Abstract
Steroidal saponins are widely distributed among monocots, including the Amaryllidaceae family to which the Allium genus is currently classified. Apart from sulfur compounds, these are important biologically active molecules that are considered to be responsible for the observed activity of Allium species, including antifungal, cytotoxic, enzyme-inhibitory, and other. In this paper, literature data concerning chemistry and biological activity of steroidal saponins from the Allium genus has been reviewed.
Electronic supplementary material
The online version of this article (doi:10.1007/s11101-014-9381-1) contains supplementary material, which is available to authorized users.
Keywords: Allium, Steroidal saponins, Saponins activity
Introduction
The genus Allium (Amaryllidaceae) is one of the largest monocot genera comprising more than 800 species (Li et al. 2010; APG 2009). It is widely distributed in nature and has adapted to diverse habitats across the Holarctic region, with the exception of A. dregeanum, which is native to South Africa (Li et al. 2010). Some Allium species, such as garlic, onion and leek, are widely cultivated as vegetable products, spices and for medical purposes. The most characteristic constituents in Allium plants are sulfur compounds, which are the most important substances both in terms of chemotaxonomic value and biological activity (Rose et al. 2005). However, various researchers tend to attribute the potential pharmacological benefits of Allium plants to constituents other than sulfur compounds, such as steroidal saponins. Also, polyphenolic compounds, especially flavonoids, as well as fructans, N-cynnamic amides, and antioxidative enzymes are considered to be equally important (Matsuura 2001; Lanzotti 2005; Štajner et al. 2006; Amagase 2006; Lanzotti 2012).
Apart from the Amaryllidaceae family, steroidal saponins are widely distributed in other monocot families: Asparagaceae (Agave, Asparagus, Convallaria, Hosta, Nolina, Ornithogalum, Polygonatum, Sansevieria, Yucca), Costaceae (Costus), Dioscoreaceae (Dioscorea), Liliaceae (Lilium), Melanthiaceae (Paris), Smilacaceae (Smilax). Interestingly, these compounds have been reported as well in some dicotyledonous angiosperms: Zygophyllaceae (Tribulus, Zygophyllum), Solanaceae (Solanum, Lycopersicon, Capsicum), Plantaginaceae (Digitalis) and Fabaceae (Trigonella).
There are numerous reports referring to pharmacological activities of steroidal saponins. Some of them showed promising antifungal, cytotoxic, anti-inflammatory, antithrombotic, and hypocholesterolemic effects (Sparg et al. 2004; Lanzotti 2005; Güçlü-Üstündağ and Mazza 2007). Most importantly, these compounds are used as substrates in the production of steroid hormones and drugs.
Steroidal sapogenins and saponins have been identified so far in over 40 different Allium species. The earliest reports on Allium saponins date back to the 1970s and dealt with identification of diosgenin in A. albidum (Kereselidze et al. 1970) and alliogenin in the bulbs of A. giganteum (Khristulas et al. 1970). Further studies performed worldwide in the following years led to the isolation of a large number of new compounds. The first chemical survey of saponins from the genus Allium was published by Kravets in 1990, and this was followed by an update by Lanzotti in 2005 (Kravets et al. 1990; Lanzotti 2005). Since then, a large number of new compounds has been discovered, and there were also some that have not been included in the previous surveys.
A recent review by Lanzotti et al. (2014) compiled data on various compounds identified in Allium species with a reported cytotoxic and antimicrobial activity.
The present review is predominantly focused on the chemistry of Allium steroidal saponins and their biological activities.
Chemistry of Allium saponins
Steroidal saponins from the genus Allium can be divided into three groups on the basis of the sapogenin structure: spirostanols, furostanols, and open-chain saponins. The latter group is often referred to in the literature as “cholestane saponins” (Challinor and De Voss 2013). Allium saponins are mostly mono- or bidesmosides, however a tridesmodic cholestane glycoside has been reported in the bulbs of A. macleanii (Inoue et al. 1995). The sugar residue in Allium saponins consists of linear or branched chains made up most often of glucose (Glc), rhamnose (Rha), galactose (Gal), xylose (Xyl), and arabinose (Ara) units.
Spirostane-type saponins
A vast structural diversity of Allium spirostanols is associated with the differences in the structure of aglycones, especially their oxygenation patterns and stereochemistry (Table 1). In spirostane-type sapogenins, the steroid A/B ring junction is found mostly in a trans (5α), or more rarely in a cis (5β) fusion (e.g. anzurogenin A [48] and C [58]). Δ5(6) unsaturation is considered to be a quite common feature (diosgenin [4], ruscogenin [17], yuccagenin [19], lilagenin [20], cepagenin [44], karatavigenin C [45]). However, a double bond located at C25(27) was reported in the aglycones of saponins present in A. macrostemon and in one of the sapogenins identified in A. ursinum bulbs (He et al. 2002; Sobolewska et al. 2006; Cheng et al. 2013). The C-25 methyl group is found with either S or R absolute configuration. In many cases the isolated sapogenins appear to be a mixture of diastereomers R and S.
Table 1.
Spirostane-type sapogenins identified in the genus Allium
| No. | Common name | Structure | Species |
|---|---|---|---|
| [1] | Tigogenin | (25R)-5α-spirostane-3β-ol | A. affine, A. chinense, A. fistulosum, A. macleanii, A. macrostemon, A. rotundum, A. sativum |
| [2] | Neotigogenin | (25S)-5α-spirostane-3β-ol | A. chinense, A. tuberosum |
| [3] | Smilagenin | (25R)-5β-spirostane-3β-ol | A. macrostemon |
| [4] | Diosgenin | (25R)-spirost-5(6)-ene-3β-ol | A. affine, A. albidum, A. ampeloprasum, A. angulosum, A. cepa, A. cernuum, A. fistulosum, A. flavum, A. fuscoviolaceum, A. giganteum, A. gramineum, A. karataviense, A. narcissiflorum, A. nutans, A. porrum, A. rotundum, A. schoenoprasum, A. senescens, A. ursinum, A. vineale, A. waldsteinii |
| [5] | (25R)-spirost-5(6),25(27)-diene-3β-ol | A. ursinum | |
| [6] | Laxogenin | (25R)-5α-spirostane-3β-ol-6-one | A. chinense, A. schoenoprasum |
| [7] | Hecogenin | (25R)-5α-spirostane-3β-ol-12-one | A. albidum, A. rotundum |
| [8] | (25S)-5β-spirostane-1β,3β-diol | A. tuberosum | |
| [9] | Gitogenin | (25R)-5α-spirostane-2α,3β-diol | A. aflatunense, A. chinense, A. cyrillii, A. elburzense, A. fistulosum, A. hirtifolium, A. jesdianum, A. macrostemon, A. porrum, A. rotundum, A. sativum, A. sativum L. var. Voghiera, A. victorialis var. platyphyllum |
| [10] | Neogitogenin | (25S)-5α-spirostane-2α,3β-diol | A. chinense, A. tuberosum |
| [11] | (25S)-5β-spirostane-2β,3β-diol | A. tuberosum | |
| [12] | β-Chlorogenin | (25R)-5α-Spirostane-3β,6β-diol | A. erubescens, A. giganteum, A. gramineum, A. leucanthum, A. porrum, A. rotundum, A. sativum, A. waldsteinii |
| [13] | 25-Epi-ruizgenin | (25S)-5β-spirostane-3β,6α-diol | A. tuberosum |
| [14] | (25R)-5α-Spirostane-3β,11α-diol | A. schoenoprasum | |
| [15] | (25R)-5β-spirostane-3β,12β-diol | A. macrostemon | |
| [16] | (25S)-5α-spirostane-3β,24β-diol | A. chinense | |
| [17] | Ruscogenin | (25R)-spirost-5(6)-ene-1β,3β-diol | A. affine, A. albidum, A. nutans |
| [18] | (25S)-ruscogenin | (25S)-spirost-5(6)-ene-1β,3β-diol | A. cepa |
| [19] | Yuccagenin | (25R)-spirost-5(6)-ene-2α,3β-diol | A. ampeloprasum, A. fistulosum, A. flavum, A. giganteum, A. karataviense, A. rotundum, A. turcomanicum |
| [20] | Lilagenin | (25S)-spirost-5(6)-ene-2α,3β-diol | A. tuberosum |
| [21] | 5β-Spirost-25(27)-ene-2β,3β-diol | A. macrostemon | |
| [22] | 5β-spirost-25(27)-ene-3β,12β-diol | A. macrostemon | |
| [23] | Porrigenin B | (25R)-5α-spirostane-3β,6β-diol-2-one | A. ampeloprasum, A. porrum |
| [24] | Neoporrigenin B | (25S)-5α-spirostane-3β,6β-diol-2-one | A. porrum |
| [25] | Neoagigenone | (25S)-5α-spirostane-2α,3α-diol-6-one | A. turcomanicum |
| [26] | Anzurogenin B | (25R)-5α-spirostane-2α,5α-epoxy-3β,6β-diol | A. stipitatum/A. suvorovii |
| [27] | Nuatigenin | (22S,25S)-22,25-epoxy-furost-5(6)-ene-3β,26-diol | A. vineale |
| [28] | Izonuatigenin | (25R)-spirost-5(6)-ene-3β,25β-diol | A. vineale |
| [29] | 12-Ketoporrigenin | (25R)-5α-spirostane-3β,6β-diol-12-one | A. porrum |
| [30] | Porrigenin C | (25R)-5α-spirostane-3β,6β-diol-2,12-dione | A. porrum |
| [31] | Agapanthagenin | (25R)-5α-spirostane-2α,3β,5α-triol | A. aflatunense, A. elburzense, A. hirtifolium |
| [32] | (25S)-5β-spirostane-2β,3β,5β-triol | A. tuberosum | |
| [33] | Gantogenin | (25R)-5α-spirostane-2α,3β,6α-triol | A. giganteum, A. jesdianum |
| [34] | Agigenin | (25R)-5α-spirostane-2α,3β,6β-triol | A. albopilosum, A. ampeloprasum, A. atroviolaceum, A. giganteum, A. gramineum, A. hirtifolium, A. leucanthum, A. macleanii, A. ostrowskianum, A. porrum, A. rotundum, A. sativum var. Voghiera, A. schubertii |
| [35] | 2-O-[(S)-3-hydroxy-3-methylglutaryl]-agigenin | 2-O-[(S)-3-hydroxy-3-methylglutaryl]-(25R)-5α-spirostane-2α,3β,6β-triol | A. albopilosum |
| [36] | Neoagigenin | (25S)-5α-spirostane-2α,3β,6β-triol | A. albopilosum, A. ampeloprasum ssp. persicum, A. giganteum, A. minutiflorum, A. nigrum, A. porrum, A. schubertii, A. turcomanicum |
| [37] | 6-O-benzoyl-neoagigenin | 6-O-benzoyl-(25S)-5α-spirostane-2α,3β,6β-triol | A. turcomanicum |
| [38] | Porrigenin A | (25R)-5α-spirostane-2β,3β,6β-triol | A. porrum |
| [39] | Neoporrigenin A | (25S)-5α-spirostane-2β,3β,6β-triol | A. porrum |
| [40] | (25R)-5α-spirostane-2α,3β,27-triol | A. tuberosum | |
| [41] | Anzurogenin D | (25R)-5α-spirostane-3β,5α,6β-triol | A. stipitatum/A. suvorovii |
| [42] | (25S)-5α-spirostane-2α,3β,27-triol | A. tuberosum | |
| [43] | (25S)-5β-spirostane-3β,5β,6α-triol | A. tuberosum | |
| [44] | Cepagenin | (24S,25R)-spirost-5(6)-ene-1β,3β,24-triol | A. cepa |
| [45] | Karatavigenin C | (24S,25S)-spirost-5(6)-ene-2α,3β,24-triol | A. karataviense |
| [46] | (20S,25S)-spirost-5(6)-ene-3β,11α,21-triol | A. schoenoprasum | |
| [47] | (20S,25S)-spirost-5(6)-ene-3β,12β,21-triol | A. schoenoprasum | |
| [48] | Anzurogenin A | (25R)-5β-spirostane-2α,3β,5β-triol-6-one | A. stipitatum/A. suvorovii |
| [49] | Alliogenin | (25R)-5α-spirostane-2α,3β,5α,6β-tetrol | A. aflatunense, A. albopilosum, A. elburzense, A. giganteum, A. hirtifolium, A. karataviense, A. macleanii, A. minutiflorum, A. turcomanicum |
| [50] | Neoalliogenin | (25S)-5α-spirostane-2α,3β,5α,6β-tetrol | A. turcomanicum |
| [51] | 3-O-acetyl-alliogenin | 3-O-acetyl-(25R)-5α-spirostane-2α,3β,5α,6β-tetrol | A. albopilosum, A. giganteum, A. karataviense |
| [52] | Karatavigenin (3-O-benzoyl-alliogenin) | 3-O-benzoyl-(25R)-5α-spirostane-2α,3β,5α,6β-tetrol | A. giganteum, A. karataviense, A. macleanii |
| [53] | Karatavigenin B (2-O-benzoyl-alliogenin) | 2-O-benzoyl-(25R)-5α-spirostane-2α,3β,5α,6β-tetrol | A. karataviense |
| [54] | 3-O-(2-hydroxybutyryl)-alliogenin | 3-O-(2-hydroxybutyryl)-(25R)-5α-spirostane-2α,3β,5α,6β-tetrol | A. karataviense |
| [55] | (24S,25S)-5β-spirostane-2α,3β,5β,24-tetrol | A. tuberosum | |
| [56] | (24S,25S)-5β-spirostane-2β,3β,5β,24-tetrol | A. tuberosum | |
| [57] | Atroviolacegenin | (25R)-5α-spirostane-2α,3β,6β,27-tetrol | A. atroviolaceum |
| [58] | Anzurogenin C | (24S,25S)-5β-spirostane-2α,3β,5,24-tetrol-6-one | A. stipitatum/A. suvorovii |
| [59] | Luvigenin | (25R)-4-methyl-19-norspirosta-l,3,5(10)-triene | A. giganteum |
| [60] | (24S,25R)-5α-spirostane-2α,3β,5α,6β,24-pentaol | A. giganteum | |
| [61] | (24S,25S)-5α-spirostane-2α,3β,5α,6β,24-pentaol | A. karataviense | |
| [62] | 3-O-acetyl-(24S,25S)-5α-spirostane-2α,3β,5α,6β,24-pentaol | A. giganteum | |
| [63] | 3-O-benzoyl-(24S,25S)-5α-spirostane-2α,3β,5α,6β,24-pentaol | A. karataviense | |
| [64] | 2,3-Seco-porrigenin | (25R)-5α-2,3-secospirostane-2,3-dioic acid-6β-hydroxy-3,6-γ-lactone | A. porrum |
The most common spirostanol sapogenins identified in Allium plants are: diosgenin [4], tigogenin [1], gitogenin [9], agigenin [34], alliogenin [49], and β-chlorogenin [12]. It was claimed that β-chlorogenin, a genin present in common garlic A. sativum, could be considered as a chemical marker for its identification in various food products, as the characteristic garlic sulfur compounds are very unstable (Itakura et al. 2001).
Until now, over 130 spirostanol glycosides have been identified in various Allium species. It should be mentioned however that some of these compounds were obtained as a result of enzymatic hydrolysis of furostanol saponin fraction by β-glucosidase (Ikeda et al. 2000).
Allium spirostane-type saponins are typically monodesmodic with the sugar residue usually at C-3 position. In rare cases, the sugar moiety was reported to be linked at other positions, such as C-1 (e.g. alliospirosides A-D [169, 170, 178, 179]) (Kravets et al. 1986a, b, 1987), C-2 (compounds from A. giganteum and A. albopilosum) (Sashida et al. 1991), C-24 (chinenoside VI [116], karatavioside F [181], and anzuroside [190]), or C-27 (tuberoside L [104]) (Jiang et al. 1998; Vollerner et al. 1984; Vollerner et al. 1989; Sang et al. 2001a).
Table 3 of ESM summarizes chemical structures of spirostane-type saponins that were reported in Allium species.
Furostane-type saponins
Furostanol aglycones possess either a cis or a trans fusion between ring A and B, or a double bond between C-5 and C-6 leading to 5α, 5β or Δ5(6) series. In the case of furostane-type sapogenins a double bond may also be located at 20(22) (e.g. ascalonicoside B [220], ceparoside C [230], chinenoside II [234]) or 22(23) (four furostanols from A. tuberosum) (Fattorusso et al. 2002; Yuan et al. 2009; Peng et al. 1996b; Sang et al. 2001b). The 27-Me group may be in either R or S configuration. Furostane-type compounds isolated from Allium species usually possess an OH or OMe group at C-22. However, sapogenins with a C-22 methyl ether are considered to be artifacts resulting from the use of methanol in the extraction/isolation procedures.
From among 140 furostanol glycosides identified in the Allium genus, sixteen compounds were found to be such methoxy-derivatives.
Furostanol saponins in Allium plants are bidesmodic glycosides with sugar chains attached usually at C-3 and C-26 positions. A rare glycosylation at C-1 with a galactose unit was reported in ascalonicosides A1/A2 [217, 218] (Fattorusso et al. 2002). A vast majority of furostanol saponins possess an O-linked glucose residue attached at position C-26. In compounds such as ceposides, persicoside C [205, 206], ascalonicosides A1/A2 [217, 218] a disaccharide chain was reported at C-26 (Lanzotti 2012; Sadeghi et al. 2013; Fattorusso et al. 2002).
Cholestane-type (open-chain) saponins
A review of available literature data shows that as much as 18 cholestane-type compounds have been identified in ten different Allium species.
