Abstract
HLDA10 is the Tenth Human Leukocyte Differentiation Antigen (HLDA) Workshop. The HLDA Workshops provide a mechanism to allocate cluster of differentiation (CD) nomenclature by engaging in interlaboratory studies. As the host laboratory, we invited researchers from national and international academic and commercial institutions to submit monoclonal antibodies (mAbs) to human leukocyte surface membrane molecules, particularly those that recognised molecules on human myeloid cell populations and dendritic cells (DCs). These mAbs were tested for activity and then distributed as a blinded panel to 15 international laboratories to test on different leukocyte populations. These populations included blood DCs, skin-derived DCs, tonsil leukocytes, monocyte-derived DCs, CD34-derived DCs, macrophage populations and diagnostic acute myeloid leukaemia and lymphoma samples. Each laboratory was provided with enough mAb to perform five repeat experiments. Here, we summarise the reactivity of different mAb to 68 different cell-surface molecules expressed by human myeloid and DC populations. Submitted mAbs to some of the molecules were further validated to collate data required to designate a formal CD number. This collaborative process provides the broader scientific community with an invaluable data set validating mAbs to leukocyte-surface molecules.
Monoclonal antibodies (mAbs) are one of the most widely applied products in biomedical research and clinical diagnostics. The research and development process has generated mAbs that have translated from the research laboratory to clinical applications. However, the criterion for a ‘good' antibody depends on its characterisation and often its intended use. While mAbs that recognise a molecule on the cell surface are invaluable for flow cytometry studies, the same mAb is often not useful in pathology laboratories using immunohistochemistry on paraffin sections for diagnostic purposes. Furthermore, many mAbs are available from various sources; however, their characterisation is often limited. The Human Leukocyte Differentiation Antigen (HLDA) Workshops provide the opportunities to broaden the characterisation of mAb in a collaborative manner. Unfortunately, in this era of science, there is a perception that ‘publish or perish' is the only way forward. Funding bodies are almost universally reluctant to provide the means to ‘validate reagents'. Consequently, it is only through generosity and good will that many of these reagents are tested in an unbiased study.
The classification of myeloid lineage cells including monocytes and dendritic cells (DCs) is the subject of much debate.1 Transcriptomic and proteomic analysis of myeloid populations provides a plethora of data to aid this discussion. The availability of mAbs to molecules identified through these studies will substantially aid our understanding of these cell lineages. The studies reported in this paper add to the empirical testing of mAb binding to molecules expressed on the cell surface of human myeloid lineage cells.
The potential of cell therapies using DCs to treat cancers is well known. Most clinical studies have opted to use in vitro generated monocyte-derived DCs rather than peripheral blood DCs.2 To date, the use of monocyte-derived DCs has failed to provide strong clinical data to argue that these should be the cells of choice.3 Indeed, a number of system analysis of DC-like and DC populations have consistently demonstrated differences in the two cell types. Trials are now investigating the naturally occurring blood DCs. Having an array of mAbs that are well characterised for their ability to bind to DCs will aid in the development of DC-based therapies.
Our aims for this HLDA10 (Tenth HLDA) Workshop were to provide empirical validation of mAbs to cell-surface molecules whose expression is found on DCs and myeloid cells from a variety of tissues, further clarify the cell surface of human DCs and assess the binding of mAbs to samples of haematological malignancies. This followed our work in HLDA7, which described the major human blood DC subsets leading to the description of the CD141+ DC as the counterpart of the cross-presenting mouse DC.4, 5, 6 Here, we present the collated work of different groups that contributed to studies in the HLDA10. The groups were provided with enough mAbs to perform three to five repeat tests on a particular cell population in which their laboratory had significant expertise. The outcomes provide the wider community with a broader range of mAbs with documented expression on DC and DC-like populations.
Results
Myeloid-, B-cell- and Hodgkin-derived cell lines
The HLDA10 panel of test mAbs, listed in Table 1, were initially tested by flow cytometry for binding to at least three of six cell lines representing commonly used myeloid-derived cells (U937, HL-60, THP-1 and NB4), a T-cell-derived line (Jurkat), a B-cell-derived line (Raji) and two Hodgkin lymphoma (HL)-derived lines (KM-H2 and HDLM2).7 Testing was performed in groups based on the mAb format (purified or direct conjugate). Results were assessed as the percentage of cells with a mean fluorescent index greater than the mean fluorescent index of the isotype binding the same cells (Table 2). As the results in this paper were collated from multiple groups using different technology and different flow cytometers, we have presented the data in the scale outlined in the methods. Very few mAbs showed no reactivity with any of the cell lines and these were not tested further in the larger studies unless they showed reactivity to a transfectant.
Table 1. Details of the mAb included in the blinded HLDA10 antibody panel.
| Code | Clone | Molecule (named as indicated on submission) | Label | Isotype | Provider |
|---|---|---|---|---|---|
| 10-01 | GE2 | Clec7A/Dectin-1/BGR | Purified | m IgG1 | G Brown |
| 10-02 | 8F9 | DNGR-1 | Purified | m IgG2aK | G Brown |
| 10-03 | 010e | CD1a | Ascites | m IgG1 | A Bensussan |
