Abstract
Introduction
Self-efficacy has been found to have a direct relation with self-care in diabetes. Several tools have been developed and used for evaluating self-efficacy of diabetic patients, the most widely used being the Diabetes Management Self-Efficacy Scale (DMSES). The aim of the present study was to translate, culturally adapt, and validate the Greek DMSES (GR-DMSES) in order for it to be used in the ATTICA pilot study of the SmartCare EU-funded project.
Methods
Using standard procedures, the original version of DMSES was translated and culturally adapted into Greek. Content validity was assessed by an expert panel with the calculation of a content validity index of the overall scale. Α convenient sample was recruited to complete the questionnaire. Psychometric testing of the produced instrument included internal consistency test (Cronbach’s alpha), construct validity (factor analysis), and stability (intraclass correlation coefficient).
Results
One hundred and sixteen patients, aged 36–86 years, with type 2 diabetes (T2D) participated in the study. There were no items excluded from the original scale after the content validity procedure. The coefficient Cronbach's alpha for the internal consistency was 0.93 and the intraclass correlation coefficient for the stability with a 5-week time interval was 0.87 (P < 0.001). Factor analysis yielded four factors related to diet, medical therapy, medication and feet check, and physical activity.
Conclusion
The findings supported that the GR-DMSES was reliable and valid in measuring self-efficacy related to diabetes self-management, thus providing a quick and easy-to-use tool for health professionals dealing with Greek adults with T2D.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-015-0278-1) contains supplementary material, which is available to authorized users.
Keywords: Diabetes, Diabetes Management Self-Efficacy Scale, Greece, Self-efficacy, Validation
Introduction
Diabetes mellitus (DM) is among the 10 leading causes of death globally, according to World Health Organization (WHO) data [1]. The prevalence of DM for adults was estimated to be 6.4% in 2010 and is projected to rise to 7.7% by the year 2030 [2]. European data published in 2013, show an 8.5% prevalence of diabetes in the age group of 20–79 years [3]. Consisting an emerging pandemic, diabetes imposes a large economic burden on the individual, the national healthcare system, and the economy [4]. This is not the consequence of just diabetes per se, but also of its complications, which additionally contribute to premature mortality rates, and social and economic burdens [5].
In order to prevent diabetes, manage existing diabetes, and prevent or at least slow down the rate of development of diabetes complications, medical nutrition therapy (MNT) is recommended [6]. MNT consists of healthy eating, regular physical activity, and often pharmacotherapy [7]. These behavioral changes need to be achieved and maintained, given the fact that diabetes is a chronic condition, in order to improve clinical outcomes, health status, and quality of life [8]. In recognition of diabetes being a largely self-managed condition, care for those people is focused on diabetes self-management education and support, and aims at facilitating the aforementioned behavior changes [7, 8]. However, behavioral changes are complex processes that are influenced by various factors, among which self-efficacy has been identified as a core one [9].
Self-efficacy is a concept introduced by Bandura within the context of social learning theory, which was progressed into the social cognitive theory [10–12]. Social cognitive theory postulates that an individual’s behavior is determined by personal, behavioral, and environmental factors [10–12]. These factors are not of equal strength, do not occur simultaneously and the interaction between them differs based on the individual, the particular behavior being examined, and the specific situation in which the behavior occurs [12]. However, humans contribute to their own motivation, behavior, and development due to five capabilities: symbolizing, vicarious, forethought, self-regulatory, self-reflective [11, 12]. Self-efficacy is a major determinant of self-regulation and is defined as “people’s beliefs about their capabilities to produce designated levels of performance that exercise influence over events that affect their lives” [13].
In diabetes, self-efficacy has been found to have a direct relation with self-care, in a way that this construct owns the predictability power of self-care behavior [14]. This finding has also been replicated in low-income diabetic populations, where higher self-efficacy has been associated with improved glycemic control, medication adherence, self-care behavior, and mental health-related quality of life [15]. However, even though self-efficacy has been found to positively affect adherence to treatment, mixed results have been presented regarding the impact on clinical outcomes [16–18]. This may be partly attributed to the fact that there is not only one tool available for assessing self-efficacy.
In fact, several tools have been developed and used for evaluating self-efficacy of diabetic patients, the most widely used being the Diabetes Management Self-Efficacy Scale (DMSES) [19]. This tool was originally developed in 1999 and consists of 20 items that reflect the tasks a person with type 2 diabetes (T2D) has to carry out in the context of managing this condition [20]. These diabetes self-care activities can be grouped as follows [21]:
Performing activities essential for the treatment of diabetes (i.e., use of medication, dietary adherence);
Self-observation (i.e., of body weight, feet condition);
Self-regulating activities (i.e., correction of hypo and/or hyperglycemia).
