Abstract
The southwestern region of China, along the Myanmar border, has accounted for the highest number of cases of imported malaria since China shifted from a malaria control program to an elimination strategy in 2010. We conducted a retrospective study, in which 623 medical charts were analyzed to provide an epidemiological characterization of malaria cases that were diagnosed and treated at the People's Hospital of Tengchong County (PHTC), located in southwestern China, from 2008 to 2013. Our aim was to understand the characteristics of malaria in this region, which is a high-endemic region with imported cases. The majority of patients were male (91.7%), and the average age was 32.4 years. Most of the patients (86.4%) had visited Myanmar; labor was the purpose of travel for 63.9% of the patients. Plasmodium vivax and Plasmodium falciparum were responsible for 53.8% and 34.9% of the infections, respectively. The number of hospitalized patients rose gradually from 2008 to 2010 and reached its peak in 2010 (191). After 2010, the number of hospitalized cases fell rapidly from 191 (2010) to 45 (2013), and the proportion of patients who lived in the forest and the number infected with P. falciparum also fell. In conclusion, the number of hospitalized patients in the southwestern region of China, Tengchong county, decreased after China implemented a malaria elimination strategy in 2010. However, migrant workers returning from Myanmar remained important contributors to cases of imported malaria. The management of imported malaria should be targeted by the malaria elimination program in China.
Keywords: China, imported malaria, malaria elimination strategy, migrant labor, Myanmar
Introduction
Malaria is curable and preventable, but it remains an important cause of illness and death in children and adults in endemic countries. In China, malaria has historically been considered the parasitic disease that has had the greatest impact on social-economic development, with more than 30 million cases and a fatality rate of approximately 1% per year. However, reported cases of malaria have declined dramatically in recent years.1,2 As a result, China now aims to eliminate malaria by 2020.2,3,4
In contrast to the reduction in cases of autochthonous malaria, the number of imported malaria cases has increased in mainland China.5 Most of these cases were imported from African (60%) or Southeast Asian countries (36.9%). Myanmar has the heaviest malaria burden in the Greater Mekong Sub-region and is a major source of imported cases of malaria in China.6,7,8
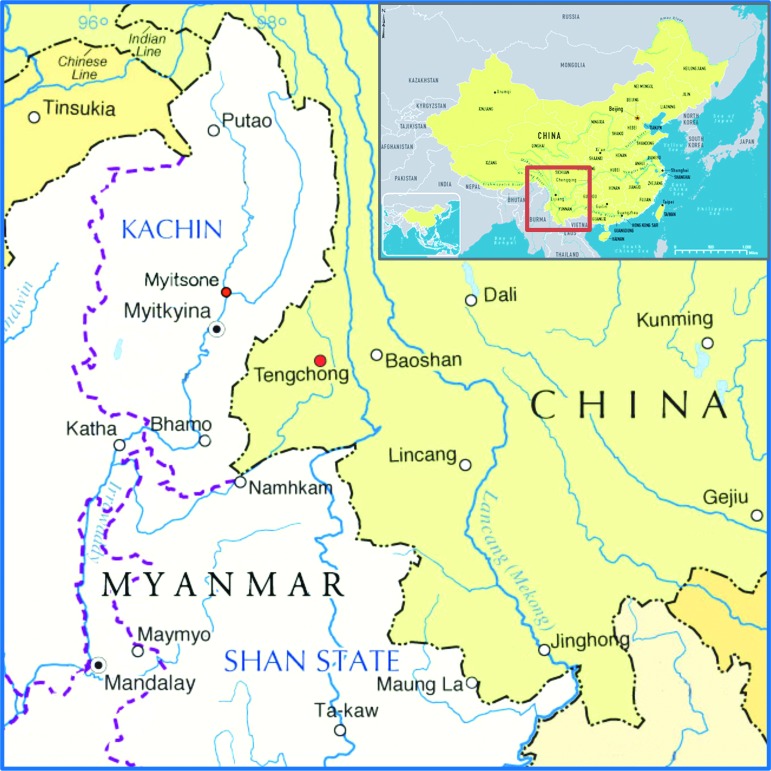
In southwestern China, especially in Yunnan province, malaria is particularly problematic among border crossers and ethnic minority groups in the Yunnan–Myanmar border areas.9 These areas are the most underdeveloped in China, and poor, marginalized, ethnic minority groups are particularly at risk of malaria, especially migrant workers who frequently engage in activities such as logging, mining, farming, and construction. Tengchong is a county in Yunnan province with a population of over 660 000. This county is in the southwestern border area of China (98.05°–98.46°E, 24.38°–25.52°N), covers an area of 5845 km2 and shares a 148-km-long border with Myanmar. Tengchong county town is located only 189.5 km from Myanmar's second largest city, Myitkyina (Figure 1). Tengchong county has had one of the highest imported malaria incidences in Yunnan province in recent years.10 In 2010, China revised the national malaria strategy for 2010–2015 from a control strategy to a strategy with the goal of elimination. One of the most important components of this strategy is to provide easy access to high-quality treatment for Chinese nationals and foreign nationals in border areas. As a result, county hospitals have become some of the main participants in implementing this strategy.