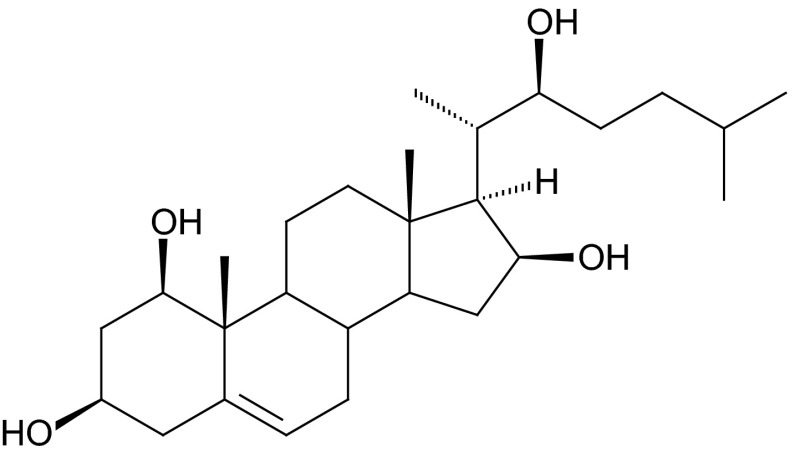
Allium open-chain aglycones possess Δ5(6) unsaturation with an exception of schubertoside A [329]—Δ4(5), and one of the glycosides found in A. albopilosum with a saturated aglycone (Kawashima et al. 1991b; Mimaki et al. 1993). Glycosides based on alliosterol—(22S)-cholest-5(6)-ene-1β,3β,16β,22-tetrol (Fig. 1 [196]), or related sapogenins showing the same oxygenation pattern at C-1, C-3, C-16 and C-22 are most common (Challinor and De Voss 2013). Sugar units are attached at one, two or, more seldom, at three separate positions (in A. macleanii) (Inoue et al. 1995). Most of these compounds are glycosylated at C-16, whereas in contrast to spirostanol and furostanol saponins, the attachment of sugar chain at position C-3 is almost unique (tuberoside U [353]) (Sang et al. 2003).
Fig. 1.
Alliosterol—(22S)-cholest-5(6)-ene-1β,3β,16β,22-tetrol [196]
Table 2 lists steroidal saponins/sapogenins identified in Allium species. Plant names are cited exactly as they were referred to in the original report. It is almost certain that some of them are synonyms but as the authors of the present review are not specialists in plant taxonomy no amendments have been made.
Table 2.
List of steroidal saponins/sapogenins reported in Allium species
| Species | Glycoside common name [no.] | Sapogenin [no.] | Sugar residue | References |
|---|---|---|---|---|
| A. affine Ledeb. | Tigogenin [1] | Kravets et al. (1990) | ||
| Diosgenin [4] | Kravets et al. (1990) | |||
| Ruscogenin [17] | Kravets et al. (1990) | |||
| A. aflatunense B. Fedtsch. | [70] | Gitogenin [9] | 3-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1999c) |
| [75] | Gitogenin [9] | 3-O-β-d-Glc-(1 → 2)-[4-O-(S)-3-hydroxy-3-methylglutaryl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1999c) | |
| [84] | Agapanthagenin [31] | 2-O-β-d-Glc | Mimaki et al. (1999c) | |
| [105] | Alliogenin [49] | 2-O-β-d-Glc | Kawashima et al. (1991a) | |
| A. albanum Grossh | Saponins present | Ismailov et al. (1976) | ||
| A. albidum Fisch. Ex M. Bieb. | Diosgenin [4] | Kereselidze et al. (1970) | ||
| Hecogenin [7] | Kravets et al. (1990) | |||
| Ruscogenin [17] | Pkheidze et al. (1971) | |||
| A. albiflorus | Saponins present | Ismailov and Aliev (1976) | ||
| A. albopilosum C.H. Wright | Agigenin [34] | Mimaki et al. (1993) | ||
| Neoagigenin [36] | Mimaki et al. (1993) | |||
| Alliogenin [49] | Mimaki et al. (1993) | |||
| AGINOSIDE [93] | Agigenin [34] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1993) | |
| [94, 123] | (25R,S)-5α-spirostane-2α,3β,6β-triol [34, 36] | 3-O-β-d-Glc-(1 → 2)-[3-O-acetyl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1993) | |
| [101] | 2-O-[(S)-3-hydroxy-3-methylglutaryl]-agigenin [35] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1993) | |
| [105] | Alliogenin [49] | 2-O-β-d-Glc | Mimaki et al. (1993) | |
| [184] | 3-O-acetyl-alliogenin [51] | 2-O-β-d-Glc | Mimaki et al. (1993) | |
| [197, 198] | (25R,S)-5α-furostane-2α,3β,6β,22,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1993) | |
| [199] |
Alliosterol [(22S)-cholest-5(6)-ene-1β,3β,16β,22-tetrol] [196] |
1-O-α-l-Rha 16-O-α-l-Rha-(1 → 3)-O-β-d-Glc | Mimaki et al. (1993) | |
| [200] | Cholest-5(6)-ene-1β,3β,16β-triol-22-one | 1-O-α-l-Rha 16-O-α-l-Rha-(1 → 3)-O-β-d-Glc | Mimaki et al. (1993) | |
| [201] | 5α-Cholestane-1β,3β,16β-triol-22-one | 1-O-α-l-Rha 16-O-α-l-Rha-(1 → 3)-O-β-d-Glc | Mimaki et al. (1993) | |
| A. ampeloprasum L. | Agigenin [34] | Morita et al. (1988) | ||
| [87] | Agigenin [34] | 3-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Morita et al. (1988) | |
| AMPELOSIDE Bs1 [90] | Agigenin [34] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Morita et al. (1988) | |
| AGINOSIDE [93] | Sata et al. (1998) | |||
| YAYOISAPONIN C [95] | Agigenin [34] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Sata et al. (1998) | |
| YAYOISAPONIN A [96] | Agigenin [34] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Sata et al. (1998) | |
| DIOSCIN [135] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sata et al. (1998) | |
| KARATAVIOSIDE A [151] | Yuccagenin [19] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Uchida et al. (2009) | |
| YAYOISAPONIN B [174] | Porrigenin B [23] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Sata et al. (1998) | |
| AMPELOSIDE Bf1 [202] | (25R)-5α-furostane-2α,3β,6β,22,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Morita et al. (1988) | |
| AMPELOSIDE Bf2 [203] | (25R)-5α-furostane-2α,3β,6β,22,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Morita et al. (1988) | |
| [204] | (25R)-5α-furostane-3β,26-diol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1999b) | |
| A. ampeloprasum L. ssp. persicum | PERSICOSIDE A [120] | Neoagigenin [36] | 3-O-β-d-Glc-(1 → 3)-[β-d-Xyl-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Sadeghi et al. (2013) |
| PERSICOSIDE B [121] | Neoagigenin [36] | 3-O-β-d-Xyl-(1 → 3)-[α-l-Rha-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Sadeghi et al. (2013) | |
| PERSICOSIDE C (C1/C2) [205, 206] | Furost-5(6)-ene-1β,3β,22ξ,26-tetrol | 26-O-α-l-Rha-(1 → 2)-β-d-Gal 1-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Sadeghi et al. (2013) | |
| PERSICOSIDE D (D1/D2) [207, 208] | Furostane-2α,3β,22ξ,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Sadeghi et al. (2013) | |
| CEPOSIDES A1/A2 [209, 210] | (25R)-furost-5(6)-ene-1β,3β,22ξ,26-tetrol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Gal 1-O-β-d-Xyl | Sadeghi et al. (2013) | |
| CEPOSIDES C1/C2 [211, 212] | (25R)-furost-5(6)-ene-1β,3β,22ξ,26-tetrol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Gal 1-O-β-d-Gal | Sadeghi et al. (2013) | |
| TROPEOSIDES A1/A2 [213, 214] | Furost-5(6)-ene-3β,22ξ-diol | 26-O-α-l-Rha 1-O-β-d-Gal | Sadeghi et al. (2013) | |
| TROPEOSIDES B1/B2 [215, 216] | Furost-5(6)-ene-3β,22ξ-diol | 26-O-α-l-Rha 1-O-β-d-Xyl | Sadeghi et al. (2013) | |
| ASCALONICOSIDES A1/A2 [217, 218] | Furost-5(6)-ene-3β,22ξ-diol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 1-O-β-d-Gal | Sadeghi et al. (2013) | |
| PERSICOSIDE E [219] | Alliosterol [196] | 1-O-α-l-Rha 16-O-α-l-Rha (1 → 2)-O-β-d-Gal | Sadeghi et al. (2013) | |
| A. angulosum Lour. | Diosgenin [4] | Azarkova et al. (1974) | ||
| A. ascalonicum L. | ASCALONICOSIDE A1 [217] | Furost-5(6)-ene-3β,22α-diol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 1-O-β-d-Gal | Fattorusso et al. (2002) |
| ASCALONICOSIDE A2 [218] | Furost-5(6)-ene-3β,22β-diol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 1-O-β-d-Gal | Fattorusso et al. (2002) | |
| ASCALONICOSIDE B [220] | Furost-5(6),20(22)-diene-3β-ol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 1-O-β-d-Gal | Fattorusso et al. (2002) | |
| ASCALONICOSIDE C [221] | (25R)-5α-furostane-3β,5α,6β,22,26-pentaol-2-one | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Kang et al. (2007) | |
| ASCALONICOSIDE D [222] | (25R)-22-methoxy-5α-furostane-3β,5α,6β,26-tetrol-2-one | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Kang et al. (2007) | |
| DICHOTOMIN [223] | (25R)-furost-5(6)-ene-3β,22α,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Kang et al. (2007) | |
| PARISAPONIN I [224] | (25R)-furost-5(6)-ene-3β,22,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Ara-(1 → 4)]-O-β-d-Glc | Kang et al. (2007) | |
| A. atroviolaceum Boiss. | Atroviolacegenin [57] | Zolfaghari et al. (2006) | ||
| [87] | Agigenin [34] | 3-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Zolfaghari et al. (2006) | |
| ATROVIOLACEOSIDE [108] | Atroviolacegenin [57] | 3-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Zolfaghari et al. (2006) | |
| [225] | Furostane-2α,3β,6β,22α-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Zolfaghari et al. (2006) | |
| [226] | Furostane-2α,3β,6β,22α-tetrol | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Zolfaghari et al. (2006) | |
| A. cepa L. | Diosgenin [4] | Kravets et al. (1990) | ||
| (25S)-ruscogenin [18] | Kravets et al. (1986a, b) | |||
| Cepagenin [44] | Kravets et al. (1987) | |||
| [132] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 2)-O-α-l-Ara | Kravets et al. (1990) | |
| [133] | Diosgenin [4] | 3-O-β-d-Gal-(1 → 4)-O-α-l-Rha-(1 → 2)-O-α-l-Ara | Kravets et al. (1990) | |
| [143] | Diosgenin [4] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Gal-(1 → 4)-O-α-l-Rha-(1 → 2)-O-α-l-Ara | Kintya and Degtyareva (1989) | |
| ALLIOSPIROSIDE A [169] | (25S)-ruscogenin [18] | 1-O-α-l-Rha-(1 → 2)-O-α-l-Ara | Kravets et al. (1986a) | |
| ALLIOSPIROSIDE B [170] | (25S)-ruscogenin [18] | 1-O-α-l-Rha-(1 → 2)-O-α-d-Gal | Kravets et al. (1986b) | |
| ALLIOSPIROSIDE C [178] | Cepagenin [44] | 1-O-α-l-Rha-(1 → 2)-O-α-l-Ara | Kravets et al. (1987) | |
| ALLIOSPIROSIDE D [179] | Cepagenin [44] | 1-O-α-l-Rha-(1 → 2)-O-α-d-Gal | Kravets et al. (1987) | |
| ALLIOFUROSIDE A [227] | (25S)-furost-5(6)-ene-1β,3β,22α,26-tetrol | 26-O-β-d-Glc 1-O-α-l-Rha-(1 → 2)-O-α-l-Ara | Kravets et al. (1986a) | |
| CEPAROSIDE A [228] | (25R)-22-methoxy-furost-5(6)-ene-1β,3β,22α,26-tetrol | 26-O-β-d-Glc 1-O-α-l-Rha-(1 → 2)-O-α-l-Ara | Yuan et al. (2008) | |
| CEPAROSIDE B [229] | (25R)-furost-5(6)-ene-1β,3β,22α,26-tetrol | 26-O-β-d-Glc 1-O-α-l-Rha-(1 → 2)-O-α-l-Ara | Yuan et al. (2008) | |
| CEPAROSIDE C [230] | (25R)-furost-5(6),20(22)-diene-3β,26-diol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Gal | Yuan et al. (2009) | |
| CEPAROSIDE D [231] | (25S)-furost-5(6),20(22)-diene-3β,26-diol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Gal | Yuan et al. (2009) | |
| CEPOSIDE A1 [209] | (25R)-furost-5(6)-ene-1β,3β,22α,26-tetrol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Gal 1-O-β-d-Xyl | Lanzotti et al. (2012b) | |
| CEPOSIDE B [232] | (25R)-furost-5(6)-ene-1β,3β,22α,26-tetrol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 1-O-β-d-Xyl | Lanzotti et al. (2012b) | |
| CEPOSIDE C1 [211] | (25R)-furost-5(6)-ene-1β,3β,22α,26-tetrol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Gal 1-O-β-d-Gal | Lanzotti et al. (2012b) | |
| A. cepa L. var. tropea | TROPEOSIDE A1 [213] | Furost-5(6)-ene-3β,22α-diol | 26-O-α-l-Rha 1-O-β-d-Gal | Corea et al. (2005) |
| TROPEOSIDE A2 [214] | Furost-5(6)-ene-3β,22β-diol | 26-O-α-l-Rha 1-O-β-d-Gal | Corea et al. (2005) | |
| TROPEOSIDE B1 [215] | Furost-5(6)-ene-3β,22α-diol | 26-O-α-l-Rha 1-O-β-d-Xyl | Corea et al. (2005) | |
| TROPEOSIDE B2 [216] | Furost-5(6)-ene-3β,22β-diol | 26-O-α-l-Rha 1-O-β-d-Xyl | Corea et al. (2005) | |
| ASCALONICOSIDES A1/A2 [217, 218] | Corea et al. (2005) | |||
| ASCALONICOSIDE B [220] | Corea et al. (2005) | |||
| A. cepa L. var. aggregatum (Aggregatum group) | ALLIOSPIROSIDE A [169] | Teshima et al. (2013) | ||
| ALLIOSPIROSIDE B [170] | Teshima et al. (2013) | |||
| A. cernuum Roth. | Diosgenin [4] | Azarkova et al. (1983) | ||
| A. chinense G. Don | Tigogenin [1] | Matsuura et al. (1989b) | ||
| Neotigogenin [2] | Sapogenins [1,2,6,9] obtained on acid hydrolysis of the crude saponin fraction | Matsuura et al. (1989b) | ||
| Laxogenin [6] | Matsuura et al. (1989b) | |||
| Gitogenin [9] | Matsuura et al. (1989b) | |||
| [65, 110] | (25R,S)-5α-spirostane-3β-ol [1, 2] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Kuroda et al. (1995), Jiang et al. (1998) | |
| [66, 111] | (25R,S)-5α-spirostane-3β-ol [1, 2] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)-(6-O-acetyl-β-d-Glc)]-(1 → 4)-O-β-d-Gal | Jiang et al. (1998) | |
| [71, 113] | (25R,S)-5α-spirostane-2α,3β-diol [9, 10] | 3-O-β-d-Glc-(1 → 2)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Jiang et al. (1998) | |
| [73, 115] | (25R,S)-5α-spirostane-2α,3β-diol [9, 10] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Kuroda et al. (1995) | |
| NEOMACROSTEMONOSIDE D [111] | Neotigogenin [2] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)-(6-O-acetyl-β-d-Glc)]-(1 → 4)-O-β-d-Gal | Jiang et al. (1999) | |
| CHINENOSIDE VI [116] | (25S)-5α-spirostane-3β,24β-diol [16] | 3-O-α-l-Ara-(1 → 6)-O-β-d-Glc 24-O-β-d-Glc | Jiang et al. (1998) | |
| [157] | Laxogenin [6] | 3-O-α-l-Ara-(1 → 6)-O-β-d-Glc | Kuroda et al. (1995), Peng et al. (1996b), Baba et al. (2000) | |
| [159] | Laxogenin [6] | 3-O-(2-O-acetyl-α-l-Ara)-(1 → 6)-O-β-d-Glc | Kuroda et al. (1995) | |
| [161] | Laxogenin [6] | 3-O-β-d-Xyl-(1 → 4)-[α-l-Ara-(1 → 6)]-O-β-d-Glc | Peng et al. (1995, 1996b); Baba et al. (2000) | |
| CHINENOSIDE I [233] | (25R)-5α-furostane-3β,22,26-triol-6-one | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 4)-[α-l-Ara-(1 → 6)]-O-β-d-Glc | Matsuura et al. (1989b) | |
| CHINENOSIDE II [234] | (25R)-5α-furost-20(22)-ene-3β,26-diol-6-one | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 4)-[α-l-Ara-(1 → 6)]-O-β-d-Glc | Peng et al. (1996b) | |
| CHINENOSIDE III [235] | (25R)-5α-furost-20(22)-ene-3β,26-diol-6-one | 26-O-β-d-Glc 3-O-α-l-Ara-(1 → 6)-O-β-d-Glc | Peng et al. (1996b) | |
| CHINENOSIDE IV [236] | (25R)-23-methoxy-5α-furost-20(22)-ene-3β,26-diol-6-one | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 4)-[α-l-Ara-(1 → 6)]-O-β-d-Glc | Peng et al. (1996c) | |
| CHINENOSIDE V [237] | (25R)-23-methoxy-5α-furost-20(22)-ene-3β,26-diol-6-one | 26-O-β-d-Glc 3-O-α-l-Ara-(1 → 6)-O-β-d-Glc | Peng et al. (1996c) | |
| A. cyrillii Ten. | F-GITONIN [72] | Gitogenin [9] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Tolkacheva et al. (2012) |
| [75] | Gitogenin [9] | 3-O-β-d-Glc-(1 → 2)-[4-O-(S)-3-hydroxy-3-methylglutaryl-O-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Tolkacheva et al. (2012) | |
| A. elburzense Wendelbo | Agapanthagenin [31] | Barile et al. (2004) | ||
| Alliogenin [49] | Barile et al. (2004) | |||
| [85] | Agapanthagenin [31] | 3-O-β-d-Glc | Barile et al. (2004) | |
| [69] | Gitogenin [9] | 3-O-β-d-Glc-(1 → 4)-O-β-d-Glc | Barile et al. (2004) | |
| [106] | Alliogenin [49] | 3-O-β-d-Glc | Barile et al. (2004) | |
| ELBURZENSOSIDE A1 [238] | Furostane-2α,3β,5α,6β,22α-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc | Barile et al. (2004) | |
| ELBURZENSOSIDE A2 [239] | Furostane-2α,3β,5α,6β,22β-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc | Barile et al. (2004) | |
| ELBURZENSOSIDE B1 [240] | Furostane-2α,3β,5α,6β,22α-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 4)-O-β-d-Glc | Barile et al. (2004) | |
| ELBURZENSOSIDE B2 [241] | Furostane-2α,3β,5α,6β,22β-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 4)-O-β-d-Glc | Barile et al. (2004) | |
| ELBURZENSOSIDE C1 [242] | Furostane-2α,3β,5α,22α-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc | Barile et al. (2004) | |
| ELBURZENSOSIDE C2 [243] | Furostane-2α,3β,5α,22β-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc | Barile et al. (2004) | |
| ELBURZENSOSIDE D1 [244] | Furostane-2α,3β,5α,22α-tetrol | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Barile et al. (2004) | |
| ELBURZENSOSIDE D2 [245] | Furostane-2α,3β,5α,22β-tetrol | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Barile et al. (2004) | |
| A. erubescens C. Koh. | β-Chlorogenin [12] | Chincharadze et al. (1979) | ||
| ERUBOSIDE B [79] | β-Chlorogenin [12] | 3-O-β-Glc-(1 → 3)-[β-d-Glc-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Chincharadze et al. (1979) | |
| A. fistulosum L. | Yuccagenin [19] | Kim et al. (1991) | ||
| Tigogenin [1] | Lai et al. (2012) | |||
| Gitogenin [9] | Lai et al. (2012) | |||
| (25R)-19-norspirosta-1,3,5(10)-triene-4-methyl-2-ol [246] | Lai et al. (2012) | |||
