Abstract
Inflammatory bowel diseases (IBD) are associated with an altered systemic immune response leading to inflammation-mediated damage to the gut and other organs. Oral immune therapy is a method of systemic immune modulation via alteration of the gut immune system. It uses the inherit ability of the innate system of the gut to redirect the systemic innate and adaptive immune responses. Oral immune therapy is an attractive clinical approach to treat autoimmune and inflammatory disorders. It can induce immune modulation without immune suppression, has minimal toxicity and is easily administered. Targeting the systemic immune system via the gut immune system can serve as an attractive novel therapeutic method for IBD. This review summarizes the current data and discusses several examples of oral immune therapeutic methods for using the gut immune system to generate signals to reset systemic immunity as a treatment for IBD.
Interactions between susceptibility genes, the environment, the gut microbiome and the systemic immune system have a role in the pathogenesis of inflammatory bowel disease (IBD).1 Currently available treatments for IBD, which target the systemic immune system, induce immunosuppression, thereby exposing the patient to the risk of infections and malignancy. The interplay between the gut and the systemic immune system determines the final effect on target organs, including the bowel mucosa. Therefore, the gut immune system was suggested as a potential target for immune modulatory agents that act locally at the level of the bowel as a means for altering the systemic immune response. Oral immune therapy is a method for altering the systemic immune system via an effect on the gut immune system to generate a signal that will affect the systemic immune system. This method does not involve generalized immune suppression. This review summarizes several oral immune modulatory methods that can be used to alter systemic immunity as a means of treatment of IBD.
Oral immunomodulation to target the systemic immune system
Oral immune therapy is a method for altering the systemic immune system via an effect on the gut immune system. It is based on an inherent mechanism in which the gut immune system inhibits or promotes its reactions towards orally administered antigens.2, 3 The capability of the gut mucosa-associated immune system to mount an immune response against pathogenic antigens, while maintaining ignorance or active suppression against non-pathogenic antigens undines this phenomenon. It is associated with the ability of the innate immune system of the gut to generate signals that promote systemic adaptive responses. Oral immune therapy uses a physiological system that responds to food or pathogenic antigens, gut microbiome-derived epitopes, or any other type of adjuvants or orally administered antigens to which the gut mucosa is exposed.4 Oral tolerance can be viewed as one category of oral immune therapy, and is defined as a specific suppression of humoral and/or cellular immune responses to an antigen, by the administration of the same antigen, or towards bystander orally administered epitopes.5
Oral immune therapy is a valid approach to prevent and treat unwanted immune responses that cause a variety of diseases or that complicate the treatment of a disease, and can be used for the treatment of immune-mediated or immune-related disorders.6
The mechanism of action of oral immune therapy is not fully elucidated. For oral tolerance, low doses of orally administered antigens favor active suppression with the generation of regulatory T cells (Tregs), whereas high doses favor clonal anergy/deletion.7, 8, 9 For other methods of oral immune therapy, promotion of Tregs is one potential mechanism for the suppression of systemic inflammation at target organs via an effect on gut mucosal surfaces.10 Crosstalk between antigen-presenting cells in the gut, including dendritic cells (DCs) and T cells, has a role in the generation of the immune signals between the gut and the systemic immune systems. Oral immune therapy is not necessarily antigen specific and can suppress inflammation at the site of inflammation via the induction of suppressor cells, or Tregs, in an antigen-independent manner.11, 12 The non-antigen specificity of oral immune therapy may be associated with a bystander effect at the level of the gut or in target organs in which the disease-associated antigen is being presented.8, 13, 14 Adjuvants in the gut have an important role in oral immune therapy.12 These are critical for appropriate activation of the innate immune system in the gut, thereby affecting the type of signal being delivered to the systemic immune system.15
Oral immune therapy has several clinical advantages (Table 1). It uses the inherent ability of the gut immune system to control unwanted systemic immune responses and as such is not associated with generalized immune suppression. It preferentially induces Tregs. It can promote systemic tolerance in an antigen-dependent or -independent manner. In most cases, the compounds used for oral immune therapy do not reach the blood, making this method nontoxic and with minimal side effects. It is not associated with the harmful cytokine release syndrome that is noted for some of the intravenously administered immunomodulatory agents. Oral immune therapy is effective both for preventive therapy and for treatment at the peak of disease. In most cases, as no systemic absorption is required, a relatively low dose is sufficient for a clinically meaningful effect. Oral immune therapy provides a platform that can be used for many disorders. For the patients, it is easily tolerated, and eliminates safety concerns and pain related to needles. Trained medical personnel are not required to administer these drugs, and they are inexpensive.
Table 1. Advantages of oral immune therapy for the treatment of inflammatory bowel disease in contrast with the disadvantages of systemic immunomodulatory agents.
| Oral immune therapy | Systemic immune modulators | |
|---|---|---|
| Mechanism | Takes advantage of the inherent ability of the gut's immune system to control unwanted systemic immune responses | Act from the outside of inflammatory pathways |
| Generalized immune suppression | Not associated with general immune suppression | Induces generalized immune suppression |
| Induction of regulatory T cells | Preferentially induces regulatory T cells | May reduce regulatory T cells |
| Induction of tolerance | Can induce systemic tolerance | Does not induce tolerance |
| Target antigen dependency | Can be induced in an antigen-dependent or -independent manner | Not antigen-dependent |
| Reach the blood | Most compounds used do not reach the blood system | Needs to reach the blood |
| Toxicity | Minimal side effects | Significant toxicity including risks of infection and malignancy. Toxicity and side effects limit their use in a major proportion of patients |
| Cytokine release syndrome | Not associated with a harmful cytokine release syndrome | May be associated with cytokine release syndrome |
| Prevention or treatment | Effective both for preventive therapy and for treatment at the peak of disease | Many of the compounds used are effective for an established disease, and due to potential toxicity, they are not ideal for prevention. |
| Maintenance therapy | Can be used for maintenance | For several compounds, the toxicity prohibits their use for maintenance therapy |
| Dose | No absorption is required; therefore, a relatively low dose is sufficient for achievement of a clinically meaningful effect | Relatively high dosages are required depending on bioavailability |
| Platform | A platform that can be used for many disorders | Some compounds are disease specific |
| Patient advantages | Easily tolerated | Toxicity may limit tolerability |
| Safety concerns and pain | Eliminates safety concerns and pain related to needles. | Safety concerns and pain related to use of needles may limit their use for some patients |
| Requirement for trained personnel | Trained medical personnel not required for administration | For some compounds, intravenous or subcutaneous administration along with trained personnel are required |
| Cost | Relatively low cost | Expensive |
In contrast, most immune modulatory agents have significant side effects and are associated with some type of generalized immune suppression and an increased risk of infection and malignancy (Table 1). Relatively higher dosages of these drugs are required for the induction of immune suppression. In most cases, treatments are effective only after the onset of the disease, and due to the potential side effects, they cannot be used as preventive measures. Their toxicity and side effects limit their use in a large proportion of patients. Safety concerns and pain related to use of needles limit their use in some patients, and the requirement for trained medical personnel for administration may limit their use in some settings.
Most importantly, the immune modulatory agents used today for IBD do not achieve remission in many patients.16, 17 Not all IBD patients benefit from currently available drugs.18 Young people with IBD do not want to be on long-term drug therapy. Oral immune therapy, while not yet studied in large cohorts of patients, may provide an answer to this unmet need.
The interplay between the gut immune system and the systemic immune system: transfer of signals from the bowel is relevant for the pathogenesis of IBD
The gut immune system generates immune signals that can alter the systemic immune response. A complex interplay between many distinct intestinal immune cell types occurs at the gut level, affecting the interplay with the systemic immune system. Several of these components either generate or serve as signals, which alter the response of the systemic immune system.19 The immune gastrointestinal barrier is designed to distinguish between beneficial and harmful components in the gut to maintain systemic immune tolerance.1 It is composed of intestinal epithelial cells (IECs); cells of the innate immune system, including macrophages, monocytes, neutrophils and DCs; and cells of the adaptive immune system, including T and B lymphocytes and their secreted mediators, the cytokines and chemokines.1 Organized lymphoid structures and mucosal cells in the gut wall and beneath the epithelium, and the interaction of many types of cells, including DCs, natural killer T (NKT) cells, M cells, Paneth cells, mast cells, goblet cells and columnar epithelial cells, take part in the gut–systemic immune system interaction.20, 21 Secondary lymphoid tissue, such as Peyer's patches, and tertiary lymphoid tissue (the lymphoid follicles) respond to antigenic stimuli by releasing cytokines or producing secretory IgA.22 The IECs are in close cooperation with intraepithelial lymphocytes and possess Toll-like receptors on their surface and Nod-like receptors, which sense pathogens or pathogen-associated molecular patterns. All of these components of the gut immune system take part in the interplay with the systemic immune system.