This original tool consists of four factors: five items in the first factor (nutrition specific and weight), nine items in the second factor (nutrition general and medical treatment), three items in the third factor (physical exercise), and three items in the fourth factor (blood sugar). Two versions of the tool are available, both of them consisting of these 20 items and differing mainly in the responses available. The first one asks the respondent to select from a five-point scale (yes definitely, probably yes, maybe yes/maybe no, probably no, definitely not) the answer that best suits him/her to the question of how convinced he/she is that he/she is able to perform the task described in each item [20]. This version has been translated and validated in Turkish [22] and Iranian [23]. The second version uses an 11-point scale which ranges from 0 (cannot do at all) to 10 (certainly can do) as response options to the statement “I am confident that I am able to…” which ends with each one of the items included in the scale. This version has been adapted for use in China [24] and Australia [25]. In both occasions, response grades are summed to produce a single score for self-efficacy.
In accordance to European data, a 7% prevalence of diabetes is reported for Greece [3], but no tools measuring self-efficacy related to diabetes self-management are available for this population. Therefore, the aim of the present study was to translate and culturally adapt the DMSES into Greek (GR-DMSES) and to test the psychometric properties of the produced instrument in a Greek population of middle-aged and elderly patients with T2D. This validated version of DMSES will be used in the ATTICA pilot of the EU-funded project SmartCare, aiming to investigate whether computer-based self-management support can contribute to improve health outcomes of patients with T2D aged over 50 years. Given the need for a tool suitable for use in the aforementioned study and the high prevalence of T2D in older adults [26], the simplest five-point answer scale [20] was chosen to be translated and validated.
Methods
To ensure the quality and the efficiency of the adapted instrument in the Greek T2D population, the validation of the GR-DMSES was based on the procedure that has been previously described in the literature [27]. This procedure was accomplished in two phases.
First Phase: Translation, Cultural Adaptation, and Content Validity Measurement
Sample and Setting
A convenient sample of patients with T2D was recruited from the Diabetic Association of Piraeus and Islands in Attica, Greece, and from a public, military hospital in the same area. In total, 150 people were recruited and given a consent form. To determine the sufficient size of the sample for factor analytic procedures, the following two criteria were used: (1) the sample should have 51 more cases than the number of variables to support Chi-square testing in Bartlett’s test of sphericity [28]; (2) the sample should include at least 100 cases and a subjects to variables (STV) ratio should be no less than 5 [29]. One of the authors (VE) collected all the data through face-to-face interviews with the participants, during a week. The information collected was confidential and the study procedures were according to the Declaration of Helsinki of 1964, describing the Ethical Principles for Medical Research Involving Human Subjects, as revised in 2013.
Translation
Given the fact that there is no perfect method for translating an instrument, multiple techniques were used, as proposed for cross-cultural research, namely forward translation, consensus meeting, back translation, and pre-test piloting [30, 31]. Forward translation: two bilingual (Greek and English) translators worked independently to produce a Greek version of the DMSES. Consensus meeting: according to the WHO Scientific Advisory Committee of the Medical Outcomes Trust, the 8th requirement when developing or translating an instrument is the cultural adaption which also involves the revision of the translated instrument by a panel of experts [32]. To resolve any ambiguities and discrepancies between the two forward translated versions and to culturally adapt the instrument, the items were revised from a consensus group. The group consisted of the two forward translators, a third bilingual translator and one endocrinologist. Back translation: the version produced by the group was then back translated in English by two bilingual translators whose mother tongue is English and who had never before seen the original English version of the scale.
Content Validity
To test content validity, the translated version was sent to a panel consisting of eight experts in the area of diabetes self-management, who were familiarized with the conceptual underpinnings and measurement model of the instrument. This multidisciplinary team comprised three endocrinologists, one diabetes specialist pathologist, two registered dietitians, and two registered nurses. Each of the health professionals were asked to assess the necessity of each item of the instrument through a three-response Likert scale (1 = “necessary”, 2 = “useful but not necessary”, and 3 = “unnecessary”). A content validity index of individual items (I-CVI) was calculated for each item as well as an overall score; average of the I-CVIs for all items on the scale (S-CVI/Ave) for the GR-DMSES. The I-CVI is computed as the number of experts giving a rating of 1 (thus dichotomizing the ordinal scale into necessary and not necessary), divided by the total number of experts. S-CVI/Ave is computed as the average of the I-CVIs for all items on the scale. According to Davis (1992), a content valid instrument should have a minimum content validity index of 0.80 [33]. During this procedure, item 18 “I am able to visit my doctor once a year to monitor my diabetes”, was changed to “I am able to visit my doctor every three months to monitor my diabetes” to reflect local best practice. Additionally, the word “blood” from the phrase “blood sugar” in items 1, 2, and 3, was not translated into Greek at all, as it is not used in every day speaking when someone is referring to blood sugar.