Figure 1.
Map of the Myanmar–China border area near Tengchong county.
The Myitsone Dam project, formally started in 2009, is a large dam and hydroelectric power development project in Myanmar. Myitsone is located approximately 42 kilometers north of Myitkyina (Figure 1). From 2009 to 2011, Chinese workers were employed in the construction of Myitsone. These workers engaged in advance earthworks for the dam. They worked and lived in areas where malaria was highly endemic, and most of them did not take chemoprophylaxis. Malaria was the most common origin of fever in this group. These workers went to Tengchong, the nearest county in China, if they became sick. Does this population influence the malaria incidence in Tengchong county?
We conducted this retrospective study to determine whether malaria cases in Tengchong have decreased since 2010 and to characterize malaria cases in this county.
Materials and methods
Study setting
We conducted this study at the 480-bed PHTC, which is the largest public hospital in Tengchong county, Yunnan province, China, from January 1, 2008, to December 31, 2013. PHTC is a secondary hospital that serves all of the Chinese nationals and foreign nationals in the county, and it is the only hospital in Tengchong county with the capacity to admit malaria cases. The laboratory technicians in PHTC have been trained to identify Plasmodium species from peripheral blood smears or rapid diagnostic tests (RDT).
Case identification
We evaluated all of the admitted cases by reviewing the malaria records in the Department of Infectious Diseases, PHTC, for the stated period. A positive malaria case was defined as any patient with clinical manifestation (i.e., fever) and pathology confirmed by microscopy or RDT. The hospital medical records system was accessed to review and extract predefined demographic, clinical, and laboratory data for all identified malaria cases. Severe cases were defined according to the World Health Organization (WHO) criteria.11
Data management and analysis
The characteristics of all hospitalized malaria cases were described according to geographical and temporal distribution, age, gender, purpose of travel, country in which the infection was acquired, and Plasmodium species. To compare the changes that occurred after the Chinese government revised its malaria strategy in 2010, we divided all of the cases into two groups by stages (2008–2010 and 2011–2013).
We used Microsoft Excel to calculate the constituent ratio of total, autochthonous and imported malaria cases and to analyze the seasonal and precipitation-related trends. Differences in species were evaluated using the χ2/Fisher exact test with the Stata 11 software (StataCorp LP, College Station, TX, USA), and P < 0.05 was considered statistically significant.
Results
General and clinical characteristics
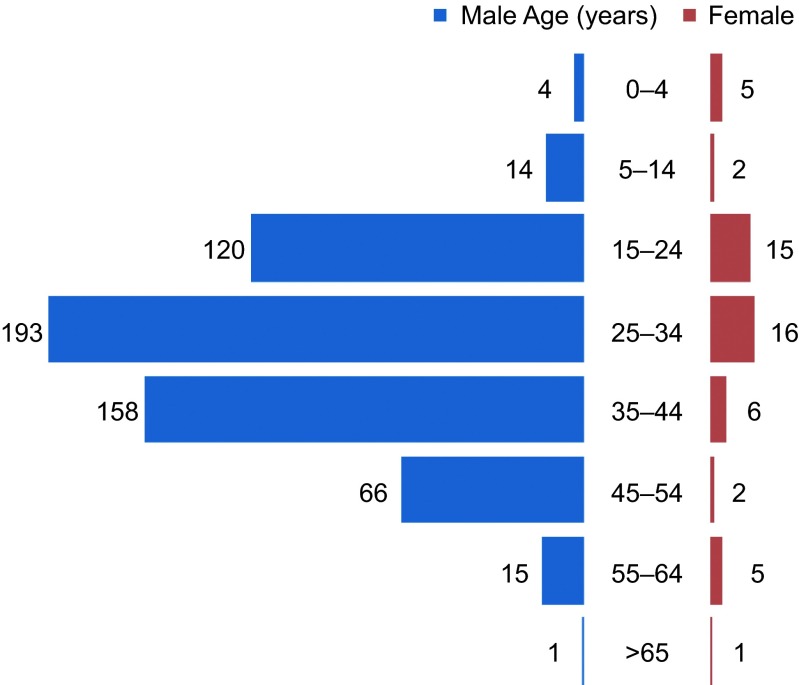
A total of 623 confirmed cases of malaria were admitted to PHTC from January 1, 2008, to December 31, 2013. Patient demographics for the included cases are summarized in Table 1. Most of the infected people were males (571, 91.7%). The median age of all cases was 24 years (range 1 month–67 years), and most (373, 59.9%) were 25–44 years of age (Figure 2). Chinese citizens accounted for 598 cases (96%), and 25 cases (4%) were Burmese. Among the Chinese citizens, more than half of the cases (338, 54.3%) resided in Tengchong county, and the remainder (260, 41.7%) were from other areas in China.