| 10-04 | CL3 | LPAP | Purified | m IgG1 | Filatov |
| 10-05 | 7C5 | Unknown | S/N | m IgG1 | H-J Bühring |
| 10-06 | FAB3480P | Clec2D/OCIL | PE labelled | m IgG1 | R&D Systems |
| 10-07 | FAB17291P | Trem-2 | PE labelled | Rat IgG2b | R&D Systems |
| 10-08 | FMU-FAT-6 | FAT1 cadherin | Purified | m IgG1 | B Jin |
| 10-09 | 9A11 | Clec9A/DNGR | Purified | m IgG1 | C Reis e Sousa |
| 10-10 | 0619 | CD1a | Ascites | m IgG2a | Bensussan |
| 10-11 | CL4 | LPAP | Purified | m IgG1 | Filatov |
| 10-12 | 58B1A2 | CD276 (NIH-3T3) | S/N | m IgG1 | H-J Bühring |
| 10-13 | FAB1748P | Clec4A/DCIR | PE labelled | m IgG1 | R&D Systems |
| 10-14 | FAB1750P | TIM-1 | PE labelled | m IgG2b | R&D Systems |
| 10-15 | FAB812P | Flt-3/Flk-2 | PE labelled | m IgG1 | R&D Systems |
| 10-16 | FMU-FAT1-7 | FAT1 cadherin | Purified | m IgG1 | B Jin |
| 10-17 | HB3 | CLEC12A/MICL | Purified | m IgG1 | R&D Systems |
| 10-18 | O249 | CD1b | Ascites | m IgG1 | Bensussan |
| 10-19 | CL7 | LPAP | Purified | m IgG2a | Filatov |
| 10-20 | 59A3B3 | Unknown | S/N | m IgG1 | H-J Bühring |
| 10-21 | FAB2806P | Clec4D/SF8 | PE labelled | m IgG2b | R&D Systems |
| 10-23 | FMU-CRT-2 | Calreticulin | Purified | m IgG1 | B Jin |
| 10-24 | FAB2365P | TIM-3 | PE labelled | Rat IgG2a | R&D Systems |
| 10-25 | W4A5B3 | Unknown | S/N | m IgG1 | H-J Bühring |
| 10-26 | L161 | CD1c | Ascites | m IgG1 | A Bensussan |
| 10-27 | 56A1C2 | Unknown | S/N | m IgG1 | H-J Bühring |
| 10-28 | FAB238P | Clec5A/MDL-1 | PE labelled | m IgG2b | R&D Systems |
| 10-29 | FMU-CRT-8 | Calreticulin | Purified | m IgG1 | B Jin |
| 10-30 | FMU-IL-13RA2-7 | IL-13 Ra2 | Purified | m IgG1 | B Jin |
| 10-31 | FAB1900P | Clec5C/NKp80/KLRF1 | PE labelled | Rat IgG2a | R&D Systems |
| 10-32 | W5C4C5 | Unknown | S/N | m IgG1 | H-J Bühring |
| 10-33 | 66E2D11 | Unknown | S/N | m IgG1 | H-J Bühring |
| 10-34 | BB27 | CD101 | Ascites | m IgG1 | A Bensussan |
| 10-35 | FAB1859P | Clec7A/Dectin-1 | PE labelled | m IgG2b | R&D Systems |
| 10-36 | FAB3479P | FPRL1/FPRL/2 | PE labelled | m IgG2b | R&D Systems |
| 10-37 | FMU-IL-13RA2-8 | IL-13 Ra2 | Purified | m IgG1 | R&D Systems |
| 10-38 | BGA69 | Unknown | Ascites | m IgG1 | B Jin |
| 10-39 | F9-3C2F1 | Unknown | S/N | m IgM | H-J Bühring |
| 10-40 | FAB1798P | Clec8A/LOX-1 | PE labelled | m IgG2b | R&D Systems |
| 10-41 | FMU-IL-13RA2-14 | IL-13 Ra2 | Purified | m IgG1 | B Jin |
| 10-42 | FMU-CRT-17 | Calreticulin | Purified | m IgM | B Jin |
| 10-43 | DY12 | CD245 | Purified | m IgG1 | A Bensussan |
| 10-44 | HEK5-1B3A3 | CD276 | S/N | m IgG1 | H-J Bühring |
| 10-45 | FAB6049P | Clec9A/DNGR | PE labelled | m IgG1 | R&D Systems |
| 10-46 | IPS-K-2B6A8 | CD276 | S/N | m IgG1 | H-J Bühring |
| 10-47 | FAB3744P | FPR1 | PE labelled | m IgG2a | R&D Systems |
| 10-48 | DY35 | CD245 | Ascites | m IgG1 | A Bensussan |
| 10-49 | 58A2B11 | Unknown | S/N | m IgG1 | H-J Bühring |
| 10-50 | FAB154P | Axl | PE labelled | m IgG1 | R&D Systems |
| 10-51 | FAB2946P | CLEC12A/MICL/CCL-1 | PE labelled | m IgG2b | R&D Systems |
| 10-52 | FAB1517P | ULBP-3 | PE labelled | m IgG2a | R&D Systems |
| 10-53 | AY19 | IL-1RAcP | Purified | m IgG1 | B Jin |
| 10-54 | FAB637P | Clec13A/CD302 | PE labelled | m IgG1 | R&D Systems |
| 10-55 | SC5 | Vimentin | Ascites | m IgM | A Bensussan |
| 10-56 | FAB3131P | Tie-2 | PE labelled | m IgG1 | R&D Systems |
| 10-57 | FAB7436P | Clec14A | PE labelled | m IgG2b | R&D Systems |
| 10-58 | T-1A5 | CD276 | S/N | m IgG1 | H-J Bühring |
| 10-59 | MDR64 | Unknown | Ascites | m IgG1 | A Bensussan |
| 10-60 | 825724 | Human PIgR | Purified | m IgG3 | R&D Systems |
| 10-61 | ANC8D12 | CD273, B7-DC | Purified | m IgG1K | Ancell |
| 10-62 | ANC8C9 | GARP | Purified | m IgG2aK | Ancell |
| 10-63 | ANC10G10 | GARP | Purified | m IgG1K | Ancell |
| 10-64 | MIH43 | B7-H4 | PE labelled | m IgG1K | R&D Systems |
| 10-65 | 8F9 | Clec9A (DNGR-1) | PE labelled | mIgG2aK | R&D Systems |
| 10-66 | 17G10.2 | CD85g (ILT7) | PE labelled | m IgG1K | R&D Systems |
| 10-67 | 1D12 | TIM-1 | PE labelled | m IgG1K | R&D Systems |
| 10-68 | 1B4 | TSLP-R | PE labelled | m IgG1K | R&D Systems |
| 10-69 | CMRF-56 | Unknown | Purified | m IgG1 | D Hart |
| 10-70 | L4 | P2X7 | Purified | m IgG2b | R Slutyer |
| 10-71 | 111F8.04 | DCIR | Alexa 488 | m IgG1 | Dendritics |
| 10-72 | 9E8 | Clec4A (DCIR) | PE labelled | m IgG1K | R&D Systems |
| 10-73 | 50C1 | Clec12A | PE labelled | m IgG2aK | R&D Systems |
| 10-74 | 24 | CD85h (ILT1) | PE labelled | m IgG2bK | R&D Systems |
| 10-75 | F38-2E2 | Tim-3 | PE labelled | m IgG1K | R&D Systems |
| 10-76 | HEK/1/85 | CCR5 | PE labelled | Rat IgG2aK | R&D Systems |
| 10-77 | 104A10.01 | DORA | Alexa 488 | m IgG1 | Dendritics |
| 10-78 | 9B9 | Clec4D (Dectin-3) | PE labelled | m IgG2bK | R&D Systems |
| 10-79 | 15 E 2 | Clec7A (Dectin-1) | PE labelled | m IgG2aK | R&D Systems |
| 10-80 | TX45 | MAIR II | PE labelled | m IgG1K | R&D Systems |
| 10-81 | 9F4 | Tim-4 | PE labelled | m IgG1K | R&D Systems |
| 10-82 | CMRF-44 | Unknown | Purified | m IgM | D Hart |
| 10-83 | 118A8.05 | DC-SIGN like | Alexa 488 | m IgG1 | Dendritics |
| 10-84 | 36H2 | FDF03 | Alexa 488 | Rat IgG2a | Dendritics |
| 10-85 | CMRF-81 | Tetanus toxoid | m IgG1K | D Hart | |
| 10-86 | EX32 | Allergin | Purified | m IgG2a | A Shibuya |
| 10-87 | EX33 | Allergin | Purified | m IgG2b | A Shibuya |
| 10-88 | Tx93 | CD300H | Purified | m IgG2a | A Shibuya |
Abbreviations: HLDA10, Tenth Human Leukocyte Differentiation Antigen; Ig, immunoglobulin; m, mouse; PE, phycoerythrin; S/N, supernatant.
Addresses of providers are as follows: G Brown, Aberdeen, UK. A Bensussan, Paris, France. Filatov, Moscow, Russia. H-J Bühring, Tübingen, Germany. R&D Systems, Minneapolis, MN, USA. B Jin, Xi'an, China. C Reis e Sousa, London, UK. Ancell, Bayport, MN, USA. D Hart, Sydney, NSW, Australia. R Slutyer, Auckland, New Zealand. Dendritics, Lyon, France. A Shibuya, Tsukuba Science-City, Japan.
Table 2. Binding of the panel to cell lines.