Pre-test Piloting
The instrument was pilot tested with 10 patients with T2D from the Diabetic Association of Piraeus and Islands in Attica, Greece, to ensure that future users will comprehend all questions and procedures [34]. Statement of doubts and suggestions regarding each item of the scale was encouraged by open-ended questions, asked by one of the authors. No difficulties in understanding the items were recorded. Completion of the questionnaire took 6–12 minutes.
Second Phase: Psychometric Testing
Internal Consistency
To determine internal consistency, Cronbach’s alpha was used, which is expressed as a number between 0 and 1 [35]. Values that fall between 0.80 and 0.90 are considered a very good indicator [36]. Except for overall alpha, two other indicators are important for internal consistency; corrected item–total correlation and Cronbach’s alpha if item deleted. The first indicator is a way to assess how well one item’s score is internally consistent with composite scores from all other items that remain. If this correlation is weak (anything less than 0.30 is a weak correlation for item-analysis purposes) [37], then that item should be removed and not used to form a composite score for the variable in question. The other indicator is the resulted Cronbach’s alpha if a given item is deleted and it is valuable for determining which of a set of items contributes to the total alpha.
Construct Validity
Construct validity was examined by undertaking Principal-Component Factor Analysis with a varimax rotation. To attain the best fitting structure and the correct number of factors, the following criteria were used: (1) Kaiser rule, (2) the percentage of variation that is explained, (3) scree plot. According to Kaiser rule [38], the factors that should remain are those with eigenvalue greater than 1.0, since this essentially shows that this value is the amount of variation explained by a factor and the eigenvalue which is equal to 1.0 shows a substantial change. A second selection method based on similar conceptual structure is to retain the number of factors that account for a certain percent of variance extracted. There is no unanimity in the literature regarding the degree of the variance explained needed before the number of factors is sufficient. The majority suggest that 75–90% of the variance should be accounted for [39]; however, some indicate that as little as 50% of the variance explained is acceptable [40], especially when the researcher’s goal emphasizes parsimony (explaining variance with as few factors as possible). The third criterion is the Cattell’s scree plot, which helps to determine the number of factors seeing the breaking point or elbow [41]. Ultimately, the decision regarding the number of factors to retain should be made based on comprehensibility and interpretability in the context of the research [29].
Additionally, it is determined that only factors with loadings >0.30 were included in each factor. Prior to conducting factor analysis, the following conditions were examined to determine if it was appropriate to continue with the specific analysis: correlation matrix, communalities, reproduced correlations, measures of sampling adequacy (MSA). Kaiser–Meyer–Olkin measure of sampling adequacy (KMO) and Bartlett’s test were calculated to evaluate whether the sample was large enough to perform a satisfactory factor analysis. The KMO should be greater than 0.5 to proceed with a satisfactory factor analysis and values greater than 0.8 consist a really good indicator [38]. The P value of Bartlett’s test of sphericity (which tests the null hypothesis that the original correlation matrix is an identity matrix) should be significant and lower than 0.05 [38].
Stability
For examining test–retest reliability and stability, 25 participants, randomly selected from the total sample, completed for the second time the questionnaire 5 weeks after the first completion. Based on a code that each respondent had, the respondent’s data of the first and second measurement could be detected and matched. Then, by means of the intraclass correlation coefficient (ICC), the test–retest reliability was calculated, with appropriate transformation of data.
Statistical Analysis
Data analysis was conducted using SPSS statistical software version 21 (SPSS Inc., Chicago, IL, USA). All variables of the study were calculated with descriptive analysis including mean, standard deviation (SD), frequency and percentage. The psychometric properties of the GR-DMSES were determined with item analysis, reliability, and validity. Item analysis were conducted with item–total correlations. To test the reliability of the GR-DMSES, internal consistency using Cronbach’s alpha for each subscale and the overall DMSES. Construct validity was examined by undertaking Principal-Component Factor Analysis with a varimax rotation. To test stability of the GR-DMSES, test–retest reliability was evaluated using ICC using a two-way random effects model and absolute agreement with a 5-week interval between assessments.
Results
Research Population
From the 150 people initially recruited to participate in the study, only 117 returned completed questionnaires (response rate 78%) and consent forms. One of the questionnaires was excluded due to substantial missing data, thus leaving 116 tools for analysis. This number of participants produced an STV ratio of 5.8. Demographic characteristics of the participants are summarized in Table 1. The age of participants ranged from 36 to 86 years with a mean age of 64.4 years (SD = 10.42). The research group was 41.8% male and 58.2% female. Most of the patients had graduated from high school (33.6%), 30.9% had secondary school education, 25.5% elementary education level, and 10% had university or other education. The majority of patients were married (66.7%), 8.8% were divorced, 6.1% were unmarried, and 18.4% co-habited with someone else. Most of the patients had public insurance (90%), 6.4% had private, and 3.6% had both. The average number of years depicting diabetes duration was 12.9 years (SD = 8.05) ranging from 1 to 38 years.
Table 1.
