Abstract
We investigated the effect of resistant maltodextrin (RMD), a non-viscous soluble dietary fiber, on intestinal immune response and its mechanism in mice. Intestinal and fecal immunoglobulin A (IgA) were determined as indicators of intestinal immune response, and changes in the intestinal environment were focused to study the mechanism. BALB/c mice were fed one of three experimental diets, a control diet or a diet containing either 5% or 7.5% RMD, for two weeks. Continuous intake of RMD dose-dependently increased total IgA levels in the intestinal tract. Total IgA production from the cecal mucosa was significantly increased by RMD intake, while there were no significant differences in mucosal IgA production between the control group and experimental groups in the small intestine and colon. Continuous intake of RMD changed the composition of the cecal contents; that is, the composition of the cecal microbiota was changed, and short-chain fatty acids (SCFAs) were increased. There was an increased trend in Bacteroidales in the cecal microbiota, and butyrate, an SCFA, was significantly increased. Our study demonstrated that continuous intake of RMD enhanced the intestinal immune response by increasing the production of IgA in the intestinal tract. It suggested that the increase in total SCFAs and changes in the intestinal microbiota resulting from the fermentation of RMD orally ingested were associated with the induction of IgA production in intestinal immune cells, with the IgA production of the cecal mucosa in particular being significantly increased.
Keywords: resistant maltodextrin, IgA, cecal fermentation, cecal microbiota, short-chain fatty acid
INTRODUCTION
The intestine is responsible for the absorption of nutrients, which is a critical function for life, whereas it is always at risk of invasion by bacterial pathogens and viruses from the external world. Moreover, there are massive amounts of antigenic substances in the intestine such as undigested foods and intestinal microbiota. In order to deal with the massive amounts of such foreign substances, gut-associated lymphoid tissue (GALT) is developed, and it accounts for about 70% of the lymphatic tissue in the entire body. The characteristic immune responses of GALT include blocking of microbial mucoadhesion and neutralization of viruses by producing IgA antibodies.
There have been many reports indicating that ingestion of prebiotics increases production of IgA, which is important for protection against infection in the intestinal mucosa. Indigestible carbohydrates, such as fructooligosaccharides (FOS) [1], galactooligosaccharides (GOS) [2], and lactosucrose [3] have immunomodulating properties in the intestine that are associated with an increase in short-chain fatty acids and change in the composition of the intestinal microbiota in the intestine. Additionally, it has been reported that probiotic bacteria and beta-glucans sensitize the immune cells in the GALT and enhance production of antibodies; that is, intestinal contents other than absorbable nutrients are also deeply involved in the modulation of vital functions.
Resistant maltodextrin (RMD), a non-viscous soluble fiber, is a prebiotics and is known to regulate the function of the intestine. It has been reported that continuous ingestion of RMD for 3 weeks increased Bifidobacterium in feces in humans [4]. It has been also reported that short-chain fatty acids (SCFAs) produced as a result of fermentation of RMD lowered the pH of the cecal content and enhanced the absorption of minerals in rats [5]. The changes in the intestinal environment resulting from fermentation of food constituents in the intestine would affect the regulation of vital functions, and fermentation of RMD is expected to have a positive influence on immune responses as in the case of FOS and GOS. Since RMD contains beta linkages in its structure, it might have a direct immunomodulating effect like beta-glucans, however, the effect of RMD on immune response has not yet reported. In this study, we investigated the effect of dietary RMD on the intestinal immune response in mice. Intestinal and fecal IgA were determined as indicators of intestinal immune response, and changes in intestinal environment were focused on to study the mechanism responsible for the effect of RMD.
MATERIALS AND METHODS
Animals and diets
Eight-week-old female BALB/c mice were purchased from CLEA Japan, Inc. (Tokyo, Japan), and were housed in a room at 23–25°C with a relative humidity of 50 ± 10% and a 12-hour light-dark cycle. The mice were divided into plastic cages by group and were given free access to experimental diets and drinking water. A purified diet prepared based on AIN-93G was used as the control diet, and diets with either 5% or 7.5% RMD in replace of corn starch were used as the experimental diets. The control and experimental diets were solidified in pellets and sterilized with gamma irradiation at Funabashi Farm Co., Ltd. (Chiba, Japan). RMD was manufactured by Matsutani Chemical Industry Co., Ltd. (Hyogo, Japan). All experiments were conducted in accordance with the internal regulations of the Nihon University Animal Care and Use Committee.