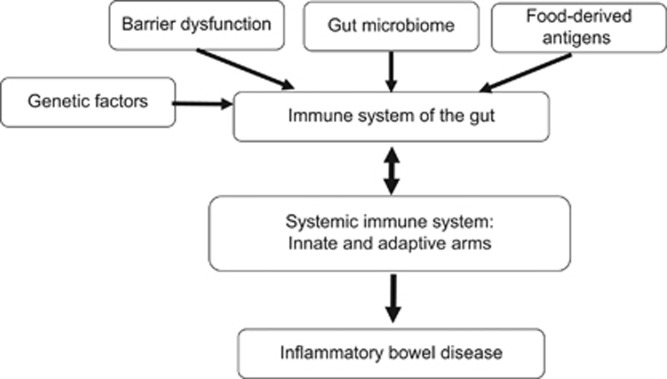
Components of the mucosal immune system are implicated in the pathogenesis of IBD affecting both the innate and adaptive arms of the systemic immune system.23 Disruption of mucosal homeostasis can alter the systemic immune response leading to bowel inflammation such as that seen in IBD (Figure 1).21 Barrier dysfunction, the gut microbiome, food-derived antigens and adjuvants are all relevant for activation of the gut immune system thereby affecting the systemic immune response.23 Examples for several components of the gut immune system and their role in the pathogenesis of IBD are described below.
Figure 1.
Schematic description of the interaction between the gut and systemic immune systems in the pathogenesis of inflammatory bowel disease.
Mucosal barrier
(i) The IECs constitute the first barrier in the gut against the lumen and are required for the maintenance of barrier integrity. They participate in food degradation and absorption, and have a role in intestinal inflammation.24 They translate signals coming from the outside world, and deliver information/signals about the gut lumen to immune cells.25 The communication occurs from the epithelial cells to the immune system and also in the opposite direction. By producing antimicrobial peptides, IECs alter the gut microbial community. IECs also respond to cytokines and other mediators of immune cells in the lamina propria.25 They interact with DCs and other immune cells to drive tolerogenic responses under the steady state, and they release immune mediators to recruit inflammatory cells and to elicit immunity to infectious agents. Dysregulation within the epithelial layer increases intestinal permeability, alters the interactions between IECs and immune cells in the lamina propria, disturbs the intestinal immune homeostasis and can lead to immune derangement and IBD.24, 26
(ii) The mucosal barrier has three major components: the mucus layer, the epithelial glycocalyx and the surface epithelium, whose integrity depends on tight junctions.27 Large highly glycosylated gel-forming mucins, MUC2, MUC5 and MUC5AC, are major components of the mucus that covers the intestine and stomach, respectively. The mucus limits the number of bacteria that reach the epithelium and the Peyer's patches.28 Goblet cells secrete mucin and mucus components and can prevent the presentation of oral antigens to the immune system. These cells deliver small intestinal luminal material to tolerogenic type DCs in the lamina propria.28 In addition to gel-forming mucins, the transmembrane mucins MUC3, MUC12 and MUC17 form the enterocyte glycocalyx, which extends a micrometer out from the brush border. The MUC17 mucin shuttles from a surface to an intracellular vesicle controlling the microbiota.28 Epithelial tight junction regulates paracellular trafficking of macromolecules.29 It is a multi-protein complex that forms a selective permeable seal between adjacent epithelial cells, creating a border between apical and basolateral membrane domains. Patients with IBD have a weakened mucosal barrier. They demonstrate increased intestinal paracellular permeability and increased intestinal tight junctions disruption. Disruption of the intestinal tight junction barrier followed by permeation of luminal toxic molecules induces a perturbation of the mucosal immune system and inflammation which exacerbates IBD.30 These changes also affect the transepithelial transport of macronutrients and micronutrients and the gut microbiome in these patients.29
(iii) Peyer's patches are lymphoid structures overlain by the epithelium, in which 5% of the cells are specialized M (microfold) epithelial cells, which are a major portal of entry for bacteria. There are no goblet cells in the dome epithelium, and M cells have a scarce glycocalyx allowing easy microbial interaction.27 Peyer's patches are sites of lesions in Crohn's disease (CD), and the 'anti-pancreatic' antibody associated with CD is targeting glycoprotein 2, the receptor for type 1 bacterial fimbrial protein (fimH) on M cells.
Cellular stress
(iv) Autophagy in the intestine, including macroautophagy and xenophagy, has a role in generating an intestinal immune response and antimicrobial protection in some of the patients.26 A dysfunctional autophagic mechanism leads to chronic intestinal inflammation in IBD. Genome-wide association studies have identified roles for numerous autophagy genes in IBD. Mucosal susceptibility or defects in sampling of gut luminal antigens via autophagy and crosstalk between the innate immune system and the microbiota activate the innate immune response mediated by enhanced Toll-like receptor activity.1, 31, 32
(v) The maintenance of gut mucosal equilibrium requires a balance between enterocyte loss by apoptosis and the generation of new cells by proliferation from stem cell precursors at the base of the intestinal crypts. Receptors of the innate immune system, including Toll-like receptors 2, 4 and 9 and the intracellular pathogen recognition receptor NOD2/CARD15, are associated with the initiation of enterocyte apoptosis. Induction of enterocyte apoptosis in response to activation of these innate immune receptors has a role in the development of IBD.33
Subsets of cells involved in the immune response
(vi) The intestinal mucosa contains numerous DCs that exert protective immunity to infectious agents or tolerance to innocuous antigens, including food and commensal bacteria.12, 19, 34 DCs in the gut actively sample both pathogenic and non-pathogenic antigens, including those derived from the microbiota, followed by migration to secondary lymphoid organs in the gut to activate naive T cells.19 DCs in the gut induce gut-homing properties on T cells upon activation, enabling T-cell migration back to intestinal sites. Specialized CD103+ intestinal DCs promote the differentiation of Foxp3+ Tregs via a retinoic acid-dependent process.35 DCs dysfunction contributes to IBD development.34 Both gut microbiota and food-derived antigens alter intestinal DCs function, and contribute to a loss of tolerance and to induction and progression of IBD.19 In patients with IBD, the tolerance/immunity balance is disturbed, leading to chronic intestinal inflammation driven by aberrant T-cell reactivity to intestinal bacteria.36 Tolerogenic DCs act by promoting differentiation and expansion of Tregs that efficiently modulate gut inflammation, and they are disturbed in IBD.37
(vii) NKT cells are a subset of non-conventional T cells recognizing endogenous and/or exogenous glycolipid antigens when presented by the major histocompatibility complex class I-like antigen-presenting molecules CD1d.38 NKT cells are abundant in the gut immune system. Upon T-cell receptor engagement, gut NKT cells rapidly produce cytokines, thus affecting mucosal immunity.39 Mucosal and systemic NKT cell development is under the control of the commensal microbiota.40 NKT cells in the bowel recognize microbial lipid antigens presented by CD1d. These cells exhibit effector functions in antimicrobial defense and in the modulation of inflammation in the gut. CD1d controls the composition of the intestinal microbiota via regulation of Paneth cell function.41 In animal models of IBD, NKT cells make both protective and pathogenic contributions to disease. In patients with ulcerative colitis and in a mouse model in which both CD1d expression and the frequency of subsets of NKT cells are increased, they promote intestinal inflammation.42 Oral immune therapy was reported to be associated with promotion of NKT cells in both animal models and humans.13, 43, 44, 45, 46, 47
(viii) Immature myeloid cells, known as myeloid-derived suppressor cells (MDSCs), including neutrophilic and monocytic myeloid cells, are found in inflammatory loci and secondary lymphoid organs in mice with intestinal inflammation and in patients with IBD.48 They interact with Th17 cells, and their function is determined by the ER stress.48 Their pro-inflammatory or immunosuppressive role in IBD is not well defined.