| Molecule | KM-H2 | Raji | HEL | NB4 | HL-60 | THP-1 | U937 | |
|---|---|---|---|---|---|---|---|---|
| 10-01 | CLEC7A | − | + | + | − | +/− | − | + |
| 10-02 | CLEC9A | +/− | − | − | ++ | ++ | ++ | ++ |
| 10-03 | CD1a | + | + | ++ | − | ++ | ++ | ++ |
| 10-04 | LPAP | − | − | − | ++ | ++ | ++ | ++ |
| 10-05 | Unknown | Limited supply | ||||||
| 10-06 | CLEC2D | + | − | + | − | − | − | + |
| 10-07 | Trem-2 | − | − | + | + | ++ | ++ | ++ |
| 10-08 | FAT1 cadherin | ++ | ++ | ++ | + | ++ | ++ | ++ |
| 10-09 | CLEC9A | − | − | − | − | − | − | +/− |
| 10-10 | CD1a | NT | NT | NT | NT | ++ | NT | NT |
| 10-11 | LPAP | − | − | − | − | − | − | + |
| 10-12 | CD276 | NT | NT | NT | NT | NT | NT | NT |
| 10-13 | CLEC4A | − | + | +/− | − | − | − | + |
| 10-14 | TIM-1 | +* | − | − | − | − | − | − |
| 10-15 | Flt-3/Flk-2 | − | − | − | − | + | ++ | ++ |
| 10-16 | FAT1 cadherin | ++ | +/− | + | + | ++ | ++ | ++ |
| 10-17 | CLEC12A | − | − | − | + | ++ | − | ++ |
| 10-18 | CD1b | + | − | − | + | +/− | ++ | ++ |
| 10-19 | LPAP | − | + | + | ++ | ++ | ++ | ++ |
| 10-20 | Unknown | ++ | ++ | ++ | NT | ++ | ++ | ++ |
| 10-21 | Clec4D | − | − | − | − | − | − | − |
| 10-23 | Calreticulin | ++ | + | ++ | ++ | ++ | ++ | ++ |
| 10-24 | TIM-3 | − | − | − | − | − | − | − |
| 10-25 | Unknown | NT | NT | NT | NT | NT | NT | NT |
| 10-26 | CD1c | − | +/− | − | − | − | + | + |
| 10-27 | Unknown | − | − | ++ | NT | ++ | ++ | ++ |
| 10-28 | CLEC5A | − | − | − | − | + | ++ | |
| 10-29 | Calreticulin | + | + | + | ++ | ++ | ++ | ++ |
| 10-30 | IL-13 Ra2 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 10-31 | CLEC5C | − | − | − | − | − | − | − |
| 10-32 | Unknown | NT | NT | NT | NT | NT | NT | NT |
| 10-33 | Unknown | − | − | ++ | Nt | ++ | ++ | ++ |
| 10-34 | CD101 | +/− | + | +/− | − | ++ | + | + |
| 10-35 | CLEC7A | − | − | − | − | − | − | − |
| 10-36 | FPRL1 | − | − | − | − | − | − | − |
| 10-37 | IL-13 Ra2 | ++ | + | ++ | + | ++ | ++ | ++ |
| 10-38 | Unknown | − | +/− | − | + | +/− | ++ | + |
| 10-39 | Unknown | NT | NT | NT | NT | NT | NT | NT |
| 10-40 | CLEC8A | − | − | − | − | − | − | − |
| 10-41 | IL-13 Ra2 | ++ | + | ++ | + | ++ | ++ | ++ |
| 10-42 | Calreticulin | ++ | + | − | + | ++ | ++ | ++ |
| 10-43 | CD245 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 10-44 | CD276 | NT | NT | NT | NT | NT | NT | NT |
| 10-45 | CLEC9A | − | − | +/− | − | − | − | +/− |
| 10-46 | CD276 | NT | NT | NT | NT | NT | NT | NT |
| 10-47 | FPR1 | − | − | +/− | +/− | + | ++ | ++ |
| 10-48 | CD245 | − | + | − | + | ++ | ++ | ++ |
| 10-49 | Unknown | NT | NT | NT | NT | NT | NT | NT |
| 10-50 | Axl | − | − | − | − | − | − | − |
| 10-51 | CLEC12A | − | − | − | − | + | − | ++ |
| 10-52 | ULBP-3 | − | − | − | + | + | ++ | ++ |
| 10-53 | IL-1RAcP | ++ | + | + | ++ | ++ | + | ++ |
| 10-54 | CLEC13A | − | − | +/− | − | − | − | + |
| 10-55 | Vimentin | ++ | − | +/− | + | + | ++ | ++ |
| 10-56 | Tie-2 | − | − | + | − | − | − | + |
| 10-57 | CLEC14A | − | − | − | − | − | − | − |
| 10-58 | CD276 | NT | NT | NT | NT | NT | NT | NT |
| 10-59 | Unknown | − | − | − | + | ++ | ++ | |
| 10-60 | PIg receptor | ++ | +/− | NT | NT | ++ | NT | ++ |
| 10-61 | CD273 | ++ | + | + | ++ | ++ | NT | ++ |
| 10-62 | GARP | ++ | − | + | ++ | + | NT | ++ |
| 10-63 | GARP | ++ | − | +/− | − | + | NT | +/− |
| 10-64 | B7-H4 | − | NT | − | NT | − | NT | − |
| 10-65 | CLEC9A | − | − | − | NT | ++ | NT | ++ |
| 10-66 | ILT7 | − | − | − | NT | − | NT | − |
| 10-67 | TIM-1 | +/− | +/− | − | NT | − | NT | − |
| 10-68 | TSLP-R | − | − | − | NT | − | NT | − |
| 10-69 | Unknown | ++ | ++ | − | NT | − | NT | − |
| 10-70 | P2X7 | ++ | − | − | NT | − | NT | − |
| 10-71 | CLEC4A | − | − | − | NT | − | NT | +/− |
| 10-72 | CLEC4A | − | − | − | NT | − | NT | +/− |
| 10-73 | Clec12A, | − | − | − | NT | ++ | NT | ++ |
| 10-74 | CD85h (ILT1) | − | +/− | − | NT | +/− | NT | ++ |
| 10-75 | Tim-3 | − | − | − | NT | − | NT | +/− |
| 10-76 | CCR5 | + | − | − | NT | − | NT | +/− |
| 10-77 | DORA | − | − | − | NT | − | NT | + |
| 10-78 | Clec4D (Dectin-3) | − | − | − | NT | +/− | NT | + |
| 10-79 | Clec7A (Dectin-1) | − | +/− | − | NT | ++ | NT | ++ |
| 10-80 | MAIR II | +/− | − | +/− | NT | +/− | NT | +/− |
| 10-81 | Tim-4 | − | +/− | − | NT | − | NT | +/− |
| 10-82 | Unknown | ++ | NT | − | NT | − | NT | NT |
| 10-83 | DC-SIGN like | − | − | − | NT | − | NT | − |
| 10-84 | FDF03 | − | − | − | NT | − | NT | +/− |
| 10-85 | Tetanus toxoid | + | NT | − | NT | − | NT | NT |
| 10-86 | Allergin | − | − | − | NT | − | NT | − |
| 10-87 | Allergin | − | − | − | NT | − | NT | − |
| 10-88 | CD300H | − | − | +/− | NT | ++ | NT | ++ |
Abbreviation: NT, not tested.
As this was a screening excersie, a record was made whether the antibody bound well to >30% of the population (++), bound to <30% of the population (+), bound to <5% or did not bind to the cell line (−).
Transient transfectants expressing cDNA for antigen
MAbs to well-described antigens were validated by testing for binding to transient transfectants expressing the cDNA of interest in CHOK1 fibroblasts. Where possible, the efficiency of transfections was tested with a tagged protein. The binding of each mAb to transfectants was tested by flow cytometry, and positive signals were compared with isotype controls and untransfected cells. The mAbs that were shown to bind to transfectants were TIM-1 (10-14, 10-67), TIM-3 (10-24), CLECSF6 (10-13, 10-72), CLEC4D (10-21, 10-78), CLEC7A (10-01, 10-35, 10-79), CLEC9A (10-02, 10-09, 10-45, 10-65) and CLEC12A (10-51, 10-73). These results can be seen on the HCDM website (www.hcdm.org). As all mAbs to CLEC9A (10-02, 10-09, 10-45 and 10-65) bound to transfectants expressing the CLEC9A cDNA despite not binding to cell lines or most in vitro derived populations, studies were continued with this group of mAbs.
In vitro derived cell populations
Directly labelled mAbs from the HLDA10 panel were tested for binding to CD34-derived LC from two donors. Immature cells from both donors bound to 10-13, 10-15, 10-24, 10-28, 10-34, 10-51, 10-53, 10-69 and 10-73. Mature cells from both donors bound to 10-03, 10-10, 10-28, 10-34, 10-47, 10-61, 10-68, 10-69, 10-72, 10-73, 10-82 and 10-84. There was variation in the binding of mAbs to CD34-derived DCs from different donors. Of those tested, mAbs to DCIR (10-13) bound to immature LCs, but the binding decreased following activation. Few mAbs showed distinctive binding to the immature or mature LC population.
Fresh blood DC and monocyte populations
The HLDA10 panel was tested on fresh blood DC and monocyte populations from between three to five different donors by at least three laboratories. As mAbs were submitted to the Workshop as tissue culture supernatant, purified, biotinylated or directly conjugated to either fluorescein isothiocyanate or phycoerythrin (PE), we tested the panel in groups depending on the type of conjugates required. Practically, this allowed us to complete testing on cells from a limited number of donors. MAbs were tested by flow cytometry for binding to the lineage-negative (Lin−) (CD3, CD19, CD56) HLA-DR+ fraction of peripheral blood mononuclear cells (PBMCs). The data in Table 3 represents a combined spanning tree progression analysis (SPADE) of primary blood monocyte and DC populations. A number of mAbs within the panel showed variable results between laboratories, which may reflect differences in staining intensities, as a result of the different flow cytometer configurations. Results were provided to the collating laboratory by participating laboratories in tabular formats with examples of profiles. MAbs with similar profiles were compared directly. An interlaboratory analysis of the data was subsequently performed by SPADE on FCS files provided by the participating groups (results from HLDA10, in preparation). The summary results presented in Table 3 are derived from the SPADE analysis based on data from nine donors. The mAbs 10-07, 10-14, 10-40, 10-45, 10-50, 10-52, 10-56, 10-64, 10-67, 10-79 and 10-81 had variable backgrounds in the different laboratories.
