Abstract
Background
Atopic dermatitis (AD) is an inflammatory skin condition that can occur in early life, predisposing to asthma development in a phenomenon known as the atopic march. Although genetic and environmental factors are known to contribute to AD and asthma, the mechanisms underlying the atopic march remain poorly understood. Filaggrin loss-of-function mutations are a major genetic predisposer for the development of AD and progression to AD-associated asthma.
Objective
We sought to experimentally address whether filaggrin mutations in mice lead to the development of spontaneous eczematous inflammation and address the aberrant immunologic milieu arising in a mouse model of filaggrin deficiency.
Methods
Filaggrin mutant mice were generated on the proallergic BALB/c background, creating a novel model for the assessment of spontaneous AD-like inflammation. Independently recruited AD case collections were analyzed to define associations between filaggrin mutations and immunologic phenotypes.
Results
Filaggrin-deficient mice on a BALB/c background had profound spontaneous AD-like inflammation with progression to compromised pulmonary function with age, reflecting the atopic march in patients with AD. Strikingly, skin inflammation occurs independently of adaptive immunity and is associated with cutaneous expansion of IL-5–producing type 2 innate lymphoid cells. Furthermore, subjects with filaggrin mutations have an increased frequency of type 2 innate lymphoid cells in the skin in comparison with control subjects.
Conclusion
This study provides new insights into our understanding of the atopic march, with innate immunity initiating dermatitis and the adaptive immunity required for subsequent development of compromised lung function.
Key words: Allergy, asthma, atopic dermatitis, atopy, eczema, filaggrin, flaky tail, type 2 innate lymphoid cells, innate immunity, mouse, mutation
Abbreviations used: AD, Atopic dermatitis; AHR, Airway hyperresponsiveness; Cdyn, Dynamic lung compliance; CFP, Cerulean fluorescent protein; dLN, Draining lymph node; eGFP, Enhanced green fluorescent protein; FLG, Human filaggrin gene; Flg, Murine filaggrin gene; iILC2, Inflammatory type 2 innate lymphoid cells; ILC2, Type 2 innate lymphoid cell; IL-7Rα, IL-7 receptor α; NBNT, Non-B/non-T cell; NF-κB, Nuclear factor κB; nILC2, Natural type 2 innate lymphoid cell; RL, Lung resistance; ROR, Retinoic acid–related orphan receptor; TSLP, Thymic stromal lymphopoietin; WT, Wild-type
There has been a profound increase in the incidence of atopic disease morbidity in developed societies in recent decades. Atopic individuals, who are characterized by increased serum IgE levels, are predisposed to having allergies such as atopic dermatitis (AD) and asthma. AD is heritable and characterized by pruritic eczematous lesions, with approximately 20% of children affected in the developed world.1 The cause of AD is multifactorial, with interplay between genetic predisposition and environmental factors initiating aberrant inflammation.2 The term atopic march encapsulates the predisposition of patients with AD in infancy to progress to secondary allergic disorders, such as asthma.3 Although AD as the first manifestation of atopic diathesis in early life is well established, how AD development primes progression to secondary allergies is not known.
Loss-of-function mutations in the human filaggrin gene (FLG) have been identified as the major genetic predisposing factor for AD development,4, 5, 6 and in the context of the atopic march, patients with AD with FLG mutations are predisposed to the development of asthma.7, 8 We previously identified a mutation in the murine filaggrin gene (Flg) in the “flaky tail” double-mutant (Mattma/maFlgft/ft) mouse strain, resulting in a lack of filaggrin protein in the skin.9 We recently separated the matted and filaggrin mutations present in Mattma/maFlgft/ft flaky tail mice.10 We now show that filaggrin-deficient mice, analogous to FLG mutations in human subjects, have spontaneous dermatitis, become atopic and progress to lung inflammation with age. By using a mouse with a mutation in a gene implicated in the atopic march in human subjects, the roles of innate versus adaptive immunity are shown in the initial development of dermatitis and progression to aberrant lung inflammation. Filaggrin-deficient mice on a BALB/c background have a spontaneous expansion of IL-5–producing type 2 innate lymphoid cells (ILC2s) into the skin, with an increase in skin ILC2 numbers also seen in patients with FLG mutations, reinforcing the role of innate immunity in the development of AD.
Methods
Mice
All mice were congenic BALB/c strain, with BALB/c mice used as wild-type (WT) control animals. The Flgft and ma mutations in flaky tail (Mattma/maFlgft/ft) mice (Stock a/a ma ft/ma ft/JSun; JR#9078; Jackson Laboratories, Bar Harbor, Me) were separated, and the Flgft mutation was backcrossed to the congenic C57BL/6J background in accordance with previously published methods.10 Flgft/ft C57BL/6J congenic mice were subsequently backcrossed to the congenic BALB/c background, and these mice were used in this study. Il4KN2,11 Il5CFP, Il13eGFP,12 Il17eGFP (Biocytogen, Worcester, Mass), and Rag1-deficient (Jackson Laboratories) mice were crossed with Flgft/ft mice in house. Mice expressing the luciferase transgene under the control of a nuclear factor κB (NF-κB) promoter (NF-κB-Luc; Caliper Life Sciences, Hopkinton, Mass) were crossed to Flgft/ft mice.
Mice were housed in specific pathogen-free conditions, with irradiated diet and bedding and water ad libitum. All animal experiments were performed in compliance with the Irish Department of Health and Children regulations and approved by Trinity College Dublin's BioResources ethical review board.
Clinical scoring
The severity of skin inflammation was clinically scored longitudinally by using a system based on the macroscopic diagnostic criteria described by Saunders et al10 and adapted from assessment of skin inflammation in the Nc/Nga mouse model.13 In brief, a scoring system (0, none; 1, mild; 2, moderate; and 3, severe) was applied to the signs of edema, erythema, scaling, and erosion. The total score for each mouse was calculated from the sum of individual scores for each parameter.
Analysis of airway hyperresponsiveness
Lung function or airway hyperresponsiveness (AHR) was analyzed in 32-week-old mice by using an invasive method in which mice were tracheostomized and ventilated with whole-body plethysmography14 by using a pneumotachograph linked to a transducer (EMMS, Hants, United Kingdom). Changes in lung resistance (RL) and dynamic lung compliance (Cdyn) in response to increasing doses of nebulized and inhaled methacholine (10, 30, 60, and 100 mg/mL; Sigma-Aldrich, St Louis, Mo) were recorded, as previously described.9, 15
Flow cytometric and cytokine analyses of human suction blisters
Suction blistering was performed on patient donors after obtaining informed written consent, and sample use was given ethical approval from the NRES Committee South Central, United Kingdom. Patients with moderate-to-severe AD were recruited and genotyped for FLG mutations (see this article's Online Repository at www.jacionline.org).5 Patients with WT, heterozygous, and compound heterozygous FLG status were included in the study. Suction blister cups were applied to the skin of patients with a vacuum pressure of 200 to 400 mm Hg, as previously described.16 Blisters were formed within 60 to 90 minutes, and suction was then removed. Twenty-four hours later, fluid was aspirated with a 30-gauge needle. Fluids were centrifuged at 1500 rpm for 5 minutes at 4°C, and cell pellets were resuspended in RPMI 1640 supplemented with 10% human serum.
