Evidence for prognosis and treatment of elderly patient with primary central nervous system is limited. High-dose methotrexate should be applied whenever possible, especially combination with oral alkylating agents is a promising approach. Further combinations with other intravenous drugs do not seem to improve outcome. More prospective trials designed for elderly PCNSL patients are warranted.
Keywords: primary central nervous system lymphoma, PCNSL, elderly patients, individual patient data meta-analysis, systematic review
Abstract
Background
To investigate prognosis and effects of first-line therapy in elderly primary central nervous system lymphoma (PCNSL) patients.
Patients and methods
A systematic review of studies about first-line therapy in immunocompetent patients ≥60 years with PCNSL until 2014 and a meta-analysis of individual patient data from eligible studies and international collaborators were carried out.
Results
We identified 20 eligible studies; from 13 studies, we obtained individual data of 405 patients, which were pooled with data of 378 additional patients (N = 783). Median age and Karnofsky Performance Score (KPS) was 68 years (range: 60–90 years) and 60% (range: 10%–100%), respectively. Treatments varied greatly, 573 (73%) patients received high-dose methotrexate (HD-MTX)-based therapy. A total of 276 patients received whole-brain radiotherapy (median 36 Gy, range 28.5–70 Gy). KPS ≥ 70% was the strongest prognostic factor for mortality [hazard ratio (HR) 0.50, 95% confidence interval (CI) 0.41–0.62]. After a median follow-up of 40 months, HD-MTX-based therapy was associated with improved survival (HR 0.70, 95% CI 0.53–0.93). There was no difference between HD-MTX plus oral chemotherapy and more aggressive HD-MTX-based therapies (HR 1.39, 95% CI 0.90–2.15). Radiotherapy was associated with an improved survival, but correlated with an increased risk for neurological side-effects (odds ratio 5.23, 95% CI 2.33–11.74).
Conclusions
Elderly PCNSL patients benefit from HD-MTX-based therapy, especially if combined with oral alkylating agents. More aggressive HD-MTX protocols do not seem to improve outcome. WBRT may improve outcome, but is associated with increased risk for neurological side-effects. Prospective trials for elderly PCNSL patients are warranted.
introduction
Primary central nervous system lymphoma (PCNSL) is an aggressive non-Hodgkin lymphoma (NHL) mostly of B-cell origin, which exclusively invades the central nervous system compartment. It accounts for 3%–4% of all primary brain tumors and 4%–6% of extranodal lymphomas [1]. The incidence of PCNSL in immunocompetent patients has been steadily increasing over the last 30 years [2, 3]. High-dose methotrexate (HD-MTX) in combination with HD-cytarabine (HD-AraC) is the backbone of current treatment [4]. A recent randomized, controlled trial investigated the role of whole-brain radiotherapy (WBRT) as consolidation compared with no consolidation therapy, suggesting that WBRT does not prolong survival but enhances disease control [5]. However, despite treatment improvement, the prognosis of PCNSL patients is still poor compared with systemic NHL [6].
Patients older than 60 years account for 50% of all PCNSL cases. Although elderly patients are able to tolerate aggressive systemic chemotherapy [7], they have an inferior prognosis compared with younger patients and are more seriously affected by iatrogenic toxicity, especially neurological side-effects following WBRT [8]; therefore, they represent a unique and vulnerable treatment subgroup [9, 10]. An US registry study of 579 elderly patients diagnosed with PCNSL in the 1990s revealed that the median survival was only 7 months and WBRT alone was the most common treatment modality (46%) [11]. To date, a systematic summary of the research efforts to optimize therapy for elderly PCNSL patients is missing. Therefore, we conducted a systematic review and individual patient data meta-analysis to comprehensively investigate prognosis and treatment strategies for elderly patients with newly diagnosed PCNSL.
patients and methods
systematic review of the literature
We included any study that focused on first-line therapy and outcome in exclusively immunocompetent PCNSL patients ≥60 years. We searched Medline, EMBASE, and the Cochrane Library from database initiation until November 2014. We also searched annual meeting abstracts of the following societies (2010–2014): European hematology association (EHA), American Society of Clinical Oncology (ASCO), and American Society of Hematology (ASH). Two investigators (VG and BK) independently reviewed potentially eligible titles, abstracts and full texts if eligible. We asked all primary investigators of eligible studies to provide individual patient data.
individual patient data meta-analysis
Individual patient data from the identified studies were pooled with published and unpublished data of elderly PCNSL patients from international collaborators to maximize generalizability and statistical power. Anonymized patient data were collected using a prespecified case report form. The Research Ethics Committee of the University of Basel approved this study.
end point definitions
Main end points of our individual patient data meta-analysis were: (i) overall survival (OS, defined as time from diagnosis until death due to any cause or date of last follow-up visit) and (ii) progression-free survival (PFS, defined as time from diagnosis until progression, relapse, or death; whichever occurred first, or date of last follow-up visit). Further end points included: complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). We considered the response status as reported in the publications or as provided by the cooperating investigators. Clinical apparent neurological side-effects were defined as any significant and progressive neurological deterioration in the absence of disease progression developing at any time after start of treatment.
investigated treatments
Patients were categorized as follows: (i) HD-MTX-based therapies [defined as any therapy that contained HD-MTX (>1 g/m2)] versus no HD-MTX (including those only receiving WBRT); (ii) HD-MTX monotherapy versus HD-MTX plus any other chemotherapy, (iii) HD-MTX in combination with oral chemotherapy only versus HD-MTX plus at least two other i.v. agents (aggressive) (steroids were not considered as a single agent); and (iv) HD-MTX-based therapy with WBRT versus HD-MTX-based therapy without WBRT. We restricted our investigations to these four categories to address basic therapeutic principles and to preserve sufficient numbers of patients and events. We did not investigate the association between rituximab and the respective outcomes, because the follow-up times in patients receiving rituximab was much shorter compared with those not receiving it, preventing reliable conclusion.
