Abstract
The mononuclear phagocyte system, consisting of monocytes, macrophages and dendritic cells (DCs), has an important role in tissue homeostasis as well as in eliciting immune responses against invading pathogens. Blood monocytes have been viewed for decades as precursors of tissue macrophages. Although the newest data show that in the steady state resident macrophages of many organs are monocyte independent, blood monocytes critically contribute to tissue macrophage and DC pools upon inflammation. To better understand the relationship between these populations and their phenotype, we isolated and differentiated human blood CD14+ monocytes in vitro into immature and mature monocyte-derived dendritic cells (MoDCs) as well as into seven different monocyte-derived macrophage subsets. We used the panel of 70 monoclonal antibodies (mAbs) submitted to the 10th Human Leukocyte Differentiation Antigen Workshop to determine the expression profiles of these 10 populations by flow cytometry. We now can compile subpanels of mAbs to differentiate the 10 monocyte/macrophage/MoDC subsets, providing the basis for novel diagnostic and therapeutic tools.
Monocytes, macrophages and dendritic cells (DCs) have an important role in tissue homeostasis, innate immune responses and initiation of adaptive immunity and traditionally comprise the mononuclear phagocyte system.1, 2 Recent data based predominantly on experiments in the mouse suggest that their ontogeny is rather diverse: monocytes and DCs are short-lived bone marrow-derived leukocytes that share the monocyte–macrophage DC progenitor as their last common haematopoietic precursor.1, 3 Afterwards, the development is split into two separate lineages: monocytes (and monocyte-derived macrophages) develop through a Flt3L-independent common monocyte progenitor, while a Flt3L-dependent common DC progenitor gives rise to all DC lineages.1, 2, 3, 4 A further restricted pre-cDC progenitor gives rise to conventional CD1c+ and CD141+ DCs.2, 3, 5 In contrast, most tissue-resident macrophages as well as Langerhans cells are seeded before birth from embryonic precursors originating in either yolk sac or foetal liver, are long-lived and maintained in the steady state by self-proliferation.1, 6 Only in skin and intestine, adult circulating monocytes substantially contribute to the resident macrophage pool in homeostasis.1, 6, 7
In humans, all three main DC subsets can be found in the peripheral blood: conventional CD1c+ DCs (or cDC2s, according to a new nomenclature8), conventional CD141+ DCs (or cDC1s) and plasmacytoid DCs (pDCs).3, 9 All DC subsets are also detectable in lymphoid and non-lymphoid tissues, although pDCs are abundant in non-lymphoid tissues only upon inflammation.3 CD1c+ DCs are characterized by high expression of CD1c, CD11c and CD172α (SIRPα), production of proinflammatory cytokines upon stimulation, migration to draining lymph nodes and efficient priming of CD4+ T-cell responses.2, 3 CD141+ DCs are distinguishable by the lack (or low expression) of CD14, CD1c, CD11c, CD11b and CD172α. Instead, they express CD370 (Clec9A/DNGR-1) and the chemokine receptor XCR1, produce tumour necrosis factor α, interferon λ (IFNλ), but little interleukin-12 (IL-12) when stimulated, and are superior at cross-presentation of antigens to CD8+ T cells.3, 9 pDCs, characterized by CD123, CD303 and CD304 and low expression of CD11c and CD14, produce high levels of IFNα in response to viruses and are thought to be important in antiviral immunity.2, 9
Human blood monocytes form three subsets based on the differential expression of CD14 and CD16: a CD14+CD16-, a CD14loCD16+ and an intermediate CD14+CD16+ subset. The most abundant classical CD14+CD16- monocytes readily extravasate into tissues in response to inflammation, where they can differentiate into macrophage-like or DC-like cells.1, 2, 9, 10 This ability of blood monocytes to differentiate to DCs or macrophages was explored in vitro already 20 years ago11, 12 and since then, most studies of the human mononuclear phagocyte system utilised in vitro generated monocyte-derived macrophages and monocyte-derived DCs (MoDCs) due to the inability to sample primary cells in sufficient amounts.9 This strategy unveiled an incredible plasticity of macrophages in response to various stimuli, ranging from most extreme classical M1 stimuli (such as IFNγ or toll-like receptor ligands) to an alternative activation (M2) using IL-4.13, 14, 15 M1-activated macrophages are highly proinflammatory with strong microbicidal and tumoricidal activity and potently stimulate Th1 responses. Activation with Th2 cytokines IL-4 or IL-13, sometimes referred to as an M2a regime, yields Th2-promoting macrophages with high phagocytic and tissue-remodelling capabilities. In contrast, activation with IL-10 (sometimes called an M2c activation) results in an immunosuppressive and tissue-remodelling phenotype.14
Here, we explored the flexibility of human blood CD14+ monocytes to differentiate and polarise into seven different macrophage subsets as well as into immature and mature MoDCs (Figure 1a) and analysed their surface markers by flow cytometry. Using monoclonal antibodies (mAbs) submitted to the 10th Human Leukocyte Differentiation Antigen Workshop (HLDA10), we show that each of the differentiated subsets is unique. Second, our results confirm that these cells are different from primary DC subsets found in human blood.
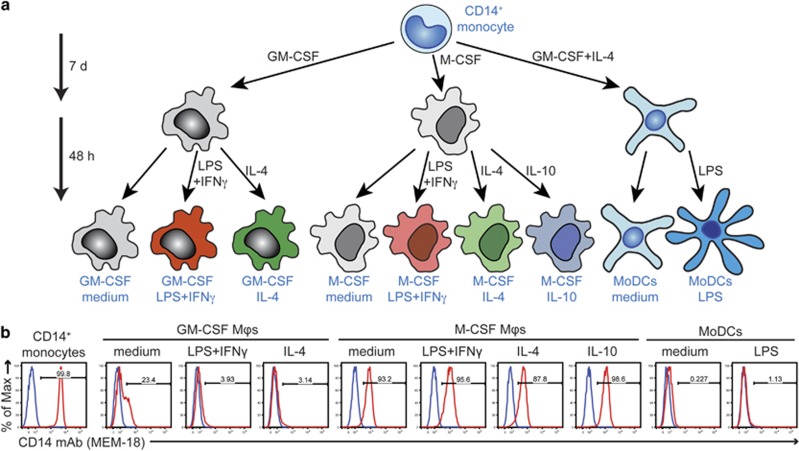
Figure 1.