Table 1. Characteristics of all hospitalized malaria cases during the period 2008 to 2013, Tengchong county.
| Patient characteristic | Total (n = 623) | Total (%) | |
|---|---|---|---|
| Age, Median (IQR) | 24(32–40) | ||
| Range | 0–67 | ||
| Gender | Male | 571 | 91.7 |
| Nationality | China | 598 | 96.0 |
| Myanmar | 25 | 4.0 | |
| Ethnicity | Han | 592 | 95.0 |
| Ethnic minority | 31 | 5.0 | |
| Region visited | Myanmar | 538 | 86.4 |
| Laos | 7 | 1.1 | |
| Congo | 1 | 0.2 | |
| Domestic epidemic area | 21 | 3.4 | |
| No travel history | 56 | 9.0 | |
| Purpose of travel | Migrant workers | 399 | 64.0 |
| Chinese citizen living abroad | 30 | 4.8 | |
| Business | 10 | 1.6 | |
| Visiting friends and relatives | 4 | 0.6 | |
| Sightseeing | 1 | 0.2 | |
| Other purpose | 130 | 21.7 | |
| Autochthonous cases | 44 | 7.1 | |
| Living environment | Forest | 309 | 49.6 |
| Countryside | 136 | 21.8 | |
| City | 68 | 10.9 | |
| Unknown | 110 | 17.7 | |
| HIV | Positive | 6 | 1.0 |
| Chemoprophylaxis | Yes | 8 | 1.3 |
| No or Unknown | 615 | 98.7 | |
| Diagnosis | Plasmodium falciparum | 218 | 35.0 |
| Plasmodium vivax | 336 | 53.9 | |
| Mixed | 14 | 2.3 | |
| Unidentified | 55 | 8.8 | |
| Types | Severe | 151 | 24.2 |
| Uncomplicated | 472 | 75.8 | |
| Outcome | Death | 3 | 0.5 |
| Recovery | 620 | 99.5 |
Figure 2.
Distribution of all the hospitalized malaria cases in Tengchong county by age and gender, 2008–2013.
Of the 623 people infected with malaria, 538 (86.4%) had returned from Myanmar within the previous six months, seven from Laos, and one from the Democratic Republic of the Congo. Only 21 patients had traveled to the endemic area in China, and 56 patients without a history of traveling to any malaria-endemic area in the previous six months were identified as autochthonous cases. Among the 568 patients with a history of travel, the most frequently reported purpose for travel was labor (64%). Other purposes for travel included residence (4.8%), business (1.6%), and visiting friends and relatives (0.6%). Only one patient's purpose was sightseeing. Most of the patients had lived in the forest (49.6%) or countryside (21.8%) when they were in the malaria-endemic areas. Only 10.9% of the patients had lived in the city.
Eight patients (1.3%) had used chemoprophylaxis throughout the duration of their travels. The remainder did not take any chemoprophylaxis or related medicine during their travels. Six patients were co-infected with HIV (6/623, 1.0%). There were nine pregnant women among the 52 female cases.
Plasmodium vivax was the dominant species, accounting for 336 cases (53.9%). Plasmodium falciparum was responsible for 218 (34.9%) cases; fourteen patients were co-infected with both P. vivax and P. falciparum (Table 1). No P. ovale, P. malariae or P. knowlesi cases were identified during the research stage.
