinical outcome and health care utilization. Pancreas. 2014;43:1334–1339. doi: 10.1097/MPA.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neoptolemos JP, London NJ, Carr-Locke DL. Assessment of main pancreatic duct integrity by endoscopic retrograde pancreatography in patients with acute pancreatitis. Br J Surg. 1993;80:94–99. doi: 10.1002/bjs.1800800131. [DOI] [PubMed] [Google Scholar]
- 106.Howard TJ, Moore SA, Saxena R, Matthews DE, Schmidt CM, Wiebke EA. Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery. 2004;136:909–916. doi: 10.1016/j.surg.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 107.Nealon WH, Bhutani M, Riall TS, Raju G, Ozkan O, Neilan R. A unifying concept: pancreatic ductal anatomy both predicts and determines the major complications resulting from pancreatitis. J Am Coll Surg. 2009;208:790–799; discussion 799-801. doi: 10.1016/j.jamcollsurg.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 108.Trevino JM, Tamhane A, Varadarajulu S. Successful stenting in ductal disruption favorably impacts treatment outcomes in patients undergoing transmural drainage of peripancreatic fluid collections. J Gastroenterol Hepatol. 2010;25:526–531. doi: 10.1111/j.1440-1746.2009.06109.x. [DOI] [PubMed] [Google Scholar]
- 109.Lau ST, Simchuk EJ, Kozarek RA, Traverso LW. A pancreatic ductal leak should be sought to direct treatment in patients with acute pancreatitis. Am J Surg. 2001;181:411–415. doi: 10.1016/s0002-9610(01)00606-7. [DOI] [PubMed] [Google Scholar]
- 110.Iso Y, Kubota K. Intragastric stapled pancreatic pseudocystgastrostomy under endoscopic guidance. Surg Laparosc Endosc Percutan Tech. 2013;23:330–333. doi: 10.1097/SLE.0b013e31828e3675. [DOI] [PubMed] [Google Scholar]