Demographic characteristics of participants
| Characteristics | Total sample (n = 116) | Retest sample (n = 25) |
|---|---|---|
| Gender (%) | ||
| Male | 41.8 | 44.0 |
| Female | 58.2 | 56.0 |
| Education level (%) | ||
| Primary | 25.5 | 32.0 |
| Secondary | 30.9 | 12.0 |
| High | 33.6 | 34.0 |
| University | 9.1 | 12.0 |
| Other | 0.9 | 0.0 |
| Family status (%) | ||
| Unmarried | 6.1 | 8.0 |
| Married | 66.7 | 64.0 |
| Divorced | 8.8 | 8.0 |
| Widower | 18.4 | 20.0 |
| Health insurance (%) | ||
| Yes, public | 90.0 | 92.0 |
| Yes, private | 6.4 | 8.0 |
| Yes, both | 3.6 | 0.0 |
| Age (years), mean ± SD | 64.4 ± 10.42 | 64.1 ± 14.05 |
| Disease duration (years), mean ± SD | 12.9 ± 8.05 | 11.0 ± 6.72 |
SD standard deviation
Content Validity
As it is already mentioned, the translated scale was judged by an expert panel of eight healthcare professionals on relevance and phrasing of the instrument items. S-CVI/Ave for GR-DMSES was computed and found to be 0.85.
Internal Consistency
The GR-DMSES had an overall coefficient alpha of 0.93 (Table 2). The corrected item–total correlations indicated good correlations (different from zero) for all the items. Removal of one or more weakly correlated items did not result in major consequences on the alpha value. The mean corrected item–total correlation for the GR-DMSES was 0.61 (minimum = 0.46, maximum = 0.74; Table 3). Cronbach’s alpha coefficients, for each of the subscales identified during construct validity testing of GR-DMSES, were 0.92 for diet, 0.76 for medical therapy, 0.70 for medication and feet check, and 0.79 for physical activity.
Table 2.
Cronbach’s alpha for GR-DMSES (n = 116)
| Number of items | Cronbach’s alpha | |
|---|---|---|
| GR-DMSES | 20 | 0.93 |
| Diet (items 4, 5, 9, 10, 13, 14, 15, 16, 17) | 9 | 0.92 |
| Medical therapy (items 1, 2, 3, 6, 18) | 5 | 0.76 |
| Medication and feet check (items 7, 19, 20) | 3 | 0.70 |
| Physical activity (items 8, 11, 12) | 3 | 0.79 |
GR-DMSES Greek version of the Diabetes Management Self-Efficacy Scale
Table 3.
Reliability analysis
| Scale mean if item deleted | Scale variance if item deleted | Corrected item–total correlation | Cronbach’s alpha if item deleted | |
|---|---|---|---|---|
| DMSES1 | 40.48 | 230.419 | 0.458 | 0.928 |
| DMSES2 | 40.33 | 227.771 | 0.591 | 0.926 |
| DMSES3 | 40.46 | 228.846 | 0.602 | 0.926 |
| DMSES4 | 40.09 | 226.658 | 0.613 | 0.925 |
| DMSES5 | 40.04 | 224.749 | 0.647 | 0.925 |
| DMSES6 | 39.91 | 226.134 | 0.517 | 0.927 |
| DMSES7 | 40.28 | 224.467 | 0.580 | 0.926 |
| DMSES8 | 39.85 | 222.417 | 0.572 | 0.926 |
| DMSES9 | 40.22 | 223.080 | 0.732 | 0.923 |
| DMSES10 | 40.24 | 225.444 | 0.708 | 0.924 |
| DMSES11 | 40.05 | 225.188 | 0.590 | 0.926 |
| DMSES12 | 40.12 | 223.296 | 0.639 | 0.925 |
| DMSES13 | 39.72 | 220.848 | 0.649 | 0.924 |
| DMSES14 | 39.80 | 221.019 | 0.677 | 0.924 |
| DMSES15 | 39.86 | 217.242 | 0.745 | 0.922 |
| DMSES16 | 39.76 | 223.182 | 0.627 | 0.925 |
| DMSES17 | 39.40 | 221.171 | 0.618 | 0.925 |
| DMSES18 | 40.25 | 229.879 | 0.473 | 0.928 |
| DMSES19 | 40.76 | 231.849 | 0.584 | 0.926 |
| DMSES20 | 40.41 | 229.031 | 0.562 | 0.926 |
Cronbach’s alpha = 0.929
DMSES Diabetes Management Self-Efficacy Scale
Construct Validity
Regarding the criteria needed to be satisfied in order to proceed with factor analysis, Bartlett’s test of sphericity was found to be significant (χ2 = 1041.94, P < 0.001) and the KMO measure of sampling adequacy was found to be acceptable (0.87). Based on the results of these two tests and other conditions which were satisfied (correlation matrix, communalities, reproduced correlations, MSA), it was determined that a factor analysis could be computed on this data set.
