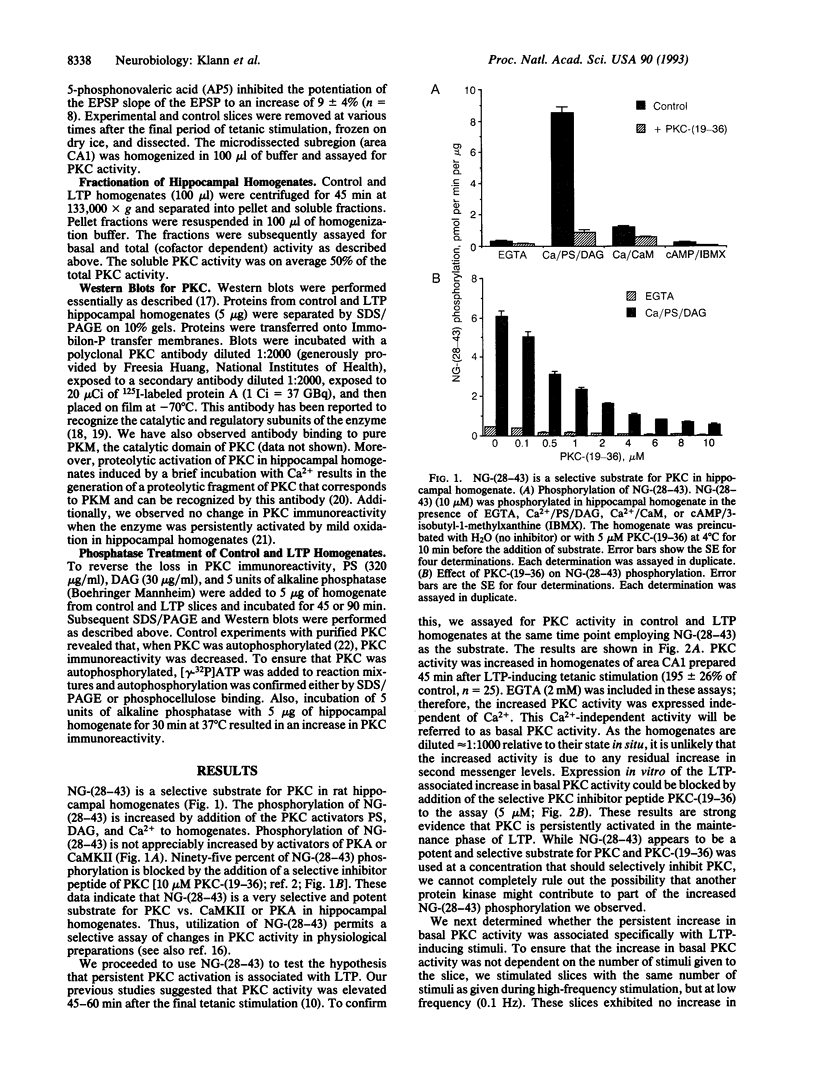
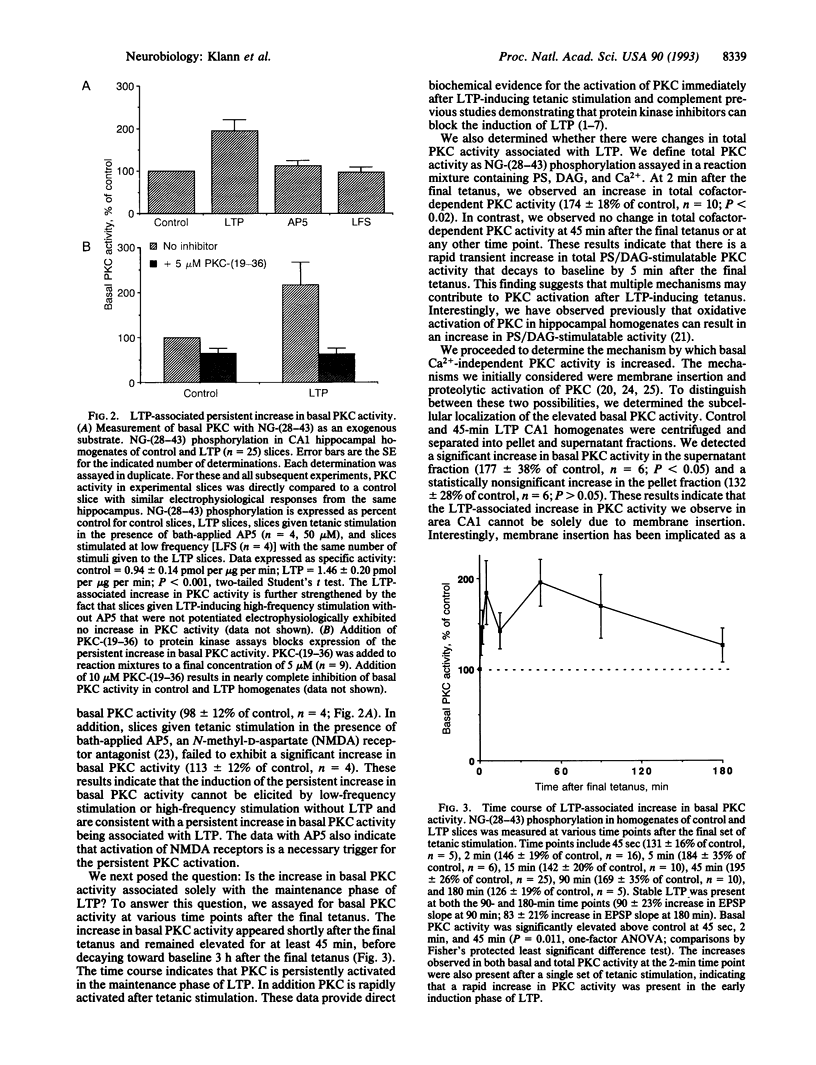
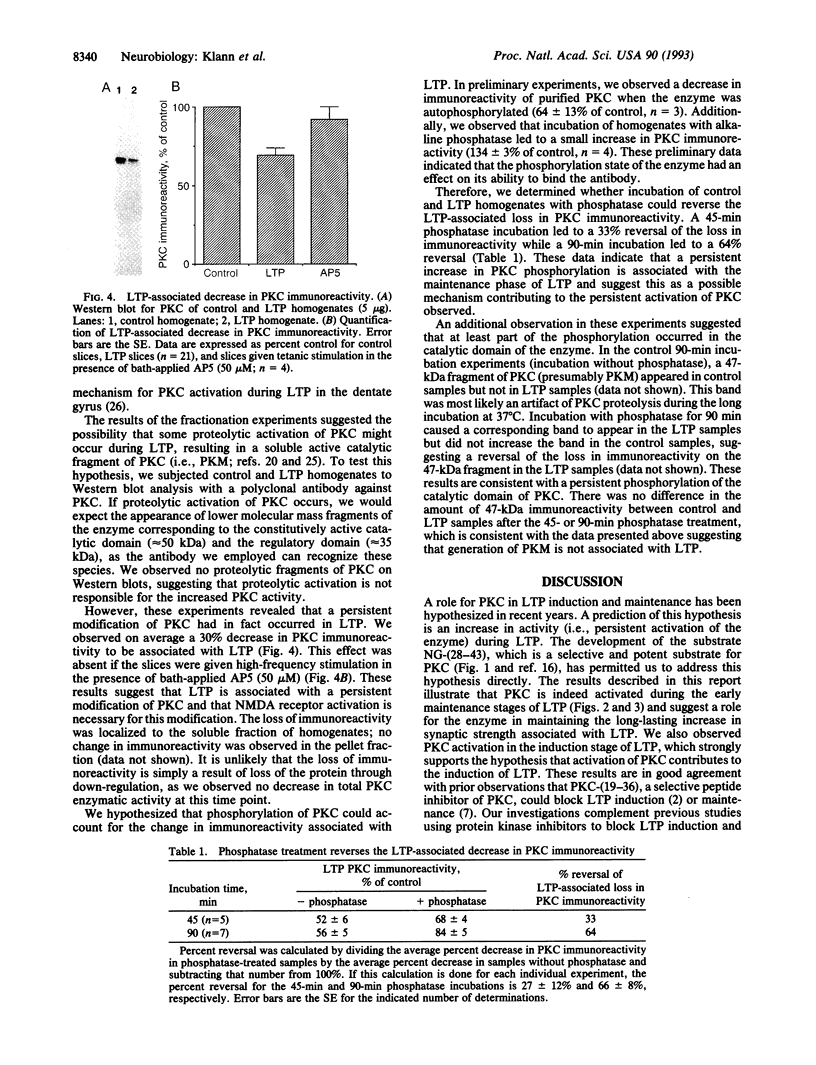
Abstract
Previous reports using various protein kinase inhibitors have suggested that protein kinase activity is necessary for both the induction and maintenance of hippocampal long-term potentiation (LTP), a cellular phenomenon likely to contribute to mammalian memory formation. We designed and characterized a selective peptide substrate for protein kinase C (PKC), corresponding to amino acids 28 to 43 of the neuronal protein neurogranin, and used the substrate to obtain direct biochemical evidence for activation of PKC in both the induction and maintenance phases of LTP. As the effect cannot be accounted for by either of two well-known mechanisms for persistent PKC activation, membrane insertion, or proteolysis, the persistent activation of PKC in the maintenance phase of LTP appears to occur via another mechanism. The maintenance phase of LTP is associated with decreased immunoreactivity of PKC, an effect that can be reversed with phosphatase treatment. Thus, PKC appears to be both phosphorylated and persistently activated in the maintenance phase of LTP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Aquino A., Hartman K. D., Knode M. C., Grant S., Huang K. P., Niu C. H., Glazer R. I. Role of protein kinase C in phosphorylation of vinculin in adriamycin-resistant HL-60 leukemia cells. Cancer Res. 1988 Jun 15;48(12):3324–3329. [PubMed] [Google Scholar]
- Baudier J., Bronner C., Kligman D., Cole R. D. Protein kinase C substrates from bovine brain. Purification and characterization of neuromodulin, a neuron-specific calmodulin-binding protein. J Biol Chem. 1989 Jan 25;264(3):1824–1828. [PubMed] [Google Scholar]
- Baudier J., Deloulme J. C., Van Dorsselaer A., Black D., Matthes H. W. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem. 1991 Jan 5;266(1):229–237. [PubMed] [Google Scholar]