There were three cases of death (0.5%), all of which were due to P. falciparum or co-infection with P. vivax and P. falciparum. Among the confirmed cases, 551 cases (88.4%) were diagnosed by microscopy; the remainder were diagnosed by RDT after a negative result under microscopy. Eighty-eight percent of the patients were diagnosed with malaria for the first time; the remainder (11.4%) had suffered from malaria previously.
Malaria incidence variation by season
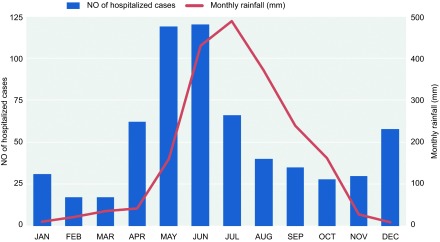
Although the peak in the incidence of cases occurred during the monsoon season, the risk of acquiring malaria from Myanmar was distributed year-round. The monthly distribution of malaria cases over this period is graphically represented in Figure 3. The majority of the malaria cases (442/623, 70.9%) were admitted in the monsoon rainy season from April to September, and only 29.1% were diagnosed from October to March. May and June were the peak months, accounting for 38.4% of the cases (239/623). The rainfall distribution in the Tengchong area is affected by monsoons. The trend of case numbers generally followed that of the seasonal rainfall, which typically begins in April and ends in October. The trend was also partly affected by seasonal workers returning to China to perform agricultural work during this period. December and January was another small peak period (89/623, 14.3%).
Figure 3.
Number of hospitalized malaria cases in Tengchong by month and average monthly rainfall in Tengchong, 2008–2013.
Changes after implementation of the elimination strategy
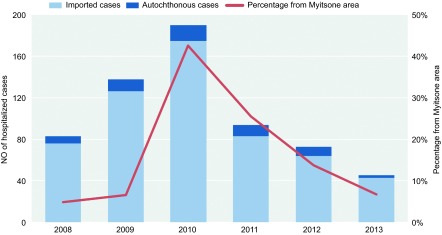
The number of annual cases ranged from 45 to 191 (Figure 4). The number of hospitalized patients rose gradually from 2008 to 2010 and reached its peak in 2010 (191 per year). After 2010, the number of cases fell rapidly from 191 cases (2010) to 45 cases (2013) per year. Compared with 2010, there was a 50% decline in malaria cases in 2011. Imported malaria accounted for the vast majority of the hospitalized cases throughout the six-year period (Figure 4). The proportion of patients from Myitsone and surrounding areas also began to fall in 2011.
Figure 4.
All hospitalized malaria cases in Tengchong county, 2008–2013, and the proportion of patients returning from the Myitsone area.
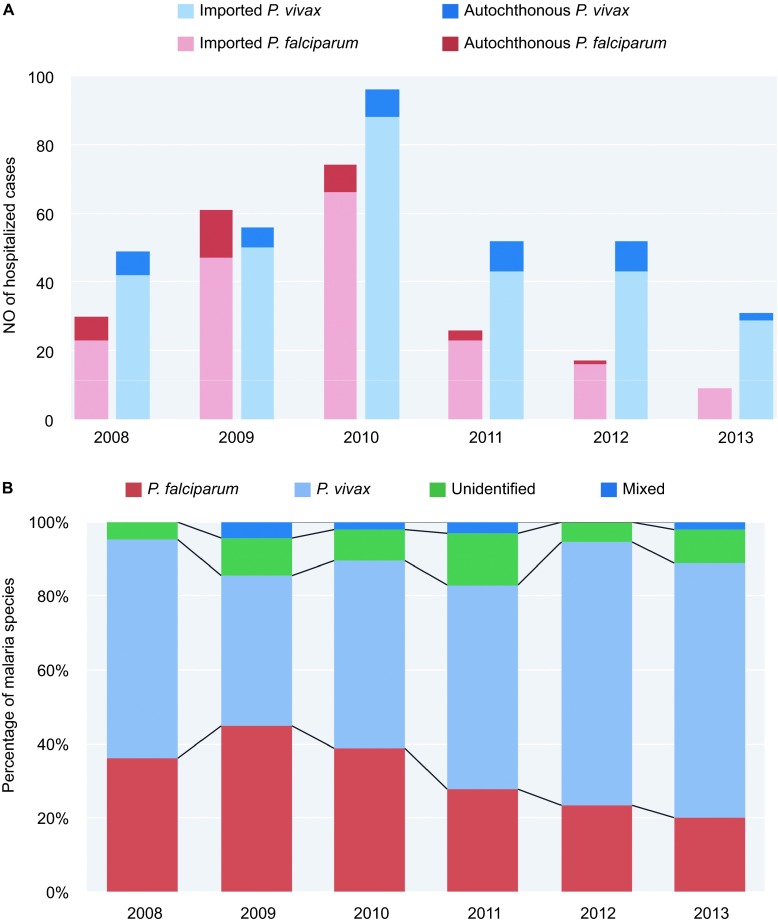
The number of P. falciparum cases exceeded that of P. vivax cases (61 vs. 56), and the number of autochthonous P. falciparum cases reached its peak in 2009. The numbers of cases of the two species both reached their highest in 2010. After 2010, the number of autochthonous P. falciparum cases drastically decreased, and no autochthonous P. falciparum cases were found in 2013 (Figure 5A). The number of autochthonous P. vivax cases also declined after 2010. There were only two autochthonous P. vivax cases in 2013.