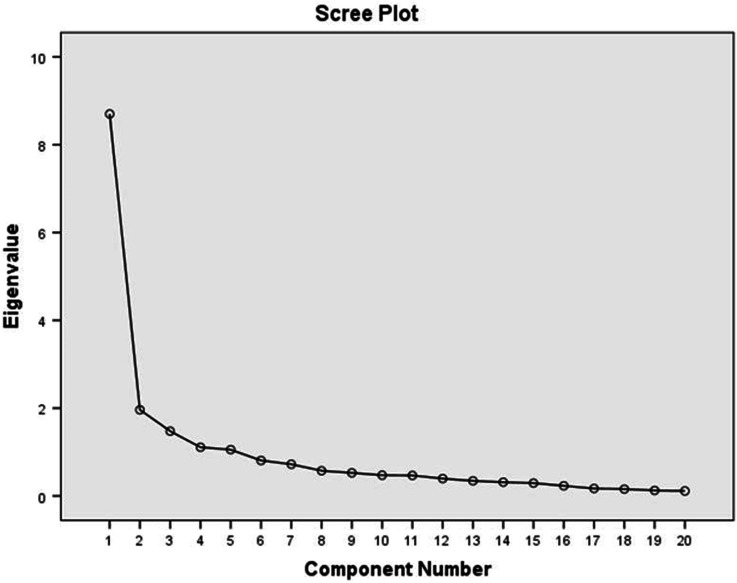
The three criteria that were used in order to obtain the best fitting structure and the correct number of factors produced different number of factors. Specifically, components with eigenvalues greater than 1.0, produced five factors, Cattell’s scree test plot, indicated four factors (Fig. 1) and the percentage of total variance explained by each factor, revealed at least two factors (Table 4). Due to disagreement of methods, four factors were decided to be the optimum solution, based on comprehensibility and interpretability in the context of the research [29]. These four factors explained the 66.2% of the total sample variance.
Fig. 1.
Scree plot for determination of number of factors
Table 4.
The results of the Principal-Component Factor Analysis
| Eigenvaluea | % of variance | Cumulative % | |
|---|---|---|---|
| 1 | 8.701 | 43.503 | 43.503 |
| 2 | 1.961 | 9.806 | 53.309 |
| 3 | 1.474 | 7.370 | 60.680 |
| 4 | 1.108 | 5.541 | 66.221 |
aThe latent dimension is taken to be equal to the number of eigenvalues which are >1.0
The principal components that were chosen follow the statement that the first component accounts for the maximum part of the variance which was 43.5% with eigenvalue 8.7 (Table 4). This factor describes the nutritional adjustments a diabetic person is recommended to do in order to manage the disease. On this factor, loadings are found for items which concern the ability of the patient to follow dietary recommendations when he/she is under specific circumstances, such as being ill, away from home, on holiday, at a party, or in a specific psychological pressure like stress (items 4, 5, 9, 10, 13, 14, 15, 16, 17). The second component explains the maximum part of the remaining variance (9.8%) with an eigenvalue of 2.0. On this factor, loadings are found about blood sugar control, weight control, and visiting doctor regularly (items 1, 2, 3, 6, 18). The third factor explains 7.4% of the variance with an eigenvalue of 1.5 and has to do with the medication and feet check, including taking and adjusting medication and self-examination of feet (items 7, 19, 20). The last factor explains 5.5% of the variance with an eigenvalue of 1.1. This factor refers to items related to physical activity and it includes three items concerning the ability to take enough exercise, more exercise if doctor suggests that and the ability to adjust eating plan when taking more exercise (items 8, 11, 12). Fourteen items had double loading, but for the majority of them (9 items) the bigger factor loading was kept (Table 5). One item had almost equal factor loadings (ability to adjust eating plan when ill) and in this case the loading taken into account was chosen based on better interpretation of the analysis. The same applied for the remaining four items, which the smaller factor loading was taken into account for the same reason.
Table 5.