(a) Differentiation regime to obtain human monocyte-derived macrophages (Mφs) and human MoDCs. MACS-isolated CD14+ human monocytes were differentiated into macrophages by cultivation with either GM-CSF or M-CSF for 7 days. Afterwards, macrophages were re-seeded and activated as indicated for 48 h. MoDCs were generated from CD14+ monocytes by 7-day cultivation with GM-CSF and IL-4. Their maturation was induced by stimulation with LPS for additional 48 h. (b) Control staining of the isolated CD14+ monocytes and the nine monocyte-derived cell populations using CD14 mAb MEM-18 (red histograms; overlaid with the background staining of the isotype control mAb (blue histograms)).
RESULTS
In vitro generation of human macrophages and dendritic cells from human peripheral blood monocytes
To screen the large number of mAbs submitted to the HLDA10 workshop, we isolated 70 million CD14+ monocytes from human peripheral blood mononuclear cells (PBMCs) by magnetic-activated cell sorting using CD14 microbeads. The purity of isolated monocytes was 99.8%, as determined by CD14 staining (Figure 1b). The CD14+ monocytes were then differentiated into macrophages by cultivation with either granulocyte–macrophage (GM-CSF) or macrophage (M-CSF) colony-stimulating factor for 7 days (d). Afterwards, both lineages were activated with the prototype M1 (lipopolysaccharide (LPS)+IFNγ) or M2a (IL-4) stimuli or left untreated for 48 h (Figure 1a). M-CSF-differentiated macrophages were additionally activated with IL-10 to obtain the M2c subtype.14 MoDCs were generated from CD14+ monocytes by 7-day cultivation with GM-CSF and IL-4. Their maturation was induced with LPS for additional 48 h. Although MoDCs were non-adherent, all types of monocyte-differentiated macrophages were predominantly adherent with a fried-egg-shaped (GM-CSF-differentiated subtypes) or round-to-rod-like (M-CSF-differentiated subtypes) morphology (data not shown). To check for proper differentiation, we also measured expression of several monocyte–macrophage markers by flow cytometry (Figure 1b and data not shown). In comparison with freshly isolated monocytes, the LPS coreceptor CD14 was only moderately downregulated from the surface of all types of M-CSF-differentiated macrophages, but strong downregulation was observed in GM-CSF-differentiated macrophages and especially in MoDCs, which is consistent with previous reports.16, 17 Nevertheless, CD14 expression on GM-CSF-differentiated macrophages activated with either LPS+IFNγ or IL-4 was lower compared with the levels we usually observed.
HLDA10 mAbs that failed to bind any of the generated cell types or bound weakly to freshly isolated monocytes only
We received 70 mAbs submitted to the HLDA10 workshop and tested them with freshly purified CD14+ monocytes and all nine differentiated cell types described above. The staining parameters (geometric mean of fluorescence intensity, corrected for the background staining using isotype control mAbs, and percentage of positive cells with respect to a 0.1% cutoff of the negative control) of all tested mAbs are summarised in Supplementary Table. Thirty mAbs failed to substantially bind to any tested cell type. These included mAbs specific to tetanus toxoid or to proteins normally localised intracellularly, such as vimentin or calreticulin.18 Others recognised antigens known to be specifically expressed outside the myeloid lineage: Clec5C, also known as NKp80, expressed on NK cells and some T-cell subsets,19 Glycoprotein A Repetitions Predominant (GARP, also known as LRRC32) expressed by activated regulatory T cells,20 protein tyrosine phosphatase, receptor type, C-associated protein (PTPRCAP, also known as LPAP) expressed on T and B cells,21 or decoy IL-13 receptor α 2 (IL13Ra2) and Fat-1 cadherin, expressed on neoplastic cells.22, 23 Similarly, negligible binding, not exceeding 1% positive events in any of the population tested, was detected using mAbs specific to Axl, CD365 (also known as TIM-1 or HAVCR1), CD135 (also known as Flt-3 (FMS-like tyrosine kinase 3) or Flk-2), CD85g (also known as ILT7) and with three (clones 9A11 and 8F9, the latter used in two different formats) out of four mAbs specific to CD370 (also known as Clec9A or DNGR-1). In summary, negativity of these mAbs might be due to the lack of expression of the respective antigens by the generated cells.
However, activities of the following mAbs may have been lost during labelling or transport, because the respective antigens were described to be expressed on at least one of the tested cell types or other mAbs of the same specificity scored positively: no binding was detected with one mAb (clone GE2) out of three specific to CD369 (also known as Clec7A or Dectin-1) and one mAb (clone 111F8.04) out of three to CD367 (also known as Clec4A or dendritic cell immunoreceptor (DCIR)). Furthermore, the HLDA10 mAbs to the C-type lectin domain family members Clec2D (also known as LLT1 or OCIL) and Clec14A, to the B7 family member B7-H4 and to the immunoglobulin superfamily member DORA (DOwn-Regulated by Activation, also known as IGSF6) were negative, though previous studies showed that B7-H424 and Clec2D25 were expressed on human MoDCs or even on monocytes. Furthermore, mRNAs for scarcely characterised Clec14A26 and DORA27 were detected in MoDCs.