(ix) Regulatory T cells (Tregs) maintain self-tolerance and control excessive immune responses to foreign antigens.49 Tregs inhibit effector cells by several mechanisms, including: promotion of inhibitory cytokines; induction of death by cytokine deprivation or cytolysis; local metabolic perturbation by changes in extracellular nucleotide/nucleoside fluxes; alterations in intracellular signaling molecules, such as cyclic AMP; and inhibition of DCs.49, 50, 51 The lamina propria constitutes an effector site that actively influences Tregs-cell function.52 Tregs must be in the proximity of their target cells within lymphoid organs and the lamina propria in the intestine.52 Foxp3(+) Tregs maintain immune balance in the gut via IL-10- and TGF-β-dependent mechanisms.53 Their differentiation and function are modulated by intestinal microbiota.54, 55 Inflammation in IBD is mediated by inappropriate production of pro-inflammatory cytokines by CD4+ T-effector cells, which are not suppressed by Tregs.56, 57 Activation of Tregs inhibits the inflammatory response to commensal bacteria and is central for mucosal tolerance. Loss of this mechanism leads to inappropriate immune reactivity toward commensal organisms, contributing to mucosal inflammation in IBD.51, 58, 59
(x) Th17 cells infiltrate the intestine of IBD patients, producing IL-17 and amplifying the inflammatory process. Th17 can be converted into either IFN-γ-producing Th1 cells or Tregs.60 Antigen presenting cells mediate differentiation of naive T cells into effector T-helper cells, including Th1, Th2 and Th17, and can alter gut homeostasis leading to IBD.1, 31, 32
(xi) Macrophages functions change during infection and inflammation. The intestinal macrophage pool requires continual renewal from circulating blood monocytes, unlike most other tissue macrophages, which derive from primitive precursors that subsequently self-renew.61, 62, 63 Macrophages in the gut have a role in Tregs function.52 As regulatory cells in the gut, macrophages also have a role in the pathogenesis of IBD.62
(xii) The function of both T and B cells is required for proper interplay between the gut and the systemic immune systems. Plasticity of CD4+ helper T cells is important for the correct function of the gut immune system.1, 31, 32
Taken together, these studies suggest that each of the components of the gut immune system is pertinent for the induction and/or progression of IBD. Most of these subsets of cells are involved directly or indirectly in the signaling between the gut and the systemic immune systems, a process that is relevant for the generation and maintenance of the inflammatory process in IBD. Therefore, oral immune therapy, which affects these types of signals between the gut and the systemic immune systems, may aim at several of these targets.
The gut microbiome in the interplay between the gut and the systemic immune system in IBD
The gut microbiota is required for proper development of the host and maintenance of intestinal homeostasis.64 Continuous exposure of the intestinal mucosa to diverse microorganisms and to food-derived products and metabolites is required for proper function of the gut immune regulatory system.21 The gut microbiome is important for the generation of the signals between the gut and the systemic immune systems.65 Both positive and negative stimulation by luminal products incite the assembly of inflammasomes involved in maintaining the integrity of the intestinal epithelium and a favorable environment for both the host and the microbiota. Indigenous bacteria stimulate the immune system to protect against commensal and exogenous pathogens.66 Gut microbiota, mainly Bacteroidetes and Firmicutes, generate a tolerogenic response via acting on DCs and inhibiting the Th17 pathway.22 Bacteroides fragilis leads to the production of anti-inflammatory IL-10 by Tregs and lamina propria macrophages. Fragmented filamentous bacteria promote gut inflammation via the induction of Th17 cells.22 Increased gut permeability, bacterial translocation and increased lipopolysaccharide levels have been described in patients with immune-associated disorders.67, 68, 69 Intestinal barrier loss alone is insufficient to initiate disease.70
Dysbiosis and alterations in the intestinal microbiome are associated with IBD.32 Gut microbes can induce and sustain the disease.71 The loss of normal tolerance to intestinal microbiota and/or to food or environment-derived antigens leads to mucosal damage.22 Both in active and in quiescent disease, the fecal- and mucosal-associated microbiomes show reduced diversity. Various cells from IBD patients show increased susceptibility to bacterial products, including flagellin, pili and lipopolysaccharide.72 Mucosa-associated adherent, invasive Escherichia coli (E. coli), which are pro-inflammatory and resistant to killing by mucosal macrophages, may be associated with the pathogenesis of CD.73 Bacteria and their products trigger cytokine expression, including tumor necrosis factor alpha (TNF-α) and IL-8 by macrophages and epithelial cells, respectively, in patients with CD.72 Bacteria coated with IgA can induce gut inflammation in patients with IBD.74 Intestinal microbiota and its toxic components act on Nod1 and Nod2 receptors leading to defective signaling, which accounts for the development of IBD.22 Microbiota can also induce anti-inflammatory or regulatory immunological mechanisms. The balance between these opposing processes is relevant for IBD.32, 75 IL-10 from macrophages, T cells and B cells, and TGFβ1 from epithelial cells and other non-lymphoid/myeloid cells, are relevant for the anti-inflammatory pattern. The neutralization of TGFβ1 increases Th1 and Th17 responses in IBD; however, exogenous TGFβ1 does not inhibit inflammation because of a block in intracellular signaling mediated by Smad7.76 On the basis of the above, fecal transplantation was developed as a mean for the treatment of IBD.77
The liver–gut axis in IBD
The liver is a site where immune signals derived from the gut interact with the systemic immune system. It is at the juncture of the peripheral circulation and the portal circulation leading to interaction between naive T cells and hepatic cells. This interaction results in the generation or disruption of the development of tolerance to commensal bacteria and other environmental agents.78 The liver–gut crosstalk is the basis for the hepatobiliary manifestations of IBD.79 Gut microbiota are associated with the development of intestinal, hepatic and extra-intestinal manifestations of IBD.80 The pathogenesis of the liver manifestation of IBD is related to gut inflammation that results in inflamed portal tracts of the enterohepatic circulation of lymphocytes from the gut to the liver.79 It involves multiple gut-derived inflammatory cell types and cytokines, chemokines and other molecules that lead to the destruction of normal liver architecture.78 Both pathogenic and commensal microbiota trigger these events. Products of the microbiota activate the innate immune system to drive pro-inflammatory gene expression, inducing chronic inflammatory disease of the liver.81 The crosstalk has a role in the pathogenesis and outcome of primary sclerosing cholangitis and in immunoglobulin G4-associated cholangitis in patients with IBD.
Reduced intestinal availability of bile salts reduces stimulation of the farnesoid X receptor, inducing bile salt overload and hepatotoxicity through reduced action of intestinal fibroblast growth factor 19.80 Enteral lipids reduce inflammation and liver damage during stress or systemic inflammation, whereas parenteral lipid is associated with liver damage.80 CD8+ cells primed in the GALT acquire effector function and can migrate to the liver, leading to cholangitis in an antigen-dependent manner.82
Targeting the gut immune system as a means for altering the systemic immune system for the treatment of IBD
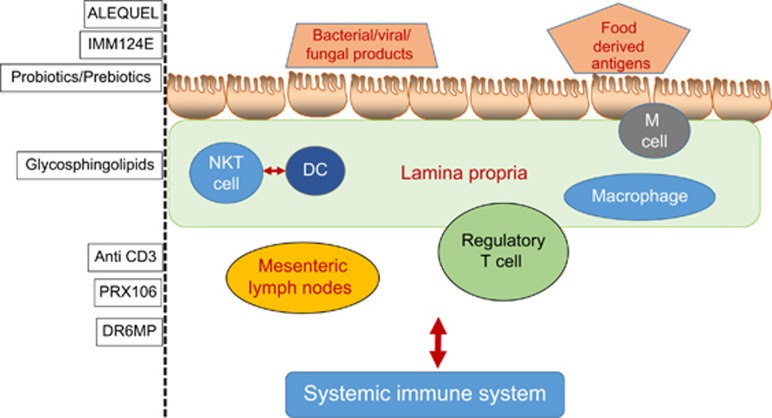
Diverse regulatory mechanisms cooperate to maintain intestinal homeostasis, and a breakdown in these pathways precipitates the chronic inflammatory pathology in IBD.83 Orally administered agents that alter the gut immune system and/or target the signals between the gut and the systemic immune systems can serve as means for altering the systemic immune system for the treatment of IBD. Described below are several examples of compounds being developed for oral immune therapy in IBD, some of which have been tested in humans (Figures 1 and 2).
Figure 2.
Several potential targets for oral immune therapy in inflammatory bowel disease at different levels of the immune system of the gut.
Oral administration of an extract of autologous colonic protein-derived antigens, Alequel
Oral immune therapy based on the oral administration of extracts of colonic proteins alleviated immune-mediated colitis in animal models of IBD.45 A marked reduction in the fraction of injured colonic areas and colon weights were observed, along with reductions in the inflammatory response and mucosal ulcerations. These effects were associated with an increase in TGFβ1 and a decrease in IFN-γ serum levels. TNBS-induced colitis was attenuated in naive recipients of splenocytes from tolerized rats compared with rats that received splenocytes from control donors.45
In humans, oral administration of Alequel, an extract of autologous colonic-derived proteins, was safe in patients with CD.84 In a phase I trial, 10 CD patients were orally treated with Alequel for 16 weeks. Seven patients achieved clinical remission and improvement in their IBD questionnaire scores. The high levels of colitis-extracted protein-specific IFN-γ-spot-forming colonies decreased with therapy. The beneficial effect was associated with alteration of the CD4+/CD8+ lymphocyte ratio, increased peripheral NKT cell numbers and increased serum IL-10 and IL-4 levels.84 In a phase II randomized, double-blind, placebo-controlled trial, 31 patients with moderate-to-severe CD were enrolled in a 27-week study.85 Clinical remission was achieved in 58% of the patients in the treated group compared with 29% in the placebo group. A clinical response was observed in 67% and 43% of the treated vs placebo groups, respectively. An improved IBD questionnaire score was observed in 43% of the treated vs 12% of placebo groups. A decrease in the number of subject-specific, antigen-directed-IFN-γ spot-forming colonies and an increased percentage of peripheral blood NKT cells were observed in drug-treated patients who achieved remission. In a subsequent phase II trial, 43 CD patients were enrolled in a randomized, placebo-controlled, double-blind trial. Remission was achieved in 43% of treated versus 33% of the placebo group in weeks 6–9. For weeks 9–12, the remission rate was 50% in the drug-treated vs 33% for the placebo groups. Altered NKT cell numbers and CD4+/CD8+ T-lymphocyte ratios were noted in treated patients. No treatment-related adverse events were noted in the studies.86 Alterations in CD4+, CD8+ and NKT lymphocytes cells support the notion that oral immune therapy using non-absorbable autologous proteins affects the systemic immune system.
