As a different entity of diffuse large B-cell lymphoma (DLBCL) according to the current World Health Organization classification,1 primary mediastinal large B-cell lymphoma (PMBL) is different from DLBCL in terms of clinical, immunohistochemical and genetic features.2 Despite a remarkable advance with first-line treatment of PMBL patients in the rituximab era,3, 4, 5, 6, 7 10–30% of patients still experience progression or relapse. Although patients with relapsed or refractory PMBL are often treated with high-dose therapy followed by autologous stem cell transplantation (HDT/ASCT) after salvage treatment, the progression-free survival (PFS) at 5 years of 27% is unsatisfactory compared with DLBCL in the pre-rituximab era.8, 9, 10 Moreover, information regarding outcomes after HDT/ASCT for relapsed or refractory PMBL is limited in the rituximab era. Therefore, clarifying the current role of HDT/ASCT is vital to establish the optimal treatment strategy.
Recently, we published the results of a multicenter retrospective study for newly diagnosed PMBL patients in Japan and described the outcome following first-line treatment.6 This report describes subgroup analyses of our recent retrospective study, focusing on the primary end point for patients treated with HDT/ASCT after relapse or refractory disease to clarify the clinical outcomes and the role of HDT/ASCT for PMBL patients with relapsed or refractory disease. Detailed information about the patients, data collection and central pathological review of our analysis were described previously.6 Information about treatment and assessment for patients with relapse and refractory disease and statistical method are also described in Supplementary Method 1. The study protocol was approved by the Institutional Review Board of Nagoya Daini Red Cross Hospital (where this study was organized) and each participating hospital based on the Ethical Guideline for Epidemiologic Research from the Ministry of Health, Labor and Welfare in Japan. The study complied with all the provisions of the Declaration of Helsinki.
We identified a total of 44 PMBL patients treated with HDT/ASCT after first relapse or primary refractory disease between 1996 and 2012, and retrospectively analyzed. Patient characteristics are summarized in Table 1. The median time from initial diagnosis to the first relapse or refractory disease was 8 months. Relapse or refractory disease occurred <12 months from initial diagnosis in 66% of patients. The patients with primary refractory disease comprised 41% of the population. The median age at relapse was 26.5 (range, 17–59) years, and female patients were predominant (59%). Stage I/II at relapse was also predominant (60%). Of 44 PMBL patients with relapse or refractory disease, 34 (79%) and 2 (5%) patients had a relapse in the mediastinum or central nervous system, respectively. Twenty-nine patients (66%) had received rituximab-containing chemotherapy as the first-line treatment. Ten patients (23%) had received radiotherapy (RT) as part of the first-line treatment. Eleven patients (25%) had received RT as part of the second-line treatment.
