Abstract
There is currently no licensed vaccine that protects foals against Rhodococcus equi–induced pneumonia. Oral administration of live, virulent R. equi to neonatal foals has been demonstrated to protect against subsequent intrabronchial challenge with virulent R. equi. Electron beam (eBeam)-inactivated R. equi are structurally intact and have been demonstrated to be immunogenic when administered orally to neonatal foals. Thus, we investigated whether eBeam inactivated R. equi could protect foals against developing pneumonia after experimental infection with live, virulent R. equi. Foals (n = 8) were vaccinated by gavaging with eBeam-inactivated R. equi at ages 2, 7, and 14 days, or gavaged with equal volume of saline solution (n = 4), and subsequently infected intrabronchially with live, virulent R. equi at age 21 days. The proportion of vaccinated foals that developed pneumonia following challenge was similar among the vaccinated (7/8; 88%) and unvaccinated foals (3/4; 75%). This vaccination regimen did not appear to be strongly immunogenic in foals. Alternative dosing regimens or routes of administration need further investigation and may prove to be immunogenic and protective.
Introduction
Rhodococcus equi is a Gram-positive, facultative, intracellular pathogen that causes a pyogranulomatous pneumonia in foals approximately 1 to 6 months of age [1, 2]. Virulence of R. equi in foals is attributable to the presence of an 85- to 90-kilobase (kb) plasmid, including the vapA gene which encodes the virulence-associated protein A (VapA) [3–5]. Mature horses are generally not susceptible unless immunocompromised [6, 7]. The reasons for this age-related susceptibility are not fully understood; however, immaturity or naivety of the immune system of foals have been proposed as principal determinants of the outcome of infection [8].
Pneumonia induced by Rhodococcus equi occurs worldwide, and virulent isolates can be found at horse farms in the air, soil, and feces [9–11]. The disease is problematic for several reasons. First, the insidious progression of R. equi pneumonia in foals results in marked pathology by the time clinical signs are manifested [12]. Consequently, treatment is generally prolonged, expensive, and not always successful. Screening for earlier detection of disease has been demonstrated to have limited accuracy [13, 14]. Methods for chemo- or immuno-prophylaxis have either been inadequately effective (at best) or unacceptable (e.g., macrolide chemoprophylaxis because of concerns for promoting antimicrobial resistance) [15–18]. Moreover, prophylactic strategies such as transfusion of hyperimmune plasma can be expensive, labor-intensive, and carry some risk for foals [19–23]. Thus, great need exists for an effective vaccine to prevent R. equi pneumonia in foals.
Currently, no commercial vaccine against R. equi pneumonia is licensed in the United States, Canada, or European Union. Several vaccines against R. equi pneumonia have been investigated, including maternal vaccination [24–26], subunit vaccines [27, 28], genetically-modified organisms [29, 30], and DNA vaccines [31–33]. To date, the only method that has been repeatedly documented to protect foals against experimental intrabronchial infection with R. equi has been oral administration (gavage) of live, virulent R. equi [34, 35]. While these results are greatly encouraging, the administration of live, virulent organisms as a vaccine is not feasible because of safety concerns for the environment and for foals. Thus, alternative approaches to the use of live, virulent R. equi should be considered. Recently, our laboratory demonstrated that irradiating live, virulent R. equi with an electron beam (eBeam) inhibited bacterial replication while maintaining cell wall integrity [36]. Moreover, when administered intragastrically these eBeamed bacteria induced both mucosal and cell-mediated immunity (CMI) [36]. Further studies have shown that eBeam-inactivated bacteria remain metabolically active [37]. Thus, we hypothesized that vaccinating foals with eBeam-inactivated R. equi, using the same vaccination schedule as was most recently demonstrated to protect foals using orally-administered live, virulent organisms [35], would protect foals against intrabronchial infection with live, virulent R. equi.
Materials and Methods
Ethics Statement
All procedures for this study were reviewed and approved by the Texas A&M University Institutional Animal Care and Use Committee (protocol number AUP# IACUC 2013–0171) and the Texas A&M University Institutional Biosafety Committee (permit number 014132-Cohen). The foals used in this study were owned by Texas A&M University, and permission for their use was provided in compliance with the Institutional Animal Care and Use Committee procedures. No foals were euthanized or died during the course of this study.
Preparation of Bacteria and Electron Beam Irradiation
Rhodococcus equi strain EIDL 5–331 (a virulent, vapA-gene-positive isolate recovered from a pneumonic foal in Texas) was used for this study. The method for culture and inactivation of R. equi for vaccine preparation has been described in an earlier publication from our laboratory [36]. Briefly, one colony-forming unit (CFU) was incubated overnight at 37°C in 25 ml of brain-heart infusion (BHI) broth and sub-cultured in 1,000 ml of BHI broth for an incubation of another 24 hr. The bacterial suspension was washed with phosphate-buffered saline (PBS), and resuspended in sterile 0.9% NaCl solution. For eBeam preparation, 25 ml of bacterial suspensions of approximately 1x109 CFU/ml were exposed to a target irradiation dose of 5 kGy using a 10-MeV, 18-kW linear accelerator. Inactivated R. equi were cultured immediately after irradiation to confirm absence of bacterial replication [36].
Study Animals
Twelve healthy Quarter Horse foals were used for this study. All foals had age-appropriate results of complete blood count (CBC) on day 2 of life. Individual foals were randomly assigned to a vaccinated group, Group 1 (N = 8), or a control group, Group 2 (N = 4). Group 1 foals received 1 x 1011 CFU of R. equi inactivated by 5 kGy of eBeam irradiation, adjuvanted with 100 μg of the mucosal adjuvant cholera toxin B (CTB, List Biological Laboratories, Campbell, CA, USA), and suspended to a final volume of 100 ml in 0.9% NaCl solution by gavage on days 2, 7, and 14 of life. This dose was previously demonstrated to be immunogenic in foals [36] and represents 10 times the dose of live organisms administered orally in previous studies [34,35]. The frequency of administration was selected to match that used for oral administration of live, virulent R. equi [35]. Group 2 foals (N = 4) received 100 ml of 0.9% NaCl solution intragastrically at ages 2, 7, and 14 days. The foals in Groups 1 and 2 were housed separately.
Experimental Infection
Foals from Group 1 and 2 were experimentally infected at age 21 days with 1 x 106 CFU of live R. equi (strain EIDL 5–331, the same strain used for the vaccine). Prior to experimental infection with R. equi, each foal’s lungs were evaluated by auscultation and thoracic ultrasonography to document absence of pre-existing lung disease. Foals were sedated using intravenous injection of romifidine (0.8 mg/kg; Sedivet, Boehringer-Ingelheim Vetmedica, Inc., St. Joseph, MO, USA) and butorphanol (0.02 mg/kg; Zoetis, Florham Park, New Jersey, USA) to facilitate endoscopy. An aseptically-prepared, videoendoscope with outer diameter of 9-mm was inserted via the nares into the trachea and passed to the bifurcation of the main-stem bronchi. A 40-mL suspension of virulent EIDL 5–331 R. equi containing approximately 1 x 106 viable bacteria was administered transendoscopically, with 20 ml infused into the right mainstem bronchus and 20 ml into the left mainstem bronchus. The channel was flushed twice with 20 ml of air after each 10 ml bacterial infusion.
