Abstract
Background
Despite the widespread use of noninvasive testing prior to invasive coronary diagnostic the diagnostic yield of elective coronary angiography has been reported low in subjects with suspected obstructive CAD.
Objective
To determine the predictive value of noncoronary atherosclerosis (NCA) in subjects with suspected stable coronary artery disease (CAD) intended to invasive coronary angiography.
Methods
Ultrasound-based assessment of carotid artery plaque (CAP), carotid intima-media thickness (CIMT) and ankle-brachial index (ABI) was performed in 2216 subjects with suspected CAD prior to coronary angiography. Logistic regression and c-statistics were used to analyze the diagnostic value of NCA for the presence of obstructive CAD and the intention to revascularization.
Results
Percentage of positive results of elective coronary angiography was low but comparable to other studies (41 % obstructive CAD). We identified 1323 subjects (60 %) with NCA, most of them were characterized by CAP (93 %). CAP independently predicted obstructive CAD in addition to traditional risk factors and clinical factors while CIMT and ABI failed to improve the prediction. The presence of NCA and typical angina were the strongest predictors for obstructive CAD (OR 4.0 and 2.4, respectively). A large subgroup of patients (n = 703, 32 %) with atypical clinical presentation and lack of NCA revealed a low indication for revascularization <15 % indicating a large proportion of subjects with non-obstructive CAD in this subgroup.
Conclusion
The evaluation of noncoronary atherosclerosis has the potential to impact clinical decision making and to direct subsequent diagnostic procedures in subjects with suspected coronary artery disease.
Clinical trial registration
Electronic supplementary material
The online version of this article (doi:10.1007/s00392-015-0900-x) contains supplementary material, which is available to authorized users.
Keywords: Coronary artery disease; Noncoronary atherosclerosis, carotid artery plaque; Intima-media thickness; Ankle-brachial index
Background
Noninvasive testing is recommended in patients with suspected stable ischemic heart disease (SIHD) prior to invasive coronary angiography to identify subjects at risk [1, 2]. Functional or stress testing to detect inducible ischemia has been the gold standard and is the most common noninvasive test used to diagnose SIHD. However, its high predictive value shown in myriad diagnostic studies is not translated into clinical reality. In less than half of the patients intended to elective coronary angiography significant obstructive CAD is confirmed [3]. The diagnostic yield of current noninvasive testing before heart catheterization is low and patients with a positive result on a noninvasive test exhibit only a slightly higher likelihood to have obstructive CAD than those who did not undergo any testing [3].
The generalized nature of atherosclerosis is expressed in the concomitant occurrence of CAD with noncoronary atherosclerotic diseases [4]. Noninvasive imaging tests aiming at detecting noncoronary atherosclerotic disease might be helpful in addition to the clinical presentation and functional/stress testing to direct diagnostic strategies in subjects with unknown but suspected CAD.
In the last two decades, measures of noncoronary atherosclerosis [NCA, e.g., carotid artery plaque, carotid intima-media thickness (CIMT), ankle-brachial index (ABI)] became surrogate marker broadly accepted for the prediction of incident cardiovascular events [5–7]. Recent studies showed that especially ultrasound-based carotid artery plaque assessment is also predictive for prevalent CAD [8–17]. The appropriate use of CIMT and ABI testing and its impact as measures for risk assessment have been defined for several scenarios [18, 19]. The clinical impact of the ultrasound-based assessment of noncoronary atherosclerosis in subjects with suspected CAD considered for invasive cardiac catheterization has not been defined so far.
In the present study, we investigate the association of carotid artery ultrasound measures (CIMT, carotid plaque), the ankle-brachial index (ABI) and the patients history of peripheral or cerebrovascular disease with obstructive CAD as confirmed by coronary angiography in subjects with suspected CAD intended for invasive coronary diagnostic.
Methods
Study cohort
The Leipzig Heart Study is designed as an observational study to evaluate biomarkers and their ability to assess the presence and severity of CAD in subjects with suspected CAD. A detailed description of study design and baseline characteristics has been published elsewhere [20]. The study meets the ethical standards of the Declaration of Helsinki, and written informed consent was obtained from all participants.
The present study included 2552 subjects with suspected stable CAD. Patients were admitted to the Leipzig University Heart Center (2006–2011) for invasive coronary angiography due to the presence of clinical symptoms (e.g., chest pain, shortness of breath) and positive noninvasive testing (e.g., cardiopulmonary exercise test, echocardiographic, nuclear or magnetic resonance imaging). Patients with known CAD and any previous coronary revascularization in form of percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) were excluded in order to recruit only those with first onset of symptoms and untreated coronary arteries. We excluded 195 cases in which documentation of their medical history was incomplete and 141 cases in which ultrasound was not available or with insufficient quality. Finally, 2216 subjects were included into the analyses.
Risk factors and clinical presentation
Risk factors and clinical presentation were obtained from a standardized interview, a standardized biometric assessment and laboratory analyses as published before [20]. Chest pain was inquired using the WHO Rose Angina Questionnaire [21]. The classification of chest pain in nonanginal chest pain, atypical angina and typical angina was performed according to the guidelines [1]. High-sensitive C-reactive protein (hs-CRP) and n-terminal pro brain natriuretic peptide (nt-proBNP) have been shown associated with cardiovascular phenotypes and coronary events in stable subjects and were integrated in the analyses [22–25].
Evaluation of noncoronary atherosclerosis
History of peripheral vascular disease (PVD)
Information about interventions due to lower extremity artery disease (LEAD) and cerebrovascular disease (CVD) were obtained from the interview.