Experiment 1: Effect of dietary RMD on total IgA secretion into the intestine and excretion into feces
The mice were divided into 3 groups and were fed one of the experimental diets for 2 weeks. Each group was divided into two subgroups, and fecal and intestinal samples were collected after 1- and 2-week feeding periods. Feces were collected for 24 hours at the ends of the 1st and 2nd week and freeze-dried. The intestines were excised by dissection from the site immediately below the stomach to the colon. Feces were ground and homogenized in PBS solution containing 50 mM EDTA and 0.1 mg/ml trypsin inhibitor. The homogenate solutions were centrifuged, and the supernatants were appropriately diluted and used for analysis. The intestines were homogenized with their contents in the same manner as the feces.
The total IgA levels in the supernatants of feces and intestinal homogenates were determined by sandwich enzyme-linked immunosorbent assay (ELISA). For the determination of total IgA levels, MaxiSorp Immuno Plates (Thermo Scientific Nunc, Waltham, MA, USA) were coated with goat anti-mouse IgA, and after blocking, standard mouse IgA and appropriately diluted samples were added to the plates. Then, the plates were incubated with alkaline phosphatase-labeled goat anti-mouse IgA antibody. After disodium 4-nitrophenyl phosphate was added, the optical density was measured at 405 nm, and total IgA concentration was determined.
Experiment 2: Effect of dietary RMD on total IgA production from the intestinal mucosa by segment
The mice were divided into 3 groups and fed one of the experimental diets for 2 weeks. Each group was divided into two subgroups, and intestines were excised after 1- and 2-week feeding periods. The intestines were excised by dividing them into 3 segments: the small intestine, cecum and colon. The intestines were inverted or incised to remove the contents, washed in PBS solution and then homogenized in the same manner as described in Experiment 1. The soluble fractions were extracted, and total IgA levels in the intestinal mucosa were determined.
Experiment 3: Effect of dietary RMD on cecal contents
The mice were divided into 3 groups, and each group was fed one of the experimental diets for 2 weeks. The ceca were excised by dissection, and the cecal contents were stored at −20°C until analyses of intestinal microbiota, SCFAs and pH. The analysis of cecal contents was conducted at the Central Institute for Experimental Animals (Kanagawa, Japan). The cecal microbiota was analyzed by the terminal restriction fragment length polymorphism (T-RFLP) method. Regarding SCFAs, formic acid, acetic acid, propionic acid, butyric acid, succinic acid and lactic acid were analyzed by the high-performance liquid chromatography (HPLC) method.
Statistical analysis
Results were expressed as means ± SD. Statistical differences were calculated using SPSS software (Ver. 13.0 J, SPSS Japan). Data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison. Differences were considered significant when the p value was lower than 0.05.
RESULTS
Experiment 1
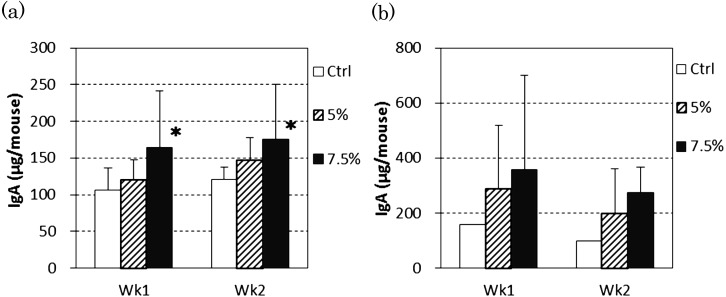
At the ends of both 1-week and 2-week feeding periods, the total IgA amounts in the intestinal tracts including their contents were increased dose-dependently by RMD intake, and the 7.5% RMD group showed a significantly higher value compared with the control group (Fig. 1-a). The total IgA levels in feces were not significantly different among groups (Fig. 1-b).
Fig. 1.
Effect of dietary RMD on total IgA amount in the intestinal tracts and feces of mice.
(a) Total IgA amount in the intestinal tract including intestinal content (n=12). (b) Total IgA amount in feces excreted for 24 hours (n=3). BALB/c mice were fed diets containing 5% or 7.5% RMD and the IgA levels in the intestinal tract and feces were determined. Data are presented as the mean ± SD. *Significant difference from the control group (p<0.05).
