Abstract
Introduction
Obstructive sleep apnoea (OSA), the most common type of sleep-disordered breathing, is associated with significant immediate and long-term morbidity, including fragmented sleep and impaired daytime functioning, as well as more severe consequences, such as hypertension, impaired cognitive function and reduced quality of life. Perioperatively, OSA occurs frequently as a consequence of pre-existing vulnerability, surgery and drug effects. The impact of OSA on postoperative respiratory complications (PRCs) needs to be better characterised. As OSA is associated with significant comorbidities, such as obesity, pulmonary hypertension, myocardial infarction and stroke, it is unclear whether OSA or its comorbidities are the mechanism of PRCs. This project aims to (1) develop a novel prediction score identifying surgical patients at high risk of OSA, (2) evaluate the association of OSA risk on PRCs and (3) evaluate if pharmacological agents used during surgery modify this association.
Methods
Retrospective cohort study using hospital-based electronic patient data and perioperative data on medications administered and vital signs. We will use data from Partners Healthcare clinical databases, Boston, Massachusetts. First, a prediction model for OSA will be developed using OSA diagnostic codes and polysomnography procedural codes as the reference standard, and will be validated by medical record review. Results of the prediction model will be used to classify patients in the database as high, medium or low risk of OSA, and we will investigate the effect of OSA on risk of PRCs. Finally, we will test whether the effect of OSA on PRCs is modified by the use of intraoperative pharmacological agents known to increase upper airway instability, including neuromuscular blockade, neostigmine, opioids, anaesthetics and sedatives.
Ethics and dissemination
The Partners Human Research Committee approved this study (protocol number: 2014P000218). Study results will be made available in the form of manuscripts for publication and presentations at national and international meetings.
Keywords: EPIDEMIOLOGY
Strengths and limitations of this study.
This work uses a large clinical database consisting of preoperative, intraoperative and postoperative patient data.
Our prediction model draws on well-established clinical characteristics associated with obstructive sleep apnoea (OSA) as well as new measures aimed at improving dynamic risk assessment in a perioperative setting.
The results of this study may enable perioperative clinicians to identify adult surgical patients at highest risk for OSA, optimise preoperative interventions, and appropriately triage care postoperatively based on intraoperative events.
Potential limitations relate to the need for validation studies in data sets from other institutions to determine generalisability of prediction score.
Introduction
Background
Obstructive sleep apnoea (OSA) is a common disorder characterised by recurrent collapse of the upper airway. This chronic condition may be diagnosed by the presence of symptoms and, depending on the specific criteria used for making the diagnosis, more than five episodes of apnoea, hypopnoea or respiratory effort-related arousal per hour of sleep (apnoea hypopnoea index, AHI, ≥5/h).1 2 Daytime symptoms refer to excessive daytime sleepiness, morning headaches, decreased concentration, memory loss, decreased libido and irritability. Other OSA-related symptoms include witnessed apnoea, snoring, non-refreshing sleep, and gasping or choking at night.3
Recent epidemiological data report that an estimated 70 million people in the USA alone are affected by OSA, making it the most common type of sleep-disordered breathing (SDB).4 5 In the general adult population, approximately 13% of men and 6% of women have moderate-to-severe SDB, defined as AHI ≥15/h.5 It is also estimated that 14% of men and 5% of women have AHI ≥5/h plus daytime symptoms.5 The prevalence of SDB without daytime symptoms is even higher and reaches values of up to 9% in women and 24% in men.2 6 It is possible that such epidemiological data underestimate the frequency of OSA among today's general population since obesity, a major driver of OSA,7 has greatly increased in the last decade.5 8 Furthermore, studies have shown that OSA is commonly undiagnosed, suggesting an even higher prevalence of adults who suffer from this sleep disorder.9–11
Surgical patients with OSA are at a higher risk of developing postoperative respiratory complications (PRCs), such as reintubation and requirement of non-invasive ventilation.12–14 Upper airway collapse in the perioperative setting results in hypoventilation and is an important component of the mechanism of PRCs. In studies previously reported by our laboratory, independent of OSA, reintubation and unplanned ICU admission result in a 70-fold to 90-fold increase in in-hospital mortality.15 16 However, despite an increased rate of PRCs, SDB, as identified by diagnostic codes, was paradoxically associated with lower mortality, hospital length of stay and costs among certain surgical specialties.12 The mechanisms of the opposed effects of OSA on respiratory complication rate and mortality are unclear. We speculate that reintubation in patients with OSA is typically the consequence of upper airway dysfunction rather than pulmonary pathology, and the former can be treated more efficiently.
Mechanism of perioperative obstructive sleep apnoea
Quantification of perioperative vulnerability to upper airway collapse requires consideration of preoperative and perioperative risk factors that affect the balance between collapsing forces and dilating forces of the upper airway. Perioperative anatomical and physiological factors need to be taken into account.
Anatomical abnormalities increase collapsing forces
Anatomical risk factors in patients with OSA include a reduction in the size of the retropalatal and retroglossal airway.17 18 Perioperatively, anatomical vulnerability is augmented, thereby increasing upper airway instability.
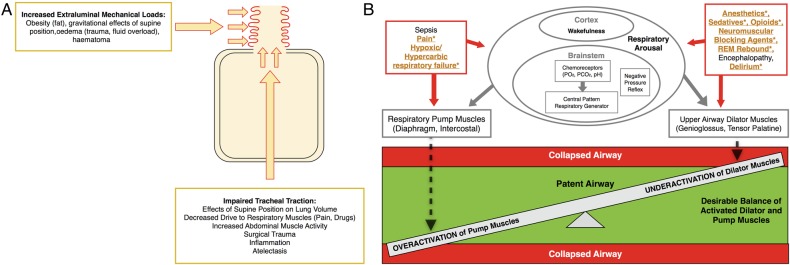
Figure 1A summarises perioperative risk factors that can compromise upper airway anatomy. Mechanical loads to the collapsible segments of the retropalatal and retropharyngeal upper airway lead to physical compression of the airway. Clinically, such an extraluminal mechanical load can occur as a consequence of a postoperative haematoma following cervical, otolaryngology or thyroid surgery.19 20 In addition, peripharyngeal oedema may occur in perioperative medicine as a consequence of fluid overload. Bradley and colleagues studied the effects of antishock trouser inflation on upper airway size, and reported narrowed pharynx and enlarged neck circumference measured by acoustic pharyngometry.21 Congestive heart failure increases the AHI, which presumably, is the consequence of nocturnal rostral fluid shift.22 Airway patency may also be affected by peripharyngeal inflammation and oedema in the setting of intubation and extubation.
Figure 1.
Pathophysiology of perioperative obstructive sleep apnoea. (A) Pathological anatomy. This schematic of the respiratory system demonstrates the anatomical forces (red arrows) increasing collapsibility of the upper airway (red curly lines). Caudal tracheal traction stabilises the upper airway such that it is less vulnerable to collapse. CPAP treatment can evoke caudal tracheal traction and increase end-expiratory lung volume. Collapsing physical forces are those that increase the mechanical load on the upper airway (haematoma, oedema, fat) and those that reduce caudal tracheal traction (atelectasis, supine, flat position). (B) Pathological physiology. The vulnerable perioperative upper airway physiology is illustrated as a scale, demonstrating the fragile balance between activation of respiratory pump muscles and upper airway dilator muscles (green zone). When activated, pump muscles generate negative inspiratory pressure and tip the balance to upper airway collapse (red zone). In normal physiology, upper airway dilator muscles activate to counterbalance the negative inspiratory pressure and dilate the upper airway. Underactivation of airway dilator muscles, such as the tongue muscle, will result in collapse (red zone). A variety of perioperative events affect respiratory arousal, which can impair airway patency by overactivating pump or underactivating dilator muscles, respectively. Patients with OSA are at higher vulnerability towards collapse, and the specific pathophysiological mechanism of the increased perioperative vulnerability to collapse in OSA are emphasised in yellow colour and denoted with an asterisk. CPAP, continuous positive airway pressure; OSA, obstructive sleep apnoea.