Table 3. Summary of the binding of the HDLA10 panel to fresh blood monocyte and DC populations and overnight cultured blood DCs.
| HLDA10 code | Molecule | CD14++ CD16− monocyte | CD14+ CD16++ monocyte | CD1c+DC | CD141+ DC | CD16+ DC | pDC | CD34+ DC | CMRF−56+ DC |
|---|---|---|---|---|---|---|---|---|---|
| 10-01 | CLEC7A | ++ | +/− | +/− | +/− | − | − | − | NT |
| 10-02 | CLEC9A | +/− | − | − | ++ | − | − | − | NT |
| 10-03 | CD1a | − | − | − | − | − | − | − | NT |
| 10-04 | LPAP | ++ | ++ | − | − | +/− | − | − | NT |
| 10-06 | CLEC2D | + | + | − | − | − | − | − | NT |
| 10-07 | Trem-2 | ++ | ++ | +/− | +/− | + | + | − | NT |
| 10-08 | FAT1 cadherin | + | +/− | +/− | − | +/− | − | − | + |
| 10-09 | CLEC9A | − | − | − | ++ | − | − | − | NT |
| 10-10 | CD1a | − | − | − | − | − | − | − | NT |
| 10-11 | LPAP | − | − | − | − | − | +/− | − | NT |
| 10-13 | CLEC4A | ++ | + | ++ | + | + | ++ | − | + |
| 10-14 | TIM-1 | − | − | − | − | − | − | − | NT |
| 10-15 | Flt-3/Flk-2 | − | − | +/− | + | − | − | − | NT |
| 10-16 | FAT1 cadherin | +/− | − | − | − | − | − | − | NT |
| 10-17 | CLEC12A | ++ | + | + | + | + | +/− | − | + |
| 10-18 | CD1b | − | − | +/− | − | − | − | − | NT |
| 10-19 | LPAP | +/− | − | − | − | − | − | − | + |
| 10-20 | Unknown | NT | NT | NT | NT | NT | NT | NT | ++ |
| 10-21 | CLEC4D | + | +/− | − | − | − | − | − | NT |
| 10-23 | Calreticulin | + | − | − | − | − | − | − | + + |
| 10-24 | TIM-3 | + | + | ++ | ++ | − | +/− | − | NT |
| 10-25 | Unknown | − | − | ++ | − | − | − | − | ++ |
| 10-26 | CD1c | − | − | ++ | − | − | − | − | − |
| 10-27 | Unknown | NT | NT | NT | NT | NT | NT | NT | ++ |
| 10-28 | Clec5a | + | +/− | − | − | − | − | − | NT |
| 10-29 | Calreticulin | − | − | − | − | − | − | − | NT |
| 10-30 | IL-13 Ra2 | + | − | − | − | − | − | − | NT |
| 10-31 | CLEC5C | − | − | − | − | − | − | − | NT |
| 10-33 | Unknown | NT | NT | NT | NT | NT | NT | NT | ++ |
| 10-34 | CD101 | ++ | ++ | ++ | ++ | ++ | − | − | + |
| 10-35 | CLEC7A | ++ | ++ | − | − | − | − | − | NT |
| 10-36 | FPRL1/FPRL/2 | + | + | − | − | − | − | − | NT |
| 10-37 | IL-13 Ra2 | − | − | − | − | − | − | − | NT |
| 10-38 | Unknown | − | − | − | +/− | − | − | − | NT |
| 10-40 | CLEC8A | +/− | +/− | − | − | − | − | − | NT |
| 10-41 | IL-13 Ra2 | + | + | − | − | − | − | − | + |
| 10-42 | Calreticulin | − | − | − | − | − | − | − | NT |
| 10-43 | CD245 | + | + | + | − | − | + | − | ++ |
| 10-45 | CLEC9A | − | − | − | + | − | − | − | NT |
| 10-47 | FPR1 | + | + | − | − | − | − | − | NT |
| 10-48 | CD245 | + | + | + | − | +/− | + | − | + |
| 10-50 | Axl | − | − | − | +/− | − | − | − | NT |
| 10-51 | CLEC12A | ++ | + | ++ | + | − | − | − | + |
| 10-52 | ULBP-3 | − | − | − | − | − | − | − | NT |
| 10-53 | IL-1RAcP | + | + | + | − | − | − | − | ++ |
| 10-54 | CLEC13A | + | − | − | − | − | − | − | NT |
| 10-55 | Vimentin | ++ | ++ | + | + | + | +/− | − | + |
| 10-56 | Tie-2 | +/− | − | − | − | − | − | − | NT |
| 10-57 | CLEC14A | − | − | − | − | − | − | − | NT |
| 10-58 | CD276 | − | − | − | − | − | − | −/+ | NT |
| 10-59 | Unknown | − | − | − | − | − | − | −/+ | NT |
| 10-60 | PIg receptor | +/− | − | − | − | − | − | − | NT |
| 10-61 | CD273 | +/− | − | − | − | − | − | − | + |
| 10-62 | GARP | − | − | − | − | − | − | − | + |
| 10-63 | GARP | − | − | + | − | − | − | − | NT |
| 10-64 | B7-H4 | − | − | − | − | − | − | − | NT |
| 10-65 | CLEC9A | +/− | − | − | ++ | − | − | − | NT |
| 10-66 | ILT7 | − | − | − | − | − | ++ | − | NT |
| 10-67 | TIM-1 | + | + | − | − | − | − | − | NT |
| 10-68 | TSLP-R | − | − | − | − | − | − | − | ++ |
| 10-69 | Unknown | + | + | + | − | − | − | − | ++ |
| 10-70 | P2X7 | − | − | +/− | − | − | +/− | − | ++ |
| 10-71 | CLEC4A | +/− | − | +/− | − | − | − | − | ++ |
| 10-72 | CLEC4A | ++ | + | + | + | +/− | + | − | + |
| 10-73 | Clec12A, | ++ | ++ | ++ | ++ | + | + | − | + |
| 10-74 | ILT1 | ++ | ++ | + | + | +/− | − | − | NT |
| 10-75 | Tim-3 | + | + | ++ | ++ | − | − | − | ++ |
| 10-76 | CCR5 | − | − | ++ | − | − | ++ | − | NT |
| 10-77 | DORA | − | − | − | − | − | − | − | NT |
| 10-78 | CLEC4D | + | − | − | − | − | − | − | NT |
| 10-79 | CLEC7A | + | + | +/− | + | − | − | − | NT |
| 10-80 | MAIR II | ++ | + | + | + | − | − | − | NT |
| 10-81 | Tim-4 | − | − | − | − | − | − | − | NT |
| 10-82 | Unknown | − | − | − | − | − | − | − | ++ |
| 10-83 | DC-SIGN like | − | − | − | − | − | − | − | + |
| 10-84 | FDF03 | ++ | + | +/− | − | − | − | − | ++ |
| 10-85 | Tetanus toxoid | − | − | − | − | − | − | − | NT |
Abbreviations: HLDA10, Tenth Human Leukocyte Differentiation Antigen; mAb, monoclonal antibody; mDC, murine dendrtic cell; NT, not tested; PBMC, peripheral blood mononuclear cell; pDC, plasmacytoid dendritic cell.
Monocyte populations include the CD14++CD16− classical and CD14+CD16++ non-classical cell monocytes. DC populations include the Lin−CD14−HLA-DR+CD1c+ mDC, Lin−CD14−HLA-DR+CD141+ mDC, Lin−CD14−HLA-DR+CD16+ mDC, Lin−CD14−HLA-DR+CD304+ pDC and the Lin−CD14−HLA-DR+CD34+ cells. The data are scaled from the combined analysis by SPADE for the fresh PBMCs. PBMCs were cultured overnight and the Lin−HLA-DR+ CMRF-56+ DC cells were tested for binding of HLDA10 mAb.
The mAbs that did not bind to any cells in the Lin−HLA-DR+ gate were 10-06, 10-11, 10-14, 10-31, 10-40, 10-52, 10-56, 10-57, 10-64, 10-67, 10-68, 10-77, 10-81, 10-82, 10-83.
The remaining mAbs bound to monocyte or DC populations within the peripheral blood Lin−HLA-DR+ fraction. The Lin−HLA-DR+ fraction was further gated into the CD14+ myeloid and CD14− DC populations. The myeloid populations included the CD14hiCD16− (classical) and CD14loCD16+ non-classical monocytes. The DC populations included the CD14−CD11c+ myeloid DC (mDC) and the CD304+ plasmacytoid DC (pDC). mDCs were subdivided into CD1c+, CD16+, CD141+ and CD34+ populations.
Markers that bound to cells in all myeloid PBMCs were 10-13, 10-17, 10-24, 10-43, 10-48, 10-51, 10-53, 10-54 (weak on CD14hi), 10-55, 10-69, 10-71, 10-72 and 10-73 (with a bifid pattern on CD16+ DC).
Markers that were only found on monocytes included 10-21 (only CD14 monocytes), 10-28, 10-29 (weak), 10-36, 10-42 (weak mAb so may be on DC), 10-47, 10-54, 10-62, 10-63 (weak on the CD16+ monocyte subset) and 10-78 (on CD16+ monocytes but not CD16+ DC).