- Bazzi M. D., Nelsestuen G. L. Constitutive activity of membrane-inserted protein kinase C. Biochem Biophys Res Commun. 1988 Apr 15;152(1):336–343. doi: 10.1016/s0006-291x(88)80719-8. [DOI] [PubMed] [Google Scholar]
- Borner C., Filipuzzi I., Wartmann M., Eppenberger U., Fabbro D. Biosynthesis and posttranslational modifications of protein kinase C in human breast cancer cells. J Biol Chem. 1989 Aug 15;264(23):13902–13909. [PubMed] [Google Scholar]
- Charriaut-Marlangue C., Otani S., Creuzet C., Ben-Ari Y., Loeb J. Rapid activation of hippocampal casein kinase II during long-term potentiation. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10232–10236. doi: 10.1073/pnas.88.22.10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992 Apr 9;356(6369):521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron. 1991 Aug;7(2):319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- Chen S. J., Klann E., Gower M. C., Powell C. M., Sessoms J. S., Sweatt J. D. Studies with synthetic peptide substrates derived from the neuronal protein neurogranin reveal structural determinants of potency and selectivity for protein kinase C. Biochemistry. 1993 Feb 2;32(4):1032–1039. doi: 10.1021/bi00055a006. [DOI] [PubMed] [Google Scholar]
- Colley P. A., Sheu F. S., Routtenberg A. Inhibition of protein kinase C blocks two components of LTP persistence, leaving initial potentiation intact. J Neurosci. 1990 Oct;10(10):3353–3360. doi: 10.1523/JNEUROSCI.10-10-03353.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg J., de Graan P. N., Schrama L. H., Gispen W. H. Dioctanoylglycerol and phorbol diesters enhance phosphorylation of phosphoprotein B-50 in native synaptic plasma membranes. Biochem Biophys Res Commun. 1986 May 14;136(3):1007–1012. doi: 10.1016/0006-291x(86)90433-x. [DOI] [PubMed] [Google Scholar]