Table 1. Patient characteristics.
| Characteristics |
HDT/ASCT |
Chemo-sensitive |
Chemo-refractory |
||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | P-value | |
| No. of patients | 44 | 100 | 30 | 68 | 14 | 32 | |
| Age at relapse (years) | |||||||
| Median | 26.5 | 29.5 | 26.5 | ||||
| Range | 17–59 | 19–59 | 17–48 | ||||
| ⩾30 years | 14 | 32 | 10 | 33 | 4 | 12 | >0.99 |
| Sex | |||||||
| Male | 18 | 41 | 12 | 40 | 6 | 43 | 0.748 |
| Female | 26 | 59 | 18 | 60 | 8 | 57 | |
| Stage at relapse | |||||||
| I/II | 26 | 60 | 8 | 27 | 9 | 69 | 0.016 |
| Relapse at mediastinum | 34 | 79 | 23 | 77 | 11 | 85 | 0.699 |
| CNS relapse | 2 | 5 | 2 | 6.6 | 0 | 0 | >0.99 |
| PS at diagnosis | |||||||
| ⩾2 | 14 | 33 | 9 | 31 | 5 | 38 | 0.729 |
| LDH at diagnosis | |||||||
| Greater than ULN | 36 | 88 | 25 | 86 | 11 | 92 | |
| Extranodal sites >1 | 10 | 24 | 8 | 28 | 2 | 17 | 0.694 |
| IPI at diagnosis | |||||||
| IPI ⩾3 | 9 | 22 | 5 | 17 | 4 | 33 | 0.408 |
| Low | 17 | 41 | 12 | 43 | 5 | 38 | |
| Low intermediate | 15 | 37 | 11 | 39 | 4 | 31 | |
| High intermediate | 7 | 17 | 3 | 11 | 4 | 31 | |
| High | 2 | 5 | 2 | 7 | 0 | 0 | |
| Bulky tumor at diagnosis, cm | |||||||
| ⩾10 | 26 | 70 | 19 | 73 | 7 | 64 | 0.699 |
| Presence of pleural or pericardial effusion at diagnosis | |||||||
| Yes | 26 | 60 | 18 | 62 | 8 | 57 | >0.99 |
| Rituximab-containing therapy as first-line treatment | 33 | 72 | 22 | 54 | |||
| Yes | 29 | 66 | 22 | 73 | 7 | 50 | 0.177 |
| Prior RT as first-line treatment | |||||||
| Yes | 10 | 23 | 8 | 27 | 2 | 14 | 0.462 |
| First-line treatment | |||||||
| R-CHOP | 27 | 63 | 21 | 70 | 6 | 43 | 0.107 |
| CHOP | 12 | 28 | 7 | 23 | 5 | 36 | 0.475 |
| The second-/third-generation regimens | 4 | 9 | 2 | 7 | 2 | 14 | 0.581 |
| Primary refractory disease | |||||||
| Yes | 18 | 41 | 10 | 33 | 8 | 57 | 0.191 |
| Relapse time | |||||||
| Relapse <12 months | 29 | 66 | 19 | 63 | 10 | 71 | 0.738 |
| Relapse ⩾12 months | 15 | 34 | 2 | 22 | 12 | 32 | |
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine and prednisolone; CNS, central nervous system; HDT/ASCT, high-dose chemotherapy followed by autologous stem cell transplantation; IPI, international prognostic index; LDH, lactate dehydrogenase; PS, performance status; R, rituximab; RT, radiotherapy; ULN, upper limit of normal.
As a salvage regimen, a high-dose (⩾2 g/m2) cytarabine-based regimen and an ICE (ifosfamide, etoposide and carboplatin)-based regimen were used in 49% and 19% of patients, respectively. As conditioning regimen, the BEAM (carmustine, etoposide, cytarabine and melphalan)-based protocol was the most frequently used (41%), followed by the MCEC (ranimustine, cyclophosphamide, etoposide and carboplatin)-based regimen11 (25%) and the LEED regimen (cyclophosphamide, etoposide, melphalan and dexamethasone)12 (20%).
Patient characteristics according to chemo-sensitivity are shown in Table 1. Advanced-stage patients were significantly predominant in chemo-refractory group than the chemo-sensitive group (69% vs 27%, P=0.016). No other significant differences were found between the two groups.