Markers that were found on monocytes and DCs included 10-01, 10-04, 10-07 (monocytes and CD16 DC), 10-08, 10-10, 10-15 (weak), 10-16 (weak), 10-18, 10-19, 10-26, 10-30, 10-37, 10-38, 10-41 (monocytes and CD1c DC), 10-23, 10-33, 10-34, 10-59, 10-60, 10-61 (on CD1c DC and not on the CD16 subset), 10-76 (on CD16+ monocytes but not CD16+ DC) and 10-84 (not CD141hi). Antibodies that bound to mDCs and not pDCs were 10-35, 10-74, 10-75, 10-79 and 10-80.
In our gating strategy, CD16 is expressed on CD14lo and CD14− cells. These populations were interrogated as CD14loCD16+ monocytes (non-classical monocytes) and CD14−CD16+ DC. Two antibodies were observed to bind to CD16+ monocytes but not CD16+ DC. They were 10-47 and 10-36. Of interest, these antibodies also failed to bind to CD1c+ DC.
There were seven antibodies in the panel that only bound to DCs. The antibodies 10-20 and 10-27 were bound to CD1c and pDCs, 10-02, 10-09 and 10-45 bound strongly to CD141 DCs with weaker binding to other populations and 10-03 bound to CD1c+. One antibody 10-66 (ILT7) bound to a molecule expressed only on pDCs.
A number of mAbs were tested on both pDCs in PBMCs from freshly donated venous samples and pDCs purified indirectly from peripheral blood leucocyte from healthy human buffy coats. There were some differences in the intensity of binding of some mAbs to pDCs in these two preparations with mAb binding more intensely to the indirectly purified cells.
The mAbs 10-4, 10-48, 10-53 and 10-69 were found to bind monocytes and CD1c DCs, but notably were not expressed by CD141+ DC. CD34+ DC can be identified in the Lin−HLA-DR+CD14− population in the peripheral blood. However, this small population did not bind any of the mAbs with high intensity recognised on the SPADE analysis.
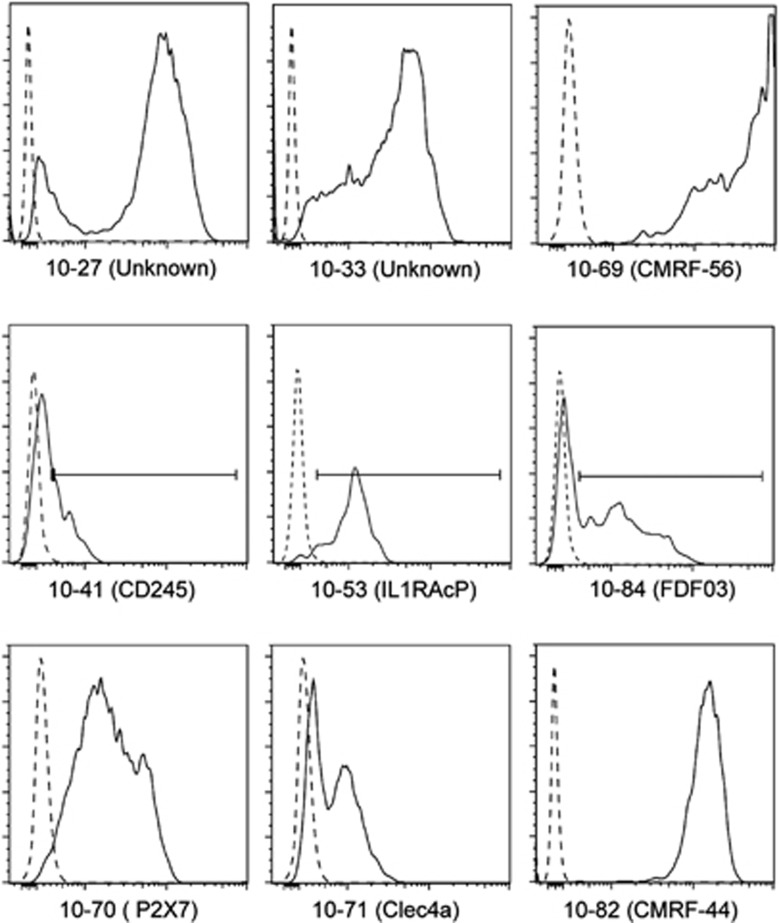
Overnight cultured PBMCs induce expression of the CMRF-56 antigen on Lin−DC populations.8 A clinical human-chimeric CMRF-56 antibody was used to identify blood-derived DCs following overnight culture. The binding of 28 mAbs from the HLDA10 panel to HLA-DR+ CMRF-56+ DC was tested and the results are summarised in Table 3. The mAbs tested were selected based on previously recorded >5% positive expression on CD11c+ mDC in freshly isolated PBMCs. A total of 28 mAbs displayed positive binding on CMRF-56+ blood-derived DCs. The mAbs that bound most brightly to CMRF-56+ DC were 10-27, 10-33 to unknown molecules, 10-53 (IL-1RAcP), 10-70 (P2XR7), 10-71 (DCIR), 10-82 (CMRF-44), 10-84 (FDF03) and 10-41 (CD245) (Figure 1). These results identified a small number of mAbs that bind to molecules that are spontaneously upregulated on DCs with overnight culture in the absence of Toll-like receptors.
Figure 1.
Screening of hCMRF-56+ blood-derived DCs (BDCs) to Tenth Human Leukocyte Differentiation Antigen (HLDA10) monclonal antibodies (mAbs). A clinical human anti-human-chimeric IgG4 CMRF-56 was used to identify BDCs in PBMCs cultured for 15 h. In the absence of a Toll-like receptor (TLR) stimulus HLA-DR+ hCMRF-56+ BDC bound to mAb 10-27 (unknown specificity), 10-33 (unknown specificity), 10-69 (CMRF-56), 10-41 (CD245), 10-53 (IL-1RAcP), 10-84 (FDF03), 10-70 (P2X7), 10-71 (CLEC4a) and 10-82 (CMRF-44).
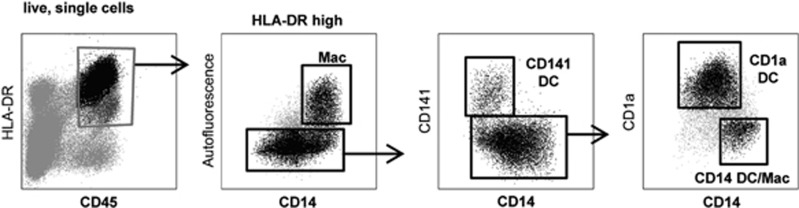
Skin-derived DCs
DCs purified from skin biopsies were tested for binding to the mAb panel (Table 4). The CD45+ HLA-DR+ cells that migrated from the skin were selected as CD14loCD141+, CD14loCD1a− or CD14loCD1a+ DC (Figure 2). As the DC populations purified from skin biopsies were limiting, only mAbs to CLECs and adhesion molecules were tested. The results were normalised to 10-31 (CLEC5C). The CD1a+ population generally bound to mAb tested more intensely.
Table 4. Binding of mAb from HLDA10 panel to skin-derived DC populations.
| Code | Molecule | CD141+ | CD14+ | CD1a+ |
|---|---|---|---|---|
| 10-06 | CLEC2D | + | + | ++ |
| 10-07 | Trem-2 | + | ++ | ++ |
| 10-13 | CLEC4A | ++ | ++ | ++ |
| 10-14 | TIM-1 | ++ | + | ++ |
| 10-15 | Flt-3 | + | + | ++ |
| 10-21 | CLEC4D | + | + | + |
| 10-24 | TIM-3 | + | − | + |
| 10-28 | CLEC5A | + | ++ | ++ |
| 10-31 | CLEC5C | − | − | − |
| 10-35 | CLEC7A | + | ++ | ++ |
| 10-36 | FPRL1 | + | +/− | + |
| 10-40 | CLEC8A | + | + | + |
Abbreviations: DC, dendritic cell; HLDA10, Tenth Human Leukocyte Differentiation Antigen; mAb, monoclonal antibody.
Data are represented as normalised to CLEC5A.
Figure 2.
Gating strategy used to select dendrtic cell (DC) that migrated from skin samples.
Acute myeloid leukaemia samples
The panel included mAbs derived from mice immunised with acute myeloid leukaemia (AML) and a number of mAbs that have been reported in the literature to be in clinical assessment as antileukemic therapeutics (TIM-3, CLL-1).9 This led us to test the mAbs on a sample of three AMLs that were in our donor bank. Seventeen mAbs bound to more than one AML sample (Table 5) with three mAbs, 10-24 (TIM-3), 10-33 and 10-53 (IL-1RAcP), showing the brightest binding to all three AMLs.