statistical analysis
We used multivariable Cox regression analysis with a random effect for study/database for all prognostic analyses, which were conducted on the complete dataset. In sensitivity analyses, we restricted our analysis to patients being diagnosed after 1997 or to prospective studies. For exploratory purposes, we additionally analyzed survival within different time periods of diagnosis (periods of 10 years). Follow-up was estimated using the inverse Kaplan–Meier method [12]. We used Kaplan–Meier plots, logistic, and Cox regression models to compare the different treatment regimens with respect to response rate, PFS, and OS. Each analysis was adjusted for age, KPS (≥70% versus <70%), and a random effect for study/database was added. The impact of WBRT on OS and PFS was not investigated using the Cox regression model, because of severe violation of the proportional hazards assumption. For all treatment regimens, we additionally investigated whether the association between treatment and outcome differed by KPS status and tested possible interactions. The impact of WBRT on neurological side-effects was investigated using random-effect logistic regression adjusted for the factors mentioned above. P < 0.05 (two-sided) was considered significant. Further details about methods and statistical analyses are provided in the supplementary Material S1, available at Annals of Oncology online.
results
studies identified by the systematic review
We identified 20 eligible studies published between 1996 and 2014 including 1103 patients (supplementary Figure S2, available at Annals of Oncology online) [13–32]. Detailed characteristics of identified studies, applied therapies, and outcomes are summarized in Table 1. In 13/20 (65%) studies, patient data were collected from more than one center [13, 15–18, 21, 23, 25–27, 29–31]; of those, 8 (62%) were planned prospectively [15, 16, 18, 25–27, 30, 31]. The assessment of the methodological quality of studies is provided in supplementary Material S3, available at Annals of Oncology online. Only three studies (15%) reported neurocognitive outcomes as measured with the mini-mental-state examination tool [13, 30, 33]. Patient recruitment for the only identified randomized trial (N = 98) has recently been completed and preliminary data were presented in abstract form [25]. This phase II trial suggests that HD-MTX plus procarbazine and vincristine followed by cytarabine (MPV-A) may be more effective compared with HD-MTX plus temozolomide regarding response, PFS, and OS (Table 1) with similar toxicities (≥grade 3 toxicities 72% versus 71%); however, the differences were not statistically significant. The investigators also evaluated neurocognitive outcomes and quality of life, but they were not reported in the abstract [25]. The publication by Roth et al. reported data of the subgroup of elderly PCNSL patients (≥70 years) who were enrolled in the randomized G-PCNSL-SG-1 trial, which was not specifically designed for elderly patients [26]. One German multicenter single-arm study including patients ≥65 years of age recently completed recruitment of 112 patients, but no data have yet been published (NCT00989352).
Table 1.
Characteristics of included studies
| References | In IPDMA | No. of patients | Median age | Design | HD-MTX | CTX components | Response evaluation | Response rate (CR rate) % | Median FU (months) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Freilich et al. [13] | Yes | 12 | 76 | Retro multi | Yes | 1. HD-MTX, IT-MTX, PCB, VINCR; 2. HD-MTX, IT-MTX, AraC; 3.HD-MTX, PCB, IT-MTX, TT | CT or MRI | 91 (75) | 13 | n.r. | 31 |
| Fritsch et al. [24] | Yes | 28 | 75 | Pros single | Yes | R, HD-MTX, CCNU, PCB | MRI | 82 (64) | 36 | 16 | 18 |
| Ghesquieres et al. [18], Ab | Yes | 36 | 66 | Pros multi | Yes | COP, MCOPA, low-dose CYM. | CT or MRI | 61 (53) | n.r. | 16 | 16 |
| Ghesquieres et al. [18], Bb | Yes | 18 | 73 | Pros multi | Yes | MCVP | CT or MRI | 56 (28) | n.r. | 7 | 15 |
| Hoang-Xuan et al. [33] | Yes | 50 | 72 | Pros multi | Yes | HD-MTX, CCNU, PCB, IT-MTX, steroids | CT or MRI | 48 (42) | 36 | 7 | 14 |
| Illerhaus et al. [19] | Yes | 30 | 70 | Pros single | Yes | HD-MTX, CCNU, PCB | MRI | 70 (44) | 78 | 6 | 15 |
| Kurzwelly et al. [21] | Yes | 17 | 75 | Retro multi | No | TMZ | MRI | 53 (47) | 25 | 5 | 21 |
| Laack et al. [16] | No | 19 | 76 | Pros multi | No | WBRT, steroids | MRI | 42 (16) | 6 | 3 | 6 |
| Lee et al. [29]$c | Yes | 38 | 69 | Retro single | Yes | HD-MTX, RANIMST, PCB, steroids | MRI | 74 (42) | 36.5 | 18 | 43 |
| Makino et al. [32] | No | 63 | n.r. | Retro single | Yes | HD-MTX, RANIMST, PCB, steroids | MRI | n.r. | n.r. | 7 | 31 |
| Ney et al. [22] | No | 174 | 72 | Retro single | Yes | HD-MTX, WBRT | CT or MRI | 76 (n.r.) | 34 | 24 | 25 |
| Ng et al. [14] | Yes | 10 | 73 | Retro unclear | Yes | HD-MTX | n.r. | 90 (60) | 23 | 18 | 36 |
| Olivier et al. [31] | No | 35 | 65 | Pros multi | Yes | HD-MTX, VIND, IDA, steroids | MRI | 51 (17) | 57 | 13 | 19 |
| Omuro et al. [17] | Yes | 23 | 68 | Retro multi | Yes | HD-MTX, TMZ | n.r. | 70 (55) | 26 | 8 | 35 |
| Omuro et al. [25]a | No | 98 | 72 | RCT multi | Yes | MPV-A versus HD-MTX TMZ | n.r. | 82 (62) versus 71 (45) | n.r. | 9.5 versus 6.1 | 31 versus 13.8 |
| Pulczynski et al. [30]d | Yes | 42 | 66 | Pros multi | Yes | HD-MTX, ARAC, TMZ | MRI | 88 (60) | 24 | 13 | Not reached |
| Roth et al. [26] | No | 192 | n.r. | RCT multi | Yes | HD-MTX (IFO) | CT or MRI | 44 (30) | n.r. | 4 | 13 |
| Schlegel et al. [27]a | No | 89 | 68 | Pros multi | Yes | HD-MTX, ARAC, DEXA, IF, CYLCO, VINCR, IT-ARAC | n.r. | 57 (49) | 12 | 8 | 22 |
| Schuurmans et al. [23] | Yes | 74 | 65 | Retro multi | Yes | 1. WBRT only; 2. HD-HD-MTX; 3. MBVP | n.r. | 74 (n.r.) | 20 | 7 | 21 |
| Welch et al. [28] | No | 24 | 82 | Retro single | Yes | MVP | CT or MRI | 63 (58) | 15 | 7 | 8 |
| Zhu et al. [20] | Yes | 31 | 74 | Retro single | Yes | HD-MTX | MRI | 96 (60) | 28 | 7 | 37 |
aOnly published in abstract form.
bResults from one single study that stratified therapy according to age (A, 61–69 years; B, ≥70 years).