Figure 5.
(A) Distribution of P. vivax and P. falciparum malaria cases by year. (B) Composition of malaria species by year, 2008–2013.
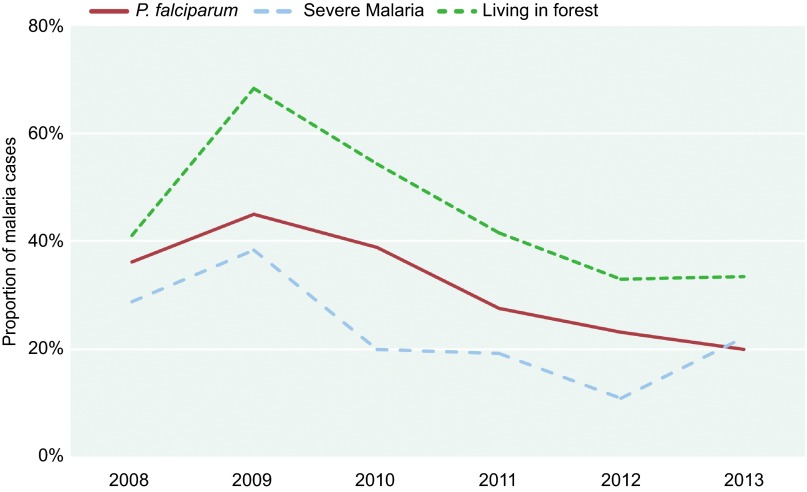
The ratios of P. falciparum in the hospitalized cases declined after 2009. With the decrease in P. falciparum cases, the ratio of P. vivax cases increased (Figure 5B). We also found that the proportion of patients who had been living in the forest declined with the proportion of P. falciparum and severe malaria (Figure 6).
Figure 6.
Proportions of related characteristics in all the hospitalized malaria cases in Tengchong county, 2008–2013.
We divided 2008–2013 into two groups: the first three years comprised group 1, and the next three years comprised group 2. We compared the characteristics of the two groups (Table 2) to evaluate the effects of the implementation of the elimination strategy. We found that the main populations of hospitalized patients were still young male workers with a history of travel to Myanmar. The proportion of patients who had been to a domestic epidemic area decreased after 2010. However, the proportion of patients who had been to a forest fell after 2010 (P < 0.005). The rate of cases infected with P. falciparum was lower in group 2 than in group 1 (P < 0.005). The proportion of severe malaria cases also declined (P < 0.005). No cases of death were recorded after 2010 in PHTC.
Table 2. Characteristics of patients between 2008–2010 and 2011–2013.