Carotid artery plaque
The ultrasound procedure of carotid arteries has been described in detail before [20] (brief description in supplemental material). Carotid artery plaque was defined as recommended by the American Society of Echocardiography Intima-Media Thickness Task Force [26]: echogenic thickening of intimal reflection that extends into the arterial lumen at least 0.5 mm or 50 % of the surrounding CCA-IMT value or an intimal + medial thickness of >1.5 mm. Plaque presence was documented as ‘present’ or ‘absent’ for the common part and bulb of the right and left carotid artery, respectively. A simple plaque score (PS) was calculated by counting segmental plaque presence of the common carotid artery and bulb. As the extracranial length of internal carotid artery and the quality of its imaging is variable, we restricted plaque score determination to the common part and the bulb resulting in values of 0–4. Intra- and inter-reader reliability of carotid artery plaque assessment were tested in scans of 60 subsequent subjects being read by 4 sonographers, each blinded from the other’s findings. Krippendorff’s alpha was 0.90 for intra-reader reliability and 0.65 for interreader reliability (further data in Supplemental Table 1).
Carotid intima-media thickness (CIMT)
The mean and maximum of the combined thickness of the intimal and medial layer of the far wall of the CCA were measured with a semiautomated border detection program (EchoPAC Dimension 06, GE Medical Systems, Munich, Germany). The detecting area of CIMT was defined as the distal 1 cm (about 250 single measure points) of the common carotid arteries, proximal to the origin of the bulb (Supplemental Fig. 2a–c). Measurements of the left and right sides were averaged to obtain the CIMTmean, the higher value of the left and right maximum CIMT was used to obtain CIMTmax. Intra- and inter-reader reliability of CIMT were tested in scans of 60 subsequent subjects being read by 4 sonographers, each blinded from the other’s findings. Concordance correlation coefficients (CCC) for intra-reader reliability were 0.95 (CIMTmean) and 0.91 (CIMTmax), CCC for interreader reliability were 0.90 (CIMTmean) and 0.87 (CIMTmax), further data in the Supplemental Table 1.
Ankle-brachial index (ABI)
Systolic blood pressures of the right arm and both ankles are measured twice by gold standard method Doppler ultrasound using sphygmomanometer cuffs and a handheld Doppler probe (Huntleigh Mini-Dopplex, Kempen, Germany) [27]. ABI was calculated by dividing the averaged systolic blood pressure at the posterior tibial artery by the averaged systolic blood pressure in the arm. The lower ABI of the right and left side was used for analyses. Subjects were categorized in subgroups of normal (≥1.1), low-normal (1.00–1.09), borderline (0.9–0.99), pathological (0.5–0.89) and critical (<0.5) ABI.
Coronary angiography
Coronary angiography was performed following the standards of our institution [20]. Patients were classified in subsets with (1) normal angiogram or nonobstructive CAD with luminal reduction <50 % and (2) obstructive CAD with at least one stenosis ≥50 % in a major coronary vessel. Additionally, subjects were classified with respect to subsequently induced therapeutic interventions namely conservative therapy or intention to coronary revascularization [percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)].
Statistics
Categorical data are presented as numbers or percentages and were compared using χ2 or Fisher’s exact test, as appropriate. For continuous variables, we present arithmetic mean ± standard deviation for normally distributed variables and median ± standard deviation for non-normally distributed variables. Continuous variables were compared using Student’s t test and Mann–Whitney U test, as appropriate. Odds ratios (OR) of CIMTmean, CIMTmax (per 0.1 mm), carotid artery plaque (per point of plaque score), ABI and clinical factors were obtained from logistic regression analyses, adjusted for traditional risk factors (age, gender, diabetes, tobacco use, hypertension, dyslipidemia). Stepwise forward logistic regression (Wald Chi square) was performed to identify major predictors. Receiver operating characteristic (ROC) was performed for:
Traditional risk factors (TRF),
TRF + clinical factors [clinical presentation (angina, NYHA), history of PVD, reduced left-ventricular ejection fraction <50 %, hsCRP, ntproBNP] and the incremental impact of carotid ultrasound measures to 2):
+CIMTmean,
+Carotid artery plaque,
+ABI.
Sensitivity and specificity were calculated for a positive carotid plaque test (≥1 plaque in CCA or bulb). P values <0.05 were considered statistically significant. IBM SPSS Statistics 20 was used for statistical analyses of models. The statistical software package “R” (www.r-project.org”) was used to perform concordance analyses.
Results
Baseline characteristics of the participants
Demographic and clinical characteristics of the study subjects are summarized in Table 1. The median age was 64 years (interquartile range 54–70 years); 35.3 % were female; 31.7 % were diabetics. Chest pain was the reason for cardiac diagnostic assessment in 64.2 % of participants. 25.0 % presented with typical angina and 39.2 % were classified as atypical angina or non-cardiac chest pain. The remaining subjects presented with other atypical clinical presentation but a positive result in former noninvasive testing. The distribution of the clinical presentation in our German cohort intended for invasive coronary diagnostic is comparable with the American NCDR CathPCI Registry. In this large-scale study, 33 % subjects were graded as stable angina and the remaining subjects with atypical or asymptomatic presentation [3].
Table 1.