Table 5. Summary of the binding of the HLDA10 panel to three AML samples.
| Code | Molecule | AML 1 | AML 2 | AML 3 |
|---|---|---|---|---|
| 10-02 | DNGR-1 | +/− | + | − |
| 10-15 | Flt-3 | +/− | ++ | +/− |
| 10-17 | CLEC12A | ND | ++ | +/− |
| 10-20 | Unknown | + | +/− | + |
| 10-24 | TIM-3 | ++ | ++ | ++ |
| 10-27 | Unknown | ++ | +/− | ++ |
| 10-33 | Unknown | ++ | ++ | ++ |
| 10-43 | CD245 | +/− | +/− | + |
| 10-45 | CLEC9A | +/− | ++ | − |
| 10-51 | CLEC12A | +/− | ++ | +/− |
| 10-52 | ULBP-3 | + | ++ | − |
| 10-53 | IL-1RACP | ++ | ++ | ++ |
| 10-54 | CLEC13A | +/− | ++ | − |
| 10-65 | CLEC9A | +/− | + | − |
| 10-70 | P2X7 | + | ++ | +/− |
| 10-73 | CLEC12A | +/− | ++ | + |
| 10-75 | TIM-3 | ++ | ++ | + |
Abbreviations: AML, acute myeloid leukaemia; HLDA10, Tenth Human Leukocyte Differentiation Antigen.
Immunohistochemistry of paraffin sections
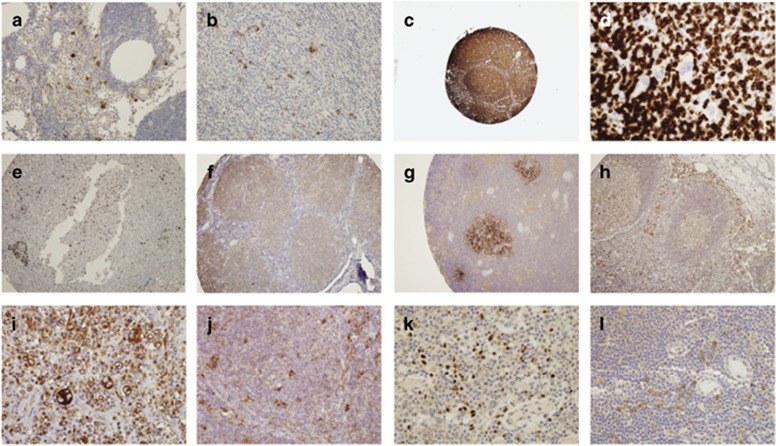
Purified or biotinylated mAbs that were submitted in larger quantities were tested for their ability to stain paraffin-embedded sections from representative lymphoma and normal non-lymphoid tissue. Seven mAbs within the panel showed interesting staining on lymphoma cells. A CD1a antibody (10-03) showed distinct intense membrane/cytoplasmic staining of scattered cells in the interfollicular regions of reactive lymph nodes (Figure 3a) and lymph nodes involved with HL (Figure 3b) suggestive of histiocyte staining.
Figure 3.
Lymphoma samples were tested for the expression of Tenth Human Leukocyte Differentiation Antigen (HLDA10) monoclonal antibodies (mAbs) by immunohistochemistry of paraffin sections. (a) mAb to CD1a (10-03) on reactive lymph nodes and (b) lymph nodes involved with Hodgkin lymphoma (HL). (c) mAb to LPAP (10-04) on lymphomas and reactive lymph nodes. (d) LPAP (10-19) did not stain the Reed–Sternberg cells in HL (arrow). (e) The 10-04 mAb did not stain non-lymphoid cells in the liver tissue. (f) LPAP (10-19) weakly stained all lymphoid cells in lymphomas. (g) FAT1 cadherin (10-16) on reactive lymph node specimens. (h) Calreticulin (10-23) on reactive lymph nodes and (i) showing increased staining intensity in Reed–Sternberg cells in HL and (j) scattered large cells in HL and follicular lymphoma (FL). (k) The IL-13-Ra2 (10-37) mAb on HL and (l) on reactive lymph nodes.
There were three mAbs to the LPAP (10-04, 10-19 and 10-11). These mAbs showed intense membrane/cytoplasmic staining of all lymphoid cells in all lymphoma cases and reactive lymph nodes (Figure 3c). However, LPAP did not stain the Reed–Sternberg cells in HL (Figure 3d, arrow). No LPAP mAb stained non-lymphoid cells, although scattered lymphoid cells were positive in the liver tissue (Figures 3e and f). FAT1 cadherin (10-16) showed membrane/cytoplasmic staining of the follicles in one out of two reactive lymph node specimens (Figure 3g), but showed no staining in lymphoma or non-haematopoietic tissues.
Calreticulin (10-23) showed positive membrane/cytoplasmic staining of most cells in reactive lymph nodes (Figure 3h) and all lymphoma samples. There was increased staining intensity in the Reed–Sternberg cells in HL (Figure 3i) and scattered large cells in HL and follicular lymphoma (Figure 3j), which may represent immunoblasts or histiocytes. Nonspecific staining of thyroid tissue, gastric, prostate and colorectal adenocarcinoma by 10-23 was evident.
The IL-13-Ra2 (10-37) mAb showed intense nuclear staining of a small number of lymphoid cells in HL (Figure 3k) and weaker membrane/cytoplasmic staining of a small population of cells located around the high-endothelial venules in other lymphomas and reactive lymph nodes, possibly reflecting DCs or histiocyte staining (Figure 3l).
Discussion
The HLDA10 panel of mAb was gathered to reflect the growing interest in identifying DC and myeloid lineage sub-populations. The mAbs were expected to bind to one of these populations directing the type of studies that were performed. Further results of the studies can be found on the HCDM website (www.hcdm.org).
The strategy that was used to test these mAbs was to firstly identify those that worked in our hands on at least one cell line ensuring that activity had not been lost in transit. Active mAbs were included in the panels dispatched to colleagues for testing in external laboratories. Those mAbs that demonstrated binding to DC- or myeloid-like cell lines were assayed for binding to fresh DCs in the peripheral blood from three healthy donors. Some of these mAbs that bound to fresh DCs were further characterised for binding to DCs with an activated phenotype.
The process of testing large number of mAbs in cross-laboratory protocols is cumbersome and fraught with interlaboratory variability. Consequently, we have approached these problems by reporting positive data and not the negative data. For example, we report which mAb did bind to transfectants. We have not said which mAb did not bind transfectants in our hands. We decided that this approach would indicate that a negative result may be because of variables that were outside the scope of the workshop. Such variables include subsaturating levels of mAb, only CHO cells were used for transfections, transfections were performed with cDNAs cloned into different expression vectors, mAbs were well travelled and so on. Thus, the results provide a report of well-validated mAbs and have no bearing on the reactivity of unreported mAbs. In fact, many of these mAbs will be validated in ongoing subsequent studies.
The HLDA10 provided the opportunity to further test a large selection of mAbs on a number of DC populations. The classification of DCs and their distinction from other myeloid cell populations continues to be debated.1 In these flow cytometry studies on PBMCs, we used a backbone of mAbs that allowed us to distinguish monocyte and DC subsets. Our gating strategy demonstrated the presence of CD16+ cells in both the CD14lo monocyte gate and the CD14− DC gate. This is consistent with our earlier description of a CD16+ DC population from the HLDA8 Workshop.4 Although the origin of these cells may be related, we identified differential binding of at least two mAbs, 10-36 and 10-47, to the two CD16+ populations indicating their further distribution.
CMRF-56 is an mAb that recognises a cell surface activation marker found on human blood DCs.8 A human-chimeric CMRF-56 mAb has been developed for purification of DC immunotherapy.10 Although the CMRF-56 mAb remains, after inclusion in five Workshops, an orphan mAb that binds to an unknown antigen, we were able to further phenotype these cells. Eight mAbs in the panel bound to the CMRF-56-activated cells including CMRF-56 itself (10-69). mAbs to two unknown antigens (10-27 and 10-33) bound to these cells as did 10-43 (CD245), 10-70 (P2X7), 10-71 (DCIR), 10-82 (CMRF-44) and 10-84 (FDFO3). The P2X7 molecule is an ATP-gated cation channel. Extracellular ATP activates P2X7 resulting in the uptake of organic cations. It has previously been shown to be on in vitro derived DCs.11 In these studies, 10-70 mAb bound weakly to fresh blood DCs but did bind to the cultured blood DCs.