Nine mAbs bound to freshly isolated monocytes but not to any of the macrophage or MoDC subtypes. These were mAb FAB6367P recognising the CD302 molecule (Clec13A), mAb 24 to the CD85h molecule (ILT1), mAb FAB1798P to the lectin-like oxidised LDL receptor (LOX-1/Clec8A), mAb FAB3131P to the CD202b molecule (Tie-2/TEK), mAb 9F4 to TIM-4, mAb FAB1517P to ULBP-3, mAb FAB6049P to CD370/Clec9A and mAbs FAB3744P and FAB3479P against the formyl peptide receptors FPR1 and FPR2, respectively. The majority of these stainings were very modest, with 1.6–10.2% positive events. Only CD85h mAb 24 stained positively >33% of isolated monocytes.
As CD370 mAb FAB6049P showed a positive staining with monocytes in comparison with the two other tested CD370 mAbs 9A11 and 8F9, we analysed monocytes of two other donors using this mAb. The staining was negative (data not shown), corroborating that the CD370 molecule (Clec9A) is most likely not expressed by monocytes and monocyte-derived cells. These results also showed that CD370 mAb FAB6049P might recognise a sub-population of blood monocytes in a donor-dependent manner.
HLDA10 mAbs recognising only GM-CSF-differentiated macrophages and MoDCs
Seven mAbs specifically recognised all types of the GM-CSF-differentiated macrophages and both immature and mature MoDCs, but did not bind monocytes or any of the M-CSF-differentiated macrophages. These included two CD1a mAbs (clones 010e and 0619), one CD1b mAb (clone O249), one CD1c mAb (clone L161) and three mAbs (clones CMRF-44, CMRF-56 and BGA69) with unknown specificity. The strong binding of all CD1 mAbs was enhanced in response to LPS+IFNγ or LPS. A typical expression pattern of the CD1 mAbs is shown by CD1a mAb 0619 in Figure 2a (top). Two of the three mAbs with unknown specificity (BGA69 and CMRF-44) stained weaker immature MoDCs than the third mAb CMRF-56. However, the overall staining patterns of these three mAbs were very similar, which raised the assumption that they might recognise the same antigen (Figure 2a, bottom).
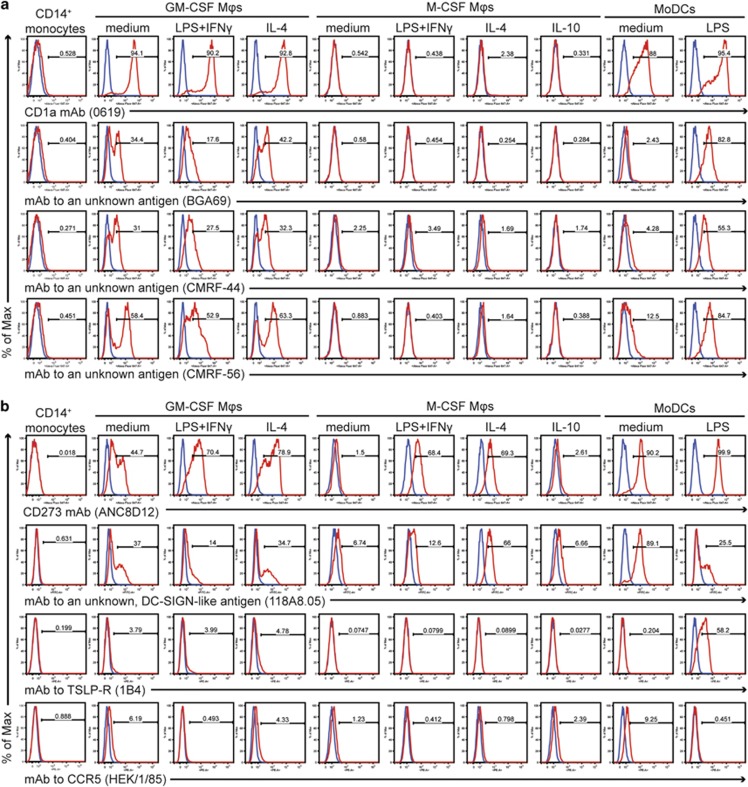
Figure 2.
(a) Expression profiles of HLDA10 mAbs that stained GM-CSF-differentiated macrophages and MoDCs. The expression profile of CD1a mAb 0619 (code 10-10) is shown at the top. Identical patterns were seen with another CD1a mAb (clone 010e, code 10-03), CD1b mAb O249 (code 10–18) and CD1c mAb L161 (code 10–26). Three mAbs with unknown specificity (code 10–38, clone BGA69; code 10–82, clone CMRF-44 and code 10–69, clone CMRF-56) showed a similar pattern but in contrast to the CD1 mAbs recognised poorly immature MoDCs. The expression profiles of these mAbs are shown at the bottom. (b) HLDA10 mAbs reactive with certain monocyte-derived macrophage and DC populations but not with freshly isolated monocytes. In all cases, the specific staining shown by the red histograms is overlaid with the background staining of the isotype control mAbs (shown in blue).
HLDA10 mAbs recognising at least one differentiated cell type but not monocytes
Four mAbs reacted with at least one differentiated cell type but not with monocytes (Figure 2b). The CD273 mAb ANC8D12 recognising the immunoinhibitory molecule B7-DC (also known as PD-L2) stained strongly not only immature and mature MoDCs but also all types of the GM-CSF-differentiated macrophages. Within the M-CSF-differentiated macrophages it stained only those that were activated with LPS+IFNγ or IL-4. The same populations were recognised by mAb 118A8.05, specific to an unknown antigen similar to DC-SIGN/CD209 (therefore termed DC-SIGN-like), although the staining of the LPS+IFNγ- or LPS-activated populations was weaker. More discrete stainings were observed with mAbs specific to cytokine receptors: mAb 1B4 specific to thymic stromal lymphopoietin receptor (TSLP-R/CRLF2) strongly reacted only with mature MoDCs and stained very weakly all GM-CSF-differentiated macrophages. The CCR5 mAb HEK/1/85 stained weakly immature MoDCs and all macrophage populations that were not activated with proinflammatory stimuli.