Sample Collection
Blood samples were collected from foals and their dams on day 2 (prior to vaccination), on day 21 (post-vaccination, pre-challenge), and day 84 (post-challenge) of foals’ age. A total of 43 ml of blood was collected from each foal from a jugular vein into VacutainerTM tubes: 16 ml of blood was collected into 2 tubes without anticoagulant and centrifuged at 3,000 x g for 5 min to harvest serum, which was separated and frozen at -80°C until assayed; 24 ml of blood was collected into 3 tubes with sodium heparin as an anticoagulant for isolation of peripheral blood mononuclear cells (PBMCs); and, 3 ml of blood was collected into a tube with 5.4 mg EDTA as an anticoagulant to perform a CBC. A total of 24 ml of blood was collected from each mare from a jugular vein into 3 VacutainerTM tubes with sodium heparin as anticoagulant for isolation of peripheral blood mononuclear cells (PBMCs). Naso-pharyngeal samples were collected from foals on days 2, 21, and 84 by inserting a 26-mm white plastic foam plug (Identi-Plug, Jaece Industries Inc, North Tonawanda, NY, USA), introduced with an equine insemination pipet, to the nasal ventral meatus. The foam plug was left in the nasal cavity for 5 min, and the naso-pharyngeal liquid was collected by centrifugation in a 50-ml conical tube at 500 x g for 10 min and frozen at -80°C until assayed. Transendoscopic tracheobronchial aspirate (T-TBA) fluid was collected 3 times from foals in both groups: on day 21 (pre-challenge); at the time of clinical diagnosis with R. equi pneumonia; and, either when clinical signs of R. equi pneumonia ceased to exist or at age 84 days (i.e., end of study) if foals remained healthy. The T-TBAs were performed using a 1-meter endoscope which was disinfected with glutaraldehyde, and then rinsed with 1 X PBS prior to the procedure. The T-TBA was obtained by sedating foals with romifidine (0.8 mg/kg; Sedivet, Boehringer-Ingelheim Vetmedica, Inc., St. Joseph, MO, USA) and washing the tracheobronchial tree with 0.9% NaCl solution delivered through a triple-lumen, double-guarded sterile tubing system (MILA International, Inc, Erlanger, KY, USA).
Foal Monitoring and Diagnostic Criteria
Beginning with the day of infection, rectal temperature, heart rate, and respiratory rate of the foals were monitored and recorded twice daily. Clinical signs of abnormal lung sounds, coughing, lethargy, respiratory effort, nasal discharge, and polysynovitis also were monitored and recorded twice daily. The lungs were monitored weekly using thoracic ultrasonography for evidence of peripheral pulmonary consolidation or abscess formation.
Foals were considered to manifest clinical signs of pneumonia when they had ultrasonographic evidence of pulmonary abscessation or consolidation with maximal lesion diameter ˃ 2.0 cm and either a rectal temperature of ˃ 39.7°C, a cough at rest, or both fever (˃ 39.7°C) and cough. On the day a foal was diagnosed with pneumonia, a CBC, thoracic ultrasonography, and T-TBA were performed. The T-TBA fluid was submitted to the Texas Veterinary Medical Diagnostic Laboratory, College Station, for cytologic examination and for microbiologic culture. Foals were considered to have a diagnosis of R. equi pneumonia if they met the aforementioned criteria for clinical signs of pneumonia and had cytologic evidence of septic inflammation with Gram-positive pleomorphic rods and R. equi recovered by microbiologic culture of T-TBA fluid. Foals were monitored through 84 days of age. Foals that did not have clinical signs of disease, but had ultra-sonographic evidence of lung lesions, were monitored through 84 days of age.
Treatment of Study Foals
Foals diagnosed with R. equi pneumonia were treated with either the combination of clarithromycin (7.5 mg/kg; PO; q 12 hr) and rifampin (5 mg/kg; PO, q 12 hr) or liposomal gentamicin solution (6.6 mg/kg, q 24 hr) [38] diluted in 250 ml of 0.9% NaCl given via slow intravenous infusion over 15 min. Foals were treated as deemed necessary by attending veterinarians (AIB; NDC; MCC) with flunixin meglumine (0.6 to 1.1 mg/kg; PO) for discomfort and fever. Foals were treated until clinical signs and thoracic ultrasonographic lesions resolved.
Cell-Mediated Immune Response
The CMI response to vaccination was assessed by interferon-γ (IFN-γ) production by peripheral blood mononuclear cells (PBMCs) following specific stimulation with an R. equi antigen (strain EIDL 5–331). The protocol for preparation of R. equi antigen has been described previously [39]. The PBMCs were isolated using a Ficoll-Paque gradient separation (GE Healthcare, Piscataway, NJ, USA) and resuspended in 1X RPMI-1640 media with L-glutamine (Gibco, Life Technologies, Grand Island, NY, USA), 15% fetal bovine serum (Gibco, Life Technologies, Grand Island, NY, USA), and 1.5% penicillin-streptomycin (Gibco, Life Technologies, Grand Island, NY, USA). The PBMCs were cultured for 48 h at 37°C with 5% CO2 with either media only, the mitogen Concanavalin A (positive control; 2.5 mg/ml, Sigma-Aldrich, St. Louis, MO, USA), or R. equi antigen representing multiplicity of infection of 10. After 48 h, supernatants from each group were harvested and frozen at -80°C until examined for IFN-γ production using an equine IFN-γ enzyme linked immunosorbent assay (ELISA) kit (Mabtech AB, Nacka Strand, Stockholm, Sweden) according to manufacturer’s instructions. Optical densities (OD) were determined using a microplate reader Synergy 2 (Biotek, Winooski, VT, USA); standard curves were generated and IFN-γ concentrations in each sample were calculated for each isotype using the software Gen 5 (Biotek, Winooski, VT, USA). Mare PBMCs were tested along with each sample from their foals as controls to ensure that positive (i.e., Concanavilin A) and negative (i.e., media only) stimuli were functioning as expected because we anticipated that PBMCs of younger foals might be less responsive to ConA stimulation.
Mucosal and Systemic Humoral Immune Responses
Mucosal humoral immune responses were assessed by quantifying R. equi-specific IgA in naso-pharyngeal eluates. Systemic humoral response was assessed among foals by quantifying serum concentrations of R. equi-specific IgA and IgG sub-isotypes. Concentrations R. equi-specific IgA and IgG sub-isotypes were determined by ELISA as previously described [36]. Briefly, ELISA plates (Maxisorp, Nalge Nunc International, Rochester, NY) were coated with 2.5 mg/ml of R. equi antigen (same R. equi antigen as mentioned above) diluted in coating buffer (Carbonate-bicarbonate buffer, Sigma-Aldrich, St. Louis, MO) overnight at 4°C. Plates were washed 5 times with Tris buffered saline (TBS) with 0.005% Tween 20, blocked with 200 ml TBS with 1% BSA for 30 min at room temperature (RT), and washed again. Serum samples (diluted 1:32) from study foals, a positive control of R. equi hyperimmune plasma (Mg Biologics, Ames, IA) diluted at concentrations of 1:40, 1:320, 1:10, and 1:320 for IgGa, IgGb, IgG(T), and IgA, respectively, and a negative control of undiluted fetal horse serum (Biowest, Miami, FL, USA) were added in duplicates to the wells. Naso-pharyngeal (NP) swab eluates were assayed undiluted. After another washing, goat anti-horse IgA (Bethyl Laboratories, Montgomery, TX, USA), IgGb (Lifespan Biosciences, Seattle, WA), or IgG(T) (Bethyl Laboratories, Montgomery, TX, USA) peroxidase conjugated, or mouse anti-horse IgGa peroxidase conjugated (AbD Serotec, Raleigh, NC, USA) were added to the wells and incubated for 60 min at RT. Plates were washed again, and TMB One Component HRP Microwell Substrate (Bethyl Laboratories, Montgomery, TX) was added to the wells and incubated for 15 min at 22°C in the dark. The reaction was stopped by adding sulfuric acid solution to the wells. Optical densities were determined by using microplate reader Synergy 2 (Biotek, Winooski, VT, USA).