| (25R)-spirost-1(2),4(5)-diene-2,6-diol-3-one [247] | Sapogenins [1, 9, 246, 247, 248, 249] obtained from the acid hydrolysis product of the whole glycoside mixture of Welsh onion seeds | Lai et al. (2012) | ||
| (25R)-spirost-1(2),4(5)-diene-2-ol-3-one [248] | Lai et al. (2012) | |||
| (25R)-spirost-4(5)-ene-2-ol-3-one [249] | Lai et al. (2012) | |||
| DIOSCIN [135] | Jung et al. (1993) | |||
| [141] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Jung et al. (1993) | |
| FISTULOSIDE A [148] | Yuccagenin [19] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Gal | Do et al. (1992) | |
| FISTULOSIDE B [149] | Yuccagenin [19] | 3-O-α-l-Rha-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Gal | Do et al. (1992) | |
| FISTULOSIDE C [150] | Yuccagenin [19] | 3-O-β-d-Glc-(1 → 3)-[β-d-Glc-(1 → 4)]-O-β-d-Gal | Do et al. (1992) | |
| FISTULOSAPONIN A [250] | (25R)-furost-5(6),20(22)-diene-3β,26-diol-2-one | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Lai et al. (2010) | |
| FISTULOSAPONIN B [251] | (25R)-furost-5(6)-ene-3β,22α,26-triol-2-one | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Lai et al. (2010) | |
| FISTULOSAPONIN C [252] | (25R)-furost-5(6)-ene-3α,22α,26-triol-2-one | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Lai et al. (2010) | |
| FISTULOSAPONIN D [253] | (25R)-furost-5(6)-ene-3β,22α,26-triol-2-one | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Glc-(1 → 2)-O-β-d-Glc | Lai et al. (2010) | |
| FISTULOSAPONIN E [254] | (25R)-furost-5(6),20(22)-diene-2α,3β,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Glc-(1 → 2)-O-β-d-Glc | Lai et al. (2010) | |
| FISTULOSAPONIN F [255] | (25R)-furost-5(6)-ene-2α,3β,22α,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Glc-(1 → 2)-O-β-d-Glc | Lai et al. (2010) | |
| PROTOGRACILLIN [256] | (25R)-furost-5(6)-ene-3β,22α,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc | Lai et al. (2010) | |
| [257] | (25R)-furost-5(6)-ene-3β,22α,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Lai et al. (2010) | |
| DICHOTOMIN [223] | Lai et al. (2010) | |||
| A. flavum L. | [137] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 4)-[β-d-Glc-(1 → 2)]-O-β-d-Glc | Rezgui et al. (2014) |
| [153] | Yuccagenin [19] | 3-O-β-d-Xyl-(1 → 3)-[β-d-Gal-(1 → 2)]-O-β-d-Gal-(1 → 4)-O-β-d-Gal | Rezgui et al. (2014) | |
| [154] | Yuccagenin [19] | 3-O-β-d-Xyl-(1 → 3)-[β-d-Glc-(1 → 2)]-O-β-d-Gal-(1 → 4)-O-β-d-Gal | Rezgui et al. (2014) | |
| A. fuscoviolaceum L. | Diosgenin [4] | Kravets et al. (1990) | ||
| A. giganteum Regel | Diosgenin [4] | Kravets et al. (1990) | ||
| β-Chlorogenin [12] | Kelginbaev et al. (1974) | |||
| Yuccagenin [19] | Kelginbaev et al. (1974) | |||
| Gantogenin [33] | Kelginbaev et al. (1975) | |||
| Agigenin [34] | Kelginbaev et al. (1974) | |||
| Neoagigenin [36] | Kelginbaev et al. (1973, 1974) | |||
| Alliogenin [49] | Khristulas et al. (1970), Gorovits et al. (1971) | |||
| Luvigenin [59] | Kravets et al. (1990) | |||
| AGINOSIDE [93] | Kelginbaev et al. (1976), Kawashima et al. (1991a) | |||
| [98] | Agigenin [34] | 3-O-β-d-Glc-(1 → 2)-[4-O-(S)-3-hydroxy-3-methylglutaryl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1994) | |
| [105] | Alliogenin [49] | 2-O-β-d-Glc | Sashida et al. (1991) | |
| [106] | Alliogenin [49] | 3-O-β-d-Glc | Gorovits et al. (1971) | |
| [184] | 3-O-acetyl-alliogenin [51] | 2-O-β-d-Glc | Sashida et al. (1991) | |
| [186] | Karatavigenin [52] | 2-O-β-d-Glc | Sashida et al. (1991) | |
| [191] | (24S,25R)-5α-spirostane-2α,3β,5α,6β,24-pentaol [60] | 24-O-β-d-Glc | Kawashima et al. (1991a) | |
| [193] | 3-O-acetyl-(24S,25S)-5α-spirostane-2α,3β, 5α,6β,24-pentaol [62] | 2-O-β-d-Glc | Mimaki et al. (1994) | |
| [258] | (25R)-22-methoxy-5α-furostane-2α,3β,6β,22ξ,26-pentaol | 26-O-β-d-Glc 3-O-β-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1994) | |
| [259] | (25R)-3-O-benzoyl-22-methoxy-5α-furostane-2α,3β,5α,6β,22ξ,26-hexol | 26-O-β-d-Glc 2-O-β-d-Glc | Mimaki et al. (1994) | |
| [260] | (25R)-3-O-acetyl-22-methoxy-5α-furostane-2α,3β,5α,6β,22ξ,26-hexol | 26-O-β-d-Glc 2-O-β-d-Glc | Mimaki et al. (1994) | |
| A. gramineum C. Koch. | Diosgenin [4] | Kravets et al. (1990) | ||
| β-Chlorogenin [12] | Kravets et al. (1990) | |||
| Agigenin [34] | Kravets et al. (1990) | |||
| ERUBOSIDE B [79] | Kravets et al. (1990) | |||
| A. hirtifolium Boiss. | [69] | Gitogenin [9] | 3-O-β-d-Glc-(1 → 4)-O-β-d-Glc | Barile et al. (2005) |
| [85] | Agapanthagenin [31] | 3-O-β-d-Glc | Barile et al. (2005) | |
| HIRTIFOLIOSIDE D [92] | Agigenin [34] | 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Barile et al. (2005) | |
| [106] | Alliogenin [49] | 3-O-β-d-Glc | Barile et al. (2005) | |
| HIRTIFOLIOSIDE A1 [261] | Furostane-2α,3β,22α-triol | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Barile et al. (2005) | |
| HIRTIFOLIOSIDE A2 [262] | Furostane-2α,3β,22β-triol | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Barile et al. (2005) | |
| HIRTIFOLIOSIDE B [263] | Furost-20(22)-ene-2α,3β-diol | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Barile et al. (2005) | |
| HIRTIFOLIOSIDE C1 [264] | Furostane-2α,3β,22α-triol | 26-O-β-d-Glc | Barile et al. (2005) | |
| HIRTIFOLIOSIDE C2 [265] | Furostane-2α,3β,22β-triol | 26-O-β-d-Glc | Barile et al. (2005) | |
| A. jesdianum Boiss. | F-GITONIN [72] | Mimaki et al. (1999c) | ||
| [86] | Gantogenin [33] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1999c) | |
| [266] | Alliosterol [196] | 1-O-β-d-Glc 16-O-β-d-Glc | Mimaki et al. (1999c) | |
| [267] | Alliosterol [196] | 1-O-α-l-Rha 16-O-β-d-Glc | Mimaki et al. (1999c) | |
| A. karataviense Regel | Diosgenin [4] | Gorovits et al. (1973) | ||
| Yuccagenin [19] | Gorovits et al. (1973) | |||
| Karatavigenin c [45] | Vollerner et al. (1983b) | |||
| Alliogenin [49] | Gorovits et al. (1973), Mimaki et al. (1999c) | |||
| Karatavigenin [52] | Gorovits et al. (1973) | |||
| Karatavigenin B [53] | Khristulas et al. (1974) | |||
| [105] | Alliogenin [49] | 2-O-β-d-Glc | Mimaki et al. (1999c) | |
| [106] | Alliogenin [49] | 3-O-β-d-Glc | Gorovits et al. (1973) | |
| [107] | Alliogenin [49] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d--d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1999c) | |
| KARATAVIOSIDE A [151] | Vollerner et al. (1978), Mimaki et al. (1999c) | |||
| KARATAVIOSIDE B [152] | Yuccagenin [19] | 3-O-β-d-Glc-(1 → 2)-[4-O-β-hydroxy-β-methylglutaryl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Vollerner et al. (1983a) | |
| KARATAVIOSIDE E [180] | Karatavigenin C [45] | 3-O-β-d-Xyl-(1 → 3)-[β-d-Glc-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Vollerner et al. (1984) | |
| KARATAVIOSIDE F [181] | Karatavigenin C [45] | 3-O-β-d-Xyl-(1 → 3)-[β-d-Glc-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal 24-O-β-d-Glc | Vollerner et al. (1984) | |
| [184] | 3-O-acetyl-alliogenin [51] | 2-O-β-d-Glc | ||
| [185] | 3-O-(2-hydroxybutyryl)-alliogenin [54] | 2-O-β-d-Glc | Mimaki et al. (1999c) | |
| [186] | Karatavigenin [52] | 2-O-β-d-Glc | Mimaki et al. (1999c) | |
| [187] | Karatavigenin B [53] | 3-O-β-d-Glc | Khristulas et al. (1974) | |
| [192] | (24S,25S)-5α-spirostane-2α,3β,5α,6β,24-pentaol [61] | 2-O-β-d-Glc 24-O-β-d-Glc-(1 → 2)-O-β-d-Glc | Mimaki et al. (1999c) | |
| [194] | 3-O-benzoyl-(24S,25S)-5α-spirostane-2α,3β,5α,6β,24-pentaol [63] | 2-O-β-d-Glc | Mimaki et al. (1999c) | |
| [195] | 3-O-benzoyl-(24S,25S)-5α-spirostane-2α,3β,5α,6β,24-pentaol [63] | 2-O-β-d-Glc 24-O-β-d-Glc | Mimaki et al. (1999c) | |
| KARATAVIOSIDE C [268] | (25R)-furost-5(6)-ene-2α,3β,22α,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Vollerner et al. (1980) | |
| [269] | (25R)-22-methoxy-5α-furostane-2α,3β,5,6β,22ξ-pentaol | 26-O-β-d-Glc 2-O-β-d-Glc | Mimaki et al. (1999c) | |
| A. leucanthum C. Koch | ERUBOSIDE B [79] | Mskhiladze et al. (2008b) | ||
| [80] | β-Chlorogenin [12] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mskhiladze et al. (2008b) | |
| [81] | β-Chlorogenin [12] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mskhiladze et al. (2008b) | |
| [91] | Agigenin [34] | 3-O-β-d-Glc-(1 → 2)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mskhiladze et al. (2008b) | |
| AGINOSIDE [93] | Mskhiladze et al. (2008b) | |||
| YAYOISAPONIN C [95] | Mskhiladze et al. (2008b) | |||
| LEUCOSPIROSIDE A [97] | Agigenin [34] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mskhiladze et al. (2008b) | |
| A. macleanii Baker | [67] | Tigogenin [1] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Xyl-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Inoue et al. (1995) |
| AGINOSIDE [93] | Inoue et al. (1995) | |||
| [98] | Agigenin [34] | 3-O-β-d-Glc-(1 → 2)-[4-O-(S)-3-hydroxy-3-methylglutaryl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Inoue et al. (1995) | |
| [100] | Agigenin [34] | 3-O-β-d-Glc-(1 → 2)-[4-O-benzoyl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Inoue et al. (1995) | |
| [105] | Alliogenin [49] | 2-O-β-d-Glc | Inoue et al. (1995) | |
| [186] | Karatavigenin [52] | 2-O-β-d-Glc | Inoue et al. (1995) | |
| [270] | Alliosterol [196] | 1-O-α-l-Rha 3-O-α-l-Rha 16-O-β-d-Glc | Inoue et al. (1995) | |
| A. macrostemon Bunge (A. grayi Regel) | Tigogenin [1] | Okanishi et al.(1975), He et al. (2002) | ||
| Smilagenin [3] | Okanishi et al.(1975) | |||
| Gitogenin [9] | Okanishi et al.(1975) | |||
| MACROSTEMONOSIDE A [65] | Tigogenin [1] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Peng et al. (1992) | |
| MACROSTEMONOSIDE D [66] | Tigogenin [1] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)-(6-O-acetyl-β-d-Glc)]-(1 → 4)-O-β-d-Gal | Peng et al.(1992) | |
| [129] | (25R)-5β-spirostane-3β,12β-diol [15] | 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Cheng et al. (2013) | |
| [172] | 5β-Spirost-25(27)-ene-2β,3β-diol [21] | 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | He et al. (2002), Cheng et al. (2013) | |
| MACROSTEMONOSIDE S [173] | 5β-Spirost-25(27)-ene-3β,12β-diol [22] | 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Cheng et al. (2013) | |
| MACROSTEMONOSIDE B [271] | (25R)-5β-furostane-3β,22,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Chen et al. (2007) | |
| MACROSTEMONOSIDE E [272] | 5α-Furost-20(22)-ene-3β,26-diol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Peng et al. (1993) | |
| MACROSTEMONOSIDE F [273] | 5β-Furost-20(22)-ene-3β,26-diol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Peng et al.(1993) | |
| MACROSTEMONOSIDE G [274] | 5β-Furost-25(27)-ene-3β,12β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Peng et al. (1995) | |
| MACROSTEMONOSIDE H [275] | 22-Methoxy-5β-furost-25(27)-ene-3β,12β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Peng et al. (1995) | |
| MACROSTEMONOSIDE I [276] | 5β-Furost-25(27)-ene-3β,22,26-triol-12-one | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Peng et al. (1995) | |
| MACROSTEMONOSIDE J [277] | (25R)-5β-furostane-2β,3β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Peng et al. (1994) | |
| MACROSTEMONOSIDE K [278] | (25R)-22-methoxy-5β-furostane-2β,3β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Peng et al. (1994) | |
| MACROSTEMONOSIDE L [279] | (25R)-5β-furost-20(22)-ene-2β,3β,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Peng et al. (1994) | |
| [280] | 5β-Furost-25(27)-ene-1β,3β,3β,22α,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Gal | He et al. (2002) | |
| MACROSTEMONOSIDE M [281] | (25R)-5β-furostane-1β,2β,3β,6α,22-pentaol | 26-O-β-d-Glc | Chen et al. (2006) | |
| MACROSTEMONOSIDE N [282] | 5β-Furost-25(27)-ene-1β,2β,3β,6α,22-pentaol | 26-O-β-d-Glc | Chen et al. (2006) | |
| MACROSTEMONOSIDE O [283] | 5β-Furost-25(27)-ene-3β,22,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Chen et al. (2007) | |
| MACROSTEMONOSIDE P [284] | (25R)-5β-furostane-1β,3β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Chen et al. (2007) | |
| MACROSTEMONOSIDE Q [285] | (25R)-5β-furostane-1α,2β,3β,22,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Chen et al. (2007) | |
| MACROSTEMONOSIDE R [286] | (25R)-5β-furostane-2α,3β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Chen et al. (2007) | |
| [287] | (25R)-furostane-3β,22,26β-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Chen et al. (2006) | |
| [288] | (25S)-furostane-3β,22,26β-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Chen et al. (2006) | |
| [289] | (25R)-5α-furostane-3β,12β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Chen et al. (2010) | |
| [290] | (25R)-5α-furostane-3β,12α,22,26-tetrol | 26-O-β-d-bGlc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Chen et al. (2010) | |
| [291] | (25R)-5β-furostane-3β,12α,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Chen et al. (2010) | |
| [292] | 5α-Furost-25(27)-ene-3β,12β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Chen et al. (2009) | |
| [293] | 5β-Furost-20(22),25(27)-diene-3β,12β,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Chen et al. (2009) | |
| [294] | 5β-Furostane-3β,12α,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 2)-O-β-d-Gal | Ou et al. (2012) | |
| A. minutiflorum Regel | Neoagigenin [36] | Barile et al. (2007) | ||
| Alliogenin [49] | Barile et al. (2007) | |||
| MINUTOSIDE B [119] | Neoagigenin [36] | 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Barile et al. (2007) | |
| MINUTOSIDE A [295] | (25R)-furostane-2α,3β,6β,22α,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Barile et al. (2007) | |
| MINUTOSIDE C [296] | (25R)-furostane-2α,3β,5α,6β,22α,26-hexol | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 3)-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Barile et al. (2007) | |
| A. narcissiflorum Vill. | TRILLIN (ALLIUMOSIDE A) [130] | Diosgenin [4] | 3-O-β-d-Glc | Krokhmalyuk and Kintya (1976b) |
| DELTONIN [134] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 2)-[β-d-Glc-(1 → 4)]-O-β-d-Glc | Mimaki et al. (1996) | |
| [139] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 2)-[β-d-Xyl-(1 → 4)]-O-β-d-Glc | Mimaki et al. (1996) | |
| [141] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Mimaki et al. (1996) | |
| ALLIUMOSIDE B [297] | (25R)-furost-5(6)-ene-3β,22α,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 6)-O-β-d-Glc | Krokhmalyuk and Kintya (1976b) | |
| ALLIUMOSIDE C [298] | (25R)-furostane-3β,22α,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 6)-O-β-d-Gal-(1 → 6)-O-β-d-Glc | Lazurevski et al. (1975) | |
| ALLIUMOSIDE D [299] | (25S)-furost-5(6)-ene-3β,22α,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 6)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc | Krokhmalyuk and Kintya (1976a) | |
| ALLIUMOSIDE E [300] | (25S)-furost-5(6)-ene-3β,22α,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 4)-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 6)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc | Krokhmalyuk and Kintya (1976a) | |
| [301] | (25R)-22-methoxy-furost-5(6)-ene-3β,22ξ,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Mimaki et al. (1996) | |
| [302] | (25R)-22-methoxy-furost-5(6)-ene-3β,22ξ,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-O-[β-d-Glc-(1 → 4)]-O-β-d-Glc | Mimaki et al. (1996) | |
| A. nigrum L. | NIGROSIDES A1/A2 [89, 117] | (25R,S)-5α-spirostane-2α,3β,6β-triol [34, 36] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Jabrane et al. (2011) |
| NIGROSIDES B1/B2 [88, 118] | (25R,S)-5α-spirostane-2α,3β,6β-triol [34, 36] | 2-O-β-d-Glc 3-O-β-d-Gal | Jabrane et al. (2011) | |
| AGINOSIDE [93] | Mostafa et al. (2013) | |||