Baseline characteristics of the study participants
| Characteristic | Total (n = 2216) | Obstructive CAD (n = 913) | No obstructive CAD (n = 1303) | P value |
|---|---|---|---|---|
| Age (years) | 64 ± 11 | 66 ± 11 | 62 ± 11 | <0.001 |
| Female sex (%) | 35.3 | 23.5 | 43.5 | <0.001 |
| Body mass index (kg/m2) | 29.2 ± 5.0 | 29.0 ± 4.6 | 29.4 ± 5.2 | 0.190 |
| Waist-hip ratio | 0.98 ± 0.08 | 1.01 ± 0.07 | 0.96 ± 0.09 | <0.001 |
| Diabetes (%) | 31.7 | 35.9 | 28.7 | <0.001 |
| Hypertension (%) | 83.3 | 84.1 | 82.7 | 0.387 |
| Dyslipidemia (%) | 47.8 | 58.3 | 41.7 | <0.001 |
| Tobacco use | ||||
| Former (%) | 38.3 | 42.4 | 35.5 | <0.001 |
| Current (%) | 17.0 | 20.4 | 14.7 | |
| Family history (%) | 32.4 | 33.1 | 31.8 | 0.553 |
| Ejection fraction <50 % | 15.3 | 19.5 | 12.3 | <0.001 |
| hsCRP (mg/l) | 2.2 ± 9.7 | 2.6 ± 11.8 | 2.0 ± 7.7 | <0.001 |
| Nt-proBNP (ng/l) | 149 ± 1255 | 226 ± 1480 | 120 ± 1056 | <0.001 |
| Medication | ||||
| ASA (%) | 50.6 | 59.5 | 44.0 | <0.001 |
| Beta blocker (%) | 57.9 | 58.4 | 57.6 | 0.783 |
| RAAS antagonist (%) | 67.6 | 69.3 | 66.3 | 0.293 |
| Calcium antagonist (%) | 24.0 | 24.5 | 23.6 | 0.717 |
| Diuretic (%) | 38.1 | 38.4 | 37.8 | 0.849 |
| Statin (%) | 36.6 | 42.7 | 32.2 | <0.001 |
| Clinical presentation | ||||
| NCP/AA/TA (%) | 15.7/23.5/25.0 | 11.0/22.0/35.0 | 19.1/24.6/18.0 | <0.001 |
| NYHA II/III (%) | 41.4/11.0 | 40.4/9.4 | 42.1/12.3 | 0.009 |
P value refers to the comparison of the obstructive CAD/no obstructive CAD groups
hsCRP high-sensitive C-reactive protein, NCP nonanginal chest pain, AA atypical angina, TA typical angina. P value refers to the comparison of the obstructive CAD/no obstructive CAD groups
Prevalence of noncoronary atherosclerosis
Findings of noncoronary atherosclerosis are summarized in Table 2. History of peripheral artery disease was reported in 4.5 % of the subjects. Carotid arterial lesions were present in 55.7 % (men 61.8 %, women 44.1 %). Carotid artery plaque more often occurs in the bulb (54.0 %) than in the common part of the carotid artery (24.0 %, P < 0.001). The majority of subjects had a normal and low-normal ABI (51.4 and 29.7 %, respectively). Significant ABI values <0.9 were found in 254 subjects (11.5 %).
Table 2.
Prevalence of noncoronary atherosclerosis
| Characteristic | Total | Obstructive CAD | No obstructive CAD | P value |
|---|---|---|---|---|
| History of atherosclerotic disease | ||||
| LEAD (%) | 3.4 | 5.9 | 1.7 | <0.001 |
| CVD (%) | 1.3 | 2.0 | 0.8 | 0.022 |
| PVD (%) | 4.5 | 7.3 | 2.5 | <0.001 |
| Presence of carotid arterial lesions | ||||
| Carotid plaque (%) | 55.7 | 73.9 | 43.0 | <0.001 |
| CPS 1/2/3/4 (%) | 16.8/19.9/10.2/8.9 | 17.9/25.1/15.7/15.2 | 16.0/16.2/6.3/4.5 | <0.001 |
| CIMTmean (mm) | 0.78 ± 0.15 | 0.82 ± 0.15 | 0.76 ± 0.14 | <0.001 |
| CIMTmax (mm) | 0.94 ± 0.20 | 0.99 ± 0.21 | 0.92 ± 0.19 | <0.001 |
| Presence of lower extremity arterial disease | ||||
| ABI ≥ 1.1 | 51.4 | 43.8 | 56.7 | <0.001 |
| 1.0–1.09 | 29.7 | 27.5 | 31.3 | |
| 0.9–0.99 | 7.4 | 9.9 | 5.7 | |
| 0.5–0.89 | 9.3 | 14.5 | 5.6 | |
| <0.5 | 2.2 | 4.4 | 0.7 | |
| Cumulative NCA (%) | 59.7 | 78.1 | 46.8 | <0.001 |
P value refers to the comparison of the obstructive CAD/no obstructive CAD groups
LEAD lower extremity artery disease, CVD cerebrovascular disease, PVD peripheral vascular disease, CPS carotid plaque score, CIMT carotid intima-media thickness, ABI ankle-brachial index, NCA noncoronary atherosclerosis
Cumulative noncoronary atherosclerotic disease was identified in 1323 subjects (59.7 %). Thereof, carotid artery plaque was present in the majority of subjects (93.3 %), the other PVD characteristics were less present (PVD in history 7.5 %, CIMTmax > 1.5 mm 2.3 %, ABI < 0.9 19.2 % of subjects with NCA).
Prevalence of coronary artery disease
Angiographically significant CAD was present in 41.2 % of the subjects (48.7 % of men, 27.5 % of women) resulting in assignment to PCI or CABG in 37.5 % of males and 20.8 % of females. The remaining subjects were free of angiographically visible CAD or showed wall irregularities <50 % luminal reduction. These findings of diagnostic angiography show high accordance with the American NCDR CathPCI Registry (41.0 % with obstructive CAD) [3].
Predictors of obstructive CAD
Known conventional risk factors higher age, male sex, diabetes, dyslipidemia and tobacco use were independently associated with obstructive coronary artery disease and revascularization. An echocardiographic reduction of the left-ventricular ejection fraction, higher levels of high-sensitive C-reactive protein and nt-pro brain natriuretic peptide were also independently associated with obstructive coronary artery disease (Table 3) and revascularization (Supplemental Table 2).
Table 3.