| Patient characteristics | 2008–2010 n = 411 | 2011–2013 n = 212 | P Value | |
|---|---|---|---|---|
| Age, Median (IQR) | 31(24–40) | 33(25–40) | 0.498 | |
| Range | 0–67 | 4–56 | ||
| Gender | Male | 375(91) | 196(92) | 0.604 |
| Nationality | China | 398(97) | 200(94) | 0.132 |
| Myanmar | 13(3) | 12(6) | ||
| Ethnicity | Han | 395(96) | 197(93) | 0.083 |
| Ethnic minority | 16(4) | 15(7) | ||
| Region travelled | Myanmar | 356(87) | 182(86) | 0.004 |
| Laos | 1(0) | 6(3) | ||
| Congo | 1(0) | 0(0) | ||
| Domestic epidemic area | 19(5) | 2(1) | ||
| No travel history | 34(8) | 22(10) | ||
| Type | Imported | 358(87) | 188(89) | 0.572 |
| Migrant workers | 273(66) | 126(59) | ||
| Other | 85(21) | 62(48) | ||
| Autochthonous | 53(13) | 24(11) | ||
| Living environment | Forest | 231(56) | 78(37) | <0.001 |
| Countryside | 73(18) | 63(30) | ||
| City | 45(11) | 23(11) | ||
| Unknown | 62(15) | 48(23) | ||
| Diagnosis | Plasmodium falciparum | 166(40) | 52(25) | 0.001 |
| Plasmodium vivax | 201(49) | 135(64) | ||
| Mixed | 10(2) | 4(2) | ||
| Unidentified | 34(8) | 21(10) | ||
| Types | Severe | 115(28) | 36(17) | 0.002 |
| Uncomplicated | 296(72) | 176(83) |
Discussion
In this study, we found that most of the hospitalized malaria cases in Tengchong were Chinese males. These patients had worked in Myanmar as migrant workers and lived in forests or rural areas before contracting malaria. They did not take standard chemoprophylaxis drugs to prevent malaria. Among the cases, P. vivax and P. falciparum were the main species of malaria. The mortality of the cases was less than 1%. The proportion of pregnant cases was high in the childbearing age group.12
Hospitalized cases were distributed year-round, but there were two peaks. The greatest peak was in the summer, which coincided with the period of peak rainfall in Tengchong and surrounding areas (Figure 3). The other, smaller peak was in the winter, when most of the local workers came back to Tengchong to harvest their fields and participate in the Spring Festival. Similar trends were found in another study in this region.13
The number of cases fell after 2010 (Figure 4). The proportion of P. falciparum cases and the proportion of mobilized workers who had worked in forests also decreased. The percentage of severe malaria among all of the cases and the cases of death declined after 2010. However, the basic demography of the malaria patients did not change after 2010 (Table 2). Young males who traveled to Myanmar were the predominant malaria patients in PHTC, and approximately half of the patients were Tengchong local residents.
Political and economic factors in northern Myanmar may also influence the incidence of malaria in Tengchong county. In 2011, an armed conflict erupted between the Kachin Independence Army and the Myanmar government in northern Myanmar, and the Myanmar government suspended the Myitsone dam project. In our study, the admitted malaria cases from Myitsone reached their peak in 2010. After this event, the number of Chinese workers in Myitsone and surrounding areas decreased dramatically. The proportion of patients from Myitsone and surrounding areas also fell (Figure 4).
Zhou et al. consider P. vivax malaria to be endemic in areas of Yunnan province along the China–Myanmar border, whereas cases of P. falciparum malaria are most likely imported from Myanmar.14 Thus, when the population of individuals returning from Myanmar declines, the number of P. falciparum patients in Tengchong also declines. In our study, we found that the proportion of patients who had been living in the forest declined with the proportion of P. falciparum and severe malaria (Figure 6). Thus, travel to a forest may be one high risk factor for P. falciparum infection.
There are both similarities and differences in characteristics between eastern and southwestern China. The annual number of imported malaria cases increased dramatically in both eastern and southwestern China. Most cases occurred in males, and the main purpose for travel was labor. However, in eastern China, most cases were acquired from Africa. P. falciparum was the dominant species in Jiangsu province (82.1%).15 In Tengchong, P. vivax was the dominant species, and most patients had been in Myanmar before their malaria was diagnosed.
Imported malaria has become a common problem in many countries and a major public health challenge in China.16,17,18,19,20,21,22,23,24,25,26 Chinese workers returning from Africa account for the majority of cases of imported malaria. Southeast Asia, including Myanmar, is another major source of imported cases. Imported malaria can also increase the number of local cases. Because imported malaria is widely distributed throughout China, the disease can be introduced into malaria-free localities during the transmission season, especially when a large number of cases are clustered in areas where the Anopheles species of mosquitoes are prevalent.
The proportion of chemoprophylaxis use in the migrant labor force is low. Unlike short-term travelers, migrant workers may remain for several months in an endemic rural area.27,28,29 These workers lack the necessary knowledge regarding how to avoid contracting malaria. Determining how to increase chemoprophylaxis compliance is a major issue. Pre-referral anti-malaria treatment in this group is another possible method to control infection rates.30,31,32 However, more clinical trials should be applied in these high-risk populations.