| AGINOSIDE/TUROSIDE A [93, 122] | (25R,S)-5α-spirostane-2α,3β,6β-triol [34, 36] | 3-O-β-d-Xyl-(1 → 3)-[β-d-Glc-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Jabrane et al. (2011) | |
| [98, 124] | (25R,S)-5α-spirostane-2α,3β,6β-triol [34, 36] | 3-O-β-d-Glc-(1 → 2)-[4-O-(S)-3-hydroxy-3-methylglutaryl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Jabrane et al. (2011) | |
| NIGROSIDE C (SCHUBERTOSIDE D) [303] | Alliosterol [196] | 1-O-α-l-Rha 16-O-α-l-Rha-(1 → 3)-O-β-d-Gal | Jabrane et al. (2011) | |
| NIGROSIDE D [304] | Alliosterol [196] | 16-O-α-l-Rha-(1 → 3)-O-β-d-Gal | Jabrane et al. (2011) | |
| A. nutans L. | Diosgenin [4] | Azarkova et al. (1983) | ||
| [138] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 2)-[β-d-Glc-(1 → 4)]-O-β-d-Gal | Akhov et al. (1999) | |
| [147] | Ruscogenin [17] | 1-O-β-d-Gal | Akhov et al. (1999) | |
| NOLINOFUROSIDE D [305] | (25S)-furost-5(6)-ene-1β,3β,22α,26-tetrol | 26-O-β-d-Glc 1-O-β-d-Gal | Akhov et al. (1999) | |
| DELTOSIDE [306] | (25R)-furost-5(6)-ene-3β,22α,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[β-d-Glc-(1 → 4)]-O-β-d-Glc | Akhov et al. (1999) | |
| A. ostrowskianum Regel | Agigenin [34] | Mimaki et al. (1993) | ||
| F-GITONIN [72] | Mimaki et al. (1993) | |||
| AGINOSIDE [93] | Mimaki et al. (1993) | |||
| [196, 197] | (25R,S)-5α-furostane-2α,3β,6β,22,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Mimaki et al. (1993) | |
| [307] | Alliosterol [196] | 16-O-β-d-Glc-(1 → 3)-O-β-d-Glc | Mimaki et al. (1993) | |
| A. porrum L. (A. ampeloprasum L. var. porrum) | Diosgenin [4] | Fattorusso et al. (1998) | ||
| β-Chlorogenin [12] | Fattorusso et al. (1998) | |||
| Porrigenin B [23] | Carotenuto et al. (1997b) | |||
| Neoporrigenin B [24] | Carotenuto et al. (1997b) | |||
| 12-Ketoporrigenin [29] | Carotenuto et al. (1997b) | |||
| Porrigenin C [30] | Fattorusso et al. (2000) | |||
| Agigenin [34] | Carotenuto et al. (1997b) | |||
| Neoagigenin [36] | Carotenuto et al. (1997b) | |||
| Porrigenin A [38] | Carotenuto et al. (1997b) | |||
| Neoporrigenin A [39] | Carotenuto et al. (1997b) | |||
| 2,3-Seco-porrigenin [64] | Carotenuto et al. (1997b) | |||
| F-GITONIN [72] | Carotenuto et al. (1999) | |||
| [74] | Gitogenin [9] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Carotenuto et al. (1999) | |
| [78] | β-Chlorogenin [12] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Gal 6-O-β-d-Glc | Adão et al. (2011a) | |
| [80] | β-Chlorogenin [12] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Carotenuto et al. (1999) | |
| [82] | β-Chlorogenin [12] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Carotenuto et al. (1999) | |
| AGINOSIDE [93] | Harmatha et al. (1987) | |||
| LEUCOSPIROSIDE A [97] | Adão et al. (2011b) | |||
| [162] | 12-Ketoporrigenin [29] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Fattorusso et al. (2000) | |
| [175] | Porrigenin B [23] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Adão et al. (2012) | |
| [177] | Porrigenin C [30] | 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Fattorusso et al. (2000) | |
| [267] | Alliosterol [196] | 1-O-α-l-Rha 16-O-β-d-Glc | Fattorusso et al. (2000) | |
| [308] | Alliosterol [196] | 1-O-β-d-Glc-(1 → 4)-O-α-l-Rha 16-O-β-d-Gal | Fattorusso et al. (2000) | |
| A. rotundum L. | Tigogenin [1] | Maisashvili et al. (2007) | ||
| Diosgenin [4] | Sapogenins [1, 4, 7, 9, 12, 19, 34] were isolated by hydrolyzing saponins directly in the raw material | Maisashvili et al. (2007) | ||
| Hecogenin [7] | Maisashvili et al. (2007) | |||
| Gitogenin [9] | Maisashvili et al. (2007) | |||
| β-Chlorogenin [12] | Maisashvili et al. (2007) | |||
| Yuccagenin [19] | Maisashvili et al. (2007) | |||
| Agigenin [34] | Maisashvili et al. (2007) | |||
| [74] | Gitogenin [9] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Maisashvili et al. (2012) | |
| DIDEGLUCOERUBOSIDE B [77] | β-Chlorogenin [12] | 3-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Maisashvili et al. (2008) | |
| ERUBOSIDE B [79] | Maisashvili et al. (2008) | |||
| AGINOSIDE [93] | Maisashvili et al. (2008) | |||
| YAYOISAPONIN C [95] | Maisashvili et al. (2008) | |||
| TRILLIN (ALLIUMOSIDE A) [130] | Maisashvili et al. (2008) | |||
| [309] | (25R)-5α-furostane-2α,3β,22α,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Maisashvili et al. (2012) | |
| A. sativum L. | SATIVOSIDE-R2 [68] | Tigogenin [1] | 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Matsuura et al. (1989a) |
| F-GITONIN [72] | Matsuura et al. (1989a) | |||
| [76] | β-Chlorogenin [12] | 3-O-β-d-Gal | Matsuura et al. (1988) | |
| DIDEGLUCOERUBOSIDE B [77] | β-Chlorogenin [12] | 3-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Matsuura et al. (1988) | |
| ERUBOSIDE-B [79] (obtained by enzymatic hydrolysis of proto-eruboside B) | Matsuura et al. (1988) | |||
| ISO-ERUBOSIDE-B [310] | (25S)-5α-spirostane-3β,6β-diol | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Peng et al. (1996a) | |
| SATIVOSIDE-B1 [311] | (25R)-5α-furostane-3β,6β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Matsuura et al. (1989a) | |
| SATIVOSIDE-R1 [312] | (25R)-5α-furostane-3β,22,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Matsuura et al. (1989a) | |
| PROTO-ERUBOSIDE-B [313] | (25R)-5α-furostane-3β,6β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Matsuura et al. (1988) | |
| PROTO-ISO-ERUBOSIDE-B [314] | (25S)-5α-furostane-3β,6β,22,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Peng et al. (1996a), Ma et al. (2011) | |
| PROTO-DESGALACTOTIGONIN [315] | Matsuura et al. (1989a) | |||
| [316] | (25S)-22-methoxy-5α-furostane-3β,6β,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Ma et al. (2011) | |
| [317] | (25R)-22-methoxy-5α-furostane-3β,6β,26-triol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Ma et al. (2011) | |
| [318] | (25R)-22-methoxy-5α,6β-furostane-3β,26-diol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Ma et al. (2011) | |
| A. sativum L. var. Voghiera | [73] | Gitogenin [9] | 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) |
| AMPELOSIDE Bs1 [90] | Lanzotti et al. (2012a) | |||
| VOGHIEROSIDE A1 [319] | Furostane-2α,3β,5α,22α,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-O-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| VOGHIEROSIDE A2 [320] | Furostane-2α,3β,5α,22β,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| VOGHIEROSIDE B1 [321] | Furostane-2α,3β,5α,22α,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| VOGHIEROSIDE B2 [322] | Furostane-2α,3β,5α,22β,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| VOGHIEROSIDE C1 [323] | Furostane-2α,3β,6β,22α,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| VOGHIEROSIDE C2 [324] | Furostane-2α,3β,6β,22β,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| VOGHIEROSIDE D1 [325] | Furostane-2α,3β,22α,26-tetrol | 26-O-α-l-Rha 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| VOGHIEROSIDE D2 [326] | Furostane-2α,3β,22β,26-tetrol | 26-O-α-l-Rha 3-O-β-d-Glc-(1 → 3)-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| VOGHIEROSIDE E1 [327] | Furostane-2α,3β,22α,26-tetrol | 26-O-α-l-Rha 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| VOGHIEROSIDE E2 [328] | Furostane-2α,3β,22β,26-tetrol | 26-O-α-l-Rha 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Lanzotti et al. (2012a) | |
| A. schoenoprasum L. | [83] | (25R)-5α-spirostane-3β,11α-diol [14] | 3-O-β-d-Glc-(1 → 3)-[β-d-Glc-(1 → 4)]-O-β-d-Gal | Timité et al. (2013) |
| [131] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Timité et al. (2013) | |
| DELTONIN [134] | Timité et al. (2013) | |||
| [158] | Laxogenin [6] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Timité et al. (2013) | |
| [160] | Laxogenin [6] | 3-O-α-l-Rha-(1 → 2)-[β-d-Glc-(1 → 4)]-O-β-d-Glc | Timité et al. (2013) | |
| [182] | (20S,25S)-spirost-5(6)-ene-3β,11α,21-triol [46] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Timité et al. (2013) | |
| [183] | (20S,25S)-spirost-5(6)-ene-3β,12β,21-triol [47] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Timité et al. (2013) | |
| DELTOSIDE [306] | Timité et al. (2013) | |||
| A. schubertii Zucc. | [100, 126] | (25R,S)-5α-spirostane-2α,3β,6β-triol [34, 36] | 3-O-β-d-Glc-(1 → 2)-[4-O-benzoyl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Kawashima et al. (1993) |
| [99, 125] | (25R,S)-5α-spirostane-2α,3β,6β-triol [34, 36] | 3-O-β-d-Glc-(1 → 2)-[3-O-benzoyl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Kawashima et al. (1993) | |
| [98, 124] | (25R,S)-5α-spirostane-2α,3β,6β-triol [34, 36] | 3-O-β-d-Glc-(1 → 2)-[4-O-(S)-3-hydroxy-3-methylglutaryl-β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Kawashima et al. (1993) | |
| [194, 195] | (25R,S)-5α-furostane-2α,3β,6β,22,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Kawashima et al. (1993) | |
| SCHUBERTOSIDE D (NIGROSIDE C) [303] | Alliosterol [196] | 1-O-α-l-Rha 16-O-α-l-Rha-(1 → 3)-O-β-d-Gal | Kawashima et al. (1991b) | |
| SCHUBERTOSIDE A [329] | (22S)-cholest-4(5)-ene-16β,22-diol-3-one | 16-O-α-l-Rha-(1 → 3)-O-β-d-Gal | Kawashima et al. (1991b) | |
| SCHUBERTOSIDE B [330] | (22S)-cholest-5(6)-ene-3β,16β,22-triol | 16-O-α-l-Rha-(1 → 3)-O-β-d-Gal | Kawashima et al. (1991b) | |
| SCHUBERTOSIDE C [331] | (22S)-cholest-5(6)-ene-3β,16β,22-triol | 3-O-β-d-Glc 16-O-α-l-Rha-(1 → 3)-O-β-d-Gal | Kawashima et al. (1991b) | |
| A. senescens L. | Diosgenin [4] | Inoue et al. (1995) | ||
| [140] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc | Inoue et al. (1995) | |
| [141] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Inoue et al. (1995) | |
| A. stipitatum Regel./A. suvorovii Regel. | Diosgenin [4] | Sapogenins [4,19] obtained from the acid hydrolysis of the purified combined glycosides | Vollerner et al. (1988a) | |
| Yuccagenin [19] | Vollerner et al. (1988a) | |||
| Anzurogenin B [26] | Vollerner et al. (1988a, b) | |||
| Anzurogenin D [41] | Kravets (1994) | |||
| Anzurogenin A [48] | Vollerner et al. (1988a) | |||
| Alliogenin [49] | Vollerner et al. (1988a) | |||
| Anzurogenin C [58] | Vollerner et al. (1989) | |||
| Alliosterol [196] | Vollerner et al. (1991) | |||
|
Alliogenone [359] [(25R)-5α-spirostane-2α,3β,5α-triol-6-one] |
Kravets (1994) | |||
| KARATAVIOSIDE A [151] | Kravets (1994) | |||
| KARATAVIOSIDE B [152] | Kravets (1994) | |||
| ANZUROSIDE [190] | Anzurogenin C [58] | 24-O-β-d-Glc | Vollerner et al. (1989) | |
| ALLIOSIDE A [333] | Alliosterol [196] | 16-O-β-d-Gal | Vollerner et al. (1991) | |
| ALLIOSIDE B [334] | Alliosterol [196] | 1-O-β-d-Glc 16-O-β-d-Gal | Vollerner et al. (1991) | |
| A. triquetrum L. | ASCALONICOSIDES A1/A2 [217, 218] | Corea et al. (2003) | ||
| TRIQUETROSIDE A1 [335] | Furost-5(6)-ene-1β,22α-diol |
26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc |
Corea et al. (2003) | |
| TRIQUETROSIDE A2 [336] | Furost-5(6)-ene-1β,22β-diol |
26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc |
Corea et al. (2003) | |
| TRIQUETROSIDE B [337] | Furost-5(6),20(22)-diene-1β-ol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Corea et al. (2003) | |
| TRIQUETROSIDE C1 [338] | Furost-5(6)-ene-1β,22α-diol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 3-O-β-d-Glc | Corea et al. (2003) | |
| TRIQUETROSIDE C2 [339] | Furost-5(6)-ene-1β,22β-diol | 26-O-α-l-Rha-(1 → 2)-O-β-d-Glc 3-O-β-d-Glc | Corea et al. (2003) | |
| A. tuberosum Rottl. ex Spreng | Neotigogenin [2] | Saponins [165, 168, 171] obtained after enzymatic hydrolysis of furostanol saponin fraction by β-glucosidase | ||
| TUBEROSIDE J [102] | (25R)-5α-spirostane-2α,3β,27-triol [40] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Sang et al. (2001a) | |
| TUBEROSIDE K [103] | (25R)-5α-spirostane-2α,3β,27-triol [40] | 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (2001a) | |
| TUBEROSIDE L [104] | (25R)-5α-spirostane-2α,3β,27-triol [40] | 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc 27-O-β-d-Glc | Sang et al. (2001a) | |
| NICOTIANOSIDE C [109] | Neotigogenin [2] | 3-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Sang et al. (2000) | |
| TUBEROSIDE D [112] | Neogitogenin [10] | 3-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Sang et al. (1999a, b), Ikeda et al. (2000) | |
| TUBEROSIDE E [114] | Neogitogenin [10] | 3-O-β-d-Glc-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (1999a) | |
| TUBEROSIDE [128] | (25S)-5α-spirostane-2α,3β,27-triol [42] | 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Zou et al. (2001) | |
| TUBEROSIDE M [163] | (25S)-5β-spirostane-1β,3β-diol [8] | 3-O-α-l-Rha-(1 → 4)-O-β-d-Glc | Sang et al. (2002) | |
| TUBEROSIDE N [164] | (25S)-5β-spirostane-2β,3β-diol [11] | 3-O-β-d-Glc-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (2003) | |
| [165] | 25-Epi-ruizgenin [13] | 3-O-α-l-Rha-(1 → 4)-O-β-d-Glc | Ikeda et al. (2000) | |
| TUBEROSIDE O [166] | (25S)-5β-spirostane-2β,3β,5β-triol [32] | 3-O-β-d-Glc | Sang et al. (2003) | |
| TUBEROSIDE P [167] | (25S)-5β-spirostane-2β,3β,5β-triol [32] | 3-O-α-l-Rha-(1 → 4)-O-β-d-Glc | Sang et al. (2003) | |
| [168] | (25S)-spirostane-3β,5β,6α-triol [43] | 3-O-α-l-Rha-(1 → 4)-O-β-d-Glc | Ikeda et al. (2000) | |
| [171] | Lilagenin [20] | 3-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Ikeda et al. (2000) | |
| [188] | (24S,25S)-5β-spirostane-2α,3β,5β,24-tetrol [55] | 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Hu et al. (2014) | |
| TUBEROSIDE Q [189] | (24S,25S)-5β-spirostane-2β,3β,5β,24-tetrol [56] | 3-O-α-l-Rha-(1 → 4)-O-β-d-Glc | Sang et al. (2003) | |
| [340] | (24S,25S)-5β-spirostane-2β,3β,24-triol | 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Hu et al. (2009) | |
| TUBEROSIDE A [341] | (25S)-5α-furost-20(22)-ene-2α,3β,26-triol | 26-O-β-d-Glc 26-O-β-d-Glc | Sang et al. (1999b) | |
| TUBEROSIDE B [342] | (25S)-5α-furost-20(22)-ene-2α,3β,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (1999b) | |
| TUBEROSIDE C [343] | (25S)-5α-furost-20(22)-ene-2α,3β,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc | Sang et al. (1999b) | |
| TUBEROSIDE R [344] | (25S)-5β-furost-20(22)-ene-2β,3β,5,26-tetrol | 26-O-β-d-Glc 3-O-β-d-Glc | Sang et al. (2003) | |
| TUBEROSIDE S [345] | (25S)-5β-furost-20(22)-ene-3β,26-diol | 26-O-β-d-Glc 3-O-β-d-Glc-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (2003) | |
| TUBEROSIDE T [346] | (25S)-5α-furost-20(22)-ene-3β,26-diol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (2003) | |
| [347] | (25S,20R)-5α-furost-22(23)-ene-2α,3β,20,26-tetrol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (2001b) | |
| [348] | (25S,20R)-20-methoxy-5α-furost-22(23)-ene-2α,3β,20,26-tetrol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (2001b) | |
| [349] | (25S,20S)-5α-furost-22(23)-ene-2α,3β,20,26-tetrol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (2001b) | |
| [350] | (25S,20S)-5α-furost-22(23)-ene-3β,20,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc | Sang et al. (2001b) | |
| [351] | (25R)-5α-furostane-3β,22,26-triol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Ikeda et al. (2004) | |
| [352] | (25S)-5β-furostane-3β,5β,6α,22,26-pentaol | 26-O-β-d-Glc 3-O-α-l-Rha-(1 → 4)-O-β-d-Glc | Ikeda et al. (2004) | |
| [267] | Alliosterol [196] | 1-O-α-l-Rha 16-O-β-d-Glc | Sang et al. (2000) | |
| TUBEROSIDE U [353] | (22S,25S)-cholest-5(6)-ene-3β,16β,22,26-tetrol | 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc 16-O-β-d-Glc | Sang et al. (2003) | |
| A. turcomanicum Regel | Yuccagenin [19] | Pirtskhalava et al. (1977a) | ||