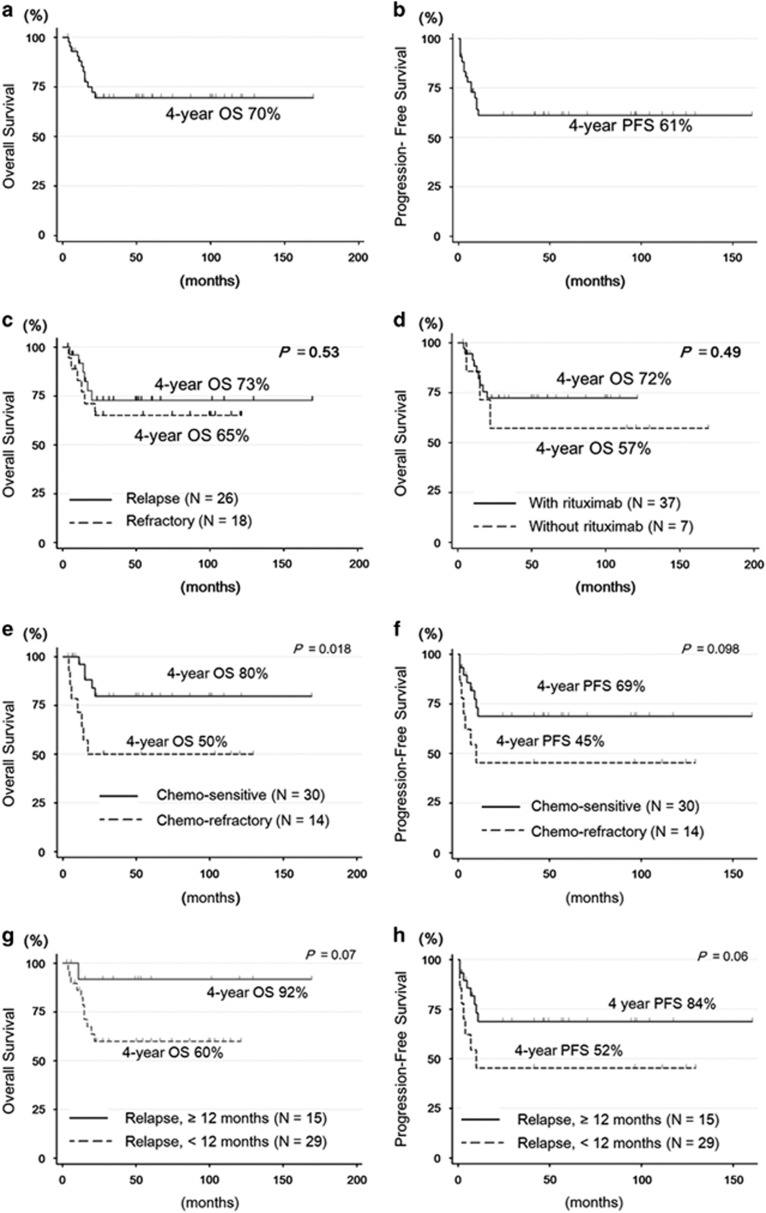
The overall response rate after HDT/ASCT was 77.2% (complete remission, 63.6%). With a median follow-up of 53.5 months in surviving patients, the overall survival (OS) and PFS at 4 years were 70% and 61%, respectively (Figures 1a and b). The median OS after relapse or progression was not reached. The OS at 4 years was 73% in relapsed patients and 65% in patients with primary refractory disease (P=0.53, Figure 1c). OS did not significantly differ between patients irrespective of rituximab-containing salvage treatment (P=0.49, Figure 1d).
Figure 1.
Survival after first relapse or progression in PMBL patients treated with HDT/ASCT. (a) OS of all patients, (b) PFS of all patients, (c) OS according to disease status, (d) OS according to rituximab-containing treatment, (e) OS according to chemotherapy responsiveness at transplantation, (f) PFS according to chemotherapy responsiveness at transplantation, (g) OS according to relapse <12 months after diagnosis and (h) PFS according to relapse <12 months after diagnosis.
A chemo-refractory relapse (N=13) was associated with a shorter OS and PFS when compared with chemo-sensitive relapse (N=31; 4-year OS: 80% vs 50%, chemo-sensitive vs chemo-refractory, respectively, P=0.018, Figure 1e; 4-year PFS: 69% vs 45%, P=0.098, Figure 1f). Meanwhile, patients who experienced a late relapse after 12 months from initial treatment (N=15) showed excellent outcomes when compared with those who experienced an early relapse (N=29) (4-year OS: 60% vs 92%, early relapse vs late relapse, respectively, P=0.07; Figure 1g; 4-year PFS: 52% vs 84%, P=0.06; Figure 1h). For five patients who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) as salvage treatment after HDT/ASCT (N=5), about half the patients achieved a durable response with allo-HSCT (Supplementary Figure S1).