- Gianotti C., Nunzi M. G., Gispen W. H., Corradetti R. Phosphorylation of the presynaptic protein B-50 (GAP-43) is increased during electrically induced long-term potentiation. Neuron. 1992 May;8(5):843–848. doi: 10.1016/0896-6273(92)90198-m. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992 Dec 18;258(5090):1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Hu G. Y., Hvalby O., Walaas S. I., Albert K. A., Skjeflo P., Andersen P., Greengard P. Protein kinase C injection into hippocampal pyramidal cells elicits features of long term potentiation. 1987 Jul 30-Aug 5Nature. 328(6129):426–429. doi: 10.1038/328426a0. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Chan K. F., Singh T. J., Nakabayashi H., Huang F. L. Autophosphorylation of rat brain Ca2+-activated and phospholipid-dependent protein kinase. J Biol Chem. 1986 Sep 15;261(26):12134–12140. [PubMed] [Google Scholar]
- Huang K. P., Huang F. L. Immunochemical characterization of rat brain protein kinase C. J Biol Chem. 1986 Nov 5;261(31):14781–14787. [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Kemp B. E., Benjamini E., Krebs E. G. Synthetic hexapeptide substrates and inhibitors of 3':5'-cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E., Chen S. J., Sweatt J. D. Increased phosphorylation of a 17-kDa protein kinase C substrate (P17) in long-term potentiation. J Neurochem. 1992 Apr;58(4):1576–1579. doi: 10.1111/j.1471-4159.1992.tb11382.x. [DOI] [PubMed] [Google Scholar]
- Klann E., Chen S. J., Sweatt J. D. Persistent protein kinase activation in the maintenance phase of long-term potentiation. J Biol Chem. 1991 Dec 25;266(36):24253–24256. [PubMed] [Google Scholar]
- Lovinger D. M., Wong K. L., Murakami K., Routtenberg A. Protein kinase C inhibitors eliminate hippocampal long-term potentiation. Brain Res. 1987 Dec 8;436(1):177–183. doi: 10.1016/0006-8993(87)91573-3. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., Waxham M. N. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989 Aug 17;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Koshland D. E., Jr Domain structure and phosphorylation of protein kinase C. J Biol Chem. 1987 Feb 15;262(5):2291–2297. [PubMed] [Google Scholar]