Experiment 2
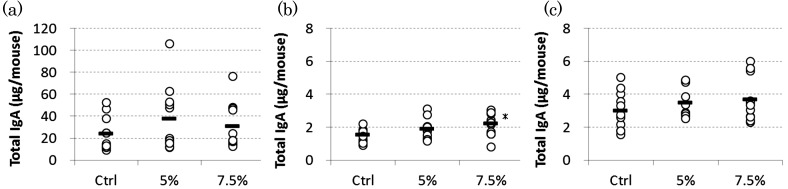
The largest amount of total IgA was produced from the mucosa of the small intestine, which accounted for about 80% of the total IgA produced in the entire intestine (Fig. 2). In the small intestines and colon, there were no significant differences in the amount of total IgA between the control group and experimental groups. In the cecal mucosa, however, the amount of total IgA produced was dose-dependently increased by RMD intake, and the 7.5% RMD group had a significantly higher level compared with the control group (p<0.05).
Fig. 2.
Effect of dietary RMD on total IgA production from the intestinal mucosa in mice.
(a) Small intestine, (b) Cecum, (c) Colon
BALB/c mice were fed diets containing 5% or 7.5% RMD. Intestines were divided into 3 segments, the small intestine, cecum and colon, and IgA levels in mucosal extracts were determined. Each circle represents an individual mouse (n=11). *Significant difference from the control group (p<0.05).
Experiment 3
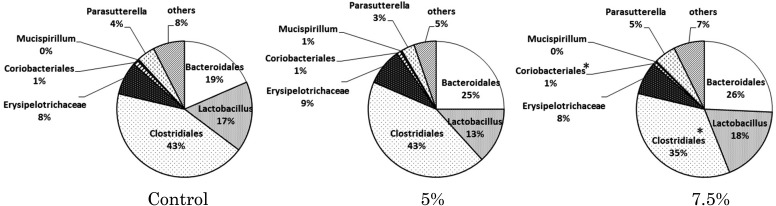
The composition of the cecal microbiota was shifted in association with RMD intake. Clostridiales and Coriobacteriales were decreased by RMD intake, and the decreases were significant in the 7.5% RMD group compared with the control group. In the 7.5% RMD group, Bacteroidales tended to be increased (p=0.141), but the difference was not significant (Fig. 3). The occupancy of Lactobacillus was not influenced by RMD intake. Regardless of the amount of RMD intake, Clostridiales showed the highest occupancy.
Fig. 3.
Effect of dietary RMD on the composition of the cecal microbiota analyzed by the T-RFLP method.
BALB/c mice were fed diets conctaining 5% or 7.5% RMD for 2 weeks. Data are presented as the mean and are expressed in percentage. *Significant difference from the control group (p<0.05).
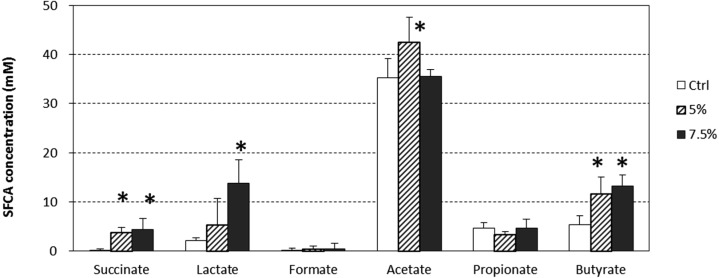
The total SCFA level in the cecal contents was significantly and dose-dependently increased in association with RMD intake, and pH level was significantly and dose-dependently decreased in association with RMD intake (p<0.05) (Table 1). The concentrations of succinic acid, lactic acid and butyric acid were significantly increased by continuous intake of RMD (p<0.05) (Fig. 4). The levels of formic acid and propionic acid were fairly constant, regardless of the amount of RMD intake.
Table 1. Effect of dietary RMD on total SCFAs and pH in cecal contents.
| Control | 5% | 7.5% | p-value | |
|---|---|---|---|---|
| Total SCFAs (mg/cecum) | 0.50 ± 0.12 | 1.22 ± 0.53* | 1.67 ± 0.33* | <0.01 |
| pH | 7.77 ± 0.05 | 7.05 ± 0.18* | 6.77 ± 0.20* | <0.01 |
BALB/c mice were fed diet containing 5% or 7.5% RMD for 2 weeks. Data are presented as the mean ± SD (n=6). *Significant difference from the control group (p<0.05)
Fig. 4.
Effect of RMD on SCFAs in the cecal contents.
BALB/c mice were fed diets containing 5% or 7.5% RMD for 2 weeks. Data are presented as the mean ± SD (n=6). *Significant difference from the control group (p<0.05).