Rotated factor matrix of the Greek version of the Diabetes Management Self-Efficacy Scale (n = 116)
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
|---|---|---|---|---|
| Factor 1: diet | ||||
| 4. I am able to choose the correct food | 0.566 | 0.455 | ||
| 5. I am able to choose different foods and stick to a healthy eating pattern | 0.547 | 0.508 | ||
| 9. I am able to adjust my eating plan when ill | 0.537 | 0.540 | ||
| 10. I am able to follow a healthy eating pattern most of the time | 0.445 | 0.668 | ||
| 13. I am able to follow a healthy eating pattern when I am away from home | 0.832 | |||
| 14. I am able to adjust my eating plan when I am away from home | 0.826 | |||
| 15. I am able to follow a healthy eating pattern when I am on holiday | 0.798 | 0.347 | ||
| 16. I am able to follow a healthy eating pattern when I am eating out or at a party | 0.768 | 0.387 | ||
| 17. I am able to adjust my eating plan when I am feeling stressed or anxious | 0.684 | 0.324 | ||
| Factor 2: medical therapy | ||||
| 1. I am able to check my blood sugar if necessary | 0.651 | |||
| 2. I am able to correct my blood sugar when the sugar level is too high | 0.358 | 0.576 | ||
| 3. I am able to correct my blood sugar when the blood sugar level is too low | 0.496 | 0.620 | ||
| 6. I am able to keep my weight under control | 0.414 | 0.333 | ||
| 18. I am able to visit my doctor every three months to monitor my diabetes | 0.748 | |||
| Factor 3: medication and feet check | ||||
| 7. I am able to examine my feet for cuts | 0.736 | 0.357 | ||
| 19. I am able to take my medication as prescribed | 0.663 | 0.512 | ||
| 20. I am able to adjust my medication when I am ill | 0.662 | |||
| Factor 4: physical activity | ||||
| 8. I am able to take enough exercise, for example, walking the dog or riding a bicycle | 0.302 | 0.802 | ||
| 11. I am able to take more exercise if the doctor advises me to do so | 0.891 | |||
| 12. When taking more exercise I am able to adjust my eating plan | 0.389 | 0.696 | ||
| Variance explained (%) | 43.5 | 9.8 | 7.4 | 5.5 |
Extraction method: Principal-Component Analysis, rotation method: varimax with Kaiser normalization
Stability
Twenty-two percent of the sample was completed the scale for the second time after 5 weeks from initial approach. ICC was found to be 0.87 (95% confidence interval 0.085–0.965, P < 0.001).
Discussion
The objective of this study was to translate, culturally adapt and evaluate the validity and reliability of the DMSES among a Greek population. According to the results of the present study, the items were homogenous to the scale; as the mean and variance scores of each item and item–total correlation confirmed this homogeneity.
The sample size in the present study was larger than that of the Australian (n = 88) and Turkish (n = 101) validation studies and smaller than that of the Iranian (n = 332) and Chinese (n = 230) validation studies [22–25]. For sample size determination, all criteria that were suggested in the methods section were satisfied.
The content validity of this scale seems sufficiently secured. Specifically, the S-CVI/Ave score depicted acceptable agreement [42, 43], but was found to be smaller than that calculated in the validation study for the Chinese version (0.86) [24]. In the present study, the expert panel consisted of professionals working with diabetic patients coming from different disciplines (nurses, dietitians, endocrinologists), all being aware that self-efficacy consists a major determinant of self-management. However, there were no self-efficacy experts in the panel as there is no such specialty in Greece.
Reliability of the GR-DMSES was high with a value of 0.93 of the total scale and ranged from 0.70 to 0.92 for the subscales. This is similar to findings reported for the Iranian (0.92), English (0.91), and Chinese versions (0.93), and higher than the Dutch (0.81) and Turkish versions (0.88) [20, 22–25].
It is of note that the various versions differ in the number of factors revealed. In the present study, four factors were generated, in concordance to the Chinese and Dutch tools [20, 24], whereas in the Turkish version there were three factors [22] and in the Iranian version, five factors [23]. The difference in the number of generated factors between the present version and other versions, could be related either to the various response scales of the instruments or to different sample sizes. The present tool is closer to the original regarding sample size and uses the same response scale, parameters that may explain the fact that in both tools four factors were revealed. Differences were also apparent regarding the content of factors. Factor 1 in GR-DMSES is the same to factor 1 of the Chinese version and similar to the sum of factors 1 and 2 of the Dutch version. Factor 2 in GR-DMSES is similar to factor 2 and two items present in factors 3 and 4 of the Chinese version, and to factor 4 plus two items present in factors 1 and 2 of the Dutch version. Factor 3 is similar to factor 4 of the Chinese version and there is no similarity with any of the factors found in the Dutch version. Finally, factor 4 is similar to factor 3 of the Chinese version, which has an extra item and identical to factor 3 of the Dutch version.
The findings with regard to stability of the instrument, measured with a time interval of 5 weeks, can be judged as excellent good with an ICC of 0.87. This finding on the test–retest reliability indicated good stability of the GR-DMSES according to Rosner’s recommendation, who suggested that an ICC >0.75 means excellent reproducibility [44]. This finding is similar to that of the Turkish version of DMSES where the ICC was found to be 0.91. There are two common methods to calculate test–retest reliability: Pearson’s product-moment correlation and ICC [45]. However, the Pearson’s product-moment correlation, which is used in other studies, has limitations such as an inability to detect a systematic error [45]. On the other hand, ICC has been reported to be a more sensitive method to assess test–retest reliability.
Nevertheless it should be noted that this tool was validated in middle and old age urban convenient population suffering from T2D. This may limit the generalizability of the findings in other age groups or rural populations.