Clinical laboratories continue to search for characterized mAbs that bind antigens embedded in paraffin sections for diagnostic purposes. The HLDA10 panel was tested on a small number of HLs. Antibodies to the LPAP molecule were identified as having a similar expression to CD45 corroborating the reports in the literature that LPAP associates with CD45.12 However, in line with the criteria for CD designation, LPAP as an intracellular molecule was not eligible for a CD.
Therapeutic antibodies to treat haematological and other malignancies are one of the fastest growing groups of biologics. The difficulty in their development is often identifying suitable markers to target. We tested the HLDA10 panel on a small number of AML. Three mAbs showed convincing reactivity to all three AMLs tested. These included mAbs to 10-24 and 10-75 (TIM-3), 10-33 (unknown antigen) and 10-53 (IL-1RAcP). Although the sample size is too small for any statistical analysis, the finding that TIM-3 bound to all three AMLs is consistent with the reports of TIM-3 suitability as a target for Ab therapeutics in AML.9
New CDs were designated on strict criteria. Two independent mAbs assessed in the HLDA Workshop arena are required to show identical patterns of reactivity by flow cytometry and reactivity against transfected cells to be provided with a CD. These studies need to have demonstrated that the mAbs have the following characteristics. The antibody bound to cells transfected with cDNA encoding the given protein, the antibody had the predicted (reported in the literature) binding profile on primary cell populations as assessed by flow cytometry, the antibody recognised a well-described protein and had been characterised in work published in peer-reviewed journals. Although biochemistry is desirable, many of the mAbs were submitted as directly conjugated to fluorochrome in limiting amounts. As a number of mAbs targeted DCs and did not bind to cell lines, limited biochemistry was performed. At the HLDA10, seven new CDs were designated: CD365 (TIM-1; mAbs 10-14, 10-67), CD366 (TIM-3; mAbs 10-24, 10-75), CD367 (DCIR/CLECSF6; mAbs 10-13, 10-71, 10-72), CD368 (CLEC4D; 10-21, 10-78), CD369 (CLEC7A/Dectin-1; mAbs 10-01, 10-35, 10-79), CD370 (CLEC9A; mAbs 10-02, 10-09, 10-45, 10-65) and CD371 (CLEC12A; mAbs 10-17, 10-51, 10-73).
C-type lectin family members are important pathogen recognition molecules expressed widely on myeloid and DC lineage cells. The nomenclature for these molecules is confusing with the same molecule referenced to in the literature as different CLECs. For example, the HUGO gene name CLEC4A encodes the molecule CLECSF6. A number of mAbs submitted to the HLDA10 panel were to CLECs, and CD numbers were designated to mAbs that bound five CLECs. This should make the literature focussed on these CLEC molecules more consistent. CD370 (CLEC9A, 10-02, 10-09, 10-45, 10-65) antibodies only recognised the rare CD141hi subset of peripheral blood DCs. All antibodies to CLEC 4A, CLEC7A and CLEC12A bound to monocytes and DC subsets. However, CLEC4D antibodies only recognised the CD14hi monocyte population. The mAb to CLEC molecules CD367, CD368, CD369 and CD370 were shown to bind to the CD1a+ skin DC.
Overall, these data provide a substantial list of available mAb that bind to different human myeloid and DC populations. This will contribute to our ability to identify and target specific cell types in future studies. The new CDs will add consistency to reporting data.
Methods
HLDA10 panel
The panel of mAbs was collected from those reported to bind to monocytes or DC populations and were obtained from individual researchers and commercial companies. The panel included unsolicited mAbs. Contributors to the HLDA10 supplied mAbs to the DCR group at the ANZAC Research Institute (Sydney, NSW, Australia). The mAbs, their suppliers and the blinded code number for each mAb are listed in Table 1. mAbs were diluted to 100 μg ml−1 and were used at saturating concentrations determined by information provided by supplier and titration on cell lines. The panel included a control mAb; CMRF-81 (10-85) was a purified mAb to tetanus toxoid.
Participating laboratories
Blood DCs: DCR, Sydney, Australia; Anna Brooks, Auckland, New Zealand; Muzzafilla Haniffa, Newcastle, UK; pDCs: Daniel Benítez Ribas, Barcelona, Spain; B-cell subsets (tonsil and spleen): Pablo Engel Rocamora, Barcelona, Spain; monocyte macrophage subtypes: Richard Kroczek, Berlin, Germany; Hannes Stockinger, Vienna, Austria; monocyte-derived DC: Julia Almeida, Salamanca, Spain; Hans-Jörg Bühring, Tübingen, Germany; CD34-derived LC: James Young, New York, NY, USA; AML: DCR, Sydney, NSW, Australia; Langerhans cells: Andrew Harman, Sydney, NSW, Australia; lLymphomas: Kenneth Lee, Sydney, NSW, Australia.
Primary cell populations
Venous blood from healthy donors and clinical samples was collected with informed consent approved by the Sydney Local Health District Human Research Ethics Committee (HREC/11/CRGH61, HREC/12/CRGH/59 and HREC/07/RPAH/28), consistent with the Declaration of Helsinki. Mononuclear cells were isolated using Ficoll-Paque density gradient centrifugation (GE Healthcare, Little Chalfont, UK).
In a separate analysis, pDCs were purified from peripheral blood leukocytes from healthy buffy coats using indirect magnetic isolation (Miltenyi Biotech, Sydney, NSW, Australia) of CD303+ cells.
Diagnostic samples were collected from patients newly diagnosed with AML. The mononuclear fraction was prepared using Ficoll-Paque density gradient centrifugation and stored frozen in liquid nitrogen until use. Cells were thawed immediately before use and then stained as for fresh blood samples. A viability dye was included in all analyses.
CMRF-56+ blood DCs
PBMCs were cultured in X-Vivo 15 media (Lonza, Mount Waverley VIC, Australia) for 15 h. This incubation period results in the upregulation of the CMRF-56 antigen and an activated-like phenotype.
Skin DCs
This study was approved by the Western Sydney Local Area Health District (WSLHD) Human Research Ethics Committee (HREC); reference number HREC/2013/8/4.4(3777) AU RED HREC/13/WMEAD/232. Healthy human tissue was obtained from a range of plastic surgeons and written consent was obtained from all donors. Skin was processed by enzymatic digestion as described previously.13 The dermal cells were enriched for CD45- expressing cells using CD45 magnetic bead separation (Miltenyi Biotech). Cell suspensions were labelled for flow cytometric phenotyping of surface expression markers. Cells were labelled in aliquots of 1 × 106 cells per 100 μl of buffer, according to standard protocols. Nonviable cells were excluded by staining with Live/Dead Near-IR Dead Cell Stain Kit (Life Technologies, Sydney, NSW, Australia). Flow cytometry was performed on Becton Dickenson (BD, San Jose, CA, USA) LSR Fortessa flow cytometer and data analysed by FlowJo (Treestar, Ashland, OR, USA).
Flow cytometric labelling of blood DCs
Saturating amounts of unconjugated antibodies were incubated with 5 × 106 PBMCs for 20 min on ice before being washed with FACS buffer (phosphate-buffered saline/0.5% bovine serum albumin) and incubated with Alexa-Fluor 488 goat anti-mouse F(ab)2 antibody. After washing, cells were blocked with 10% normal mouse serum for 10 min followed by the addition of the directly labelled antibodies for the DC backbone and incubation on ice for 20 min. After washing, DAPI (4',6-diamidino-2-phenylindole) was added to the samples and cells were analysed on a BD Influx. Three million events were collected in the live gate for each sample.
Directly conjugated antibodies were added to the DC backbone panel (described in detail in Fromm et al., submitted) and incubated with 5 × 106 PBMCs. After washing, DAPI was added to the samples as they were analysed using a BD Influx. Three million events were collected in the live gate for each sample.
Data presentation
As the Workshop testing is multiple laboratories providing data on the same antibody samples using different experimental cells, protocols and equipment, the quantification of results will vary. For this reason, the code is normalised to all work presented. Tables have been collated with the following scale. A minus (−) indicates no staining above background isotype control, +/− indicates limited staining (<10% of the population) above background isotype control, + indicates staining between 10 and 50% above background isotype control and ++ indicates 50-100% staining above background isotype staining.