The value of traditional risk factors and clinical characteristics for predicting obstructive CAD
| Variable | Unadj. odds ratio (95 % CI) | P value | Adj. odds ratio (95 % CI) | P Value |
|---|---|---|---|---|
| Traditional risk factors | ||||
| Age, per 5 years increase | 1.13 (1.08–1.17) | <0.001 | ||
| Male sex | 2.50 (2.07–3.02) | <0.001 | ||
| Diabetes | 1.39 (1.16–1.66) | <0.001 | ||
| Dyslipidemia | 1.95 (1.64–2.31) | <0.001 | ||
| Hypertension | 1.11 (0.88–1.39) | 0.389 | ||
| Former tobacco use | 1.60 (1.32–1.93) | <0.001 | ||
| Current tobacco use | 1.86 (1.46–2.37) | <0.001 | ||
| Additional clinical characteristics | ||||
| Family history of CAD | 1.06 (0.87–1.30) | 0.553 | 1.19 (0.96–1.48) | 0.118 |
| LV-EF <50 % | 1.73 (1.37–2.19) | <0.001 | 1.31 (1.02–1.69) | 0.034 |
| hsCRP, per 5 mg/l | 1.11 (1.06–1.17) | <0.001 | 1.08 (1.03–1.14) | 0.004 |
| Nt-proBNP, per 500 ng/l | 1.11 (1.06–1.16) | <0.001 | 1.06 (1.01–1.10) | 0.009 |
| Clinical presentation | ||||
| Nonanginal chest pain | 0.69 (0.52–0.90) | 0.007 | 0.85 (0.64–1.14) | 0.290 |
| Atypical angina | 1.07 (0.86–1.35) | 0.543 | 1.38 (1.08–1.76) | 0.011 |
| Typical angina | 2.33 (1.86–2.91) | <0.001 | 3.09 (2.42–3.95) | <0.001 |
| Dyspnea NYHA II | 0.83 (0.68–0.99) | 0.042 | 0.90 (0.73–1.09) | 0.274 |
| NYHA III | 0.67 (0.50–0.90) | 0.008 | 0.64 (0.46–0.88) | <0.001 |
The clinical presentation of typical angina (OR 3.1) as well as atypical angina (OR 1.4) was associated with obstructive CAD and revascularization. In contrast, the classification ‘nonanginal chest pain’ and shortness of breath neither did predict obstructive CAD nor revascularization.
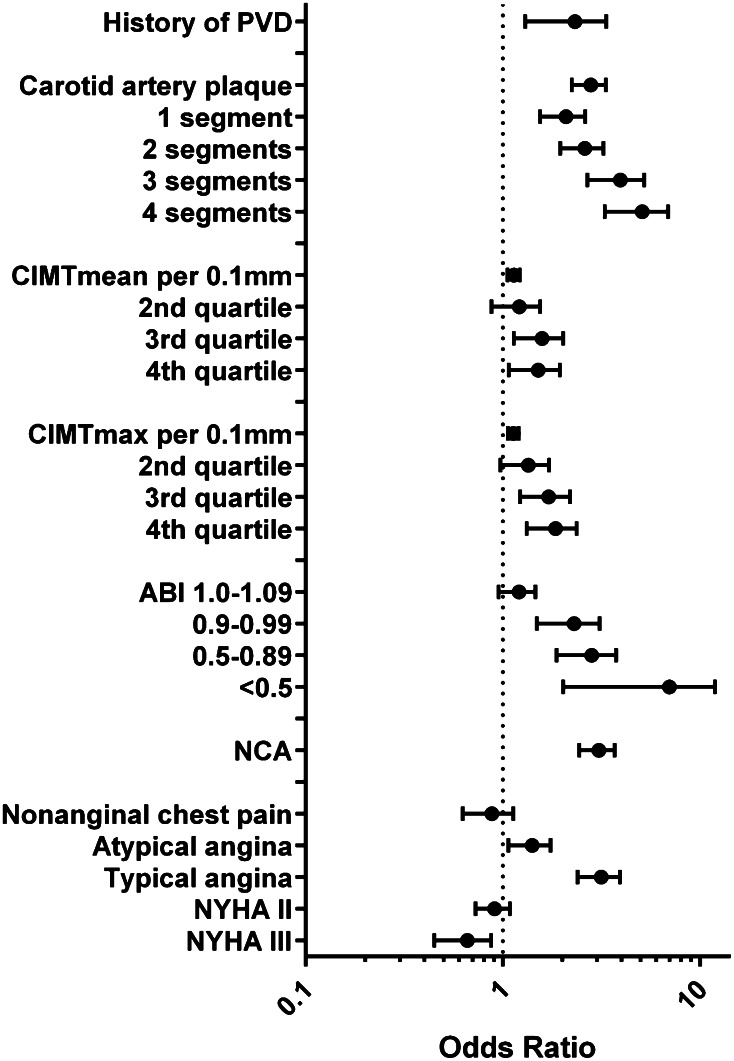
All noncoronary atherosclerotic disease characteristics were independently (i.e., after adjustment for traditional risk factors) associated with obstructive coronary artery disease (Fig. 1; Table 4) and revascularization (Supplemental Table 2). The presence of carotid artery plaque predicted obstructive CAD with OR 2.8. Multisegmental affection of the carotid arterial system linearly increased the predictive value to OR 4.9. Mean and maximum CIMT were independently associated with CAD (per 0.1 mm increase OR 1.13). ABI values <1.0 predicted obstructive CAD with OR 2.5. In summary, any noncoronary atherosclerosis predicted obstructive CAD and revascularization with OR 3.0.
Fig. 1.
Odds ratios and error bars for measures of noncoronary atherosclerosis and features of clinical presentation predicting obstructive CAD in subjects with suspected CAD
Table 4.