At present, the vulnerable populations should be targeted by the elimination strategy. The following measures may be included: the use of “malaria packs” that contain long-lasting insecticide-treated nets; prophylactic medications; and information, education and communication materials for border crossers in Yunnan. Health education information on malaria risks and protection should be provided to all migrant workers both before their travel abroad and upon their return home.33 Labor agencies should provide travelers with essential preventive measures. Training should also be provided to physicians to ensure the provision of accurate diagnosis and appropriate treatment. For local health agencies, prompt case verification and response are required to ensure the elimination of residual potential reservoirs and the prevention of local transmission caused by imported pathogens.34
In summary, the malaria situation at the Myanmar–Yunnan border was severe before the implementation of the elimination strategy in 2010. Malaria cases fell after 2010, but the proportion of imported malaria cases is still high. Most of these cases are migrant workers who have been to Myanmar. These workers rarely take chemoprophylaxis or use proper personal protective equipment in areas that are highly endemic for malaria. Some of these workers contract malaria in Myanmar and then return to China. This imported malaria poses a severe threat to the malaria elimination program in China. China will continue to strengthen its strategy to eliminate imported malaria cases by cooperating with bordering countries and non-governmental organizations.
PHTC is the only authorized hospital that can admit malaria patients. In spite of this, the limitations of this study include its retrospective methodology and the inclusion of patients only from a secondary hospital. In addition, only admitted cases were included in the study, which may not reflect the entire population in this area. There were no reliable data on length of stay or detailed data about repeat presentation, so we were unable to determine confidently whether each case was a relapse, recrudescence, or a re-infection.
Furthermore, it was not possible to exclude the presence of concomitant Salmonella infection from the medical charts in all of the cases. Salmonella infection is another common disease that causes fever in local hospitals, although few cases were confirmed by a positive culture result. This study only focused on the epidemiological factors in these cases. A detailed clinical data analysis will be conducted in future studies.
References
- 1World Health Organization. World malaria report 2014. Geneva: WHO, 2014. Available at http://www.who.int/malaria/publications/world_malaria_report_2014/en/ (accessed 16 November 2015). [Google Scholar]
- 2Zhang Q, Lai S, Zheng C et al. The epidemiology of Plasmodium vivax and Plasmodium falciparum malaria in China, 2004–2012: from intensified control to elimination. Malar J 2014; 13: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Cao J, Zhou SS, Zhou HY, Yu YB, Tang LH, Gao Q. [Malaria from control to elimination in China: transition of goal, strategy and interventions]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2013; 25: 439–443. Chinese. [PubMed] [Google Scholar]
- 4Feachem RG, Phillips AA, Hwang J et al. Shrinking the malaria map: progress and prospects. Lancet 2010; 376: 1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Yin JH, Yang MN, Zhou SS, Wang Y, Feng J, Xia ZG. Changing malaria transmission and implications in China towards national malaria elimination programme between 2010 and 2012. PLoS One 2013; 8: e74228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Yang DC, Cai WB, Gong SH. [Malaria incidence and the importance of floating population in malaria control in Tengchong County 1997–2001]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2003; 21: 33. Chinese. [PubMed] [Google Scholar]
- 7Liu H, Xu JW, Guo XR et al. Coverage, use and maintenance of bed nets and related influence factors in Kachin Special Region II, northeastern Myanmar. Malar J 2015; 14: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Feng J, Yan H, Feng XY et al. Imported malaria in China, 2012. Emerg Infect Dis 2014; 20: 1778–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Xu J, Liu H. The challenges of malaria elimination in Yunnan Province, People's Republic of China. Southeast Asian J Trop Med Public Health 2012; 43: 819–824. [PubMed] [Google Scholar]
- 10Hui FM, Xu B, Chen ZW et al. Spatio-temporal distribution of malaria in Yunnan Province, China. Am J Trop Med Hyg 2009; 81: 503–509. [PubMed] [Google Scholar]