For surface staining, single-cell suspensions were prepared in flow cytometry buffer. Live/dead violet (Invitrogen, Carlsbad, Calif) was used to determine cell viability. Directly conjugated antibodies with fluorescein isothiocyanate, phycoerythrin, phycoerythrin–Texas Red, peridinin-chlorophyll-protein complex, peridinin-chlorophyll-protein complex–Cy5.5, PeCy7, V450, allophycocyanin, and allophycocyanin-Cy7 were used. Human cells were stained with the BioLegend (San Diego, Calif) mAbs CD4 (MEM-241), CD8 (RPA-T8), CD11b (DCIS1/18), CD45 (H130), CD56 (B159), FcεRI (AER-37 [CRA-1]), and IL-7 receptor α (IL-7Rα; A019D5); the BD Biosciences (San Jose, Calif) mAbs CD3 (SK7), CD19 (SJ25C1), and CD14 (MφP9); the Abcam (Cambridge, United Kingdom) mAb CD11c (BU15); the Miltenyi Biotec (Bergisch Gladbach, Germany) mAb chemoattractant receptor–homologous molecule expressed on TH2 lymphocytes (BM16); and the R&D Systems (Minneapolis, Minn) mAb CD123 (FAB301C). Cells were acquired by using FACSDiva (BD Biosciences) or Summit software (Beckman Coulter, High Wycombe, United Kingdom) on an LSRFortessa or CyAn flow Cytometer, respectively. Lineage gating included CD3, CD4, CD8, CD14, CD19, CD56, CD11c, CD11b, FcεRI, and CD123. ILC2s were defined as Lin−CD45+IL-7Rα+ chemoattractant receptor–homologous molecule expressed on TH2 lymphocytes positive. FlowJo (TreeStar, Ashland, Ore) and Summit software were used for further data analysis. Blister fluid was analyzed with the MAGPIX Multiplex Array (Luminex, Austin, Tex), according to the manufacturer's instructions. Quantification of ILC2s and IL-1β levels in patient samples was performed in a blinded manner.
Statistical analyses
Data are expressed as means ± SEMs and analyzed by using 2-way ANOVA or the unpaired Student t tests (Prism 6; GraphPad Software, La Jolla, Calif).
Results
Filaggrin deficiency leads to spontaneous dermatitis and atopy
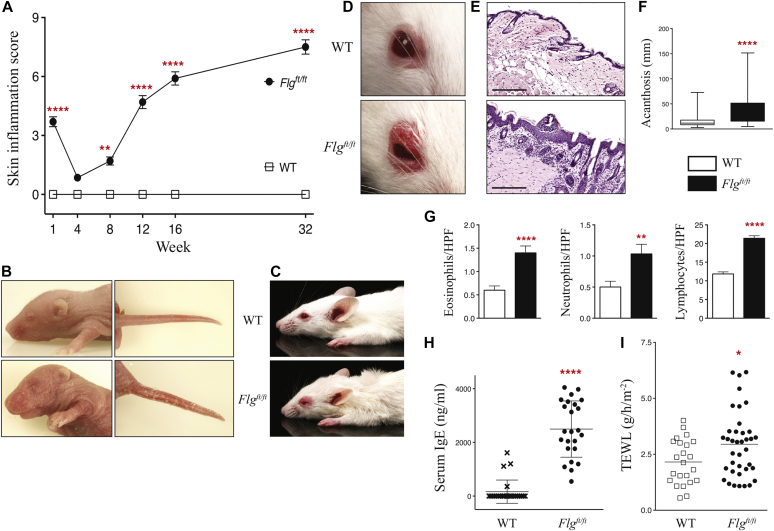
Single mutant Flgft congenic mice without the Mattma mutation were generated (see Fig E1 in this article's Online Repository at www.jacionline.org) on the proallergic BALB/c background.17, 18, 19 Flgft/ft mice have attenuated profilaggrin expression in the epidermis and absent functional filaggrin monomer (see Fig E2 in this article's Online Repository at www.jacionline.org), which is similar to what is seen in FLG-null patients.5 As neonates, Flgft/ft mice spontaneously have marked ichthyosis-like dermatitis with edema, erythema, hyperlinearity, and scaling compared with WT control animals (Fig 1, A and B). Longitudinal clinical scoring of skin inflammation shows that the early ichthyosis-like dermatitis observed in neonatal Flgft/ft mice dissipates by 4 weeks, with significant (P < .01) spontaneous eczematous-like dermatitis developing in Flgft/ft mice from 8 weeks (Fig 1, A and C). By 12 weeks, all Flgft/ft mice have overt dermatitis, with eczematous lesions occurring initially in eyelid skin (Fig 1, A and D). The dermatitis, which is characterized by edema, erythema, scaling, and lichenification (Fig 1, D), progresses with age to excoriation and severe pathology, with pruritic erythematous lesions progressing beyond the eyelid skin to around the eye and rostrum at 32 weeks (see Fig E3, A, in this article's Online Repository at www.jacionline.org). Histopathologic analysis of skin at 12 weeks demonstrates profound acanthosis (P < .0001) in Flgft/ft mice (Fig 1, F), and significant infiltration of eosinophils (P < .0001), neutrophils (P < .01), and lymphocytes (P < .0001) into the dermis (Fig 1, G). By 32 weeks, an increasing incidence of erythema and edema is evident in tail skin (see Fig E3, B) and the ear pinnae (see Fig E3, C), indicating a spectrum of pathology at these sites. Ear histopathology in Flgft/ft mice (see Fig E3, C) shows significantly increased acanthosis (see Fig E3, D) and inflammatory cell infiltrates in the dermis (data not shown). Thus Flgft/ft mice on a BALB/c background spontaneously have ichthyosis as neonates and frank eczematous dermatitis in adulthood.
Fig 1.
Development of dermatitis and atopy in filaggrin-deficient mice. A, Macroscopic clinical scoring of Flgft/ft versus WT mice. Data are from 25 to 30 mice per strain (scored longitudinally). Statistical significance was determined with 2-way ANOVA. B, Gross phenotype of a representative Flgft/ft neonate in comparison with a WT littermate. C, Gross phenotype of Flgft/ft and WT mice (age matched at 12 weeks). D, Representative image of the eczematous inflammation that develops in the eyelid skin of Flgft/ft mice at 12 weeks. E, Representative hematoxylin and eosin–stained biopsy specimens of eyelid skin from 12-week-old Flgft/ft and WT mice. Scale bar = 200 μm. F and G, Epidermal acanthosis scoring (Fig 1, F) and dermal eosinophil, neutrophil, and lymphocyte counts per high-power field (HPF; Fig 1, G) in lesional skin. Data are from 6 to 10 mice per strain. H, Total serum IgE levels from adult mutant and 12-week-old age-matched WT mice. I, Transepidermal water loss (TEWL) at 12 weeks. Data are from 23 to 38 mice per strain. *P < .05, **P < .01, and ****P < .0001.
Filaggrin-deficient mice are atopic with an altered immunologic cutaneous environment
An increased IgE level is a cardinal marker of AD.20 Flgft/ft mice had significantly (P < .0001) increased serum IgE levels at 12 weeks (Fig 1, H), indicating AD-like dermatitis. Addressing skin barrier integrity, the significantly (P < .05) increased transepidermal water loss21 demonstrated skin barrier dysregulation in Flgft/ft mice (Fig 1, I). By using NF-κB reporter mice, NF-κB activation was observed in the skin of Flgft/ftNF-κB–Luc neonates (see Fig E3, E), and the level of NF-κB activation was significantly increased in nonlesional skin of 12-week-old adult Flgft/ftNF-κB–Luc mice (see Fig E3, F and G). Furthermore, Flgft/ft mice have significantly increased contact hypersensitivity skin inflammation (P < .01) in response to oxazolone hapten22 at a dose evoking limited skin inflammation in WT mice (see Fig E4 in this article's Online Repository at www.jacionline.org). Therefore filaggrin deficiency leads to a defective skin barrier, with subclinical cutaneous inflammation in nonlesional skin, and is accompanied by a lower threshold for skin inflammation after exposure to hapten.