| Neoagigenone [25] | Pirtskhalava et al. (1977a) | |||
| Neoagigenin [36] | Pirtskhalava et al. (1977a) | |||
| 6-O-benzoyl neoagigenin [37] | Pirtskhalava et al. (1977a) | |||
| Alliogenin [49] | Pirtskhalava et al. (1977a) | |||
| Neoalliogenin [50] | Pirtskhalava et al. (1977b) | |||
| TUROSIDE A [122] | Neoagigenin [36] | 3-O-β-d-Xyl-(1 → 3)-[β-d-Glc-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Pirtskhalava et al. (1978) | |
| TUROSIDE A 6-O-BENZOATE [127] | 6-O-benzoyl-neoagigenin [37] | 3-O-β-d-Xyl-(1 → 3)-[β-d-Glc-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Pirtskhalava et al. (1979a) | |
| TUROSIDE C [354] | (25S)-5α-furostane-2α,3β,6β,22,26-pentaol | 26-O-β-d-Glc 3-O-β-d-Xyl-(1 → 3)-[β-d-Glc-(1 → 2)-O-β-d-Glc-(1 → 2)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal | Pirtskhalava et al. (1979b) | |
| A. ursinum L. | [141] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Sobolewska et al. (2006) |
| [156] | (25R)-spirost-5(6),25(27)-diene-3β-ol [5] | 3-O-α-l-Rha-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Sobolewska et al. (2006) | |
| DICHOTOMIN [223] | Sobolewska (2004) | |||
| A. vavilovii M. Popov | ASCALONICOSIDES A1/A2 [217, 218] | Zolfaghari et al. (2013) | ||
| VAVILOSIDE A1 [355] | (25R)-furost-5(6)-ene-1β,3β,22α,26-tetrol | 26-O-α-l-Rha 1-O-α-l-Rha-(1 → 2)-O-β-d-Gal | Zolfaghari et al. (2013) | |
| VAVILOSIDE A2 [356] | (25R)-furost-5(6)-ene-1β,3β,22β,26-tetrol | 26-O-α-l-Rha 1-O-α-l-Rha-(1 → 2)-O-β-d-Gal | Zolfaghari et al. (2013) | |
| VAVILOSIDE B1 [357] | (25R)-furost-5(6)-ene-1β,3β,22α,26-tetrol | 26-O-α-l-Rha 1-O-α-l-Rha-(1 → 2)-O-β-d-Xyl | Zolfaghari et al. (2013) | |
| VAVILOSIDE B2 [358] | (25R)-furost-5(6)-ene-1β,3β,22β,26-tetrol | 26-O-α-l-Rha 1-O-α-l-Rha-(1 → 2)-O-β-d-Xyl | Zolfaghari et al. (2013) | |
| A. victorialis var. platyphyllum L. | F-GITONIN [72] | Lee et al. (2001) | ||
| A. vineale L. | Diosgenin [4] | Chen and Snyder (1989) | ||
| Nuatigenin [27] | Chen and Snyder (1989) | |||
| Isonuatigenin [28] | Chen and Snyder (1989) | |||
| [131] | Diosgenin [4] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Chen and Snyder (1989) | |
| DELTONIN [134] | Chen and Snyder (1989) | |||
| [136] | Diosgenin [4] | 3-O-β-d-Glc-(1 → 4)-O-α-l-Rha-(1 → 4)-O-β-d-Glc | Chen and Snyder (1989) | |
| [142] | Diosgenin [4] | 3-O-β-d-Glc-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Chen and Snyder (1989) | |
| [144] | Diosgenin [4] | 3-O-β-d-Glc-(1 → 3)-[β-d-Glc-(1 → 6)]-O-β-d-Glc-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Chen and Snyder (1989) | |
| [145] | Diosgenin [4] | 3-O-β-d-Glc-(1 → 6)-O-β-d-Glc-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Chen and Snyder (1989) | |
| [146] | Diosgenin [4] | 3-O-β-d-Glc-(1 → 4)-[β-d-Glc-(1 → 6)]-O-β-d-Glc-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc | Chen and Snyder (1989) | |
| [155] | Isonuatigenin [28] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Chen and Snyder (1989) | |
| [176] | Nuatigenin [27] | 3-O-α-l-Rha-(1 → 2)-O-β-d-Glc | Chen and Snyder (1989) | |
| A. waldsteinii Don. | Diosgenin [4] | Eristavi et al. (1973) | ||
| β-Chlorogenin [12] | Eristavi et al. (1973) | |||
| DIDEGLUCOERUBOSIDE B [77] | Gugunishvili et al. (2006) | |||
| TRILLIN (ALLIUMOSIDE A) [130] | Gugunishvili et al. (2006) |
Biological and pharmacological properties of Allium saponins
Saponins are considered responsible for numerous pharmacological properties of many plants, and they are recognized as active constituents of Allium species as well. It should be mentioned, however, that Allium plants are not rich sources of these compounds. Results from quantitative studies indicate that saponin content is usually very low, for example A. nigrum total saponin content in different parts of the plant was determined as: 19.38 mg/g dw in the roots, 15.65 mg/g dw—bulbs, and 10.48 mg/g dw—leaves (Mostafa et al. 2013). Quantitative densitometric determination of diosgenin—the main sapogenin of A. ursinum, revealed some differences in its accumulation with respect to the vegetation period, nevertheless its highest percentage observed in the bulbs collected in March did not exceed 0.0029 % of fresh weight (Sobolewska et al. 2009). A significant exception, in terms of saponin content, is A. nutans, where the concentration of these compounds in the underground parts was established to be about 4 % of dry matter (Akhov et al. 1999).
It should be emphasized however that the results from many pharmacological in vitro and in vivo studies revealed several interesting activities of Allium saponins, for example antifungal, cytotoxic, antispasmodic, hypocholesterolemic, and other.
Cytotoxic properties
Cytotoxic activity of saponins was discussed in a number of experimental papers on Allium species. In vitro studies were performed on several human and animal cell cancer lines, including IGR-1—human melanoma cell line; HL-60—promyelotic leukemia cells; HCT-116, HT-29, and SW480—human colorectal cancer cell lines; DLD-1—human colon adenocarcinoma, HA549—lung cancer cell line, NCI-H460—human large-cell lung carcinoma, SF-268—human glioblastoma; MCF-7—human breast adenocarcinoma, HepG2—human hepatocellular liver carcinoma cell line; WEHI 164—murine fibrosarcoma cell line; J-774—murine monocyte/macrophage cell line; P-388 and L-1210—murine leukemia cell lines (Table 4 of ESM). Amongst tested spirostane saponins dioscin [135], isolated from A. ampleloprasum, seemed to be most potent, with an IC50 = 0.092 μg/mL against P388 cell line (Sata et al. 1998). This compound, which is widely distributed in species of the family Dioscoreaceae and Asparagaceae, revealed significant in vitro activity in tests performed on many other cancer cell lines (Podolak et al. 2010). Some authors claim that apart from the type of the cell line, the structure of the oligosaccharide chain, especially the site of interglycosidic linkages, rather than the sapogenin, are the modulating factors of cytotoxic properties (Rezgui et al. 2014). Some evidence that may substantiate such claims comes from the results obtained for a mixture of diosgenin tetrasaccharide and (25R)-spirost-5(6),25(27)-diene-3β-ol tetrasaccharide [141, 156] (A. ursinum) (Sobolewska et al. 2006). The sugar chain of these compounds differs from that of dioscin [135] (3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-O-β-d-Glc) by an additional terminal rhamnose moiety. Both exhibited 100 % effect already at the concentration of 2 μg/mL on melanoma B16 and sarcoma XC. Similarly, deltonin [134] (diosgenin 3-O-β-d-Glc-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc) isolated from A. schoenoprasum showed significant activity against HCT 116 and HT-29 cell lines with an IC50 = 0.40 and 0.75 μM, respectively (Timité et al. 2013). These results corroborate with those obtained by Mimaki et al. (2001), who suggested that an α-l-Rha-(1 → 2)-O-β-d-Glc sugar sequence attached to diosgenin is crucial for activity (Mimaki et al. 2001).
The most potent spirostanol glycosides include also eruboside B [79], leucospiroside A [97], yayoisaponin C [95] and aginoside [93] isolated from A. leucanthum, which showed in vitro cytotoxic activity, with relatively similar IC50 values against A549 WS1, and DLD-1 cells (Mskhiladze et al. 2008b). The two latter compounds, that were isolated from A. ampeloprasum, showed in vitro cytotoxicity against P388 cells at 2.1 μg/mL (Sata et al. 1998). Tigogenin pentasaccharide [67] (A. macleanii) and diosgenin 3-O-α-l-Rha-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc [140] (A. senescens) were cytotoxic towards HeLa cells at the concentration of 50 μg/mL, whereas already at 5 μg/mL they exhibited 64.7 and 11.5 % inhibition, respectively (Inoue et al. 1995). Several spirostanol glycosides, that were isolated from different Allium species, revealed fairly high cytotoxic activity in tests on promyelotic leukemia cells HL-60. Yuccagenin tetrasaccharide (karatavioside A [151]) from the bulbs of A. karataviense exhibited considerable cytostatic activity with an IC50 value of 2.4 μg/mL as compared with etoposide (IC50 0.3 μg/mL) (Mimaki et al. 1999c). Tuberoside M [163] from the seeds of A. tuberosum inhibited the cells growth with IC50 = 6.8 μg/mL, while F-gitonin [72] isolated from the fresh bulbs of A. jesdianum—with an IC50 value of 1.5 μg/mL (Sang et al. 2002; Mimaki et al. 1999a). Other compounds isolated from this latter species were considered to be inactive. The authors concluded that the presence of an additional OH group at C-6 in gitogenin skeleton is detrimental to activity, while cholestane glycosides showed no effect. It is probable that the presence of a carbonyl at C-6 in a laxogenin glycoside [158] isolated by Timité et al. (2013) from the whole plant of A. schoenoprasum could be responsible for the loss of activity against two cancer cell lines HCT 116 and HT-29, an effect similar to that seen by Mimaki et al. when an additional OH group was introduced at C-6 of gitogenin (Timité et al. 2013; Mimaki et al. 1999c). In accordance with the studies of Mimaki et al. (1999a, b, c) were also the results obtained for cholestane glycosides, nigrosides C [303] and D [304] isolated from the bulbs of A. nigrum, which showed no effect (IC50 > 100 μM) on the HT-29 and HCT-116 cancer cell lines in the MTT assay (Jabrane et al. 2011). Opposite results were obtained however with two cholestane glycosides isolated from A. porrum—alliosterol 1-O-α-l-Rha 16-O-β-d-Glc [267] and alliosterol 1-O-β-d-Glc-(1 → 4)-O-α-l-Rha 16-O-β-d-Gal [308], which exhibited in vitro cytotoxic properties (IC50 4.0–5.8 μg/mL) against two murine cell lines: WEHI 164 and J-774 (Fattorusso et al. 2000).
Results of cytotoxicity assays of several spirostanol sapogenins indicated their weak activity or lack of it. Agigenin [34], porrigenin A [38] and porrigenin B [23] identified in A. porrum tested in vitro for their growth-inhibitory activity on four different cell lines (IGR-1, WEHI 164, J-774, and P-388) exhibited much weaker activity when compared with 6-MP and were virtually inactive (>100 μg/mL) (Carotenuto et al. 1997a). However, some of the steroidal glycosides isolated from the same plant exhibited quite a good activity towards J-744 and WEHI-164 cells, the most active being gitogenin and porrigenin C derivatives (IC50 ranging from 1.9 to 5.8 μg/mL) (Fattorusso et al. 2000).
From among tested furostanoles the majority of compounds showed weak activity or lack of it, for example two glycosides isolated from A. tuberosum showed no activity at concentrations below 5 μM against PC-12 and HCT-116 (Ikeda et al. 2004). Among numerous furostanoles obtained from A. macrostemon which were tested against NCI-H460, SF-268, MCF-7, and HepG2 cell lines, exclusively 26-O-β-d-Glc 5α-furost-25(27)-ene-3β,12β,22,26-tetrol 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal [292] was found cytotoxic towards SF-268 cell line, while 26-O-β-d-Glc 5β-furost-20(22),25(27)-diene-3β,12β,26-triol 3-O-β-d-Glc-(1 → 2)-O-β-d-Gal [293] showed cytotoxicity towards SF-268 and NCI-H460 cell lines (Chen et al. 2009).
The differences in activity between compounds having the same aglycone but differing in sugar chain was observed by Zolfaghari et al. (2013). The equilibrated mixture of furostanols: vavilosides A1/A2–B1/B2 [355–358] and ascalonicosides A1/A2 [217, 218] isolated from A. vavilovii were tested against cell lines: J-774 and WEHI-164. The activity of all saponins was dose-dependent and varied in the following order: vavilosides B1/B2 > ascalonicosides A1/A2 > vavilosides A1/A2 (Zolfaghari et al. 2013). The substitution of a galactose residue (vavilosides A1/A2) with a xylose unit (vavilosides B1/B2) caused an increase in cytotoxic activity.
Antifungal activity
Numerous steroidal saponins isolated from different plant sources have been reported to have antifungal/antiyeast activity, particularly against agricultural pathogens. Antifungal saponins require particular attention as there is a constant need for new agents that would be effective against opportunistic fungal infections and could provide an alternative to chemical fungicides used in the fight against plant pathogens. Unfortunately, only a few studies have been performed so far on Allium steroidal glycosides.
Antifungal activity of Allium saponins was modulated by both the sapogenin type and the number and structure of the sugar residue. Generally saponins with spirostanol skeleton exhibited higher antifungal activity than furostanols. Yu et al. (2013) observed several biochemical changes which could be involved in the possible mechanism of antimicrobial activity of saponins, such as reduced glucose utilization rate, decrease of catalase activity and protein content in microorganisms.
The results from in vitro assays against different plant and human pathogen strains are provided in Table 5 of ESM.
Studies by Barile et al. (2007), Lanzotti et al. (2012a, b), and Sadeghi et al. (2013) provide evidence for significant differences in the potency of saponins belonging to furostane or spirostane groups. Minutosides A-C [295, 119, 296] (A. minutiflorum) showed concentration-dependent antifungal activity against a number of pathogens: Alternaria alternata, A. porri, B. cinerea, Fusarium oxysporum, F. solani, Pythium ultimum, R. solani, Trichoderma harzianum P1, T. harzianum T39 (Barile et al. 2007). The most pronounced effect was seen with a spirostanol minutoside B [119], as compared to both furostanols (minutosides A [295] and C [296]). Persicosides A [120] and B [121]—compounds isolated from A. ampeloprasum ssp. persicum, showed a statistically significant activity against P. italicum, A. niger and T. harzianum, higher than furostanol and cholestane compounds (Sadeghi et al. 2013). The antifungal activity of isolated compounds against B. cinerea was not significant. Interestingly, all saponins inhibited the growth of P. italicum. Antifungal properties of persicosides A [120] and B [121], ceposides A1/A2 [209, 210], tropeosides A1/A2 [213, 214] and B1/B2 [215, 216] were dose dependent. Ceposides A-C [209, 232, 211] (isolated from A. cepa) showed antifungal activity, dependent on their concentration and the fungal species used: soil-borne pathogens (Fusarium oxysporum sp. lycopersici, Rhizoctonia solani and Sclerotium cepivorum), air-borne pathogens (A. alternata, A. niger, B. cinerea, Mucor sp., and Phomopsis sp.), antagonistic fungi (Trichoderma atroviride and T. harzianum), and a pathogen specific to the Allium genus—S. cepivorum (Lanzotti et al. 2012b). Their activity varied in the following order: ceposide B > ceposide A ~ ceposide C. The authors observed a significant synergism of action between those three saponins against B. cinerea and T. atroviride. Ceposide B [232] showed significant activity against all fungi with the exception of F. oxysporum sp. lycopersici, S. cepivorum and R. solani. Ceposides A [209] and C [211] were active against all fungi with the exception of A. niger, S. cepivorum and F. oxysporum sp. lycopersici. Agigenin 3-O-trisaccharide [90] and gitogenin 3-O-tetrasaccharide [73], isolated from the bulbs of A. sativum var. Voghiera, were more active against B. cinerea and T. harzianum than furostanol voghierosides isolated from that plant (Lanzotti et al. 2012a). All the compounds were effective towards T. harzianum in a dose dependent manner, but only spirostanol saponins and voghieroside C [323, 324]—against B. cinerea.