HLDA10 mAbs binding to monocytes and to at least one of the differentiated cell types
Thirteen mAbs intensely stained monocytes and reacted with at least one macrophage or MoDC population (Figure 3). Out of those, mAb FAB2384P specific to the C-type lectin receptor (CLR) Clec5A (also known as myeloid DAP12-associating lectin or MDL-1) stained beside monocytes all macrophages but not MoDCs.
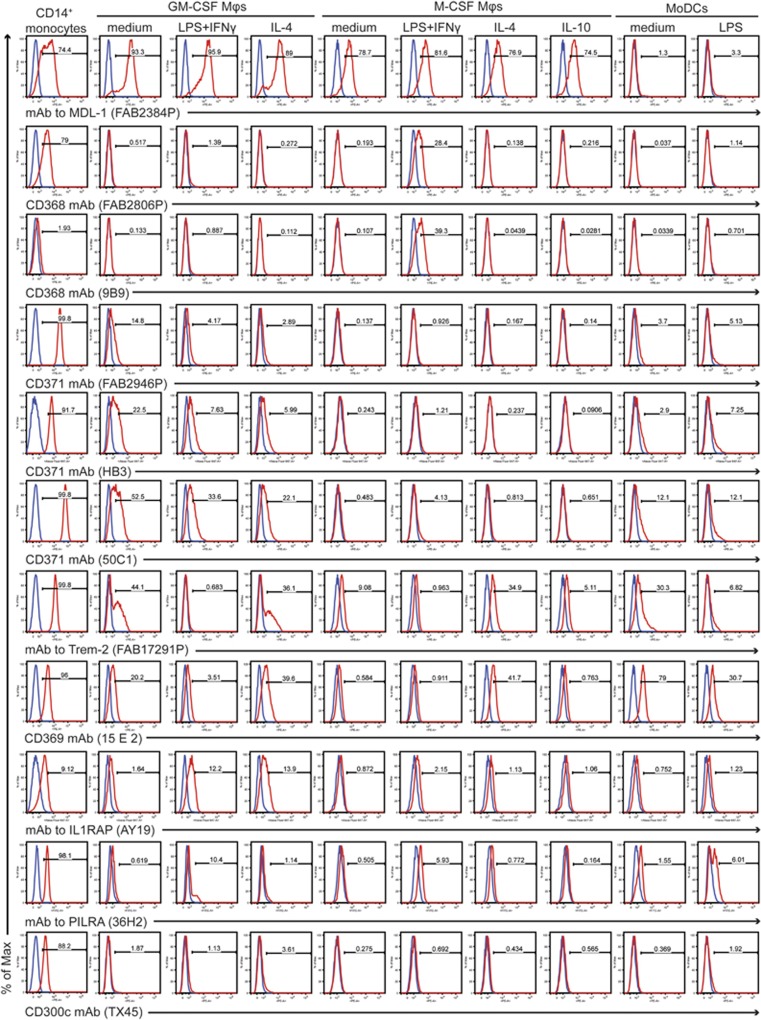
Figure 3.
Expression profiles of HLDA10 mAbs that stained monocytes and at least one of the differentiated cell types. Specific staining shown in red histograms is overlaid with the background staining of the isotype control mAbs (shown in blue).
The most distinctive staining was observed with two CD368 mAbs (FAB2806P and 9B9) recognising another CLR family member—Clec4D (also known as Dectin-3 or macrophage C-type lectin, MCL), which stained only M-CSF-differentiated and LPS+IFNγ-stimulated macrophages. However, while mAb FAB2806P stained strongly also monocytes, we detected only a weak staining using mAb 9B9, suggesting that its epitope might be largely hidden on monocytes. All three CD371 mAbs (HB3, FAB2946P and 50C1) against the myeloid inhibitory C-type lectin-like receptor (MICL, also known as Clec12A) scored highly positively with freshly isolated monocytes. Expression of MICL was downregulated through each differentiation regime with the strongest downregulation upon M-CSF treatment. The lowest downregulation was seen on the GM-CSF-differentiated cells but upon activation of these cells with both LPS+IFNγ and IL-4, MICL expression was further suppressed. Although having the same staining profile, the three CD371 mAbs stained especially monocytes and GM-CSF-differentiated macrophages with different intensities (Figure 3).
In comparison with CD371, a very similar staining we observed with mAb FAB17291P specific to the Trem-2 (triggering receptor expressed on myeloid cells 2), though this receptor was upregulated by IL-4 in the M-CSF-differentiated macrophages. Two CD369 mAbs (FAB1859P and 15 E 2), recognising Clec7A/Dectin-1, stained predominantly monocytes, MoDCs and IL-4-activated macrophages of both lineages and barely reacted with the other M-CSF macrophage subtypes. mAb AY19 to the interleukin 1 receptor accessory protein (IL1RAP, also known as IL-1RAcP) substantially stained monocytes and both classically and alternatively activated macrophages of the GM-CSF lineage but no other macrophage or MoDC type.
Finally, mAb 36H2 specific to the paired immunoglobulin-like type 2 receptor (PILRA, also known as FDF03) and mAb TX45 to CD300c (also known as MAIR II) stained predominantly monocytes, while binding to any of the differentiated/activated cells was barely detectable. The only difference was that mAb 36H2 displayed a slightly stronger binding with both LPS+IFNγ-treated macrophages and LPS-treated MoDCs.