Data Analysis
Clinical data
Data were analyzed using descriptive and inferential methods. For descriptive purposes, categorical data were summarized in contingency tables and continuous data were summarized as medians and ranges. Categorical data were compared using Fisher’s exact tests and continuous variables were compared using a Wilcoxon rank-sum test.
CMI data (IFN-γ expression by cultured PBMCs)
Data were analyzed using linear mixed-effects (LME) models to account for repeated measures on foals. The outcome variable was IFN-γ expression, which was transformed using the function log10 (concentration + 1); the addition of 1 was necessary because there were values of 0 for some foals at some times.
Serum and nasal antibody concentration data
The optical density (OD) values for a given foal at a given age were divided by the plate positive control value to account for plate-to-plate variation; each foal’s serum samples from all 3 time-points were tested on a single plate (i.e., the same plate). Data were analyzed using linear mixed-effects modeling. The outcome (dependent) variable was the relative OD (i.e., sample OD/positive control); age, vaccine group, and their interaction terms (i.e., age x group) were modeled as fixed, categorical effects, and foal was modeled as a random effect to account for repeated measures. Model fit was assessed graphically using diagnostic residual plots. When model fit appeared poor, data were transformed (log10) to improve fit, and model fit was again assessed using diagnostic residual plots.
Results
Clinical Outcomes
All foals developed lesions ≥ 2 cm in their peripheral lungs identified by thoracic ultrasonography. The primary study outcome was the proportion of foals that developed clinical signs of pneumonia as defined above. Ten of 12 infected foals (83%) developed clinical signs of pneumonia, and isolation of virulent R. equi and cytologic evidence of sepsis was observed for the TBA fluid of all foals at the time clinical signs of pneumonia were observed. There was no significant (P = 0.9999; Fisher’s exact test) difference in the proportion of foals that developed clinical signs of pneumonia among the control foals (75%; 3/4) and the vaccinated foals (88%; 7/8). All affected foals had fever, cough, abnormal lung sounds or tracheal rattle, and ultrasonographic evidence of pulmonary consolidations. There were no significant differences in foals in the age at onset of clinical signs, duration of clinical signs, or lesion sizes (Tables 1 and 2. Of the study foals, 1 of the 4 control foals (25%) and 4 of the 8 vaccinated foals (50%) developed polysynovitis associated with experimental R. equi pneumonia; there was no significant difference in these proportions (P = 0.5758; Fisher’s exact test).
Table 1. Ages at onset of clinical signs and ultrasonographic lesions of vaccinated and control foals.
There were no significant differences between the two groups.
| Variable | Controls (N = 4) Median (Range) | Vaccinates (N = 8) Median (Range) | P# |
|---|---|---|---|
| Age at onset of coughing (days) | 34 (26 to 39) | 43 (27 to 37) | 0.7758 |
| N = 4 | N = 7 | ||
| Age at onset of tachypnea* (days) | 21.5 (20 to 27) | 23.5 (21 to 35) | 0.3031 |
| N = 4 | N = 8 | ||
| Age at onset of fever (days) | 34 (30 to 37) | 36 (33 to 42) | 0.9076 |
| N = 3 | N = 7 | ||
| Age at onset of ultrasound lesions (days) | 36.5 (34 to 39) | 37 (33 to 46) | 0.9999 |
| N = 4 | N = 8 | ||
| Age of maximal diameter of ultrasound lesions (days) | 39.5 (36 to 49) | 42 (33 to 54) | 0.8649 |
| N = 4 | N = 8 |
# P values from Wilcoxon rank-sum test
* Tachypnea defined as respiratory rate > 60
Table 2. Duration of clinical signs and ultrasonographic lesions of vaccinated and control foals.
There were no significant differences between the 2 groups.
| Variable | Controls (N = 4) Median (Range) | Vaccinated (N = 8) Median (Range) | P# |
|---|---|---|---|
| Duration of coughing (days) | 7.5 (4 to 19) | 13 (4 to 24) | 0.2964 |
| N = 4 | N = 7 | ||
| Duration of tachypnea* (days) | 36.5 (27 to 57) | 33.5 (15 to 59) | 0.7972 |
| N = 4 | N = 7 | ||
| Duration of fever (days) | 2 (1 to 13) | 5 (1 to 17) | 0.9079 |
| N = 3 | N = 7 | ||
| Duration of fever (days) including the foal that had 0 days | 1.5 (0 to 13) | 3.5 (0 to 17) | 0.6678 |
| N = 3 | N = 8 | ||
| Duration of ultrasound lesions (days) | 21 (7 to 42) | 17.5 (7 to 43) | 0.9999 |
| N = 4 | N = 8 | ||
| Duration of abnormal lung sounds (days) | 5.5 (1 to 29) | 25 (2 to 34) | 0.2183 |
| N = 4 | N = 7 | ||
| Duration of abnormal lung sounds (days) including the foal that had 0 days. | 5.5 (1 to 29) | 21.5 (0 to 34) | 0.4439 |
| N = 4 | N = 8 | ||
| Duration of depressed attitude (days) | 4.5 (0 to 16) | 11 (0 to 33) | 0.2654 |
| N = 4 | N = 8 | ||
| Duration of antimicrobial treatment (days) | 35 (13 to 41) | 25 (12 to 42) | 0.5676 |
| N = 3 | N = 7 | ||
| Duration of antimicrobial treatment (days) including the foal that had 0 days | 24 (0 to 41) | 24.5 (0 to 42) | 0.9321 |
| N = 4 | N = 8 | ||
| Duration of diarrhea (days) | 3 (1 to 8) | 3 (2 to 8) | 0.8778 |
| N = 3 | N = 5 |
# P values from Wilcoxon rank-sum test
* Tachypnea defined as respiratory rate > 40
Serum and Nasal R. equi-Specific Antibody Responses
Serum IgGa
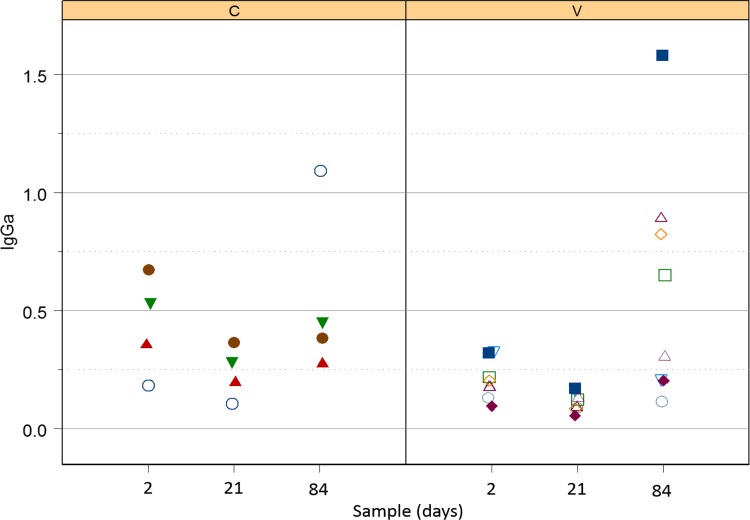
There was no significant effect of either vaccination or the interaction of vaccination and time (i.e., no modification of effects of time by vaccination group; Fig 1) on serum concentrations of anti-R. equi-specific IgGa. For these data, log10 transformation was deemed appropriate for analysis to ensure good model fit. Concentrations of IgGa were significantly (P = 0.0120; LME) lower for foals in both groups at age 21 days (controls: mean relative OD = 0.19; 95% confidence interval = 0.11 to 0.29; vaccinates: mean relative OD = 0.11; 95% confidence interval = 0.07 to 0.19), than at age 2 days (controls: mean relative OD = 0.34; 95% confidence interval = 0.20 to 0.57; vaccinates: mean relative OD = 0.20; 95% confidence interval = 0.11 to 0.36). Concentrations of R. equi-specific IgGa at day 84 were not significantly greater than baseline, although it appeared that IgGa values were higher than baseline for the eBeam vaccinated foals (Fig 1). Concentrations of IgGa were significantly (P < 0.05; LME) greater at age 84 days than age 21 days for foals in both groups (controls: mean relative OD = 0.62; 95% CI = 0.40 to 0.97; vaccinates: mean relative OD = 0.37; 95% CI = 0.24 to 0.58).