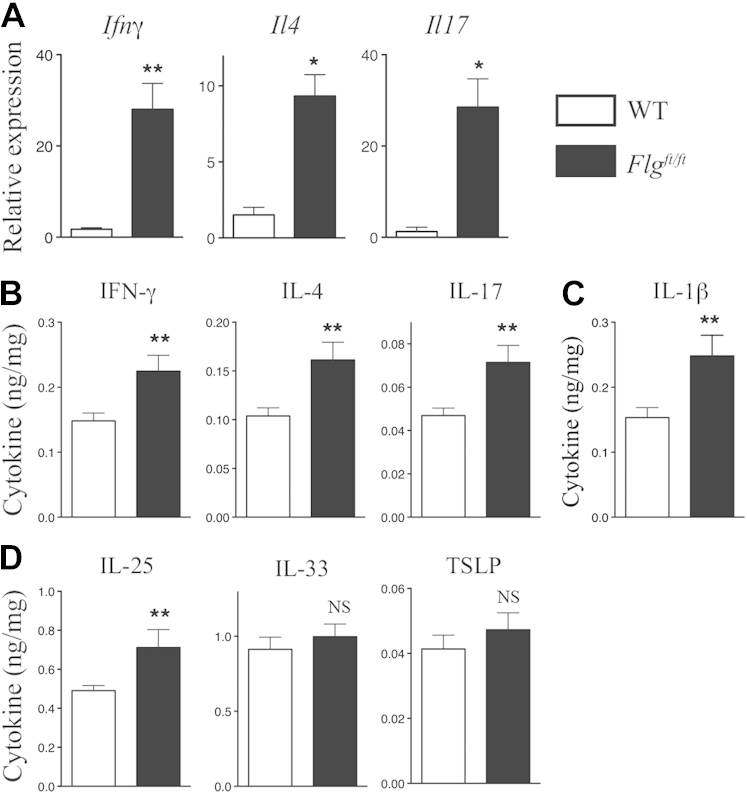
Gene expression analysis of lesional eyelid skin in 12-week-old Flgft/ft mice (Fig 2, A) demonstrated increased Ifng, Il4, and Il17 transcripts, which are typical of mixed type 1, 2, and 17 cutaneous cytokine responses in lesional inflamed skin. Given the dysregulated skin barrier and increased NF-κB activity in the uninvolved skin of Flgft/ft mice, the basal inflammatory state of nonlesional skin was addressed by quantifying cytokines in Flgft/ft and WT skin (Fig 2, C-E, and see Fig E5 in this article's Online Repository at www.jacionline.org). In nonlesional Flgft/ft skin there was a significant (P < .01) approximately 50% upregulation in the levels of IL-4, IL-17, and IFN-γ (Fig 2, B) in addition to IL-1β (P < .01; Fig 2, C). Because the alarmin cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) are implicated in the pathogenesis of allergic skin inflammation in experimental models and patients with AD,16, 23, 24, 25, 26 we evaluated alarmin expression in Flgft/ft mice. IL-25 was significantly (P < .05) upregulated in Flgft/ft skin, whereas IL-33 and TSLP levels were not (Fig 2, D). There were no differences between Flgft/ft and WT mice for other cytokines assayed (see Fig E5). These data demonstrate that filaggrin-deficient mice spontaneously become atopic, with dysregulated skin barrier function. Flgft/ft mice have cutaneous subclinical inflammation characterized by a generalized immune response with increased cardinal TH1, TH2, and TH17 cytokine levels, as well as selective upregulation of IL-1β and IL-25 in nonlesional skin.
Fig 2.
Skin inflammation in filaggrin-deficient mice. A, Fold change in Ifng, Il4, and Il17 mRNA expression (n = 6-8 per group) in skin. Data are representative of 3 experiments. B-D, Cytokine quantification in nonlesional skin expressed as nanograms of cytokine per milligram of protein. Data are from 25 to 35 mice per strain. Statistical significance was determined with the Student t test. *P < .05 and **P < .01. NS, Nonsignificant.
Filaggrin-deficient mice have an expansion of type 2 innate lymphoid cells in the skin
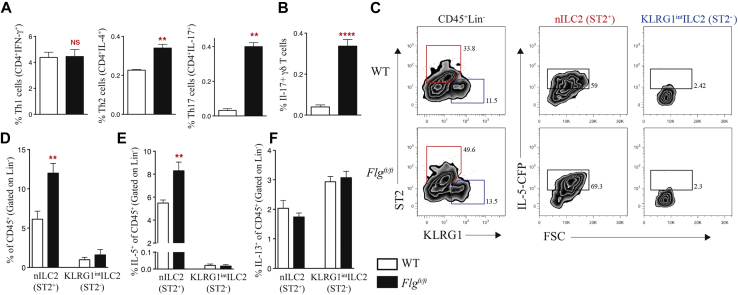
Because IFN-γ, IL-4, and IL-17 levels were increased in the skin of Flgft/ft mice, we examined the cellular source of these cytokines. IFN-γ+CD4+ TH1 cell frequency in the draining lymph nodes (dLNs) was comparable between Flgft/ft and WT mice (Fig 3, A). Generating dual Flgft/ft-IL-4–KN211 reporter mice demonstrated a significantly (P < .01) increased frequency of TH2 cells in Flgft/ft mice (Fig 3, A). The use of Flgft/ftIL-17–enhanced green fluorescent protein (eGFP) reporter mice showed increased frequency (P < .01) of both IL-17–eGFP+CD4+ TH17 cells (Fig 3, A) and IL-17–eGFP+ γδ T cells (Fig 3, B) in the dLNs of Flgft/ft mice.
Fig 3.
Filaggrin deficiency leads to an increase in ILC2 numbers in the skin. A, Frequency of TH1 (CD4+IFN-γ+), TH2 (CD4+IL-4+), and TH17 (CD4+IL-17+) cells as a percentage of total cells in skin dLNs. B, Frequency of IL-17+ γδ T cells as a percentage of total cells in skin dLNs. C, Expression of ST2 and KLRG1 on CD45+Lin− cells in the skin; outlined areas indicate nILC2s (red) or KLRG1int ILC2s (blue). D, Frequency of nILC2s (ST2+) and KLRG1int ILC2s (ST2−) of CD45+ cells (gated on Lin− cells) between Flgft/ft and WT mice. E, Frequency of IL-5+ nILC2s (ST2+) and IL-5+ KLRG1int ILC2s (ST2−) of CD45+ cells (gated on Lin− cells) between Flgft/ft and WT mice. F, Frequency of IL-13+ nILC2s (ST2+) and IL-13+ KLRG1int ILC2 (ST2−) of CD45+ cells (gated on Lin− cells) between Flgft/ft and WT mice. Data are representative of 3 experiments (n = 6-10 mice per group). Statistical significance was determined with the Student t test. **P < .01 and ****P < .0001. NS, Nonsignificant.