DISCUSSION
We investigated the influence of RMD, a starch-derived soluble dietary fiber, on intestinal immune responses in an animal model. In experiment 1, total IgA levels in the intestines were increased dose-dependently by continuous intake of RMD, suggesting increased intestinal immune responses. IgA secreted in the intestine is effective for protection against infection in the intestine. Peyer’s patches (PPs) present in the small intestine are involved in IgA production from the intestinal mucosa as the inductive site. Besides PPs in the small intestine, there are also lymph nodes in the cecum and colon. Continuous intake of RMD might affect these lymph nodes, and IgA secretion from the intestinal mucosa might be increased. In order to identify the site in the intestine responsible for increased IgA secretion, the intestine was divided into 3 segments, the small intestine, cecum and colon, and mucosal IgA production from each segment was determined in experiment 2. The total IgA production in the small intestine was remarkably higher than those in the cecum and colon, indicating that the small intestine played a major role in IgA secretion in GALT, which is also supported by the fact that the small intestine has the largest surface area in the intestinal tract. In the small intestine and colon, there were no significant differences in the amount of total IgA between the control group and experiment groups; however, mucosal IgA production in the cecum was significantly increased by continuous intake of RMD. Nakamura et al. reported that the increase of IgA secretion in the mucosa of the lower intestine was remarkable in mice fed FOS [1] and indicated that the changes in the intestinal environment mediated by the intestinal microbiota were involved. Actually, feeding of indigestible carbohydrates such as FOS [6], raffinose [7] and RMD [5] resulted in a severalfold increase of cecum weight due to fermentation in the cecum in rodents. Since consumption of RMD increased the cecal content and it is speculated that the intestinal content stays longer in the cecum of rodents, it is likely that increased IgA production by the cecal mucosa also contributed to the increase in IgA amount in the intestinal tract in the 7.5% RMD group.
Meanwhile, B cell stimulation by polysaccharides via Toll-like receptor and/or Dectin-1 is considered one of the factors that enhance IgA secretion in the intestine. To investigate whather a similar effect occurs with RMD, PP cells were co-cultured with RMD, but enhancement of IgA secretion was not observed (data not shown). The result indicates that RMD has no direct stimulating effect on cells from the intestinal immune system, unlike beta-glucans and lipopolysaccharides (LPSs). The immunostimulatory components of beta-glucans contain a structure of the beta-1,3-linked main chain with the beta-1,6-linked side chains [8, 9]. By contrast, the main chain of starch-derived RMD has an alpha-1,4 linkage, and beta linkages may be produced in the manufacturing process. However, it is considered that all the beta linkages of RMD are all present in the side chains. The structural difference is one of the reasons why the beta-glucan-like direct immunostimulatory effect was not observed with RMD. Hence, similar to the case of FOS, it is suggested that the increase in total IgA secretion induced by RMD intake might be mediated by the changes in the intestinal environment.
We presumed that the changes in the intestinal environment resulting from RMD intake affected the induction and secretion of IgA, and we analyzed the intestinal microbiota and SCFAs in the cecal contents in experiment 3. Analysis of the cecal microbiota by the T-RFLP method showed a possibility that continuous intake of RMD might increase the percentage of Bacteroidales (p=0.141). It has been reported that continuous intake of FOS, one of the indigestible carbohydrates, increased Bacteroides in feces and the cecum in mice [10] and increased IgA production of PP cells [11]. Bacteroides highly induce IgA production in B cells in the PP [12]. Further, it was reported that in Bacteroides mono-associated mice, the IgA production in the large intestine was significantly higher than those in germ-free mice and Lactobacillus mono-associated mice and that Bacteroides colonization in the intestine enhanced formation of the germinal center, enhanced development of IgA-producing precursor B cells and activated induction of IgA production [13]. The IgA-producing precursor B cells, which are induced in the germinal center of lymphoid tissues such as PPs, differentiate into IgA plasma cells after homing into the lamina propria and then produce and secrete IgA in the intestine. In our study, therefore, the changes in the intestinal microbiota, such as increased Bacteroidales, appeared to be greatly involved in the mechanism of increased IgA production from the intestinal mucosa resulting from continuous intake of RMD.