Fig 1. Relative OD of IgGa against R. equi in control foals (left panel labeled C) and vaccinated foals (right panel labeled V).
Both groups tended to decrease between days 2 and 21, but this difference was not significant; however, both groups increased significantly between days 21 and 84.
Serum R. equi-specific IgGb
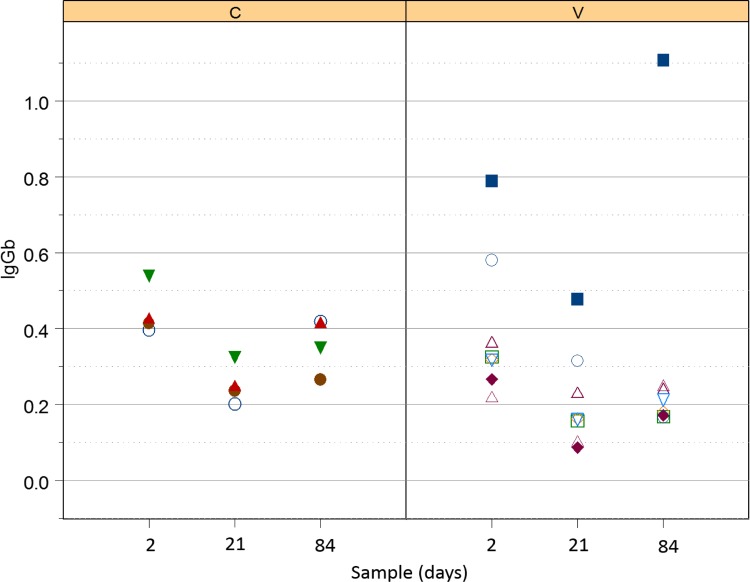
There was no significant effect of either vaccination or the interaction of vaccination and time (i.e., no modification of effects of time by vaccination group; Fig 2) on serum concentrations of IgGb. Concentrations of IgGb were, however, significantly (P = 0.0003; LME) lower for foals in both groups at age 21 days (controls: mean relative OD = 0.25; 95% confidence interval = 0.16 to 0.34; vaccinates: mean relative OD = 0.21; 95% confidence interval = 0.12 to 0.30), than at age 2 day (controls: mean relative OD = 0.44; 95% confidence interval = 0.26 to 0.63; vaccinates: mean relative OD = 0.40; 95% confidence interval = 0.21 to 0.58). Although values for both groups appeared (Fig 2) to be lower at day 84 than day 2, this difference was not significant. Although OD values tended to increase from day 21 to day 84, this difference also was not significant.
Fig 2. Relative OD of IgGb against R. equi in control foals (left panel labelled C) and vaccinated foals (right panel labelled V).
Both groups decreased significantly between days 2 and 21; although values tended to be lower at day 84, this difference was not significant.
Serum R. equi-specific IgG(T)
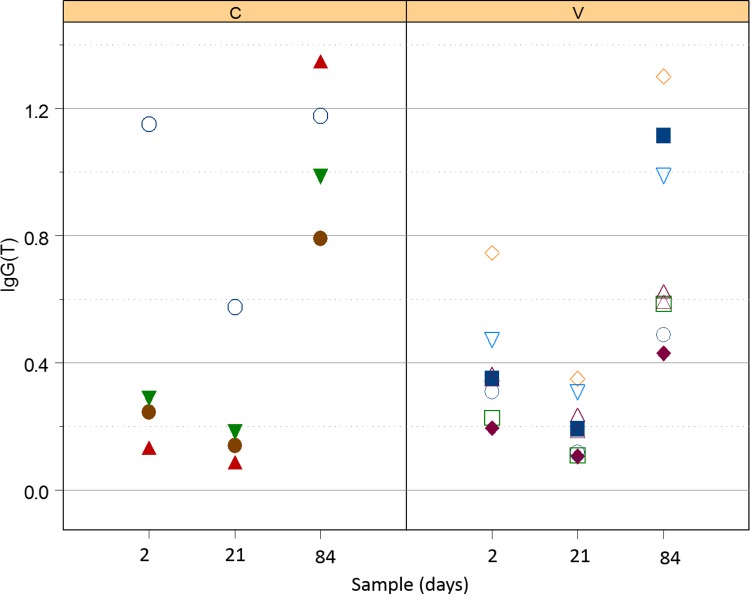
There was no significant effect of either vaccination or the interaction of vaccination and time (i.e., no modification of effects of time by vaccination group; Fig 3) on serum concentration of IgG(T). Concentrations of IgG(T) were, however, significantly (P = 0.0230; LME) lower for foals in both groups at age 21 days (controls: mean relative OD = 0.31; 95% confidence interval = 0.15 to 0.47; vaccinates: mean relative OD = 0.17; 95% confidence interval = 0.01 to 0.33), than at age 2 days (controls: mean relative OD = 0.50; 95% confidence interval = 0.26 to 0.73; vaccinates: mean relative OD = 0.36; 95% confidence interval = 0.07 to 0.64). Values for both groups at day 84 (controls: 0.96; 95% CI = 0.81 to 1.12; vaccinates: 0.82; 95% CI = 0.66 to 0.98) were significantly (P < 0.05; LME) different than values for foals in that group at ages 2 or 21 days.
Fig 3. Relative OD of IgG(T) against R. equi in control foals (left panel labelled C) and vaccinated foals (right panel labelled V).
Both groups decreased significantly between days 2 and 21; values at day 84 were significantly greater than those at either day 2 or 21.
Serum R. equi-specific IgA
There was no significant effect of either vaccination or the interaction of vaccination and time (i.e., no modification of effects of time by vaccination group) on serum concentration of IgA. Although concentrations of IgA were higher for foals in both groups at age 21 days (controls: mean relative OD = 0.69; 95% confidence interval = 0.34 to 1.04; vaccinates: mean relative OD = 0.80; 95% confidence interval = 0.45 to 1.15), than at age 2 days (controls: mean relative OD = 0.39; 95% confidence interval = 0.03 to 0.76; vaccinates: mean relative OD = 0.51; 95% confidence interval = 0.15 to 0.88), the difference was not significant. Values for both groups at day 84 were similar to age 2 days (controls: mean relative OD = 0.42; 95% confidence interval = 0.07 to 0.77; vaccinates: mean relative OD = 0.54; 95% confidence interval = 0.18 to 0.91) and not significantly different than values for foals in that group at age 21 days.
Nasal R. equi-specific IgA
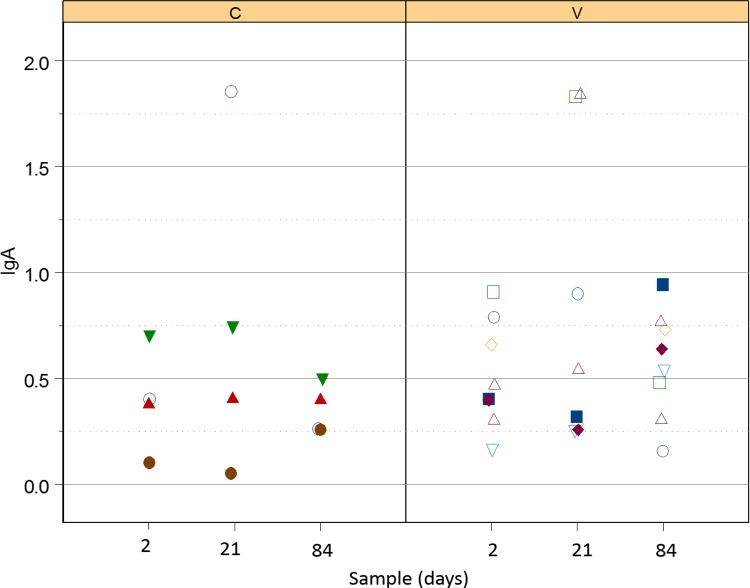
Although values of the relative OD (Fig 4) tended to increase at day 21 for several foals, there were no significant effects of time, group, or their interaction on nasal IgA values.