Impaired caudal traction on the trachea increases collapsibility
Isono and colleagues have conducted extensive investigations of position-dependent effects on airway obstruction. In anaesthetised and paralysed patients with OSA, the authors found that the lateral and sitting positions improve the collapsibility of the passive pharyngeal airway.23 24
Among patients with OSA, the supine position not only promotes a more obstructive orientation of the pharyngeal soft tissues, but also reduces caudal traction, thereby increasing vulnerability to upper airway collapse.
During inspiration, caudal traction on the airway due to lung expansion dilates and stabilises the upper airway, a force that opposes the negative intraluminal pressure and prevents collapse.25 The supine position during surgery, immediate postoperative period, and transition to sleep impairs tracheal traction on the airway and promotes collapse,23 24 as illustrated in figure 1A. Tracheal traction is also impaired by any event that reduces lung volume, often secondary to diaphragmatic dysfunction. Impaired function of the respiratory pump muscles (diaphragm and intercostal muscles) results in ineffective expansion of the lung and occurs in the setting of surgery and trauma.26 Pain-induced splinting and pharmacological agents, such as opioids, decrease drive to the respiratory pump muscles, thereby preventing full lung inflation and reducing tracheal traction.27 Studies in the intensive care unit have demonstrated how systemic inflammation and mechanical ventilation dramatically disrupts diaphragmatic function.28 29
Neuromuscular mechanisms of perioperative airway collapse
A balance between the upper airway dilator muscles (genioglossus, tensor palatine) and the respiratory pump muscles (diaphragm, intercostal muscles) exist to maintain upper airway patency during wakefulness and sleep, as illustrated in figure 1B. Respiratory pump muscles generate inspiratory airflow associated with negative intraluminal pressure, which is detected by mechanoreceptors and transmitted to the upper airway dilator muscles via the hypoglossal nerve. As a result, the genioglossus contracts and stabilises the upper airway. Respiration is also stimulated by hypoxia and hypercarbia, which are detected by chemoreceptors. In addition to wakefulness, information transmitted by mechanoreceptors and chemoreceptors stimulate respiratory arousal, which has been previously defined as arousal from sleep and other drug-induced or endogenous impairments of consciousness.30 Cortical effects on respiratory arousal are important, and any decrease in arousal can impair the voluntary effort to breathe spontaneously through a patent upper airway.31
A variety of pharmacological and non-pharmacological perioperative factors affect respiratory arousal. While the specific effects of perioperative pharmacological agents depend on agent, dose and specific muscle group, studies have shown that such agents largely dampen stimulation to the nerves controlling respiratory muscles.
Anaesthetics and sedatives
Studies in humans and animals have demonstrated the effects of anaesthetics on the upper airway by a variety of mechanisms. Anaesthetics decrease muscle and neural activity important for respiration as well as wakefulness through varying mechanisms.32 Propofol, an agent commonly used for induction and maintenance of anaesthesia, dose-dependently increases collapsibility of the upper airway through depressed respiratory drive to and direct inhibition of upper airway dilator muscle activity in humans.33 In humans, anaesthetised with isoflurane, reflexive activity or the responsiveness of upper airway dilator muscles to negative pressure, was found to be greatly reduced.34 The diminishing effects of anaesthetics on neuronal activity also differ between hypoglossal and phrenic nerve.35 With a focus on neural mechanisms for altered upper airway activity, Nishino et al36 investigated the differential effects of anaesthetics and found greater dampening of hypoglossal nerve input relative to the phrenic nerve. This effect may result in greater anaesthesia-induced impairment of upper airway dilators compared to respiratory pump muscles, increasing the upper airway's propensity for collapse. While this effect was observed across three classes of drugs (volatile, barbiturate and benzodiazepine), ketamine reduced neural input to the upper airway dilator muscles and respiratory pump muscles equally. Furthermore, ketamine's effect on the upper airway dilator muscles was less relative to GABAergic anaesthetics.36 Such findings are corroborated by mechanistic studies in rats that demonstrate a dissociation between loss of consciousness and upper airway dilator muscle function under ketamine anaesthesia.37 Taken together, studies suggest that patients with OSA, who have preoperative upper airway instability, may be at a heightened risk of upper airway collapse when under the influence of anaesthetics. The unique effects associated with ketamine, however, suggest that this drug may be a safer choice for patients with OSA.
Opioids
The use of opioids for postoperative pain management has been increasingly identified as a contributor to postoperative exacerbation of SDB.38 39 Studies in human and animal subjects have investigated the mechanism by which patients with preoperative OSA may be vulnerable to the effects of perioperative opioids. Patients with OSA have increased sensitivity to pain40–42 as well as increased sensitivity to the respiratory depressant effects of opioids.43 Such findings are particularly relevant to the patient with postoperative OSA given the effects of opioids on upper airway patency. Animal studies have shown that opioids increase upper airway resistance, resulting in obstruction.44 Opioids directly inhibit hypoglossal motoneurons, which leads to suppressed genioglossus activity.45 Thus, the use of opioids during and immediately after surgery is an important perioperative factor to consider in patients with OSA when assessing the risk of upper airway instability and the PRCs that may arise as a consequence.
Neuromuscular blocking agents and reversal agents
Neuromuscular blockade agents act longer than the duration of surgery and postoperative residual curarisation affects postoperative respiratory outcome.46 Upper airway dilators are more vulnerable to minimal effects of neuromuscular blocking agents compared to the respiratory pump muscles.47 48 This differential activation of pump versus dilator muscles may set off an unwanted chain of events such that the relatively more active respiratory pump muscles generate excessive negative intrathoracic pressure, resulting in negative pressure pulmonary oedema.49 Even at levels producing minimal blockade, as measured by train-of-four ratio 0.5–1, neuromuscular blocking agents increased upper airway collapsibility and impaired compensatory genioglossus response to negative pharyngeal pressure challenges.50 Studies in surgical patients have demonstrated the dose-dependent association between intermediate-acting neuromuscular blocking agents and PRCs, an effect shown to be unyielding despite neostigmine-based reversal at end of surgery.16 51 52 On the basis of the pathophysiology of the disease, patients with OSA should have an increased vulnerability to the effects of neuromuscular blocking agents and reversal agents.47 50 53 However, population-based studies aiming to quantify the effects of residual neuromuscular blockade in patients with and without risk of OSA are currently missing.
The impact of such pharmacological agents commonly used in anaesthesia care on the risk of respiratory outcomes in patients with OSA has yet to be determined. Our study will address the unmet need of evaluating the perioperative effect of neuromuscular blocking agents, reversal agents, opioids, sedatives and anaesthetics in patients at risk of OSA.
Non-pharmacological events
Non-pharmacological perioperative events, such as rapid eye movement (REM) rebound, encephalopathy, delirium, can disrupt respiratory arousal and result in upper airway collapse.30 In the immediate postoperative period, patients commonly experience poor quality, disrupted and reduced sleep, resulting in a deficit of REM sleep.54 Sleep studies in surgical patients have identified an REM rebound effect, in which REM sleep returns acutely and suddenly.54 55 Increased amounts of REM during sleep is associated with impaired respiratory arousal and more frequent episodes of nocturnal hypoxaemia.56 Patients with OSA also have diminished or lost airway reflex during non-REM sleep, so patients with OSA may be at an even greater propensity for upper airway collapse and hypoxaemia with phenomenon of REM rebound. While patients with OSA have been shown to compensate for diminished airway sizes with higher basal genioglossus muscle activity,57 this neuromuscular compensation has been found to be present only during wakefulness, and thus, futile in the setting of REM-predominant sleep. Recent prospective studies have demonstrated a significant reduction in REM sleep in patients with and patients without OSA during the early postoperative period.58 Postoperatively, time spent in REM sleep did not consistently predict postoperative OSA severity,38 which may be the consequence of REM suppression secondary to postoperative pain, as well as administration of opioids and sedatives. Of note, studies have also identified other important contributors to SDB. Events that impair a patient's level of consciousness also disrupt respiratory arousal and result in upper airway instability. Such events include delirium, stroke, septic encephalopathy, systemic inflammation and metabolic disturbances, such as hypoglycaemia and hypothyroidism.30
Study rationale
In order to evaluate the perioperative risk of patients presenting with OSA, it is important to take into account the ‘true’ prevalence of the disease in the perioperative cohort. An important limitation of the existing literature relates to the focus on patients who carry the clinical diagnosis of OSA. As a consequence of analysing only those patients with an International Classification of Diseases 9 (ICD-9) diagnostic code for SDB, a large subpopulation with undiagnosed OSA remain undetected.