$cThis is an updated study of previously reported patients by Taoka et al. [34].
dStudy also included patients below 60 years of age, in total N = 66.
ARAC, cytarabine; CCNU, lomustine; CR, complete remission; CT, computed tomography; CTX, chemotherapy; FU, follow-up; HD-MTX, high-dose methotrexate; IDA, idarubicine; IFO, ifosfamide; IT, intrathecal; IPDMA, individual patient data meta-analysis; multi, multicenter study; MBVP, MTX–BCNU–teniposide–dexamethason; MVP, high-dose methotrexate–vincristine–procarbazine; MRI, magnetic resonance imaging; n.r., not reported; PBC, procarbazine; PR, partial remission; RANIMST, ranimustine; single, single-center study; pros, prospective study; retro, retrospective study; R, rituximab; RCT, randomized clinical trial; TMZ, temozolomide; TT, thiotepa; VINCR, vincristine; VIND, vindesin; WBRT, whole-brain radiotherapy.
individual patient data meta-analysis
patient characteristics
From the identified 20 studies, 405 (40%) individual patient data [13, 14, 17–21, 23, 24, 30, 33, 34] were available for our individual patient data meta-analysis and pooled with 378 published and unpublished patient data from 6 other databases (Milan N = 9 [35], N = 18 [36], N = 183 [37], N = 36 [4]; Boston N = 22 [not published]; Freiburg, N = 67 [not published]; Tel Aviv, N = 16 [not published]; Rochester N = 27 [not published]). Altogether 783 patients diagnosed from 1977 to 2014 were included in our individual patient data meta-analysis (supplementary Figure S2, available at Annals of Oncology online). Two hundred sixty-one of 783 (33%) patient data were collected in prospective studies, 67% were collected in retrospective studies. Patients' characteristics are summarized in Table 2. Applied treatment regimens varied widely and are listed in detail in the supplementary Material S4, available at Annals of Oncology online) (only patients included in the individual patient data meta-analysis). Overall, WBRT monotherapy was the most common treatment modality (13%), followed by HD-MTX monotherapy (9%). Patients being diagnosed after 1997 (N = 552) were mostly treated with HD-MTX monotherapy (10%), second most frequent treatment was HD-MTX plus oral chemotherapy (9%).
Table 2.
Patient’s characteristics
| Baseline characteristics | IPD not from review (N = 378) | IPD from review (N = 405) | All (N = 783) |
|---|---|---|---|
| Sex | |||
| Female | 174 (46) | 218 (53.8) | 392 (50.1) |
| Age at diagnosis | |||
| Median (IQR) | 67 (63, 72) | 70 (65, 74) | 68 (64, 73) |
| <65 | 151 (40) | 105 (26) | 256 (33) |
| 65–70 | 118 (31) | 115 (28) | 233 (30) |
| 71–75 | 72 (19) | 109 (27) | 181 (23) |
| >75 | 37 (10) | 76 (19) | 113 (14) |
| KPS | |||
| Median % (IQR) | 60 (40, 80) | 60 (50, 80) | 60 (50, 80) |
| KPS ≥ 70% | 150 (40) | 182 (45) | 332 (42) |
| KPS < 70% | 178 (47) | 193 (48) | 371 (47) |
| Missing values | 50 (13) | 30 (7) | 80 (10) |
| Serum LDH at diagnosis | |||
| Elevated | 98 (26) | 70 (17) | 168 (21) |
| Normal | 158 (42) | 92 (23) | 250 (32) |
| Missing values | 122 (32) | 243 (60) | 365 (47) |
| Involvement of deep brain structures | |||
| Yes | 193 (51) | 152 (38) | 345 (44) |
| No | 144 (38) | 110 (27) | 254 (32) |
| Missing values | 41 (11) | 143 (35) | 184 (24) |
| Elevated CSF protein | |||
| Yes | 106 (28) | 63 (16) | 169 (22) |
| No | 83 (22) | 29 (7) | 112 (14) |
| Missing values | 189 (50) | 313 (77) | 502 (64) |
| Year of diagnosis | |||
| 1977–1986 | 31 (8) | 0 | 31 (4) |
| 1987–1996 | 151 (40) | 30 (7) | 181 (23) |
| 1997–2007 | 116 (31) | 262 (65) | 378 (48) |
| After 2007 | 78 (21) | 96 (24) | 174 (22) |
| Missing values | 2 (1) | 17 (4) | 19 (2) |
Values are frequencies (percentages) unless otherwise specified.
CSF, cerebrospinal fluid; IPD, individual patient data; IQR, interquartile range; KPS, Karnofsky performance score; LDH, serum lactate dehydrogenase.
prognostic factors
After a median follow-up of 40 months [95% confidence interval (CI) 36.0–47.0], 507 (65%) patients died. Median PFS and OS were 10 (95% CI 8.0–12.0) and 19 months (95% CI 16.0–21.0), respectively (Figure 1A). Grouping patients by time of diagnosis revealed improvement over time regarding OS; patients younger than 65 years seemed to have the best survival prognosis (Figure 1B and C). Table 3 summarizes the results from the multivariable analyses for OS. A KPS ≥70% was significantly associated with improved survival. Other factors such as involvement of deep brain structures, cerebrospinal fluid protein elevation, and serum lactate dehydrogenase had no impact on OS or PFS (supplementary Material S5, available at Annals of Oncology online). Our sensitivity analyses conditional on time of diagnosis (diagnosed after 1997) and study design also supported the strong prognostic value of KPS (data not shown).
Figure 1.