The value of noncoronary atherosclerosis for predicting obstructive CAD
| Variable | Unadj. odds ratio (95 % CI) | P value | Adj. odds ratio (95 % CI) | P value |
|---|---|---|---|---|
| History of PVD | 3.14 (2.05–4.83) | <0.001 | 2.17 (1.38–3.41) | 0.001 |
| Carotid artery plaque | 3.76 (3.13–4.52) | <0.001 | 2.75 (2.26–3.35) | <0.001 |
| 1 Segment | 2.43 (1.89–3.13) | <0.001 | 2.04 (1.57–2.64) | <0.001 |
| 2 Segments | 3.39 (2.67–4.30) | <0.001 | 2.54 (1.98–3.27) | <0.001 |
| 3 Segments | 5.48 (4.03–7.46) | <0.001 | 3.81 (2.75–5.27) | <0.001 |
| 4 Segments | 7.48 (5.33–10.5) | <0.001 | 4.87 (3.40–6.98) | <0.001 |
| CIMTmean per 0.1 mm | 1.31 (1.23–1.40) | <0.001 | 1.13 (1.06–1.22) | 0.001 |
| CIMTmax per 0.1 mm | 1.28 (1.21–1.35) | <0.001 | 1.13 (1.06–1.20) | <0.001 |
| ABI 1.0–1.09 | 1.14 (0.93–1.39) | 0.207 | 1.19 (0.96–1.47) | 0.111 |
| 0.9–0.99 | 2.25 (1.61–3.13) | <0.001 | 2.20 (1.54–3.14) | <0.001 |
| 0.5–0.89 | 3.34 (2.45–4.56) | <0.001 | 2.71 (1.93–3.80) | <0.001 |
| <0.5 | 8.21 (3.94–17.1) | <0.001 | 5.80 (2.71–12.4) | <0.001 |
| ABI <1.0 | 2.96 (2.37–3.69) | <0.001 | 2.48 (1.95–3.16) | <0.001 |
| NCA | 4.05 (3.35–4.90) | <0.001 | 3.02 (2.46–3.71) | <0.001 |
Diagnostic benefit of NCA testing
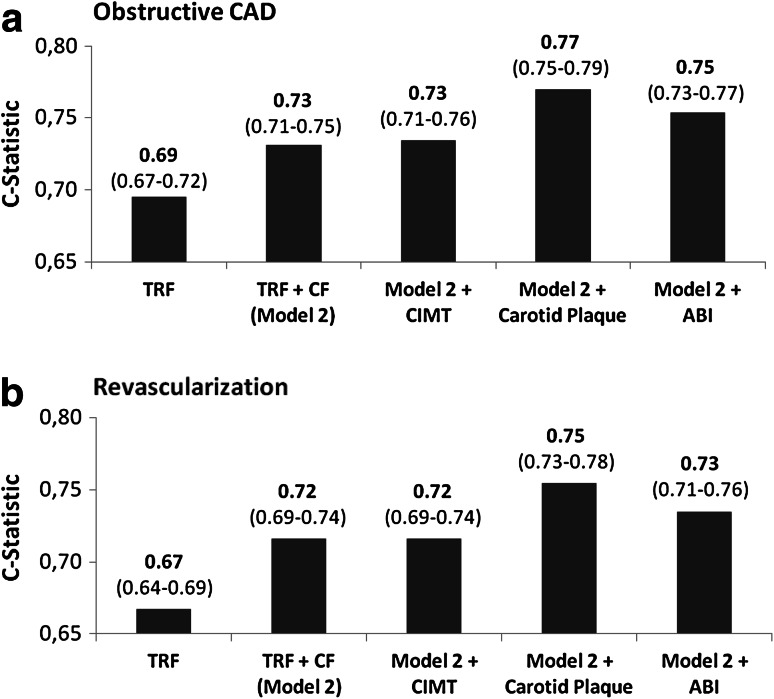
To investigate which method of NCA testing is best suited to improve diagnostic of CAD and revascularization, we analyzed C-statistics of carotid plaque, ABI and CIMT. C-statistic showed that carotid artery plaque assessment best improved the prediction of obstructive CAD and the intention for revascularization while ABI was inferior and CIMT failed to give independent impact to a model including TRF and clinical factors (CF, including angina, NYHA, history of PVD, reduced left-ventricular ejection fraction <50 %, hsCRP, ntproBNP), Fig. 2. The area under the curve (AUC) to predict obstructive CAD improved from 0.69 (TRF) and 0.73 (TRF + CF) to 0.77 (TRF + CF + carotid plaque), P < 0.001. The AUC to predict the need for revascularization improved from 0.67 (TRF) and 0.72 (TRF + CF) to 0.75 (TRF + CF + carotid plaque), P < 0.001.
Fig. 2.
Bars indicate the area under the ROC curve of various models to predict obstructive CAD (a) and coronary revascularization (b). The simplest model (TRF) includes the traditional risk factors (age, gender, diabetes status, hypertension, tobacco use, serum LDL-cholesterol, HDL-cholesterol adjusted for lipid lowering medication). In model 2 further clinical factors (angina, NYHA, history of PVD, left-ventricular ejection fraction) and biomarker (hsCRP, ntproBNP) were included. Finally, vascular ultrasound measures (CIMT, carotid artery plaque, ABI) were added to model 2, respectively
Calculating the optimal cut-off for the number of carotid artery segments diseased to predict obstructive CAD, Youden’s index reached its maximum at one segment diseased. The strength of NCA testing is the high negative predictive value especially in subjects with atypical clinical presentation reaching negative predictive values of 81 % for obstructive CAD and 87 % for revascularization (detailed data of specificity, sensitivity, positive and negative predictive value in Supplemental Table 3).