The alterations in type 2 and type 17 responses led us to examine the role of the recently described innate lymphoid cells, which were classified as negative for lineage markers and expressing IL-7Rα (CD127), CD25, and CD90, which have been investigated in a number of inflammatory diseases.27, 28, 29 ILC2s are implicated in allergy; are regulated by IL-25, IL-33, and TSLP; are characterized by expression of the transcription factors GATA3 and retinoic acid–related orphan receptor (ROR) α; and produce the type 2 cytokines IL-5, IL-9, and IL-13.27, 29, 30 Recently ILC2 numbers have been shown to be increased in the skin of patients with AD and also in mouse skin after chemical (MC903)– and allergen (house dust mite)–elicited cutaneous inflammation.16, 25 In the skin of WT mice, numbers of resident ILC2s (Lin−ST2+KLRG1loIL-7Rα+Thy-1loSca-1hi; see Fig E6, A, in this article's Online Repository at www.jacionline.org) correspond to the natural type 2 innate lymphoid cell (nILC2) classification that has recently been defined as distinct from the IL-25–elicited inflammatory type 2 innate lymphoid cell (iILC2) population in the lung.31 In the skin of Flgft/ft mice, there is a significant (P < .01) increase in the frequency of nILC2s compared with the frequency in WT animals (Fig 3, C and D). In addition, there is a KLRG1int ILC2 in mouse skin (see Fig E6, A), which lacks ST2 expression consistent with iILC2s31; however, iILC2s are KLRG1hi and are a distinct population. There was no difference in the frequency of KLRG1int ILC2s in Flgft/ft mice compared with WT mice (Fig 3, C and D). Consistent with the absence of the iILC2s in the skin of Flgft/ft mice, we also do not see this population after 4 days of treatment of WT ear skin with MC903 (see Fig E7, A, in this article's Online Repository at www.jacionline.org). However, as reported,31 after 3 days of intraperitoneal treatment with recombinant IL-25 but not IL-33, iILC2 numbers are increased in the lungs of WT mice (see Fig E7, B). In contrast to the lung, after 3 days of intradermal treatment in ear skin with recombinant IL-25, there is a negligible influx of iILC2s (see Fig E7, C), indicating that iILC2s might not be upregulated in the skin on inflammation.
Previously, a study demonstrated, by using quantitative RT-PCR, that activated skin ST2+ ILC2s produced more IL-5, leading to eosinophil influx and development of spontaneous dermatitis.26 We have generated a novel IL-5–cerulean fluorescent protein (CFP) reporter mouse to accurately quantify IL-5–expressing ILC2s in the skin (see Fig E8, A-C, in this article's Online Repository at www.jacionline.org). In WT mice nILC2s in the skin produce IL-5 in the steady state (see Fig E6, B and C). Strikingly, having generated Flgft/ftIL-5–CFP reporter mice, there was a significant (P < .01) increase in the frequency of IL-5–producing nILC2s in Flgft/ft mice compared with levels in WT control animals (Fig 3, C and E). In addition, numbers of nILC2s (see Fig E9, A, in this article's Online Repository at www.jacionline.org) and IL-5–producing nILC2s (see Fig E9, B) were also increased in the skin dLNs of Flgft/ft relative to levels in WT mice. Because ILC2s express IL-13,12 IL-13–eGFP reporter mice were used to look at IL-13–expressing ILC2s in the skin. In the skin of WT mice, both nILC2s and KLRG1int ILC2s constitutively express IL-13 in the steady state, with KLRG1int ILC2s having marginally higher IL-13 expression (see Fig E6, D). However, Flgft/ftIL-13–eGFP reporter mice demonstrated no differences in the frequency of IL-13–producing nILC2s and KLRG1int ILC2s between Flgft/ft and WT mice (Fig 3, F). Using Flgft/ftIL-17–eGFP reporter mice, we examined whether the marked increase in IL-17–producing CD45+ cells in the skin of Flgft/ft mice (see Fig E9, C) correlated with an increase in IL-17 production by innate lymphoid cells. We found no IL-17–producing population in Flgft/ft mice (see Fig E9, D). No differences were observed in numbers of ILC3s (Lin−IL-7Rα+ST2−RORγT+) in the skin (see Fig E9, E). Similarly, there were no differences in numbers of ILC1s (Lin−IL-7Rα+ T-box transcription factor [T-bet]+; see Fig E9, F). Using Flgft/ftKN2 IL-4 reporter mice, we investigated whether increased numbers of IL-4–producing CD45+ cells in the skin of Flgft/ft mice (see Fig E9, G) corresponded to increased numbers of IL-4–producing ILC2s. No ILC2 population produced IL-4 in the skin of Flgft/ft mice (see Fig E9, H). In addition to IL-5–producing nILC2 expansion, there was significantly increased skin infiltration of eosinophils (P < .05; non-B/non-T-cells [NBNT] cells SiglecF+CD11b+), mast cells (P < .01; NBNT ckit+FcεR1+ cells), and basophils (P < .0001; NBNT FcεR1+ckit− cells) in Flgft/ft mice (see Fig E9, I). Collectively, Flgft/ft mice on a BALB/c background have a cutaneous expansion of IL-5–producing nILC2s, with a mixed type 2 and type 17 inflammatory milieu.
Filaggrin-deficient mice have spontaneous pulmonary inflammation
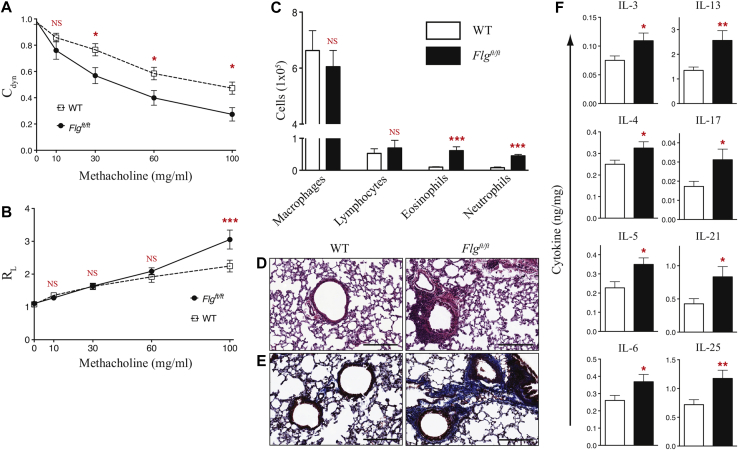
FLG mutations in human subjects predispose to asthma development after AD occurrence, exemplifying the atopic march.7, 8 Therefore we analyzed AHR in Flgft/ft mice. Similar to studies on flaky tail mice,9 16-week-old Flgft/ft mice had AHR comparable with that seen in WT control animals, with no pulmonary inflammation (data not shown) despite having dermatitis (Fig 1, A). When analyzing 32-week-old Flgft/ft mice with marked dermatitis, significantly altered Cdyn was observed (Fig 4, A). Flgft/ft mice had no differences in RL apart from at the highest methacholine concentration (Fig 4, B). Significant changes in dynamic lung compliance, but not resistance in Flgft/ft mice, suggests that aberrant lung function is predominately caused by peripheral alterations, such as lung parenchyma elasticity, with lesser effects on central airway function.32 In agreement with this altered lung function, there were significantly (P < .01) more cells in bronchoalveolar lavage fluid of Flgft/ft mice (see Fig E10, A, in this article's Online Repository at www.jacionline.org), with a significant (P < .001) increase in neutrophil and eosinophil numbers (Fig 4, C).