HLDA10 mAbs that stained all mononuclear phagocyte populations generated
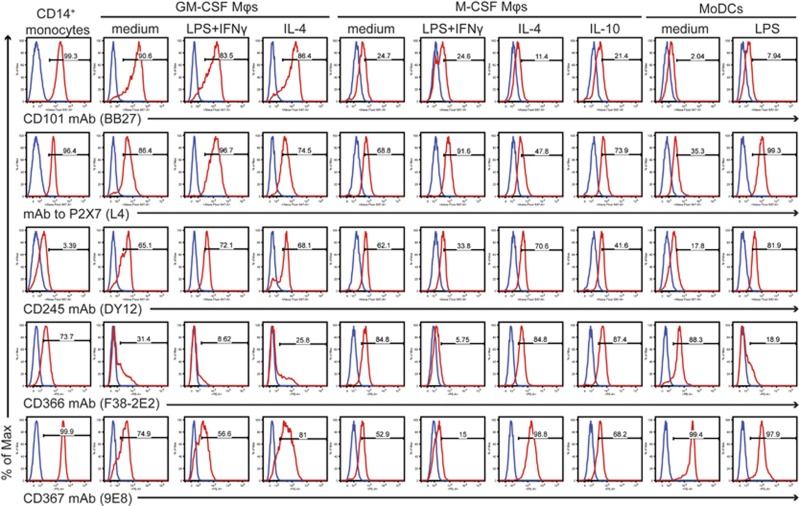
Seven mAbs reacted with all cell types analysed (Figure 4). CD101 mAb BB27 showed the most robust expression of its antigen on monocytes and GM-CSF-differentiated macrophages. mAb L4 specific to the purinergic receptor P2X7 (also known as P2RX7) and the CD245 mAbs DY12 and DY35 exhibited a more uniform staining with moderately increased staining on the LPS+IFNγ-activated GM-CSF lineage and mature MoDCs. Additionally, increased P2X7 staining was observed in the M-CSF lineage upon LPS+IFNγ stimulation. Further, mAbs FAB2365P and F38-2E2 recognising the CD366 molecule (also known as TIM-3 or HAVCR2) revealed a downregulation of TIM-3 in response to the proinflammatory stimuli in both macrophage lineages and also in MoDCs. mAbs FAB1748P and 9E8 specific to the CD367 molecule (also known as Clec4A or DCIR) showed an identical staining pattern: they highly stained monocytes and MoDCs, while staining of macrophages was weaker with downregulation upon LPS+IFNγ stimulation. On the other hand, IL-4 upregulated the CD367 molecule.
Figure 4.
Expression profiles of HLDA10 mAbs that stained all cell types analysed. Specific staining shown in red histograms is overlaid with the background staining of the isotype control mAbs (blue histograms).
DISCUSSION
The difficulty to access human tissue-resident macrophages as well as a rarity of human DCs in the blood (<0.1% of PBMCs) have prompted researchers to rely on in vitro differentiation of human blood monocytes with GM-CSF or M-CSF to obtain monocyte-derived macrophages or with GM-CSF+IL-4 to obtain MoDCs. These studies revealed many important functions of the mononuclear phagocyte system.9 However, one needs to be aware of the limitations of this approach and that a lot of work is still ahead of us.
In this study, we evaluated the expression profile of human monocytes and nine different types of monocyte-derived cells including seven macrophage subtypes as well as immature and mature MoDCs by using the panel of mAbs submitted to HLDA10. HLDA10 focused on DC antigens and thus the call was to submit DC-specific mAbs. Therefore, we were puzzled when we did not observe binding of several mAbs specific to known or suspected DC antigens in any of the populations tested, although our generated MoDCs expressed high levels of the CD1a, CD1b and CD1c molecules and were negative for CD14, characteristics of DCs. It may be possible that some mAbs lost their functionality during the labelling procedure or during transport. Alternatively, this result may reflect the inability of monocytes to differentiate into true DCs using the GM-CSF+IL-4 regime or may indicate that pathways, which these cells underwent from the common monocyte–macrophage DC progenitor, are too divergent. As noted previously for blood monocytes,7 also our monocytes and all monocyte-derived populations were devoid of the CD135 molecule (Flt-3) that restricts the DC development along the Flt3L gradient.9 We also did not observe expression of pDC-restricted CD85g/ILT728 or the C-type lectin receptor CD370 (Clec9A also known as DNGR-1) that is specifically expressed on CD141+ DCs (cDC1s) and crucial for their cross-presenting capacity of necrotic cell-derived antigens.3, 29 Similarly, we did not detect expression of Axl, a TAM receptor tyrosine kinase involved in apoptotic cell clearance, which is specifically expressed by Langerhans cells, but not by monocyte-derived cells unless stimulated with transforming growth factor-β1.30 We also did not detect the CD365 molecule (also known as Tim-1 or HAVCR1). This receptor was initially described as T-cell restricted and mediating Th2 inflammation and also to be expressed in kidney proximal tubule cells,31 but one report has shown low levels of TIM-1 mRNA also in cDC2s and pDCs.32 However, we cannot exclude the possibility that we were not able to detect some DC markers due to polymorphisms in the human population.
Eight antigens we found to be expressed on freshly isolated monocytes but not on any differentiated macrophage or MoDC subset. Among these, the CD202b molecule (angiopoietin-1 receptor Tie-2) was already described as a marker of a proangiogenic subset of monocytes.2, 33 Similarly, mAbs to the CD302 molecule (C-type lectin receptor DCL-1 or Clec13A) involved in phagocytosis, endocytosis and migration, or to the FPR2 (formyl peptide receptor 2), which regulates chemotaxis and cell activation, stained only monocytes and their antigens were already reported as downregulated during in vitro differentiation to macrophages or MoDCs.34, 35 We failed to detect expression of the efferocytosis-mediating proteins TIM-4 and LOX-1/Clec8A on differentiated macrophages or MoDCs. Since both were described to be expressed by these cells in vivo,31, 36 we presume that their expression might depend on factors poorly recapitulated in vitro.