Fig 4. Relative OD of IgA against R. equi in control foals (left panel labelled C) and vaccinated foals (right panel labelled V).
There were no significant effects of time or group.
CMI Responses
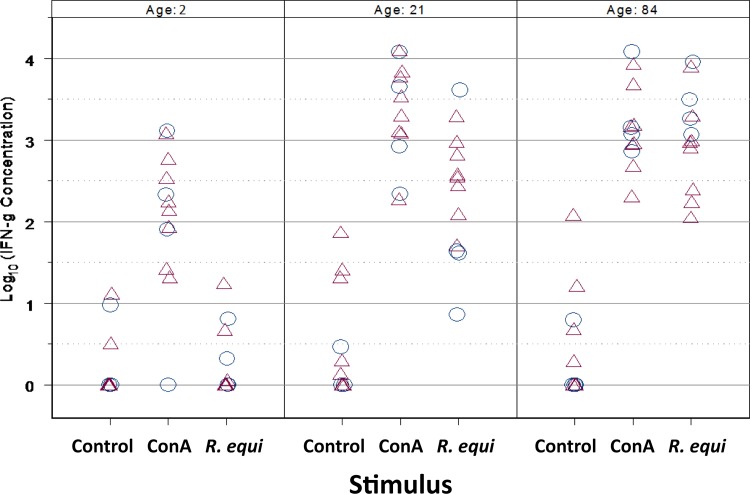
Stimulation with ConA resulted in significant (P < 0.0001; LME) induction of IFN-γ expression by PBMCs of foals at all ages (Fig 5). Responses to ConA were significantly greater on day 21 (P = 0.0060; LME) and on day 84 (P = 0.0160; LME) than on day 2; however the responses to ConA did not differ between foals at day 21 and day 84. Basal expression of INF-γ did not change among ages (Fig 5). Although R. equi antigen did not stimulate a significant increase in IFN-γ expression on day 2, responses on day 21 and day 84 were significantly (P < 0.0001; LME) greater than those observed on day 2 (Fig 5). Moreover, the magnitude of expression of IFN-γ on day 84 was significantly (P = 0.0196; LME) greater than that of day 21 (Fig 5).
Fig 5. Production of IFN-γ by PBMCs exposed to stimuli at ages 2, 21, and 84 days in 12 foals.
Circles represent unvaccinated foals (N = 4) and triangles represent vaccinated foals (N = 8). The mitogen (concavalin A) induced significantly (P < 0.0001; LME) greater expression of IFN-γ relative to the unstimulated PBMCs (Control) at each age; moreover, expression induced at days 21 and 84 was significantly (P = 0.0060 and 0.0160, respectively; LME) greater than that induced on day 2. There was no significant difference among ages for the unstimulated control samples. Stimulation with R. equi did not induce expression of IFN-γ relative to unstimulated control cells on day 2, but did induce significant expression on days 21 and 84 (P < 0.0001; LME) relative to baseline, and expression was significantly greater (0.0196; LME) on day 84 (following infection) than 21 (prior to infection).
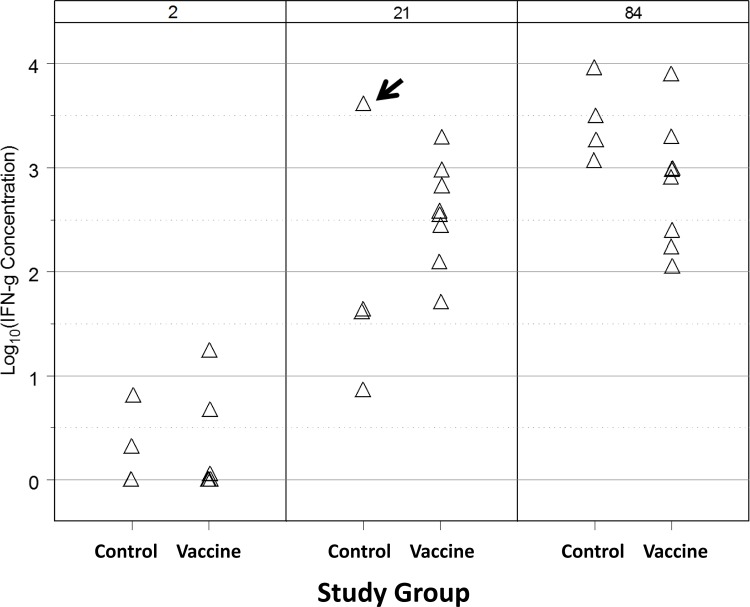
Our primary objective for CMI testing was to compare IFN-γ production by stimulated PBMCs by age between vaccinated and unvaccinated foals. For a given age, there was no significant effect of vaccination on IFN-γ production (Fig 6). Significant effects of age, however, were observed: stimulation with R. equi antigen generated significantly (P < 0.0001; LME) greater IFN-γ production at ages 21 and 84 days relative to day 2, and responses on day 84 were significantly (P < 0.0095; LME) greater on day 84 than on day 21. Graphically, it appeared that there was an outlier among control foals at age 21 days and evaluation of diagnostic residual plots indicated this observation was highly influential. When this observation was excluded, significant effects of age, vaccination, and their interaction were observed. Excluding this foal, the IFN-γ responses were significantly greater at age 21 days (P < 0.05; LME) for the vaccinated foals than the controls, but there was no significant difference between groups at either age 2 days (prior to vaccination) or age 84 days (after intrabronchial infection). Among the control foals, IFN-γ expression in response to treatment was significantly (P < 0.05; LME) greater on day 84 than either days 2 or 21, but values on days 2 and 21 were not significantly different. Among the vaccinated foals, values of IFN-γ were significantly (P < 0.05; LME) greater at day 21 and 84 than day 2, but did not differ significantly between days 21 and 84. These data indicate that, when the outlier was excluded, CMI was significantly (P < 0.05; LME) greater in the vaccinated foals than control foals on day 21 (prior to infection). Interestingly, the foal with the outlier observation was the control foal that did not develop clinical signs of pneumonia following infection.
Fig 6. Production of IFN-γ by PBMCs exposed to R. equi antigen at ages 2, 21, and 84 days in 12 foals: 4 control foals that were unvaccinated and 8 foals vaccinated intragastrically with an eBeam vaccine.
No significant effect of vaccine was observed, but concentrations of IFN-γ increased significantly (P < 0.0001; LME) at ages 21 and 84 days relative to controls, and values were significantly higher on day 84 than 21. A value for 1 control foal on day 21 was considered to be an outlier (arrow). When this value was excluded, significant effects of vaccination that varied by age were observed: excluding this value, the vaccinated group had significantly (P < 0.05; LME) higher IFN-γ expression than control foals on day 21 (but not at the other ages; see text for details).