The gold standard for the diagnosis of OSA is polysomnography. According to current clinical guidelines for OSA evaluation, patients are prompted to undergo this sleep study if determined to be high risk by their physician.3 As a routine evaluation for OSA, polysomnography is impractical because of its limited availability, discomfort to the patient and high cost.59 60 The use of screening tools for OSA helps identify patients at risk of OSA. Widely used scores include the Perioperative Sleep Apnea Prediction Score,61 the STOP-Bang62 and Berlin Questionnaires,63 and the Epworth Sleepiness Scale.64 Such scores rely on a clinical exam to determine neck circumference and/or patient questionnaire of daytime OSA symptoms. Not all patients are able to have their necks measured, and many patients are asymptomatic or unaware of their symptoms, limiting the ability of the existing scores to assess true prevalence of OSA. Anaesthesiologists have also used scores, such as the Mallampati Score and the American Society of Anesthesiologists (ASA) Checklist, to assess difficulty of intubation as related to a narrow upper airway,65 but there is inconsistency in reported sensitivity and specificity of the Mallampati score as a predictor of OSA.64 Furthermore, the currently available scores require data not routinely available from clinical databases, such as history of snoring and witnessed apnoea. This proposal is based on the consideration that other data available in the patient's electronic medical record may be sufficient to predict OSA and its associated increased risk of PRCs. Application of our prediction score on large perioperative data sets will permit research endeavours, such as the evaluation of the effect of OSA on patient outcomes and the justification of healthcare resource usage.
Furthermore, understanding how pharmacological agents commonly used in perioperative care impact postoperative outcomes among patients with high risk of OSA will improve our ability to provide better care for this vulnerable surgical population. Traditionally, anaesthesia providers have determined dosing of various drugs based on standard parameters of age, gender, height and weight. However, such practices may not sufficiently guide providers in optimal drug administration, especially in a subpopulation more vulnerable to the effects of those drugs as already demonstrated in the literature. More specifically, we would like to better understand the interaction between the disease OSA and opioids, neuromuscular blocking agents, neostigmine, sedatives and anaesthetics to optimally predict postoperative respiratory outcomes. Using our prediction score for OSA in a large perioperative database, we will evaluate how the use of pharmacological agents modifies the risk of PRCs in patients with OSA.
Objectives
The primary objectives are to:
Develop and validate a novel prediction score of OSA to identify patients at high risk of OSA based on markers of the disease easily available from clinical databases.
Evaluate the effect of being at high risk of OSA, as defined by the prediction score, on the primary outcome of PRCs among patients undergoing surgery at Massachusetts General Hospital.
Evaluate if use of neuromuscular blockade, neostigmine-based reversal of neuromuscular blockade, opioids, sedatives and anaesthetics modify the risk of OSA on PRCs.
The secondary objective is to:
Investigate whether the association between OSA risk and PRCs is modified by age, gender, body mass index (BMI) and major comorbidities.
Hypotheses for the primary outcome
On the basis of previous data,12 we hypothesise that patients with a high risk of OSA, as identified by our new prediction instrument, are more vulnerable to acute postoperative upper airway failure that leads to reintubation. We further hypothesise that such patients will experience less favourable outcomes depicted as intensive care unit admission rate, hospital length of stay and hospital costs.
As a departure from the current literature on the perioperative effects of OSA, we believe that perioperative variables, which increase the vulnerability to airway collapse, will give us clinically meaningful information in order to predict which patient with OSA will develop PRCs.
Methods and analysis
Study overview
The proposed study is a retrospective cohort analysis using hospital-based electronic patient data and perioperative data on medications administered and patient vital signs. We will use data from major clinical databases at Massachusetts General Hospital, a tertiary care facility and teaching hospital of Harvard Medical School in Boston, Massachusetts. In addition, polysomnography data will be extracted from clinical databases at several hospitals affiliated with Partners Healthcare.
As previously used for epidemiological studies by our group, data from two clinical databases will be retrieved and combined to provide de-identified preoperative, intraoperative and postoperative information: the Research Patient Data Registry and the Anesthesia Information Management System.15 16 51 66 The Research Patient Data Registry contains demographic and billing data regarding patient comorbidities and postoperative outcome and survival. The Anesthesia Information Management System contains physiological data from patient monitors as well as information on medical history and documentation of important surgery and anaesthesia-related events, including adverse events, perioperative procedures, and drug and fluid therapy. In addition, we will extract data related to hospital length of stay, discharge, and cost of care from our institution's administrative database, EPSi. Patient data from these databases are linked through unique patient identifiers, and the variables described in this protocol will be abstracted to form one database. The present database spans January 2007 to August 2014, and includes 140 000 surgical cases. On the basis of previous work, we will conservatively anticipate that 25% of the cases will not satisfy inclusion criteria due to patient's age, emergency status and missing data.15 51 Thus, we estimate 100 000 patient cases will meet our inclusion criteria.
Subject selection
For the three primary objectives, we will include all adult surgical patients who underwent general anaesthesia and received endotracheal intubation or airway management by supraglottic airway device at our institution, for whom inpatient admission was planned, between January 2007 and August 2014. Because reintubation is a component of our composite outcome of PRCs, we will only include those patients who have had removal of all airway management devices within the operating room after the procedure. Surgical procedures followed by reintubation for an additional scheduled surgical procedure in the operating room after initial extubation or removal of airway device will be excluded from the study, as we presume that such cases did not require reintubation in the setting of adverse postoperative respiratory status. Patients who underwent surgery in the 4 weeks prior to the study case will be excluded. Finally, all patients with an intraoperative death will be excluded from the study since OSA is not a biological mechanism of intraoperative death when a patient's airway is secure by an airway device. Patients will be identified using anaesthesia data obtained from Research Patient Data Registry and Anesthesia Information Management System.
The study methods are outlined in three sections to address the three primary objectives.
Objective 1: Development of prediction model for OSA
Prediction model reference standard
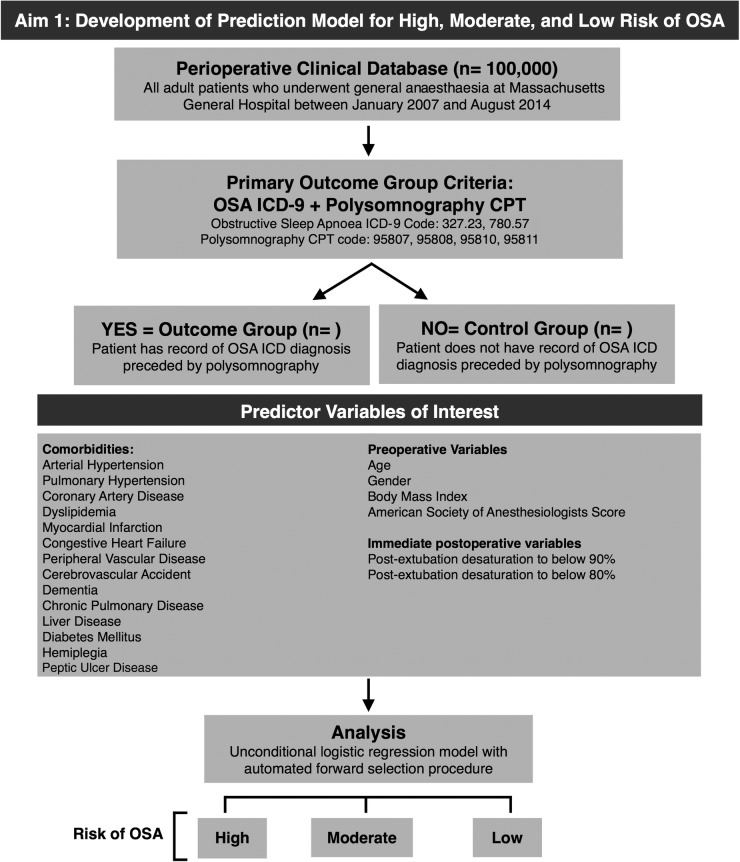
The reference standard for the prediction model will be defined as patients with an ICD-9 OSA diagnosis following the appearance of a polysomnography procedural (CPT, Current Procedural Terminology) code in our medical databases (figure 2). From this specific sequence of events, we infer that these patients had their clinically suspected OSA diagnosis confirmed by polysomnography.