Regarding non-relapse mortality after HDT/ASCT, one patient died <100 days as a result of toxicity from transplantation due to infection. Second malignancy developed in one patient after 14 months from HDT/ASCT.
Analysis of prognostic factors for PFS and OS in PMBL patients with relapsed or refractory disease is shown in Supplementary Table S1. Although there was a trend for shorter OS and PFS for patients with early relapse <12 months (P=0.097 and 0.097, respectively), no significant prognostic factor was identified in this study. Prior rituximab treatment and RT did not affect the survival of PMBL patients with relapsed or refractory disease in this study.
The present study demonstrated that HDT/ASCT was effective and could be curative after relapse or refractory disease in a substantial number of PMBL patients. In a past study, PMBL patients with relapsed or refractory disease had inferior outcomes compared with DLBCL patients.8 However, the OS in PMBL patients treated with HDT/ASCT after relapsed or refractory disease in this study was comparable to that in DLBCL patients with relapsed or refractory disease.13 In the absence of randomized trials, a relatively large retrospective study, such as the present study, represents an important source of evidence that can contribute to the establishment of rational treatment recommendations for relapsed or refractory PMBL.
In the present study, there was a trend of shorter OS and PFS for patients who experienced early relapse <12 months after diagnosis although it was not significant probably owing to small number of patients. Notably, >80% of patients treated with HDT/ASCT showed a curative potential when they experienced a late relapse. This result is consistent with findings from the CORAL (Collaborative Trial in Relapsed Aggressive Lymphoma) study of relapsed DLBCL.13
Response to salvage chemotherapy is another important issue. The OS at 4 years for patients with chemo-sensitive disease was significantly higher than that of patients with chemo-refractory disease (80% and 50%, respectively) in the present study. These results are consistent with those of a previous study.10 Therefore, if stem cell transplantation is considered, outcomes are expected to be best in chemo-sensitive patients at the time of second-line therapy. Novel drugs targeting CD30 or PD-1 have been developed recently,14 and an innovative approach including these novel drugs should be explored to increase the response rate of salvage regimens for PMBL patients with relapsed or refractory disease.
Treatment-related toxicities are another important issue to consider when weighing the benefits of HDT/ASCT. In the current study, treatment-related mortality at <100 days was 2.3% (N=1) in the HDT/ASCT group, which is consistent with prior reports in DLBCL.13 Moreover, only one patient developed a second malignancy in the HDT/ASCT group in the present study. However, longer follow-up is required to evaluate the incidence of late toxicity such as a second malignancy.
The role of allo-HSCT for relapsed and refractory PMBL after HDT/ASCT has not been fully investigated. Nath et al.9 described the efficacy of allo-HSCT as salvage therapy for a relapsed PMBL patient as a case report. In the present study, about half of the patients who underwent allo-HSCT as salvage treatment after HDT/ASCT achieved durable remission. Therefore, allo-HSCT could be curative, at least in a portion of patients failing HDT/ASCT.
Although the present study provides novel information regarding PMBL, several limitations should be discussed. First, this was a retrospective study with possible unrecognized biases. Second, patients received various regimens of chemotherapy according to each institution's preferred strategy; therefore, treatment outcomes may have been overestimated or underestimated. Finally, we used computed tomography (CT) for response assessment because a large number of PMBL patients in this study were treated before positron emission tomography/CT (PET/CT) became widespread in Japan. PET/CT images may be superior compared with CT for distinguishing the tumor activity of the residual mediastinal mass.15 Therefore, the response assessed with CT in this study may have been underestimated for patients with a residual mediastinal mass after treatment.
In conclusion, HDT/ASCT is a good treatment option for relapsed or refractory PMBL patients, especially those who experienced a relapse ⩾12 months after diagnosis. However, considering the poor outcome of chemo-refractory patients and patients who experience an early relapse (<12 months after diagnosis), efforts should be made to improve the response rate to salvage chemotherapy before administering HDT/ASCT. These findings require validation in future prospective studies.