Mskhiladze et al. (2008a) in their studies on anti-yeast effects of saponins from A. leucanthum observed that β-chlorogenin as aglycone and the branched oligosaccharide chain substituted by xylose rather than glucose are beneficial for the activity. Yayoisaponin C [95], eruboside B [79], aginoside [93], agigenin 3-O-β-d-Glc-(1 → 2)-O-β-d-Glc-(1 → 4)-O-β-d-Gal [91] and β-chlorogenin 3-O-β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal [80] exhibited antifungal activity on several Candida strains, including C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, C. kefyr, C. krusei, C. lusitaniae, and also on Cryptococcus neoformans, however β-chlorogenin glycoside was the most active compound with MFC from ≤6.25 to 25 μg/mL (as compared to amphotericin B 0.78–12.5 μg/mL). In another study the same compound isolated from A. porrum showed antifungal activity towards Fusarium culmorum (ED50 = 30 μg/mL) (Carotenuto et al. 1999). Eruboside B [79] (A. sativum), β-chlorogenin glycoside as well, inhibited in vitro the growth of C. albicans (MIC 25 μg/mL) (Matsuura et al. 1988).
Agigenin glycosides: aginoside [93] together with yayoisaponins A [96] and C [95] isolated from A. ampeloprasum showed antifungal activity against Mortierella ramanniana at 10 μg/disc (Sata et al. 1998). None of the saponins was active against Penicillium chrysogenum at concentrations up to 100 μg/disc. Ampeloside Bs1 [90], agigenin 3-O-β-d-Glc-(1 → 4)-O-β-d-Gal [87], and furostane-type ampeloside Bf1 [202] isolated from the same species did not inhibit the growth of Aspergillus niger; spirostanols showed weak activity against Candida albicans (Morita et al. 1988). Aginoside [93] at 400 ppm completely inhibited the growth of C. gloeosporioides, Fusarium verticillioides, and Botrytis squamosa and partially suppressed F. oxysporum f. sp. cepae and F. oxysporum f. sp. radicis-lycopersici (Mostafa et al. 2013). The influence of the structure of the sugar chain on the observed anti-fungal activity of compounds bearing the same aglycone was revealed in studies by Teshima et al. (2013).
Alliospirosides A [169] and B [170] (both (25S)-ruscogenin glycosides), which are present mainly in the basal plates and roots of A. cepa Aggregatum group, to a different extent inhibited in vitro a wide range of plant pathogenic fungi: Alternaria ssp., Botrytis ssp., Colletotrichum spp., Curvularia lunata, Epicoccum nigrum, Fusarium ssp., Magnaporthe oryzae, S. cepivorum, and Thanatephorus cucumeris (Teshima et al. 2013). Alliospiroside A [169] strongly inhibited (>80 % growth inhib.) the growth of Colletotrichum spp. isolates. It was also more effective against M. oryzae and S. cepivorum compared to alliospiroside B [170], however, its antifungal activity against B. cinerea, F. oxysporum and F. solani was relatively low.
Enzyme inhibitory properties
Saponin fraction isolated from the methanol extract of A. chinense inhibited cAMP PDE (43.5 %) and Na+/K+ATP-ase (59.3 %) at the concentration of 100 μg/mL (Kuroda et al. 1995). Both enzymes were also inhibited by (25R,S)-5α-spirostane-3β-ol tetrasaccharide [65, 110] (IC50 7.0 × 10−5 and 4.0 × 10−5 M respectively). Laxogenin glycosides exhibited significant activity only on cAMP phosphodiesterase, one of which, with an acetyl group in the saccharide moiety, was almost as potent as papaverine used as a positive control (IC50 3.3 × 10−5 and 3.0 × 10−5 M respectively).
Also, saponins isolated from A. giganteum bulbs inhibited cAMP phosphodiesterase (Mimaki et al. 1994) and in concordance with previously cited results, an acetyl derivative—3-O-acetyl-(24S,25S)-5α-spirostane-2α,3β,5α,6β,24-pentaol 2-O-β-d-Glc [193] exhibited inhibitory activity almost equal to that of papaverine (IC50 4.1 × 10−5 and 3.0 × 10−5 M respectively). In the same study, furostanol saponins were revealed to be much more potent than the corresponding spirostanol glycosides. The results were in contrast to the previous studies of these authors which showed that furostanol glycosides were less active, exhibiting only weak inhibitory activity or none. The authors concluded that the anti-enzyme activity could be dependent on the number of hydroxyls in the A and B rings as in the present study the tested furostanol saponins contained several OH groups.
Saponins isolated from the fruits of A. karataviense and A. cepa as well as the products of chemical modifications of karatavioside A, were studied on a highly purified porcine kidney Na+/K+ATP-ase, in the concentration range from 1 × 10−4 to 1 × 10−7 M (Mirsalikhova et al. 1993). All the compounds affected the enzyme activity being capable of its inhibition, and/or activation. As was showed, the presence of a hydroxyl group in the F-ring at C-24 led to a decrease in the percentage inhibition of Na+/K+ATP-ase. At the concentration of 1 × 10−4 M the inhibitory effect of karatavioside A [151] was 19.8 %, karatavioside B [152]—32.4 %, karatavioside C [268]—4.9 %, karatavioside E [180]—1.7 %, karatavioside F [181]—7.5 %, alliospiroside A [169]—99.7 %, alliospiroside B [170]—76.3 %, alliospiroside D [179]—67.1 %; while alliospiroside C [178] activated the enzyme by 13.4 %. A keto group at C-6 of sapogenin slightly increased the inhibition level of Na+/K+ATP-ase.
Moreover, it was revealed that alliospirosides A [169] and B [170] were both uncompetitive enzyme inhibitors, while alliospiroside D [179]—competitive. Interestingly, alliospiroside C [178], although bearing the same aglycone as alliospiroside D—cepagenin [44], did not inhibit Na+/K+ATP-ase at all.
Drugs acting via inhibition of the activity of this transport enzyme may be of potential use in the treatment of many diseases of the cardiovascular system, the kidneys, the immune system, which are connected with disturbances in the active transport of ions.
Cardioprotective activity
Three saponins from A. chinense and their aglycones were tested for the protective effects against oxidative stress-induced cardiac damage (Ren et al. 2010). Their activities were evaluated on H2O2-injured cardiac H9C2 cells. The cytotoxicity was measured using MTT assay while the oxidative damage by determination of MDA and NO contents. All tested compounds protected cultured H9C2 cells from death in the concentration range of 5–20 μΜ. It was shown that glycosides exhibited less protective efficacy than sapogenins. Among these, laxogenin [6] and tigogenin [1] displayed stronger effects than furostane-type aglycones. The authors concluded that the presence of F ring in spirostanols may enhance their protective activity whereas oxidation in the B ring might be detrimental as laxogenin was less active than tigogenin.
Nine furostane saponins isolated by Lai et al. from A. fistulosum were tested for antihypoxic activity against hypoxia/reoxygenation (H/R)-induced human umbilical vein endothelial cell (HUVEC) injury (Lai et al. 2010). Cell viability was determined by MTT assay. It was observed that the saponin treatment significantly improved the survival of H/R-treated HUVEC (P < 0.05) in a dose-dependent manner. Fistulosaponin A [250] was the most effective compound with a cell viability of 59.5 ± 3.0, 76.3 ± 3.3, 80.1 ± 3.6, 82.7 ± 4.1, 86.3 ± 4.6, and 78.2 ± 2.8 % for the six dose groups (0.5, 1, 5, 10, 50, and 100 μM), respectively.
In animal studies, alloside B [334], isolated from fruits of A. suvorovii and A. stipitatum, exhibited a statistically reliable hypotriglyceridemic activity in experimental hyperlipidemia caused by 1-day starvation, Triton WR-1339 and vitamin D2–cholesterol, when compared with lipanthyl (Aizikov et al. 1995).
The hypocholesterolemic activity of saponins was reported in many animal studies.
The cholesterol-lowering effect of garlic is probably partially due to the steroid saponin presence. In a rat model of experimental hyperlipidemia induced by feeding a 0.5 % cholesterol-enriched diet saponin-rich fraction from raw garlic administrated at 10 mg/kg/day led to a decrease of plasma total and LDL cholesterol concentration level without affecting HDL cholesterol levels after 16 weeks (Matsuura 2001). It was claimed that the reduction of concentration of plasma cholesterol concentration is the result of inhibition of cholesterol absorption by saponins in the intestine or a direct effect on cholesterol metabolism.
Antispasmodic effect
Furostanol saponins hirtifoliosides C1/C2 [264, 265] and a spirostanol glycoside agapanthagenin 3-O-Glc [85] isolated from A. hirtifolium, along with four saponins elburzensosides A1/A2 [238, 239] and C1/C2 [242, 243] and the sapogenin agapanthagenin [31], from A. elburzense, were subjected to biological assays on the guinea-pig isolated ileum in order to evaluate their possible antispasmodic activity (Barile et al. 2005). Apart from the agapanthagenin glycoside, all the tested compounds were able to reduce induced contractions, as measured by the reduction of histamine release, in a concentration-dependent manner. Elburzensosides C1/C2 [242, 243] and agapanthagenin [31] showed the highest activity with a maximum effect at 10−5M (approx. 50 % inhibition).
The authors concluded that the positive effect is associated with the presence of a hydroxyl group at position C-5 and of a glucose unit at position C-26. On the other hand, hydroxylation at C-6 and glucose attachment at C-3 seem to be structural features responsible for the loss of activity. Furostane-type saponins that were isolated from A. cepa var. tropea, namely tropeosides A1/A2 [213, 214] and B1/B2 [215, 216] were able to dose-dependently relieve acetylocholine- and histamine-induced contractions (50 % inhibition of contractions was seen at the concentration of 10−5 M) (Corea et al. 2005). Interestingly, other furostanols identified in this plant, such as ascalonicosides A1/A2 [217, 218], were inactive.
Other activities
Macrostemonoside A [65] inhibited ADP-induced rabbit ertythrocyte aggregation with IC50 = 0.065 mM (Peng et al. 1992). An in vitro inhibitory activity of ADP-induced platelet aggregation was also reported for macrostemonosides E [272], F [273] and G [274] (IC50 = 0.417; 0.020; 0.871 mM, respectively) (Peng et al. 1993, 1995). 26-O-β-d-Glc (25R)-5α-furostane-3β,12β,22,26-tetrol 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal [289] and 26-O-β-d-Glc (25R)-5α-furostane-3β,12α,22,26-tetrol 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Glc-(1 → 4)-O-β-d-Gal [290] exhibited significant inhibitory activity on CD40L expression on the membrane of ADP stimulated platelets (Chen et al. 2010).
β-chlorogenin 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-O-β-d-Gal 6-O-β-d-Glc [78], isolated from the bulbs of A. ampeloprasum var. porrum, demonstrated in vivo antiinflammatory and gastroprotective effects in a carrageenan-induced oedema assay and by measuring acute gastric lesions induced by acidified ethanol (Adão et al. 2011a). Saponin administrated orally (100 mg/kg) inhibited oedema formation similar to dexamethasone (25 mg/kg). Cytoprotective activity of β-chlorogenin glycoside resulted in a significant reduction in gastric hyperemia and also in the severity and number of lesions.
Macrostemonoside A [65] increased the synthesis and release of visfatin in 3T3-L1 adipocytes and elevated mRNA levels in this cytokine in a dose- and time-dependent mode (Zhou et al. 2007). In a study on C57BL/6 mice fed on a high-fat diet, this saponin when administered at the dose of 4 mg/kg/day for 30 days moderately inhibited glucose level, glycogen hepatic content, total plasma cholesterol level and abdominal adipose tissue (Xie et al. 2008).
In the molluscicidal bioassay with Biomphalaria pfeifferei diosgenin 3-O-β-d-Glc-(1 → 4)-[β-d-Glc-(1 → 6)]-O-β-d-Glc-(1 → 4)-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-O-β-d-Glc [146], isolated from A. vineale, exhibited 100 % effect at 25 ppm in <24 h (Chen and Snyder 1989). The authors observed that the molluscicidal activity of isolated compounds increased with an increasing number of monosaccharides in a sugar moiety.
Aginoside [93] was found to be toxic to leek-moth larvae Acrolepiopsis assectella (Harmatha et al. 1987). The compound caused mortality and ecdysial failures 56 ± 10 and 19 % respectively in larvae of A. assectella reared on semisynthetic diet at a concentration of 0.9 mg/g of diet.
Conclusions
In this paper steroidal saponins reported in various Allium species from early 1970 to March 2014 are reviewed, including their skeletal structures and sugar chains.
Until now, as many as 290 saponins have been identified, including a certain number of methoxyl derivatives originating from furostanol compounds, that should be considered as artifacts resulting from the use of methanol in the extraction/isolation procedures.