Figure 2.
Aim 1: Development of prediction model for high, moderate, and low risk of OSA (CPT, Current Procedural Terminology; ICD, International Classification of Diseases; OSA, obstructive sleep apnoea).
Validation of reference standard for the diagnosis of OSA
Prior to the development of the prediction model, we will conduct a medical chart review of 100 randomly selected patients in order to determine whether or not such patients actually have evidence of OSA in the time between their polysomnography and surgery. This cohort of patients will consist of 50 cases of OSA, according to our criteria of ICD 9 diagnostic code and polysomnography CPT code, and 50 cases without OSA. A blinded chart review will be performed on this mixed group of 100 cases. Confirmatory evidence of OSA would include a reported AHI ≥5 as documented in a patient's medical chart,2 or treatment with continuous positive airway pressure. The predictive model will be performed if the ICD-9 and CPT code combination has an acceptable positive predictive value (≥0.8).
Predictor variables
A number of variables have been found to be associated with an increased prevalence of OSA and are currently used for different screening tools for OSA in surgical patients.62 65 67 From the Anesthesia Information Management System and Research Patient Data Registry databases, we will obtain and include the following data in our prediction score: age, BMI, gender and the ASA physical status classification (figure 2). We will incorporate medical comorbidities using ICD-9 diagnostic codes, some of which are defined by the Deyo-Charlson Comorbidity Index (table 1).68 All covariates included in the prediction model must be present within 1 year of surgery date. In addition, as a departure from current literature on developing OSA screening scores, we will consider oxygen desaturation immediately after extubation as a predictor. This strategy will most likely increase the predictive value of our score—patients with OSA are very vulnerable to desaturation after surgery, and we have the unique opportunity to use this characteristic of OSA desaturation after anaesthesia that has not yet been used in existing prediction scores. Postextubation oxygen desaturation will be defined as an oxyhaemoglobin reading <90%, and <80% for at least 1 min, as measured by pulse oximetry during the first 10 min after extubation in the operating room.
Table 1.
Diagnostic (ICD-9) and procedural (CPT) codes used to generate predictor and outcome variables
| Variable | Diagnostic or procedure name | Code type | Code |
|---|---|---|---|
| Reference standard outcome for prediction model of aim 1 | |||
| Obstructive sleep apnoea | Obstructive sleep apnoea (adult or paediatric) | ICD-9 | 327.23 |
| Unspecified sleep apnoea | ICD-9 | 780.57 | |
| Polysomnography | Sleep study, simultaneous recording of ventilation, respiratory effort, ECG or heart rate, oxygen saturation, attended by a technologist | CPT | 95807 |
| Any age, sleep staging with 1–3 additional parameters of sleep, attended by a technologist | CPT | 95808 | |
| Age 6 years or older, sleep staging with 4 or more additional parameters of sleep, attended by a technologist | CPT | 95810 | |
| Age 6 years or older, sleep staging with 4 or more additional parameters of sleep, with continuous positive airway pressure therapy or bi-level ventilation, attended by a technologist | CPT | 95811 | |
| Medical comorbidities | |||
| Arterial hypertension | Malignant essential hypertension | ICD-9 | 401.0 |
| Benign essential hypertension | ICD-9 | 401.1 | |
| Unspecified essential hypertension | ICD-9 | 401.9 | |
| Other malignant secondary hypertension | ICD-9 | 405.09 | |
| Other benign secondary hypertension | ICD-9 | 405.19 | |
| Other unspecified secondary hypertension | ICD-9 | 405.99 | |
| Pulmonary hypertension | Pulmonary hypertension | ICD-9 | 416.0 |
| Coronary artery disease | Coronary atherosclerosis of unspecified type of vessel native or graft | ICD-9 | 414.00 |
| Coronary atherosclerosis of native coronary artery | ICD-9 | 414.01 | |
| Coronary atherosclerosis of autologous vein bypass graft | ICD-9 | 414.02 | |
| Coronary atherosclerosis of non-autologous biological bypass graft | ICD-9 | 414.03 | |
| Coronary atherosclerosis of artery bypass graft | ICD-9 | 414.04 | |
| Coronary atherosclerosis of unspecified bypass graft | ICD-9 | 414.05 | |
| Coronary atherosclerosis of native coronary artery of transplanted heart | ICD-9 | 414.06 | |
| Coronary atherosclerosis of bypass graft (artery) (vein) of transplanted heart | ICD-9 | 414.07 | |
| Aneurysm of heart (wall) | ICD-9 | 414.10 | |
| Aneurysm of coronary vessels | ICD-9 | 414.11 | |
| Dissection of coronary artery | ICD-9 | 414.12 | |
| Other aneurysm of heart | ICD-9 | 414.19 | |
| Chronic total occlusion of coronary artery | ICD-9 | 414.20 | |
| Coronary atherosclerosis due to lipid rich plaque | ICD-9 | 414.30 | |
| Coronary atherosclerosis due to calcified coronary lesion | ICD-9 | 414.40 | |
| Other specified forms of chronic ischaemic heart disease | ICD-9 | 414.80 | |
| Chronic ischaemic heart disease unspecified | ICD-9 | 414.90 | |
| Dyslipidemia | Pure hypercholesterolaemia | ICD-9 | 272.0 |
| Pure hyperglyceridaemia | ICD-9 | 272.1 | |
| Mixed hyperlipidaemia | ICD-9 | 272.2 | |
| Hyperchylomicronemia | ICD-9 | 272.3 | |
| Other and unspecified hyperlipidaemia | ICD-9 | 272.4 | |
| Other disorders of lipoid metabolism | ICD-9 | 272.8 | |
| The following medical comorbidities are derived from ICD-9 codes, as defined by the Deyo Charlson Comorbidity Index:68 Myocardial Infarction, Congestive Heart Failure, Peripheral Vascular Disease, Cerebrovascular Accident, Dementia, Chronic Pulmonary Disease, Mild Liver Disease, Moderate to Severe Liver Disease, Diabetes with Chronic Complications, Diabetes without Chronic Complications, Hemiplegia or Paraplegia, Peptic Ulcer Disease | |||
| Primary Outcome for Aim 2 and Aim 3 | |||
| Pneumonia | Pneumococcal pneumonia (Streptococcus pneumonia) | ICD-9 | 481 |
| Pneumonia due to Klebsiella pneumoniae | ICD-9 | 482.0 | |
| Pneumonia due to Pseudomonas | ICD-9 | 482.1 | |
| Pneumonia due to Streptococcus, unspecified | ICD-9 | 482.30 | |
| Pneumonia due to Staphylococcus, unspecified | ICD-9 | 482.40 | |
| Pneumonia due to Staphylococcus aureus | ICD-9 | 482.41 | |
| Methicillin resistant pneumonia due to Staphylococcus aureus | ICD-9 | 482.42 | |
| Pneumonia due to Escherichia coli | ICD-9 | 482.82 | |
| Pneumonia due to other Gram-negative bacteria | ICD-9 | 482.83 | |
| Pneumonia due to other specified bacteria | ICD-9 | 482.89 | |
| Bacterial pneumonia, unspecified | ICD-9 | 482.9 | |
| Pneumonia, organism unspecified | ICD-9 | 486 | |
| Pneumonia due to other specified organism | ICD-9 | 483.8 | |
| Pneumonia in aspergillosis | ICD-9 | 484.6 | |
| Bronchopneumonia, organism unspecified | ICD-9 | 485 | |
| Pneumonitis due to inhalation of food or vomitus | ICD-9 | 507.0 | |
| Pulmonary oedema | Pulmonary congestion and hypostasis | ICD-9 | 514 |
| Acute oedema of lung, unspecified | ICD-9 | 518.4 | |
| Congestive heart failure | ICD-9 | 428.0 | |
| Fluid overload | ICD-9 | 276.6 | |
| Other fluid overload | ICD-9 | 276.69 | |
| Reintubation | Intubation, endotracheal, emergency procedure | CPT | 31500 |
| Ventilation assist and management, initiation of pressure or volume preset ventilators for assisted or controlled breathing; hospital inpatient/observation, initial day | CPT | 94002 | |
| Respiratory failure | Pulmonary insufficiency following trauma and surgery | ICD-9 | 518.5 |
| Acute respiratory failure following trauma and surgery | ICD-9 | 518.51 | |
| Other pulmonary insufficiency, not elsewhere classified, following trauma and surgery | ICD-9 | 518.52 | |
| Respiratory failure | ICD-9 | 518.81 | |
| Other pulmonary insufficiency, not elsewhere classified | ICD-9 | 518.82 | |
| Acute and chronic respiratory failure | ICD-9 | 518.84 | |
CPT, Current Procedural Terminology; ICD, International Classification of Diseases.
