Abstract
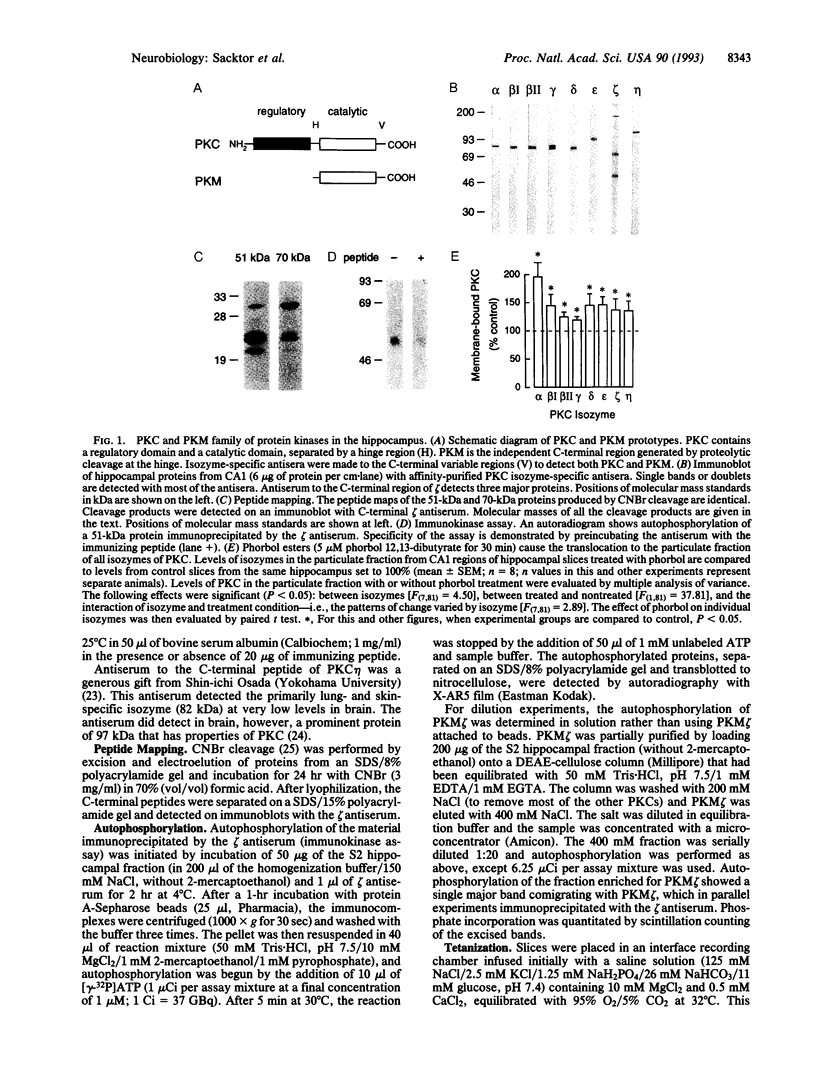
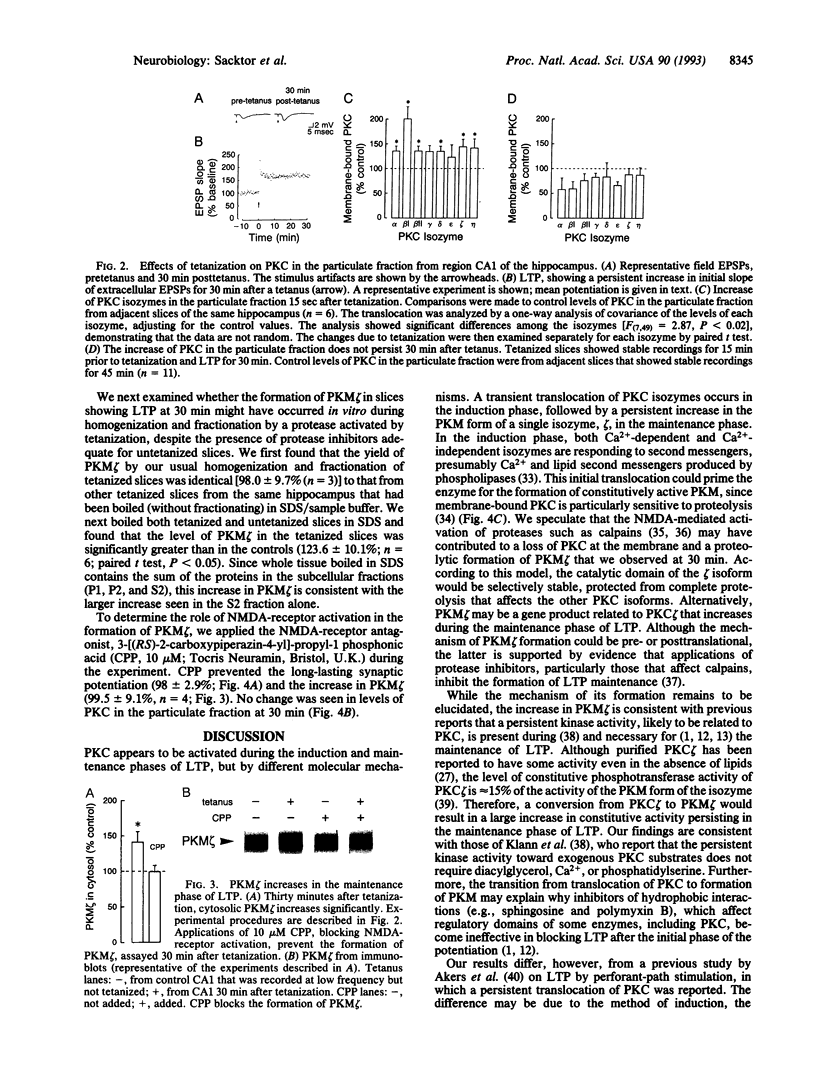
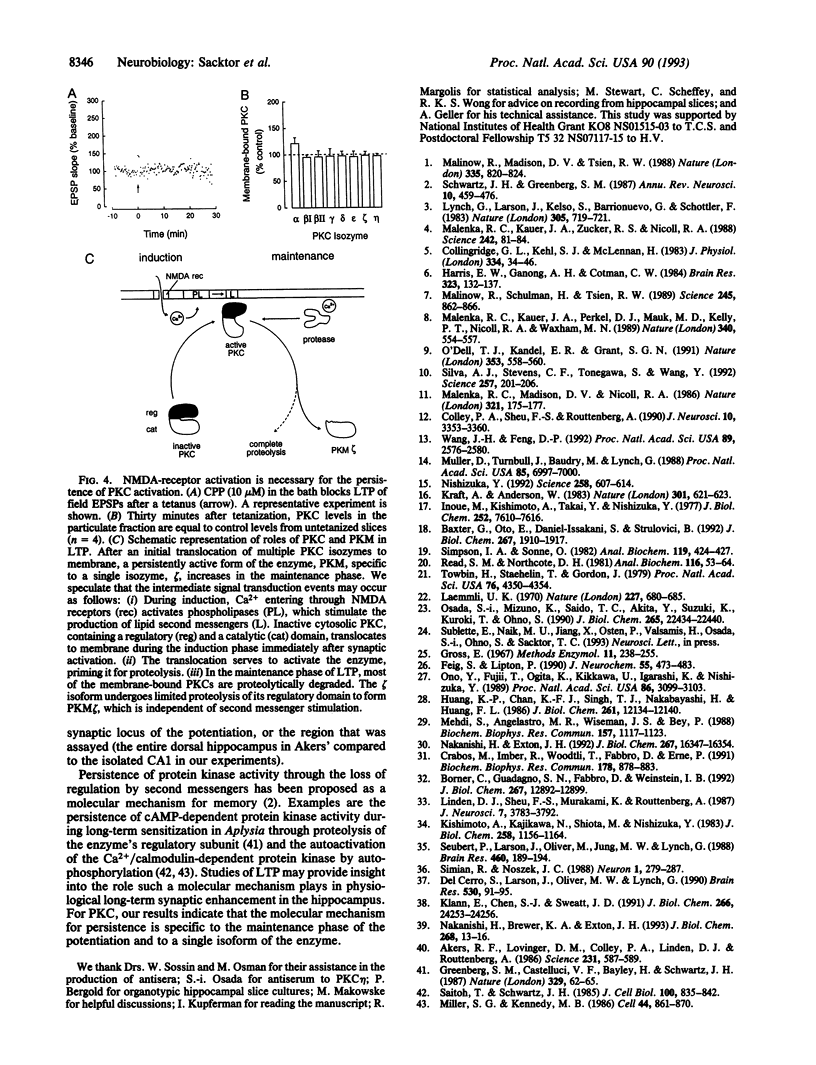
Long-term potentiation in the CA1 region of the hippocampus, a model for memory formation in the brain, is divided into two phases. A transient process (induction) is initiated, which then generates a persistent mechanism (maintenance) for enhancing synaptic strength. Protein kinase C (PKC), a gene family of multiple isozymes, may play a role in both induction and maintenance. In region CA1 from rat hippocampal slices, most of the isozymes of PKC translocated to the particulate fraction 15 sec after a tetanus. The increase of PKC in the particulate fraction did not persist into the maintenance phase of long-term potentiation. In contrast, a constitutively active kinase, PKM, a form specific to a single isozyme (zeta), increased in the cytosol during the maintenance phase. The transition from translocation of PKC to formation of PKM may help to explain the molecular mechanisms of induction and maintenance of long-term potentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Baxter G., Oto E., Daniel-Issakani S., Strulovici B. Constitutive presence of a catalytic fragment of protein kinase C epsilon in a small cell lung carcinoma cell line. J Biol Chem. 1992 Jan 25;267(3):1910–1917. [PubMed] [Google Scholar]
- Borner C., Guadagno S. N., Fabbro D., Weinstein I. B. Expression of four protein kinase C isoforms in rat fibroblasts. Distinct subcellular distribution and regulation by calcium and phorbol esters. J Biol Chem. 1992 Jun 25;267(18):12892–12899. [PubMed] [Google Scholar]
- Colley P. A., Sheu F. S., Routtenberg A. Inhibition of protein kinase C blocks two components of LTP persistence, leaving initial potentiation intact. J Neurosci. 1990 Oct;10(10):3353–3360. doi: 10.1523/JNEUROSCI.10-10-03353.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabos M., Imber R., Woodtli T., Fabbro D., Erne P. Different translocation of three distinct PKC isoforms with tumor-promoting phorbol ester in human platelets. Biochem Biophys Res Commun. 1991 Aug 15;178(3):878–883. doi: 10.1016/0006-291x(91)90973-b. [DOI] [PubMed] [Google Scholar]