Typical angina and noncoronary atherosclerosis—the major signs of stable obstructive CAD
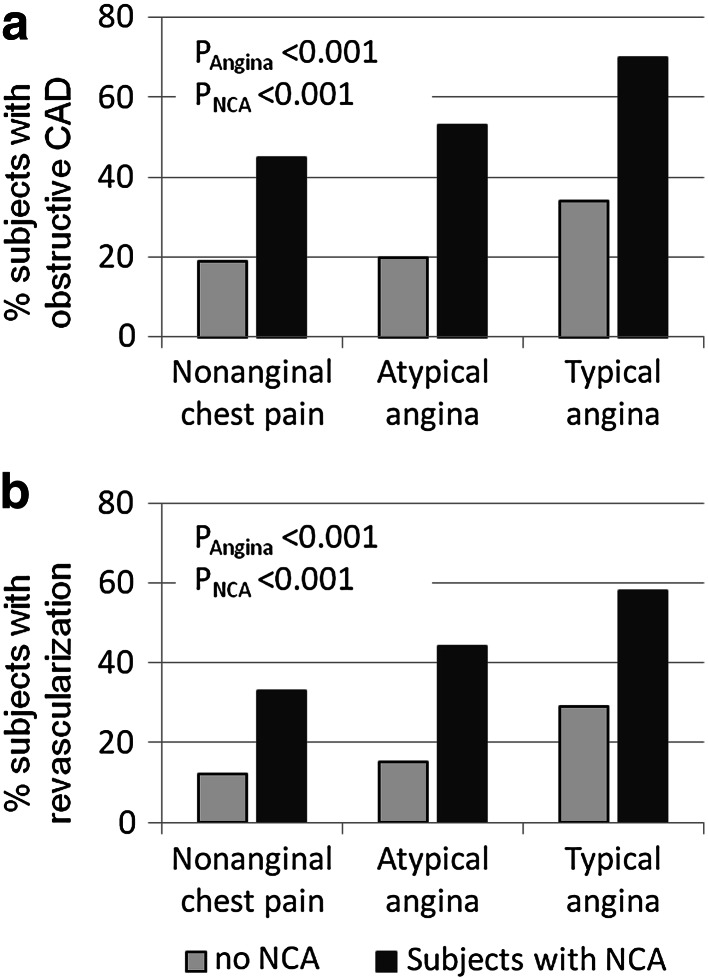
Stepwise logistic regression identified two major predictors for stable obstructive CAD: the presence of noncoronary atherosclerosis (OR 4.0, CI 3.3–4.8) and typical angina (OR 2.4, CI 1.9–3.0). Both were also the strongest predictors for revascularization: NCA OR 3.6 (CI 2.9–4.5) and typical angina OR 2.7 (CI 2.1–3.5). The impact of the assessment of NCA to predict CAD and revascularization was evident independently of age and gender (Supplemental Table 4). The strong predictive value of NCA was demonstrated independently of the clinical presentation (Fig. 3). However, the relevance of noncoronary atherosclerosis is pivotal in subjects with atypical clinical presentation. While subjects with typical angina already have a higher pretest probability for obstructive CAD and revascularization, subjects with atypical clinical presentation demonstrated a lower pretest probability with questionable necessity for invasive coronary angiography. Under this condition, the detection of noncoronary atherosclerosis significantly increased the pretest probability to higher than 40 %, while subjects without detectable noncoronary atherosclerosis were unlikely to have obstructive CAD (<20 %) and were even more unlikely to get coronary revascularization (<15 %, Fig. 3). Atypical clinical presentation and lacking detection of NCA applies to a large group of patients in our cohort (n = 703, 32 %).
Fig. 3.
Percentage of subjects with obstructive CAD (a) and coronary revascularization (b) depending on the absence (lightly colored) and presence (dark colored) of noncoronary atherosclerotic disease (NCA) and the clinical presentation (x-axis, subset of nonanginal chest pain includes subjects without chest pain)
Discussion
The objective of our study was to evaluate the predictive value of noncoronary atherosclerosis assessment for identifying coronary artery disease and the need for revascularization in a cohort of patients with clinical symptoms indicating first-time elective invasive coronary angiography. Our major results are:
A simple ultrasound-based screening for noncoronary atherosclerosis improved the pretest probability of obstructive CAD and the intention to revascularization prior to invasive coronary angiography. Its strength is the high negative predictive value in subjects with atypical clinical presentation identifying a large group of subjects with low probability of obstructive CAD.
Especially ultrasound-based carotid artery plaque assessment was identified as a useful measure of noncoronary atherosclerosis. The presence of carotid artery plaque and its extent were independently associated with the first-time diagnosis of stable obstructive CAD after adjustment for traditional cardiovascular risk factors and further CAD-associated factors such as clinical presentation, known noncoronary vascular disease, reduced left-ventricular ejection fraction and additional biochemical markers.
CIMT and ABI also independently predicted obstructive CAD after adjustment for traditional risk factors but CIMT and ABI failed to add significant diagnostic value to a model including of established risk factors and carotid artery plaque.
The observation that carotid artery plaque is able to predict prevalent CAD has been reported in some small angiographic studies [11–17]. Our much larger study additionally investigated the relationship between carotid ultrasound findings and the intention for coronary revascularization. Furthermore, our analyses were interpreted in the context of important clinical factors including the clinical presentation, cardiac function and biochemical biomarkers which were disregarded in most of the previous studies.
Although typical angina is the major clinical presentation of stable CAD, and, as also demonstrated in our study, one of the strongest predictors of obstructive CAD, only a quarter to a third of subjects intended for elective coronary angiography due to suspected obstructive CAD presents with typical angina. The majority of patients have an atypical clinical presentation resulting in a high chance of negative angiographic results. For these patients, the evidence of noncoronary atherosclerosis is the most important predictor of obstructive CAD. Conversely, in the subset of subjects with atypical clinical presentation and no detectable noncoronary atherosclerosis the probability of obstructive CAD is low (<20 %) and the rate of coronary revascularization is even lower (<15 %). Interestingly, this applies to a relatively large group of patients in our cohort, about one third of patients. When cardiologists and other clinicians consider whether a patient with atypical presentation has significant coronary artery disease, they are less likely to want to determine whether noncoronary atherosclerosis disease is present, unless there is a strong suspicion that the presence of severe disease could determine the patient’s near future prognosis, before deciding on carrying out coronary angiography. However, the clinicians should consider the increased likelihood of CAD in the type of patients included in this study if there is evidence of noncoronary atherosclerosis and the non-invasive tests, particularly for the presence of carotid artery disease, are positive.
The superiority of carotid artery plaque assessment compared to CIMT is explained by its carotid-wide screening area, while CIMT measurement is limited to the far wall of the common part of the carotid arteries. Indeed, atherosclerotic disease occurs predominantly downstream to the region of CIMT measurement namely in the bulb and the proximal parts of the internal/external branches of the carotid artery [28]. We did not consider IMT measurements of other carotid artery regions due to the limited standardization of the measurements and focused on that recommended by the Carotid Intima-Media Thickness Task Force of the American Society of Echocardiography [26]. In line with our results, a recent meta-analysis showed that carotid artery plaque has a higher diagnostic accuracy compared with CIMT for the prediction of future CAD events [9]. Further studies also showed that CIMT added only little predictive value to conventional risk factors [8, 9, 11]. Today there is strong evidence for the superiority of carotid artery plaque assessment for the prediction of incident cardiovascular events, and as here shown also for prevalent CAD, which evokes increasing debate regarding the IMT paradigm [29–31].