Fig 4.
Spontaneous lung inflammation in filaggrin-deficient mice. A and B, Measurement of AHR as assessed by Cdyn (Fig 4, A) and RL (Fig 4, B) in response to increasing doses of methacholine. Data are representative of 3 experiments (n = 6-8 mice per group). Statistical significance was determined with 2-way ANOVA. C, Differential cell counts from bronchoalveolar lavage fluid. Data are representative of 3 experiments (n = 6-8 mice per group). Statistical significance was determined with the Student t test. D and E, Representative hematoxylin and eosin–stained (Fig 4, D) and Masson trichrome–stained (Fig 4, E) lung tissue. Scale bars = 200 μm. F, Cytokine quantification in the lung expressed as nanograms of cytokine per milligram of protein. Data are from 16 to 20 mice per strain. Statistical significance was determined with the Student t test. *P < .05, **P < .01, and ***P < .001. NS, Nonsignificant.
The compromised lung function in Flgft/ft mice older than 24 weeks was reflected in significant lung pathology with mixed peribronchial cellular infiltrates observed in hematoxylin and eosin–stained sections (Fig 4, D). Flgft/ft mice did not have goblet cell hyperplasia, peribronchial eosinophilia, or marked airway occlusion (data not shown). Consistent with altered peripheral changes to the lungs of Flgft/ft mice, there was marked collagen deposition (Fig 4, E), with significantly increased (P < .05) collagen levels in the lungs of Flgft/ft mice (see Fig E10, B). Quantification of pulmonary eosinophil peroxidase and myeloperoxidase enzymatic activity (see Fig E10, C and D) indicated increased eosinophil and neutrophil activity in the lungs of Flgft/ft mice, which is in agreement with the increased numbers of eosinophils and neutrophils in bronchoalveolar lavage fluid (Fig 4, C). With respect to increased eosinophil numbers in the skin and lungs of deficient mice, we also noted inflammation in the upper esophagus of Flgft/ft mice (see Fig E11, A, in this article's Online Repository at www.jacionline.org) with significant (P < .05) eosinophil infiltration (see Fig E11, B). However, Flgft/ft mice do not have the overt esophageal pathology reported in food allergen–induced models of eosinophilic esophagitis.33
In the inflamed lungs of Flgft/ft mice, levels of the type 2 cytokines IL-4, IL-5, and IL-13 were significantly (P < .05-.01) increased (Fig 4, F). IL-17 levels were significantly (P < .05) increased in lung homogenates (Fig 4, F), as were levels of IL-3 (P < .05), IL-6 (P < .05), and IL-21 (P < .05). We also observed an increase in IL-25 levels in the lungs of Flgft/ft mice (Fig 4, F), whereas levels of the other epithelial cytokines (ie, IL-33 and TSLP) were unchanged (see Fig E10, E). Levels of other cytokines assayed were comparable in the lungs of WT and Flgft/ft mice (see Fig E10, E). These data demonstrate the development of marked pulmonary inflammation with age in Flgft/ft mice on a BALB/c background secondary to dermatitis development, with decreased lung compliance, increased parenchymal collagen deposition, and eosinophil and neutrophil infiltration with mixed type 2 and type 17 pulmonary inflammation.
Cutaneous inflammation occurs in filaggrin-deficient mice in the absence of adaptive immunity
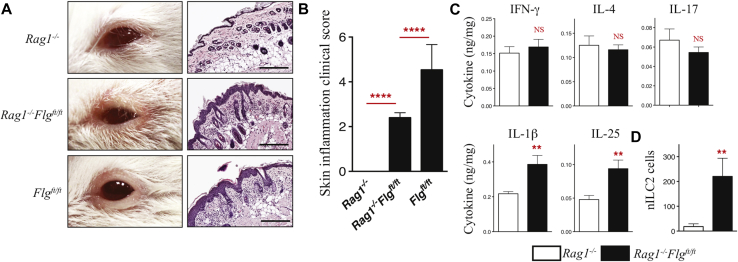
To assess the relative role of innate versus adaptive immunity in the context of spontaneous skin and lung inflammation caused by filaggrin deficiency, we crossed Flgft/ft and Rag1−/− mice, generating T cell– and B cell–deficient Rag1−/−Flgft/ft mice. Neonatal Rag1−/−Flgft/ft mice retain the erythematous scaly skin phenotype typical of Flgft/ft mice. Adult Rag1−/−Flgft/ft mice had eczematous eyelid lesions similar to Flgft/ft mice (Fig 5, A), with a significant clinical score (Fig 5, B). Histopathology reveals that Rag1−/−Flgft/ft mice have marked inflammation (Fig 5, A), with significantly increased epidermal acanthosis, and infiltration of eosinophils (P < .001) and neutrophils (P < .01) into the dermis (see Fig E12, A, in this article's Online Repository at www.jacionline.org) relative to Rag1−/− mice.
Fig 5.
Dermatitis develops in filaggrin-deficient mice independent of adaptive immunity. A, Representative images of inflammation in eyelid skin and representative hematoxylin and eosin–stained eyelid skin. Scale bars = 200 μm. B, Clinical scoring of eyelids at 12 weeks. Data are from 20 to 25 mice per strain. Statistical significance was determined with the Student t test. C, Cytokine quantification in nonlesional skin expressed as nanograms of cytokine per milligram of protein. Data are from 16 to 20 mice per strain. Statistical significance was determined with the Student t test. D, nILC2 numbers in the skin. Data are representative of 3 experiments (n = 6-10 mice per group). Statistical significance was determined with the Student t test. **P < .01 and ****P < .0001. NS, Nonsignificant.
Cytokine protein levels were assessed in nonlesional skin biopsy specimens of Rag1−/−Flgft/ft mice. Consistent with the cutaneous innate immune milieu observed in the skin of Flgft/ft mice, both IL-1β and IL-25 levels were significantly (P < .01) upregulated in nonlesional skin of Rag1−/−Flgft/ft mice relative to those in Rag1−/− control skin (Fig 5, C). However, IL-4, IL-17, and IFN-γ levels were unchanged in the skin (Fig 5, C), as were IL-33 and TSLP levels (see Fig E12, B). No increases were observed in the levels of other cytokines assayed (see Fig E12, B). Importantly, inflammation observed in Rag1−/−Flgft/ft mice is associated with a significant (P < .01) cutaneous nILC2 expansion relative to that seen in Rag1−/− mice (Fig 5, D). Rag1−/−Flgft/ft mice do not have lung inflammation, as measured based on compliance and resistance (see Fig E13, A and B, in this article's Online Repository at www.jacionline.org) and other parameters (data not shown), as observed in Flgft/ft mice. Although pulmonary IL-1β levels were increased (P < .05) in Rag1−/−Flgft/ft mice, there were no differences in the levels of other cytokines analyzed (see Fig E13, C).