The long-term learning ability of the gut immune system was recently published in these patients. A total of 92 patients treated with Alequel were followed. In patients who responded, the mean disease-free interval was 7.3±3.96 months. The opposite effect was noted for patients who received placebos.87 These results suggested that short-term oral administration of autologous colonic extracts exerts a long-term beneficial memory effect in moderate-to-severe CD.87 The long-term effect was associated with the promotion of regulatory/suppressor cells with memory phenotypes.
Taken together, the results suggest that oral immune therapy of non-absorbable Alequel is safe and effective for the treatment of moderate-to-severe CD. It induces a long-term beneficial memory effect in these patients.
Oral administration of non-absorbable delayed-release 6-mercaptopurine in patients with CD
The purine analogs azathioprine and 6-mercaptopurine (6-MP) are a mainstay of long-term maintenance therapy for IBD patients.88 Their use is somewhat limited by systemic side effects that are associated with low tolerability and low compliance.89, 90, 91, 92 Adverse events are associated with discontinuation or dose reduction of therapy in 9–28% of patients.90, 91, 92 Serious adverse events were reported in 14% of patients receiving azathioprine. Dosing strategies to improve therapeutic response and reduce adverse reactions are being used.93
A novel formulation of low-dose, delayed-release 6-mercaptopurine (DR-6MP) was developed for oral immune therapy.94 Pharmacokinetic and proof-of-concept open-label studies showed that DR-6MP is not absorbed significantly. Administration of a single dose of DR-6MP increased systemic CD4+CD25+Foxp3+ Tregs 24 h after ingestion. In a phase I proof-of-concept trial, administration of DR-6MP in active CD patients for 12 weeks resulted in remission in 3 out of 10 vs one out of three patients treated with Purinethol.94 Seventy patients with moderately active CD were enrolled in a 12-week double-blind controlled phase II trial compared with Purinethol. DR-6MP had similar efficacy to Purinethol following 12 weeks of treatment. However, the time to maximal clinical response was 8 weeks for DR-6MP vs 12 weeks for Purinethol. A higher proportion of patients on DR-6MP achieved clinical remission at week 8 and showed improvements in IBD questionnaire score. DR-6MP led to a decrease of CD62+ expression in T cells, implying a reduction of lymphocyte adhesion to the site of inflammation. DR-6MP was safer than Purinethol, with significantly fewer adverse events. There was no evidence of drug-induced leukopenia in the DR-6MP group, and a much lower proportion of hepatotoxicity.94
The data suggest that oral immune therapy using a non-absorbable DR-6MP formulation is safe and biologically active in the gut. It is clinically effective, exerting a systemic immune response with low systemic bioavailability and a low incidence of side effects. A possible effect of absorbed metabolites was not ruled out in the above studies. However, the lack of an effect on the number of peripheral blood leukocytes supports a local action on the gut immune system that led to alteration of the systemic immune system documented by changes in CD62+ T cells.
Oral administration of non-absorbable anti-CD3 antibodies in humans and in an animal model of colitis
Intravenous administration of anti-CD3 antibodies was shown effective in the transplantation setting and in several immune-mediated disorders owing to their ability to induce tolerance.95 In mouse models of autoimmune diabetes, parenteral administration of anti-CD3 antibodies induced disease remission by restoring tolerance to pancreatic β-cells in part of the treated animals or patients. These antibodies arrest ongoing disease by clearing pathogenic T cells from target organs. In humans with recently diagnosed diabetes, preservation of β-cell function was achieved by short-term administration of a CD3-specific antibody.95 Clinical trials using two distinct humanized Fc-mutated antibodies to human CD3, ChAglyCD3 (otelixizumab) and OKT3-γ-1 Ala-Ala (teplizumab), demonstrated that parenteral CD3 antibodies preserved β-cell function, maintaining high levels of endogenous insulin secretion in treated patients.96 Parenteral administration of visilizumab, a humanized low-Fc receptor binding anti-CD3 antibody, induced a rapid clinical response in patients with steroid-refractory ulcerative colitis. This antibody induced apoptosis of lamina propria CD4+ T cells isolated from non-IBD individuals, ulcerative colitis and CD patients.97 Incubation of the inflamed mucosal biopsy specimens from patients with IBD with otelixizumab reduced inflammation-associated tyrosine phosphoprotein of proteins associated with T-cell receptor activation.98 Encouraging results from phase 1/2 clinical trials have been reported for visilizumab and foralumab in patients with IBD.99 However, these parenteral CD3-based therapies have high rates of adverse events. Studies reveal a narrow therapeutic window of anti-CD3-based therapies, in which low doses are ineffective and higher pharmacologically active doses cause intolerable levels of adverse effects.
One of the goals for the immunotherapy of autoimmune diseases is the induction of Tregs that mediate tolerance while omitting generalized immune suppression. Oral administration of anti-CD3 has been tested as a means to promote Tregs via stimulation of the gut immune system which preferentially induces these cells.100 Orally delivered antibodies do not have side effects associated with generalized immune suppression, and do not induce cytokine release syndromes, making them clinically applicable both for chronic therapy and for preventive treatment.101
Orally administered anti-CD3 monoclonal antibody is biologically active in the gut and suppressed experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis, in a dose-dependent fashion, both before disease induction and at the height of disease. Oral anti-CD3 antibody acts by inducing a unique type of Tregs characterized by latency-associated peptide (LAP) on its cell surface functioning via a TGF-β-dependent mechanism.101, 102 It suppressed the incidence of type 1 diabetes in an animal model via conversion of Th1 responses into Th2/Th3 in the periphery, including in pancreatic lymph nodes.103 Oral and nasal administration of anti-CD3 to a NZB and SNF-1 mouse model of lupus suppressed autoantibody production and prevented kidney damage.104, 105 Oral anti-CD3 alleviated type 2 diabetes and nonalcoholic steatohepatitis (NASH) in an animal model.15 Animals fed the anti-CD3 antibody with the gut adjuvant β-glucosylceramide (GC) showed reductions in pancreatic hyperplasia, hepatic fat accumulation and muscle inflammation; alleviation of type 2 diabetes; and reductions in liver enzymes and cholesterol and triglyceride levels.15 The effect of this type of oral immune therapy on the gut immune system was associated with the promotion of systemic CD4+LAP+ T cells, a decrease in NKT cells and an increase in TGF-β and IL-10 secretion from DCs and from anti-CD3-activated PBLs.
In mice with colitis, oral administration of anti-CD3 induced changes in the mucosal immune response that prevented colitis by affecting the systemic immune system.106 The effect was independent of a specific antigen and was associated with reduced T-cell activation in an IL-10-dependent manner. Oral anti-CD3 protected severe combined immunodeficient mice injected with CD4+CD45RBhigh T cells from colitis.106 No differences in total cell counts or percentages of CD4+ and forkhead box P3+ Tregs was noted between mice given anti-CD3 or controlled immunoglobulin. In mice with enteropathy, oral anti-CD3 reduced levels of inflammatory cytokines and increased levels of the anti-inflammatory cytokines IL-10 and TGF-β.106
In an open-label Phase I clinical trial comprising 18 healthy males, oral anti-CD3 was biologically active and well tolerated. No systemic treatment-related adverse effects were noted.107 Specifically, there was no change in the CD3+ lymphocyte count and no human anti-mouse antibodies were detected, implying non-absorption of the antibodies and a local effect on the gut immune system. The local effect was associated with an effect on the systemic immune system manifested by suppression of the Th1/Th17 and IFN-γ responses and increased CD4+CD25+ and CD8+CD25+ T cells.107 Treatment was associated with a decrease in IFN-γ and IL-17 and an increase in TGF-β secretion from anti-CD3-stimulated PBLs. In addition, decreased IL-23 and IL-6 expression and increased IL-10 and TGF expression in DCs, along with decreased IFN-γ and IL-17 secretion from anti-CD3-stimulated PBLs, and decreased IL-23 expression in DCs, were noted.107
In a Phase-IIa trial of patients with type 2 diabetes and NASH, oral administration of several dosages of anti-CD3 was biologically active and well tolerated without treatment-related adverse events. While exerting its effect on the gut immune system, it promoted Tregs systemically, manifested by an increase in CD4+LAP+ and CD4+CD25+LAP+ cells, with an increase in TGF-β serum levels. Decreases in plasma glucose and liver enzymes were noted in a dose-dependent manner.108
Taken together, the animal models and the human studies support an oral immune therapy effect on the systemic immune system for the non-absorbable anti-CD3. A lack of systemic absorption or an effect on leukocyte numbers supports the high safety profile of this method.