Allium genus is characterized by a great diversity of structures. Apart from spirostane- and furostane-type compounds, a rare group of open-chain saponins has been identified in several species. Allium genus is also a source of unique steroidal sapogenins, such as 25(S)-5β-spirostane-1β,3β-diol [8] and 2,3-seco-porrigenin [64]. Despite a relatively low content of steroidal glycosides in Allium species, they are considered to contribute, in addition to sulfur compounds, to the overall biological activity of these plants. Undoubtedly, stability of saponins is their advantage as compared to fairly unstable sulfur compounds, thus, they in fact may be predominant active constituents of Allium products. Bearing this aspect in mind it seems highly feasible to develop antifungal Allium preparations against animal and plant pathogens. Also, reports on high in vitro cytotoxic activity of steroidal saponins from Allium species makes them potential candidates for further development as anti-cancer agents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
References
- Adão CR, Da Silva BP, Parente JP. A new steroidal saponin with antiinflammatory and antiulcerogenic properties from the bulbs of Allium ampeloprasum var. porrum. Fitoterapia. 2011;82:1175–1180. doi: 10.1016/j.fitote.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Adão CR, Da Silva BP, Parente JP. A new steroidal saponin from Allium ampeloprasum var. porrum with antiinflammatory and gastroprotective effects. Phytochem Lett. 2011;4(3):306–310. doi: 10.1016/j.phytol.2011.05.009. [DOI] [Google Scholar]
- Adão C, Da Silva B, Tinoco L, et al. Haemolytic activity and immunological adjuvant effect of a new steroidal saponin from Allium ampeloprasum var. porrum. Chem Biodivers. 2012;9(1):58–67. doi: 10.1002/cbdv.201100005. [DOI] [PubMed] [Google Scholar]
- Aizikov MI, Kravets SD, Prokhorova IR, et al. Structure and hypolipidemic activity of alloside B extracted from anzur onions. Pharm Chem J. 1995;29(8):547–548. doi: 10.1007/BF02219011. [DOI] [Google Scholar]
- Akhov LS, Musienko MM, Piacente S, et al. Structure of steroidal saponins from underground parts of Allium nutans L. J Agric Food Chem. 1999;47:3193–3196. doi: 10.1021/jf9901800. [DOI] [PubMed] [Google Scholar]
- Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136(3):716S–725S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- APG An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 2009;161:105–121. doi: 10.1111/j.1095-8339.2009.00996.x. [DOI] [Google Scholar]
- Azarkova AF, Glyzina GS, Melnikova TM, et al. Diosgenin from Allium angulosum. Chem Nat Comp. 1974;10(3):412. doi: 10.1007/BF00563918. [DOI] [Google Scholar]
- Azarkova AF, Stikhin VA, Cherkasov OA, et al. Diosgenin from Allium nutans and Allium cernuum. Chem Nat Comp. 1983;19(5):621. doi: 10.1007/BF00576113. [DOI] [Google Scholar]
- Baba M, Ohmura M, Kishi N, et al. Saponins isolated from Allium chinense G. Don and antitumor-promoting activities of isoliquiritigenin and laxogenin from the same drug. Biol Pharm Bull. 2000;23(5):660–662. doi: 10.1248/bpb.23.660. [DOI] [PubMed] [Google Scholar]
- Barile E, Zolfaghari B, Sajjadi SE, et al. Saponins of Allium elburzense. J Nat Prod. 2004;67:2037–2042. doi: 10.1021/np0497752. [DOI] [PubMed] [Google Scholar]
- Barile E, Capasso R, Izzo AA, et al. Structure–activity relationships for saponins from Allium hirtifolium and Allium elburzense and their antispasmodic activity. Planta Med. 2005;71(11):1010–1018. doi: 10.1055/s-2005-873134. [DOI] [PubMed] [Google Scholar]
- Barile E, Bonanomi G, Antignani V, et al. Saponins from Allium minutiflorum with antifungal activity. Phytochemistry. 2007;68:596–603. doi: 10.1016/j.phytochem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Carotenuto A, Fattorusso E, Lanzotti V, et al. Porrigenins A and B, novel cytotoxic and antiproliferative sapogenins isolated from Allium porrum. J Nat Prod. 1997;60(10):1003–1007. doi: 10.1021/np960657r. [DOI] [PubMed] [Google Scholar]
- Carotenuto A, Fattorusso E, Lanzotti V, et al. 12-keto-porrigenin and the unique 2,3-seco-porrigenin, new antiproliferative sapogenins from Allium porrum. Tetrahedron. 1997;53(9):3401–3406. doi: 10.1016/S0040-4020(97)00062-8. [DOI] [Google Scholar]
- Carotenuto A, Fattorusso E, Lanzotti V, et al. Spirostanol saponins of Allium porrum L. Phytochemistry. 1999;51:1077–1082. doi: 10.1016/S0031-9422(98)00712-2. [DOI] [PubMed] [Google Scholar]
- Challinor VL, De Voss JJ. Open-chain steroidal glycosides, a diverse class of plant saponins. Nat Prod Rep. 2013;30:429–454. doi: 10.1039/c3np20105h. [DOI] [PubMed] [Google Scholar]
- Chen S, Snyder JK. Diosgenin-bearing, molluscicidal saponins from Allium vineale: an NMR approach for the structural assignment of oligosaccharide units. J Org Chem. 1989;54:3679–3689. doi: 10.1021/jo00276a033. [DOI] [Google Scholar]
- Chen HF, Wang NL, Sun HL, et al. Novel furostanol saponins from the bulbs of Allium macrostemon B. and their bioactivity on [Ca2+] increase induced by KCl. J Asian Nat Prod Res. 2006;8(1–2):21–218. doi: 10.1080/10286020500172533. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang G, Wang N, et al. New furostanol saponins from the bulbs of Allium macrostemon Bunge and their cytotoxic activity. Pharmazie. 2007;62(7):544–548. [PubMed] [Google Scholar]
- Chen H-F, Wang G-H, Luo Q, et al. Two new steroidal saponins from Allium macrostemon Bunge and their cytotoxicity on different cancer cell lines. Molecules. 2009;14:2246–2253. doi: 10.3390/molecules14062246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ou W, Wang G, et al. New steroidal glycosides isolated as CD40L inhibitors of activated plateletes. Molecules. 2010;15:4589–4598. doi: 10.3390/molecules15074589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S-B, Wang Y, Zhang Y-F, et al. Steroidal saponins from Allii macrostemonis bulbs. Chin Tradit Herb Drugs. 2013;44(9):1078–1081. [Google Scholar]
- Chincharadze DG, Kelginbaev AN, Gorovits MB, et al. Steroidal saponins and sapogenins of Allium. XV. Eruboside B from Allium erubescens. Khim Prir Soedin. 1979;4:509–514. [Google Scholar]
- Corea G, Fattorusso E, Lanzotti V. Saponins and flavonoids of Allium triquetrum. J Nat Prod. 2003;66(11):1405–1411. doi: 10.1021/np030226q. [DOI] [PubMed] [Google Scholar]
- Corea G, Fattorusso E, Lanzotti V, et al. Antispasmodic saponins from bulbs of red onion, Allium cepa L. var. tropea. J Agric Food Chem. 2005;53:935–940. doi: 10.1021/jf048404o. [DOI] [PubMed] [Google Scholar]
- Do JC, Jung KY, Son KH. Steroidal saponins from subterranean part of Alliumfistulosum. J Nat Prod. 1992;55:168–172. doi: 10.1021/np50080a003. [DOI] [Google Scholar]
- Eristavi LI, Gorovits MB, Abubakirov NK. Steroid sapogenins of Allium waldsteinii. Chem Nat Comp. 1973;9:124. doi: 10.1007/BF00580922. [DOI] [Google Scholar]
- Fattorusso E, Lanzotti V, Magno S, et al. Sapogenins of Allium porrum L. J Agric Food Chem. 1998;46:4904–4908. doi: 10.1021/jf980849n. [DOI] [Google Scholar]
- Fattorusso E, Lanzotti V, Taglialatela-Scafati O, et al. Cytotoxic saponins from bulbs of Allium porrum L. J Agric Food Chem. 2000;48(8):3455–3462. doi: 10.1021/jf000331v. [DOI] [PubMed] [Google Scholar]
- Fattorusso E, Iorizzi M, Lanzotti V. Chemical composition of shallot (Allium ascalonicum Hort.) J Agric Food Chem. 2002;50(20):5686–5690. doi: 10.1021/jf020396t. [DOI] [PubMed] [Google Scholar]
- Gorovits MB, Khristulas FS, Abubakirov NK. Alliogenin and alliogenin β-D-glucopyranoside from Allium giganteum. Chem Nat Comp. 1971;7(4):412–417. doi: 10.1007/BF00564727. [DOI] [Google Scholar]
- Gorovits MB, Khristulas FS, Abubakirov NK. Steroid saponins and sapogenins of Allium IV. Karatavigenin—a new sapogenin from Allium karataviense. Chem Nat Comp. 1973;9(6):715–717. doi: 10.1007/BF00565793. [DOI] [Google Scholar]
- Güçlü-Üstündağ Ö, Mazza G. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr. 2007;47(3):231–258. doi: 10.1080/10408390600698197. [DOI] [PubMed] [Google Scholar]
- Gugunishvili DN, Eristavi LI, Gvazava LN, et al. Steroidal saponins from Allium waldsteinii. Chem Nat Comp. 2006;42(4):499–500. doi: 10.1007/s10600-006-0194-3. [DOI] [Google Scholar]
- Harmatha J, Mauchamp B, Arnault C, et al. Identification of a spirostane-type saponin in the flowers of leek with inhibitory effects on growth of leek-moth larvae. Biochem Syst Ecol. 1987;15(1):113–116. doi: 10.1016/0305-1978(87)90089-5. [DOI] [Google Scholar]
- He X, Qiu F, Shoyama Y, et al. Two new steroidal saponins from “Gualou-xiebai-baijiu-tang” consisting of Fructus Trichosanthis and Bulbus Allii macrostemi. Chem Pharm Bull. 2002;50(5):653–655. doi: 10.1248/cpb.50.653. [DOI] [PubMed] [Google Scholar]
- Hu G, Mao R, Ma Z. A new steroidal saponin from the seeds of Allium tuberosum. Food Chem. 2009;113:1066–1068. doi: 10.1016/j.foodchem.2008.08.061. [DOI] [Google Scholar]
- Hu G, Lu Y, Yu W, et al. A steroidal saponin from the seeds of Allium tuberosum. Chem Nat Comp. 2014;49(6):1082–1086. doi: 10.1007/s10600-014-0825-z. [DOI] [Google Scholar]
- Ikeda T, Tsumagari H, Nohara T. Steroidal oligoglycosides from the seeds of Allium tuberosum. Chem Pharm Bull. 2000;48(3):362–365. doi: 10.1248/cpb.48.362. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Tsumagari H, Okawa M, et al. Pregnane- and furostane-type oligoglycosides from the seeds of Allium tuberosum. Chem Pharm Bull. 2004;52:142–145. doi: 10.1248/cpb.52.142. [DOI] [PubMed] [Google Scholar]
- Inoue T, Mimaki Y, Sashida Y, et al. Steroidal glycosides from Allium macleanii and A. senescens, and their inhibitory activity on tumor promoter-induced phospholipid metabolism of HeLa cells. Phytochemistry. 1995;40(2):521–525. doi: 10.1016/0031-9422(95)00223-T. [DOI] [PubMed] [Google Scholar]
- Ismailov AI, Aliev AM. Determination of steroid saponins in Allium albiflorus of Azerbaijan (Russian) Farmatsiya. 1976;25(2):17–20. [PubMed] [Google Scholar]
- Ismailov AI, Tagiev SA, Rasulov EM. Steroidal saponins and sapogenins from Alliumrubellum and A. albanm. Khim Prir Soedin. 1976;4:550–551. [Google Scholar]
- Itakura Y, Ichikawa M, Mori Y, et al. How to distinguish garlic from the other Allium vegetables. J Nutr. 2001;131(3):963S–967S. doi: 10.1093/jn/131.3.963S. [DOI] [PubMed] [Google Scholar]
- Jabrane A, Ben Jannet H, Miyamoto T, et al. Spirostane and cholestane glycosides from the bulbs of Allium nigrum L. Food Chem. 2011;125(2):447–455. doi: 10.1016/j.foodchem.2010.09.028. [DOI] [Google Scholar]
- Jiang Y, Wang N-L, Yao X-S, et al. Structural elucidation of the anticoagulation and anticancer constituents from Allium chinense. Yao Xue Xue Bao. 1998;33(5):355–361. [PubMed] [Google Scholar]
- Jiang Y, Wang N-L, Yao X-S, et al. Steroidal saponins from the bulbs of Allium chinense. Stud Plant Sci. 1999;6(C):212–219. doi: 10.1016/S0928-3420(99)80029-9. [DOI] [Google Scholar]
- Jung K-Y, Do J-C, Son K-H. The structures of two diosgenin glycosides isolated from the subterranean parts of Allium fistulosum. J Korean Soc Food Nutr. 1993;22(3):313–316. [Google Scholar]
- Kang L-P, Liu Z-J, Zhang L, et al. New furostanol saponins from Allium ascalonicum L. Magn Reson Chem. 2007;45:725–733. doi: 10.1002/mrc.2037. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Mimaki Y, Sashida Y. Steroidal saponins from Allium giganteum and A. aflatunense. Phytochemistry. 1991;30(9):3063–3067. doi: 10.1016/S0031-9422(00)98253-0. [DOI] [Google Scholar]
- Kawashima K, Mimaki Y, Sashida Y. Schubertosides A-D, new (22S)-hydroxycholestane glycosides from Allium schubertii. Chem Pharm Bull. 1991;39:2761–2763. doi: 10.1248/cpb.39.2761. [DOI] [Google Scholar]
- Kawashima K, Mimaki Y, Sashida Y. Steroidal saponins from the bulbs of Allium schubertii. Phytochemistry. 1993;32(5):1267–1272. doi: 10.1016/S0031-9422(00)95103-3. [DOI] [PubMed] [Google Scholar]
- Kelginbaev AN, Gorovits MB, Khamidkhodzbaev SA, et al. Steroid saponins of Allium V. Neoagigenin from Allium giganteum. Chem Nat Comp. 1973;9:416. doi: 10.1007/BF00565719. [DOI] [Google Scholar]
- Kelginbaev AN, Gorovits MB, Abubakirov NK. Steroid saponins and sapogenins of Allium VII. The structure of neoagigenin and agigenin. Chem Nat Comp. 1974;10:829–830. doi: 10.1007/BF00564025. [DOI] [Google Scholar]
- Kelginbaev AN, Gorovits MB, Abubakirov NK. Steroid saponins and sapogenins of Allium VIII. Structure of gantogenin. Chem Nat Comp. 1975;11(4):546–547. doi: 10.1007/BF00566817. [DOI] [Google Scholar]
- Kelginbaev AN, Gorovits MB, Gorovits TT, et al. Steroid saponins and sapogenins of Allium IX. The structure of aginoside. Chem Nat Comp. 1976;12(4):422–427. doi: 10.1007/BF00564801. [DOI] [Google Scholar]
- Kereselidze EV, Pkheidze TA, Kemertelidze EP. Diosgenin from Allium albidum. Khim Prir Soedin. 1970;6(3):378. [Google Scholar]
- Khristulas FS, Gorovits MB, Luchanskaya VN, et al. A new steroid sapogenin from Allium giganteum. Khim Prir Soedin. 1970;6(4):489. [Google Scholar]
- Khristulas FS, Gorovits MB, Abubakirov NK. Steroid saponins and sapogenins of Allium VI. The 3-O-β-D-glucopyranoside of karatvigenin B. Chem Nat Comp. 1974;10(4):544–545. doi: 10.1007/BF00563845. [DOI] [Google Scholar]
- Kim CM, Son KH, Kim SH, et al. Steroidal sapogenin content in some domestic plants. Arch Pharm Res. 1991;14(4):305–310. doi: 10.1007/BF02876875. [DOI] [Google Scholar]
- Kintya PK, Degtyareva LP. Steroid glycosides of garden onion seeds. Structure of ceposide D. Chem Nat Comp. 1989;25:124–125. doi: 10.1007/BF00596720. [DOI] [Google Scholar]
- Kravets SD. Steroids of the spirostan and furostan series from plants of the genus Allium. XXVIII. Alliogenone, anzurogenin D and karataviosides A and B from Allium suvorovii and A.stipitatum. Chem Nat Comp. 1994;30(3):408–409. doi: 10.1007/BF00629988. [DOI] [Google Scholar]
- Kravets SD, Vollerner YS, Gorovits MB, et al. Steroids of the spirostan and furostan series from plants of the genus Allium XXI. Structure of alliospiroside A and alliofuroside A from Allium cepa. Chem Nat Comp. 1986;22(2):174–181. [Google Scholar]
- Kravets SD, Vollerner YS, Gorovits MB, et al. Steroids of the spirostan and furostan series from plants of the genus Allium XXII. The structure of alliospiroside B from Allium cepa. Chem Nat Comp. 1986;22(5):553–556. doi: 10.1007/BF00599259. [DOI] [Google Scholar]
- Kravets SD, Vollerner YS, Shashkov AS, et al. Steroids of the spirostan and furostan series from plants of the genus Allium XXIII. Structure of cepagenin and of alliospirosides C and D from Allium cepa. Chem Nat Comp. 1987;23(6):700–706. doi: 10.1007/BF00596646. [DOI] [Google Scholar]
- Kravets SD, Vollerner YS, Gorovits MB, et al. Steroids of the spirostan and furostan series from plants of the genus Allium. Chem Nat Comp. 1990;26(4):359–373. doi: 10.1007/BF00598985. [DOI] [Google Scholar]
- Krokhmalyuk VV, Kintya PK. Steroid saponins XIII. The structure of alliumosides D and E from Allium narcissiflorum. Chem Nat Comp. 1976;12(2):165–168. doi: 10.1007/BF00566337. [DOI] [Google Scholar]
- Krokhmalyuk VV, Kintya PK. Steroid saponins X. Glycosides of Allium narcissiflorum. The structure of glycosides A and B. Chem Nat Comp. 1976;12(1):46–48. doi: 10.1007/BF00570179. [DOI] [Google Scholar]
- Kuroda M, Mimaki Y, Kameyama A, et al. Steroidal saponins from Allium chinense and their inhibitory activities on cyclic AMP phosphodiesterase and Na+K+ ATPase. Phytochemistry. 1995;40(4):1071–1076. doi: 10.1016/0031-9422(95)00423-5. [DOI] [PubMed] [Google Scholar]
- Lai W, Wu Z, Lin H, et al. Anti-ischemia steroidal saponins from the seeds of Allium fistulosum. J Nat Prod. 2010;73:1053–1057. doi: 10.1021/np900815p. [DOI] [PubMed] [Google Scholar]
- Lai W, Yang YB, Li X, et al. New steroidal sapogenins from the acid hydrolysis product of the whole glycoside mixture of Welsh onion seeds. Chin Chem Lett. 2012;23(2):193–196. doi: 10.1016/j.cclet.2011.11.014. [DOI] [Google Scholar]
- Lanzotti V. Bioactive saponins from Allium and Aster plants. Phytochem Rev. 2005;4:95–110. doi: 10.1007/s11101-005-1254-1. [DOI] [Google Scholar]
- Lanzotti V. Bioactive polar natural compounds from garlic and onions. Phytochem Rev. 2012;11(2–3):179–196. doi: 10.1007/s11101-012-9247-3. [DOI] [Google Scholar]
- Lanzotti V, Barile E, Antignani V, et al. Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera. Phytochemistry. 2012;78:126–134. doi: 10.1016/j.phytochem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Lanzotti V, Romano A, Lanzuise S, et al. Antifungal saponins from bulbs of white onion, Allium cepa L. Phytochemistry. 2012;74:133–139. doi: 10.1016/j.phytochem.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Lanzotti V, Scala F, Bonanomi G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem Rev. 2014 [Google Scholar]
- Lazurevski GV, Krokhmalyuk VV, Kintya PK. Structure of steroidal glycosides of Alliumnarcissiflorum. Dokl Akad Nauk SSSR. 1975;221:744. [PubMed] [Google Scholar]