A significant number of mAbs (eighteen) within the HLDA10 workshop panel recognised members of the CLR family, which are widely expressed on myeloid cells. CLRs contain one or more conserved structural motifs called the C-type lectin-like domain that gave the name to the family.36 The prototypical function of the C-type lectin-like domain is calcium-dependent binding of lectins (carbohydrates) present on pathogens or self-antigens, but several CLRs bind lectins or other ligands, such as proteins or lipids, in a calcium-independent manner.36 CLRs then signal to engage the cell's phagocytic or endocytic machinery and ligand uptake. Furthermore, many CLRs are involved in immune cell responses, including reactive oxygen species production, inflammasome activation, chemotaxis, cytokine production or DC maturation.37 CLR signalling can either synergize or antagonise signals from other receptors, leading to fine-tuning of innate immune response to infection or tissue damage.36, 37
The CD369 molecule (Clec7A/Dectin-1) is a β-glucan pattern recognition receptor. Upon binding of β-1,3-linked glucans present in the cell wall of fungi, some bacteria and plants, Dectin-1 engages signalling cascades that drive innate and adaptive immunity through its phosphorylated cytoplasmic ITAM (immune-receptor tyrosine-based activation motif, also referred to as hemITAM, since it contains only one YxxL motif).36, 37 Previous studies have shown that human Dectin-1 is expressed on granulocytes, monocytes, DCs, monocyte-derived macrophages and MoDCs.38, 39 The expression pattern we observed during macrophage differentiation, including its further downregulation by proinflammatory stimuli and upregulation by IL-4, is in line with the expression pattern observed for mouse Dectin-1.40 In contrast, the CD368 molecule (also known as Dectin-3, Clec4D or macrophage C-type lectin, MCL) is highly expressed by neutrophils and monocytes and weakly by DCs in the peripheral blood; and its expression is lost upon in vitro differentiation of monocytes into macrophages or MoDCs.41 Our results confirmed this phenotype, but our observation that Dectin-3 was profoundly and specifically upregulated in the M-CSF- and LPS+IFNγ-stimulated macrophages has not been reported earlier. It is interesting to speculate that this macrophage subset might be pivotal for host defence against fungi or mycobacteria, since Dectin-3, paired with other CLRs, namely Dectin-2 (Clec6A) or Mincle (Clec4E), respectively, has been shown to serve as a pattern recognition receptor for these pathogens.42, 43 Clec5A, also known as MDL-1, represents another member of the family involved in myeloid cell activation. MDL-1 serves as a dengue virus receptor44 and MDL-1 modulating mAbs revealed its involvement in a mouse model of arthritis, though its endogenous ligand remained unidentified.45 Our data confirm the previously published results showing that MDL-1 was exclusively expressed by monocytes, macrophages and osteoclasts, but not by MoDCs.36, 44 The last two CLRs we found expressed in our monocyte-derived cells are the CD367 molecule (Clec4A, also known as DCIR) and the CD371 molecule (Clec12A, also known as MICL). Both are immunoinhibitory receptors that upon engagement recruit phosphatases SHP-1 and SHP-2 to their phosphorylated cytoplasmic immune-receptor tyrosine-based inhibition motifs.36, 37 DCIR is predominantly expressed by DCs, including MoDCs, while MICL by monocytes and granulocytes, but both CLRs have been shown to be expressed in other myeloid populations and downregulated by proinflammatory stimuli.46, 47, 48, 49 In line with the published data, we observed downregulation of the CD367 and CD371 molecules in response to the LPS+IFNγ/LPS stimulation. Additionally, we extend these findings by reporting of a loss of CD371 expression from the surface of the M-CSF-differentiated but not GM-CSF-differentiated macrophages.
In conclusion, using mAbs submitted to the HLDA10 workshop, we provide here a comprehensive analysis of antigens expressed on the surface of blood monocytes, immature and mature MoDCs and on seven monocyte-derived macrophage subsets. We found that several known DC markers (for example, CD135, CD370 and CD85g) were not expressed by monocytes or in vitro derived cells, while other antigens (for example, CD366, CD367, CD245 and CD101) we found highly expressed by all cell types analysed. In addition, we observed clear differences in the expression of several markers including CD273, CD1a, CD101, CD369, CD371 and IL1RAP between the similarly activated GM-CSF- and M-CSF-differentiated macrophage populations that corroborate the distinct action of these two CSFs onto monocyte differentiation observed by the transcriptome analysis.50 In summary, using the HLDA10 mAb panel we show that we can subtly discriminate different types of macrophages and DCs, which is the basis and prerequisite to specifically target pathogenic subtypes of macrophages and DCs driving autoimmune diseases or malignancies.
METHODS
Reagents and antibodies
LPS (Escherichia coli serotype O55:B5) was purchased from Sigma-Aldrich (Munich, Germany). Recombinant human M-CSF, IFNγ and IL-10 were obtained from Peprotech (Rocky Hill, NJ, USA). Recombinant human GM-CSF and IL-4 were from Novartis AG (Basel, Switzerland). The HLDA10 mAb panel, tested within the 10th Human Leukocyte Differentiation Antigen Workshop, in Wollongong, Australia, is detailed at http://www.hcdm.org. Alexa Fluor 647-conjugated monomeric streptavidin used at a final concentration of 2.5 μg ml−1 was prepared in-house. Allophycocyanin-conjugated goat anti-mouse IgG+IgM Ab used at a final dilution of 1:2500 was from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Control antibodies included: Pacific Blue-, FITC- or PE-labelled mouse IgG1 isotype control mAb, clone MOPC-21 (Biolegend, San Diego, CA, USA), Pacific Blue-conjugated CD14 mAb, clone MEM-18, unlabelled mouse IgG1 isotype control mAb, clone PPV06 (both from EXBIO, Prague, Czech Republic) and biotin-labelled mouse IgG1 isotype control mAb MCA928B (AbD Serotec, Oxford, UK).
Monocyte isolation
Leukocytes of a healthy donor were collected using a leukocyte reduction system chamber and a Trima Accel automated blood collection system (Terumo BCT, Lakewood, CA, USA). PBMCs were isolated from these preparations by density gradient centrifugation using Lymphoprep (Axis-Shield, Oslo, Norway). CD14+ monocytes were then purified from the PBMCs by magnetic-activated cell sorting using CD14 microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). The study, performed in accordance with the Declaration of Helsinki, was approved by the ethics committee of the Medical University of Vienna (2177/2013).