Summarizing, self-efficacy is a major determinant of self-regulation and diabetes consists a highly prevalent, among Greeks, chronic disease demanding self-management. Health professionals are in position to help people with diabetes improve their self-efficacy. However, the first step of nutrition care process, a systematic approach to providing high-quality nutrition care [46], is assessment. Having a validated tool appropriate for the population to assess self-efficacy is really important in the realm of providing the best nutritional support. Moreover, applying an already widely used tool gives the opportunity to health professionals to compare the effectiveness of their intervention, regarding self-efficacy, to that of other studies and further improve their techniques. This study showed that GR-DMSES is a valid and reliable scale and could be used to measure self-efficacy related to diabetes self-management of Greek diabetic patients, thus providing a quick and easy-to-use tool to health professionals dealing with Greek adults with T2D.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank Mrs. Golfo Gemistou, president of Diabetic Association of Piraeus and Islands in Attica, Greece, and all the diabetic patients who participated in the study. No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Evaggelia Fappa, Vasiliki Efthymiou, George Landis, Anastasios Rentoumis, and John Doupis have nothing to disclose.
Compliance with Ethics Guidelines
The information collected was confidential and the study procedures were according to the Declaration of Helsinki of 1964, describing the Ethical Principles for Medical Research Involving Human Subjects, as revised in 2013. Informed consent was obtained from all study participants.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.WHO. The top 10 causes of death. 2014. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/. Cited July 20, 2014.
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.IDF . IDF diabetes atlas. 6. Brussels: International Diabetes Federation; 2013. [PubMed] [Google Scholar]
- 4.IDF. The economic impacts of diabetes 2009. Available from: http://www.idf.org/diabetesatlas/economic-impacts-diabetes. Cited July 20, 2014.
- 5.van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(Suppl 1):S3–S8. doi: 10.1097/01.hjr.0000368191.86614.5a. [DOI] [PubMed] [Google Scholar]
- 6.Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 7.Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821–3842. doi: 10.2337/dc13-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas L, Maryniuk M, Beck J, et al. National standards for diabetes self-management education and support. Diabetes Care. 2013;36(Suppl 1):S100–S108. doi: 10.2337/dc13-S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holloway A, Watson HE. Role of self-efficacy and behaviour change. Int J Nurs Pract. 2002;8:106–115. doi: 10.1046/j.1440-172x.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 10.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037/0033-295X.84.2.191. [DOI] [PubMed] [Google Scholar]
- 11.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice Hall; 1986. [Google Scholar]
- 12.Bandura A. Social cognitive theory. In: Annals of child development. Greenwich: Jai Press LTD; 1989.
- 13.Bandura A. Self-efficacy. In: Ramachaudran VS, editor. Encyclopedia of human behavior. vol. 4. New York: Academic Press; 1994. p. 71–81 (Reprinted in Friedman H, editor. Encyclopedia of mental health. San Diego: Academic; 1998).
- 14.Mohebi S, Azadbakht L, Feizi A, Sharifirad G, Kargar M. Review the key role of self-efficacy in diabetes care. J Educ Health Promot. 2013;2:36. doi: 10.4103/2277-9531.115827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker RJ, Smalls BL, Hernandez-Tejada MA, Campbell JA, Egede LE. Effect of diabetes self-efficacy on glycemic control, medication adherence, self-care behaviors, and quality of life in a predominantly low-income, minority population. Ethn Dis. 2014;24:349–355. [PMC free article] [PubMed] [Google Scholar]
- 16.Mishali M, Omer H, Heymann AD. The importance of measuring self-efficacy in patients with diabetes. Fam Pract. 2011;28:82–87. doi: 10.1093/fampra/cmq086. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Wang J, Zheng P, et al. Effects of self-care, self-efficacy, social support on glycemic control in adults with type 2 diabetes. BMC Fam Pract. 2013;14:66. doi: 10.1186/1471-2296-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chlebowy DO, Garvin BJ. Social support, self-efficacy, and outcome expectations: impact on self-care behaviors and glycemic control in Caucasian and African American adults with type 2 diabetes. Diabetes Educ. 2006;32:777–786. doi: 10.1177/0145721706291760. [DOI] [PubMed] [Google Scholar]
- 19.Mohammad Rezal H, Emma M, Julinawati S, Suffian A, Husna MY. Systematic review: the measurement of health self-efficacy to diabetes. Austr J Basic Appl Sci. 2013;7:295–302.
- 20.Bijl JV, Poelgeest-Eeltink AV, Shortridge-Baggett L. The psychometric properties of the diabetes management self-efficacy scale for patients with type 2 diabetes mellitus. J Adv Nurs. 1999;30:352–359. doi: 10.1046/j.1365-2648.1999.01077.x. [DOI] [PubMed] [Google Scholar]
- 21.Pennings-van der Eerden L. Self-care behavior in the treatment of diabetes mellitus. Amsterdam: University of Utrecht; 1992.