Adult Rag1−/−Flgft/ft mice had eczematous eyelid lesions with significant clinical scoring (Fig 5, B) but no lung inflammation (see Fig E13, A-C). Rag1−/−Flgft/ft mice were reconstituted with B and T cells to address whether adaptive immunity exacerbated skin or lung inflammation. B cell– and T cell–reconstituted Rag1−/−Flgft/ft mice have more severe dermatitis relative to that seen in Rag1−/−Flgft/ft mice (see Fig E14, A, in this article's Online Repository at www.jacionline.org), with significantly increased clinical scores (see Fig E14, B). Furthermore, reconstituted Rag1−/−Flgft/ft mice had marked atopy, which was significantly greater than that seen in Rag1−/− mice receiving B and T cells (see Fig E14, C). In addition to more marked skin inflammation, B cell– and T cell–reconstituted Rag1−/−Flgft/ft mice had compromised lung function with a specific significant alteration in Cdyn, indicating progression to secondary lung inflammation (see Fig E14, D). B cell– and T cell–reconstituted Rag1−/−Flgft/ft mice had no differences in RL (see Fig E14, E). These data demonstrate that the spontaneous development of dermatitis caused by filaggrin deficiency is mediated by innate immunity involving upregulation of IL-1β, IL-25, and nILC2s, with adaptive immunity required for the development of severe skin pathology and progression to lung inflammation.
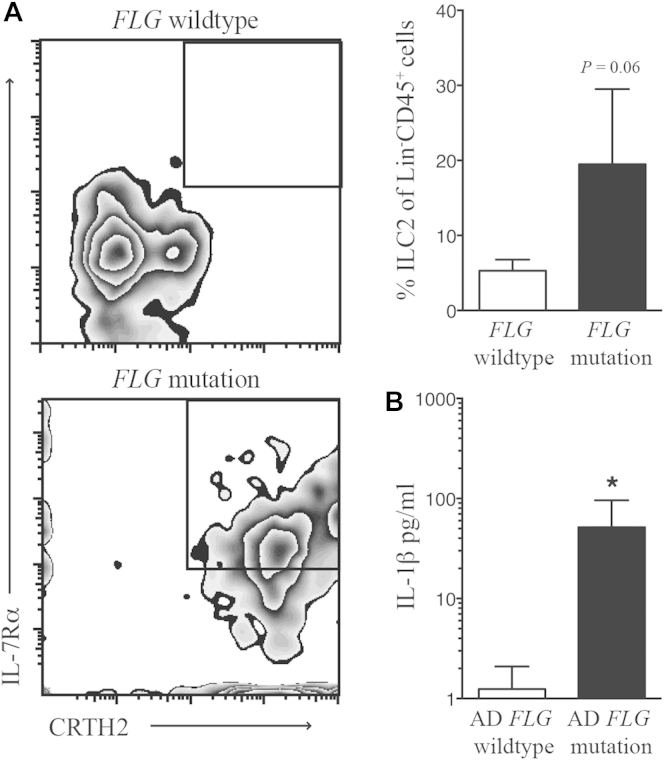
ILC2s are expanded in the skin of patients with FLG mutations
It has been reported recently that ILC2s are present in the skin of patients with AD.16, 25 Given the spontaneous expansion in ILC2 frequency in the skin of filaggrin-deficient mice (Fig 3), we investigated whether ILC2 frequency was altered in the skin of patients with mutations in FLG. We now show that there are increased ILC2 numbers (P = .06) in skin blisters taken from nonlesional skin of patients with FLG mutations5 compared with the skin of FLG WT subjects (Fig 6, A). Furthermore, similar to the increase in IL-1β levels in the skin of filaggrin-deficient mice (Fig 2), IL-1β levels are significantly upregulated within the blister fluid of acute lesional skin from patients with moderate-to-severe AD with FLG mutations compared with levels seen in those without FLG mutations (Fig 6, B).
Fig 6.
ILC2s are expanded in the skin of patients with FLG mutations. A, Chemoattractant receptor–homologous molecule expressed on TH2 lymphocytes (CRTH2)+IL-7Rα+ ILC2s (gated on Lin−CD45+ cells) are upregulated in skin suction blisters of patients with FLG mutations in comparison with those of patients without FLG mutations. B, IL-1β is upregulated in the blister fluid of acute lesional skin from patients with AD with FLG mutations compared with those without FLG mutations. Statistical significance was determined with the Mann-Whitney test. *P < .05.
Discussion
Filaggrin mutations have been identified as the major genetic predisposer to AD development and in the context of the atopic march, the subsequent progression to AD-associated asthma. We now show that filaggrin-deficient mice, which have a mutation analogous to the filaggrin mutations found in human subjects, are atopic, have spontaneous AD-like inflammation, and progress to pulmonary inflammation with age. Emerging evidence from genome-wide association studies and Immunochip and transcriptome analyses34, 35, 36, 37, 38, 39 has highlighted the complexity of genetic predisposition in human AD. The polygenetic nature of dermatitis is also evident in mice, with marked differences between BALB/c and C57BL/6J strains in the magnitude of skin inflammation and the functional genes involved.16, 25 Herein filaggrin-deficient mice were generated on a BALB/c background, a strain predisposed to type 2/type 17–associated inflammation.17, 18, 19, 40, 41, 42 BALB/c Flgft/ft mice have spontaneous AD-like skin inflammation and pulmonary inflammation unlike the filaggrin mutant on the C57BL/6J background10 and the Flg deletion knockout C57BL/6 mouse.43 These contrasting phenotypes in mice, highlighting the gene-modifying effects of strain background, are indicative of the complexity of how loss-of-function FLG mutations in human subjects can lead to AD.
Flgft/ft mice have neonatal ichthyosis, with eczematous AD inflammation developing with 100% penetrance in Flgft/ft mice by 12 weeks. Skin barrier dysregulation in the nonlesional skin of Flgft/ft mice, as evidenced by transepidermal water loss, reflects the epidermal barrier dysfunction that has been shown in nonlesional skin in patients with AD.44, 45 Furthermore, the increased susceptibility of Flgft/ft mice to contact hypersensitivity inflammation indicates that the dysregulated barrier can facilitate allergenic sensitization. Eczematous inflammation in filaggrin-deficient mice is characterized by increased type 1, type 2, and type 17 cytokines, indicating a generalized inflammatory response in lesional skin. Expansion of TH2 and TH17 cells in skin dLNs and IL-17–eGFP+ γδ T cells indicates that the mixed inflammatory cutaneous milieu resembles the dual TH2 and TH17 T-cell response in patients with AD.46 Importantly, development of spontaneous lesions in Rag1−/−Flgft/ft mice demonstrates that dermatitis can occur in the absence of adaptive immunity. However, we demonstrate that adaptive immunity is required for progression to secondary lung inflammation. A recent study demonstrated that Rag2−/−Flgft/ft mice (homozygous for Mattma) did not have dermatitis in the dorsal flank.47 Differences in terms of the development of skin inflammation between both studies may be due to the presence of the matted mutation (Mattma), which is still present in the Rag2−/−/Flgft/ft mice in the study by Leisten et al,47 confounding direct comparison with our findings, where only the Flgft mutation is present in mice; animal housing conditions might also be a factor. However, similar to our findings, this letter reports an increase in the frequency of Lin−CD3−Thy1+IL7R+ innate lymphoid cells in Rag2−/−Flgft/ft mice in comparison with that in Rag2−/− control animals.47
Increased NF-κB activity in nonlesional skin of Flgft/ft mice indicates basal subclinical cutaneous inflammation. Indeed, although relatively modest, the increased IL-4, IL-17, and IFN-γ protein levels in nonlesional skin demonstrated a generalized subclinical inflammatory milieu in the skin. Nonlesional skin was assessed to investigate inflammatory mechanisms in the barrier-dysregulated skin of Flgft/ft mice, avoiding potential complications of secondary inflammation associated with lesional skin. These data correlate with recent transcriptomic studies analyzing the uninvolved skin of patients with AD and filaggrin mutations, which demonstrated upregulation of TH1- and TH2-associated transcripts.39 Importantly, upregulation of IL-1β in the nonlesional skin of both Flgft/ft and Rag1−/−Flgft/ft mice is consistent with our previous work in which epidermal IL-1β levels were increased in Flgft/ft mice and also in patients with AD with filaggrin mutations,48 indicating a key role for IL-1β in the dysregulated cutaneous environment arising from filaggrin deficiency. Interestingly, we now show that IL-1β levels are upregulated in the blister fluid of acute lesional skin of patients with AD with FLG mutations in comparison with those in patients with AD with WT FLG.