Discussion
The eBeam-inactivated R. equi vaccine was not effective in protecting foals against experimental infection with live virulent R. equi. Ten of the 12 foals became clinically affected with pneumonia, and there was no significant difference in the proportion developing pneumonia among the vaccinated foals (88%; 7/8) or the control foals (75%; 3/4). Furthermore, clinical parameters such as age at onset or duration of cough, fever, abnormal lung sounds, and ultrasonographic lesions did not differ significantly between groups. Only age at onset of tachypnea differed significantly between groups. In light of other findings, we consider this finding to be the result of chance. It should be noted that all foals in all groups developed at least 1 thoracic ultrasonographic lesion > 1 cm in diameter (including the 2 foals that remained free of clinical pneumonia). In the clinical experience of the authors, the observed clinical signs and thoracic ultrasonographic lesions were similar to those observed with naturally-occurring R. equi pneumonia in foals. As observed with other relatively low-dose challenge models [40,41], not all foals developed clinical signs following intrabronchial infection. This is consistent with observations that many foals naturally infected may develop sub-clinical infection [40,42,43].
The eBeam vaccine did not elicit either systemic humoral or mucosal humoral immune responses. A previous report from our laboratory similarly documented that oral administration of eBeamed virulent R. equi failed to elicit systemic humoral immune responses [36]; however, in that study there was evidence that vaccination stimulated a significant increase in nasal IgA against R. equi. Reasons for this discrepancy in IgA responses between these studies are unknown. One possibility is that the previous study used 4 intragastric vaccinations during the first 21 days of age, whereas in this study we performed only 3 intragastric vaccinations during the first 14 days of age. Conceivably, the responses observed in our previous report might have resulted from the additional vaccine dose. Alternatively, foals might have developed stronger and thus detectable responses at an older age (i.e., at 30 days as in the previous study rather than at age 21 days as for this study). Our rationale for decreasing from 4 to 3 doses of vaccine for this study were to mimic the design of the most recent report documenting the efficacy of oral administration of live R. equi to protect against challenge at 21 days of age [35], to evaluate responses to challenge earlier in life than 30 days, and because our belief that giving fewer vaccines would be associated with better adaptation and compliance by veterinarians and farm staff.
All serum sub-isotypes of IgG (i.e., IgGa, IgGb, and IgG(T)) decreased from day 2 to day 21 in foals of both groups. This decrease was attributed to decay of maternal transfer of antibodies [44]. Values of serum R. equi-specific IgGa increased from day 21 (pre-infection) to day 84 (post-infection), indicating that serum IgGa might be a marker of infection; however, the values for age 84 weren’t significantly greater than those for day 2, indicating that this response was neither very robust nor consistent. Serum concentrations of IgGb did not appear to increase following infection and thus appeared to be a poor indicator of exposure and disease. In contrast, IgG(T) increased significantly between day 21 and 84 and the values at day 84 were also significantly greater than those at day 2. These results using a whole-cell R. equi antigen are consistent with a recent report indicating that IgGb specific for VapA was not useful either for diagnosis or prediction (screening) in foals with naturally-occurring or experimental pulmonary infection with R. equi [45], but that VapA-specific IgGa and IgG(T) were significantly increased following maternal decay, and that the magnitude of increase was greater for IgG(T) [46, 47]. IgGa is thought to be induced by a Th1-biased immune response, which is vital for intracellular pathogenic elimination, whereas IgGb and IgG(T) are thought to result from a Th2-type immune response [48]. Because Th1-type immune responses are considered to be important for control of intracellular pathogens, it has been suggested that those foals that develop R. equi pneumonia are biased towards an ineffective Th2-type response [48]. Alternatively, progression of the disease process might result in sub-isotype class switching. High titers of IgG(T) are important as they opsonize R. equi and engage the Fc receptors in neutrophils, which have decreased phagocytic and killing capacities in the neonatal foal [49–51]. This engagement of IgG(T) to the Fc receptors increases phagocytosis of R. equi by opsonization [46]. As noted previously [47], further evaluation of IgG(T) for diagnostic and screening purposes is warranted.
A significant CMI response to the R. equi strain used for the vaccine as measured by IFN-γ expression by cultured PBMCs was observed when an influential statistical outlier was excluded: IFN-γ responses were not significantly different on day 2 (prior to vaccination) for both control and vaccinated foals, but by day 21 vaccinated foals showed a significantly greater IFN-γ response than the controls. At age 84 days, neither vaccinated nor unvaccinated foals differed in their IFN-γ production. These findings are consistent with our previous results documenting evidence of intragastric vaccination of eBeamed R. equi eliciting CMI responses. Our latest CMI results must be interpreted with caution because statistical significance was only observed when an outlier was excluded, and this outlier was a control (unvaccinated foal) with a very high CMI response. Interestingly, this control foal was the 1 control foal that did not develop clinical pneumonia following challenge. Regardless, the CMI responses observed in this study in response to vaccination were not protective against intrabronchial infection. As expected, we observed increased production of IFN-γ with age in response to either stimulus (the mitogen ConA [positive control] or the R. equi antigen). Foals express less IFN-γ early in life [52–54]. This inability to express IFN-γ likely impairs the ability of young foals to mount a robust Th1-based cell mediated immune response, thereby contributing to their susceptibility to intracellular pathogens such as R. equi. This might also contribute to inefficient antigen priming that impairs the foal’s ability to respond to antigens [55, 56].
There are a number of reasons why this vaccine might have failed in this study to protect foals despite previous evidence of immunogenicity. Whereas live organisms multiply, the eBeam inactivated bacterial cells do not: thus, it is possible that the magnitude of the antigenic load delivered to the gastrointestinal tract might have been inadequate to stimulate immune responses that protected against infectious challenge. Conceivably, a larger dose or more frequent administration of intragastric eBeamed R. equi might have provided protection. In our previous study of the immunogenicity of oral, virulent R. equi in foals, we used a similar dose of vaccine but administered 4 doses rather than the 3 doses administered for this project [36]. Alternatively, some degree of intracellular replication might be required to stimulate protective immunity. It is also possible that the route of vaccine administration may not have been optimal.
Although it is generally considered that CMI responses are essential for immunity to intracellular pathogens, evidence exists that humoral systemic immune responses can provide protection. In our previous study [36] we noted that oral administration of live, virulent R. equi stimulated a systemic R. equi-specific IgGa response whereas the oral eBeam vaccine did not. Conceivably, antibody responses might be essential for protection against R. equi. Evidence exists that transfusion of hyperimmune plasma can protect foals against experimentally-induced and naturally-occurring R. equi pneumonia,[19–23] although conflicting evidence exists [23, 57].
In summary, our eBeam-inactivated vaccine was neither strongly immunogenic nor protective against experimental infection with live, virulent R. equi. Despite these initial disappointing results, this type of vaccine approach should not be entirely dismissed because a higher dose, stronger dose, more frequent administration, or an alternative method of administration needs to be evaluated. Further understanding of the mechanisms that provide protection to foals when administered live, virulent R. equi and availability of a small animal model of R. equi pneumonia would further facilitate vaccine development.
Acknowledgments
The authors would like to thank Ms. Laura Bilke, Skye Broyles, Kara Colwell, Amber Telscher, and Krissy Johnson Schroeder for technical assistance with animal work, Mr. Mickey Speakmon for electron beam irradiation, and Boehringer-Ingelheim Vetmedica, Inc for donation of romifidine. Funding for this study was provided by the Grayson-Jockey Club Research Foundation. Additional support was provided by the Link Equine Research Endowment at Texas A&M University.
Data Availability
All 2 files are available from the Figshare (https://figshare.com) database (DOIs: 10.6084/m9.figshare.2062566 and 10.6084/m9.figshare.2062575).
Funding Statement
Funded by Grayson-Jockey Club Research Foundation (https://www.grayson-jockeyclub.org/) (Grant to NDC) and Link Equine Research Endowment (Support to NDC). The open access publishing fees for this article have been covered by the Texas A&M University Online Access to Knowledge (OAK) Fund, supported by the University Libraries and the Office of the Vice President for Research.