Development of prediction model
We will use an unconditional logistic regression model with an automated forward selection procedure to select for predictors of our a priori defined reference standard. To determine the goodness-of-fit of the final prediction model, we will use the Hosmer-Lemeshow test, which indicates that there is no significant difference between observed and expected OSA status if p value ≥0.05. A point value will be assigned to each predictor variable proportional to the estimates from the logistic regression. The predictive value of the score for OSA will be assessed using c-statistics, which is equivalent to the area under the ROC curve.69 We will aim to achieve a minimum c-statistic of 0.8. In addition, we will evaluate if the addition of a variable that can be obtained by anaesthesiologists at the end of the surgical case, for example, postextubation desaturation, improves the predictive ability of the score. For this purpose, we will use risk reclassification analysis to compare the clinical impact of these two models.70 71 The net reclassification improvement will be generated by balancing the proportion of subjects whose risk was more accurately classified using the expanded prediction model with postextubation desaturation compared with the prediction model without postextubation desaturation against the proportion of participants whose risk was less accurately classified.70
We will calculate positive and negative likelihood ratios for each stratum of the score. We will use bootstrap techniques to determine the robustness of included variables, which are close to the p value cut-off of 0.05. We will then use classification tables to determine the best cut-off value for the prediction score to classify patients at high risk for OSA. We will also use cross-validation to evaluate any potential overfitting of our prediction model.
Objective 2: Effect of high OSA risk on postoperative respiratory complications
Exposure variables
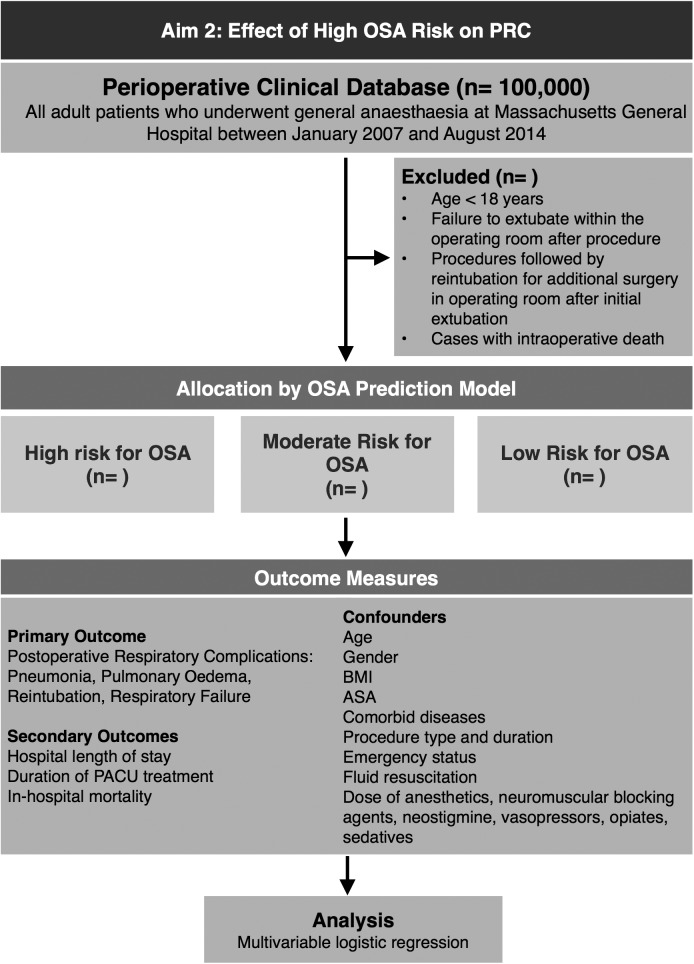
Our primary exposure variable of interest is OSA risk, as defined by our prediction model developed in aim 1. We will identify patients in our population as having a high, moderate and low risk for OSA using our prediction model, and produce three cohorts of patients, which we will follow for the occurrence of outcome events.
Outcome variables
The primary outcome of this part of the study is a composite outcome defined as the incidence of reintubation, pulmonary oedema, pneumonia and respiratory failure within the first three postoperative days. Secondary outcomes include the aforementioned individual outcomes as well as hospital length of stay, duration of postanaesthesia care unit treatment and in-hospital mortality. Hospital length of stay will be defined as the postoperative length of hospital stay following surgery. The primary outcome has been previously used and validated by chart review.51 66 The outcomes events for the primary analysis will be identified by ICD-9 diagnostic and CPT procedural codes obtained from the Research Patient Data Registry database (table 1).
Outcome model
We will perform multivariable logistic regression analyses to evaluate the effect of estimated OSA risk on our respiratory outcomes. Results will be presented as an age-adjusted and multivariable-adjusted OR with 95% CIs. We will consider a two-tailed p value of <0.05 as statistically significant.
To control for confounding effects, we will consider a priori the following risk factors: age, gender, BMI, ASA physical status classification, comorbidities, surgical specialty, duration of the surgical procedure, admission type and emergency status.16 We will additionally control for dose of anaesthesia (median dose of anaesthetic agents corrected for age),72 opioids (calculated as total morphine equivalent dose),73 vasopressors, sedatives, neuromuscular blocking agents and neostigmine use (figure 3).
Figure 3.
Aim 2: Effect of high OSA risk on postoperative respiratory complications (ASA, American Society of Anesthesiologists; BMI, body mass index; OSA, obstructive sleep apnoea; PRC, postoperative respiratory complication).
The effect of surgery type will be analysed in greater detail by grouping similar types of surgery (eg, cardiovascular, laparoscopic) to determine if surgery type is an effect modifier and not a confounder. If this is found to be the case, surgical specialty will no longer be included as a covariate, and the previously described model will be stratified by surgery type.
Sample size and power calculations
On the basis of previous work with data from surgical patients in our institution, we expect approximately 100 000 patients undergoing surgery to meet our inclusion criteria during the observational period. Studies on prevalence of OSA in the general surgical population provide a range of estimates: one study found 17% of surgical patients as having severe OSA (AHI>30).11 Other studies relying on screening scores found anywhere from 4.8%74 to 41.6%75 of surgical patients at high risk of OSA. Thus, we conservatively estimate 3% (n=3000) patients in our surgical population to have a high likelihood of OSA. Basing on our prediction score, we will classify patients as high, moderate and low OSA risk.
Previous work by our laboratory51 found an overall incidence of 3.7% for our primary outcome of PRCs. Data on differences in postoperative outcomes between OSA and non-OSA groups provide us with estimates for our predicted intergroup differences. Liao et al13 found an intergroup (OSA vs non-OSA) difference of 11% for their composite outcome of total respiratory complications. Mokhlesi et al12 investigated the incidence of emergent intubation following elective surgery among patients with and without SDB. Emergent intubation occurred at a rate of 3.5–11.4% among patients with SDB versus 0.3–7% among patients without SDB across four categories of elective surgery.12 The intergroup difference observed was approximately 3%.12 Basing on this data, we will conservatively estimate an intergroup difference of 10% for our composite outcome, with smaller differences observed for outcomes with lower frequencies. Power is calculated based on comparing proportions of outcome rates between expected patients with OSA and the reference population without OSA. Our fixed sample size of 100 000 will provide us with a power >90% to identify a 10% intergroup difference with an α error of 0.05.