In our study, analysis of the intestinal microbiota was performed by the T-RFLP method, and the total bacterial count could not be quantified, but an increase in cecum weight dependent on the amount of RMD intake was observed. It was previously reported that the total bacterial count in feces was increased dose-dependently by RMD intake [14], and thus, the total bacterial count in the cecum in this study was also considered to be increased. In the cecal microbiota, the percentage of Coriobacteriales was significantly decreased by RMD intake, but this bacteria was very minor, accounting for around 1% of the cecal bacteria, and its involvement in health is not of great interest [15], indicating less influence on animals. Clostridiales was dominant in the cecal microbiota regardless of the amount of RMD intake. Recent studies have revealed that Clostridium bacteria are helpful in maintenance of the homeostasis of the intestinal immune system. Kawamoto et al. [16] reported that Clostridium bacteria maintained the diversity and balance of microbiota in the intestine and led to efficient IgA production by Foxp3-positive T cells and that the IgA produced in that mechanism maintained the diversity and balance of the intestinal microbiota. IgA plays an important role in protection against infection in the mucosal immune system, not by eliminating all the bacteria but by maintaining the diversity and balance of the intestinal microbiota. That is, interaction between immune cells and the intestinal microbiota maintains the homeostasis of intestinal immunity. In our study, although RMD intake altered the composition of the cecal microbiota, Clostridiales were the most dominant, and thus, it is suggested that the healthy state of the intestine was maintained in terms of IgA production.
We observed a significant and dose-dependent increase in the total SCFA concentrations in the cecal contents as a result of RMD intake. Cecal SCFAs, which are the metabolites of indigestible food component, are produced by the intestinal bacteria and known to be an energy source for proliferation of epithelial cells [17]. It has been determined in recent years that SCFAs produced by the intestinal bacteria regulate immune function. Butyric acid produced by Clostridium bacteria induces differentiation of regulatory T cells and prevents colitis [18], and acetic acid produced by Bifidobacterium protects intestinal epithelial cells and prevents E.coli O157 infection [19]. According to these studies, there is a possibility that intestinal immune function is enhanced by alteration of the intestinal microbiota and their metabolites as a result of ingestion of probiotics and/or prebiotics. Hence, changes in the cecal contents by continuous RMD intake may also affect the IgA production in the intestine.
Intake of prebiotics increases SCFAs in the lower intestine, but the patterns of their changes vary depending on the kinds of prebiotics. FOS intake increases acetic acid and butyric acid [1], and raffinose intake increases formic acid and acetic acid and decreases butyric acid [7]. RMD intake significantly increased butyric acid in the cecum in this study, and it has been reported that RMD intake resulted in increased butyric acid in feces in humans [4]. Continuous intake of RMD was characterized by an increase in butyric acid in addition to an increase in the total amount of SCFAs and maintenance of Clostridiales as the dominant bacteria in the cecal microbiota in this study. Thus, RMD may have the potential to alleviate inflammation in the colon, as Furusawa et al. [18] mentioned the importance of Clostridia and butyrate in ameliorating the development of colitis.
We determined the IgA production responses of intestinal immune cells in the PP and cecal patch (CeP) by co-culture with LPS or Concanavalin A, but there were no differences in IgA levels between the control and experimental groups (data not shown). In vivo, various substances including undigested foods present in the intestine and particularly in the cecum are considered to include large amounts of SCFAs. Therefore, the influence of SCFAs on the IgA production responses in immune cells should be further investigated, including the influence on IgA plasma cells in the intestinal lamina propria.
Our study demonstrated that continuous oral intake of RMD enhanced the intestinal immune response by increasing the production of IgA in the intestinal tract. It is suggested that the increase in total SCFAs and changes in the intestinal microbiota resulting from the fermentation of RMD orally ingested were associated with the induction of IgA production in intestinal immune cells, with the IgA production of the cecal mucosa in particular being significantly increased. Although further studies are required for the detailed mechanism of the induction of IgA production, given that RMD is already widely applied in commercial foods and beverages in many countries, it is expected that RMD could contribute to people staying healthy through maintenance of intestinal health.