Oral administration of non-absorbable anti-lipopolysaccharide antibodies with adjuvants in humans and in an animal model of colitis
The gut microbiome and bacteria-derived products are relevant to the pathogenesis of IBD and can serve as targets for oral immune therapy. Imm124E is an IgG-enriched fraction of enterotoxigenic E. coli-containing colostrum that contains anti-lipopolysaccharide and several glycosphingolipid adjuvants (Immuron, Melbourne, VIC, Australia). Induction of oral immune therapy using the non-absorbable Imm124E formulation altered the systemic immune system. In the ob/ob model of diabetes and NASH, oral administration of Imm124E decreased serum TNF-α levels, and increased the number of splenic CD4+CD25+, CD4+CD25+Foxp3+ Tregs, and NKT cells. The effects on the systemic immune system were associated with a decrease in ALT serum levels and hepatic triglycerides, and improved glucose intolerance.109
In humans, in an open-label trial, subjects with biopsy-proven NASH and type 2 diabetes were orally administered Imm124E110 for 30 days. No treatment-related adverse events were observed and no human anti-bovine antibodies were detected. Increased serum levels of GLP-1 and adiponectin, along with the promotion of CD25+ and CD4+CD25+Foxp3+ Tregs, were noted. These effects on the systemic immune system were associated with the alleviation of insulin resistance as determined by a decrease in fasting glucose levels, an increase in early insulin secretion following glucose administration, and improvements in the glucose tolerance test and HBA1C levels. Treated patients showed a decrease in the serum levels of triglycerides, total cholesterol and low-density lipoprotein cholesterol, and a decrease in liver enzymes.110
In the mouse-TNBS colitis model, oral administration of Imm124E increased serum levels of the anti-inflammatory cytokine IL-10 and promoted both CD4+CD25+ and CD4+Foxp3+ Tregs. These effects were associated with an amelioration of body weight loss and improved bowel histology. The extent of the disease, and the inflammation, colitis damage and regeneration scores decreased in treated mice.111
The animal models and the human studies suggest that oral immune therapy using non-absorbable Imm124E alters the immune-mediated clinical manifestations by exerting a local effect on the gut immune system. The lack of absorption supports the high safety profile of this immune modulatory method.
Oral administration of non-absorbable glycosphingolipids in humans and in an animal model of colitis
Oral administration of β-glycosphingolipids has been tested as a means to alter NKT cells in immune-mediated disorders.112 Glucocerebroside (β-glucosylceramide, GC) and lactosylceramide are metabolic intermediates in the metabolic pathways of complex glycosphingolipids.113 Their immune modulatory effects were shown in several studies.112 GC inhibited NKT lymphocyte proliferation in the presence of DCs.114 It increased the peripheral/intrahepatic NKT lymphocyte ratio, decreased serum IFN-γ levels and increased serum IL-10 levels, exerting a beneficial immune modulatory effect alleviating inflammation in several animal models of immune-mediated disorders.44, 112, 114 In a leptin-deficient ob/ob mice model of diabetes and NASH, oral administration of GC altered NKT cell distribution and the cytokine profile in an anti-inflammatory direction.115 The effects were associated with a decrease in liver size and hepatic fat content, near-normalization of glucose tolerance and decreased serum triglyceride levels.115 A synergistic beneficial effect was noted for the combination of GC and lactosylceramide in animal models of diabetes and NASH.116, 117
In the TNBS colitis model, oral administration of GC led to an increased peripheral/intrahepatic CD4/CD8 lymphocyte ratio, decreased STAT-1 and -4 expression, and overexpression of STAT-6, along with decreased IFN-γ serum levels.44 The effects on the systemic immune system were associated with the alleviation of colitis manifested by improvement of both the macroscopic and microscopic scores.
In humans, oral administration of GC to patients with diabetes and NASH was tested in a randomized, double-blind placebo-controlled trial.118 No treatment-related adverse events were noted. An increase in peripheral NKT regulatory lymphocytes was observed. This effect was associated with a decrease in HBA1C levels, improvement in the glucose tolerance test, increased HDL levels and a decrease of hepatic fat by magnetic resonance imaging.118
The data suggest that non-absorbable glycosphingolipids exert an effect on the innate immune system of the gut thereby altering the systemic immune system.
Oral administration of non-absorbable PRX106 in an animal model of colitis
Parenteral administration of etanercept is successfully used in the treatment of rheumatoid arthritis but is not effective for the treatment of CD.119, 120, 121 In some cases, it was associated with exacerbation of the disease.122 A different effect on lamina propria lymphocytes was suggested as a possible explanation.123
PRX-106 is a non-absorbable orally administered BY-2 plant cell that expresses a recombinant anti-TNF fusion protein that consists of the soluble form of the human TNF receptor fused to the Fc component of a human antibody IgG1 domain. PRX-106 has an amino acid sequence identical to etanercept. In vitro, PRX-106 binds TNF alpha, inhibiting it from binding to cellular TNF receptors and blocking its downstream effects.124 Oral administration of BY-2 plant cells expressing PRX-106 resulted in altered distribution of hepatic Tregs with a significant alteration in the distribution of CD4+CD25+FOXP3+ cells and in CD8+CD25+FOXP3+ cells. A change in the spleen/liver ratio for CD4+CD25+FOXP3+ was noted. The effects on the systemic immune system of the non-absorbable formulation were associated with alleviation of immune-mediated colitis in a mouse model.124 Improvements in weight loss and of bowel histology were noted in the PRX-106-treated mice. A reduction in I-IkB-alpha phosphorylation was noted in colon samples, indicating a lower level of apoptosis in the inflamed tissues.
The data support the notion that oral administration of a non-absorbable formulation of plant cells expressing recombinant anti-TNF fusion protein has biological activity in the gut, and can alter the systemic immune system to exert a beneficial immune modulatory clinical effect. It may provide a new, safer and more effective anti-TNF alpha-based immune therapy for IBD.124
Oral administration of Lactococcus lactis secreting an anti-TNF nanobody in animal models of colitis
Lactococcus lactis is a lactic acid Gram-positive food-grade bacteria that is safely consumed. It was genetically engineered and orally formulated to deliver therapeutic proteins in the bowel for immune modulation.125 A tolerogenic live Lactococcus lactis bacteria was engineered for controlled secretion of the type 1 diabetes autoantigen GAD65370-575 and IL-10 in the gut. In combination with short-course low-dose anti-CD3, this treatment increased systemic CD4+Foxp3+CD25+ Tregs, improved insulitis and functional β-cell mass and restored glucose levels in recent-onset NOD mice.126 Similarly, Lactococcus lactis was engineered to secrete monovalent and bivalent murine (m)TNF-neutralizing nanobodies. Its oral administration resulted in local delivery of anti-mTNF nanobodies at the colon and significantly reduced inflammation in mice with dextran sulfate sodium-induced chronic colitis, and improved established enterocolitis in IL-10−/− mice.127 The data supports the concept that systemic tolerogenic effects can be achieved via an effect on the gut immune system.
Nutraceuticals, functional foods, prebiotics, probiotics, polyunsaturated fatty acids, amino acids and polyphenols, and other gut-associated adjuvants, can re-establish gut tolerance by altering the gut immune system and/or via modulation of intestinal microbiota
Amino acids (glutamine, arginine, tryptophan and citrulline), fatty acids (short-chain and omega-3 fatty acids and conjugated linoleic acids) and probiotics (Bifidobacterium, Saccharomyces and Lactobacillus) can restore the intestinal barrier, supporting gut barrier integrity and function.128 Probiotics prevent pathogen adherence and invasion of the epithelium by blocking adherence sites and upregulating the gene expression of MUC2 and antimicrobial peptides.129 Probiotics restore eubiosis and potentially restore the deleterious effects of bacterial metabolites and of unabsorbed dietary constituents with the production of free radicals and phenols associated with cell damage. Probiotics affect the innate inflammatory response of epithelial cells to stimuli from the gut lumen reducing inflammation. They exert an effect on DCs and on epithelial cells to affect naive T lymphocytes in the lamina propria, thereby affecting adaptive immunity.129, 130
In both CD and ulcerative colitis, the advantage of probiotics remains unproven.131 Some studies, however, did show a beneficial effect. E. coli Nissle 1917 maintained remission, suggesting that it can serve as an alternative in patients intolerant or resistant to 5-aminosalicylic acid preparations. In pouchitis, small controlled trials suggest a benefit from VSL no. 3 in the primary and secondary prevention of pouchitis.131 Taken together, the data support the possibility that probiotics and prebiotics may alter the systemic immune system via a gut signal.