Objective 3: Risk modification by pharmacological agents
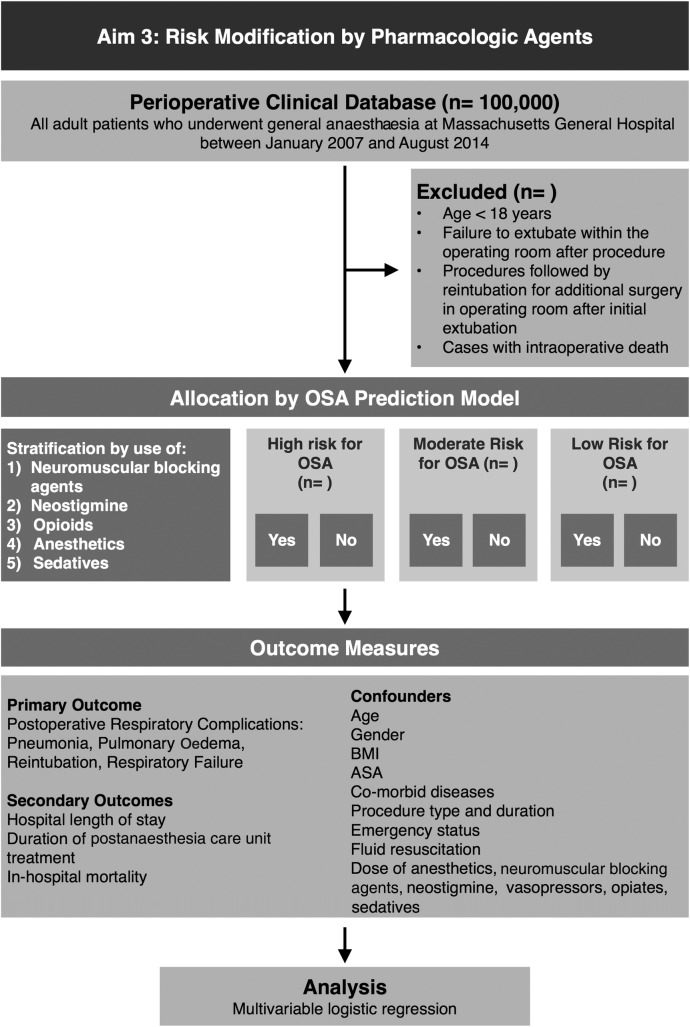
Exposure variable and rationale
We will obtain data on the intraoperative use of intermediate-acting neuromuscular blocking agents, neostigmine-based reversal of neuromuscular blockade, opioids, anaesthetics and sedatives as additional independent variables in the analysis to test whether or not such pharmacological agents modify the effect of OSA on the risk for PRCs (figure 4). We have previously studied the use of intermediate-acting neuromuscular blocking agents and found that their use was associated with an increased risk of respiratory complications.16 In addition, we have observed that the use of the reversal agent neostigmine does not decrease but increase the risk of PRCs.16 52 However, recent work demonstrates that such effects could be mitigated by neostigmine only at low doses and with simultaneous careful monitoring of neuromuscular transmission (train-of-four).51
Figure 4.
Aim 3: Risk modification by pharmacological agents (ASA, American Society of Anesthesiologists; BMI, body mass index; OSA, obstructive sleep apnoea; REM, rapid eye movement).
Patients with OSA should be at high risk of respiratory complications induced by pharmacological agents because such agents can affect upper airway patency.35 44 48 76 We thus expand our investigation to include the risk modification effect of pharmacological agents (neuromuscular blocking agents, neostigmine, opioids, anaesthetics and sedatives) on PRCs in a subpopulation of surgical patients who may be at an inherent higher vulnerability towards upper airway collapse and subsequent poor respiratory outcomes. Similar to previous work, we will extract information on administration of pharmacological agents from the Anesthesia Information Management System database.51
Outcome variables
The primary outcome is the composite variable of PRCs, consisting of: reintubation, pulmonary oedema, pneumonia and respiratory failure. Secondary outcomes include hospital length of stay, duration of postanaesthesia care unit treatment, inhospital mortality, as well as the aforementioned outcomes. These outcomes are defined by ICD-9 and CPT codes located in the Research Patient Data Registry database, and have been previously validated by chart review by our laboratory (table 1).51
Stratified analysis to assess for effect modification by pharmacological agents
To evaluate potential effect modification by neuromuscular blockade, neostigmine, opioid, anaesthetic and sedative use, we will run stratified analyses of the association between OSA and the outcome events based on intraoperative use of pharmacological agents. We will use the likelihood ratio test to contrast our main model to a model including interaction terms between OSA and the following variables: neuromuscular blocking agent dose, opioid dose and median effective dose of anaesthetics. To control for confounding effects, we will consider a priori the following risk factors: age, gender, BMI, ASA physical status classification, comorbidities, surgical specialty, duration of the surgical procedure and emergency status.16 The stratified analyses for neuromuscular blockade, opioid, anaesthetic and sedative use will be performed independently using stratified versions of the previously described model. The potential for risk modification of neostigmine will be performed in the subset of patients receiving neuromuscular blockade.
Study cohorts
On the basis of previous work with data from surgical patients in our institution, approximately 100 000 patients will meet inclusion criteria. On the basis of data estimating OSA prevalence in the general surgical population, we conservatively expect to find approximately 3000 patients with high likelihood of OSA in our surgical population. Using our prediction model from aim 1, we will determine the risk of OSA and assign patients found to be at high, moderate and low risk of OSA.
Ethics and dissemination
This study uses internal hospital-based data routinely collected for medical documentation purposes. As it is a systematic review of the data, there is little ethical risk. Patient privacy and protection of health information will be maintained. The results of this study will be shared in the form of presentations at national and international meetings. The complete study and conclusions regarding the primary objectives will be presented in manuscript form.
Limitations and strengths
This article presents the protocol and data analysis plan for the development of a novel prediction score for OSA and application of the score to more accurately characterise the risk imparted by OSA condition on PRCs.
Our approach relies on the investigation of patient data on file. Thus, our findings depend on the quality of the database which is susceptible to measurement biases. There is potential for variability in the input of billing diagnoses and codes. This database has been used in previous studies15 16 and demonstrated to have high specificity following verification of diagnostic codes positive for study's composite outcome variable. Furthermore, we will validate the use of diagnostic and procedural codes in the development of our prediction model by medical record review. Nevertheless, it is possible that information is left out of some patients’ charts and, consequently, our database of our composite outcomes and independent variables. A second limitation involves our inability to capture those patients admitted to an outside hospital with PRCs after discharge from our institution. A third limitation rises from the multifactorial and dynamic nature of OSA: patients diagnosed with OSA, even by polysomnography, may not necessarily have evidence of OSA on the day of surgery. An example would be a patient who loses significant weight just prior to surgery. Diagnosis of OSA by polysomnography prior to weight loss may no longer be valid following weight loss.77 Thus, we are limited in our development of a prediction model since we initially rely on polysomnography procedure codes and ICD-9 diagnoses as our standard. We hope to minimise this limitation by developing a prediction model that relies on variables that are highly likely to predict OSA even in the absence of polysomnographic evidence or clinical diagnosis.
In spite of these limitations, our study derives its strengths from a number of key elements. Our database is large and includes a variety of surgical procedure types and methods of anaesthesia, thus increasing the generalisability of the study results and applicability of our prediction score models. In addition, we have a multidisciplinary team, which includes population scientists, data analysts and clinicians. Such a team provides the experience and skill level needed for efficient, accurate and precise design and analysis of the current study. Our team has also previously developed prediction scores for PRCs.15
Conclusions
The present study examines patients who we presume to have a high risk of perioperative respiratory failure: patients with OSA. The prediction score we develop to assess OSA risk will be a useful and practical tool for further OSA research and care. We believe the results of this study will provide new insight on whether or not high risk for OSA increases a patient's risk of developing PRCs, independent of other perioperative risk factors. Moreover, the results of this study might be important to evaluate the effects of interventions, such as reversing neuromuscular blockade, on respiratory outcome of OSA in the perioperative setting.