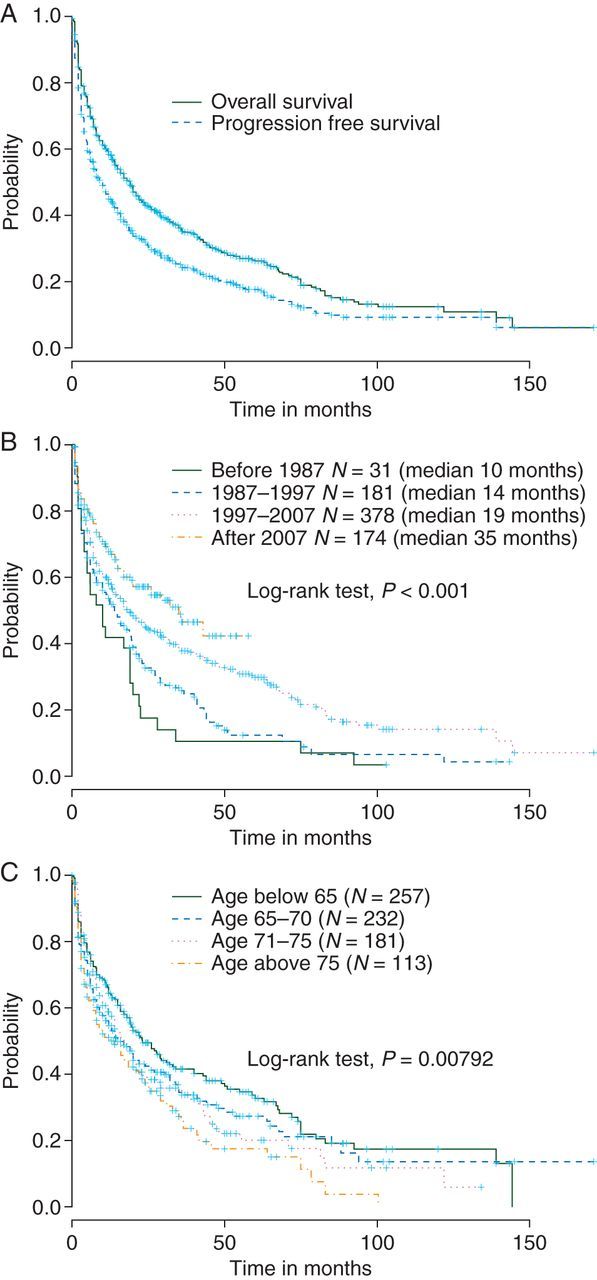
(A) Overall and progression-free survival of the whole cohort; (B) overall survival grouped by time of diagnosis (in 19 cases, exact date was missing); (C) overall survival grouped by age groups.
Table 3.
Analysis of prognostic factors for mortality (based on 692 complete cases)
| Factor | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age | |||
| <65 | Reference | n.a. | n.a. |
| 65–70 | 1.13 | 0.88–1.44 | 0.336 |
| 71–75 | 1.16 | 0.89–1.52 | 0.277 |
| >75 | 1.36 | 0.98–1.89 | 0.067 |
| KPS ≥ 70% | 0.50 | 0.41–0.62 | <0.001 |
CI, confidence interval; KPS, Karnofsky performance score.
cause of death
Of those 508 (65%) patients who died, causes of death were PCNSL in 310 (61%), treatment-related complications in 34 (7%), sepsis in 25 (5%) of patients. Twenty-one patients (4%) died of other causes. Cause of death was unknown in 118 (23%) deceased patients.
response to treatment
Overall 531 of 783 (68%) patients responded to first-line treatment [375 (48%) CR, 156 (20%) PR, 19 (2%) SD, 130 (19%) PD, and in 81 (10%) response status was missing]. Altogether, 466 of 783 (60%) patients experienced either progression or relapse. Any HD-MTX-based therapy was associated with better response compared with therapies not containing HD-MTX (73% versus 55%), but the difference was not statistically significant in the adjusted analysis. High-dose MTX plus any other chemotherapy was significantly associated with higher response rates compared with HD-MTX monotherapy (73% versus 68%) in the adjusted analysis. Overall response rates between HD-MTX combined with oral chemotherapy and HD-MTX plus at least two other i.v. agents (aggressive) were similar (73% versus 75%). Further details are given in supplementary Material S6, available at Annals of Oncology online.
treatment regimens and survival
Of 573 patients (73%) who received any HD-MTX-based chemotherapy for first-line therapy, the median number of applications was 4 (range, 1–29) with a median HD-MTX dosage of 3 g/m2 (range, 1–8 g/m2), 396/573 (77%) patients received ≥3 g/m2.
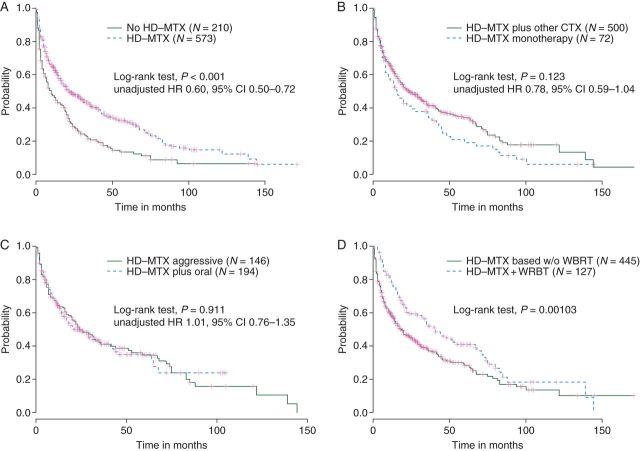
Any HD-MTX-based therapy (N = 573, HD-MTX ≥1 g/m2) was independently associated with improved OS compared with therapies without HD-MTX (N = 210), which also included WBRT monotherapy (Table 4) [unadjusted 12- and 24-month OS rates: 63% (95% CI 59% to 67%) versus 47% (95% CI 40% to 53%) and 49% (95% CI 44% to 53%) versus 29% (95% CI 23% to 36%), respectively—Figure 2A]. This association was consistent throughout KPS subgroups and in the subpopulation of patients not receiving any WBRT during first-line treatment. Sensitivity analysis based on patients being diagnosed after 1997 revealed similar results. There was also a positive association regarding PFS without statistical significance (adjusted HR 0.80, 95% CI 0.61–1.04, P = 0.100). In the unadjusted analysis, patients treated with HD-MTX plus any other chemotherapy [median HD-MTX applications 4, interquartile range (IQR) 2–5] only tended to have an improved prognosis compared with HD-MTX monotherapy (median HD-MTX applications 3, IQR 1–7) (Figure 2B) [unadjusted 12- and 24-month OS rates: 64% (95% CI 0.60% to 0.68%) versus 57% (95% CI 0.44% to 0.67%) and 50% (96% CI 0.45% to 0.55%) versus 41% (95% CI 0.29% to 0.53%)]. However, the association was statistically significant in the adjusted multivariable analysis (Table 4) and also in our sensitivity analyses restricted to patients being diagnosed after 1997 (adjusted HR 0.63, 95% CI 0.45–0.89) or treated within prospective studies (adjusted HR 0.39, 95% CI 0.21–0.71). Furthermore, HD-MTX plus other chemotherapy was significantly associated with improved PFS (adjusted HR 0.39, 95% CI 0.27–0.58, P < 0.001).