IL-25 and IL-33 are overexpressed in skin of patients with AD,16, 49, 50, 51 with TSLP overexpression associated with skin inflammation in mice.52 Flgft/ft and Rag1−/−Flgft/ft mice have an increase in IL-25 levels, but not IL-33 and TSLP levels, in nonlesional skin. Recent studies have shown roles for IL-25, IL-33, and TSLP in eliciting ILC2s during cutaneous inflammation in transgenic mice after IL-2 treatment in response to chemical and allergen challenge and in the skin of patients with AD.16, 24, 25, 26 Previously, it has been demonstrated that dermal ILC2s in the steady state constitutively produce IL-13, but on activation, this population expanded and switched to a proinflammatory phenotype characterized by increased Il5 mRNA expression, which promoted eosinophil infiltration and spontaneous dermatitis.26 Importantly, we now show a specific upregulation of these activated IL-5–producing ILC2s in the skin of Flgft/ft mice using a novel IL-5–CFP reporter mouse. These ILC2s, which also constitutively express IL-13, correspond to the nILC2s described by Huang et al.31 After intraperitoneal recombinant IL-25 treatment, we also observe an increase in the lungs of the KLRG1hi iILC2 population recently described,31 but we do not observe this population in the skin of Flgft/ft mice or in ear skin of WT mice after IL-25 or MC903 treatment.
Further work is needed to define the expansion of distinct ILC2 subpopulations in different organs. Strikingly, the ILC2 expansion in filaggrin-deficient mice translates to patients, with ILC2 frequency increased in skin suction blisters of patients with FLG mutations compared with that seen in those without FLG mutations. Collectively, the increased frequency of ILC2s in the skin of human subjects with FLG mutations is comparable with the phenotype that develops spontaneously in filaggrin-deficient mice. Importantly, nILC2 numbers are also specifically increased in the skin of Rag1−/−Flgft/ft mice, indicating the importance of ILC2s in the pathogenesis of skin inflammation arising from skin barrier dysregulation caused by filaggrin deficiency. Indeed, Rag1−/− mice have dermatitis associated with ILC2 activity after treatment with IL-2–JES6-1.26 In addition to ILC2 expansion, we also observed an increase in the numbers of eosinophils, mast cells, and basophils in the skin of Flgft/ft mice, which is similar to the phenotype seen with IL-2–JES6-1–induced inflammation.26
Carriers of filaggrin mutations have an increased risk of AD-associated asthma.7, 8 Filaggrin-deficient mice have a striking age-dependent progression to pulmonary inflammation characterized by compromised lung function and involving parenchymal alterations in lung physiologic dynamics. Decreased compliance in filaggrin-deficient mice was associated with increased collagen deposition and eosinophil and neutrophil infiltration of the lungs with mixed type 2 and type 17 inflammatory responses, reflecting aspects of pulmonary pathology associated with multiple asthma phenotypes.53
Importantly, Rag1−/−Flgft/ft mice do not have lung pathology, demonstrating that the adaptive immune response is required for the progression from dermatitis to pulmonary inflammation.
In summary, filaggrin deficiency in mice leads to the development of features of the atopic march that occur in patients with AD with FLG mutations. This study highlights how skin inflammation in the context of dysregulated skin barrier function develops independently of the adaptive immune response, whereas the subsequent progression to compromised lung function requires adaptive immunity.
Key messages.
-
•
Filaggrin-deficient mice have spontaneous AD-like inflammation and progress to compromised pulmonary function, reflecting the atopic march in patients with AD.
-
•
AD-like inflammation in the context of filaggrin deficiency is associated with a cutaneous expansion in IL-5–producing ILC2 numbers in mice, and in patients with AD with FLG mutations, there is an increase in ILC2 infiltration of the skin.
-
•
In the absence of adaptive immunity, filaggrin-deficient mice experience spontaneous skin inflammation but do not have lung pathology.
Acknowledgments
We thank Professor W. H. Irwin McLean for his support and Professor Jamie Lee for kindly providing reagents. We also thank all the patients and donors involved in the study.
Footnotes
Supported by the Wellcome Trust (Programme grant 092530/Z/10/Z [to P.G.F.] and Investigator Award 100963/Z/13/Z [to A.N.J.M.]), UK-MRC (to A.N.J.M.), Science Foundation Ireland (10/IN.1/B3004 [to P.G.F.]), the National Children's Research Centre (to P.G.F.), Misses Barrie Charitable Trust (to G.S.O.), the NIHR Biomedical Research Centre Programme (to G.S.O.), and the Comprehensive Research Network (to G.S.O.).