- 11World Health Organization. Management of severe malaria: a practical handbook. 3rd edn. Geneva: WHO, 2012. Available at http://www.who.int/malaria/publications/atoz/9789241548526/en/ (accessed 16 November 2015). [Google Scholar]
- 12Desai M, ter Kuile FO, Nosten F et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7: 93–104. [DOI] [PubMed] [Google Scholar]
- 13Li N, Parker DM, Yang Z et al. Risk factors associated with slide positivity among febrile patients in a conflict zone of north-eastern Myanmar along the China-Myanmar border. Malar J 2013; 12: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Zhou G, Sun L, Xia R et al. Clinical malaria along the China-Myanmar border, Yunnan Province, China, January 2011–August 2012. Emerg Infect Dis 2014; 20: 675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Liu Y, Hsiang MS, Zhou H et al. Malaria in overseas labourers returning to China: an analysis of imported malaria in Jiangsu Province, 2001–2011. Malar J 2014; 13: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Al-Kuwari MG. Epidemiology of imported malaria in Qatar. J Travel Med 2009; 16: 119–122. [DOI] [PubMed] [Google Scholar]
- 17Broderick C, Nadjm B, Smith V et al. Clinical, geographical, and temporal risk factors associated with presentation and outcome of vivax malaria imported into the United Kingdom over 27 years: observational study. BMJ 2015; 350: h1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Dharmawardena P, Premaratne RG, Gunasekera WM, Hewawitarane M, Mendis K, Fernando D. Characterization of imported malaria, the largest threat to sustained malaria elimination from Sri Lanka. Malar J 2015; 14: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Dos-Santos JC, Angerami RN, Castineiras CM et al. Imported malaria in a non-endemic area: the experience of the university of Campinas hospital in the Brazilian Southeast. Malar J 2014; 13: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Sturrock HJ, Roberts KW, Wegbreit J, Ohrt C, Gosling RD. Tackling imported malaria: an elimination endgame. Am J Trop Med Hyg 2015; 93: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Chen SC, Chang HL, Chen KT. The epidemiology of imported malaria in Taiwan between 2002–2013: the importance of sensitive surveillance and implications for pre-travel medical advice. Int J Environ Res Public Health 2014; 11: 5651–5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Musa IR, Gasim GI, Eltoum AO, Adam I. Imported malaria at Buraidah Central Hospital, Qassim, Saudi Arabia: a retrospective analysis. Travel Med Infect Dis 2014; 12: 733–737. [DOI] [PubMed] [Google Scholar]
- 23Odolini S, Gautret P, Kain KC et al. Imported Plasmodium vivax malaria ex Pakistan. J Travel Med 2014; 21: 314–317. [DOI] [PubMed] [Google Scholar]
- 24Stepien M, Rosinska M. Imported malaria in Poland 2003 to 2011: implications of different travel patterns. J Travel Med 2014; 21: 189–194. [DOI] [PubMed] [Google Scholar]
- 25Nilles EJ, Alosert M, Mohtasham MA et al. Epidemiological and clinical characteristics of imported malaria in the United Arab Emirates. J Travel Med 2014; 21: 201–206. [DOI] [PubMed] [Google Scholar]
- 26Fonseca AG, Dias SS, Baptista JL, Torgal J. The burden of imported malaria in Portugal 2003 to 2012. J Travel Med 2014; 21: 354–356. [DOI] [PubMed] [Google Scholar]
- 27Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med 2008; 359: 603–612. [DOI] [PubMed] [Google Scholar]
- 28Kurt TL. Malaria prevention in short-term travelers. N Engl J Med 2008; 359: 2293; author reply 2293–2294. [DOI] [PubMed] [Google Scholar]
- 29Schlagenhauf P, Petersen E. Current challenges in travelers' malaria. Curr Infect Dis Rep 2013; 15: 307–315. [DOI] [PubMed] [Google Scholar]
- 30Bello SO. Pre-referral artesunate in severe malaria. Lancet 2009; 373: 1762–1763; author reply 1763. [DOI] [PubMed] [Google Scholar]
- 31von Seidlein L, Deen JL. Pre-referral rectal artesunate in severe malaria. Lancet 2009; 373: 522–523. [DOI] [PubMed] [Google Scholar]
- 32Gomes MF, Faiz MA, Gyapong JO et al. Pre-referral rectal artesunate to prevent death and disability in severe malaria: a placebo-controlled trial. Lancet 2009; 373: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Zhang M, Liu Z, He H et al. Knowledge, attitudes, and practices on malaria prevention among Chinese international travelers. J Travel Med 2011; 18: 173–177. [DOI] [PubMed] [Google Scholar]
- 34Marsh K. Research priorities for malaria elimination. Lancet 2010; 376: 1626–1627. [DOI] [PubMed] [Google Scholar]