Acknowledgments
We thank the following physicians for providing patient cases: Yoshinobu Kanda, MD of Saitama Medical Center, Jichi Medical University; Yoshihiro Yakushizin, MD of Ehime University Hospital; Yasunori Nakagawa, MD of the Japanese Red Cross Medical Center; Taro Masunari, MD of Chugoku Central Hospital; Yoshitoyo Kagami, MD of Toyota Kosei Hospital; Kazunori Ohnishi, MD of Hamamatsu University Hospital; Naoe Goto, MD of Gifu University Hospital; Norifumi Tsukamoto, MD of Gunma University Hospital; Noriyasu Fukushima, MD of Saga University Hospital; Shigeru Kusumoto, MD of Nagoya City University Hospital; Yoshitaka Imaizumi, MD of Nagasaki University Hospital; Koji Kato, MD of Kyushu University Hospital; Takenori Takahata, MD of Hirosaki University Hospital; Yasufumi Masaki, MD of Kanazawa Medical University Hospital; Akiyo Yoshida, MD of Kanazawa University Hospital; Masanobu Nakata, MD of Sapporo Hokuyu Hospital; Akinao Okamoto, MD of Fujita Health University Hospital, Dokkyo University Hospital; Ryosuke Shirasaki, MD of Teikyo University Hospital; Ichiro Hanamura, MD of Aichi Medical University Hospital; Kensuke Naito, MD of Hamamatsu Medical Center; Shingo Kurahashi, MD of Japanese Red Cross Nagoya First Hospital; Masahide Yamamoto, MD of Medical Hospital of Tokyo Medical and Dental University; Junji Suzumiya, MD of Shimane University Hospital; Hirokazu Nagai, MD of Nagoya Medical Center; Masahiro Yokoyama, MD of Cancer Institute Hospital; Yuichi Hasegawa, MD of University of Tsukuba Hospital; Katsuhiro Miura, MD of Nihon University Itabashi Hospital; Kensuke Usuki, MD of NTT Medical Center Tokyo; Naokuni Uike, MD of Kyushu Cancer Center; Shin Fuzisawa, MD of Yokohama City University Medical Center; Yasushi Takamatsu, MD of Fukuoka University Hospital; Akinori Nishikawa, MD of Wakayama Medical University Hospital; Naoto Tomita, MD of Yokohama City University Hospital; Hidek Tsujimura, MD of Chiba Cancer Center; Takashi Miyagi, MD of Heart Life Hospital; Katsuya Fujimoto, MD of Hokkaido University Hospital; Senji Kasahara, MD of Gifu Municipal Hospital; Atsushi Wakita, MD of Nagoya City West Medical Center; Michihide Tokuhira, MD of Saitama Medical Center; Takahiko Utsumi, MD of Shiga Medical Center for Adults; Kazuhito Yamamoto, MD of Aichi Cancer Center; Kunio Kitamura, MD of Ichinomiya Municipal Hospital; Toshimasa Kamoda, MD of Tenri Hospital; Kana Miyazaki, MD of Mie University Hospital; Keiichiro Mihara, MD of Hiroshima University Hospital, Tohoku University Hospital; Yoshiko Inoue, MD of Kumamoto Medical Center; Masatoshi Kanno, MD of Nara Medical University Hospital; Kazutaka Sunami, MD of Okayama Medical Center; Noriko Usui, MD of Third Hospital, Jikei University School of Medicine; and Yoshiharu Maeda, MD of Komagome Hospital. We also thank the patients, physicians, nurses and staff members who participated in this multicenter study for their excellent cooperation. We gratefully acknowledge the Program to Disseminate Tenure Tracking System, MEXT, Japan for a grant to KS, the National Cancer Center Research and Development Fund (23-A-17) for a grant to TK and the Hematological Malignancy Clinical Study Group.