- Greenberg S. M., Castellucci V. F., Bayley H., Schwartz J. H. A molecular mechanism for long-term sensitization in Aplysia. Nature. 1987 Sep 3;329(6134):62–65. doi: 10.1038/329062a0. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Chan K. F., Singh T. J., Nakabayashi H., Huang F. L. Autophosphorylation of rat brain Ca2+-activated and phospholipid-dependent protein kinase. J Biol Chem. 1986 Sep 15;261(26):12134–12140. [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Klann E., Chen S. J., Sweatt J. D. Persistent protein kinase activation in the maintenance phase of long-term potentiation. J Biol Chem. 1991 Dec 25;266(36):24253–24256. [PubMed] [Google Scholar]
- Linden D. J., Sheu F. S., Murakami K., Routtenberg A. Enhancement of long-term potentiation by cis-unsaturated fatty acid: relation to protein kinase C and phospholipase A2. J Neurosci. 1987 Nov;7(11):3783–3792. doi: 10.1523/JNEUROSCI.07-11-03783.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., Waxham M. N. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989 Aug 17;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Zucker R. S., Nicoll R. A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988 Oct 7;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Mehdi S., Angelastro M. R., Wiseman J. S., Bey P. Inhibition of the proteolysis of rat erythrocyte membrane proteins by a synthetic inhibitor of calpain. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1117–1123. doi: 10.1016/s0006-291x(88)80989-6. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Muller D., Turnbull J., Baudry M., Lynch G. Phorbol ester-induced synaptic facilitation is different than long-term potentiation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6997–7000. doi: 10.1073/pnas.85.18.6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H., Brewer K. A., Exton J. H. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993 Jan 5;268(1):13–16. [PubMed] [Google Scholar]