Summary
Oral immune therapy may overcome many of the obstacles of the agents currently used in the treatment of IBD. Oral immune therapy methods are based on the exertion of an effect on the gut innate immune system and/or on the microbiome, thereby inducing an immune signal that can alter the systemic immune system. Oral immune therapy provides a means for immune modulation with minimal side effects, as it does not involve generalized immune suppression. While overcoming the adverse events barrier, large clinical trials are required to show the efficacy of these treatments in IBD. In the future, this type of immune modulation may present an alternative to the currently used immune suppressive and immune modulatory agents.
Acknowledgments
This work was supported in part by a grant from The Roman-Epstein Research Foundation (YI).
Footnotes
YI is the medical director of Immuron and a consultant for Teva, Enzo Biochem, Protalix, Therapix, Nasvax and Natural Shield.
References
- 1Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol 2014; 20: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Lefrancois L, Puddington L. Intestinal and pulmonary mucosal T cells: local heroes fight to maintain the status quo. Annu Rev Immunol 2006; 24: 681–704. [DOI] [PubMed] [Google Scholar]
- 3van der Heijden PJ, Stok W, Bianchi AT. Contribution of immunoglobulin-secreting cells in the murine small intestine to the total 'background' immunoglobulin production. Immunology 1987; 62: 551–555. [PMC free article] [PubMed] [Google Scholar]
- 4Guy-Grand D, Azogui O, Celli S, Darche S, Nussenzweig MC, Kourilsky P et al. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J Exp Med 2003; 197: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Wang X, Sherman A, Liao G, Leong KW, Daniell H, Terhorst C et al. Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Deliv Rev 2013; 65: 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Castro-Sanchez P, Martin-Villa JM. Gut immune system and oral tolerance. Br J Nutr 2013; 109 (Suppl 2): S3–11. [DOI] [PubMed] [Google Scholar]
- 7Faria AM, Weiner HL. Oral tolerance. Immunol Rev 2005; 206: 232–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Pabst O, Herbrand H, Friedrichsen M, Velaga S, Dorsch M, Berhardt G et al. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol 2006; 177: 6824–6832. [DOI] [PubMed] [Google Scholar]
- 9Pabst R, Rothkotter HJ. Structure and function of the gut mucosal immune system. Adv Exp Med Biol 2006; 579: 1–14. [DOI] [PubMed] [Google Scholar]
- 10Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol 2006; 13: 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Mizrahi M, Ilan Y. The gut mucosa as a site for induction of regulatory T-cells. Curr Pharm Des 2009; 15: 1191–1202. [DOI] [PubMed] [Google Scholar]
- 12Ilan Y. Oral tolerance: can we make it work? Hum Immunol 2009; 70: 768–776. [DOI] [PubMed] [Google Scholar]
- 13Shlomai A, Trop S, Gotsman I, Jurim O, Diment J, Alper R et al. Immunomodulation of experimental colitis: the role of NK1.1 liver lymphocytes and surrogate antigens—bystander effect. J Pathol 2001; 195: 498–507. [DOI] [PubMed] [Google Scholar]
- 14Brandtzaeg P, Pabst R. Let's go mucosal: communication on slippery ground. Trends Immunol 2004; 25: 570–577. [DOI] [PubMed] [Google Scholar]
- 15Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA 2010; 107: 9765–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Nguyen DL, Flores S, Sassi K, Bechtold ML, Nguyen ET, Parekh NK. Optimizing the use of anti-tumor necrosis factor in the management of patients with Crohn's disease. Ther Adv Chronic Dis 2015; 6: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Goel RM, Blaker P, Mentzer A, Fong SC, Marinaki AM, Sanderson JD. Optimizing the use of thiopurines in inflammatory bowel disease. Ther Adv Chronic Dis 2015; 6: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Moss AC. Optimizing the use of biological therapy in patients with inflammatory bowel disease. Gastroenterol Rep 2015; 3: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Mann ER, Landy JD, Bernardo D, Peake ST, Hart AL, Al-Hassi HO et al. Intestinal dendritic cells: their role in intestinal inflammation, manipulation by the gut microbiota and differences between mice and men. Immunol Lett 2013; 150: 30–40. [DOI] [PubMed] [Google Scholar]
- 20Brucklacher-Waldert V, Carr EJ, Linterman MA, Veldhoen M. Cellular Plasticity of CD4+ T Cells in the Intestine. Front Immunol 2014; 5: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Kurashima Y, Goto Y, Kiyono H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur J Immunol 2013; 43: 3108–3115. [DOI] [PubMed] [Google Scholar]
- 22Magrone T, Jirillo E. The interplay between the gut immune system and microbiota in health and disease: nutraceutical intervention for restoring intestinal homeostasis. Curr Pharm Des 2013; 19: 1329–1342. [DOI] [PubMed] [Google Scholar]
- 23Foersch S, Waldner MJ, Neurath MF. Innate and adaptive immunity in inflammatory bowel diseases. Dig Dis 2013; 31: 317–320. [DOI] [PubMed] [Google Scholar]
- 24Rescigno M. Dendritic cell-epithelial cell crosstalk in the gut. Immunol Rev 2014; 260: 118–128. [DOI] [PubMed] [Google Scholar]
- 25Wittkopf N, Neurath MF, Becker C. Immune-epithelial crosstalk at the intestinal surface. J Gastroenterol 2014; 49: 375–387. [DOI] [PubMed] [Google Scholar]
- 26Randall-Demllo S, Chieppa M, Eri R. Intestinal epithelium and autophagy: partners in gut homeostasis. Front Immunol 2013; 4: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis 2014; 32: 475–483. [DOI] [PubMed] [Google Scholar]
- 28Pelaseyed T, Bergstrom JH, Gustafsson JK, Ermund A, Birchenough GM, Schutte A et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 2014; 260: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Ghishan FK, Kiela PR. Epithelial transport in inflammatory bowel diseases. Inflamm Bowel Dis 2014; 20: 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 2015; 13: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut 2013; 62: 1653–1664. [DOI] [PubMed] [Google Scholar]
- 32Dupaul-Chicoine J, Dagenais M, Saleh M. Crosstalk between the intestinal microbiota and the innate immune system in intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 2227–2237. [DOI] [PubMed] [Google Scholar]
- 33Siggers RH, Hackam DJ. The role of innate immune-stimulated epithelial apoptosis during gastrointestinal inflammatory diseases. Cell Mol Life Sci 2011; 68: 3623–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest 2009; 119: 2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Ng SC, Kamm MA, Stagg AJ, Knight SC. Intestinal dendritic cells: their role in bacterial recognition, lymphocyte homing, and intestinal inflammation. Inflamm Bowel Dis 2010; 16: 1787–1807. [DOI] [PubMed] [Google Scholar]
- 36Magnusson MK, Wick MJ. Intestinal dendritic cell and macrophage subsets: tipping the balance to Crohn's disease? Eur J Microbiol Immunol 2011; 1: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Rutella S, Locatelli F. Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol 2011; 17: 3761–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Monteiro M, Graca L. iNKT cells: innate lymphocytes with a diverse response. Crit Rev Immunol 2014; 34: 81–90. [DOI] [PubMed] [Google Scholar]
- 39Middendorp S, Nieuwenhuis EE. NKT cells in mucosal immunity. Mucosal Immunol 2009; 2: 393–402. [DOI] [PubMed] [Google Scholar]
- 40Zeissig S, Blumberg RS. Commensal microbial regulation of natural killer T cells at the frontiers of the mucosal immune system. FEBS Lett 2014; 588: 4188–4194. [DOI] [PubMed] [Google Scholar]
- 41Zeissig S, Blumberg RS. Commensal microbiota and NKT cells in the control of inflammatory diseases at mucosal surfaces. Curr Opin Immunol 2013; 25: 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Liao CM, Zimmer MI, Wang CR. The functions of type I and type II natural killer T cells in inflammatory bowel diseases. Inflamm Bowel Dis 2013; 19: 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Trop S, Samsonov D, Gotsman I, Alper R, Diment J, Ilan Y. Liver-associated lymphocytes expressing NK1.1 are essential for oral immune tolerance induction in a murine model. Hepatology 1999; 29: 746–755. [DOI] [PubMed] [Google Scholar]