A limitation of our study is the mono-centric design which might be biased by local characteristics of allocation and accomplishment. However, both the distribution of clinical presentation and angiographic findings is comparable with the representative large-scale American NCDR CathPCI Registry including hundreds of angiographic centers [3]. Second, we did not quantify carotid artery plaque size in this initial study because our results show that the optimal Youden’s index calculated from our semiquantitative plaque score was already reached when only one carotid artery segment was affected. This indicates that the presence of atherosclerosis per se is of importance. However, the fact that multi-locus carotid artery plaque further increase the risk of CAD points towards the usefulness of a more detailed quantification of carotid atherosclerosis. Several ongoing studies announced the application of three-dimensional carotid ultrasound to improve the prediction of the incident cardiovascular risk [10, 32]. Our carotid ultrasound approach would also allow 3D-morphometric quantification; we aim at improving CAD risk prediction on the basis o these measures in the future.
Conclusion
Our study accentuates the diagnostic value of an assessment of noncoronary atherosclerosis, in particular that of carotid artery plaque, to improve the diagnostic yield of invasive coronary angiography in subjects with suspected coronary artery disease. The strength of carotid artery plaque assessment is pivotal in subjects with atypical clinical presentation, which represents the majority of subjects intended to diagnostic coronary angiography. In view of the low pretest probability within the large group of subjects with atypical clinical presentation and lack of noncoronary atherosclerosis, indication for invasive coronary diagnostic should be reassessed carefully. We conclude that carotid artery plaque detection has the potential to assess prognosis and to direct clinical decision making and subsequent diagnostic procedures in subjects with suspected coronary artery disease.
Source of funding
Initial funding of the Leipzig Heart Study was supported by the Roland-Ernst Foundation; continuation is supported by LIFE-Leipzig Research Center for Civilization Diseases, University Leipzig. LIFE is funded by the Free State of Saxony within the framework of its excellence initiative.
Electronic supplementary material
Acknowledgments
We thank Antonia Christen, Manuela Fritzsche, Kerstin Kothe, Kay Olischer, Katja Ulrich, Annegret Unger for expert technical assistance. Biochemical analyses of the LIFE Heart Study were supported by Roche Diagnostics, Germany.
References
- 1.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr, Smith SC, Jr, Spertus JA, Williams SV, American College of Cardiology F, American Heart Association Task Force on Practice G, American College of P, American Association for Thoracic S, Preventive Cardiovascular Nurses A, Society for Cardiovascular A, Interventions, Society of Thoracic S Accf/aha/acp/aats/pcna/scai/sts guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;2012(60):e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Multimodality Writing Group for Stable Ischemic Heart Disease. Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, Min JK, Patel MR, Rosenbaum L, Shaw LJ, Stainback RF, Allen JM, Technical Panel. Brindis RG, Kramer CM, Shaw LJ, Cerqueira MD, Chen J, Dean LS, Fazel R, Hundley WG, Itchhaporia D, Kligfield P, Lockwood R, Marine JE, McCully RB, Messer JV, O’Gara PT, Shemin RJ, Wann LS, Wong JB, Appropriate Use Criteria Task Force. Patel MR, Kramer CM, Bailey SR, Brown AS, Doherty JU, Douglas PS, Hendel RC, Lindsay BD, Min JK, Shaw LJ, Stainback RF, Wann LS, Wolk MJ, Allen JM. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society Of Nuclear Cardiology, Heart Failure Society of America, heart rhythm society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63(4):380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Criqui MH, Denenberg JO. The generalized nature of atherosclerosis: how peripheral arterial disease may predict adverse events from coronary artery disease. Vasc Med. 1998;3:241–245. doi: 10.1177/1358836X9800300311. [DOI] [PubMed] [Google Scholar]
- 5.Bots ML, Sutton-Tyrrell K. Lessons from the past and promises for the future for carotid intima-media thickness. J Am Coll Cardiol. 2012;60:1599–1604. doi: 10.1016/j.jacc.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 6.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality: a meta-analysis. JAMA J Am Med Assoc. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 8.Simon A, Chironi G, Levenson J. Comparative performance of subclinical atherosclerosis tests in predicting coronary heart disease in asymptomatic individuals. Eur Heart J. 2007;28:2967–2971. doi: 10.1093/eurheartj/ehm487. [DOI] [PubMed] [Google Scholar]
- 9.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Sillesen H, Muntendam P, Adourian A, Entrekin R, Garcia M, Falk E, Fuster V. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the high risk plaque bioimage study. JACC Cardiovasc Imaging. 2012;5:681–689. doi: 10.1016/j.jcmg.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Brook RD, Bard RL, Patel S, Rubenfire M, Clarke NS, Kazerooni EA, Wakefield TW, Henke PK, Eagle KA. A negative carotid plaque area test is superior to other noninvasive atherosclerosis studies for reducing the likelihood of having underlying significant coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:656–662. doi: 10.1161/01.ATV.0000200079.18690.60. [DOI] [PubMed] [Google Scholar]
- 12.Kallikazaros I, Tsioufis C, Sideris S, Stefanadis C, Toutouzas P. Carotid artery disease as a marker for the presence of severe coronary artery disease in patients evaluated for chest pain. Stroke J Cereb Circ. 1999;30:1002–1007. doi: 10.1161/01.STR.30.5.1002. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi M, Kitagawa K, Nagai Y, Yamagami H, Kondo K, Matsushita K, Oku N, Hougaku H, Ohtsuki T, Masuyama T, Matsumoto M, Hori M. Equivalence of plaque score and intima-media thickness of carotid ultrasonography for predicting severe coronary artery lesion. Ultrasound Med Biol. 2003;29:367–371. doi: 10.1016/S0301-5629(02)00743-3. [DOI] [PubMed] [Google Scholar]