Macrophage and MoDC differentiation
CD14+ monocytes were cultured in complete culture medium consisting of RPMI-1640 medium supplemented with 2 mm l-glutamine, 100 μg ml−1 streptomycin, 100 U ml−1 penicillin and 10% heat-inactivated foetal calf serum (all from Invitrogen, Carlsbad, CA, USA) at a concentration of 6.6 × 105 cells per ml (or 1 × 106 cells per ml for differentiation of M-CSF-primed macrophages). To trigger differentiation to macrophages, human recombinant cytokines were used at the following final concentrations: 25 ng ml−1 GM-CSF or 50 ng ml−1 M-CSF. Medium was supplemented every 2–3 days. After 7 days, macrophages were harvested by pipetting and the adherent cells were collected by subsequent trypsinisation. Cells were then centrifuged and resuspended in macrophage serum-free medium (Invitrogen) supplemented with 2 mm l-glutamine, 100 μg ml−1 streptomycin, 100 U ml−1 penicillin and 2% foetal calf serum. Cell density was adjusted to 3 × 105 cells per ml before seeding to 10 cm dishes. After 30 min recovery time, polarisation to the M1 state was induced by 100 ng ml−1 LPS plus 25 ng ml−1 IFNγ for 48 h. We polarised the macrophages to the M2a state by using 20 ng ml−1 IL-4 or to the M2c state by 20 ng ml−1 IL-10 (the latter for M-CSF-differentiated macrophages only).
Differentiation to MoDCs was achieved by cultivation of CD14+ monocytes for 7 days in complete culture medium supplemented with 50 ng ml−1 GM-CSF plus 50 ng ml−1 IL-4. For their maturation, the cells were further cultivated for 48 h with 100 ng ml−1 LPS.
Flow cytometry
Human macrophages were detached from the culture plates by treating them with ice-cold 1.5 mm EDTA in Hank's balanced salt solution. Also, MoDCs were treated the same way. Cells were then washed with precooled staining buffer (PBS containing 1% BSA and 0.02% NaN3) and incubated on ice for 30 min with 2.4 mg ml−1 human IgG (Beriglobin P; CSL Behring, King of Prussia, PA, USA) to prevent non-specific binding of the mAbs to Fc receptors. Monocytes were used at a concentration of 1 × 105 cells/staining, while macrophages and MoDCs were used at 8 × 104 cells/staining. Then, antibody-fluorochrome conjugates with appropriate isotype controls were added to a final concentration of 2.5 μg ml−1 in a total staining volume of 40 μl. Cells were incubated for 30 min on ice and then washed twice with the staining buffer. In case of an unconjugated mAb, or biotin-labelled mAb, the respective second step reagent was added and cells were again incubated 30 min on ice and washed again with the staining buffer. Directly before analysis, DAPI (0.5 μg ml−1, Sigma-Aldrich) was added. Samples were analysed on an LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) equipped with the FACSDiva software; and the data were further processed with the FlowJo software (Treestar, Ashland, OR, USA). Living single cells were gated according to their forward- and side-scatter characteristics, and dead cells were excluded using DAPI. The positive gate was set according to the staining with the isotype control mAbs with the cutoff of 0.1%. In Supplementary Table, geometric mean of fluorescence intensity of the specific mAb staining is corrected for background staining using matched isotype control mAb.
Acknowledgments
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement NMP4-LA-2009-228827 NANOFOL. We acknowledge our colleagues René Platzer and Johannes Huppa for providing Alexa Fluor 647-conjugated monomeric streptavidin.
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Clinical and Translational Immunology website (http://www.nature.com/cti)
Supplementary Material
References
- 1Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014; 14: 392–404. [DOI] [PubMed] [Google Scholar]
- 2Reynolds G, Haniffa M. Human and mouse mononuclear phagocyte networks: a tale of two species? Front Immunol 2015; 6: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Schlitzer A, McGovern N, Ginhoux F. Dendritic cells and monocyte-derived cells: two complementary and integrated functional systems. Semin Cell Dev Biol 2015; 41: 9–22. [DOI] [PubMed] [Google Scholar]
- 4Lee J, Breton G, Oliveira TY, Zhou YJ, Aljoufi A, Puhr S et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med 2015; 212: 385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Breton G, Lee J, Zhou YJ, Schreiber JJ, Keler T, Puhr S et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J Exp Med 2015; 212: 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014; 41: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E et al. Human dermal CD14(+) cells are a transient population of monocyte-derived macrophages. Immunity 2014; 41: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014; 14: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Haniffa M, Bigley V, Collin M. Human mononuclear phagocyte system reunited. Semin Cell Dev Biol 2015; 41: 59–69. [DOI] [PubMed] [Google Scholar]
- 10Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013; 38: 336–348. [DOI] [PubMed] [Google Scholar]
- 11Kasinrerk W, Baumruker T, Majdic O, Knapp W, Stockinger H. CD1 molecule expression on human monocytes induced by granulocyte-macrophage colony-stimulating factor. J Immunol 1993; 150: 579–584. [PubMed] [Google Scholar]
- 12Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994; 179: 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–686. [DOI] [PubMed] [Google Scholar]
- 15Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014; 40: 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA 2004; 101: 4560–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de la Rosa G, Longo N et al. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol 2002; 168: 2634–2643. [DOI] [PubMed] [Google Scholar]
- 18Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J 2010; 24: 665–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Klimosch SN, Bartel Y, Wiemann S, Steinle A. Genetically coupled receptor-ligand pair NKp80-AICL enables autonomous control of human NK cell responses. Blood 2013; 122: 2380–2389. [DOI] [PubMed] [Google Scholar]