Table 4.
Multivariable Cox regression analyses for mortality in selected therapies
| Factors | HR | 95% CI | P value |
|---|---|---|---|
| HD-MTX versus no-HD-MTX | 0.70 | 0.53–0.93 | 0.013 |
| Age | |||
| <65 | Reference | n.a. | n.a. |
| 65–70 | 1.12 | 0.88–1.43 | 0.367 |
| 71–75 | 1.16 | 0.89–1.51 | 0.286 |
| >75 | 1.30 | 0.94–1.81 | 0.113 |
| KPS ≥ 70% | 0.51 | 0.41–0.62 | <0.001 |
| HD-MTX + any other CT versus HD-MTX monotherapy | 0.54 | 0.35–0.84 | 0.006 |
| Age | |||
| <65 | Reference | ||
| 65–70 | 0.91 | 0.66–1.21 | 0.502 |
| 71–75 | 0.85 | 0.62–1.18 | 0.328 |
| >75 | 1.08 | 0.72–1.59 | 0.711 |
| KPS ≥ 70% | 0.49 | 0.38–0.62 | <0.001 |
| HD-MTX + multiagent i.v. CTa versus HD-MTX + oral CT | 1.39 | 0.90–2.15 | 0.143 |
| Age | |||
| <65 | Reference | ||
| 65–70 | 1.11 | 0.76–1.64 | 0.584 |
| 71–75 | 1.23 | 0.77–1.95 | 0.389 |
| >75 | 1.79 | 1.09–2.93 | 0.022 |
| KPS ≥ 70% | 0.48 | 0.35–0.66 | <0.001 |
aHD-MTX plus at least two other i.v. agents (aggressive).
CI, confidence interval; CT, chemotherapy; HD-MTX, high-dose methotrexate; HR, hazard ratio; KPS, Karnofsky performance score; WBRT, whole-brain radiotherapy.
Figure 2.
(A) Overall survival in patients receiving any HD-MTX-based therapy versus therapies not containing HD-MTX; (B) overall survival in patients receiving any HD-MTX + other chemotherapy versus HD-MTX monotherapy; (C) overall survival in patients receiving HD-MTX plus at least two other i.v. drugs (aggressive) versus HD-MTX plus oral chemotherapy; (D) overall survival in patients receiving HD-MTX-based chemotherapy with or without whole-brain radiotherapy. CTX, chemotherapy; HD-MTX, high-dose methotrexate; HR, hazard ratio.
Therapies combining HD-MTX plus at least two other i.v. agents (aggressive, median HD-MTX applications 3, IQR 2–4) were not associated with improved OS compared with HD-MTX plus oral chemotherapy only (e.g. HD-MTX combined with temozolomide or procarbazine, median HD-MTX applications 6, IQR 3–8) (Table 4) [unadjusted 12- and 24-month OS rates: 65% (95% CI 56% to 72%) versus 65% (95% CI 57% to 71%) and 50% (95% CI 51% to 58%) versus 50% (95% CI 42% to 58%)] (Figure 2C). In patients with a KPS ≥70% HD-MTX plus oral chemotherapy showed a trend for better OS [unadjusted 12- and 24-month OS rates: 78% (95% CI 66% to 86%) versus 73% (95% CI 64% to 83%) and 66% (95% CI 53% to 77%) versus 56% (95% CI 44% to 66%); log-rank test P = 0.0926], but the tested interaction was not significant. The sensitivity analyses restricted to patients being diagnosed after 1997 or treated in prospective studies revealed similar results. There was no difference regarding PFS (adjusted HR 1.26, 95% CI 0.80–1.99).
Overall, the addition of WBRT in those patients being treated with any HD-MTX-based chemotherapy (with WBRT, median HD-MTX applications 4, IQR 2–6; without WBRT, median HD-MTX applications 4, IQR 2–4) was associated with an OS benefit (Figure 2D) [unadjusted 12- and 24-month OS rates: 78% (95% CI 70% to 85%) versus 59% (95% CI 54% to 63%) and 59% (95% CI 50% to 68%) versus 46% (95% CI 41% to 51%); log-rank test P = 0.0010]. This association was not consistent throughout the KPS subgroups; patients with a KPS ≥ 70% did not seem to benefit [unadjusted 12 and 24-months OS rates: 81% (95% CI 69% to 88%) versus 71% (95% CI 64% to 77%) and 65% (95% CI 52% to 75%) versus 59% (95% CI 52% to 67%); log-rank test P = 0.6239]. Regarding PFS, addition of WBRT showed a positive association overall and throughout KPS subgroups in patients who were treated with HD-MTX-based chemotherapy.
When we considered ≥3 g/m2 as the cutoff to define HD-MTX therapy, results were very similar and did not change our conclusions compared with the cutoff at 1 g/m2. Protocols based on HD-MTX ≥3 g/m2 were not significantly associated with better OS compared with HD-MTX protocols with <3 g/m2 (log-rank test P = 0.116). Further details are provided in supplementary Figure S7, available at Annals of Oncology online.