- 14.Akosah KO, McHugh VL, Barnhart SI, Schaper AM, Mathiason MA, Perlock PA, Haider TA. Carotid ultrasound for risk clarification in young to middle-aged adults undergoing elective coronary angiography. Am J Hypertens. 2006;19:1256–1261. doi: 10.1016/j.amjhyper.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Morito N, Inoue Y, Urata M, Yahiro E, Kodama S, Fukuda N, Saito N, Tsuchiya Y, Mihara H, Yamanouchi Y, Saku K, Urata H. Increased carotid artery plaque score is an independent predictor of the presence and severity of coronary artery disease. J Cardiol. 2008;51:25–32. doi: 10.1016/j.jjcc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda N, Kogame N, Iijima R, Nakamura M, Sugi K. Carotid artery intima-media thickness and plaque score can predict the syntax score. Eur Heart J. 2012;33:113–119. doi: 10.1093/eurheartj/ehr399. [DOI] [PubMed] [Google Scholar]
- 17.Cohen GI, Aboufakher R, Bess R, Frank J, Othman M, Doan D, Mesiha N, Rosman HS, Szpunar S. Relationship between carotid disease on ultrasound and coronary disease on ct angiography. JACC Cardiovasc Imaging. 2013;6:1160–1167. doi: 10.1016/j.jcmg.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Society of Atherosclerosis Imaging and Prevention Developed in collaboration with the International Atherosclerosis Society Appropriate use criteria for carotid intima media thickness testing. Atherosclerosis. 2011;214:43–46. doi: 10.1016/j.atherosclerosis.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 19.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D, American Heart Association Council on Peripheral Vascular D, Council on E, Prevention, Council on Clinical C, Council on Cardiovascular N, Council on Cardiovascular R, Intervention, Council on Cardiovascular S, Anesthesia Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 20.Beutner F, Teupser D, Gielen S, Holdt LM, Scholz M, Boudriot E, Schuler G, Thiery J. Rationale and design of the leipzig (life) heart study: phenotyping and cardiovascular characteristics of patients with coronary artery disease. PLoS One. 2011;6:e29070. doi: 10.1371/journal.pone.0029070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose G, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med. 1977;31:42–48. doi: 10.1136/jech.31.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinning C, Keller T, Zeller T, Ojeda F, Schluter M, Schnabel R, Lubos E, Bickel C, Lackner KJ, Diemert P, Munzel T, Blankenberg S, Wild PS, Gutenberg Health S Association of high-sensitivity assayed troponin i with cardiovascular phenotypes in the general population: the population-based Gutenberg health study. Clinical Res Cardiol Off J Ger Cardiac Soc. 2014;103:211–222. doi: 10.1007/s00392-013-0640-8. [DOI] [PubMed] [Google Scholar]
- 23.Sinning C, Kieback A, Wild PS, Schnabel RB, Ojeda F, Appelbaum S, Zeller T, Lubos E, Schwedhelm E, Lackner KJ, Debus ES, Munzel T, Blankenberg S, Espinola-Klein C. Association of multiple biomarkers and classical risk factors with early carotid atherosclerosis: results from the Gutenberg Health Study. Clin Res Cardiol Off J Ger Cardiac Soc. 2014;103:477–485. doi: 10.1007/s00392-014-0674-6. [DOI] [PubMed] [Google Scholar]
- 24.Leistner DM, Klotsche J, Pieper L, Palm S, Stalla GK, Lehnert H, Silber S, Marz W, Wittchen HU, Zeiher AM. Prognostic value of nt-pro-bnp and hs-crp for risk stratification in primary care: results from the population-based DETECT Study. Clin Res Cardiol Off J Ger Cardiac Soc. 2013;102:259–268. doi: 10.1007/s00392-012-0530-5. [DOI] [PubMed] [Google Scholar]
- 25.Kara K, Mahabadi AA, Geisel MH, Lehmann N, Kalsch H, Bauer M, Neumann T, Dragano N, Moebus S, Mohlenkamp S, Jockel KH, Erbel R. B-type natriuretic peptide: distribution in the general population and the association with major cardiovascular and coronary events—the Heinz Nixdorf Recall Study. Clin Res Cardiol Off J Ger Cardiac Soc. 2014;103:125–132. doi: 10.1007/s00392-013-0628-4. [DOI] [PubMed] [Google Scholar]
- 26.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS, American Society of Echocardiography Carotid Intima-Media Thickness Task F Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the american society of echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Beutner F, Teren A, Gielen S, Schuler G, Wirkner K, Tiller D, Loeffler M, Scholz M. Automated photoplethysmography-based determination of ankle-brachial index: a validation study against doppler sonography. Clin Res Cardiol Off J Ger Cardiac Soc. 2012;101:875–883. doi: 10.1007/s00392-012-0471-z. [DOI] [PubMed] [Google Scholar]
- 28.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–181. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 29.Spence JD. Ultrasound measurement of carotid plaque as a surrogate outcome for coronary artery disease. Am J Cardiol. 2002;89:10B–15B. doi: 10.1016/S0002-9149(01)02327-X. [DOI] [PubMed] [Google Scholar]
- 30.Simon A, Chironi G. The relationship between carotid intima-media thickness and coronary atherosclerosis revisited. Eur Heart J. 2007;28:2049–2050. doi: 10.1093/eurheartj/ehm320. [DOI] [PubMed] [Google Scholar]
- 31.Spence JD (2012) Carotid plaque measurement is superior to imt invited editorial comment on: Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis-Yoichi Inaba, M.D., Jennifer A. Chen M.D., Steven R. Bergmann M.D., Ph.D. Atherosclerosis 220:34–35 [DOI] [PubMed]
- 32.Fernandez-Ortiz A, Jimenez-Borreguero LJ, Penalvo JL, Ordovas JM, Mocoroa A, Fernandez-Friera L, Laclaustra M, Garcia L, Molina J, Mendiguren JM, Lopez-Melgar B, de Vega VM, Alonso-Farto JC, Guallar E, Sillesen H, Rudd JH, Fayad ZA, Ibanez B, Sanz G, Fuster V. The progression and early detection of subclinical atherosclerosis (pesa) study: rationale and design. Am Heart J. 2013;166:990–998. doi: 10.1016/j.ahj.2013.08.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.