- 20Chan DV, Somani AK, Young AB, Massari JV, Ohtola J, Sugiyama H et al. Signal peptide cleavage is essential for surface expression of a regulatory T cell surface protein, leucine rich repeat containing 32 (LRRC32). BMC Biochem 2011; 12: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Schraven B, Schoenhaut D, Bruyns E, Koretzky G, Eckerskorn C, Wallich R et al. LPAP, a novel 32-kDa phosphoprotein that interacts with CD45 in human lymphocytes. J Biol Chem 1994; 269: 29102–29111. [PubMed] [Google Scholar]
- 22Sengupta S, Thaci B, Crawford AC, Sampath P. Interleukin-13 receptor alpha 2-targeted glioblastoma immunotherapy. Biomed Res Int 2014; 2014: 952128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23de Bock CE, Ardjmand A, Molloy TJ, Bone SM, Johnstone D, Campbell DM et al. The Fat1 cadherin is overexpressed and an independent prognostic factor for survival in paired diagnosis-relapse samples of precursor B-cell acute lymphoblastic leukemia. Leukemia 2012; 26: 918–926. [DOI] [PubMed] [Google Scholar]
- 24Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H et al. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol 2006; 177: 40–44. [DOI] [PubMed] [Google Scholar]
- 25Germain C, Meier A, Jensen T, Knapnougel P, Poupon G, Lazzari et al. Induction of lectin-like transcript 1 (LLT1) protein cell surface expression by pathogens and interferon-gamma contributes to modulate immune responses. J Biol Chem 2011; 286: 37964–37975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Harman AN, Bye CR, Nasr N, Sandgren KJ, Kim M, Mercier SK et al. Identification of lineage relationships and novel markers of blood and skin human dendritic cells. J Immunol 2013; 190: 66–79. [DOI] [PubMed] [Google Scholar]
- 27Bates EE, Dieu MC, Ravel O, Zurawski SM, Patel S, Bridon JM et al. CD40L activation of dendritic cells down-regulates DORA, a novel member of the immunoglobulin superfamily. Mol Immunol 1998; 35: 513–524. [DOI] [PubMed] [Google Scholar]
- 28Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G et al. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med 2006; 203: 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood 2012; 119: 2284–2292. [DOI] [PubMed] [Google Scholar]
- 30Bauer T, Zagorska A, Jurkin J, Yasmin N, Koffel R, Richter S et al. Identification of Axl as a downstream effector of TGF-beta1 during Langerhans cell differentiation and epidermal homeostasis. J Exp Med 2012; 209: 2033–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 2007; 27: 927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Sonar SS, Hsu YM, Conrad ML, Majeau GR, Kilic A, Garber E et al. Antagonism of TIM-1 blocks the development of disease in a humanized mouse model of allergic asthma. J Clin Invest 2010; 120: 2767–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell 2008; 14: 299–311. [DOI] [PubMed] [Google Scholar]
- 34Kato M, Khan S, d'Aniello E, McDonald KJ, Hart DN. The novel endocytic and phagocytic C-Type lectin receptor DCL-1/CD302 on macrophages is colocalized with F-actin, suggesting a role in cell adhesion and migration. J Immunol 2007; 179: 6052–6063. [DOI] [PubMed] [Google Scholar]
- 35Waechter V, Schmid M, Herova M, Weber A, Gunther V, Marti-Jaun J et al. Characterization of the promoter and the transcriptional regulation of the lipoxin A4 receptor (FPR2/ALX) gene in human monocytes and macrophages. J Immunol 2012; 188: 1856–1867. [DOI] [PubMed] [Google Scholar]
- 36Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol 2012; 30: 491–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Yan H, Kamiya T, Suabjakyong P, Tsuji NM. Targeting C-type lectin receptors for cancer immunity. Front Immunol 2015; 6: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Sobanov Y, Bernreiter A, Derdak S, Mechtcheriakova D, Schweighofer B, Duchler M et al. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur J Immunol 2001; 31: 3493–3503. [DOI] [PubMed] [Google Scholar]
- 39Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S et al. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol 2005; 35: 1539–1547. [DOI] [PubMed] [Google Scholar]
- 40Willment JA, Lin HH, Reid DM, Taylor PR, Williams DL, Wong SY et al. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol 2003; 171: 4569–4573. [DOI] [PubMed] [Google Scholar]
- 41Graham LM, Gupta V, Schafer G, Reid DM, Kimberg M, Dennehy KM et al. The C-type lectin receptor CLECSF8 (CLEC4D) is expressed by myeloid cells and triggers cellular activation through Syk kinase. J Biol Chem 2012; 287: 25964–25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY et al. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 2013; 39: 324–334. [DOI] [PubMed] [Google Scholar]
- 43Miyake Y, Masatsugu OH, Yamasaki S. C-type lectin receptor MCL facilitates mincle expression and signaling through complex formation. J Immunol 2015; 194: 5366–5374. [DOI] [PubMed] [Google Scholar]
- 44Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY et al. CLEC5A is critical for dengue-virus-induced lethal disease. Nature 2008; 453: 672–676. [DOI] [PubMed] [Google Scholar]
- 45Joyce-Shaikh B, Bigler ME, Chao CC, Murphy EE, Blumenschein WM, Adamopoulos IE et al. Myeloid DAP12-associating lectin (MDL)-1 regulates synovial inflammation and bone erosion associated with autoimmune arthritis. J Exp Med 2010; 207: 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Marshall AS, Willment JA, Lin HH, Williams DL, Gordon S, Brown GD. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem 2004; 279: 14792–14802. [DOI] [PubMed] [Google Scholar]
- 47Marshall AS, Willment JA, Pyz E, Dennehy KM, Reid DM, Dri P et al. Human MICL (CLEC12A) is differentially glycosylated and is down-regulated following cellular activation. Eur J Immunol 2006; 36: 2159–2169. [DOI] [PubMed] [Google Scholar]
- 48Bates EE, Fournier N, Garcia E, Valladeau J, Durand I, Pin JJ et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J Immunol 1999; 163: 1973–1983. [PubMed] [Google Scholar]
- 49Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O'Bar et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood 2010; 116: 1685–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol 2012; 188: 5752–5765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.