- 44Zigmond E, Preston S, Pappo O, Lalazar G, Margalit M, Shalev Z et al. Beta-glucosylceramide: a novel method for enhancement of natural killer T lymphoycte plasticity in murine models of immune-mediated disorders. Gut 2007; 56: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Ilan Y, Weksler-Zangen S, Ben-Horin S, Diment J, Sauter B, Rabbani E et al. Treatment of experimental colitis by oral tolerance induction: a central role for suppressor lymphocytes. Am J Gastroenterol 2000; 95: 966–973. [DOI] [PubMed] [Google Scholar]
- 46Gotsman I, Shlomai A, Alper R, Rabbani E, Engelhardt D, Ilan Y. Amelioration of immune-mediated experimental colitis: tolerance induction in the presence of preexisting immunity and surrogate antigen bystander effect. J Pharmacol Exp Ther 2001; 297: 926–932. [PubMed] [Google Scholar]
- 47Israeli E, Safadi R, Melhem A, Pappo O, Shibolet O, Klein A et al. Induction of oral immune regulation towards liver-extracted proteins for treatment of chronic HBV and HCV hepatitis: results of a phase I clinical trial. Liver Int 2004; 24: 295–307. [DOI] [PubMed] [Google Scholar]
- 48Kim YJ, Chang SY, Ko HJ. Myeloid-derived suppressor cells in inflammatory bowel disease. Intest Res 2015; 13: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Shalev I, Schmelzle M, Robson SC, Levy G. Making sense of regulatory T cell suppressive function. Semin Immunol 2011; 23: 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Pesenacker AM, Broady R, Levings MK. Control of tissue-localized immune responses by human regulatory T cells. Eur J Immunol 2015; 45: 333–343. [DOI] [PubMed] [Google Scholar]
- 51Gibson DJ, Ryan EJ, Doherty GA. Keeping the bowel regular: the emerging role of Treg as a therapeutic target in inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 2716–2724. [DOI] [PubMed] [Google Scholar]
- 52Pabst O. Trafficking of regulatory T cells in the intestinal immune system. Int Immunol 2013; 25: 139–143. [DOI] [PubMed] [Google Scholar]
- 53Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 2009; 27: 313–338. [DOI] [PubMed] [Google Scholar]
- 54Bollrath J, Powrie FM. Controlling the frontier: regulatory T-cells and intestinal homeostasis. Semin Immunol 2013; 25: 352–357. [DOI] [PubMed] [Google Scholar]
- 55Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol 2011; 4: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Hardenberg G, Steiner TS, Levings MK. Environmental influences on T regulatory cells in inflammatory bowel disease. Semin Immunol 2011; 23: 130–138. [DOI] [PubMed] [Google Scholar]
- 57Mayne CG, Williams CB. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 1772–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58Ai TL, Solomon BD, Hsieh CS. T-cell selection and intestinal homeostasis. Immunol Rev 2014; 259: 60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Xu XR, Liu CQ, Feng BS, Liu ZJ. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol 2014; 20: 3255–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60Galvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm 20142014, 928461. [DOI] [PMC free article] [PubMed]
- 61Cerovic V, Bain CC, Mowat AM, Milling SW. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol 2014; 35: 270–277. [DOI] [PubMed] [Google Scholar]
- 62Bain CC, Mowat AM. The monocyte-macrophage axis in the intestine. Cell Immunol 2014; 291: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev 2014; 260: 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog 2013; 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65Kuhn KA, Stappenbeck TS. Peripheral education of the immune system by the colonic microbiota. Semin Immunol 2013; 25: 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Kamada N, Nunez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology 2014; 146: 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Almeida J, Galhenage S, Yu J, Kurtovic J, Riordan SM. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol 2006; 12: 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis 2010; 28: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Koboziev I, Reinoso Webb C, Furr KL, Grisham MB. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Rad Biol Med 2014; 68: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol 2013; 11: 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71Dalal SR, Chang EB. The microbial basis of inflammatory bowel diseases. J Clin Invest 2014; 124: 4190–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72Klapproth JM, Sasaki M. Bacterial induction of proinflammatory cytokines in inflammatory bowel disease. Inflamm Bowel Dis 2010; 16: 2173–2179. [DOI] [PubMed] [Google Scholar]
- 73Tawfik A, Flanagan PK, Campbell BJ. Escherichia coli-host macrophage interactions in the pathogenesis of inflammatory bowel disease. World J Gastroenterol 2014; 20: 8751–8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74Shapiro JM, Cho JH, Sands BE, LeLeiko NS. Bridging the gap between host immune response and intestinal dysbiosis in inflammatory bowel disease: does immunoglobulin A mark the spot? Clin Gastroenterol Hepatol 2015; 13: 842–846. [DOI] [PubMed] [Google Scholar]
- 75Strober W. Impact of the gut microbiome on mucosal inflammation. Trends Immunol 2013; 34: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76MacDonald TT, Vossenkaemper A, Fantini M, Monteleone G. Reprogramming the immune system in IBD. Dig Dis 2012; 30: 392–395. [DOI] [PubMed] [Google Scholar]
- 77Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2014; 8: 1569–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78Ma HD, Wang YH, Chang C, Gershwin ME, Lian ZX. The intestinal microbiota and microenvironment in liver. Autoimmun Rev 2015; 14: 183–191. [DOI] [PubMed] [Google Scholar]
- 79Navaneethan U. Hepatobiliary manifestations of ulcerative colitis: an example of gut-liver crosstalk. Gastroenterology report 2014; 2: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80Visschers RG, Luyer MD, Schaap FG, Olde Damink SW, Soeters PB. The gut-liver axis. Curr Opin Clin Nutr Metab Care 2013; 16: 576–581. [DOI] [PubMed] [Google Scholar]
- 81Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology 2014; 59: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82Seidel D, Eickmeier I, Kuhl AA, Hamann A, Loddenkemper C, Schott E. CD8 T cells primed in the gut-associated lymphoid tissue induce immune-mediated cholangitis in mice. Hepatology 2014; 59: 601–611. [DOI] [PubMed] [Google Scholar]
- 83Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011; 474: 298–306. [DOI] [PubMed] [Google Scholar]
- 84Israeli E, Goldin E, Shibolet O, Klein A, Hemed N, Engelhardt D et al. Oral immune regulation using colitis extracted proteins for treatment of Crohn's disease: results of a phase I clinical trial. World J Gastroenterol 2005; 11: 3105–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85Margalit M, Israeli E, Shibolet O, Zigmond E, Klein A, Hemed N et al. A double-blind clinical trial for treatment of Crohn's disease by oral administration of Alequel, a mixture of autologous colon-extracted proteins: a patient-tailored approach. Am J Gastroenterol 2006; 101: 561–568. [DOI] [PubMed] [Google Scholar]
- 86Israeli E, Zigmond E, Lalazar G, Klein A, Hemed N, Goldin E et al. Oral mixture of autologous colon-extracted proteins for the Crohn's disease: a double-blind trial. World J Gastroenterol 2015; 21: 5685–5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87Israeli ED, Ilan A, Y, Long-term Y. Efect of short-term oral administration of a mixture of autologous proteins extracted from the colon of patients with Crohn's disease: a memory effect of oral tolerance induction. J Gastroenterol Hepatol 2015; 2: 12. [Google Scholar]
- 88Waters OR, Lawrance IC. Understanding the use of immunosuppressive agents in the clinical management of IBD. Curr Drug Targets 2011; 12: 1364–1371. [DOI] [PubMed] [Google Scholar]
- 89Gilissen LP, Wong DR, Engels LG, Bierau J, Bakker JA, Paulussen AD et al. Therapeutic drug monitoring of thiopurine metabolites in adult thiopurine tolerant IBD patients on maintenance therapy. J Crohns Colitis 2012; 6: 698–707. [DOI] [PubMed] [Google Scholar]
- 90Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD et al. Postoperative maintenance of Crohn's disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology 2004; 127: 723–729. [DOI] [PubMed] [Google Scholar]
- 91Mate-Jimenez J, Hermida C, Cantero-Perona J, Moreno-Otero R. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol 2000; 12: 1227–1233. [DOI] [PubMed] [Google Scholar]
- 92Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology 2000; 119: 895–902. [DOI] [PubMed] [Google Scholar]