WBRT and neurological side-effects
Two hundred seventy-six of 783 (35%) patients received WBRT as part of first-line therapy (median 36 Gy, range 28.5–70 Gy); 140 of 276 (51%) received a dose of more than 36 Gy. In total, 65 of 783 (8.3%) patients were reported to have clinical apparent neurological side-effects. In 91 cases (12.3%), no explicit information about neurological side-effects was available. The remaining 627 patients (80.1%) were reported as being free of neurological side-effects. Whole-brain radiotherapy was independently associated with an increased risk for neurological side-effects (adjusted odds ratio 5.23, 95% CI 2.33–11.74, P < 0.001). Of those 136 patients who were treated with <36 Gy, 38 (28%) developed neurological side-effects (missing information about neurological side-effects, 24%), whereas of those patients being treated with more than 36 Gy, 14% developed neurotoxicity (missing information about neurological side-effects, 12%).
salvage therapies
Of 466 patients (60%) with progressive or relapsing disease, 209 (45%) received salvage therapy [40 HD-MTX-based chemotherapy (19%), 83 (40%) non-HD-MTX-based chemotherapy, 62 (30%) only WBRT, in 24 (12%) cases information about salvage therapy was not available]. Further details of salvage therapies stratified by first-line treatment and time are provided in supplementary Material S8, available at Annals of Oncology online.
discussion
To our knowledge, this is the largest systematic review and individual patient data meta-analysis of the therapeutic management and outcome of elderly patients with PCNSL. Results revealed an improvement of prognosis over the last decade. Among a wide variety of treatment approaches for elderly patients with newly diagnosed PCNSL, HD-MTX-based therapies were associated with significantly better results. High-dose MTX in combination with at least two other i.v. drugs (aggressive) compared with HD-MTX plus oral chemotherapy were not associated with improved response or survival. In those patients that received HD-MTX-based chemotherapy, WBRT was associated with improved PFS, OS, but also with an increased risk of neurological side-effects. Given the lack of standardized neurological testing and poor reporting of this outcome, definite conclusions about the magnitude of this association are difficult to draw.
Although large in scale, our study has limitations. First, the predominant retrospective nature of the dataset implies inconsistent data quality throughout the different data sources. For example, information about different baseline characteristics crucial for calculation of the “International Extranodal Lymphoma Study Group (IELSG)” score was missing. Consequently, only a restricted number of patients were included in the multivariable analysis to test the prognostic discrimination of components of the IELSG score; thus, these results should be cautiously interpreted. The issue of missing data does not only apply to baseline variables, central pathology review, and information about co-morbidities, but also to acute toxicities during, reasons for treatment termination, and causes of death. In addition, we were not able to include all patients from eligible studies in our individual patient data meta-analysis and had to restrict our investigations of the associations between different treatment regimens and outcome to a limited number. This was necessary to preserve a sufficient number of patients and events in each chosen treatment category to obtain reliable conclusions. However, to maximize generalizability, we included additional patient data from other databases and accounted for the heterogeneity of data sources in our analysis. In particular, the prognostic analyses proved robust in our sensitivity analysis focusing on prospective studies and year of diagnosis. The definition of neurological side-effects was broad and not based on formal cognitive testing, because we a priori assumed that neurological testing was standardized in the minority of studies. Therefore, assuming that only severe forms of clinically apparent neurological side-effects were reported, it is likely that the actual rate of patient impairment due to neurological side-effects is higher than reported herein. Moreover, information on other risk factors, e.g. use of anticonvulsant drugs or pre-existing cerebral vasculopathy, were not available and effects on neurological status could not be addressed. Interpretation of response rates in association with different therapies also needs to consider the limitation of nonstandardized response criteria throughout pooled study data.
The cutoff of 60 years was chosen, because it is the lowest reported [7, 9]. In addition, it is also in line with other recent trials for elderly patients suffering from systemic NHL [38]. In univariable analysis, there was a clear association between increasing age and decreased survival probability. However, if adjusted for KPS, only some evidence remained indicating that age above 75 is associated with a worse survival prognosis compared with patients younger than 65 years of age. As reported previously [39], KPS equal or above 70% is of high prognostic relevance, which we could confirm in our analyses. These findings indicate, that categorization only by age alone is not informative enough to make therapeutic decisions or to define trial eligibility criteria in elderly PCNSL patients; a more appropriate approach is to at least consider both, age and clinical performance status as already implemented in an currently ongoing multicenter PCNSL trial (NCT01011920).
Methotrexate is now the most widely studied drug in the treatment of PCNSL and its efficacy has been demonstrated in several prospective trials [40–42]. Our results confirm these findings also for the subgroup of elderly PCNSL patients. However, 14% of patients in our database were treated with WBRT only, which is lower than previous reports based on US SEER registry data (46%) [11]. This discrepancy may be explained by the fact that patients included in our analyses were mostly treated at tertiary referral centers and not in the community [11]. Another explanation could be that therapy and outcome of those only being treated with WBRT were not published. Importantly, after 1997 the number of patients treated with WBRT monotherapy substantially declined and HD-MTX-based therapy was the predominant therapy of choice. A more aggressive approach consisting of HD-MTX and at least two other i.v. agents was not associated with improved response or OS compared with HD-MTX plus oral chemotherapy. This may be explained by the fact that these aggressive approaches also included anthracyclines and vincristine, both are well known to be highly active in systemic NHL, but their questionable activity in PCNSL has been reported before [43–45]. Even if some activity of these agents is assumed, the chosen combinations do not seem to be feasible, because patients receiving this aggressive approach had less HD-MTX applications (median 3) compared with HD-MTX plus oral chemotherapy (median 6).
There were some discrepancies between PFS and OS estimates in our analyses. Usually, one would rather expect significant effects on PFS, but not on OS, e.g. due to limited statistical power. In our analysis, power was not an issue, but rather the fact that patients who received HD-MTX-based chemotherapy more often received salvage treatment, which likely improved OS. Therefore, salvage therapy should always be considered, because it seems that also elderly can benefit from it even after an early relapse. However, to investigate this in further detail, more clinical information at time of relapse would have been needed.
Beside HD-MTX, there is yet no defined standard treatment of newly diagnosed PCNSL as is for instance rituximab–CHOP for systemic NHL [40]. The only phase II randomized trial specifically designed for elderly PCNSL patients suggests that MPV-A may be more effective compared with HD-MTX plus temozolomide [25]; however, the differences regarding response, PFS, or OS were not statistically significance. Still, the MPV-A regimen can be recommended for further development in the treatment of elderly PCNSL patients.
A recent US multicenter single-arm study (N = 52) showed promising results regarding long-term disease control and low rates of neurotoxicity by using the R-MPV regimen, followed by low-dose WBRT (23.4 Gy) and cytarabine as consolidation [46]. Another recent single-arm study (N = 42) investigated the combination of rituximab, HD-MTX, and temozolomide for induction followed by consolidation with etoposide and cytarabine; WBRT was omitted from the primary treatment strategy [47]. Both studies also showed good outcomes in the small subgroups of patients ≥60 years of age [46, 47].