REFERENCES
- 1.Nakamura Y, Nosaka S, Suzuki M, Nagafuchi S, Takahashi T, Yajima T, Takenouchi-Ohkubo N, Iwase T, Moro I. 2004. Dietary fructooligosaccharides up-regulate immunoglobulin A response and polymeric immunoglobulin receptor expression in intestines of infant mice. Clin Exp Immunol 137: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato T, Nakamura Y, Ozawa O. 2008. Effect of galactooligosaccharides on immune system in mice. J Jpn Soc Nutr Food Sci 61: 79–88. [Google Scholar]
- 3.Hino K, Kurose M, Sakurai T, Inoue S, Oku K, Chaen H, Fukuda S. 2007. Effect of dietary lactosucrose (4G-β-D-galactosylsucrose) on the intestinal immune functions in mice. J Appl Glycosci 54: 169–172. [Google Scholar]
- 4.Fastinger ND, Karr-Lilienthal LK, Spears JK, Swanson KS, Zinn KE, Nava GM, Ohkuma K, Kanahori S, Gordon DT, Fahey GC., Jr 2008. A novel resistant maltodextrin alters gastrointestinal tolerance factors, fecal characteristics, and fecal microbiota in healthy adult humans. J Am Coll Nutr 27: 356–366. [DOI] [PubMed] [Google Scholar]
- 5.Miyazato S, Nakagawa C, Kishimoto Y, Tagami H, Hara H. 2010. Promotive effects of resistant maltodextrin on apparent absorption of calcium, magnesium, iron and zinc in rats. Eur J Nutr 49: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohta A, Ohtsuki M, Baba S, Adachi T, Sakata T, Sakaguchi E. 1995. Calcium and magnesium absorption from the colon and rectum are increased in rats fed fructooligosaccharides. J Nutr 125: 2417–2424. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Nagura T, Sato K, Ohnishi M. 2012. Evaluation of the effects of dietary organic germanium, ge-132, and raffinose supplementation on caecal flora in rats. Biosci Microbiota Food Health 31: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanioka A, Tanabe K, Hosono A, Kawakami H, Kaminogawa S, Tsubaki K, Hachimura S. 2013. Enhancement of intestinal immune function in mice by β-D-glucan from aureobasidium pullulans ADK-34. Scand J Immunol 78: 61–68. [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y, Sumiyoshi M, Suzuki T, Sakanaka M. 2006. Antitumor and antimetastatic activity of a novel water-soluble low molecular weight beta-1, 3-D-glucan (branch beta-1,6) isolated from Aureobasidium pullulans 1A1 strain black yeast. Anticancer Res 266B: 4131–4141. [PubMed] [Google Scholar]
- 10.Nakanishi Y, Murashima K, Ohara H, Suzuki T, Hayashi H, Sakamoto M, Fukasawa T, Kubota H, Hosono A, Kono T, Kaminogawa S, Benno Y. 2006. Increase in terminal restriction fragments of Bacteroidetes-derived 16S rRNA genes after administration of short-chain fructooligosaccharides. Appl Environ Microbiol 72: 6271–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosono A, Ozawa A, Kato R, Ohnishi Y, Nakanishi Y, Kimura T, Nakamura R. 2003. Dietary fructooligosaccharides induce immunoregulation of intestinal IgA secretion by murine Peyer’s patch cells. Biosci Biotechnol Biochem 67: 758–764. [DOI] [PubMed] [Google Scholar]
- 12.Yanagibashi T, Hosono A, Oyama A, Tsuda M, Hachimura S, Takahashi Y, Itoh K, Hirayama K, Takahashi K, Kaminogawa S. 2009. Bacteroides induce higher IgA production than Lactobacillus by increasing activation-induced cytidine deaminase expression in B cells in murine Peyer’s patches. Biosci Biotechnol Biochem 73: 372–377. [DOI] [PubMed] [Google Scholar]
- 13.Yanagibashi T, Hosono A, Oyama A, Tsuda M, Suzuki A, Hachimura S, Takahashi Y, Momose Y, Itoh K, Hirayama K, Takahashi K, Kaminogawa S. 2013. IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA+ B cells. Immunobiology 218: 645–651. [DOI] [PubMed] [Google Scholar]
- 14.Baer DJ, Stote KS, Henderson T, Paul DR, Okuma K, Tagami H, Kanahori S, Gordon DT, Rumpler WV, Ukhanova M, Culpepper T, Wang X, Mai V. 2014. The metabolizable energy of dietary resistant maltodextrin is variable and alters fecal microbiota composition in adult men. J Nutr 144: 1023–1029. [DOI] [PubMed] [Google Scholar]
- 15.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. 2007. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 133: 24–33. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, Tsutsui Y, Qin H, Honda K, Okada T, Hattori M, Fagarasan S. 2014. Foxp3+ T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 41: 152–165. [DOI] [PubMed] [Google Scholar]
- 17.Sakata T, von Engelhardt W. 1983. Stimulatory effect of short chain fatty acids on the epithelial cell proliferation in rat large intestine. Comp Biochem Physiol A 74: 459–462. [DOI] [PubMed] [Google Scholar]
- 18.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543–547. [DOI] [PubMed] [Google Scholar]