- 93Amin J, Huang B, Yoon J, Shih DQ. Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm Bowel Dis 2015; 21: 445–452. [DOI] [PubMed] [Google Scholar]
- 94Israeli E, Goldin E, Fishman S, Konikoff F, Lavy A, Chowers Y et al. Oral administration of non-absorbable delayed release 6-mercaptopurine is locally active in the gut, exerts a systemic immune effect and alleviates Crohn's disease with low rate of side effects: results of double blind Phase II clinical trial. Clin Exp Immunol 2015; 181: 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95Chatenoud L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol 2003; 3: 123–132. [DOI] [PubMed] [Google Scholar]
- 96Chatenoud L, Waldmann H. CD3 monoclonal antibodies: a first step towards operational immune tolerance in the clinic. Rev Diabet Stud 2012; 9: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97Yu QT, Saruta M, Papadakis KA. Visilizumab induces apoptosis of mucosal T lymphocytes in ulcerative colitis through activation of caspase 3 and 8 dependent pathways. Clin Immunol 2008; 127: 322–329. [DOI] [PubMed] [Google Scholar]
- 98Vossenkamper A, Hundsrucker C, Page K, van Maurik A, Sanders TJ, Stagg AJ et al. A CD3-specific antibody reduces cytokine production and alters phosphoprotein profiles in intestinal tissues from patients with inflammatory bowel disease. Gastroenterology 2014; 147: 172–183. [DOI] [PubMed] [Google Scholar]
- 99Dean Y, Depis F, Kosco-Vilbois M. Combination therapies in the context of anti-CD3 antibodies for the treatment of autoimmune diseases. Swiss Med Wkly 2012; 142: w13711. [DOI] [PubMed] [Google Scholar]
- 100Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev 2011; 241: 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso A et al. New immunosuppressive approaches: oral administration of CD3-specific antibody to treat autoimmunity. J Neurol Sci 2008; 274: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25- LAP+ T cells. Nat Med 2006; 12: 627–635. [DOI] [PubMed] [Google Scholar]
- 103Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes 2007; 56: 2103–2109. [DOI] [PubMed] [Google Scholar]
- 104Wu HY, Center EM, Tsokos GC, Weiner HL. Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25-LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells. Lupus 2009; 18: 586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J Immunol 2008; 181: 6038–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106Forster K, Goethel A, Chan CW, Zanello G, Streutker C, Croitoru K. An oral CD3-specific antibody suppresses T-cell-induced colitis and alters cytokine responses to T-cell activation in mice. Gastroenterology 2012; 143: 1298–1307. [DOI] [PubMed] [Google Scholar]
- 107Ilan Y, Zigmond E, Lalazar G, Dembinsky A, Ben Ya'acov A, Hemed N et al. Oral administration of OKT3 monoclonal antibody to human subjects induces a dose-dependent immunologic effect in T cells and dendritic cells. J Clin Immunol 2010; 30: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108Lalazar G, Mizrahi M, Turgeman I, Adar T, Ben Ya'acov A, Shabat Y et al. Oral administration of OKT3 MAb to patients with NASH, promotes regulatory T-cell induction, and alleviates insulin resistance: results of a phase IIa blinded placebo-controlled trial. J Clin Immunol 2015; 35: 399–407. [DOI] [PubMed] [Google Scholar]
- 109Adar T, Ben Ya'acov A, Lalazar G, Lichtenstein Y, Nahman D, Mizrahi M et al. Oral administration of immunoglobulin G-enhanced colostrum alleviates insulin resistance and liver injury and is associated with alterations in natural killer T cells. Clin Exp Immunol 2012; 167: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110Mizrahi MLG, Shabat Y, Adar T, Ben Ya'acov A, Ilan Y. Alleviation of insulin resistance and liver damage by oral administration of etec colostrums is mediated by increased GLP-1, adiponectin serum levels and tregs: results of a phase I/II clinical trial in NASH. Hepatology 2010; 52: 163A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111Ya'acov AB, Lichtenstein Y, Zolotarov L, Ilan Y. The gut microbiome as a target for regulatory T cells-based immunotherapy: induction of regulatory lymphocytes by oral administration of anti-LPS enriched colostrum alleviates immune mediated colitis. BMC Gastroenterol 2015; 15: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112Ilan Y, Elstein D, Zimran A. Glucocerebroside: an evolutionary advantage for patients with Gaucher disease and a new immunomodulatory agent. Immunol Cell Biol 2009; 87: 514–524. [DOI] [PubMed] [Google Scholar]
- 113Radin NS, Inokuchi J. Glucosphingolipids as sites of action in the chemotherapy of cancer. Biochem Pharmacol 1988; 37: 2879–2886. [DOI] [PubMed] [Google Scholar]
- 114Margalit M, Ghazala SA, Alper R, Elinav E, Klein A, Doviner V et al. Glucocerebroside treatment ameliorates ConA hepatitis by inhibition of NKT lymphocytes. Am J Physiol Gastrointest Liver Physiol 2005; 289: G917–G925. [DOI] [PubMed] [Google Scholar]
- 115Margalit M, Shalev Z, Pappo O, Sklair-Levy M, Alper R, Gomori M et al. Glucocerebroside ameliorates the metabolic syndrome in OB/OB mice. J Pharmacol Exp Ther 2006; 319: 105–110. [DOI] [PubMed] [Google Scholar]
- 116Zigmond E, Tayer-Shifman O, Lalazar G, Ben Ya'acov A, Weksler-Zangen S, Shasha D et al. beta-glycosphingolipids ameliorated non-alcoholic steatohepatitis in the Psammomys obesus model. J Inflamm Res 2014; 7: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117Zigmond E, Zangen SW, Pappo O, Sklair-Levy M, Lalazar G, Zolotaryova L et al. Beta-glycosphingolipids improve glucose intolerance and hepatic steatosis of the Cohen diabetic rat. Am J Physiol Endocrinol Metab 2009; 296: E72–E78. [DOI] [PubMed] [Google Scholar]
- 118Lalazar GZE, Zangen SW, Pappo O, Levy Sklair M, Hemed N, Raz I et al. Treatment of insulin rsistance and non-alcoholic steatohepatitis by administration of beta glucosylceramide controlled trial. Hepatology 2009; 50: 200A (Suppl 204). [Google Scholar]
- 119Scott LJ. Etanercept: a review of its use in autoimmune inflammatory diseases. Drugs 2014; 74: 1379–1410. [DOI] [PubMed] [Google Scholar]
- 120Olivieri I, D'Angelo S, Palazzi C, Padula A. Advances in the management of psoriatic arthritis. Nat Rev Rheumatol 2014; 10: 531–542. [DOI] [PubMed] [Google Scholar]
- 121Armuzzi A, Lionetti P, Blandizzi C, Caporali R, Chimenti S, Cimino L et al. anti-TNF agents as therapeutic choice in immune-mediated inflammatory diseases: focus on adalimumab. Int J Immunopathol Pharmacol 2014; 27 (1 Suppl): 11–32. [DOI] [PubMed] [Google Scholar]
- 122Varkas G, Van Praet L, Cypers H, Elewaut D. Spondyloarthritis and inflammatory bowel disease. Comorbidity and treatment implications. Z Rheumatol 2013; 72: 524–529. [DOI] [PubMed] [Google Scholar]
- 123Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I et al. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology 2003; 124: 1774–1785. [DOI] [PubMed] [Google Scholar]
- 124Shaaltiel Y, Ya'acov AB, Shabbat Y, Zolotarov L, Gingis-Velitski S, Almon E et al. A novel method for anti-TNF based-oral immunotherapy: oral administration of a plant cell-expressed recombinant anti-TNF fusion protein for treating of Crohn's disease. Gastroenterology 2014; 146: S-901. [Google Scholar]
- 125Rottiers P, De Smedt T, Steidler L. Modulation of gut-associated lymphoid tissue functions with genetically modified Lactococcus lactis. Int Rev Immunol 2009; 28: 465–486. [DOI] [PubMed] [Google Scholar]
- 126Robert S, Gysemans C, Takiishi T, Korf H, Spagnuolo I, Sebastiani G et al. Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes 2014; 63: 2876–2887. [DOI] [PubMed] [Google Scholar]
- 127Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol 2010; 3: 49–56. [DOI] [PubMed] [Google Scholar]
- 128Andrade ME, Araujo RS, de Barros PA, Soares AD, Abrantes FA, Generoso SV et al. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin Nutr 2015; 34: 1080–1087. [DOI] [PubMed] [Google Scholar]
- 129Ramakrishna BS. Probiotic-induced changes in the intestinal epithelium: implications in gastrointestinal disease. Trop Gastroenterol 2009; 30: 76–85. [PubMed] [Google Scholar]
- 130Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 2009; 15: 300–310. [DOI] [PubMed] [Google Scholar]
- 131Sinagra E, Tomasello G, Cappello F, Leone A, Cottone M, Bellavia M et al. Probiotics, prebiotics and symbiotics in inflammatory bowel diseases: state-of-the-art and new insights. J Biol Regul Homeost Agents 2013; 27: 919–933. [PubMed] [Google Scholar]