Elderly patients exhibit increased risk for neurological side-effects when exposed to WBRT [8, 48]. In two retrospective series, WBRT in combination with chemotherapy was not associated with improved OS in PCNSL patients treated with HD-MTX, but led to improved disease control [37, 49]. Similarly, in another retrospective analysis, patients older than 60 years of age who were treated with HD-MTX did not seem to benefit from WBRT regarding OS [50]. In all mentioned studies, prevalence of neurological side-effects was not based on standardized testing, but on a broad definition similar to ours. In contrast to these studies, our data suggest that WBRT may improve OS in patients with a lower KPS. Patients with a higher KPS do not seem to benefit. Given the trade-off between a potentially small survival benefit and the risk of neurological side-effects, which still maybe underestimated in these elderly patients, we urge caution regarding the interpretation of WBRT being a superior treatment in addition to HD-MTX this subgroup.
In conclusion, this study provides a comprehensive summary of the available evidence for the first-line treatment and prognosis of elderly PCNSL patients. HD-MTX-based therapy should be offered to these frail patients, whenever possible. Especially, first-line treatment with combinations of HD-MTX with oral alkylating agents are advisable (e.g. HD-MTX plus procarbazine or temozolomide) and deserve to be further investigated. WBRT may improve outcome, but is significantly associated with increased risk for neurological side-effects. Prospective trials designed for elderly PCNSL patients are promptly needed.
funding
This study was supported by the Freiwillige Akademische Gesellschaft Basel in Switzerland (no grant number).
disclosure
EM discloses consultancies for Roche. TTB discloses consultancies for Roche/Genentech and Merck. VG and MB are supported by the Gottfried and Julia Bangerter-Rhyner Foundation and Santésuisse. EJP discloses to have received grants from Roche, Norpharma, Schering-Plough, Nordic Cancer Union, and Inge og Jørgen Larsens Mindelegat. All remaining authors have declared no conflict of interest.
Supplementary Material
acknowledgements
Thanks to the International Primary CNS Lymphoma Group (IPCG), which provided a platform for coordinating this project. Thanks to the European Organization for Research and Treatment of Cancer for permission to use the data from the EORTC study 26952 for this analysis.
references
- 1.Panageas KS, Elkin EB, DeAngelis LM, et al. Trends in survival from primary central nervous system lymphoma, 1975–1999: a population-based analysis. Cancer 2005; 104: 2466–2472. [DOI] [PubMed] [Google Scholar]
- 2.Olson JE, Janney CA, Rao RD, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer 2002; 95: 1504–1510. [DOI] [PubMed] [Google Scholar]
- 3.Makino K, Nakamura H, Kino T, et al. Rising incidence of primary central nervous system lymphoma in Kumamoto, Japan. Surg Neurol 2006; 66: 503–506. [DOI] [PubMed] [Google Scholar]
- 4.Ferreri AJ, Reni M, Foppoli M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 2009; 374: 1512–1520. [DOI] [PubMed] [Google Scholar]
- 5.Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010; 11: 1036–1047. [DOI] [PubMed] [Google Scholar]
- 6.Carrabba MG, Reni M, Foppoli M, et al. Treatment approaches for primary CNS lymphomas. Expert Opin Pharmacother 2010; 11: 1263–1276. [DOI] [PubMed] [Google Scholar]
- 7.Roth P, Hoang-Xuan K. Challenges in the treatment of elderly patients with primary central nervous system lymphoma. Curr Opin Neurol 2014; 27: 697–701. [DOI] [PubMed] [Google Scholar]
- 8.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol 2000; 18: 3144–3150. [DOI] [PubMed] [Google Scholar]
- 9.Sierra del Rio M, Rousseau A, Soussain C, et al. Primary CNS lymphoma in immunocompetent patients. Oncologist 2009; 14: 526–539. [DOI] [PubMed] [Google Scholar]
- 10.Jahnke K, Korfel A, Martus P, et al. High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma. Ann Oncol 2005; 16: 445–449. [DOI] [PubMed] [Google Scholar]
- 11.Panageas KS, Elkin EB, Ben-Porat L, et al. Patterns of treatment in older adults with primary central nervous system lymphoma. Cancer 2007; 110: 1338–1344. [DOI] [PubMed] [Google Scholar]
- 12.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–346. [DOI] [PubMed] [Google Scholar]
- 13.Freilich RJ, Delattre JY, Monjour A, DeAngelis LM. Chemotherapy without radiation therapy as initial treatment for primary CNS lymphoma in older patients. Neurology 1996; 46: 435–439. [DOI] [PubMed] [Google Scholar]
- 14.Ng S, Rosenthal MA, Ashley D, Cher L. High-dose methotrexate for primary CNS lymphoma in the elderly. Neuro Oncol 2000; 2: 40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoang-Xuan K, Chinot OL, Taillandier L. Treatment of primary central nervous system lymphoma in the elderly. Semin Oncol 2003; 30: 53–57. [DOI] [PubMed] [Google Scholar]
- 16.Laack NN, Ballman KV, Brown PB, O'Neill BP. Whole-brain radiotherapy and high-dose methylprednisolone for elderly patients with primary central nervous system lymphoma: results of North Central Cancer Treatment Group (NCCTG) 96-73-51. Int J Radiat Oncol Biol Phys 2006; 65: 1429–1439. [DOI] [PubMed] [Google Scholar]
- 17.Omuro AM, Taillandier L, Chinot O, et al. Temozolomide and methotrexate for primary central nervous system lymphoma in the elderly. J Neurooncol 2007; 85: 207–211. [DOI] [PubMed] [Google Scholar]
- 18.Ghesquieres H, Ferlay C, Sebban C, et al. Long-term follow-up of an age-adapted C5R protocol followed by radiotherapy in 99 newly diagnosed primary CNS lymphomas: a prospective multicentric phase II study of the Groupe d'Etude des Lymphomes de l'Adulte (GELA). Ann Oncol 2010; 21: 842–850. [DOI] [PubMed] [Google Scholar]
- 19.Illerhaus G, Marks R, Muller F, et al. High-dose methotrexate combined with procarbazine and CCNU for primary CNS lymphoma in the elderly: results of a prospective pilot and phase II study. Ann Oncol 2009; 20: 319–325. [DOI] [PubMed] [Google Scholar]