Author contributions
TA, KI, TK, SN and MO designed the study; TA, KS, KI, AT, YM, JT, Kinuko Mitani, TI, KS, Kana Miyazaki, Keichiro Mihara, KO, HT and HK provided cases; NN and SN reviewed the pathological materials; TA and MO collected the data; TA, KS, KI, RS, NN, SN, TK and MO analyzed and interpreted the data; KI, SN, TK and MO provided financial support; TA and RS performed the statistical analysis; and TA, KS, KI, Kana Miyazaki, KO, RS and MO wrote the manuscript. All authors have read and approved the final version of the manuscript.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
HK received research funding from Bristol-Myers Squibb, Novartis Pharma, Chugai Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Zenyaku Kogyo and Fujifilm Corporation, LTD. The remaining authors declare no conflict of interest.
Supplementary Material
References
- 1Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2008. [Google Scholar]
- 2Savage KJ, Monti S, Kutok JL, Cattoretti G, Neuberg D, De Leval L et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood 2003; 102: 3871–3879. [DOI] [PubMed] [Google Scholar]
- 3Zinzani PL, Stefoni V, Finolezzi E, Brusamolino E, Cabras MG, Chiappella A et al. Rituximab combined with MACOP-B or VACOP-B and radiation therapy in primary mediastinal large B-cell lymphoma: a retrospective study. Clin Lymphoma Myeloma 2009; 9: 381–385. [DOI] [PubMed] [Google Scholar]
- 4Rieger M, Osterborg A, Pettengell R, White D, Gill D, Walewski J et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol 2011; 22: 664–670. [DOI] [PubMed] [Google Scholar]
- 5Savage KJ, Yenson PR, Shenkier T, Klasa R, Villa D, Goktepe O et al. The outcome of primary mediastinal large b-cell lymphoma (PMBCL) in the R-CHOP treatment era. ASH Annu Meet Abstr 2012; 120: 303. [Google Scholar]
- 6Aoki T, Izutsu K, Suzuki R, Nakaseko C, Arima H, Shimada K et al. Prognostic significance of pleural or pericardial effusion and the implication of optimal treatment in primary mediastinal large B-cell lymphoma: a multicenter retrospective study in Japan. Haematologica 2014; 99: 1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Dunleavy K, Pittaluga S, Maeda LS, Advani R, Chen CC, Hessler J et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med 2013; 368: 1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Kuruvilla J, Pintilie M, Tsang R, Nagy T, Keating A, Crump M. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma 2008; 49: 1329–1336. [DOI] [PubMed] [Google Scholar]
- 9Nath SV, Seymour JF. Cure of a patient with profoundly chemotherapy-refractory primary mediastinal large B-cell lymphoma: role of rituximab, high-dose therapy, and allogeneic stem cell transplantation. Leuk Lymphoma 2005; 46: 1075–1079. [DOI] [PubMed] [Google Scholar]
- 10Sehn LH, Antin JH, Shulman LN, Mauch P, Elias A, Kadin ME et al. Primary diffuse large B-cell lymphoma of the mediastinum: outcome following high-dose chemotherapy and autologous hematopoietic cell transplantation. Blood 1998; 91: 717–723. [PubMed] [Google Scholar]
- 11Kamezaki K, Kikushige Y, Numata A, Miyamoto T, Takase K, Henzan H et al. Rituximab does not compromise the mobilization and engraftment of autologous peripheral blood stem cells in diffuse-large B-cell lymphoma. Bone Marrow Transplant 2007; 39: 523–527. [DOI] [PubMed] [Google Scholar]
- 12Han LN, Zhou J, Hirose T, Imai Y, Ishiguro T, Chou T. Feasibility and efficacy of high-dose melphalan, cyclophosphamide, etoposide, and dexamethasone (LEED) chemotherapy with or without rituximab followed by autologous stem cell transplantation for aggressive and relapsed non-Hodgkin's lymphoma. Int J Hematol 2006; 84: 174–181. [DOI] [PubMed] [Google Scholar]
- 13Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010; 28: 4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015; 125: 1394–1402. [DOI] [PubMed] [Google Scholar]
- 15Martelli M, Ceriani L, Zucca E, Zinzani PL, Ferreri AJ, Vitolo U et al. [18F]Fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large b-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol 2014; 32: 1769–1775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.