References
- 1.Takai S (1997) Epidemiology of Rhodococcus equi infections: a review. Vet Microbiol 56: 167–176. [DOI] [PubMed] [Google Scholar]
- 2.Giguère S, Prescott JF (1997) Clinical manifestations, diagnosis, treatment, and prevention of Rhodococcus equi infections in foals. Vet Microbiol 56: 313–334. [DOI] [PubMed] [Google Scholar]
- 3.Giguère S, Hondalus MK, Yager JA, Darrah P, Mosser DM, Prescott JF (1997) Role of the 85-kilobase plasmid and plasmid-encoded virulence-associated protein A in intracellular survival and virulence of Rhodococcus equi. Infect Immun 67:3548–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takai S, Sekizaki T, Ozawa T, Sugawara T, Watanabe Y, Tsubaki S (1991) Association between a large plasmid and 15- to 17-Kilodalton antigen in virulent Rhodococcus equi. Infect Immun 59:4056–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tkachuck-Saad O, Prescott JF.(1991) Rhodococus equi plasmids: isolation and partial characterization. J Clin Microbiol 29: 2696–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott JF (1991) Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev 4: 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witonsky S. (2010), CVID in a horse with chronic peritonitis: Case review and overview of other immunodeficiencies. Equine Veterinary Education, 22: 400–402. [Google Scholar]
- 8.Giguère S, Cohen ND, Chaffin MK, Hines SA, Hondalus MK, Prescott JF, et al. (2011) Rhodococcus equi: Clinical Manifestations, Virulence, and Immunity. J Vet Intern Med 25:1221–1230. 10.1111/j.1939-1676.2011.00804.x [DOI] [PubMed] [Google Scholar]
- 9.Venner M, Meyer-Hamme B, Verspohl J, Hatori F, Shimizu N, Sasaki Y, et al. (2007) Genotypic characterization of VapA positive Rhodococcus equi in foals with pulmonary affection and their soil environment on a warmblood horse breeding farm in Germany. Res Vet Sci 83: 311–317. [DOI] [PubMed] [Google Scholar]
- 10.Muscatello G (2012) Rhodococcus equi pneumonia in the foal–part 1: Pathogenesis and epidemiology. Vet J 192: 20–26. 10.1016/j.tvjl.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 11.Woolcock JB, Mutimer MD, and Bowles PM (1987) The immunological response of foals to Rhodococcus equi: a review. Vet Microbiol 14:215–224. [DOI] [PubMed] [Google Scholar]
- 12.Giguère S, Cohen ND, Chaffin KM, Hines SA, Hondalus MK, Prescott JF et al. (2011) Rhodococcus equi: Clinical manifestations, virulence, and immunity. J Vet Intern Med 25: 1221–1230. 10.1111/j.1939-1676.2011.00804.x [DOI] [PubMed] [Google Scholar]
- 13.Giguère S, Hernandez J, Gaskin J, Miller C, Bowman JL (2003) Evaluation of white blood cell concentration, plasma fibrinogen concentration, and an agar gel immunodiffusion test for early identification of foals with Rhodococcus equi pneumonia. J Am Vet Med Assoc 222: 775–781. [DOI] [PubMed] [Google Scholar]
- 14.Chaffin MK (2013) Evaluation of hematologic screening methods for predicting subsequent onset of clinically-apparent Rhodococcus equi pneumonia in foals. In: 59th Annual Convention of the American Association of Equine Practitioners, Nashville, Tennessee, USA, p 267.
- 15.Takai S, Sasaki Y, Tsubaki S (1995) Rhodococcus equi infection in foals–current concepts and implications for future research. J Equine Sci 4: 105–119. [Google Scholar]
- 16.Cohen ND, Smith KE, Ficht TA, Takai S, Libal MC, West BR, et al. (2003) Epidemiologic study of results of pulsed-field gel electrophoresis of isolates of Rhodococcus equi obtained from horses and horse farms. Am J Vet Res 64:153–161. [DOI] [PubMed] [Google Scholar]
- 17.Donecker JM, Holland RE (2006) Efficacy of the Immunomodulator Zylexis in horses challenged with equine herpes-virus under field conditions. Pfizer Animal Health. [Google Scholar]
- 18.Chaffin MK, Cohen ND, Martens RJ (2008) Chemoprophylacitic effects of azithromycin against Rhodococcus equi-induced pneumonia among foals at equine breeding farms with endemic infections. J Am Vet Med Assoc 232: 1035–1047. 10.2460/javma.232.7.1035 [DOI] [PubMed] [Google Scholar]
- 19.Madigan JE, Hietala S, Muller N (1991) Protection against naturally acquired Rhodococcus equi pneumonia in foals by administration of hyperimmune plasma. J reprod Fert 44:571–578. [PubMed] [Google Scholar]
- 20.Caston SS, McClure SR, Martens RJ, Chaffin MK, Miles KG, Griffith RW, et al. (2006) Effect of hyperimmune plasma on the severity of pneumonia caused by Rhodococcus equi in experimentally infected foals. Vet Ther 7: 361–375. [PubMed] [Google Scholar]
- 21.Martens RJ, Martens JG, and Fiske RA (1989) Rhodococcus equi foal pneumonia: Protective effects of immune plasma in experimentally infected foals. Equine Vet J 21:249–255. [DOI] [PubMed] [Google Scholar]
- 22.Wilson EM, Holcombe SJ, Lamar A, Hauptman JG, Brooks MB (2009) Incidence of transfusion reactions and retention or procoagulant and anticoagulant factor activities in equine plasma. J Vet Med 23: 323–328. [DOI] [PubMed] [Google Scholar]
- 23.Giguère S, Gaskin JM, Miller C, Bowman JL (2002) Evaluation of a commercially available hyperimmune plasma product for the prevention of naturally acquired pneumonia caused by Rhodococcus equi in foals. J Am Vet Med Assoc 220:59–63. [DOI] [PubMed] [Google Scholar]
- 24.Martens RJ, Martens JG, Fiske RA (1991) Failure of passive immunization by colostrum from immunized mares to protect foals against Rhodococcus equi pneumonia. Equine Vet J 23: 19–22. [Google Scholar]
- 25.Cauchard J, Sevin C, Ballet JJ, Taouji S (2004) Foal IgG and opsonizing anti-Rhodococcus equi antibodies after immunization of pregnant mares with a protective VapA candidate vaccine. Vet Microbiol 104: 73–81. [DOI] [PubMed] [Google Scholar]
- 26.Becú T, Polledo G, Gaskin JM (1997) Immunoprophylaxis of Rhodococcus equi pneumonia in foals. Vet Microbiol 56: 193–204. [DOI] [PubMed] [Google Scholar]
- 27.Prescott JF, Nicholson VM, Patterson MC, Zandona Meleiro MC, Caterino de Araujo A, Yager JA, et al. (1997) Use of Rhodococcus equi virulence-associated protein for immunization of foals against R. equi pneumonia. Am J Vet Res 58: 356–359. [PubMed] [Google Scholar]
- 28.Kohler AK, Stone DM, Hines MT, Byrne BA, Alperin DC, Norton LK, et al. (2003) Rhodococcus equi secreted antigens are immunogenic and stimulate a type 1 recall response in the lungs of horses immune to R. equi infection. Infect Immun 71: 6329–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashour J, Hondalus MK (2003) Phenotypic mutants of the intracellular actinomycete Rhodococcus equi created by in vivo Himar1 transposon mutagenesis. J Bacteriol 185: 2644–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pei Y, Nicholson V, Woods K, Prescott JF (2007) Immunization by intrabronchial administration to 1-week-old foals of an unmarked double gene disruption strain of Rhodococcus equi strain 103+. Vet Microbiol 125: 100–110. [DOI] [PubMed] [Google Scholar]