- Nakanishi H., Exton J. H. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem. 1992 Aug 15;267(23):16347–16354. [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989 May;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada S., Mizuno K., Saido T. C., Akita Y., Suzuki K., Kuroki T., Ohno S. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990 Dec 25;265(36):22434–22440. [PubMed] [Google Scholar]
- Schwartz J. H., Greenberg S. M. Molecular mechanisms for memory: second-messenger induced modifications of protein kinases in nerve cells. Annu Rev Neurosci. 1987;10:459–476. doi: 10.1146/annurev.ne.10.030187.002331. [DOI] [PubMed] [Google Scholar]
- Seubert P., Larson J., Oliver M., Jung M. W., Baudry M., Lynch G. Stimulation of NMDA receptors induces proteolysis of spectrin in hippocampus. Brain Res. 1988 Sep 13;460(1):189–194. doi: 10.1016/0006-8993(88)91222-x. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Sonne O. A simple, rapid, and sensitive method for measuring protein concentration in subcellular membrane fractions prepared by sucrose density ultracentrifugation. Anal Biochem. 1982 Jan 15;119(2):424–427. doi: 10.1016/0003-2697(82)90608-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. H., Feng D. P. Postsynaptic protein kinase C essential to induction and maintenance of long-term potentiation in the hippocampal CA1 region. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2576–2580. doi: 10.1073/pnas.89.7.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cerro S., Larson J., Oliver M. W., Lynch G. Development of hippocampal long-term potentiation is reduced by recently introduced calpain inhibitors. Brain Res. 1990 Oct 15;530(1):91–95. doi: 10.1016/0006-8993(90)90660-4. [DOI] [PubMed] [Google Scholar]