- Lee KT, Choi JH, Kim DH, et al. Constituents and the antitumor principle of Allium victorialis var. platyphyllum. Arch Pharm Res. 2001;24(1):44–50. doi: 10.1007/BF02976492. [DOI] [PubMed] [Google Scholar]
- Li Q-Q, Zhou S-D, He X-J, et al. Phylogeny and biogeography of Allium (Amaryllidaceae: Allieae) based on nuclear ribosomal internal transcribed spacer and chloroplast rps16 sequences, focusing on the inclusion of species endemic to China. Ann Bot. 2010;106:709–733. doi: 10.1093/aob/mcq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Luo J, Kong L. Preparative isolation of steroidal saponins from garlic (Allium sativum L.) using high-speed counter-current chromatography coupled with evaporative light scattering detection. J Liq Chromatogr Relat Technol. 2011;34:1997–2007. doi: 10.1080/10826076.2011.582911. [DOI] [Google Scholar]
- Maisashvili MR, Eristavi LI, Gvazava LN, et al. Steroidal sapogenins from Allium rotundum. Chem Nat Comp. 2007;43(6):756–757. doi: 10.1007/s10600-007-0259-y. [DOI] [Google Scholar]
- Maisashvili MR, Kuchukhidze DK, Gvazava LN, et al. Steroidal glycosides from Allium rotundum. Chem Nat Comp. 2008;44(4):545–547. doi: 10.1007/s10600-008-9116-x. [DOI] [Google Scholar]
- Maisashvili MR, Kuchukhidze DK, Kikoladze VS, et al. Steroidal glycosides of gitogenin from Allium rotundum. Chem Nat Comp. 2012;48(1):86–90. doi: 10.1007/s10600-012-0164-x. [DOI] [Google Scholar]
- Matsuura H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr. 2001;131(3):1000S–1005S. doi: 10.1093/jn/131.3.1000S. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Ushiroguchi T, Itakura Y, et al. A furostanol glycoside from garlic, bulbs of Allium sativum L. Chem Pharm Bull. 1988;36(9):3659–3663. doi: 10.1248/cpb.36.3659. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Ushiroguchi T, Itakura Y, et al. Further studies on steroidal glycosides from bulbs, roots and leaves of Allium sativum L. Chem Pharm Bull. 1989;37(10):2741–2743. doi: 10.1248/cpb.37.2741. [DOI] [Google Scholar]
- Matsuura H, Ushiroguchi T, Itakura Y, et al. A furostanol glycoside from Allium chinense G. Chem Pharm Bull. 1989;37(5):1390–1391. doi: 10.1248/cpb.37.1390. [DOI] [Google Scholar]
- Mimaki Y, Kawashima K, Kanmoto T, et al. Steroidal glycosides from Allium albopilosum and Allium ostrovskianum. Phytochemistry. 1993;34(3):799–805. doi: 10.1016/0031-9422(93)85362-U. [DOI] [PubMed] [Google Scholar]
- Mimaki Y, Nikaido T, Matsumoto K, et al. New steroidal saponins from the bulbs of Allium giganteum exhibiting potent inhibition of cAMP phosphodiesterase activity. Chem Pharm Bull. 1994;42(3):710–714. doi: 10.1248/cpb.42.710. [DOI] [PubMed] [Google Scholar]
- Mimaki Y, Satou T, Ohmura M, et al. Steroidal saponins from the bulbs of Allium narcissiflorum. Nat Med. 1996;50(4):308. [Google Scholar]
- Mimaki Y, Kuroda M, Fukasawa T, et al. Steroidal glycosides from the bulbs of Allium jesdianum. J Nat Prod. 1999;62(1):194–197. doi: 10.1021/np980346b. [DOI] [PubMed] [Google Scholar]
- Mimaki Y, Kuroda M, Sashida Y. Steroidal saponins from the bulbs of Allium aflatunense. Nat Med. 1999;53(2):88–93. doi: 10.1248/cpb.47.738. [DOI] [PubMed] [Google Scholar]
- Mimaki Y, Kuroda M, Fukasawa T, et al. Steroidal saponins from the bulbs of Allium karataviense. Chem Pharm Bull. 1999;47(6):738–743. doi: 10.1248/cpb.47.738. [DOI] [PubMed] [Google Scholar]
- Mimaki Y, Yokosuka A, Kuroda M, et al. Cytotoxic activities and structure–cytotoxic relationships of steroidal saponins. Biol Pharm Bull. 2001;24(11):1286–1289. doi: 10.1248/bpb.24.1286. [DOI] [PubMed] [Google Scholar]
- Mirsalikhova NM, Kravets SS, Sokolova SF, et al. Inhibition of highly purified porcine kidney Na, K-ATPase by steroid glycosides of the spirostan and furostan series and a study of structure–activity relationships. Chem Nat Comp. 1993;29(4):490–497. doi: 10.1007/BF00630576. [DOI] [Google Scholar]
- Morita T, Ushiroguchi T, Hayashi N, et al. Steroidal saponins from elephant garlic, bulbs of Allium ampeloprasum. Chem Pharm Bull. 1988;36(9):3480–3486. doi: 10.1248/cpb.36.3480. [DOI] [PubMed] [Google Scholar]
- Mostafa A, Sudisha J, El-Sayed M, et al. Aginoside saponin, a potent antifungal compound, and secondary metabolite analyses from Allium nigrum L. Phytochem Lett. 2013;6(2):274–280. doi: 10.1016/j.phytol.2013.03.001. [DOI] [Google Scholar]
- Mskhiladze L, Kutchukhidze J, Chincharadze D, et al. In vitro antifungal and antileishmanial activities of steroidal saponins from Allium leucanthum C.Koch a Caucasian endemic species. Georgian Med News. 2008;1(154):39–43. [PubMed] [Google Scholar]
- Mskhiladze L, Legalut J, Lavoie S, et al. Cytotoxic Steroidal Saponins from the Flowers of Allium leucanthum. Molecules. 2008;13:2925–2934. doi: 10.3390/molecules13122925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi T, Akahori A, Yasuda F, et al. Steroidal sapogenins of sixteen Liliaceae plants. Chem Pharm Bull. 1975;23(3):575–579. doi: 10.1248/cpb.23.575. [DOI] [Google Scholar]
- Ou W-C, Chen H-F, Zhong Y, et al. Inhibition of platelet activation and aggregation by furostanol saponins isolated from the bulbs of Allium macrostemon Bunge. Am J Med Sci. 2012;344(4):261–267. doi: 10.1097/MAJ.0b013e31823ea9f0. [DOI] [PubMed] [Google Scholar]
- Peng J, Wu Y, Yao XS, et al. Two new steroidal saponins from Alliummacrostemon. Yao Xue Xue Bao. 1992;27(12):918–922. [PubMed] [Google Scholar]
- Peng J-P, Wang X, Yao XS. Studies on two new furostanol glycosides from Alliummacrostemon Bunge. Yao Xue Xue Bao. 1993;28(7):526–531. [PubMed] [Google Scholar]
- Peng J-P, Yao X, Okada Y, et al. Further studies on new furostanol saponins from the bulbs of Allium macrostemon. Chem Pharm Bull. 1994;42(10):2180–2182. doi: 10.1248/cpb.42.2180. [DOI] [PubMed] [Google Scholar]
- Peng J-P, Yao X, Kobayashi H, et al. Novel furostanol glycosides from Alliummacrostemon. Planta Med. 1995;61(1):58–61. doi: 10.1055/s-2006-958000. [DOI] [PubMed] [Google Scholar]
- Peng J-P, Chen H, Qiao Y-Q, et al. Two new steroidal saponins from Allium sativum and their inhibitory effects on blood coagulability. Yao Xue Xue Bao. 1996;31(8):607–612. [PubMed] [Google Scholar]
- Peng J-P, Yao X-S, Tezuka Y, et al. Furostanol glycosides from bulbs of Allium chinense. Phytochemistry. 1996;41(1):283–285. doi: 10.1016/0031-9422(95)00553-6. [DOI] [PubMed] [Google Scholar]
- Peng J-P, Yao X-S, Tezuka Y, et al. New furostanol glycosides, chinenoside IV and V, from Allium chinense. Planta Med. 1996;62(5):456–468. doi: 10.1055/s-2006-957941. [DOI] [PubMed] [Google Scholar]
- Pirtskhalava GV, Gorovits MB, Abubakirov NK. Steroid saponins and sapogenins of Allium X. Neoagigenin 6-O-benzoate from Allium turcomanicum. Chem Nat Comp. 1977;13(4):446–448. doi: 10.1007/BF00565833. [DOI] [Google Scholar]
- Pirtskhalava GV, Gorovits MB, Abubakirov NK. Steroid saponins and sapogenins of Allium XI. Neoalliogenin from Allium turcomanicum. Chem Nat Comp. 1977;13(6):693–695. doi: 10.1007/BF00565523. [DOI] [Google Scholar]
- Pirtskhalava GV, Gorovits MB, Gorovits TT, et al. Steroid saponins and sapogenins of Allium XII Turoside A from Allium turcomanicum. Chem Nat Comp. 1978;14(3):294–298. doi: 10.1007/BF00713319. [DOI] [Google Scholar]
- Pirtskhalava GV, Gorovits MB, Abubakirov NK. Steroid saponins and sapogenins of Allium XIII: Turoside A 6-O-benzoate from Allium turcomanicum. Chem Nat Comp. 1979;14(4):459–460. doi: 10.1007/BF00565276. [DOI] [Google Scholar]
- Pirtskhalava GV, Gorovits MB, Gorovits TT, et al. Steroid saponins and sapogenins of Allium. XVI. Turoside C from Allium turcomanicum. Chem Nat Comp. 1979;15(4):446–452. doi: 10.1007/BF00565043. [DOI] [Google Scholar]
- Pkheidze TA, Kereselidze EV, Kemertelidze EP. Diosgenin, neoruscogenin, and ruscogenin from Ruscus ponticus, R. hypophyllum, and Allium albidum. Chem Nat Comp. 1971;7(6):823. doi: 10.1007/BF00567962. [DOI] [Google Scholar]
- Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev. 2010;9:425–474. doi: 10.1007/s11101-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Qiao HX, Yang J, et al. Protective effects of steroids from Allium chinense against H2O2-induced oxidative stress in rat cardiac H9C2 cells. Phytother Res. 2010;24:404–409. doi: 10.1002/ptr.2964. [DOI] [PubMed] [Google Scholar]
- Rezgui A, Mitaine-Offer A-C, Paululat T, et al. Cytotoxic steroidal glycosides from Allium flavum. Fitoterapia. 2014;93:121–125. doi: 10.1016/j.fitote.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Rose P, Whiteman M, Moore PK, et al. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potential therapeutic agents. Nat Prod Rep. 2005;22:351–368. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Zolfaghari B, Senatore M, et al. Spirostane, furostane and cholestane saponins from Persian leek with antifungal activity. Food Chem. 2013;141(2):1512–1521. doi: 10.1016/j.foodchem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Sang S, Lao A, Wang H, et al. Two new spirostanol saponins from Allium tuberosum. J Nat Prod. 1999;62(7):1028–1029. doi: 10.1021/np980486l. [DOI] [PubMed] [Google Scholar]
- Sang S, Lao A, Wang H, et al. Furostanol saponins from Allium tuberosum. Phytochemistry. 1999;52:1611–1615. doi: 10.1016/S0031-9422(99)00312-X. [DOI] [Google Scholar]
- Sang SM, Xia ZH, Mao SL, et al. Studies on chemical constituents in seeds of Allium tuberosum Rottl. Zhongguo Zhong Yao Za Zhi. 2000;25(5):286–288. [PubMed] [Google Scholar]
- Sang SM, Zou M, Xia Z, et al. New spirostanol saponins from Chiense chives (Allium tuberosum) J Agric Food Chem. 2001;49(10):4780–4783. doi: 10.1021/jf010529v. [DOI] [PubMed] [Google Scholar]
- Sang SM, Mao S, Lao A, et al. Four new steroidal saponins from the seeds of Allium tuberosum. J Agric Food Chem. 2001;49(3):1475–1478. doi: 10.1021/jf001062b. [DOI] [PubMed] [Google Scholar]
- Sang SM, Zou ML, Zhang XW, et al. Tuberoside M, a new cytotoxic spirostanol saponin from the seeds of Allium tuberosum. J Asian Nat Prod Res. 2002;4(1):69–72. doi: 10.1080/10286020290019721. [DOI] [PubMed] [Google Scholar]
- Sang SM, Mao S, Lao A, et al. New steroid saponins from the seeds of Allium tuberosum L. Food Chem. 2003;83(4):499–506. doi: 10.1016/S0308-8146(03)00131-6. [DOI] [PubMed] [Google Scholar]
- Sashida Y, Kawashima K, Mimaki Y. Novel polyhydroxylated steroidal saponins from Allium giganteum. Chem Pharm Bull. 1991;39(3):698–703. doi: 10.1248/cpb.39.698. [DOI] [Google Scholar]
- Sata N, Matsunaga S, Fusetani N, et al. New antifungal and cytotoxic steroidal saponins from the bulbs of an elephant garlic mutant. Biosci Biotechnol Biochem. 1998;62(10):1904–1911. doi: 10.1271/bbb.62.1904. [DOI] [PubMed] [Google Scholar]
- Sobolewska D (2004) Struktura oraz aktywność biologiczna glikozydów steroidowych izolowanych z czosnku niedźwiedziego Allium ursinum L. Doctoral dissertation, Jagiellonian University
- Sobolewska D, Janeczko Z, Galanty A et al (2003) Cytotoxic, antifungal and antibacterial activity of spirostanol saponin from ramson Allium ursinum L. In Abstracts of the 3rd international symposium on natural drugs, University of Naples Federico II, Naples, 2–4 Oct 2003
- Sobolewska D, Janeczko Z, Kisiel W, et al. Steroidal glycosides from the underground parts of Allium ursinum L. and their cytostatic and antimicrobial activity. Acta Pol Pharm. 2006;63(3):219–223. [PubMed] [Google Scholar]
- Sobolewska D, Janeczko Z, Podolak I, et al. Densitometric analysis of diosgenin in methanolic extracts of Allium ursinum collected at different times during plant development. J Planar Chromatogr Mod TLC. 2009;22(4):305–307. doi: 10.1556/JPC.22.2009.4.13. [DOI] [Google Scholar]
- Sohn H-Y, Kum E-J, Ryu H-Y, et al. Antifungal activity of fistulosides, steroidal saponins from Allium fistulosum L. J Life Sci. 2006;16(2):310–314. doi: 10.5352/JLS.2006.16.2.310. [DOI] [Google Scholar]
- Sparg SG, Light ME, Van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004;94:219–243. doi: 10.1016/j.jep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Štajner D, Milić N, Čanadanović-Brunet J, et al. Exploring Allium species as a source of potential medicinal agents. Phytother Res. 2006;20:581–584. doi: 10.1002/ptr.1917. [DOI] [PubMed] [Google Scholar]
- Teshima Y, Ikeda T, Imada K, et al. Identification and biological activity of antifungal saponins from shallot (Allium cepa L. Aggregatum group) J Agric Food Chem. 2013;61(31):7440–7445. doi: 10.1021/jf401720q. [DOI] [PubMed] [Google Scholar]
- Timité G, Mitaine-Offer AC, Miyamoto T, et al. Structure and cytotoxicity of steroidal glycosides from Allium schoenoprasum. Phytochemistry. 2013;88:61–66. doi: 10.1016/j.phytochem.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Tolkacheva NV, Shashkov AS, Chirva VY. Steroidal glycosides from Allium cyrillii bulbs. Chem Nat Comp. 2012;48(2):272–275. doi: 10.1007/s10600-012-0219-z. [DOI] [Google Scholar]
- Uchida A, Tao K, Ogihara J, et al. Antihepatopathic activity of foam components produced from the bulb of jumbo leek and isolation of its active saponin. Nippon Shokuhin Kagaku Kogaku Kaishi. 2009;56(12):639–646. doi: 10.3136/nskkk.56.639. [DOI] [Google Scholar]
- Vollerner YS, Gorovits MB, Gorovits TT, et al. Steroid saponins and sapogenins of Allium XIV. Structure of karatavioside A. Chem Nat Comp. 1978;14:630. doi: 10.1007/BF00937615. [DOI] [Google Scholar]
- Vollerner YS, Gorovits MB, Gorovits TT, et al. Steroid saponins and sapogenins of Allium XVII. The structure of karatavioside C. Chem Nat Comp. 1980;16:264–268. doi: 10.1007/BF00567288. [DOI] [Google Scholar]
- Vollerner YS, Abdullaev ND, Gorovits MB, et al. Steroid saponins and sapogenins of the genus Allium XVIII. The structure of karatavioside B. Khim Prirod Soedin. 1983;2:197–201. [Google Scholar]
- Vollerner YS, Abdullaev ND, Gorovits MB, et al. Steroid saponins and sapogenins of Allium XIX The structure of karatavigenin C. Chem Nat Comp. 1983;19(6):699–703. doi: 10.1007/BF00575173. [DOI] [Google Scholar]
- Vollerner YS, Abdullaev ND, Gorovits MB, et al. Steroid saponins and sapogenins of Allium XX. Structure of karataviosides E and F. Chem Nat Comp. 1984;20:64–68. doi: 10.1007/BF00574793. [DOI] [Google Scholar]
- Vollerner YS, Kravets SD, Shaskov AS, et al. Steroids of the spirostan and furostan series from plants of the genus Allium. Structure of anzurogenin A from Allium suvorovii and A.stipitatum. Chem Nat Comp. 1988;24(1):58–62. doi: 10.1007/BF00597575. [DOI] [Google Scholar]
- Vollerner YS, Kravets SD, Shashkov AS, et al. Steroids of the spirostan and furostan series from plants of the genus Allium XXV. Structure of anzurogenin B from Allium suvorovii and Allium stipitatum. Chem Nat Comp. 1988;24(2):183–186. doi: 10.1007/BF00596746. [DOI] [Google Scholar]
- Vollerner YS, Kravets SD, Shaskov AS, et al. Steroids of the spirostan and furostan series from plants of the genus Allium XXVI. Structure of anzurogenin and anzuroside from the collective fruits of Allium suvorovii and Alliumstipitatum. Chem Nat Comp. 1989;25:431–435. doi: 10.1007/BF00597651. [DOI] [Google Scholar]
- Vollerner YS, Kravets SD, Shashkov AS, et al. Steroids of the spirostan and furostan series from plants of the genus Allium XXVII. Alliosterol and allosides A and B from Allium suvorovii and Allium stipitatum—structural analogs of furostanols. Chem Nat Comp. 1991;27(2):198–207. doi: 10.1007/BF00629759. [DOI] [Google Scholar]
- Xie W, Zhang Y, Wang N, et al. Novel effects of macrostemonoside A, a compound from Allium macrostemon Bunge, on hyperglycemia, hyperlipidemia, and visceral obesity in high-fat diet-fed C57BL/6 mice. Europ J Pharamcol. 2008;599:159–165. doi: 10.1016/j.ejphar.2008.09.042. [DOI] [PubMed] [Google Scholar]
- Yu Z-H, Ding X-Z, Xia L-Q, et al. Antimicrobial activity and mechanism of total saponins from Allium chinense. Food Sci. 2013;34(15):75–80. [Google Scholar]
- Yuan L, Ji TF, Wang AG, et al. Two new furostanol saponins from the seeds of Allium cepa L. Chin Chem Lett. 2008;19:461–464. doi: 10.1016/j.cclet.2008.02.005. [DOI] [Google Scholar]
- Yuan L, Ji TF, Li CJ, et al. Two new steroidal saponins from the seeds of Allium cepa L. J Asian Nat Prod Res. 2009;11(3):213–218. doi: 10.1080/10286020802696411. [DOI] [PubMed] [Google Scholar]
- Zhou H, Yang X, Wang N-L, et al. Macrostemonoside A promotes visfatin expression in 3T3-Li cells. Biol Pharm Bull. 2007;30(2):279–283. doi: 10.1248/bpb.30.279. [DOI] [PubMed] [Google Scholar]
- Zolfaghari B, Barile E, Capasso R, et al. The sapogenin atroviolcegenin and its diglycoside atroviolaceoside from Allium atroviolaceum. J Nat Prod. 2006;69:191–195. doi: 10.1021/np0503350. [DOI] [PubMed] [Google Scholar]
- Zolfaghari B, Sadeghi M, Troiano R, et al. Vavilosides A1/A2-B1/B2, new furostane glycosides from the bulbs of Allium vavilovii with cytotoxic activity. Bioorg Med Chem. 2013;21(7):1905–1910. doi: 10.1016/j.bmc.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Zou Z-M, Yu D-Q, Yu D-Q, et al. A steroidal saponin from the seeds of Allium tuberosum. Phytochemistry. 2001;57:1219–1222. doi: 10.1016/S0031-9422(01)00216-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.