- Ocorr K. A., Schulman H. Activation of multifunctional Ca2+/calmodulin-dependent kinase in intact hippocampal slices. Neuron. 1991 Jun;6(6):907–914. doi: 10.1016/0896-6273(91)90231-n. [DOI] [PubMed] [Google Scholar]
- Ohno S., Konno Y., Akita Y., Yano A., Suzuki K. A point mutation at the putative ATP-binding site of protein kinase C alpha abolishes the kinase activity and renders it down-regulation-insensitive. A molecular link between autophosphorylation and down-regulation. J Biol Chem. 1990 Apr 15;265(11):6296–6300. [PubMed] [Google Scholar]
- Palumbo E. J., Sweatt J. D., Chen S. J., Klann E. Oxidation-induced persistent activation of protein kinase C in hippocampal homogenates. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1439–1445. doi: 10.1016/0006-291x(92)90463-u. [DOI] [PubMed] [Google Scholar]
- Reymann K. G., Brödemann R., Kase H., Matthies H. Inhibitors of calmodulin and protein kinase C block different phases of hippocampal long-term potentiation. Brain Res. 1988 Oct 4;461(2):388–392. doi: 10.1016/0006-8993(88)90274-0. [DOI] [PubMed] [Google Scholar]
- Routtenberg A., Lovinger D. M. Selective increase in phosphorylation of a 47-kDa protein (F1) directly related to long-term potentiation. Behav Neural Biol. 1985 Jan;43(1):3–11. doi: 10.1016/s0163-1047(85)91426-8. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. H., Feng D. P. Postsynaptic protein kinase C essential to induction and maintenance of long-term potentiation in the hippocampal CA1 region. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2576–2580. doi: 10.1073/pnas.89.7.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. B., Battenberg E. F., Wong K. K., Bloom F. E., Sutcliffe J. G. Subtractive cDNA cloning of RC3, a rodent cortex-enriched mRNA encoding a novel 78 residue protein. J Neurosci Res. 1990 Aug;26(4):397–408. doi: 10.1002/jnr.490260402. [DOI] [PubMed] [Google Scholar]
- Wolf M., Cuatrecasas P., Sahyoun N. Interaction of protein kinase C with membranes is regulated by Ca2+, phorbol esters, and ATP. J Biol Chem. 1985 Dec 15;260(29):15718–15722. [PubMed] [Google Scholar]