- 20.Zhu JJ, Gerstner ER, Engler DA, et al. High-dose methotrexate for elderly patients with primary CNS lymphoma. Neuro Oncol 2009; 11: 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurzwelly D, Glas M, Roth P, et al. Primary CNS lymphoma in the elderly: temozolomide therapy and MGMT status. J Neurooncol 2010; 97: 389–392. [DOI] [PubMed] [Google Scholar]
- 22.Ney DE, Reiner AS, Panageas KS, et al. Characteristics and outcomes of elderly patients with primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center experience. Cancer 2010; 116: 4605–4612. [DOI] [PubMed] [Google Scholar]
- 23.Schuurmans M, Bromberg JE, Doorduijn J, et al. Primary central nervous system lymphoma in the elderly: a multicentre retrospective analysis. Br J Haematol 2010; 151: 179–184. [DOI] [PubMed] [Google Scholar]
- 24.Fritsch K, Kasenda B, Hader C, et al. Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann Oncol 2011; 22: 2080–2085. [DOI] [PubMed] [Google Scholar]
- 25.Omuro A, Chinot O, Taillandier L, et al. Multicenter randomized phase II trial of methotrexate (MTX) and temozolomide (TMZ) versus MTX, procarbazine, vincristine, and cytarabine for primary CNS lymphoma (PCNSL) in the elderly: an Anocef and Goelams Intergroup study. J Clin Oncol 2013; 31(suppl): abstr 2032. [Google Scholar]
- 26.Roth P, Martus P, Kiewe P, et al. Outcome of elderly patients with primary CNS lymphoma in the G-PCNSL-SG-1 trial. Neurology 2012; 79: 890–896. [DOI] [PubMed] [Google Scholar]
- 27.Schlegel U, Kuhnhenn J, Kowalski S, et al. Combined systemic polychemotherapy and intrathecal treatment with liposomal cytarabine in patients with primary CNS lymphoma >= 60 years: results of a phase II-trial (meeting abstract). In Congress of the German Neurology Association (DGN), Hamburg 2012. [Google Scholar]
- 28.Welch MR, Omuro A, Deangelis LM. Outcomes of the oldest patients with primary CNS lymphoma treated at Memorial Sloan-Kettering Cancer Center. Neuro Oncol 2012; 14: 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SY, Okoshi Y, Kurita N, et al. Prognosis factors in Japanese elderly patients with primary central nervous system lymphoma treated with a nonradiation, intermediate-dose methotrexate-containing regimen. Oncol Res Treat 2014; 37: 378–383. [DOI] [PubMed] [Google Scholar]
- 30.Pulczynski EJ, Kuittinen O, Erlanson M, et al. Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: results from a phase 2 study by The Nordic Lymphoma Group. Haematologica 2014. Dec 5 [epub ahead of print], doi:10.3324/haematol.2014.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivier G, Clavert A, Lacotte-Thierry L, et al. A phase 1 dose escalation study of idarubicin combined with methotrexate, vindesine, and prednisolone for untreated elderly patients with primary central nervous system lymphoma. The GOELAMS LCP 99 trial. Am J Hematol 2014; 89: 1024–1029. [DOI] [PubMed] [Google Scholar]
- 32.Makino K, Nakamura H, Hide TI, et al. Prognostic impact of completion of initial high-dose methotrexate therapy on primary central nervous system lymphoma: a single institution experience. Int J Clin Oncol 2015; 20: 29–34. [DOI] [PubMed] [Google Scholar]
- 33.Hoang-Xuan K, Taillandier L, Chinot O, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol 2003; 21: 2726–2731. [DOI] [PubMed] [Google Scholar]
- 34.Taoka K, Okoshi Y, Sakamoto N, et al. A nonradiation-containing, intermediate-dose methotrexate regimen for elderly patients with primary central nervous system lymphoma. Int J Hematol 2010; 92: 617–623. [DOI] [PubMed] [Google Scholar]
- 35.Ferreri AJ, Licata G, Foppoli M, et al. Clinical relevance of the dose of cytarabine in the upfront treatment of primary CNS lymphomas with methotrexate-cytarabine combination. Oncologist 2011; 16: 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreri AJ, Dell'Oro S, Foppoli M, et al. MATILDE regimen followed by radiotherapy is an active strategy against primary CNS lymphomas. Neurology 2006; 66: 1435–1438. [DOI] [PubMed] [Google Scholar]
- 37.Ferreri AJ, Reni M, Pasini F, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology 2002; 58: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 38.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008; 9: 105–116. [DOI] [PubMed] [Google Scholar]
- 39.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006; 24: 5711–5715. [DOI] [PubMed] [Google Scholar]
- 40.Morris PG, Abrey LE. Therapeutic challenges in primary CNS lymphoma. Lancet Neurol 2009; 8: 581–592. [DOI] [PubMed] [Google Scholar]
- 41.Batchelor T, Carson K, O'Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96–07. J Clin Oncol 2003; 21: 1044–1049. [DOI] [PubMed] [Google Scholar]
- 42.Herrlinger U, Schabet M, Brugger W, et al. German Cancer Society Neuro-Oncology Working Group NOA-03 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma. Ann Neurol 2002; 51: 247–252. [DOI] [PubMed] [Google Scholar]
- 43.Glass J, Gruber ML, Cher L, Hochberg FH. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg 1994; 81: 188–195. [DOI] [PubMed] [Google Scholar]
- 44.Glass J, Shustik C, Hochberg FH, et al. Therapy of primary central nervous system lymphoma with pre-irradiation methotrexate, cyclophosphamide, doxorubicin, vincristine, and dexamethasone (MCHOD). J Neurooncol 1996; 30: 257–265. [DOI] [PubMed] [Google Scholar]
- 45.Mead GM, Bleehen NM, Gregor A, et al. A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer 2000; 89: 1359–1370. [PubMed] [Google Scholar]
- 46.Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 2013; 31: 3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 2013; 31: 3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001; 345: 425–432. [DOI] [PubMed] [Google Scholar]
- 49.Ekenel M, Iwamoto FM, Ben-Porat LS, et al. Primary central nervous system lymphoma: the role of consolidation treatment after a complete response to high-dose methotrexate-based chemotherapy. Cancer 2008; 113: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 50.Gavrilovic IT, Hormigo A, Yahalom J, et al. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol 2006; 24: 4570–4574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.