- 22.Kara M, van der Bijl JJ, Shortridge-Baggett LM, Asti T, Erguney S. Cross-cultural adaptation of the Diabetes Management Self-Efficacy Scale for patients with type 2 diabetes mellitus: scale development. Int J Nurs Stud. 2006;43:611–621. doi: 10.1016/j.ijnurstu.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Noroozi A, Tahmasebi R. The diabetes management self-efficacy scale: translation and psychometric evaluation of the Iranian version. Nurs Pract Today. 2014;1:9–16. [Google Scholar]
- 24.Vivienne Wu SF, Courtney M, Edwards H, McDowell J, Shortridge-Baggett LM, Chang PJ. Development and validation of the Chinese version of the Diabetes Management Self-efficacy Scale. Int J Nurs Stud. 2008;45:534–542. doi: 10.1016/j.ijnurstu.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 25.McDowell J, Courtney M, Edwards H, Shortridge-Baggett L. Validation of the Australian/English version of the Diabetes Management Self-Efficacy Scale. Int J Nurs Pract. 2005;11:177–184. doi: 10.1111/j.1440-172X.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- 26.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 27.Sousa VD, Rojjanasrirat W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. J Eval Clin Pract. 2011;17:268–274. doi: 10.1111/j.1365-2753.2010.01434.x. [DOI] [PubMed] [Google Scholar]
- 28.Lawley DN, Maxwell AE. Factor analysis as a statistical method. London: Butterworths; 1971.
- 29.Suhr DD. Exploratory or confirmatory factor analysis? 2006. Available from: http://www2.sas.com/proceedings/sugi31/200-31.pdf. Accessed Sept 9, 2014.
- 30.Maneesriwongul W, Dixon JK. Instrument translation process: a methods review. J Adv Nurs. 2004;48:175–186. doi: 10.1111/j.1365-2648.2004.03185.x. [DOI] [PubMed] [Google Scholar]
- 31.Brislin RW, Lonner WJ, Throndike RM. Cross-cultural research methods. Canada: Wiley; 1973. [Google Scholar]
- 32.Aaronson N, Alonso J, Burnam A, Lohr KN, Patrick DL, Perrin E, Stein REK. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11(3):193–205. doi: 10.1023/A:1015291021312. [DOI] [PubMed] [Google Scholar]
- 33.Davis LL. Instrument review: getting the most from your panel of experts. Appl Nurs Res. 1992;5:194–197. doi: 10.1016/S0897-1897(05)80008-4. [DOI] [Google Scholar]
- 34.Brislin RW. Back-translation for cross-cultural research. J Cross Cult Psychol. 1970;1:185–216. doi: 10.1177/135910457000100301. [DOI] [Google Scholar]
- 35.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychomerika. 1951;16:297–334. doi: 10.1007/BF02310555. [DOI] [Google Scholar]
- 36.DeVellis RF. Guidelines in scale development. In: Bickman L, Rog DJ, editors. Scale development: theory and applications. Thousand Oaks: Sage Publications; 2012. p. 73–114.
- 37.De Vaus D. Building scales. In: Surveys in social research. London and New York: Routlege; 2014. p. 179–200.
- 38.Field A. Exploratory factor analysis. In: Wright DB, editor. Discovering statistics using SPSS. London: SAGE Publications; 2005. p. 619–80.
- 39.Pett M, Lackey N, Sullivan J. Extracting the initial factors. In: Robinson S, editor. Making sense of factor analysis. Thousand Oaks: Sage Publications, Inc; 2003. p. 85–130.
- 40.Beavers AS, Lounsbury JW, Richards JK, Huck SW, Skolits GJ, Esquivel SL. Practical considerations for using exploratory factor analysis in educational research. Pract Assess Res Eval. 2013;18:1–13. [Google Scholar]
- 41.Rietveld T, Van Hout R. Factor analysis. In: De Gruyter M, editor. Statistical techniques for the study of language and language behavior. New York: Berlin; 1993. p. 251–96.
- 42.Davis LL. Instrument review: getting the most from your panel of experts. Appl Nurs Res. 1992;5:194–197. doi: 10.1016/S0897-1897(05)80008-4. [DOI] [Google Scholar]
- 43.Grant JS, Davis LL. Selection and use of content experts for instrument development. Res Nurs Health. 1997;20:269–274. doi: 10.1002/(SICI)1098-240X(199706)20:3<269::AID-NUR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 44.Rosner B. Multisample inference. In: Taylor M, editor. Fundamentals of biostatistics. Pacific Grove: Duxbury Press; 2010.
- 45.Yen M, Lo LH. Examining test-retest reliability: an intra-class correlation approach. Nurs Res. 2002;51:59–62. doi: 10.1097/00006199-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Academy of Nutrition and Dietetics. Nutrition care process. 2015. Available from: http://www.eatrightpro.org/resources/practice/nutrition-care-process. Accessed Nov 20, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.