Flow cytometry analysis
Data analysis was performed using FlowJo version 9 or X (Treestar). SPADE was performed using Cytobank (Cytobank Inc., Mountain View, CA, USA),14 following preprocessing of FCS files with FlowJo V9 to remove cell aggregates, dead cells and selected for Lin− (CD3, 19, 20, 56), HLA-DR+ cells. SPADE clustering was performed on ArcSinh-transformed fluorescent parameters using 300 nodes and 10% downsampling from 3 to 5 donors to generate unified SPADE trees based on the expression of CD11c, CD14, CD16, CD1c, CD141 and CD34. Populations were manually annotated according to the criteria used for manual analysis.
In vitro derived cell populations
Immature and mature Langerhans cells (LCs) were generated from granulocyte-colony stimulating factor-elicited CD34+ haematopoietic stem cells collected from healthy donors for allogeneic haematopoietic cell transplant, following protocols described previously.15, 16 LCs were cultured in 6-well tissue culture plates (Corning Costar, Corning, NY, USA) in X-VIVO 15 medium (BioWhittaker, Lonza, Walkersville, MD, USA). Fetal calf serum was not used in any cell cultures. Immature LCs were collected for phenotyping after 12 total days in culture. Mature LCs were immature LCs after coculture with inflammatory cytokines (IL-1-β (2 ng ml−1), IL-6 (1000 IU ml−1), TNF-α (10 ng ml−1) and prostaglandin E2 (5 mm)) for two days.
Immature and mature LCs were incubated with each of the HLDA10 antibodies. Cells incubated with biotin-conjugated antibodies were subsequently incubated with allophycocyanin-conjugated streptavidin (Molecular Probes, Life Technologies, Carlsbad, CA, USA). Cells phenotyped with unlabelled antibodies were then detected by staining with fluorescein isothiocyanate-conjugated polyclonal goat anti-mouse immunoglobulin (Dako, Carpinteria, CA, USA). A separate sample of each LC culture was stained with PE-conjugated anti-human CD83 (Beckman Coulter, Brea, CA, USA), allophycocyanin-conjugated anti-human CD86 (BD Biosciences), fluorescein isothiocyanate-conjugated anti-human CD14 (BD Biosciences), PE-Cy7-conjugated anti-human CD11b (BD Biosciences) and ECD-conjugated anti-human HLA-DR (Beckman Coulter), to ensure proper gating of LCs. Flow cytometric data were acquired on an LSR Fortessa (BD Biosciences) and analysed using FlowJo 9.6 (FlowJo LLC). Immature LCs were determined to be HLA-DR+ and CD83−, whereas mature LCs were determined to be HLA-DRbright, CD83+ and CD86+. Both immature and mature LCs were negative for CD11b and CD14.
Primary tested AML samples
Three primary AML samples were tested by flow cytometry. CRGH 3 was a peripheral blood sample from essential thrombocythemia transformed to AML of a 74-year-old male patient with cytogenetics of +8, CRGH 9 was a peripheral blood sample from an acute monoblastic leukaemia in a 59-year-old male patient with normal cytogenetics and CRGH 16 was from a leukapheresis sample of a 34-year-old male patient with AML with eosinophilia (Inv (16)). In each sample, the leukaemic blast population was gated as the viable CD45dimSSClo blasts and the CD34+CD38− ‘leukaemia stem cell'-enriched fraction was identified using the gating strategy.
Immunohistochemistry of paraffin sections
A tissue microarray was constructed from formalin-fixed paraffin-embedded blocks of selected lymphoma cases and controls. There were five cases each of HL, follicular lymphoma, diffuse large B-cell lymphoma, mantle cell lymphoma and five cases of normal lymph nodes with two tissue cores per case and an array of controls tissues. Immunohistochemical stains were performed on 3 μm sections of the tissue microarray on positive charge slides using the unknown HLDA mAb. The purified and ascities mAb immunohistochemical stains were performed on an automated Leica Bond Max or Bond III (Leica Microsystems, Melbourne, VIC, Australia) using a polymer detection system and chromogenic detection was performed using 3,3′-diaminobenzidine. All purified antibodies were initially used at a 1:100 dilution and ascities at 1:50 with antigen retrieval performed via heat-induced epitope retrieval with an EDTA-based buffer, pH 9. Any mAb that showed some staining was optimised further by adjusting the antibody concentration. All purified mAbs, which showed negative staining, were then further tested with two alternative antigen retrieval methods: HIER with a citrate-based buffer, pH 6, and with proteinase K, an enzyme-based retrieval. Neither of these alternative methods revealed any staining. Further optimisation was not performed on the ascities mAb. All of the biotin-labelled mAbs were used at a dilution of 1:50. These slides were stained manually using a BioGenex Multilink Kit (BioGenex, Fremont, CA, USA). Antigen retrieval was performed in a pressure cooker with an EDTA-based buffer and chromogenic detection was performed. None of the biotin-labelled mAb showed any staining, and further optimisation was not performed.
Acknowledgments
This work was supported by funding from the Concord Cancer Center, University of Sydney and the Australasian College of Pathologists. We would like to acknowledge the generous support of BD BioSciences (Dr Robert Balderas) and Miltenyi Biotech (Dr Katharina Winnemöller) for the provision of mAbs backbones for DC studies and to those that contributed mAb to the HLDA10 panel.
The authors declare no conflict of interest.
References
- 1Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014; 14: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Anguille S, Smits EL, Bryant C, Van Acker HH, Goossens H, Lion E et al. Dendritic cells as pharmacological tools for cancer immunotherapy. Pharmacol Rev 2015; 67: 731–753. [DOI] [PubMed] [Google Scholar]
- 3Bol KF, Tel J, de Vries IJ, Figdor CG. Naturally circulating dendritic cells to vaccinate cancer patients. Oncoimmunology 2013; 2: e23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4MacDonald KPA, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DNJ. Characterization of human blood dendritic cell subsets. Blood 2002; 100: 4512–4520. [DOI] [PubMed] [Google Scholar]
- 5Zola H, Swart B, Banham A, Barry S, Beare A, Bensussan A et al. CD molecules 2006—human cell differentiation molecules. J Immunol Methods 2007; 319: 1–5. [DOI] [PubMed] [Google Scholar]
- 6Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010; 207: 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Diehl V, Pfreundschuh M, Fonatsch C, Stein H, Falk M, Burrichter H et al. Phenotypic and genotypic analysis of Hodgkin's disease derived cell lines: histopathological and clinical implications. Cancer Surv 1985; 4: 399–419. [PubMed] [Google Scholar]
- 8Hock BD, Fearnley DB, Boyce A, McLellan AD, Sorg RV, Summers KL et al. Human dendritic cells express a 95 kDa activation/differentiation antigen defined by CMRF-56. Tissue Antigens 1999; 53: 320–334. [DOI] [PubMed] [Google Scholar]
- 9Gasiorowski RE, Clark GJ, Bradstock K, Hart DN. Antibody therapy for acute myeloid leukaemia. Br J Haematol 2014; 164: 481–495. [DOI] [PubMed] [Google Scholar]
- 10Freeman JL, Vari F, Hart DN. CMRF-56 immunoselected blood dendritic cell preparations activated with GM-CSF induce potent antimyeloma cytotoxic T-cell responses. J Immunother 2007; 30: 740–748. [DOI] [PubMed] [Google Scholar]
- 11Sluyter R, Wiley JS. Extracellular adenosine 5'-triphosphate induces a loss of CD23 from human dendritic cells via activation of P2X7 receptors. Int Immunol 2002; 14: 1415–1421. [DOI] [PubMed] [Google Scholar]
- 12Filatov AV, Meshkova TD, Mazurov DV. Epitope mapping of lymphocyte phosphatase-associated phosphoprotein. Biochem Biokhim 2014; 79: 1397–1404. [DOI] [PubMed] [Google Scholar]
- 13Harman AN, Bye CR, Nasr N, Sandgren KJ, Kim M, Mercier SK et al. Identification of lineage relationships and novel markers of blood and skin human dendritic cells. J Immunol 2013; 190: 66–79. [DOI] [PubMed] [Google Scholar]
- 14Kotecha N, Krutzik PO, Irish JM. Web-Based Analysis and Publication of Flow Cytometry Experiments. Current Protocols in Cytometry/editorial board, J Paul Robinson, managing editor, 2010, Chapter 10, Unit10.7. (Wiley Online). [DOI] [PMC free article] [PubMed]
- 15Ratzinger G, Baggers J, de Cos MA, Yuan J, Dao T, Reagan JL et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol 2004; 173: 2780–2791. [DOI] [PubMed] [Google Scholar]
- 16Romano E, Cotari JW, Barreira da Silva R, Betts BC, Chung DJ, Avogadri F et al. Human Langerhans cells use an IL-15R-alpha/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood 2012; 119: 5182–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]