Disclosure of potential conflict of interest: A. D. Irvine has received research support from the National Children's Research Centre and the Wellcome Trust. A. N. J. McKenzie has received research support from the American Asthma Foundation and Janssen Pharmaceuticals and has a patent with Janssen Pharmaceuticals. G. S. Ogg has received research support from the Medical Research Council, the Biomedical Research Centre, BMA, and Janssen; has received consultancy fees from Janssen, Novartis, and Lilly; is employed by Oxford University Hospitals and Oxford University; and has received travel support from Roche. P. G. Fallon has received research support from the Wellcome Trust, NCRC, and Science Foundation Ireland. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Deckers I.A., McLean S., Linssen S., Mommers M., van Schayck C.P., Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803. doi: 10.1371/journal.pone.0039803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 3.Spergel J.M. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. 2010;105:99–106. doi: 10.1016/j.anai.2009.10.002. quiz 7-9, 17. [DOI] [PubMed] [Google Scholar]
- 4.Irvine A.D., McLean W.H., Leung D.Y. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 5.Palmer C.N., Irvine A.D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S.P. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 6.Sandilands A., Terron-Kwiatkowski A., Hull P.R., O'Regan G.M., Clayton T.H., Watson R.M. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650–654. doi: 10.1038/ng2020. [DOI] [PubMed] [Google Scholar]
- 7.Palmer C.N., Ismail T., Lee S.P., Terron-Kwiatkowski A., Zhao Y., Liao H. Filaggrin null mutations are associated with increased asthma severity in children and young adults. J Allergy Clin Immunol. 2007;120:64–68. doi: 10.1016/j.jaci.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez E., Baurecht H., Herberich E., Wagenpfeil S., Brown S.J., Cordell H.J. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361–1370.e7. doi: 10.1016/j.jaci.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Fallon P.G., Sasaki T., Sandilands A., Campbell L.E., Saunders S.P., Mangan N.E. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders S.P., Goh C.S., Brown S.J., Palmer C.N., Porter R.M., Cole C. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J Allergy Clin Immunol. 2013;132:1121–1129. doi: 10.1016/j.jaci.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohrs K., Wakil A.E., Killeen N., Locksley R.M., Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neill D.R., Wong S.H., Bellosi A., Flynn R.J., Daly M., Langford T.K. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda H., Watanabe N., Geba G.P., Sperl J., Tsudzuki M., Hiroi J. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–466. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- 14.Finkelman F.D. Use of unrestrained, single-chamber barometric plethysmography to evaluate sensitivity to cholinergic stimulation in mouse models of allergic airway disease. J Allergy Clin Immunol. 2008;121:334–335. doi: 10.1016/j.jaci.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Amu S., Saunders S.P., Kronenberg M., Mangan N.E., Atzberger A., Fallon P.G. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124.e8. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Salimi M., Barlow J.L., Saunders S.P., Xue L., Gutowska-Owsiak D., Wang X. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oyoshi M.K., Elkhal A., Scott J.E., Wurbel M.A., Hornick J.L., Campbell J.J. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J Clin Invest. 2011;121:2210–2220. doi: 10.1172/JCI43586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spergel J.M., Mizoguchi E., Brewer J.P., Martin T.R., Bhan A.K., Geha R.S. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spergel J.M., Mizoguchi E., Oettgen H., Bhan A.K., Geha R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould H.J., Sutton B.J. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 21.Gupta J., Grube E., Ericksen M.B., Stevenson M.D., Lucky A.W., Sheth A.P. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725–730.e2. doi: 10.1016/j.jaci.2007.12.1161. [DOI] [PubMed] [Google Scholar]
- 22.Scharschmidt T.C., Man M.Q., Hatano Y., Crumrine D., Gunathilake R., Sundberg J.P. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol. 2009;124:496–506. doi: 10.1016/j.jaci.2009.06.046. e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He R., Oyoshi M.K., Garibyan L., Kumar L., Ziegler S.F., Geha R.S. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai Y., Yasuda K., Sakaguchi Y., Haneda T., Mizutani H., Yoshimoto T. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim B.S., Siracusa M.C., Saenz S.A., Noti M., Monticelli L.A., Sonnenberg G.F. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roediger B., Kyle R., Yip K.H., Sumaria N., Guy T.V., Kim B.S. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 28.Spits H., Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 29.Walker J.A., Barlow J.L., McKenzie A.N. Innate lymphoid cells—how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 30.Hams E., Fallon P.G. Innate type 2 cells and asthma. Curr Opin Pharmacol. 2012;12:503–509. doi: 10.1016/j.coph.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y., Guo L., Qiu J., Chen X., Hu-Li J., Siebenlist U. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irvin C.G., Bates J.H. Measuring the lung function in the mouse: the challenge of size. Respir Res. 2003;4:4. doi: 10.1186/rr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noti M., Wojno E.D., Kim B.S., Siracusa M.C., Giacomin P.R., Nair M.G. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellinghaus D., Baurecht H., Esparza-Gordillo J., Rodriguez E., Matanovic A., Marenholz I. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat Genet. 2013;45:808–812. doi: 10.1038/ng.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esparza-Gordillo J., Weidinger S., Folster-Holst R., Bauerfeind A., Ruschendorf F., Patone G. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009;41:596–601. doi: 10.1038/ng.347. [DOI] [PubMed] [Google Scholar]
- 36.Hirota T., Takahashi A., Kubo M., Tsunoda T., Tomita K., Sakashita M. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat Genet. 2012;44:1222–1226. doi: 10.1038/ng.2438. [DOI] [PubMed] [Google Scholar]
- 37.Paternoster L., Standl M., Chen C.M., Ramasamy A., Bonnelykke K., Duijts L. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet. 2012;44:187–192. doi: 10.1038/ng.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L.D., Xiao F.L., Li Y., Zhou W.M., Tang H.Y., Tang X.F. Genome-wide association study identifies two new susceptibility loci for atopic dermatitis in the Chinese Han population. Nat Genet. 2011;43:690–694. doi: 10.1038/ng.851. [DOI] [PubMed] [Google Scholar]
- 39.Cole C., Kroboth K., Schurch N.J., Sandilands A., Sherstnev A., O'Regan G.M. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol. 2014;134:82–91. doi: 10.1016/j.jaci.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosas L.E., Keiser T., Barbi J., Satoskar A.A., Septer A., Kaczmarek J. Genetic background influences immune responses and disease outcome of cutaneous L. mexicana infection in mice. Int Immunol. 2005;17:1347–1357. doi: 10.1093/intimm/dxh313. [DOI] [PubMed] [Google Scholar]
- 41.Alenius H., Laouini D., Woodward A., Mizoguchi E., Bhan A.K., Castigli E. Mast cells regulate IFN-gamma expression in the skin and circulating IgE levels in allergen-induced skin inflammation. J Allergy Clin Immunol. 2002;109:106–113. doi: 10.1067/mai.2002.120553. [DOI] [PubMed] [Google Scholar]
- 42.Von Stebut E., Ehrchen J.M., Belkaid Y., Kostka S.L., Molle K., Knop J. Interleukin 1alpha promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J Exp Med. 2003;198:191–199. doi: 10.1084/jem.20030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawasaki H., Nagao K., Kubo A., Hata T., Shimizu A., Mizuno H. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J Allergy Clin Immunol. 2012;129:1538–1546.e6. doi: 10.1016/j.jaci.2012.01.068. [DOI] [PubMed] [Google Scholar]
- 44.Jakasa I., Verberk M.M., Esposito M., Bos J.D., Kezic S. Altered penetration of polyethylene glycols into uninvolved skin of atopic dermatitis patients. J Invest Dermatol. 2007;127:129–134. doi: 10.1038/sj.jid.5700582. [DOI] [PubMed] [Google Scholar]
- 45.Taieb A., Hanifin J., Cooper K., Bos J.D., Imokawa G., David T.J. Proceedings of the 4th Georg Rajka International Symposium on Atopic Dermatitis, Arcachon, France, September 15-17, 2005. J Allergy Clin Immunol. 2006;117:378–390. doi: 10.1016/j.jaci.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 46.Oyoshi M.K., He R., Kumar L., Yoon J., Geha R.S. Cellular and molecular mechanisms in atopic dermatitis. Adv Immunol. 2009;102:135–226. doi: 10.1016/S0065-2776(09)01203-6. [DOI] [PubMed] [Google Scholar]
- 47.Leisten S., Oyoshi M.K., Galand C., Hornick J.L., Gurish M.F., Geha R.S. Development of skin lesions in filaggrin-deficient mice is dependent on adaptive immunity. J Allergy Clin Immunol. 2013;131:1247–1250.e1. doi: 10.1016/j.jaci.2012.12.1576. [DOI] [PubMed] [Google Scholar]
- 48.Kezic S., O'Regan G.M., Lutter R., Jakasa I., Koster E.S., Saunders S. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129:1031–1039.e1. doi: 10.1016/j.jaci.2011.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hvid M., Vestergaard C., Kemp K., Christensen G.B., Deleuran B., Deleuran M. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J Invest Dermatol. 2011;131:150–157. doi: 10.1038/jid.2010.277. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y.H., Angkasekwinai P., Lu N., Voo K.S., Arima K., Hanabuchi S. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller A.M. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoo J., Omori M., Gyarmati D., Zhou B., Aye T., Brewer A. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wenzel S.E. Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther. 2013;26:710–715. doi: 10.1016/j.pupt.2013.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.