By developing a prediction score for OSA risk, we hope to identify those patients who would benefit from specific preoperative interventions to minimise postoperative morbidity and mortality.
Footnotes
Contributors: ME and TK contributed equally as senior authors and mentors of CHS. They developed the study concept and design. CHS wrote the first draft of the manuscript and contributed to the design of the study. SD advised on the study design. CHS, SZ, TK and ME refined the protocol. MN contributed to the acquisition and analysis of data for the work. All authors revised the protocol critically for important intellectual content and approved the final manuscript.
Funding: This work is supported by Merck (grant number 224941).
Competing interests: SD is a Merck employee and Merck is the sponsor of this study.
Ethics approval: Partners Human Research Committee, protocol number: 2014P000218.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Medicine AAOS. The international classification of sleep disorders. 2nd edn Westchester, IL: American Academy of Sleep Medicine, 2005:1. [Google Scholar]
- 2.Young T, Palta M, Dempsey J et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–5. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 3.Epstein LJ, Kristo D, Strollo PJ et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Memtsoudis SG, Besculides MC, Mazumdar M. A rude awakening—the perioperative sleep apnea epidemic. N Engl J Med 2013;368:2352–3. 10.1056/NEJMp1302941 [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Barnet JH et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006–14. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapur V, Strohl KP, Redline S et al. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002;6:49–54. 10.1007/s11325-002-0049-5 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz AR, Patil SP, Laffan AM et al. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc 2008;5:185–92. 10.1513/pats.200708-137MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Carroll MD, Kit BK et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7. 10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- 9.Young T, Evans L, Finn L et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997;20:705–6. [DOI] [PubMed] [Google Scholar]
- 10.Finkel KJ, Searleman AC, Tymkew H et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 2009;10:753–8. 10.1016/j.sleep.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 11.Singh M, Liao P, Kobah S et al. Proportion of surgical patients with undiagnosed obstructive sleep apnoea. Br J Anaesth 2013;110:629–36. 10.1093/bja/aes465 [DOI] [PubMed] [Google Scholar]
- 12.Mokhlesi B, Hovda MD, Vekhter B et al. Sleep-disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest 2013;144:903–14. 10.1378/chest.12-2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao P, Yegneswaran B, Vairavanathan S et al. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth 2009;56:819–28. 10.1007/s12630-009-9190-y [DOI] [PubMed] [Google Scholar]
- 14.Vasu TS, Grewal R, Doghramji K. Obstructive sleep apnea syndrome and perioperative complications: a systematic review of the literature. J Clin Sleep Med 2012;8:199–207. 10.5664/jcsm.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brueckmann B, Villa-Uribe JL, Bateman BT et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology 2013;118:1276–85. 10.1097/ALN.0b013e318293065c [DOI] [PubMed] [Google Scholar]
- 16.Grosse-Sundrup M, Henneman JP, Sandberg WS et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ 2012;345:e6329–9. 10.1136/bmj.e6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isono S, Tanaka A, Tagaito Y et al. Pharyngeal patency in response to advancement of the mandible in obese anesthetized persons. Anesthesiology 1997;87:1055–62. 10.1097/00000542-199711000-00008 [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Isono S, Tanaka A et al. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med 2002;165:260–5. 10.1164/ajrccm.165.2.2009032 [DOI] [PubMed] [Google Scholar]
- 19.Quick E, Byard RW. Postoperative cervical soft tissue hemorrhage with acute upper airway obstruction. J Forensic Sci 2013;58(Suppl 1):S264–6. 10.1111/1556-4029.12026 [DOI] [PubMed] [Google Scholar]
- 20.Piromchai P, Vatanasapt P, Reechaipichitkul W et al. Is the routine pressure dressing after thyroidectomy necessary? A prospective randomized controlled study. BMC Ear Nose Throat Disord 2008;8:1 10.1186/1472-6815-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiota S, Ryan CM, Chiu K-L et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax 2007;62:868–72. 10.1136/thx.2006.071183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yumino D, Redolfi S, Ruttanaumpawan P et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010;121:1598–605. 10.1161/CIRCULATIONAHA.109.902452 [DOI] [PubMed] [Google Scholar]
- 23.Tagaito Y, Isono S, Tanaka A et al. Sitting posture decreases collapsibility of the passive pharynx in anesthetized paralyzed patients with obstructive sleep apnea. Anesthesiology 2010;113:812–18. 10.1097/ALN.0b013e3181f1b834 [DOI] [PubMed] [Google Scholar]
- 24.Isono S, Tanaka A, Nishino T. Lateral position decreases collapsibility of the passive pharynx in patients with obstructive sleep apnea. Anesthesiology 2002;97:780–5. 10.1097/00000542-200210000-00006 [DOI] [PubMed] [Google Scholar]
- 25.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol 1988;65:2124–31. [DOI] [PubMed] [Google Scholar]
- 26.Rademaker BM, Ringers J, Odoom JA et al. Pulmonary function and stress response after laparoscopic cholecystectomy: comparison with subcostal incision and influence of thoracic epidural analgesia. Anesth Analg 1992;75:381–5. 10.1213/00000539-199209000-00011 [DOI] [PubMed] [Google Scholar]
- 27.Ali J, Yaffe CS, Serrette C. The effect of transcutaneous electric nerve stimulation on postoperative pain and pulmonary function. Surgery 1981;89:507–12. [PubMed] [Google Scholar]
- 28.Jaber S, Petrof BJ, Jung B et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011;183:364–71. 10.1164/rccm.201004-0670OC [DOI] [PubMed] [Google Scholar]
- 29.Reid MB, Lännergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med 2002;166:479–84. 10.1164/rccm.2202005 [DOI] [PubMed] [Google Scholar]
- 30.Sasaki N, Meyer MJ, Eikermann M. Postoperative respiratory muscle dysfunction: pathophysiology and preventive strategies. Anesthesiology 2013;118:961–78. 10.1097/ALN.0b013e318288834f [DOI] [PubMed] [Google Scholar]
- 31.Lo Y-L, Jordan AS, Malhotra A et al. Influence of wakefulness on pharyngeal airway muscle activity. Thorax 2007;62:799–805. 10.1136/thx.2006.072488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eikermann M, Malhotra A, Fassbender P et al. Differential effects of isoflurane and propofol on upper airway dilator muscle activity and breathing. Anesthesiology 2008;108:897–906. 10.1097/ALN.0b013e31816c8a60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eastwood PR, Platt PR, Shepherd K et al. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology 2005;103:470–7. 10.1097/00000542-200509000-00007 [DOI] [PubMed] [Google Scholar]
- 34.Eastwood PR, Szollosi I, Platt PR et al. Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology 2002;97:786–93. 10.1097/00000542-200210000-00007 [DOI] [PubMed] [Google Scholar]
- 35.Hwang JC, St John WM, Bartlett D. Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol Respir Environ Exerc Physiol 1983;55:785–92. [DOI] [PubMed] [Google Scholar]
- 36.Nishino T, Shirahata M, Yonezawa T et al. Comparison of changes in the hypoglossal and the phrenic nerve activity in response to increasing depth of anesthesia in cats. Anesthesiology 1984;60:19–24. 10.1097/00000542-198401000-00005 [DOI] [PubMed] [Google Scholar]
- 37.Eikermann M, Grosse-Sundrup M, Zaremba S et al. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology 2012;116:35–46. 10.1097/ALN.0b013e31823d010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung F, Liao P, Elsaid H et al. Factors associated with postoperative exacerbation of sleep-disordered breathing. Anesthesiology 2014;120:299–311. 10.1097/ALN.0000000000000041 [DOI] [PubMed] [Google Scholar]