- 31.Mealey RH, Stone DM, Hines MT, Alperin DC, Littke MH, Leib SR, et al. (2007) Experimental Rhodococcus equi and equine infectious anemia virus DNA vaccination in adult and neonatal horses: effect of IL-12, and route. Vaccine 25: 7582–7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phumoonna T, Barton MD, Vanniasinkam T, Heuzenroeder MW (2008) Chimeric vapA/groEL2 DNA vaccines enhance clearance of Rhodococcus equi in aerosol challenged C3H/He mice. Vaccine 26: 2457–2465. 10.1016/j.vaccine.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 33.Giles C, Vanniasinkam T, Ndi S, Barton MD (2015) Rhodococcus equi (Prescottella equi) vaccines; the future of vaccine development. Equine Vet j 47: 510–518. 10.1111/evj.12310 [DOI] [PubMed] [Google Scholar]
- 34.Chirino-Trejo JM, Prescott JF, and Yager JA (1987) Protection of foals against experimental Rhodococcus equi pneumonia by oral immunization. Can J Vet Res 51: 444–447. [PMC free article] [PubMed] [Google Scholar]
- 35.Hooper-McGrevy KE, Wilkie BN, Prescott JF (2005) Virulence-associated protein-specific serum immunoglobulin G-isotype expression in young foals protected against Rhodococcus equi pneumonia by oral immunization with virulent R. equi. Vaccine 23: 5760–5767. [DOI] [PubMed] [Google Scholar]
- 36.Bordin AI, Pillai SD, Brake C, Bagley KB, Bourquin JR, Coleman M, et al. (2014) Immunogenicity of an electron beam inactivated Rhodococcus equi vaccine in neonatal foals. PLoS ONE 9:e105367 10.1371/journal.pone.0105367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chauhan OP, Unni LE. Pulsed electric field (PEF) processing of foods and its combination with electron beam processing In: Pillai S, Shayanfar S, editors. Electron beam pasteurization and complementary food processing technologies. Cambridge: Woodland Publishing; 2015. p. 157–168. [Google Scholar]
- 38.Burton AJ, Giguère S, Arnold RD (2014) Pharmacokinetics, pulmonary disposition and tolerability if liposomal gentamicin and free gentamicin in foals. Equine Vet J 47: 467–472. 10.1111/evj.12309 [DOI] [PubMed] [Google Scholar]
- 39.Lopez AM, Hines MT, Palmer GH, Alperin DC, Hines SA (2002) Identification of pulmonary T-lymphocyte and serum antibody isotype responses associated with protection against Rhodococcus equi. Clin Diagn Lab Immunol 9: 1270–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanz M, Loynachan A, Sun L, Oliveira A, Brehent P, Horohov DW (2013) The effect of bacterial dose and foal age at challenge on Rhodococcus equi infection. Vet Microbiol 167: 623–631. 10.1016/j.vetmic.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 41.Jacks S, Giguère S, Crawford PC, Castleman WL (2007) Experimental infection of neonatal foals with Rhodococcus equi triggers adult-like gamma interferon induction. Clin Vaccine Immunol 14: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venner M, Rödiger A, Laemmer M, Giguère (2012) Failure of antimicrobial therapy to accelerate spontaneous healing of subclinical pulmonary abscesses on a farm with endemic infections caused by Rhodococcus equi. Vet J 192: 293–298. 10.1016/j.tvjl.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 43.McQueen CM, Doan R, Dindot SV, Bourquin JR, Zlatev ZZ, Chaffin MK, et al. (2014) Identification of genomic loci associated with Rhodococcus equi susceptibility in foals. PLoS One 9: e98710 10.1371/journal.pone.0098710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sedlinská M , Krejčí J , Vyskočil M , Kudláčková H (2006) Postnatal development of blood serum concentrations of immunoglobulin IgG, IgA and IgM isotypes in suckling foal. Acta Vet Brno 75:175–182. [Google Scholar]
- 45.Sanz MG, Oliveira AF, Loynachan A, Page A, Svansson V, Giguère S, et al. (2015) Validation and evaluation of VapA-specific IgG and IgG subclass enzyme-linked immunosorbent assays (ELISAs) to identify foals with Rhodococcus equi pneumonia. Equine Vet J 10.1111/evj.12363 [DOI] [PubMed] [Google Scholar]
- 46.Lewis MJ, Wagner B, Woof JM (2008) The different effector function capabilities of the seven equine IgG subclasses have implications for vaccine strategies. Mol Immunol 45: 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanz MG, Villarino N, Ferreira-Oliveira A, Horohov DW (2015) VapA-specific IgG and IgG subclasses responses after natural infection and experimental challenge of foals with Rhodococcus equi. Vet Immunol Immunop 164: 10–15. [DOI] [PubMed] [Google Scholar]
- 48.Perkins GA and Wagner B (2015) The development of equine immunity: Current knowledge on immunology in the young horse. Equine Vet J 47: 267–274. 10.1111/evj.12387 [DOI] [PubMed] [Google Scholar]
- 49.Joller N, Weber SS, Müller AJ, Spörri R, Selchow P, Sander P, et al. (2010) Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting. PNAS 107: 20441–20446. 10.1073/pnas.1013827107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hooper-McGrevy KE, Wilkie BN, Prescott JF (2003) Immunoglobulin G subisotype responses of pneumonic and healthy, exposed foals and adult horses to Rhodococcus equi virulence-associated proteins. Clin Diag Lab Immunol 10:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bordin AI, Liu M, Nerren JR, Buntain SL, Brake CN, Kogut MH, et al. (2012) Neutrophil function of neonatal foals is enhanced in vitro by CpG oligodeoxynucleotide stimulation. Vet Immunol Immunop 145: 290–297. [DOI] [PubMed] [Google Scholar]
- 52.Boyd NK, Cohen ND, Lim WS, Martens RJ, Chaffin MK, Ball JM (2003) Temporal changes in cytokine expression of foals during the first month of life. Vet Immunol Immunopathol 92: 75–85. [DOI] [PubMed] [Google Scholar]
- 53.Sturgill TL, Giguère S, Berghaus LJ, Hurley DJ, Hondalus MK (2014) Comparison of antibody and cell-mediated immune responses of foals and adult horses after vaccination with live Mycobacterium bovis BCG. Vaccine 32: 1362–1367. 10.1016/j.vaccine.2014.01.032 [DOI] [PubMed] [Google Scholar]
- 54.Breathnach CC, Sturgill-Wright T, Stiltner JL, Adams AA, Lunn DP, Horohov DW (2006) Foals are interferon gamma-deficient at birth. Vet. Immunol. Immunopathol. 112:199–209. [DOI] [PubMed] [Google Scholar]
- 55.Sturgill TL, Strong D, Rashid C, Betancourt A, Horohov (2011) Effect of Propionibacterium acnes-containing immunostimulant on interferon-gamma (IFNγ) production in the neonatal foal. Vet Immunol Immunopathol 141: 124–127. 10.1016/j.vetimm.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 56.Ryan C and Giguère S (2010) Equine Neonates Have Attenuated Humoral and Cell-Mediated Immune Responses to a Killed Adjuvanted Vaccine Compared to Adult Horses. Clin Vaccine Immunol 17: 1896–1902. 10.1128/CVI.00328-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurley JR, Begg AP (1995) Failure of hyperimmune plasma to prevent pneumonia caused by Rhodococcus equi in foals. Aust Vet J 72: 418–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All 2 files are available from the Figshare (https://figshare.com) database (DOIs: 10.6084/m9.figshare.2062566 and 10.6084/m9.figshare.2062575).