- 39.Zaremba S, Mueller N, Heisig A et al. Elevated upper body position improves pregnancy-related OSA without impairing sleep quality or sleep architecture early after delivery. Chest 2015;148:936–44. 10.1378/chest.14-2973 [DOI] [PubMed] [Google Scholar]
- 40.Doufas AG, Tian L, Davies MF et al. Nocturnal intermittent hypoxia is independently associated with pain in subjects suffering from sleep-disordered breathing. Anesthesiology 2013;119:1149–62. 10.1097/ALN.0b013e3182a951fc [DOI] [PubMed] [Google Scholar]
- 41.Smith MT, Finan PH. Sleep, respiration, and pain: a potential nexus for chronic pain risk? Anesthesiology 2013;119:1011–13. 10.1097/ALN.0b013e3182a9521b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goksan B, Gunduz A, Karadeniz D et al. Morning headache in sleep apnoea: clinical and polysomnographic evaluation and response to nasal continuous positive airway pressure. Cephalalgia 2009;29:635–41. 10.1111/j.1468-2982.2008.01781.x [DOI] [PubMed] [Google Scholar]
- 43.Brown KA, Laferrière A, Lakheeram I et al. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology 2006;105:665–9. 10.1097/00000542-200610000-00009 [DOI] [PubMed] [Google Scholar]
- 44.Lalley PM. Mu-opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol 2003;285:R1287–304. 10.1152/ajpregu.00199.2003 [DOI] [PubMed] [Google Scholar]
- 45.Hajiha M, DuBord M-A, Liu H et al. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J Physiol 2009;587(Pt 11):2677–92. 10.1113/jphysiol.2009.171678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cammu G, De Witte J, De Veylder J et al. Postoperative residual paralysis in outpatients versus inpatients. Anesth Analg 2006;102:426–9. 10.1213/01.ane.0000195543.61123.1f [DOI] [PubMed] [Google Scholar]
- 47.Eikermann M, Vogt FM, Herbstreit F et al. The predisposition to inspiratory upper airway collapse during partial neuromuscular blockade. Am J Respir Crit Care Med 2007;175:9–15. 10.1164/rccm.200512-1862OC [DOI] [PubMed] [Google Scholar]
- 48.Eikermann M, Fassbender P, Malhotra A et al. Unwarranted administration of acetylcholinesterase inhibitors can impair genioglossus and diaphragm muscle function. Anesthesiology 2007;107:621–9. 10.1097/01.anes.0000281928.88997.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krodel DJ, Bittner EA, Abdulnour R-EE et al. Negative pressure pulmonary edema following bronchospasm. Chest 2011;140:1351–4. 10.1378/chest.11-0529 [DOI] [PubMed] [Google Scholar]
- 50.Herbstreit F, Peters J, Eikermann M. Impaired upper airway integrity by residual neuromuscular blockade: increased airway collapsibility and blunted genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology 2009;110:1253–60. 10.1097/ALN.0b013e31819faa71 [DOI] [PubMed] [Google Scholar]
- 51.McLean D, Farhan H, Diaz-Gil D et al. Dose-dependent association between intermediate-acting neuromuscular blocking agents and postoperative respiratory complications. Anesthesiology 2015;122:1201–13. 10.1097/ALN.0000000000000674 [DOI] [PubMed] [Google Scholar]
- 52.Meyer MJ, Bateman BT, Kurth T et al. Neostigmine reversal doesn't improve postoperative respiratory safety. BMJ 2013;346:f1460 10.1136/bmj.f1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasaki N, Meyer MJ, Malviya SA et al. Effects of neostigmine reversal of nondepolarizing neuromuscular blocking agents on postoperative respiratory outcomes: a prospective study. Anesthesiology 2014;121:959–68. 10.1097/ALN.0000000000000440 [DOI] [PubMed] [Google Scholar]
- 54.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. BMJ (Clin Res Ed) 1985;290:1029–32. 10.1136/bmj.290.6474.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knill RL, Moote CA, Skinner MI et al. Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology 1990;73:52–61. 10.1097/00000542-199007000-00009 [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg J, Wildschiødtz G, Pedersen MH et al. Late postoperative nocturnal episodic hypoxaemia and associated sleep pattern. Br J Anaesth 1994;72:145–50. 10.1093/bja/72.2.145 [DOI] [PubMed] [Google Scholar]
- 57.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 1992;89:1571–9. 10.1172/JCI115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung F, Liao P, Yegneswaran B et al. Postoperative changes in sleep-disordered breathing and sleep architecture in patients with obstructive sleep apnea. Anesthesiology 2014;120:287–98. 10.1097/ALN.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 59.Adesanya AO, Lee W, Greilich NB et al. Perioperative management of obstructive sleep apnea. Chest 2010;138:1489–98. 10.1378/chest.10-1108 [DOI] [PubMed] [Google Scholar]
- 60.Flemons WW, Douglas NJ, Kuna ST et al. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med 2004;169:668–72. 10.1164/rccm.200308-1124PP [DOI] [PubMed] [Google Scholar]
- 61.Ramachandran SK, Kheterpal S, Consens F et al. Derivation and validation of a simple perioperative sleep apnea prediction score. Anesth Analg 2010;110:1007–15. 10.1213/ANE.0b013e3181d489b0 [DOI] [PubMed] [Google Scholar]
- 62.Chung F, Subramanyam R, Liao P et al. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth 2012;108:768–75. 10.1093/bja/aes022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung F, Ward B, Ho J et al. Preoperative identification of sleep apnea risk in elective surgical patients, using the Berlin questionnaire. J Clin Anesth 2007;19:130–4. 10.1016/j.jclinane.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 64.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth 2010;57:423–38. 10.1007/s12630-010-9280-x [DOI] [PubMed] [Google Scholar]
- 65.Chung F, Yegneswaran B, Liao P et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology 2008;108:822–30. 10.1097/ALN.0b013e31816d91b5 [DOI] [PubMed] [Google Scholar]
- 66.Ladha KS, Vidal Melo MF, McLean D et al. Intraoperative protective mechanical ventilation and risk of postoperative pulmonary complications: a propensity score matched cohort study. BMJ BMJ 2015;351:h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med 2002;165:1217–39. 10.1164/rccm.2109080 [DOI] [PubMed] [Google Scholar]
- 68.Quan H, Sundararajan V, Halfon P et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 69.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. 10.1148/radiology.143.1.7063747 [DOI] [PubMed] [Google Scholar]
- 70.Pencina MJ, D'Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72; discussion207–12 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 71.Cook NR. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem 2008;54:17–23. 10.1373/clinchem.2007.096529 [DOI] [PubMed] [Google Scholar]
- 72.Lerou JGC. Nomogram to estimate age-related MAC. Br J Anaesth 2004;93:288–91. 10.1093/bja/aeh186 [DOI] [PubMed] [Google Scholar]
- 73.Haffey F, Brady RRW, Maxwell S. A comparison of the reliability of smartphone apps for opioid conversion. Drug Saf 2013;36:111–17. 10.1007/s40264-013-0015-0 [DOI] [PubMed] [Google Scholar]
- 74.Stierer TL, Wright C, George A et al. Risk assessment of obstructive sleep apnea in a population of patients undergoing ambulatory surgery. J Clin Sleep Med 2010;6:467–72. [PMC free article] [PubMed] [Google Scholar]
- 75.Lockhart EM, Willingham MD, Ben Abdallah A et al. Obstructive sleep apnea screening and postoperative mortality in a large surgical cohort. Sleep Med 2013;14:407–15. 10.1016/j.sleep.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herbstreit F, Zigrahn D, Ochterbeck C et al. Neostigmine/glycopyrrolate administered after recovery from neuromuscular block increases upper airway collapsibility by decreasing genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology 2010;113:1280–8. 10.1097/ALN.0b013e3181f70f3d [DOI] [PubMed] [Google Scholar]
- 77.Mitchell LJ, Davidson ZE, Bonham M et al. Weight loss from lifestyle interventions and severity of sleep apnoea: a systematic review and meta-analysis. Sleep Med 2014;15:1173–83. 10.1016/j.sleep.2014.05.012 [DOI] [PubMed] [Google Scholar]